Abstract
Lycaenidae is one of the larger of the world’s butterfly families, based on number and diversity of species, but knowledge of roosting in this group is sparse. Zizina otis riukuensis and Zizeeria maha okinawana are two small lycaenids that are commonly found in urban settings and widely distributed across much of Asia. We conducted experiments on a university campus to determine the plant species and plant structures commonly used by these two blues when roosting. We also tested the hypothesis that gregarious roosting exists in these two blues by demonstrating the non-random distribution of roosting blues and the tight mapping of their roosts to the spatial distribution of specific plant species and/or specific plant structures, as well as by demonstrating behavioral interactions among individuals during roosting-assembly. We found that both Z. otis and Z. maha roosted primarily on flowers and fruits of Tridax procumbens and Vernonia cinerea. We also found that these blues formed conspicuous roosting aggregations with significant positive associations between the flowers and fruits of both T. procumbens and V. cinerea and the blues. Moreover, our behavioral observations showed that these blues expressed various levels of interaction during roosting gatherings. Based on these findings, we conclude that gregarious roosting exists in both Z. otis and Z. maha. To our knowledge, this paper represents one of the first demonstration of nocturnal gregarious roosting in lycaenids. This study also highlights the importance of institutional estates in providing roosting resources for butterflies in urban ecosystems.
Keywords: Nocturnal roosting, Zizina otis riukuensis, Zizeeria maha, Roosting aggregation, Lycaenidae, Institutional estates, Conservation biology
BACKGROUND
Over the past few decades, the conservation of biodiversity has become an important issue at both local and global scales, with losses in biodiversity continuing unabated despite international efforts, such as the “Aichi Targets” of the Convention on Biological Diversity, to reduce declines in biodiversity (Butchart et al. 2010; Tittensor et al. 2014). Loss of biodiversity in tropical ecosystems is particularly concerning, since species diversity and abundance are particularly high in these ecosystems, as are the social and economic pressures impacting species abundance and diversity (Collen et al. 2008). For example, butterfly species of South East Asia are under particular threat due to extensive deforestation and the rapid rate at which it is proceeding (Koh 2007). Habitat degradation or loss through agricultural conversion of land and urbanization are the main factors that drive species to extinction. In Taiwan, the location of this study, much of the original broad-leaf forest below 500 m elevation has been converted into either urban or agricultural land (Li et al. 2013).
However, urban or agricultural habitats are not devoid of biodiversity, and are affected by the same factors affecting ecosystems elsewhere such as climate, substrate, resident organisms and topography (Pickett et al. 2011). While there has been much focus on how deforestation causes loss of species, much less is known about how species persist in transformed landscapes (Jain et al. 2017). A better understanding of their behavior and natural history is basic to understanding how certain species persist in agricultural and especially urban landscapes (also see Wang and Hung 2019).
When considering critical butterfly habitats, much attention is paid to larval host plants and habitats that provide food and other resources needed for breeding (Gilbert and Singer 1975). However, other critical habitats are also important to the survival of healthy adult butterfly populations, such as those needed for roosting and mating (Dennis 2004), or providing protein-rich food (i.e., pollen) for adults (Gilbert 1972; Mallet 1986). Adult butterflies are typically active during daylight hours, when ambient conditions are suitable for flight, but are inactive and roost during evening hours. Nocturnal roosting in butterflies varies widely in characteristic roosting locations and behaviors (Table 1). Individuals of most species roost alone, solitarily remaining and sleeping at night in the location where they find themselves in the late afternoon. However, some butterfly species form roosting aggregations in which individuals gather at a specific location to pass the night (Davis et al. 2012; Mallet 1986; Young and Thomason 1975). Roosting in groups is predominantly found in unpalatable species of the subfamilies Acraeinae, Danainae, Heliconiinae and Ithomiinae (Benson and Emmel 1973; Finkbeiner 2014; Finkbeiner et al. 2012; Howard and Davis 2009; Mallet 1986; Mallet and Gilbert 1995; Owen and Chanter 1969; Salcedo 2011 2010a b; Turner 1975; Urquhart and Urquhart 1979) and some palatable species of the Nymphalini (Barrett and Burns 1951) and Hesperiidae (DeVries et al. 1987) butterflies. Although there are many examples of roosting in groups from a variety of butterfly groups, reports of roosting in groups from the diverse family Lycaenidae (the “blues” or “hairstreaks”) are mainly descriptive (e.g., Heath and Emmet 1985; Thomas 1983) and no detailed case study on roosting in groups has been done in any of these species as far as we are aware.
Table 1.
Typical nocturnal roosting sites of different butterfly species
| Species | Roosting substrate | Sources |
| Hesperiidae | ||
| Celaenorrhinus fritzgaertneri | in a small cave | (DeVries et al. 1987) |
| Pyrgus malvae | at the top of dead flower-heads | (Hoskins 2018) |
| Erynnis tages | at the top of dead flower-heads | (Hoskins 2018) |
| Nymphalidae | ||
| Clossiana euphrosyne | on bracken fronds or on the flowers of rushes | (Hoskins 2018) |
| Coenonympha pamphilus | in a head-downwards posture at the top of grass heads | (Hoskins 2018) |
| Danaus plexippus | gregariously on various types of trees or shrubs (with a general preference for maples and conifers, pecans and oaks) | (Davis et al. 2012) |
| Heliconius charitonia | gregariously on leafless twigs of Anguria trees | (Waller and Gilbert 1982) |
| Heliconius erato | gregariously on leafless fine twigs or tendrils of dead vines | (Finkbeiner et al. 2012; Mallet 1986) |
| Heliconius ethilla | gregariously on leafless twigs | (Turner 1975) |
| Heliconius sara | shaded areas with plenty of thin dry vines and branches under relatively dense vegetation mats | (Salcedo 2010b) |
| Lasiommata megera | under leaves, under the lower boughs of trees or crevices in banks and walls, on fences | (Dennis 1986) |
| Manataria maculata | in shaded embankments, tree holes, and other dark hiding places | (Hanson 2000) |
| Maniola jurtina | in a head-downwards posture at the top of grass heads | (Hoskins 2018) |
| Marpesia berania | on the underside of the leaves of rubiaceous trees or other small trees | (Benson and Emmel 1973) |
| Melanargia galathea | in a head-downwards posture at the top of grass heads | (Hoskins 2018) |
| Smyrna karwinskii | gregariously in cavities of lava walls, tree trunks and on the underside of concrete slabs roofing alleys between sheds | (Muyshondt and Muyshondt 1974) |
| Papilionidae | ||
| Papilio polyxenes asterius | primarily on inflorescences, or apices, but also on stems, scapes or culms of daisies, other herbs and grasses, roost singly | (Rawlins and Lederhouse 1978) |
| Pieridae | ||
| Colias eurytherne | singly or gregariously in dense grass, in dense crown vetch, on the leaves of emergent forbs | (Clench 1970) |
| Phoobis sennae eubule | gregariously on the yellow-green leaves of vines | (Clench 1970) |
| Pieris rapae | gregariously on leaves or stems of the upper branches of Pittosporurn undulatum Vent. Var. val’iegatum | (McFarland 1971) |
| Lycaenidae | ||
| Lycaenidae icarus | on the flower-heads and stems of grasses and other plants | (Frohawk 1914) |
| Lysandra bellargus | in small groups on tall vegetation | (Thomas 1983) |
| Plebejus argus | on shrubs (bramble, gorse) and tall herbs (rank bunched grasses such as Dactylis glomerata, bracken and flowering herbs) | (Dennis 2004) |
| Pseudophilotes sinaicus | on the tips of dead stalks/dry flower heads of Jasonia montana | (James 2006) |
| Zizina otis | on low vegetation or on bushes | (Hoskins 2018) |
Moreover, roosting substrates may vary from site to site and species to species. Many species roost under leaves, some on tree trunks and some in low, dense vegetation (e.g., grasses, rushes, bushes). A few tropical species roost in caves or under cliff overhangs (Benson and Emmel 1973; Davis et al. 2012; Dennis 2004 1986; DeVries et al. 1987; Hoskins 2018; James 2006; Opler and Malikul 1992; Rawlins and Lederhouse 1978; Young and Thomason 1975). Lycaenidae is one of the larger families of the world’s butterfly species, based on the numbers and diversity (> 6000 species, 30% of all butterfly species) (Pierce et al. 2002; Zhang et al. 2019). Considering the great diversity of lycaenids and the wide range of habitats in which they occur, knowledge of roosting in this group is sparse (Table 1).
This study focuses on investigating the roosting behavior and habitats of the lesser grass blue, Zizina otis riukuensis (Matsumura, 1929), and the Japaneses pale grass blue, Zizeeria maha okinawana (Matsumura, 1929), by observating wild populations on a university campus. These two small butterflies belong to one of the largest subfamilies in Lycaenidae, the Polyommatinae (Eliot 1973). They are commonly found in urban settings and are widely distributed across much of Asia, preferring cultivated areas, abandoned lots, urban parks and gardens (Chen 2015; Chowdhury et al. 2017; Harinath et al. 2015; Lu and Chen 2014; Nidup 2016; Otaki et al. 2010; Sing et al. 2016; Tsang and Bonebrake 2017; Venkata Ramana et al. 2014; Yago et al. 2008). Given their urban setting, the main threats to these two blues is the spraying of pesticides and the physical clearance of weeds and grasses in their preferred habitats (Sing et al. 2016).
The adults of these two species are colony-forming, and swarm in great abundance in mostly open grassy habitats such as forest clearings, weedy lots, riverbanks, roadsides, parks and gardens. They are extremely active and flutter very close to the ground. They feed avidly on the nectar of daisies and other low-growing flowers, including Boerhavia, Medicago, Tridax, Trifolium and Vernonia (Hoskins 2018; Li 2007; Yuan Mou Chang, personal observation). In diffuse sunlight they will bask on the ground with their wings half open (Hoskins 2018). Around dusk, both blues settle into roosts in the vegetation, solitarily or in groups (Yuan Mou Chang, personal observation). Despite the fact that the adults of these two blues are common and easy to find, their roosting locations remain largely unreported, the one exception being a report showing that adult Z. otis roosts overnight on low vegetation or on bushes (Hoskins 2018).
Our study had two goals. The first was to determine the roosting sites of these two blues, including which plant species they use and where on these plants (flower, fruit, leaf, receptacle and stem/peduncle) these butterflies roost. We predicted that these two blues roost primarily on specific plant species and plant structures (prediction 1). The second was to test the hypothesis that gregarious roosting exists in these two blues. Two additional predictions resulted from our hypothesis that Z. otis and Z. maha roost gregariously: that the roosting individuals are distributed non-randomly and match the spatial distribution of specific plant species and/or specific plant structures within roosting aggregations (prediction 2), and since evidence of behavioral interactions among individuals in close proximity is evidence of gregarious roosting (Finkbeiner 2014 2019; Mallet 1986; Salcedo 2011), that behavioral interactions occur among individuals of these species during roost-assembly (prediction 3).
MATERIALS AND METHODS
Study site and a brief description of the experimental design
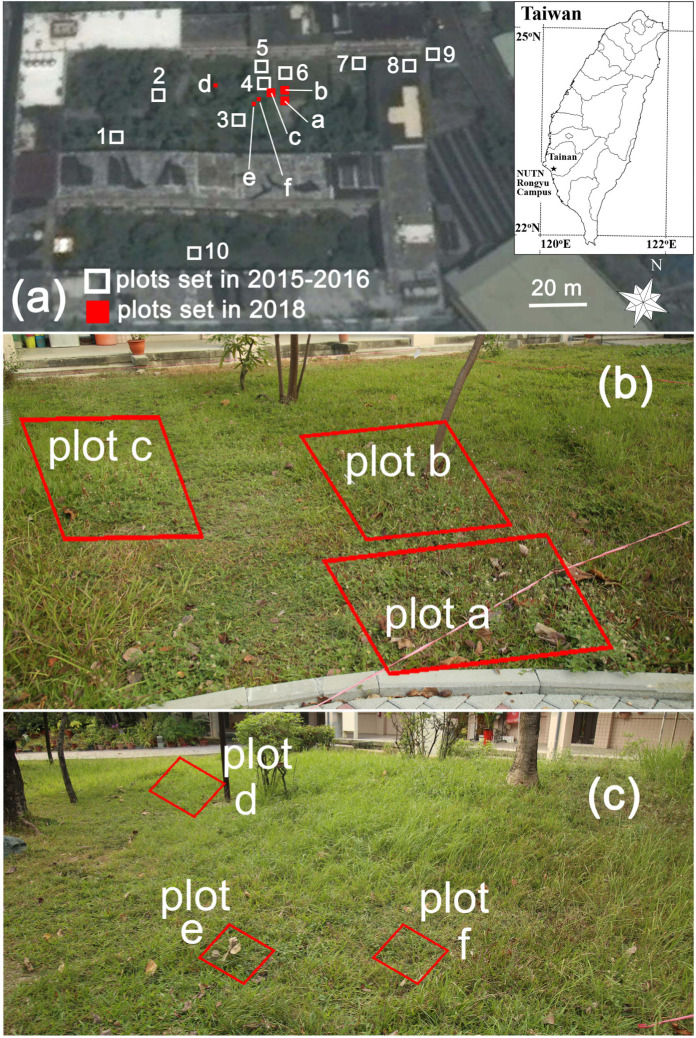
The study was conducted in a garden of the Rongyu campus of the National University of Tainan (about 2 hectares; 22°58'N, 120°13'E. About 24 m a.s.l., Tainan, Taiwan) (Fig. 1). The garden was surrounded by the concrete buildings and included lawn, groves, perennial flowering plants, herbs, shrubs and large trees. We chose the campus garden as the study site because the campus garden provides a typical habitat (grassy lawn) that these lycaenids like to use. The study site experiences a humid tropical savanna climate (Köppen climate classification) with hot summers (Temperature range: 26–33°C) and dry cool winters (Temperature range: 14–24°C) (1981–2010 records, Tainan city government, http://www.tainan.gov.tw/tainanE/).
Fig. 1.
(a) Map of Rongyu Campus, National University of Tainan. White squares indicate the plots for the night roosting surveys of butterflies from November 2015 to March 2016 (n = 10). Red squares (plots a–f) are subsites used for the random patterns test, confirming the non-random distribution of the roosting blues within roosting aggregations, as well as for observation of the social and behavioral interactions among individuals during roost-assembly. (b) A closer look of plots a, b, and c. (c) A closer look of plots d, e, and f.
The study was done in two stages. The first stage gathered data to determine the roosting sites of these two blues and address prediction 1, focusing on investigating the plant species and plant structures for roosting and took place in November 2015–March 2016. The second stage tested the hypothesis that gregarious roosting occurs in these two blues, and this stage was carried out in November–December 2018. Following prediction 2, we sought to demonstrate gregarious roosting by showing a correlation between the spatial distribution of specific plant species and that of roosting blues (i.e., a non-random distribution) in roosting aggregations. Following prediction 3, we focused on the observation of behavioral interactions among individuals during roosting-assembly.
Stage 1
Determination of plant species and structures of plants where roosting occurs (prediction 1)
Based on our preliminary observations of the behavior and locations of these butterflies, we set up ten plots (each 3 × 3 m2 in area, delineated with red plastic strings) to record the plant species and plant structures on which roosting occurs. This part of the study was conducted from late fall to early spring (November 24th, 2015 to March 31st, 2016). During this period, the nearest weather station (Tainan, 2.3 km north-west of Rongyu campus) reported a mean daily air temperature of 20°C, with mean monthly temperatures ranging from 17.3 (January 2016) to 25.1°C (November 2015) (Central Weather Bureau, http://www.cwb.gov.tw/). The overall rainfall during the study period was 556.7 mm.
We surveyed the above plots for four to five days each week during the study period. Each plot was surveyed systematically and carefully using a head lamp, beginning approximately 1 h after sunset time of 17:30–18:00, and lasting for 2–4 hours. Surveys were not conducted on days with heavy rain. Once a butterfly was located, we identified it to the species level, recorded the time, the species of plant it was on, the structure of the plant upon which the butterfly roosted, and the height above ground level of the head of the butterfly. Plant structure was recorded according to the following five categories: flower (i.e., the head inflorescence of the daisies Tridax procumbens, Vernonia cinerea, Emilia sonchifolia, Youngia japonica, Ixeris chinensis, Ageratum conyzoides; the globose heads of Mimosa pudica; the spike inflorescence of Kyllinga brevifolia and Kyllinga nemoralis; the raceme inflorescence of Axonopus compressus and Digitaria; the panicle inflorescence of Sporobolus indicus and Eragrostis amabilis; the umbel inflorescence of Hedyotis corymbosa and Fimbristylis dichotoma), fruit (the cypselas of the daisies, including ripe fruits with winged achenes ready for wind-dispersal and residual fruits after achene dispersal), leaf, receptacle (the part after the residual fruit has left), and stem/peduncle. The observations were made with the aid of a magnifying glass with LED lights, and photos were taken using a digital camera when the individuals were difficult to identify. The identities and scientific names of the butterflies are in accordance with Lu and Chen (2014). Plants were photographed and/or collected and identified in consultation with Prof. Tsung-Hsin Hsieh, Department of Ecology and Environmental Resources, National University of Tainan. The scientific names of the plants follow the Flora of Taiwan (Huang 2000). Toward the end of the experiment, we took aerial photographs and recorded the vegetation composition of each plot. In each plot, plant species were identified and the percentage of ground they covered estimated. The lawn was not mowed during the study period to allow the grasses to grow and flower.
Stage 2
1. Non-random spatial distribution of roosting blues and spatial distribution of associated plant species (prediction 2)
To understand whether the spatial distributions of roosting blues are significantly associated with the spatial distribution of the flowers and fruits of T. procumbens and V. cinerea, we examined the spatial relationship between the roosting blues and the flowers and fruits of T. procumbens and V. cinerea on six plots (plots a–f; Fig. 1, Fig. 3) from November 14th to 16th, 2018, using a Canon EOS 5D Mark III with an EF 24–105 mm f/4L IS II USM Lens (Canon Inc. Tokyo, Japan) (Fig. 1).
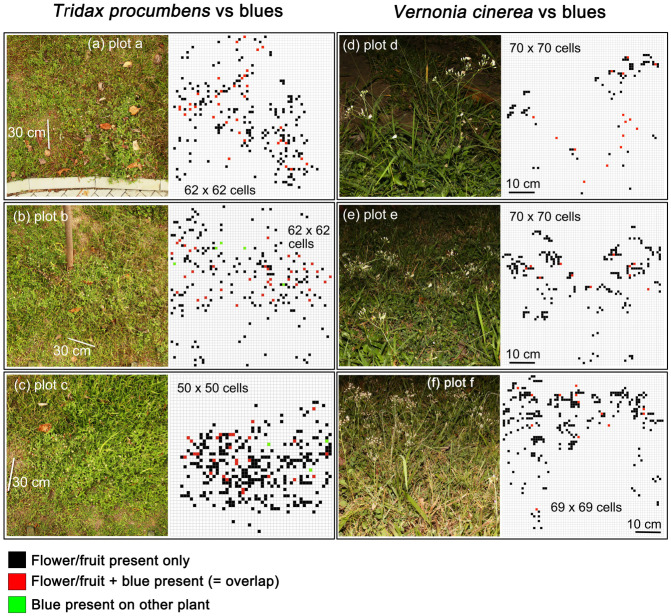
We started to search for roosting aggregations on the evening of November 3, 2018 and found aggregations in the garden. Based on the numbers of roosting blues in these aggregations, we selected the six aggregations with the most individuals (Fig. 1, plots a– f) to test the hypothesis that gregarious roosting exists in these two blues. Of these six aggregations, three (plots a–c) contained many flowers and fruits of T. procumbens, and blues were observed to roost on them. Plots d to f had many flowers and fruits of V. cinerea, and blues were observed roosting on them as well. We continued to observe roosting aggregations on these plots until November 13 to confirm that aggregations occurred each night at these plots during this period. We sampled the spatial patterns of T. procumbens and blues on the evening (about 9–10 pm) of November 14th, 2018 for plots a, b and c (Fig. 3a, 3b, 3c). Because these three plots were relatively large (about 1.5 m × 1.5 m per plot) compared to the size of the blues themselves (< 1 cm), it was difficult to identify each blue when viewing a digital photo of the entire plot on the computer, due to lack of resolution. We thus developed a “thread marking method” to make roosting sites stand out in digital photos. By marking the roosting locations of each blue within a plot with colored threads, we were able to analyze the spatial patterns of T. procumbens and blues. This approach proved to be suitable and can be used to clearly mark the roosting locations.
To accurately mark roosting locations used by blues, we first took a series of photos of each plot. These photos were taken from a squatting position, but from a variety of different angles to determine all of the locations the blues were roosting. This step was important because the act of attaching threads to plants where the butterflies were roosting typically caused the blues to fly away. Next, based on this series of photos, we tied a piece of thread to each structure on which blues had roosted. Because it was difficult to tie a thread on a flower and a fruit, we tied a thread on the “peduncle” right under a flower or a fruit where roosting occurred. We used red cotton threads for T. procumbens and yellow plastic threads for other plants where blues had roosted. On the morning (about 7–8 am) of November 15th, 2018, we took a photo of the roosting locations as indicated by the threads from the top of a ladder above each of the three plots (Fig. 3a, 3b and 3c) and used the locations of the two different color threads in a random patterns test (Roxburgh and Chesson 1998).
For V. cinerea and blues, we did not use the “thread marking method,” but directly took photos from the side of plants and blues to sample the spatial patterns of V. cinerea and blues in the evening of November 16th, 2018. Veronia cinerea is an erect herb, and blues typically roost at different heights on the flowers/fruits that are situated on the top of the slender, grooved and ribbed stems (Figs. 2b, 3d, 3e and 3f). These plots were smaller (about 0.5 m × 0.5 m per plot) than those mentioned above, so that the entire plot fit within the photo and the photo could be taken at a distance that made it is easy to identify each blue’s location when viewing a digital photo of the entire plot on the computer. The spatial patterns of plants and blues in photos were subdivided into grid cells (Fig. 3) using Pages (Pages Version 7.2, Apple Inc, CA) for the random pattern test (see below) (Roxburgh and Chesson 1998).
Fig. 2.
(a) Zizina otis and Zizeeria maha roosting gregariously on Tridax procumbens. (b) Zizina otis and Z. maha roosting gregariously on Vernonia cinerea. (c) A Z. otis roosting on a flower of T. procumbens. (d) A Z. otis roosting on a flower of V. cinerea. (e) A Z. maha roosting on a flower of T. procumbens. (f) A Z. otis roosting on the fruits of T. procumbens. (g) A Z. otis roosting on a fruit of V. cinerea (left) and a Z. otis roosting on the receptacle of T. procumbens (right). (h) A Z. otis roosting on a leaf of V. cinerea. (i) Two Z. otis roosting on a leaf of Mimosa pudica. (j) Two Z. otis roosting on a leaf of Imperata cylindrica. (k) A Z. otis roosting on a peduncle of T. procumbens. The red dots in (a) and (b) are the roosting blues.
Fig. 3.
The observed spatial patterns and the grid of the flowers and fruits of Tridax procumbens and blues at three subsites (a, b, c), and the spatial patterns and the grid processed of flowers and fruits of Vernonia cinerea and blues at three subsites (d, e, f).
2. Behavioral traits during roost-assembly
Categorization of behavioral traits of blues during roost-assembly followed Mallet (1986) and Salcedo (2011). We identified four distinct behavioral traits that are consistently exhibited by flying individuals during roost-assembly, including (1) brief approach: direct flight to a roosted individual without physical contact, (2) hovering (or fanning): hovering above a roosted individual without physical contact, (3) stopping on the same perch: briefly stopping close to a roosting individual on the same perch (flower/fruit/leave) without approaching or touching the individual, and (4) clutching: brief physical contacts between an approaching individual’s claws and the wings of a roosted individual. We recorded both approach and hovering behaviors only if they occurred within 15 cm of the roosted individual, and we recorded whether the approaching or hovering butterfly performed the behavior once or more than once. We also recorded the responses of the recipients involved: (1) no reaction, (2) movement or rotation, (3) fending off without dislodgement by vigorously flapping wings flapping without letting go of the perch, and (4) fending off and leaving: vigorously flapping the wings and then letting go of the perch. We also recorded the response of the approaching butterfly to the occurrence of fending off and taking off by the roosted individual: (1) both the approaching individual and the roosted individual leaving the perch at the same time, (2) the approaching individual usurping the perch but then flying off again to find another perch, and (3) the approaching individual usurping the perch without leaving.
Behavioral interactions were observed and recorded with the aid of binoculars during the roost-assembly in plots a-f in the afternoon hours (1510–1640) of November 28th [observation period (OP) = 34 min], 29th (OP = 49 min), and 30th (OP = 30 min), and Dec. 1st(OP=39min),5th(OP=71min),and7th(OP= 70 min), 2018. These blues began to congregate in the roost sites about one to two hours before sunset (Yuan-Mou Chang, personal observation). During this period, the blues exhibited a set of roost-assembly behavioral interactions until they were all roosted gregariously (Salcedo 2011; Yuan Mou Chang, personal observation). We observed and recorded the interactions of butterflies from a distance of about 1.5 to 2 m from the roost sites. When an interaction was detected, the observers recorded the interaction according to the above categories. We were not able to record the individual butterflies to the species level because these two species are too small and similar to distinguish with naked eyes or binoculars from these distances.
Statistics
To test the prediction that these blues roost primarily on specific plant species and structures (prediction 1), we compiled the data from the 10 plots between November 2015 and March 2016 and used a chi-square test to compare the numbers of butterflies on each of the plant species and structures. Based on the results of standard contingency table chi-square test, which assumes randomness of blues from each plant species and structures, we inferred whether there is a preference for roosting site in terms of plant species and structures (Askew 1982; Ludwig and Reynolds 1988; Rouquette and Thompson 2007). The data used for the chi-square test may have the issue of pseudoreplication if the same butterfly individuals may have been counted multiple times at their roosting sites on various evenings. However, since these blues are too small to be marked, this so far is the best statistical analysis for understanding the answers of prediction 1. We also calculated the average roosting height according to the plant structure upon which the butterfly roosted.
We used a random patterns test to test prediction 2, that roosting blues and the flowers and fruits of T. procumbens and V. cinerea will be spatially correlated, given the assumption that the butterflies exhibit patchiness in their distribution and using data collected in 2018 (Roxburgh and Chesson 1998). When organisms are patchily distributed (i.e., distributed non-randomly in space), the existence of clumped patterns (the exhibition of positive spatial autocorrelation) violates the within-species spatial randomness as well as the independence between species assumptions of traditional statistical tests for detecting interspecific associations, resulting in an elevated Type I error rate, i.e., an increase in the risk of concluding a test is statistically significant and therefore that species are associated, even when the species actually are not associated (Dale et al. 1991; Legendre 1993; Tavaré and Altham 1983). The random pattern test was developed to detect interspecific associations in which within-species patchiness is retained (Roxburgh and Chesson 1998).
We compiled the number of observations of each behavioral trait collected between November 28 and December 7, 2018 to address the prediction that these blues exhibit behavioral interactions among individuals during roost-assembly (prediction 3).
RESULTS
Determination of plant species and structures of plants where roosting occurs (Prediction 1)
A total of 1257 observations of Zizina otis and 266 observations of Z. maha were recorded. Z. otis and Z. maha roosted on 16 and 10 plant species, respectively, out of the 17 plant species present (Table 2). However, the main plant species on which these two blues roosted were T. procumbens and V. cinerea (Z. otis, χ2 = 13276.1, d.f. = 2, P < 0.001; Z. maha, χ2 = 2203.4, d.f. = 2, P < 0.001). The total percentage covered by these plants in all ten plots was only 5.9% and 1.7%, respectively. For Z. otis, 45% of individuals (n = 570) and 37% of individuals (n = 459) roosted on T. procumbens and V. cinerea, respectively. For Z. maha, 54% of individuals (n = 145) and 29% of individuals (n = 76) roosted on T. procumbens and V. cinerea, respectively (Table 2).
The main plant structures used by both Z. otis and Z. maha for roosting were flowers and fruits (Fig. 2c, 2d, 2e, 2f), although receptacles (Fig. 2g), leaves (Fig. 2h, 2i, 2j), and peduncles (Fig. 2k) were also used (Z. otis, χ2 = 1916.6, d.f. = 4, P < 0.001; Z. maha, χ2 = 227.6, d.f. = 4, P < 0.001) (Table 3). The average roosting height above ground level for Z. otis and Z. maha was 20–28 cm, ranging from 8.9 cm to 60 cm (Table 3).
Table 2.
The percentage cover of plant species and the number of Zizina otis and Zizeeria maha roosting on these species
| Percentage cover of plant species (%) | No. of roosting Zizina otis (with percentage shown, %) | No. of roosting Zizeeria maha (with percentage shown, %) | |
| Tridax procumbens | 5.9 | 570 (45.3) | 145 (54.4%) |
| Vernonia cinerea | 1.7 | 459 (36.5) | 76 (28.6%) |
| Other plant species | 92.4 | 228 (18.1) | 45 (16.9%) |
| Emilia sonchifolia | 3.5 | 59 (4.7) | 25 (9.4) |
| Mimosa pudica | 6.4 | 61 (4.9) | 2 (0.8) |
| Fimbristylis dichotoma | 0.3 | 29 (2.3) | - |
| Kyllinga brevifolia | 14.4 | 22 (1.8) | - |
| Youngia japonica | 4.7 | 21 (1.7) | 5 (1.9) |
| Axonopus compressus | 27.2 | 8 (0.6) | - |
| Digitaria | 7.7 | 7 (0.6) | 1 (0.4) |
| Ixeris chinensis | 1.4 | 7 (0.6) | 6 (2.3) |
| Imperata cylindrica | 6.8 | 6 (0.5) | 1 (0.4) |
| Eragrostis amabilis | 11.6 | 3 (0.2) | 3 (1.1) |
| Sporobolus indicus | 3.9 | 2 (0.2) | - |
| Kyllinga nemoralis | 3.4 | 1 (0.1) | 2 (0.8) |
| Ageratum conyzoides | 0.2 | 1 (0.1) | - |
| Hedyotis corymbosa | 0.1 | 1 (0.1) | - |
| Conyza bonariensis | 0.8 | - | - |
Table 3.
Plant structures used by Zizina otis and Zizeeria maha for roosting
| No. of roosting
Zizina Otis |
No. of roosting
Zizeeria maha |
||||||||||
| Flower | Fruit | Leaf | Receptacle* | Peduncle | Flower | Fruit | Leaf | Receptacle* | Stem/Peduncle | ||
| Tridax procumbens | 296 | 235 | 13 | 5 | 21 | 62 | 66 | 1 | 16 | - | |
| Vernonia cinerea | 388 | 29 | 13 | 15 | 14 | 53 | 13 | - | 6 | 4 | |
| Emilia sonchifolia | 28 | 7 | - | 24 | - | 18 | 1 | - | 4 | 2 | |
| Mimosa pudica | 49 | - | 10 | 2 | - | 1 | - | 1 | - | - | |
| Fimbristylis dichotoma | 29 | - | - | - | - | - | - | - | - | - | |
| Kyllinga brevifolia | 22 | - | - | - | - | - | - | - | - | - | |
| Youngia japonica | 20 | - | - | 1 | - | 4 | - | - | - | 1 | |
| Axonopus compressus | 2 | - | 6 | - | - | - | - | - | - | - | |
| Digitaria | 4 | - | 2 | - | 1 | 1 | - | - | - | - | |
| Ixeris chinensis | 4 | 1 | - | - | 2 | 5 | 1 | - | - | - | |
| Imperata cylindrica | - | - | 6 | - | - | - | - | 1 | - | - | |
| Eragrostis amabilis | 1 | - | 2 | - | - | 1 | - | 2 | - | - | |
| Sporobolus indicus | 1 | - | 1 | - | - | - | - | - | - | - | |
| Kyllinga nemoralis | 1 | - | - | - | - | 2 | - | - | - | - | |
| Ageratum conyzoides | 1 | - | - | - | - | - | - | - | - | - | |
| Hedyotis corymbosa | 1 | - | - | - | - | - | - | - | - | - | |
| Conyza bonariensis | - | - | - | - | - | - | - | - | - | - | |
| Total | 847 | 272 | 53 | 47 | 38 | 147 | 81 | 5 | 26 | 7 | |
| Average height (mean ± se) | 23.6 ± 0.2 | 22.3 ± 0.3 | 19.8 ± 0.8 | 24.9 ± 0.9 | 21.9 ± 0.9 | 28.7 ± 0.8 | 24.6 ± 0.6 | 22.8 ± 2.4 | 27.4 ± 1.3 | 26.3 ± 1.0 | |
*Receptacle: This specifically refers to the part after fruit dispersal.
Spatial distribution of roosting blues and plants within roosting aggregations (Prediction 2)
We found that these blues formed conspicuous roosting aggregations, typically with one individual to a flower or fruit (Figs. 2a b, 3). The total numbers of roosting individuals we sampled was 35, 40, 30, 15, 11 and 13 in plots a, b, c, d, e and f, respectively (Fig. 3). The random pattern test demonstrated significant positive associations between the flowers and fruits of T. procumbens and blues, and between the flowers and fruits of V. cinerea and blues (Table 4).
Table 4.
Results of random pattern test of the flowers and fruits of Tridax procumbens and blues at three subsites (a, b, c), and the flowers and fruits of Vernonia cinerea and blues at three subsites (d, e, f) from the data in figure 3
|
T.
procumbens vs blues |
V.
cinerea vs blues |
|||||||
| a | b | c | d | e | f | |||
| Observed overlap | 35 | 35 | 27 | 15 | 13 | 11 | ||
| Expected overlap under the null model | 17 | 20 | 14 | 7 | 8 | 7 | ||
| Number of random maps with an overlap ³ observed | 0 | 0 | 0 | 0 | 0 | 0 | ||
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||
Behavioral interactions during roosting-assembly (Prediction 3)
During roost gatherings, the most frequent behaviors of the flying individuals were the clutching and brief approach behaviors (Table 5). Less frequent behaviors were stopping on the same perch with a roosted individual or hovering near a roosted individual. Upon being clutched by the approaching individual, the primary response of the roosted individual was to fend off the approaching individual off by fluttering their wings, but without dislodgement. After fending off the approaching individual, roosted individuals sometimes left the perch, exhibited no reaction, or exhibited movement/rotation. When briefly approached, either no reaction or fending-off behavior without dislodgement were the most frequent responses of roosted individuals. Butterflies infrequently responded by fending off and leaving, or by exhibiting movement/rotation. When an approaching butterfly stopped on the same perch as a roosted butterfly, the roosted individuals exhibited fending-off behavior (28 events out of 42 observations, 66.7%) as the primary responses. When an approaching butterfly hovered near the roosted butterfly, roosted individuals exhibited no reaction as the primary response. Of the 290 behavioral interactions observed, there were 49 cases (16.9%) of fending off, then taking off. In 39 of these 49 cases, both the approaching individual and the roosted individual simultaneously left the perch after their interaction. The usurpation of the perches from the roosted individuals by flying individuals occurred in only 10 cases. In these 10 cases, only two usurpers that clutched the roosted butterflies remained on the perch while the roosted butterflies flew off. However, the remaining eight butterflies first stopped next to the roosted individuals, usurped the perch from the original individual for a given amount of time, and then flew off and found another perch.
Table 5.
Frequency of behavioral traits in blue butterflies during the formation of roosting aggregations. Numbers in the parentheses are the percentage (%)
| Flying individuals | Number of observation | Number of observation of the
response of roosted individual |
|||
| No reaction | Movement or rotation | Fending off without dislodgement | Fending off and leaving | ||
| Brief approach | 100 (34.5) | 49 (49) | 3 (3) | 45 (45) | 3 (3) |
| Hovering | 20 (6.9) | 11 (55) | 2 (10) | 6 (30) | 1 (5) |
| Stopping on the same perch | 42 (14.5) | 14 (33.3) | 0 (0) | 12 (28.6) | 16 (38.1) |
| Clutching | 128 (44.1) | 26 (20.3) | 2 (1.6) | 71 (55.5) | 29 (22.7) |
DISCUSSION
Determination of plant species and structures of plants where roosting occurs (Prediction 1)
We found a marked inequality in the number of roosting adult Zizina otis and Zizeeria maha on plants and structures. Roosting was primarily on the flowers and fruits of Tridax procumbens and Vernonia cinerea. This suggests that these two blues did not roost indiscriminately, rather they actively selected plant species and the plant structures for night roosting. T. procumbens is a straggling and procumbent hispid that flowers throughout the year, while V. cinerea is an erect herb that also flowers throughout the year. Both plant species are sources of nectar for adults of these two lycaenids during the day (Hoskins 2018; Li 2007; Personal observation). Thus, in addition to providing nectar, T. procumbens and V. cinerea play important roles in providing roosting sites for these two blues.
To our knowledge, there are only two previous studies reporting that the blues roost on flower heads: Lycaenidae icarus (Frohawk 1914) and Pseudophilotes sinaicus (James 2006), limiting opportunities for comparison. We provide some possible reasons why the two lycaenids we studied, as well as other blue species, roost on the flowers and fruits rather than other parts of the plants. One is that the head inflorescence (flowers) and the cypselas (fruits) may provide a good gripping surface that is easier to cling to or is less deflected by the butterfly’s weight. Such sites may prevent dislodgement from wind, rain, and changes in temperature during the night, when the butterflies roost (Rawlins and Lederhouse 1978).
A second possibility is that the behavior of “vesper warming,” which allows butterflies to bask in the sun as long as possible in the evening, often on the actual perch that will be used as the night roost (Clench 1966; Rawlins and Lederhouse 1978). This may prolong the period of butterfly activity and may reduce the amount of time that they are inactive and exposed to predators. Basking on roosting sites has been reported in hesperiids (Thymelicus lineola, Ancyloxypha numitor), lycaenids (Everes comyntas, Incisalia iroides, Lycaena phlaeas), nymphalines (Phyciodes tharos) and papilionids (Papilio polyxenes) (Clench 1966; Powell 1968; Rawlins and Lederhouse 1978). Our field observations indicate that the flowers and fruits of T. procumbens and V. cinerea are often located in relatively open areas of vegetation (Fig. 2A and 2B). Therefore, roosting on the tips of flowers and fruits may allow Z. otis and Z. maha to have the longest and most direct exposure to the setting sun available (perhaps the rising sun as well) in such habitats. This may prolong the period of activity in the evening, allowing the butterflies to escape predators and select a new roost if necessary (Rawlins and Lederhouse 1978).
A third possibility is that the butterflies seek to roost on the infloresence and fruits because they provide better camouflage. Roosting on or near large petals may provide better protection from predators because the butterflies might appear more cryptic, e.g., Anthocharis cardamines roost on the infloresence of Anthriscus sylvestris and Alliaria petiolata to decrease the likelihood of being seen by attackers (Courtney and Duggan 1983).
Gregarious roosting in these two blues
We demonstrated that these two blues roost gregariously in selected roosting sites. This paper represents the first demonstration of nocturnal gregarious roosting in Lycaenidae. First, we found that these blues form conspicuous aggregations and demonstrated that the roosting individuals are distributed non-randomly and match the spatial distribution of specific plant species and/or specific plant structures within roosting aggregations (prediction 2). Second, we found that these blues exhibited behavioral interactions between individuals during roosting gatherings (prediction 3).
Spatial distribution of roosting blues and plants within roosting aggregations (Prediction 2)
We found that these two blues formed conspicuous roosting aggregations in selected sites. They roosted in groups located closely together, typically with one individual to a flower or fruit. Using species of Heliconius, Mallet (1986) described various levels of gregarious roosting in butterflies. Heliconius charitonia and H. sara form tight clusters, with many individuals perching together on a twig, often holding onto each other’s legs (Mallet 1986). Clusters may be less tight. Heliconius erato roost in large groups containing some linear, nontouching arrays of as many as five individuals per twig (Mallet 1986), or roost with only one individual to a twig tip (Finkbeiner et al. 2012). On the other hand, clusters may be rather loose. Heliconius hewitsoni, melpomene, pachinus, cydno, ismenius and hecale roost in groups with individuals separated by distances as great as 1 m (Mallet 1986). The gregarious roosting of Z. otis and Z. maha in this study is similar to the roost pattern of H. erato, and likewise contains some linear, non-touching arrays.
Behavioral interactions during roosting-assembly (Prediction 3)
Our study showed that flying blues actively join other roosted individuals by the expression of various levels of behavioral interaction with roosted ones. Mallet (1986) and Crane (1957) suggest that gregarious roosting evolved through modification of courtship behavior, and the fanning (approach and hovering) and fending-off behaviors of butterflies are little more than modified courtship and mate rejection, respectively. This may act as a means by which butterflies can identify and roost near conspecifics (Finkbeiner 2014). Clutching contact was the behavior most frequently expressed by approaching blues. Clutching involves brief contacts between an approaching individual’s tarsal claws and the wings of a roosted individual. It is a physical contact that may be concurrently used to detect chemical cues from roosted individuals (Salcedo 2011). Upon being clutched by approaching individuals, the roosted individuals (the recipients) typically repelled the approaching individuals, and defended the perches by fluttering their wings. The brief approach was the second most frequently expressed behavior by approaching blues. Approach behavior is perhaps indicative of inspection of an individual or subject and may be triggered from a distance by visual cues or chemical cues released from the roosted individuals (Mallet 1986; Salcedo 2011). The roosted individuals may either not react to the flying individuals or defend the perches by fluttering their wings. We found that hovering (fanning) was not the main behavior during roosting assembly of these blues, although fanning is a frequently exhibited behavior when butterflies are identifying flowers for feeding, when females search a suitable site for oviposition, or when males court females for mating (Klein and De Araújo 2010). However, hovering is a common behavior for H. erato and H. sara during roosting interactions (Mallet 1986; Salcedo 2011).
Our data also suggested that regardless of which behavior was expressed by approaching individuals or the roosted ones, the approaching individuals typically left for other perches and only in very few cases (2 events out of 290 observations) did the approaching individuals usurp the perch and remain there. When encountered by approaching individuals, the recipients (i.e., the roosted individuals) typically flutter their wings vigorously (“fending off”). The fluttering of the wings by roosted individuals may be a visual signal used to reject the approaching individuals. In such instances, we observed that the approaching individual typically left for other perches after been rejected by the wing-fluttering behavior exhibited by roosted individuals. However, wing fluttering may also contribute to the dissemination of volatile chemical cues that may serve for intraspecific recognition or as the pheromones warn the approaching butterfly away. This may explain why blues, though roosting closely together in groups, are usually found with a single individual to a flower or fruit.
Possible function of gregariousness
Some function for gregarious roosting in these two blues can be rejected a priori. Roosting aggregations can be a behavior that facilitate mating (Bijleveld et al. 2010; Blanco and Tella 1999; Parrish and Edelstein-Keshet 1999); however, even though courtship-like behavior occurs during the roost-assembly, we failed to observe any mating events occurred on the roosts, making this function unlikely. A thermoregulatory function is also unlikely (Copp 1983; Eiserer 1984) because the aggregations are too loose to provide any microclimatic or thermoregulatory benefit. We can also rule out an aposematic function in conjunction with chemical defenses against predation. This function has been applied to explain toxic insects forming aggregations in order to enhance the effectiveness of their aposematic signal (Turner 1975; Copp 1983). However, Zizina and Zizeeria are not known to be noxious, toxic, or distasteful (Lu and Chen 2014). That roosts function as an information center (Waller and Gilbert 1982; Ward and Zahavi 1973) or for patch-sitting (Caccamise and Morrison 1986) is unlikely because foraging and roosting sites occur within several meters of each other on campus.
This leaves antipredation [selfish herd (Hamilton 1971), dilution (Treisman 1975), vigilance (Pulliam 1973)], and the safe site (Mallet 1986) as possible functions. While roosting, butterflies are fragile and vulnerable to predation, gregarious roosting may represent the selfish herd behavior in which each individual benefits by perching near other individuals, gaining from the presence and vigilance of others who provide early warning signals of predators, and thus reducing their individual threat of predation. Moreover, gregarious butterflies may also benefit from high population densities, resulting in a low per-individual attack rate within a gregarious roost (dilution) (Molles 2002; Turner 1975). On the other hand, these two blues may benefit from roosting near conspecifics to avoid disturbance (the safe site function) (Mallet 1986). If the roost site is already occupied, the site should be relatively safe and less disturbed, since the other occupants have probably roosted there for some nights. The finding that blues typically flew away from the roosting locations (i.e., the flowers and fruits of T. procumbens and V. cinerea) after we had disturbed them by tying the threads on plants supports the hypothesis of antipredation or disturbance functions.
CONCLUSIONS
We found that Zizina otis and Zizeeria maha actively select to roost on the flowers and fruits of T. procumbens and V. cinerea, showing that T. procumbens and V. cinerea are both used as roost sites and nectar sources. Our study also shows that these two lycaenids roost both singly and gregariously. This study also confirmed that blues flying near roosts exhibited a range of interactive behaviors with the roosted individuals. These interactions may depend on visual cues; though chemical cues may also be involved.
Despite the existence of anthropogenic disturbance, this study shows that urban areas can also provide important habitats for butterflies. One reason Z. otis and Z. maha are able to persist in this highly disturbed habitat is that two plant species important to them for food and roosting are also able to persist in these anthropogenic landscapes. This study highlights the importance of institutional estates, in this case a university campus, in providing resources (large open grassy areas, beds of perennial flowering plants, herbs, shrubs) for these blues (Chen 2015). In the case of Z. otis and Z. maha, the creation of habitat critical to them was an accidental byproduct of the way in which this particular university managed its grounds. This study suggests that much could be accomplished from a conservation viewpoint, if institutional estates intentionally managed their landscapes in ways that preserve or re-create landscapes with patches that provide roosting sites and other resources important to the conservation of butterflies (Chowdhury et al. 2017). We therefore call for further studies of the habitats and larval host plants needed ensure that viable populations of butterflies are retained in the urban areas. Furthermore, as loss of butterfly biodiversity becomes a conservation concern in rapidly developing countries (Koh 2007), we call on institutions to use the information provided by studies like this to intentionally manage their estates to enhance butterfly conservation.
Acknowledgments
We appreciate the help from laboratory members with the fieldwork. We are indebted to Dr. Tsung-Hsin Hsieh for his help with plant identification. We would also like to thank Dr. Wenbe Hwang for the technical assistance.
Footnotes
Authors’ contributions: There are eight authors in this manuscript. Yuan Mou Chang conceived and designed the experiments. Yuan Mou Chang, Yi Kuang Wang, Mei-Yi Ho, Stephen H. Roxburgh and Yi Ting Wu analyzed the data. Yuan Mou Chang, Mei-Yi Ho, Shuang-Ru Wang, Zi-Xuan You and Yi Ting Wu did the fieldwork. Yuan Mou Chang, Yi Kuang Wang and Kent Hatch wrote the paper.
Competing interests: We declare that we have no conflict of interest regarding this study.
Availability of data and materials: The key datasets of the manuscript are deposited as additional files in computer reader format.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Askew RR. 1982. Roosting and resting site selections by coenagrionid damselflies. Adv Odonatol 1:1–8.
- Barrett C, Burns AN. 1951. Butterflies of Australia and New Guine. N. H. Seward, Melbourn, Australia.
- Benson WW, Emmel TC. 1973. Demography of gregariously roosting populations of the nymphaline butterfly Marpesia berania in Costa Rica. Ecology 54:326–335. doi:10.2307/1934340.
- Bijleveld AI, Egas M, Van Gils JA, Piersma T. 2010. Beyond the information centre hypothesis: communal roosting for information on food, predators, travel companions and mates? Oikos 119:277–285. doi:10.1111/j.1600-0706.2009.17892.x.
- Blanco G, Tella JL. 1999. Temporal, spatial and social segregation of red-billed choughs between two types of communal roost: a role for mating and territory acquisition. Anim Behav 57:1219–1227. doi:10.1006/anbe.1999.1103. [DOI] [PubMed]
- Butchart SHM, Walpole M, Collen B, Van Strien A, Scharlemann JPW, Almond REA, Baillie JEM, Bomhard B, Brown C, Bruno J. 2010. Global biodiversity: indicators of recent declines. Science 328:1164–1168. doi:10.1126/science.1187512. [DOI] [PubMed]
- Caccamise DF, Morrison DW. 1986. Avian communal roosting: a test of the“patch-sitting” hypothesis. Condor 90:453–458. doi:10.2307/1368573.
- Chen CH. 2015. The effects of landscape composition on butterfly diversity in urban parks and green space. Chinese Culture University, Taipei, Taiwan. (in Chinese)
- Chowdhury S, Hesselberg T, Böhm M, Islam MR, Aich U. 2017. Butterfly diversity in a tropical urban habitat (Lepidoptera: Papilionoidea). Orient Insects 51:1–14. doi:10.1080/00305316.2 017.1314230.
- Clench HK. 1966. Behavioral thermoregulation in butterflies. Ecology 47:1021–1034. doi:10.2307/1935649.
- Clench HK. 1970. Communal roosting in Colias and Phoebis (Pieridae). J Lepid Soc 24:117–120.
- Collen B, Ram M, Zamin T, McRae L. 2008. The tropical biodiversity data gap: addressing disparity in global monitoring. Trop Conserv Sci 1:75–88. doi:10.1177/194008290800100202.
- Copp NH. 1983. Temperature-dependent behaviours and cluster formation by aggregating ladybird beetles. Anim Behav 31:424– 430. doi:10.1016/S0003-3472(83)80062-1.
- Courtney SP, Duggan AE. 1983. The population biology of the orange tip butterfly Anthocharis cardamines in Britain. Ecol Entomol 8:271–281. doi:10.1111/j.1365-2311.1983.tb00508.x.
- Crane J. 1957. Imaginal behavior in butterflies of the family Heliconiidae: changing social patterns and irrelevant actions. Zoologica 42:135–145.
- Dale MRT, Blundon DJ, MacIsaac DA, Thomas AG. 1991. Multiple species effects and spatial autocorrelation in detecting species associations. J Veg Sci 2:635–642. doi:10.2307/3236174.
- Davis AK, Nibbelink NP, Howard E. 2012. Identifying large-and small-scale habitat characteristics of monarch butterfly migratory roost sites with citizen science observations. Int J Zool 2012:1– 9. doi:10.1155/2012/149026.
- Dennis RLH. 1986. Selection of roost sites by Lasiommata megera (L.) (Lep., Satyridae) on fencing at Brereton Heath country park, Cheshire. Nota Lepidopterol 9:39–46.
- Dennis RLH. 2004. Just how important are structural elements as habitat components? Indications from a declining lycaenid butterfly with priority conservation status. J Insect Conserv 8:37–45. doi:10.1023/B:JICO.0000027496.82631.4b.
- DeVries PJ, Schull J, Greig N. 1987. Synchronous nocturnal activity and gregarious roosting in the neotropical skipper butterfly Celaenorrhinus fritzgaertneri (Lepidoptera: Hesperiidae). Zool J Linnean Soc 89:89–103. doi:10.1111/j.1096-3642.1987.tb01345.x.
- Eiserer LA. 1984. Communal roosting in birds. Bird Behav 5:61–80. doi:10.5040/9781472597298.ch-018.
- Eliot JN. 1973. The higher classification of the Lycaenidae (Lepidoptera): a tentative arrangement. Bulletin of the British Museum (Natural History) Entomology 28:371–505. doi:10.5962/bhl.part.11171.
- Finkbeiner SD. 2014. Communal roosting in Heliconius butterflies (Nymphalidae): roost recruitment, establishment, fidelity, and resource use trends based on age and sex. J Lepid Soc 68:10–16. doi:10.18473/lepi.v68i1.a2.
- Finkbeiner SD. 2019. Evidence for communal roost size regulation in Heliconius erato butterflies (Nymphalidae). J Lepid Soc 73:122– 128. doi:10.18473/lepi.73i2.a6.
- Finkbeiner SD, Briscoe AD, Reed RD. 2012. The benefit of being a social butterfly: communal roosting deters predation. Proc R Soc of Lond B Biol Sci 279:2769–2776. doi:10.1098/rspb.2012.0203. [DOI] [PMC free article] [PubMed]
- Frohawk FW. 1914. The sleeping attitude of Lycaenidae. Entomologist 47:212–213. doi:10.2307/j.ctt6wrb4c.6.
- Gilbert LE. 1972. Pollen feeding and reproductive biology of Heliconius butterflies. P Natl Acad Sci USA 69:1403–1407. doi:10.1073/pnas.69.6.1403. [DOI] [PMC free article] [PubMed]
- Gilbert LE, Singer MC. 1975. Butterfly ecology. Annu Rev Ecol Evol Syst 6:365–395. doi:10.2307/1939646.
- Hamilton WD. 1971. Geometry for the selfish herd. J Theor Biol 31:295–311. doi:10.1016/0022-5193(71)90189-5. [DOI] [PubMed]
- Hanson P. 2000. Insects and Spiders. In: N. M. Nadkarni and N. T. Wheelwright (eds.) Monteverde: ecology and conservation of a tropical cloud forest. Oxford University Press, New York, pp. 95–147.
- Harinath P, Suryanarayana K, Ramana SPV. 2015. Eco-biology of the dark grass blue butterfly, Zizeeria karsandra (Moore) (Lepidoptera: Rhopalocera: Lycaenidae) from the Eastern Ghats of Southern Andhra Pradesh. J Entomol Zool Stud 3:225–231.
- Heath J, Emmet AM (Ed). 1985. The moths and butterflies of Great Britain and Ireland. Harley Books, Colchester, England.
- Hoskins A. Learn about butterflies: the complete guide to the world of butterflies and moths. http://www.learnaboutbutterflies.com/index.htm. Accessed 3 February 2018.
- Howard E, Davis AK. 2009. The fall migration flyways of monarch butterflies in eastern North America revealed by citizen scientists. J Insect Conserv 13:279–286. doi:10.1007/s10841-008-9169-y.
- Huang TC (Ed). 2000. Flora of Taiwan (2nd ed.). Editorial Committee of the Flora of Taiwan, Taipei, Taiwan.
- Jain A, Lim FKS, Webb EL. 2017. Species-habitat relationships and ecological correlates of butterfly abundance in a transformed tropical landscape. Biotropica 49:355–364. doi:10.1111/btp.12435.
- James M. 2006. The natural history of the Sinai Baton Blue: the smallest butterfly in the world. Egyptian J Biol 8:67–83.
- Klein AL, De Araújo AM. 2010. Courtship behavior of Heliconius erato phyllis (Lepidoptera, Nymphalidae) towards virgin and mated females: conflict between attraction and repulsion signals? J Ethol 28:409–420. doi:10.1007/s10164-010-0209-1.
- Koh LP. 2007. Impacts of land use change on South-east Asian forest butterflies: a review. J Appl Ecol 44:703–713. doi:10.1111/j.1365-2664.2007.01324.x.
- Legendre P. 1993. Spatial autocorrelation: trouble or new paradigm? Ecology 74:1659–1673. doi:10.2307/1939924.
- Li CF, Chytrý M, Zelený D, Chen MY, Chen TY, Chiou CR, Hsia YJ, Liu HY, Yang SZ, Yeh CL, Wang JC, Yu CF, Lai YJ, Chao WC, Hsieh CF. 2013. Classification of Taiwan forest vegetation. Appl Veg Sci 16:698–719. doi:10.1111/avsc.12025.
- Li DW. 2007. Butterfly fauna and nectar plants at the Chinghsien Ecological Park, Taichung City, Central Taiwan. Endemic Species Res 9:37–49. doi:10.7064/ESR.200701.0037.
- Lu CC, Chen JJ. 2014. The life history of butterflies in Taiwan. Morning Star Publishing Inc., Taichung, Taiwan. (in Chinese)
- Ludwig JA, Reynolds JF. 1988. Statistical Ecology. John Wiley and Sons, New York, USA.
- Mallet J. 1986. Gregarious roosting and home range in Heliconius butterflies. Natl Geogr Res 2:198–215.
- Mallet J, Gilbert LE. 1995. Why are there so many mimicry rings? Correlations between habitat, behaviour and mimicry in Heliconius butterflies. Biol J Linnean Soc 55:159–180. doi:10.1111/j.1095-8312.1995.tb01057.x.
- McFarland N. 1971. A specialized case of communal roosting in Pieris rapae (Pieridae). J Lepid Soc 25:144–145.
- Molles MC. 2002. Ecology: Concepts and Applications (International ed.). The McGraw-Hill Companies, Inc., New York, USA.
- Muyshondt A, Muyshondt A. 1974. Gregarious seasonal roosting of Smyrna karwinskii adults in El Salvador (Nymphalidae). J Lepid Soc 28:224–229.
- Nidup T. 2016. Butterfly (Lepidoptera-Rhophalocera) diversity in the developing urban area of Gelephu, Bhutan. Bhutan J Nat Res Dev 3:42–46.
- Opler PA, Malikul V. 1992. A field guide to eastern butterflies. Houghton Mifflin Company, New York, USA.
- Otaki JM, Hiyama A, Iwata M, Kudo T. 2010. Phenotypic plasticity in the range-margin population of the lycaenid butterfly Zizeeria maha. BMC Evol Biol 10:252. doi:10.1186/1471-2148-10-252. [DOI] [PMC free article] [PubMed]
- Owen DF, Chanter DO. 1969. Population biology of tropical African butterflies. Sex ratio and genetic variation in Acraea encedon. J Zool 157:345–374. doi:10.1111/j.1469-7998.1969.tb01707.x.
- Parrish JK, Edelstein-Keshet L. 1999. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 284:99– 101. doi:10.1126/science.284.5411.99. [DOI] [PubMed]
- Pickett STA, Cadenasso ML, Grove JM, Boone CG, Groffman PM, Irwin E, Kaushal SS, Marshall V, McGrath BP, Nilon CH. 2011. Urban ecological systems: Scientific foundations and a decade of progress. J Environ Manage 92:331–362. doi:10.1016/j.jenvman.2010.08.022. [DOI] [PubMed]
- Pierce NE, Braby MF, Heath A, Lohman DJ, Mathew J, Rand DB, Travassos MA. 2002. The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu Rev Entomol 47:733–771. doi:10.1146/annurev.ento.47.091201.145257. [DOI] [PubMed]
- Powell JA. 1968. A study of area occupation and mating behavior in Incisalia iroides (Lepidoptera: Lycaenidae). J N Y Entomol Soc 76:47–57.
- Pulliam HR. 1973. On the advantages of flocking. J Theor Biol 38:419–422. doi:10.1016/0022-5193(73)90184-7. [DOI] [PubMed]
- Rawlins JE, Lederhouse RC. 1978. The influence of environmental factors on roosting in the black swallowtail, Papilio polyxenes asterius Stoll (Papilionidae). J Lepid Soc 32:145–159.
- Rouquette JR, Thompson DJ. 2007. Roosting site selection in the endangered damselfly, Coenagrion mercuriale, and implications for habitat design. J Insect Conserv 11:187–193. doi:10.1007/s10841-006-9030-0.
- Roxburgh SH, Chesson P. 1998. A new method for detecting species associations with spatially autocorrelated data. Ecology 79:2180–2192. doi:10.1890/0012-9658(1998)079[2180:ANMF DS]2.0.CO;2.
- Salcedo C. 2010a. Environmental elements involved in communal roosting in Heliconius butterflies (Lepidoptera: Nymphalidae). Environ Entomol 39:907–911. doi:10.1603/EN09340. [DOI] [PubMed]
- Salcedo C. 2010b. Evidence of pollen digestion at nocturnal aggregations of Heliconius sara in Costa Rica (Lepidoptera: Nymphalidae). Trop Lepid Res 20:35–37.
- Salcedo C. 2011. Behavioral traits expressed during Heliconius butterflies roost-assembly. Trop Lepid Res 21:80–83.
- Sing KW, Jusoh WFA, Hashim NR, Wilson JJ. 2016. Urban parks: refuges for tropical butterflies in Southeast Asia? Urban Ecosyst 19:1131–1147. doi:10.1007/s11252-016-0542-4.
- Tavaré S, Altham PME. 1983. Serial dependence of observations leading to contingency tables, and corrections to chi-squared statistics. Biometrika 70:139–144. doi:10.1093/biomet/70.1.139.
- Thomas JA. 1983. The ecology and conservation of Lysandra bellargus (Lepidoptera: Lycaenidae) in Britain. J Appl Ecol 20:59–83.
- Tittensor DP, Walpole M, Hill SLL, Boyce DG, Britten GL, Burgess ND, Butchart SHM, Leadley PW, Regan EC, Alkemade R. 2014. A mid-term analysis of progress toward international biodiversity targets. Science 346:241–244. doi:10.1126/science.1257484. [DOI] [PubMed]
- Treisman M. 1975. Predation and the evolution of gregariousness. I. Models for concealment and evasion. Anim Behav 23:779–800.
- Tsang TP, Bonebrake TC. 2017. Contrasting roles of environmental and spatial processes for common and rare urban butterfly species compositions. Landsc Ecol 32:47–57. doi:10.1007/s10980-016-0427-1.
- Turner JRG. 1975. Communal roosting in relation to warning colour in two heliconiine butterflies (Nymphalidae). J Lepid Soc 29:221–226.
- Urquhart FA, Urquhart NR. 1979. Breeding areas and overnight roosting locations in the northern range of the monarch butterfly (Danaus plexippus plexippus) with a summary of associated migratory routes. Can Field Nat 93:41–47.
- Venkata Ramana SP, Harinath P, Prasanna Kumar V. 2014. Life history of the dark grass blue Zizeeria Karsandra (Lepidoptera: Rhopalocera: Lycaenidae) from Southern Andhra Pradesh-India. Cibtech J Zool 3:1–6.
- Waller DA, Gilbert LE. 1982. Roost recruitment and resource utilization: observations on a Heliconius charitonia L. roost in Mexico (Nymphalidae). J Lepid Soc 36:178–184.
- Wang JS, CM Hung. 2019. Barn swallow nest predation by a recent urban invader, the Taiwan whistling thrush-implications for the evolution of urban avian communities. Zool Stud 58:1. doi:10.6620/ZS.2019.58-01. [DOI] [PMC free article] [PubMed]
- Ward P, Zahavi A. 1973. The importance of certain assemblages of birds as “information-centres” for food-finding. Ibis 115:517– 534. doi:10.1111/j.1474-919X.1973.tb01990.x.
- Yago M, Hirai N, Kondo M, Tanikawa T, Ishii M, Wang M, Williams M, Ueshima R. 2008. Molecular systematics and biogeography of the genus Zizina (Lepidoptera: Lycaenidae). Zootaxa 1746:15–38. doi:10.11646/zootaxa.1746.1.2.
- Young AM, Thomason JH. 1975. Notes on communal roosting of Heliconius charitonius (Nymphalidae) in Costa Rica. J Lepid Soc 29:243–255.
- Zhang J, Cong Q, Shen JH, Opler PA, Grishin NV. 2019. Genomics of a complete butterfly continent. bioRxiv:829887. doi:10.1101/829887.