Abstract
1. Corylifol A (CA), a phenolic compound from Psoralea corylifolia, possessed several biological properties but poor bioavailability. Here we aimed to investigate the roles of cytochromes P450s (CYPs), UDP-glucuronosyltransferases (UGTs) and efflux transporters in metabolism and disposition of CA.
2. Metabolism of CA was evaluated in HLM, expressed CYPs and UGTs. Chemical inhibitors and shRNA-mediated gene silencing of multidrug resistance-associated proteins (MRPs) and breast cancer resistance protein (BCRP) were performed to assess the roles of transporters in CA disposition.
3. Three oxidated metabolites (M1–M3) and two glucuronides (M4–M5) were detected. The intrinsic clearances (CLint) values of M1 and M4 in HLM were 48.10 and 184.03 μL/min/mg, respectively. Additionally, CYP1A1, 2C8 and 2C19 were identified as main contributors with CLint values of 13.01–49.36 μL/min/mg, while UGT1A1, 1A7, 1A8 and 1A9 were with CLint values ranging from 85.01 to 284.07 μL/min/mg. Furthermore, activity correlation analysis proved CYP2C8, UGT1A1 and 1A9 were the main active hepatic isozymes. Besides, rats and monkeys were appropriate model animals. Moreover, dipyridamole and MK571 both could significantly inhibit M4 efflux. Gene silencing results also indicated MRP4 and BCRP were major contributors in HeLa1A1 cells.
4. Taken together, CYPs, UGTs, MRP4 and BCRP were important determinants of CA pharmacokinetics.
Keywords: Corylifol A, metabolism, enzymes, efflux transporters, HeLa1A1 cells
Introduction
The signal transducers and activators of transcription 3 (STAT3), a transcription factor, is a therapeutic target for several diseases (e.g. thyroid disease, diabetes, intestinal inflammation, low blood counts, etc.), and development of STAT3 selective inhibitors as drug candidates has been a major direction to treat these diseases (Klampfer, 2006). In the past decade, the discovery of the selective or potent inhibitor of STAT3 from herbal medicines for translational applications has attracted increasing attention (Lee et al., 2012), due to most of the herbal medicines display satisfying safety during long history of use for medical treatments (Lam et al., 2010).
Corylifol A (CAS No. 775351-88-7), also known as corylinin, is a natural phenolic compound isolated from Psoralea corylifolia (Cui et al., 2015). It has been reported that CA could play an inhibitory effect on IL-6-induced STAT3 promoter activity in Hep3B cells (IC50 = 0.81 ±0.15 μM), and also inhibit STAT3 phosphorylation induced by IL-6 in Hep3B cells (Lee et al., 2012). In addition, it also exhibited cytotoxic activity against HepG2 and Hep3B hepatocellular carcinoma cell lines, with IC50 values of 4.6 and 13.5 μg/mL, respectively (Song et al., 2013). Besides, CA possessed weak inhibitory effects on severe acute respiratory syndrome coronavirus papain-like protease (32.3 ± 3.2 μM) (Kim et al., 2014). Recently, CA can be the potential uncouplers of neuronal nitric oxide synthase-postsynaptic density protein-95 (Gu et al., 2017). Correspondingly, its pharmacokinetics in vivo also attracted more attention due to remarkable bioactivities.
At present, CA were proved to be absorbed in plasma after intragastric administration of Psoralea corylifolia or Psoralea corylifolia-containing herbal prescriptions (Geng et al., 2014; Wang et al., 2015). Furthermore, the pharmacokinetics results indicated that the maximal plasma concentration (Cmax) of CA was 8.87 ng/mL at a single dose of Psoralea corylifolia extract at 2.0 g/kg (Gao et al., 2016), whereas the Cmax value was 1.70 ng/mL after oral administration of Psoralea corylifolia extract at 1.2 g/kg (Yang et al., 2018). Besides, the distribution concentrations of CA at different nuclei varied at one determined time point (ranging from 260.63 to 493.29 ng·h/g), but the overall trends were basically similar to the plasma concentration-time results (Yang et al., 2018). Poor bioavailability suggested that CA underwent the extensive first-pass metabolism. So far, only glucuronidation and sulfation were reported for the metabolism of CA (Geng et al., 2014; Wang et al., 2015). The phase I metabolism of CA still remain unknown. Additionally, the active cytochromes P450s (CYPs) and UDP-glucuronosyltransferases (UGTs) for the metabolism of CA were also unclear, and needed to be further clarified.
Because CA-O-glucuronide conjugates with a high hydro-philicity lacks the ability of passive transport, active transport of these glucuronides out of cells is a required step in drug and xenobiotic clearance via the UGT metabolism. This excretion process needs to rely on the roles of efflux transporters, such as multidrug resistance-associated proteins (MRPs) and breast cancer resistance protein (BCRP) (Qin et al., 2018a, 2019; Zhang et al., 2015). Previous studies have indicated that many compounds conjugated with glucuronide were the substrates of MRPs and BCRP transporters (Yang et al., 2018; Zhang et al., 2019). MRPs and BCRP also play an important role in determining the oral bioavailability and pharmacokinetics of the aglycones undergoing glucuronidation. Compared with glucuronidation, the disposition via efflux transporters is usually poorly characterized. Therefore, it is necessary to evaluate the effects of MRPs and BCRP transporters on the disposition of CA.
In the present study, the metabolites were characterized by ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (UHPLC/Q-TOF-MS) after incubation of CA with HLM. Furthermore, reaction phenotyping and activity correlation analysis assays were performed to identify the most important contributors for CA metabolism (Zhu et al., 2012). Additionally, several animal liver microsomes were used to determine the appropriate model animal for CA metabolism. Moreover, chemical inhibitors (dipyridamole and MK571) and shRNA-mediated gene knock-down assays were applied to evaluate a potential role of MRPs and BCRP in cellular glucuronidation of CA using UGT1A1-overexpressed HeLa cells (HeLa1A1 cells). We determine that CYPs, UGTs, MRP4 and BCRP play crucial roles in the metabolism and disposition of CA.
Materials and methods
Chemicals and reagents
Corylifol A (purity >98%) was purchased from Shanghai Winherb Medical Technology Co., Ltd (Shanghai, China). Pooled human liver microsomes (HLM), nine individual human liver microsomes (iHLM), mice liver microsomes (MLM), monkeys liver microsomes (MkLM), rabbits liver microsomes (RaLM), rats liver microsomes (RLM), dogs liver microsomes (DLM), twelve recombinant human UGT isoforms (UGT1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9, 2B4, 2B7, 2B10, 2B15 and 2B17) and twelve expressed human CYP enzymes (CYP1A1, 1A2, 1B1, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4 and 3A5) were all obtained from Corning Biosciences (Corning, NY, USA). The anti-BCRP (TA322704), anti-MRP1 (TA309559), anti-MRP3 (TA314800) and anti-MRP4 (TA327332) antibodies were purchased from OriGene Technologies (Rockville, MD). UDPGA, NADPH, MgCl2, alamethicin and D-saccharic-1, 4-lactone, dipyridamole (DIPY) and MK571 were all provided from Sigma-Aldrich (St Louis, MO, USA). Paclitaxel, 6-hydroxy-paclitaxel, β-estradiol and propofol were supplied from Aladdin Chemicals (Shanghai, China). Propofol-O-glucuronide and β-estradiol-3-O-glucuronide were obtained from Toronto Research Chemicals (Ontario, Canada). Besides, other chemicals and solvents were of analytical grade or better.
Phase I metabolism assay
As described in our previous publication (Xu et al., 2018), the incubation mixtures containing Tris-HCl buffer solution (50 mM, pH = 7.4), MgCl2 (300 mM), HLM (1 mg/mL), a series of CA solutions (0.16–80 μM) and NADPH solution (1 mM) were prepared in a total incubation volume of 100 μL. After incubation at 37 °C for 60 min, the reactions were terminated by adding 100 μL of cold acetonitrile. The negative control incubations were conducted in the absence of NADPH to confirm the metabolites produced were NADPH-dependent. Then the incubation mixtures were vortexed and centrifuged at 13,800 g for 10 min to collect the supernatant for analysis. Similarly, CA was incubated with nine iHLM (1 mg/mL) or expressed CYP enzymes (1–1.2 mg/mL) in incubation system. All experiments were performed in triplicate.
Glucuronidation assay
In this study, a series of CA solutions (0.16–80 μM) were incubated in the incubation mixtures (100 μL), contained pooled HLM (1 mg/ml), Tris-HCl buffer solution (50 mM, pH = 7.4), MgCl2 (0.88 mM), alamethicin (22 μg/mL), D-saccharic-1, 4-lactone (4.4 mM) and UDPGA (3.5 mM) as described previously (Xu et al., 2018). After incubation at 37 °C for 60 min, the reaction was terminated by adding 100 μL of cold acetonitrile. Then the incubation mixtures were vortexed and centrifuged at 13,800 g for 10 min to collect the supernatant for analysis. Incubations without UDPGA were served as the negative control to ensure that the metabolites produced were UDPGA-dependent. Analogously, CA was incubated with nine iHLM (1 mg/mL) or 12 recombinants UGT isoforms (1 mg/mL) in the incubation system. All experiments were performed in triplicate.
Analytical conditions
The separation of CA and its metabolites were performed by an Acquity UHPLC I-Class system equipped with PDA detector (Waters Corporation, Manchester, UK). Chromatographic separation was achieved on a BEH C18 column (2.1 mm × 50 mm, 1.7 μm, Waters, Ireland) while column temperature was kept at 35 °C. The mobile phase was consisted of water (A) and acetonitrile (B), both including 0.1% formic acid (V/V). The gradient elution was as follows: 10–50% B from 0 to 2.0 min, 50–100% B from 2.0 to 3.0 min, maintaining 100% B from 3.0 to 3.2 min, 100–10% B from 3.2 to 3.5 min, keeping 20% B from 3.5 to 4.0 min. The mobile phase was run at a flow rate of 0.4 mL/min and the injection volume was of 8 μL. CA and its metabolites were detected at 270 nm.
In addition, UHPLC system was coupled with a hybrid quadrupole orthogonal time-of-flight (Q-TOF) tandem mass spectrometer (SYNAPT G2 HDMS, Waters). Accurate mass measurements for CA and its metabolites were performed using electrospray ionization (ESI) in the positive ion mode with the following operating parameters settings: capillary voltage, +3000 V; sample cone voltage, 35 V; extraction cone voltage, 4 V; source temperature, 100 °C; desolvation temperature, 300 °C; cone gas flow, 50 L/h and desolvation gas flow, 800 L/h. The full scan mass range was 50–1500 Da. The method employed lock spray with leucine enkephalin (m/z 556.2771 in positive ion mode) to ensure the mass accuracy. Data acquisition, data handling and instrument control were performed by the Masslynx 4.1 software (Waters).
Because CA-related metabolites were not commercially available, their amounts were calculated based on the standard curve of CA according to the assumption that parent compound and its metabolites have closely similar UV absorbance maxima (Lu et al., 2015). The LOD and LOQ were calculated as 3-fold and 10-fold of the ratio of signal-to-noise (S/N), respectively. Calibration curves were constructed by plotting peak area ratios (Y) and versus concentrations (X) using a 1/x2 weighting factor. There was a good linearity (r2 = 0.9995) for the determination of CA from 0.01–10 μM.
Enzymes kinetic studies
Twelve commercially available expressed CYP enzymes and twelve recombinant UGT isoforms were used to identify the active enzymes involved in metabolism of CA. Metabolic rates of CA-related metabolites by CYP and UGT enzymes were expressed as the amounts of metabolites formed per minute per milligram protein (pmol/min/mg protein). To evaluate kinetic parameters, kinetic profiles of CA were performed in pooled HLM, expressed CYP and UGT isoforms, respectively. Preliminary experiments were carried out to confirm that the formation of metabolites was in the linear range over 60 min of incubation and 0.2–2.0 mg/mL of microsomal protein.
Kinetic parameters were evaluated from the suitable curves based on the profile of Eadie–Hofstee plot (Xu et al., 2018). If the Eadie–Hofstee plot was linear, the Michaelis–Menten Equation (1) was employed to analyze the kinetic data. However, when the Eadie–Hofstee plots showed characteristic profiles of atypical kinetics, including substrate inhibition and sigmoidal autoactivation kinetics, the data from these atypical profiles were fit to the Equations (2) and (3). The apparent kinetic parameters were described as mean ± SD (standard deviation) of triplicate samples. Model fitting and data analysis were performed by Graphpad Prism V5 software (San Diego, CA).
| (1) |
| (2) |
| (3) |
| (4) |
where Km is the Michaelis–Menten constant; V is the formation rates of metabolites; Vmax is the maximal rate of metabolites; Ksi is the substrate inhibition constant; S50 is the substrate concentration resulting in 50% of Vmax; and n is the Hill coefficient. The intrinsic clearance (CLint) was derived from Vmax/Km for Michaelis–Menten and substrate inhibition models and the maximal clearance (CLmax) was obtained using Equation (4) for sigmoidal autoactivation kinetics.
Activity correlation analysis
To further investigate the contribution of CYP and UGT enzymes in liver, the metabolic activities of iHLM (n = 9) toward CA, paclitaxel (a selective substrate for CYP2C8), β-estradiol (a specific substrate for UGT1A1) and propofol (a probe substrate for UGT1A9) were determined according to the previously assay protocol (Xu et al., 2018). Correlation analyses were performed between CA-oxidation and paclitaxel-6-hydroxylation, between CA-O-glucuronidation and β-estradiol-3-O-glucuronidation, and between CA-O-glucuronidation and propofol-O-glucuronidation. The incubation conditions were described as above mentioned and the substrate concentration was 10 μM (close to Km value) (Qin et al., 2018; Xu et al., 2018). The correlation analysis was performed using GraphPad Prism V5 software. The correlation parameter was estimated by the linear regression coefficient (r), and p < 0.05 was recognized as being statistically significant.
Species differences
To compare the species differences of CA metabolism, CA (0–80 μM) was incubated in phase I and glucuronidation systems described above but with liver microsomes from rat, mouse, dog, rabbit or monkey. The protein concentration of the microsomes used in phase I metabolism assay was 1 mg/mL and 0.5 mg/mL in glucuronidation assay, respectively. The incubation time was 120 min for phase I metabolism assay and 30 min for glucuronidation assay, respectively.
HeLa1A1 cells culture
HeLa1A1 cells were established and validated as described previously (Qin et al., 2018a; Yang et al., 2018; Zhang et al., 2019). Before excretion experiments, HeLa1A1 cells were cultured in DMEM containing 10% FBS, penicillin (100 U/mL) and streptomycin (100 μg/mL), maintaining at 37 °C in a humidified incubator with 5% CO2. When the engineered cells were seeded at a density of 2 × 105 cells/well in 6-well plates and reached about 90% confluence, the DMEM was removed and the cells were washed twice with pre-warmed HBSS for preparation of excretion assays.
Glucuronides excretion experiments
To evaluate the role of efflux transporters on the excretion of CA-O-glucuronide (M4), HeLa1A1 cells were incubated in HBSS solution containing CA in the absence or presence of DIPY (a selective BCRP inhibitor) or MK571 (a selective MRPs inhibitor) at 37 °C. The working concentrations were 10 μM for CA, 5 or 20 μM both for DIPY and MK571. Control assay was performed by adding the vehicle instead of inhibitors solution. At each predetermined time point (0.5, 1, 1.5 and 2 h), 200 μL of incubating medium was sampled, and replaced with the same volume of corresponding inhibitor-containing HBSS solutions immediately. Each sample was mixed with 100 μL ice-cold acetonitrile, followed by centrifugation at 13,800 g for 15 min to collecting supernatant for determination of extracellular glucuronide. At the end of the experiment (2 h), the cells were washed three times with blank HBSS buffer and then collected in 200 μL of 50% ice-cold methanol. After ultrasonication and centrifugation at 13,800 g for 15 min, the supernatant was collected to determine the intracellular amount of CA-O-glucuronide.
Moreover, to further confirm the role of individual efflux transporter, a series of constructed shRNA fragments (including shRNA-BCRP, shRNA-MRP1, shRNA-MRP3 and shRNA-MRP4) were used to establish transporter knocked-down cell lines by the lentivirus transfection following our previous publications (Qin et al., 2018a; Zhang et al., 2015). Briefly, when the cells reached about 50% confluence, the transporter shRNA plasmids (10 μg/mL) construct was transiently transfected into HeLa1A1 cells. At 6 hours after the initiation of transfection, the DMEM solution with plasmids was removed, and the fresh DMEM supplemented with 10% FBS, penicillin and streptomycin were added. Upon reaching confluence, cells were washed three times with 2mL HBSS and then treated with HBSS containing CA for excretion experiments. The control scrambled shRNA was served as a negative control.
All experiments were performed in triplicate. The excretion rate (ER), apparent efflux clearance (CLapp) of CA-O-glucuronide and the fraction of metabolized CA value (fmet) were calculated by Equations (5)-(7), where V is the volume of the incubation medium; C is the cumulative concentration of corresponding glucuronide; t is the incubation time and Ci is the intracellular concentration of CA-O-glucuronide.
| (5) |
| (6) |
| (7) |
Western blotting assays
The assays for western blotting were like the protocol as described previously (Qin et al., 2019). In brief, HeLa1A1 cell lysates (40 μg proteins) were separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA). Blots were probed with anti-BCRP, anti-MRP1, anti-MRP3 and anti-MRP4 antibodies, followed by horseradish peroxidase-conjugated rabbit anti-goat IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The protein bands were detected by enhanced chemiluminescence, and band intensities were measured by densitometry using the Quantity One software (Hercules, CA, USA).
Statistical analysis
Data are expressed as the mean ± SD (standard deviation). Mean differences between treatment and control groups were analyzed by two-tailed Student′s t-test. The level of significance was set at p < 0.05 (*), p < 0.01 (**) or p < 0.001 (***).
Results
Identification of CA and its metabolites
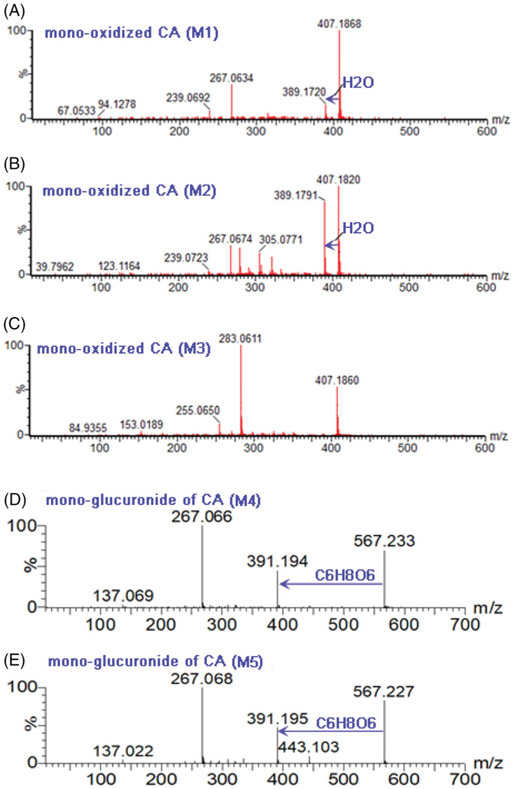
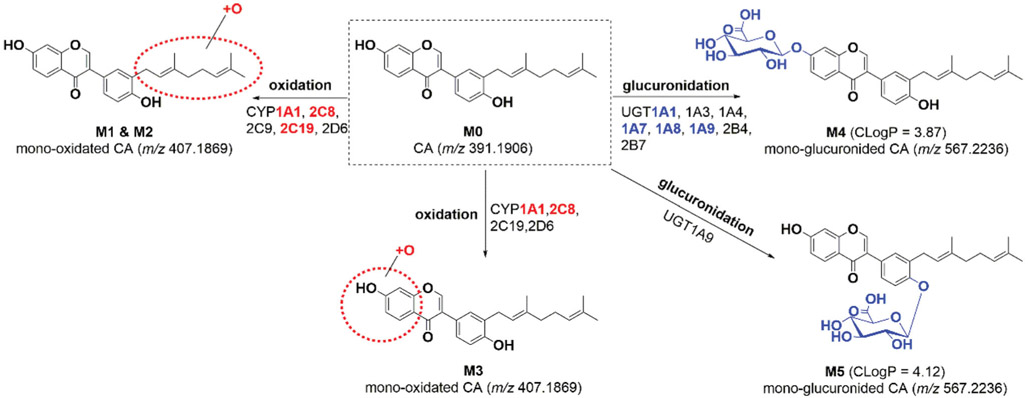
CA (M0) exhibited a typical [M + H]+ ion at m/z 391.1906 (tR = 3.03 min, C25H26O4, −3.1 ppm) as reported previously (Wang et al., 2015). The MS/MS spectra showed the characteristic fragment ions at m/z 267.069, 239.069, 183.084 and 137.030 (Supplementary Table S1). After incubation of CA in phase I system, three metabolites (M1–M3) were obviously generated, with 16 Da larger than that of M0. The MS/MS spectra of M1 (2.56 min, Figure 1(A)) and M2 (2.66 min, Figure 1(B)) gave the similar fragmentation, with the diagnostic ion at m/z 137.023, suggesting that the oxidation site was at the isopentene unit chain, which kept in line with previous studies (Wang et al., 2015; Xu et al., 2018). Likewise, M3 (2.87 min, Figure 1(C)) displayed the characteristic ion at m/z 153.019, which indicated that the oxidation reaction occurred at the A ring of M0.
Figure 1.
The MS/MS spectra of corylifol A-related metabolites, M1 (A), M2 (B), M3 (C), M4 (D) and M5 (E) after incubation of corylifol A with NADPH-supplemented pooled HLM and UDPGA-supplemented pooled HLM, respectively.
On the other hand, two additional peaks M4 (tR = 2.52 min) and M5 (tR = 2.60 min), with 176 Da larger than that of M0, suggesting the production of two glucuronides. The MS/MS analysis of M4 (Figure 1(D)) and M5 (Figure 1(E)) showed similar fragmentation pathways of M0. Based on the CLogP values (Xu et al., 2018), M4 (CLogP = 3.87) and M5 (CLogP = 4.12) were characterized as CA-7-O-glucuronide and CA-4′-O-glucuronide, respectively. The MS/MS spectra and UHPLC/Q-TOF-MS data of CA and its related metabolites were displayed in Figure 1 and Supplementary Table S1, respectively.
Phase I metabolism of CA in HLM and CYP enzymes
After incubation of CA with NADPH in pooled HLM, three mono-oxidated metabolites (M1–M3) were detected by UHPLC/Q-TOF-MS. Kinetic profiles revealed that the formation of M1 in HLM well modeled by substrate inhibition kinetics (Supplementary Figure S1(A)), whereas the complete kinetic profiles of M2 and M3 were unable to obtain because of several concentrations less than the LOQ. The Km, Vmax and CLint values of M1 were 1.51 μM, 72.68 pmol/min/mg and 48.10 μL/min/mg, respectively (Table 1).
Table 1.
Kinetic parameters derived for corylifol A-related oxidated metabolites (M1–M3) by pooled HLM, expressed CYPs enzymes, RLM, DLM, MLM, MkLM and RaLM (Mean ± SD).
| Meta. | Vmax (pmol/min/mg) | Km or S50 (μM) | Ki (μM) | n | CLint or CLmax (μL/min/mg) | Model | |
|---|---|---|---|---|---|---|---|
| HLM | M1 | 72.68 ± 1.89 | 1.51 ± 0.10 | 84.65 ± 7.86 | N.A. | 48.10 ± 3.57 | SI |
| M2 | ++ | ++ | ++ | ++ | ++ | ||
| M3 | ++ | ++ | ++ | ++ | ++ | ||
| CYP1A1 | M1 | + | + | + | + | + | |
| M2 | 37.07 ± 7.33 | 2.47 ± 0.75 | 6.24 ± 2.02 | N.A. | 15.03 ± 5.46 | SI | |
| M3 | 29.49 ± 3.72 | 2.27 ± 0.47 | 9.36 ± 2.18 | N.A. | 13.01 ± 3.17 | SI | |
| CYP2C8 | M1 | 267.4 ± 23.47 | 5.42 ± 0.75 | 18.06 ± 2.82 | N.A. | 49.36 ± 8.12 | SI |
| M2 | ++ | ++ | ++ | ++ | ++ | ||
| M3 | 99.27 ± 7.10 | 3.53 ± 0.30 | 0.89 ± 0.08 | N.A. | 28.11 ± 3.13 | SI | |
| CYP2C9 | M1 | + | + | + | + | + | |
| M2 | − | − | − | − | − | ||
| M3 | − | − | − | − | − | ||
| CYP2C19 | M1 | 411.40 ± 55.91 | 23.96 ± 3.61 | 1.87 ± 0.29 | N.A. | 17.17 ± 3.48 | SI |
| M2 | − | − | − | − | − | ||
| M3 | + | + | + | + | + | ||
| CYP2D6 | M1 | ++ | ++ | ++ | ++ | ++ | |
| M2 | − | − | − | − | − | ||
| M3 | ++ | ++ | ++ | ++ | ++ | ||
| RLM | M1 | 10,661 ± 3808 | 112.2 ± 43.95 | 9.10 ± 3.69 | N.A. | 95.01 ± 50.37 | SI |
| M2 | ++ | ++ | ++ | ++ | ++ | ||
| M3 | + | + | + | + | + | ||
| MLM | M1 | − | − | − | − | − | |
| M2 | + | + | + | + | + | ||
| M3 | + | + | + | + | + | ||
| DLM | M1 | − | − | − | − | − | |
| M2 | 50.12 ± 1.77 | 4.13 ± 0.10 | N.A. | 2.40 ± 0.36 | 6.14 ± 0.36 | Hill | |
| M3 | + | + | + | + | + | ||
| MkLM | M1 | 36.42 ± 0.57 | 1.29 ± 0.11 | N.A. | N.A. | 28.25 ± 2.54 | MM |
| M2 | + | + | + | + | + | ||
| M3 | 101.6 ± 26.54 | 10.86 ± 4.04 | 15.33 ± 5.85 | N.A. | 9.35 ± 4.25 | SI | |
| RaLM | M1 | 33.61 ± 2.35 | 3.46 ± 0.45 | 31.23 ± 5.02 | N.A. | 9.70 ± 1.43 | SI |
| M2 | 58.16 ± 6.87 | 7.71 ± 1.36 | 19.56 ± 3.98 | N.A. | 7.53 ± 1.60 | SI | |
| M3 | − | − | − | − | − |
All experiments were performed in triplicate.
Hill: sigmoidal autoactivation model; HLM: human liver microsomes; Meta.: metabolites; MM: Michaelis–Menten model; N.A.: not available; +: under the limit of quantification; ++: unable to determine the kinetic parameters in the absence of a full kinetic profile; SI: substrate inhibition model.
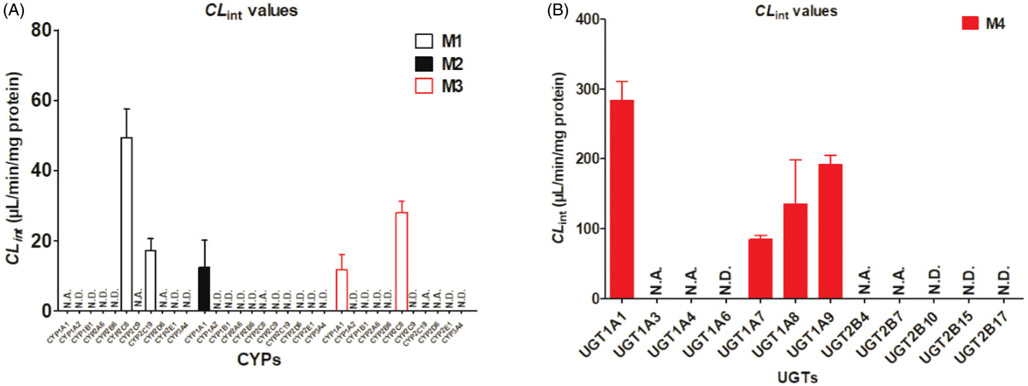
To investigate the CYP isoforms involving CA metabolism, twelve expressed CYP enzymes were incubated with CA. The results showed CYP2C8 and 2C19 primarily contributed to the formation of M1, while CYP1A1 mainly catalyzes the formation of M2 as well as the main contribution of CYP1A1 and 2C8 to the formation of M3. The detailed parameters of CA-related metabolites were listed in Table 1. Moreover, CYP1A2, 1B1, 2A6, 2B6, 2E1, 3A4 and 3A5 cannot metabolize CA at all.
Based on the reaction phenotyping results, kinetic profiles of CA-related oxidation were determined in CYP1A1, 2C8, 2C9, 2C19 and 2D6 with a series of substrate concentrations. The results indicated that kinetic profiles of M1 by CYP2C8 (Supplementary Figure S2(B)) and 2C19 (Supplementary Figure S2(C)) were well modeled by substrate inhibition kinetics as well as the kinetic profile of M2 by CYP1A1 (Supplementary Figure S2(A)) and the kinetic profiles of M3 by CYP1A1 (Supplementary Figure S2(A)) and CYP2C8 (Supplementary Figure S2(B)), which as same as the kinetics in HLM. The CLint values of M1 in CYP2C8 and CYP2C19 were 49.36 and 17.17 μL/min/mg, accompanying the Km values were 5.42 and 23.96 μM, respectively (Table 1). Similarly, the CLint value of M2 in CYP1A1 was 15.03 μL/min/mg with the Km value was 2.47 μM (Table 1). For the formation of M3, CYP1A1 and 2C8 showed the highest activity with CLint values of 13.01 and 28.11 μL/min/mg, accompanying the small variation in Km values of 2.27 and 3.53 μM, respectively (Table 1). Taken together, CYP2C8 was considered as the main CYP isozyme for the generation of M1 and M3 due to the highest CLint values (Figure 2(A); Table 2).
Figure 2.
The intrinsic clearance (CLint) values for the oxidation and glucuronidation of corylifol A by expressed CYP (A) and UGT enzymes (B), respectively. N.D.: not detected; N.A.: under the limit of quantification or unable to determine the kinetic parameters in the absence of a full kinetic profile. Error bars were the standards deviations of three measurements.
Table 2.
Kinetic parameters derived for corylifol A-related glucuronides (M4) by HLM, expressed UGTs enzymes, RLM, MLM, DLM, MkLM and RaLM (Mean ± SD).
| Meta. | Vmax (pmol/min/mg) | Km (μM) | Ki (μM) | CLint (μL/min/mg) | Model | |
|---|---|---|---|---|---|---|
| HLM | M4 | 4837 ± 595.9 | 2.64 ± 0.58 | 18.15 ± 4.51 | 1829 ± 464.3 | SI |
| M5 | + | + | + | + | ||
| UGT1A1 | M4 | 148.0 ± 4.30 | 0.52 ± 0.06 | 53.70 ± 8.03 | 284.07 ± 26.67 | SI |
| UGT1A3 | M4 | + | + | + | + | |
| UGT1A4 | M4 | + | + | + | + | |
| UGT1A7 | M4 | 440.8 ± 8.78 | 5.19 ± 0.32 | N.A. | 85.01 ± 0.06 | MM |
| UGT1A8 | M4 | 3044 ± 902.7 | 22.45 ± 7.94 | 6.98 ± 2.59 | 135.59 ± 62.56 | SI |
| UGT1A9 | M4 | 776.4 ± 23.24 | 4.05 ± 0.26 | 75.26 ± 6.26 | 191.94 ± 13.40 | SI |
| M5 | ++ | ++ | ++ | ++ | ||
| UGT2B4 | M4 | ++ | ++ | ++ | ++ | |
| UGT2B7 | M4 | ++ | ++ | ++ | ++ | |
| RLM | M4 | 2563 ± 333.6 | 2.81 ± 0.58 | 8.82 ± 1.91 | 911.1 ± 223.6 | SI |
| MLM | M4 | 6119 ± 531.3 | 4.56 ± 0.62 | 15.44 ± 2.35 | 1340 ± 218.3 | SI |
| DLM | M4 | 3054 ± 283.5 | 3.47 ± 0.62 | 51.38 ± 13.84 | 879.1 ± 177.8 | SI |
| MkLM | M4 | 6812 ± 1366 | 8.00 ± 2.08 | 5.50 ± 1.47 | 851.5 ± 279.6 | SI |
| RaLM | M4 | 2859 ± 249.4 | 3.08 ± 0.43 | 11.13 ± 1.67 | 927.6 ± 154.5 | SI |
All experiments were performed in triplicate.
HLM: human liver microsomes; Meta.: metabolites; MM: Michaelis–Menten model; N.A.: not available; +: under the limit of quantification; ++: unable to determine the kinetic parameters in the absence of a full kinetic profile; SI: substrate inhibition model.
Glucuronidation of CA in HLM and UGT isoforms
Compared to phase I metabolism, glucuronidation of CA was more efficient in both pooled HLM and UGT isoforms, as evidenced by the higher intrinsic clearance value (4-fold higher than phase I metabolism). Kinetic profiling indicated that the formation of M4 in pooled HLM (Supplementary Figure S1(B)) followed substrate inhibition kinetics, with the Km, Vmax and CLint values were 12.45 μM, 1843 pmol/min/mg proteins and 184.03 μL/min/mg proteins, respectively (Table 2).
Additionally, twelve recombinant UGT enzymes including UGT1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9, 2B4, 2B7, 2B10, 2B15 and 2B17 were applied to identify the main enzymes involving CA metabolism. As a result, UGT1A1, 1A7, 1A8 and 1A9 all showed strong catalytic activity to CA-glucuronidation, while UGT1A3, 1A4, 2B4 and 2B7 showed weak catalytic activity. The results suggested glucuronidation of CA by these UGT isoforms showed different kinetic profiles. The substrate inhibition kinetics model provides the best fit to the glucuronidation kinetic of CA in UGT1A1 (Supplementary Figure S3(A)), 1A8 (Supplementary Figure S3(C)) and 1A9 (Supplementary Figure S3(D)) with the CLint values of 284.07, 135.59 and 191.94 μL/min/mg proteins, respectively. In contrast, the generation of M4 in UGT1A7 exhibited the classical Michaelis–Menten profile (Supplementary Figure S3(B)) with minor CLint values (85.01 μL/min/mg proteins). Additionally, the Vmax of these UGT enzymes in M4 generation also followed the order of UGT1A8 (3044.0 pmol/min/mg) > UGT1A9 (776.4 pmol/min/mg) > UGT1A7 (440.8 pmol/min/mg) > UGT1A1 (148.0 pmol/min/mg) (Table 2). However, the concentrations of M5 in UGT1A9 incubated systems were too low to display a full kinetic profile and determine the kinetic parameters (Table 2). The reason may be that the side chain of 4′-OH group of CA hindered the occurrence of glucuronidation reaction, which was different from other isopentenyl flavones (Qin et al., 2018; Xu et al., 2018). To sum up, UGT1A1, 1A7, 1A8 and 1A9 were the main contributors to glucuronidation of CA (Figure 2(B)).
Activity correlation analyses
As mentioned, paclitaxel was regarded as the most widely used probe substrate for CYP2C8 (Xu et al., 2018), whereas β-estradiol and propofol were recognized as the probe substrate for UGT1A1 and 1A3, respectively (Hong et al., 2017). As shown in Supplementary Figure S4(A), a significant correlation was obtained between CA-mono-oxidation and paclitaxel-6-hydroxylation (r = 0.921, p = 0.001) in a panel of HLMs (n = 9). In addition, β-estradiol-3-O-glucuronidation (r = 0.668, p = 0.049, Supplementary Figure S4(B)) and propofol-O-glucuronidation (r = 0.771, p = 0.015, Supplementary Figure S4(C)) also had obvious correlation with CA-7-O-glucuronidation. These findings illustrated that CYP2C8 was the main contributor to the formation of M1 (Figure 2(A)), while UGT1A1 and 1A9 were the primary enzymes participated in CA glucuronidation in human liver (Figure 2(B)). Based on these data, metabolic pathways of CA involving CYP and UGT isozymes were summarized in Figure 3.
Figure 3.
Metabolic pathways of corylifol A involving in human CYP and UGT enzymes.
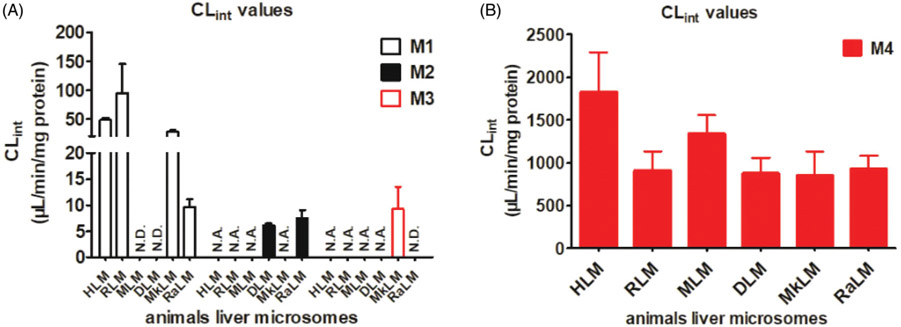
Species difference
To determine species differences in the microsomal metabolism of CA, the formation of metabolites (M1–M4) were monitored in rat, mouse, dog, monkey and rabbit liver microsomes in the presence of NADPH or UDPGA, respectively (Figure 4). Kinetics of CA-related metabolites in liver microsomes was characterized and the apparent kinetic parameters including Km, Vmax and CLint were estimated. As shown in Tables 1 and 2, M1 is the major oxidated metabolite of CA (Figure 4), while M4 is the primary glucuronide in all species tested, except mouse and dog (Figure 4). As demonstrated by Eadie–Hofstee plots, the formation of M1 in RLM (Supplementary Figure S5(A)) and RaLM (Supplementary Figure S5(D)) exhibited substrate inhibition kinetics with the CLint value of 95.01 and 9.70 μL/min/mg, while the kinetics of M1 formation in MkLM obeyed Michaelis–Menten equation with the CLint value of 28.25 μL/min/mg (Supplementary Figure S5(C)).
Figure 4.
The intrinsic clearance (CLint) values for the oxidation (A) and glucuronidation (B) of corylifol A by HLM and animal liver microsomes. N.D.: not detected; N.A.: under the limit of quantification or unable to determine the kinetic parameters in the absence of a full kinetic profile. Error bars were the standards deviations of three measurements.
Additionally, glucuronidation in all experimental animals′ liver microsomes followed substrate inhibition kinetics (Supplementary Figure S6). The CLint values for M4 by HLM (Supplementary Figure S1(B)), MLM (Supplementary Figure S6(B)), RaLM (Supplementary Figure S6(E)), RLM (Supplementary Figure S6(A)), DLM (Supplementary Figure S6(C)) and MkLM (Supplementary Figure S6(D)), were 1829, 1340, 927.6, 911.1, 879.1 and 851.5 μL/min/mg, respectively (Figure 4; Table 2). Taken together, these results implying that glucuronidation is a major metabolic pathway in all texted species, including human. However, none of the animal species are completely identical to human according to the kinetic parameters for M4. For the metabolism of CA, rats and monkeys were the best appropriate model animals.
Effect of chemical inhibitors and gene silencing on excretion of CA-O-glucuronide in HeLa1A1 cells
In this study, UGT1A1-overexpressing HeLa cells were used to elucidate the characteristics of excretion of CA-O-glucuronide (M4). The mRNA and protein levels of UGT1A1, MRP1, MRP2, MRP3, MRP4 and BCRP have been validated by Western blot assays recently (Qin et al., 2019; Zhang et al., 2019).
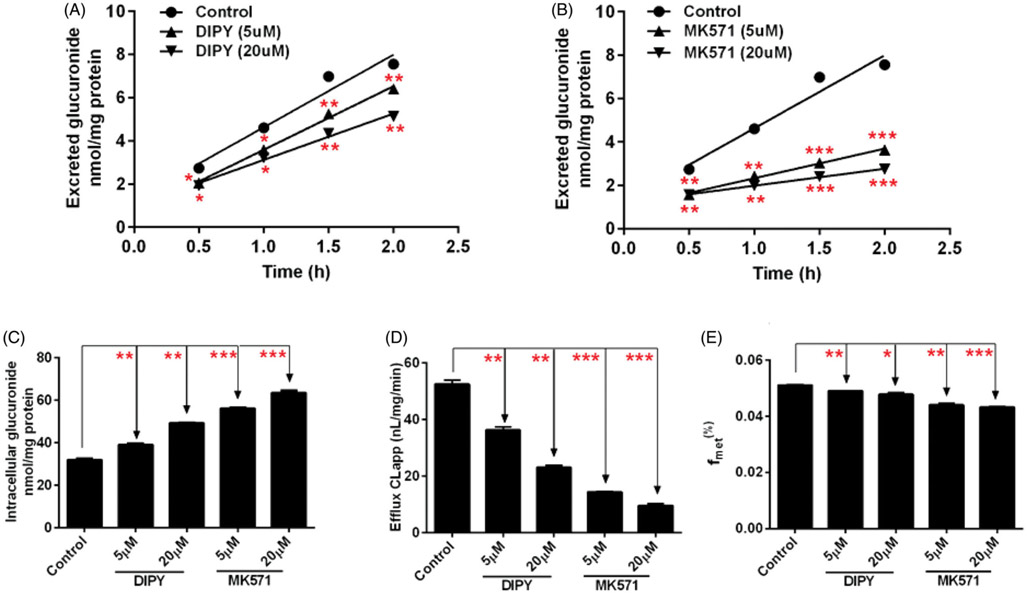
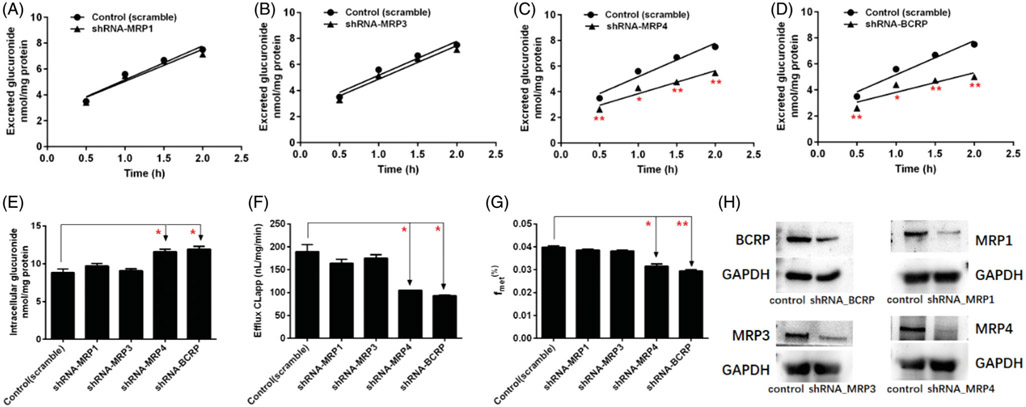
Contribution of MRPs transporters to cellular glucuronidation of CA was investigated using MK571 and shRNA-mediated gene knocked-down approach in HeLa1A1 cells. Prior to cell assays, experimental concentrations of chemical inhibitors and CA were inspected to ensure that they could not affect cell growth and UGT1A1 activity. In this study, CA (10 μM) with or without MK571 (5 or 20 μM) were incubated with HeLa1A1 cells and excretion rates of CA-O-glucuronide were determined and compared. As a result, MK571 led to 51.8%–63.6% reduction in excretion rate (ER) of CA-O-glucuronide (Figure 5(B)) but caused elevated intracellular accumulation of glucuronide (Figure 5(C)). Meantime, the CLapp value (representing the efflux efficiency of glucuronide) was substantial decreased (a 72.6%–81.7% reduction) (Figure 5(D)) and a significant reduction in the fmet value was observed (Figure 5(E)). In addition, as shown in Figure 6(H), shRNA-medicated silencing of MRP1, MRP3 and MRP4 transporters all significantly decreased the protein expression. Notably, it was found that knock-down of MRP4 transporter caused a significant decrease in ER values (Figure 6(C)) and CLapp values (Figure 6(F)) of CA-O-glucuronide, and fmet value of CA (Figure 6(G)). And MRP4 silencing also resulted in a marked reduction in intracellular levels of glucuronide (Figure 6(E)). However, shRNA-mediated silencing of MRP1 and MRP3 did not affect the excretion of CA-O-glucuronide with no significant changes in ER value, CLapp value, intracellular glucuronide and fmet value (Figure 6).
Figure 5.
Effects of chemical inhibitors on the excretion of corylifol A-glucuronide in HeLa1A1 cells. (A) Effects of DIPY (5 or 20 μM) on the excretion of glucuronide. (B) Effects of MK571 (5 or 20 μM) on the excretion of glucuronide. (C) Effects of DIPY and MK571 on the intracellular level of glucuronide. (D) Effects of DIPY and MK571 on the efflux clearance of glucuronide. (E) Effects of DIPY and MK571 on the fmet values of glucuronide. Each data point is the average of three determinations with the error bar representing the S.D. (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 6.
Effects of shRNA-mediated efflux transporters silencing on the excretion of corylifol A-glucuronide in HeLa1A1 cells. Effects of MRP1 (A), MRP3 (B), MRP4 (C) and BCRP (D) silencing on the excretion of glucuronide. (E) Effects of transporters silencing on the intracellular level of glucuronide. (F) Effects of transporters silencing on the efflux clearance of glucuronide. (G) Effects of transporters silencing on the fmet values of glucuronide. (H) Protein expression of BCRP and MRPs transporters in HeLa1A1 cells with and without shRNA-mediated gene silencing of corresponding transporters. Each data point is the average of three determinations with the error bar representing the S.D. (n = 3). *p < 0.05, **p < 0.01.
Furthermore, it was confirmed that a potent inhibitor of BCRP, DIPY (5 or 20 μM) caused a 30.8%–56.0% reduction of the CLapp value (Figure 5(D)) and significant decrease of fmet values (Figure 5(E)), and a statistical increase in the intracellular amounts of glucuronide levels (Figure 5(C)), and resulted in a moderate decrease in CA-O-glucuronide excretion (Figure 5(A)). Consistently, shRNA-mediated BCRP silencing resulted in a remarkable reduction in protein expression (Figure 6(H)), glucuronide excretion (Figure 6(D)) as well as an incremental intracellular accumulation of glucuronide (Figure 6(E)) in HeLa1A1 cells. Besides, the CLapp value with a 50.7% reduction (Figure 6(F)) and a decrease of fmet value (Figure 6(G)) also suggested that BCRP was a significant contributor to the excretion of CA-O-glucuronide.
Discussion
CA, isolated the seeds of Psoralea corylifolia (Cui et al., 2015), is a natural strong STAT3 inhibitor (Lee et al., 2012), which was associated with many diseases (Klampfer, 2006). Meanwhile, it account for 0.157–0.363% of the dried seed weight (Zhang et al., 2017). Pharmacokinetics studies indicated that CA could be rapidly absorbed into plasma and brain, and with poor bioavailability (Gao et al., 2016; Yang et al., 2018), while it could undergo massive glucuronidation and sulfation reactions (Geng et al., 2014; Wang et al., 2015). However, few researches were reported about the metabolism involving CYP and UGT enzymes. Traditionally, extensive metabolism and disposition could be a barrier to oral bioavailability as the first-pass metabolism of orally administered agents usually results in the poor oral bioavailability and lack of efficacies (Wu et al., 2011). Therefore, we performed the assays to determine the most important contributors for the metabolism and disposition of CA in this study.
Pooled HLM-mediated metabolism of CA results indicated that M1 (Figure 2(A)) and M4 (Figure 2(B)) were the most abundant metabolites. Furthermore, mono-oxidation of CA in CYP1A1 (Supplementary Figure S2(A)), 2C8 (Supplementary Figure S2(B)) and 2C19 (Supplementary Figure S2(C)), and the mono-glucuronidation by UGT1A1 (Supplementary Figure S3(A)), 1A8 (Supplementary Figure S3(C)), 1A9 (Supplementary Figure S3(D)) all followed substrate inhibition kinetics, which were similar with the kinetic profiles in pooled HLM (Supplementary Figure S1(B)). Besides, the Michaelis–Menten equation fit best to the glucuronidation of CA by UGT1A7 (Supplementary Figure S3(B)). Notably, phase I metabolic activities (ranging from 13.01 to 49.36 μL/min/mg, Table 1) were obviously weaker than the glucuronidation activities (85.01–284.07 μL/min/mg, Table 2). These results kept in line with our previous studies that glucuronidation were the main clearance pathways of isopentenyl flavones (corylin, neobavaisoflavone, icaritin, etc.) than oxidation reactions (Qin et al., 2018; Wang et al., 2018; Xu et al., 2018).
In this study, the relative catalytic activities of different CYP or UGT enzymes towards CA are evaluated based on the derived CLint values. Actually, the CLint values can be calculated not only by determining the t1/2 of substrate elimination, but also by the formation rates of produced metabolites. However, due to the possible interference of other non-metabolic pathways on the substrate consumption, there may be a large deviation in the values of CLint obtained by monitoring total substrate loss versus time using the in vitro t1/2 (Obach and Reed-Hagen, 2002). Furthermore, the use of CLint values (=Vmax/Km) as an indicator of CYP or UGT activity is advantageous, because (1) CLint represents the catalytic efficiency of the enzyme and is independent of the substrate concentration; (2) compared with other kinetic parameters such as Km and Vmax values, CLint value is more relevant in an attempt to predict hepatic clearance in vivo (Wu et al., 2013). And this calculation protocol for CLint values has been widely applied in previous studies (Qi et al., 2019; Qin et al., 2018a; Zhu et al., 2012).
Moreover, another approach, activity correlation analysis assay used specific probe substrate was applied to validate the active CYPs and UGTs of CA (Zhu et al., 2012). Our results suggested that the metabolism of CA was significantly correlated with paclitaxel-6-hydroxylation (Supplementary Figure S4(A)), β-estradiol-3-O-glucuronidation (Supplementary Figure S4(B)) and propofol-O-glucuronidation (Supplementary Figure S4(C)), which further proved that CYP2C8, UGT1A1 and 1A9 were important contributor for the metabolism of CA. Regretfully, we did not performed the activity correlation analysis by CYP1A1, 2C19, and UGT1A7, 1A8 because they were not detected in the human liver or are not widely accepted probe substrates (Xu et al., 2018).
In contrast, CA also could exhibit significant inhibitory or agitation effects on drug-metabolizing enzymes and nuclear receptors, following be catalyzed by these CYPs or UGTs enzymes (Li et al., 2015; Liu & Flynn, 2015; Ma et al., 2016; Wang et al., 2015, 2018). Psoralea corylifolia extracts showed the in vitro CYP3A4 (the most abundant liver enzyme) inhibition (IC50 = 6.0 μg/mL), which could cause potential drug-herbal medicines interactions but the roles of CA needed to be further investigated (Liu & Flynn, 2015). Besides, CA exhibited moderate inhibition on CYP2C19 and CYP2C9 isoforms with the relative activity of 1.0% and 0.8%, respectively, at a high concentration of 50 μM (Wang et al., 2018). In addition, CA also was moderate inhibitors against human UGT1A1 (IC50 = 0.65 μM & Ki = 0.79 μM) (Wang et al., 2015). And also, it was found to be naturally occurring potential inhibitor of human carboxylesterase 2, with low Ki value of 0.87 μM and IC50 value of 0.62 μM (Li et al., 2015). Moreover, CA displayed in vitro PPAR-γ activation (Ma et al., 2016). In general, nuclear receptors, including PPAR-γ activation could regulate the expression of drug-metabolizing enzymes, inducing a complex interaction among nuclear receptors, drug-metabolizing enzymes and biological natural compounds which needed to be explored in-depth.
Notably, herbal medicines–drug interactions were significant clinical safety concerns, especially the reports of liver and renal injury induced by Psoralea corylifolia (Cheung et al., 2009; Teschke and Bahre, 2009; Wang et al., 2012). Meanwhile, many cases about remarkably elevated plasma bilirubin and acute liver injury after exposure to Psoralea corylifolia and Psoralea corylifolia-containing prescriptions have been reported (Cheung et al., 2009; Teschke & Bahre, 2009; Wang et al., 2012). It seemed possible that chemical compounds or in vivo xenobiotics of Psoralea corylifolia may be responsible for the hepatotoxicity. The strong inhibitory effects on UGT1A1 enzyme of CA (IC50 = 0.65 μM) may be the reason of significant increase level of plasma bilirubin (Wang et al., 2015).
In addition, many clinical drugs, including warfarin, clopidogrel, celecoxib and so on, all were the substrates of CYP2C9 or 2C19 enzymes (https://www.pharmgkb.org/). This also added the potential risks of herbal medicines–drug interactions due to the inhibitory effects of CA on CYP2C19 and CYP2C9 isoforms (Wang et al., 2018). Similarly, several UGT1A1 substrates, like endogenous estradiol, bilirubin and clinical irinotecan, morphine, ezetimibe, all could be inhibited by CA and CA-containing herbal medicines, inducing clinical adverse reactions. Therefore, greater attention should be paid to avoid the potential risks of herbal medicines–drug interactions by considering the inhibitory effects of bioactive compounds on several human CYPs and UGTs (Lv et al., 2019; Zhou et al., 2019).
In this study, glucuronidation was the most efficient reaction of CA (Figure 2). Traditionally, the glucuronidation pathway involved at least two distinct and sequential processes, namely, glucuronide formation and excretion (Qin et al., 2018a; Zhang et al., 2015). The glucuronide formation process referred to the cellular production of glucuronides by UGT enzymes, while their transport from intracellular to extracellular compartments required the aids of efflux transporters. This phenomenon also created the interplay between UGT enzymes and efflux transporters (Qin et al., 2018a; Zhang et al., 2015). It was noted that glucuronide excretion (or production) was not always determined by UGT metabolism alone, and efflux transport occasionally could be the rate-limiting step governing the overall efficiency of glucuronidation in vivo (Jeong et al., 2005; Liu and Hu, 2007). In addition, it was widely accepted that the interplay between UGT enzymes and transporters facilitated the production and excretion of glucuronides in the intestine and liver, limiting the oral bioavailability of drugs (Qin et al., 2018a; Zhang et al., 2015). Meanwhile, our previous study have suggested BCRP and MRPs involved in efflux excretion of glucuronide conjugates in HeLa1A1 cells (Qin et al., 2018a). Our data suggested that MRP4 and BCRP as important determinants to disposition of CA in vivo (Figures 5 and 6).
Study of CYP and UGT enzymes that were responsible for metabolism of drug or bioactive compounds was also necessitated by the prevalence of CYP and UGT variants that were functionally different from the wide-types. Currently, functional UGT polymorphisms were systematically identified and newly identified variants were updated (http://www.pharmacogenomics.pha.ulaval.ca/sgc/ugt_alleles/). Most notably, CYP2C9, 2C19 and UGT1A1 were all highly polymorphic isoforms (García-Martín et al., 2006; Nagar and Blanchard, 2006; Zhao et al., 2008). Deficiency in their expressions and/or activities traditionally may lead to genetic and acquired diseases, such as Crigler-Najjar or Gilbert′s syndromes due to the complete absence of normal UGT1A1 enzyme or the association with UGT1A1*28, respectively (Beutler et al., 1998; Ritter et al., 1992). In this study, the gene expression of CYP2C9 (CYP2C9*2, CYP2C9*3, etc.), CYP2C19 (CYP2C19*2, CYP2C19*3, CYP2C19*17, etc.) and UUGT1A1 (UGT1A1*6, UGT1A1*28, etc.) should be detected to better understand the increased or decreased plasma concentrations of clinical drugs or biological natural products, causing enhanced or limited efficacy (García-Martín et al., 2006; Nagar and Blanchard, 2006; Zhao et al., 2008). Therefore, the oral bioavailability of CA could be affected by human CYP or UGT enzymes dysfunction or genetic polymorphisms.
Conclusion
In summary, this study summarized metabolic pathways of CA involving CYP and UGT enzymes (Figure 3). Three mono-oxidated metabolites and two mono-glucuronides were identified by UHPLC/Q-TOF-MS (Figure 1). In addition, two independent assays including reaction phenotyping (Figure 2) and activity correlation analysis (Supplementary Figure S4) results both suggested that CYP1A1, 2C8, 2C9, 2C19 and UGT1A1, 1A7, 1A8, 1A9 were determined as the most important contributors for CA-related metabolism. Meanwhile, glucuronidation was obviously more efficient than oxidation for CA metabolism (Figure 2). Moreover, rats and monkeys were the appropriate model animals for CA metabolism (Figure 3). Furthermore, chemical inhibition and gene knock-down of transporters suggested MRP4 and BCRP played dominant roles in disposition of CA (Figures 5 and 6). Taken together, elucidation of CA-related metabolic fates by CYP and UGT isozymes would be helpful for better understanding the pharmacokinetic behaviors and bioavailability of CA in vivo.
Supplementary Material
Acknowledgments
Funding
This work was supported by State Key Laboratory of Drug Research [SIMM1903KF-07], Guangdong Basic and Applied Basic Research Foundation [2019A1515011285], Foundation of He′nan Educational Committee [20A350012], National Natural Science Foundation of China [81903704], Program of Introducing Talents of Discipline to Universities [B13038], State Key Program of National Natural Science Foundation of China [81630097] and Major Project for International Cooperation and Exchange of the National Natural Science Foundation of China [81220108028].
Footnotes
Supplemental data for this article can be accessed here.
Disclosure statement
All the authors have reported no declarations of interest.
References
- Beutler E, Gelbart T, Demina A. (1998). Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proc Natl Acad Sci USA 95: 8170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung WI, Tse ML, Ngan T, et al. (2009). Liver injury associated with the use of Fructus Psoraleae (Bol-gol-zhee or Bu-gu-zhi) and its related proprietary medicine. Clin Toxicol (Phila) 47:683–5. [DOI] [PubMed] [Google Scholar]
- Cui Y, Taniguchi S, Kuroda T, Hatano T. (2015). Constituents of Psoralea corylifolia fruits and their effects on methicillin-resistant Staphylococcus aureus. Molecules 20:12500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Xu Z, Zhao G, et al. (2016). Simultaneous quantification of 5 main components of Psoralea corylifolia L. in rats′ plasma by utilizing ultra high pressure liquid chromatography tandem mass spectrometry. J Chromatogr B 1011:128–35. [DOI] [PubMed] [Google Scholar]
- García-Martín E, Martínez C, Ladero JM, Agúndez J. (2006). Interethnic and intraethnic variability of CYP2C8 and CYP2C9 polymorphisms in healthy individuals. Mol Diagn Ther 10:29–40. [DOI] [PubMed] [Google Scholar]
- Geng JL, Dai Y, Yao ZH, et al. (2014). Metabolites profile of Xian-Ling-Gu-Bao capsule, a traditional Chinese medicine prescription, in rats by ultra performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry analysis. J Pharm Biomed Anal 96:90–103. [DOI] [PubMed] [Google Scholar]
- Gu X, Huang J, Zhang L, et al. (2017). Efficient discovery and capture of new neuronal nitric oxide synthase-postsynaptic density protein-95 uncouplers from herbal medicines using magnetic molecularly imprinted polymers as artificial antibodies. J Sep Sci 40:3522–34. [DOI] [PubMed] [Google Scholar]
- Hong X, Zheng Y, Qin Z, et al. (2017). In vitro glucuronidation of wushanicaritin by liver microsomes, intestine microsomes and expressed human UDP-glucuronosyltransferase enzymes. Int J Mol Sci 18:1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong EJ, Liu X, Jia X, et al. (2005). Coupling of conjugating enzymes and efflux transporters: impact on bioavailability and drug interactions. Curr Drug Metab 6:455–68. [DOI] [PubMed] [Google Scholar]
- Kim DW, Seo KH, Curtis-Long MJ, et al. (2014). Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J Enzyme Inhib Med Chem 29:59–63. [DOI] [PubMed] [Google Scholar]
- Klampfer L (2006). Signal transducers and activators of transcription (STATs): novel targets of chemopreventive and chemotherapeutic drugs. Curr Cancer Drug Targets 6:561–71. [DOI] [PubMed] [Google Scholar]
- Lam W, Bussom S, Guan F, et al. (2010). The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci Transl Med 2:45ra59. [DOI] [PubMed] [Google Scholar]
- Lee SW, Yun BR, Kim MH, et al. (2012). Phenolic compounds isolated from Psoralea corylifolia inhibit IL-6-induced STAT3 activation. Planta Med 78:903–6. [DOI] [PubMed] [Google Scholar]
- Li YG, Hou J, Li SY, et al. (2015). Fructus Psoraleae contains natural compounds with potent inhibitory effects towards human carboxylesterase 2. Fitoterapia 101:99–106. [DOI] [PubMed] [Google Scholar]
- Liu Y, Flynn TJ. (2015). CYP3A4 inhibition by Psoralea corylifolia and its major components in human recombinant enzyme, differentiated human hepatoma HuH-7 and HepaRG cells. Toxicol Rep 2:530–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Hu M. (2007). Natural polyphenol disposition via coupled metabolic pathways. Expert Opin Drug Metab Toxicol 3:389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Dong D, Liu Z, et al. (2015). Metabolism elucidation of BJ-B11 (a heat shock protein 90 inhibitor) by human liver microsomes: identification of main contributing enzymes. Expert Opin Drug Metab Toxicol 11:1029–40. [DOI] [PubMed] [Google Scholar]
- Lv X, Xia Y, Finel M, et al. (2019). Recent progress and challenges in screening and characterization of UGT1A1 inhibitors. Acta Pharm Sin B 9:258–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Huang Y, Zhao Y, et al. (2016). Prenylflavone derivatives from the seeds of Psoralea corylifolia exhibited PPAR-γ agonist activity. Phytochem Lett 16:213–8. [Google Scholar]
- Nagar S, Blanchard RL. (2006). Pharmacogenetics of uridine diphosphoglucuronosyltransferase (UGT) 1A family members and its role in patient response to irinotecan. Drug Metab Rev 38:393–409. [DOI] [PubMed] [Google Scholar]
- Obach RS, Reed-Hagen AE. (2002). Measurement of Michaelis constants for cytochrome P450-mediated biotransformation reactions using a substrate depletion approach. Drug Metab Dispos 30:831–7. [DOI] [PubMed] [Google Scholar]
- Qi C, Fu J, Zhao H, et al. (2019). Identification of UGTs and BCRP as potential pharmacokinetic determinants of the natural flavonoid alpinetin. Xenobiotica 49:276–83. [DOI] [PubMed] [Google Scholar]
- Qin Z, Li S, Yao Z, et al. (2018a). Chemical inhibition and stable knock-down of efflux transporters leads to reduced glucuronidation of wushanicaritin in UGT1A1-overexpressing HeLa cells: the role of breast cancer resistance protein (BCRP) and multidrug resistance-associated proteins (MRPs) in the excretion of glucuronides. Food Funct 9: 1410–23. [DOI] [PubMed] [Google Scholar]
- Qin Z, Li S, Yao Z, et al. (2018). Metabolic profiling of corylin in vivo and in vitro. J Pharm Biomed Anal 155:157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Zhang B, Yang J, et al. (2019). The efflux mechanism of fraxetin-O-glucuronides in UGT1A9-transfected HeLa cells: identification of multidrug resistance-associated proteins 3 and 4 (MRP3/4) as the important contributors. Front Pharmacol 10:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter JK, Yeatman MT, Ferreira P, Owens IS. (1992). Identification of a genetic alteration in the code for bilirubin UDP-glucuronosyltransferase in the UGT1 gene complex of a Crigler-Najjar type I patient. J Clin Investig 90:150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Yang X-Z, Yuan J-Q. (2013). Cytotoxic constituents from Psoralea corylifolia. J Asian Nat Prod Res 15:624–30. [DOI] [PubMed] [Google Scholar]
- Teschke R, Bahre R. (2009). Severe hepatotoxicity by Indian Ayurvedic herbal products: a structured causality assessment. Ann Hepatol 8: 258–66. [PubMed] [Google Scholar]
- Wang J, Jiang Z, Ji J, et al. (2012). Evaluation of hepatotoxicity and cholestasis in rats treated with EtOH extract of Fructus Psoraleae. J Ethnopharmacol 144:73–81. [DOI] [PubMed] [Google Scholar]
- Wang L, Hai Y, Huang N, et al. (2018). Human cytochrome P450 enzyme inhibition profile of three flavonoids isolated from Psoralea corylifolia: in silico predictions and experimental validation. New J Chem 42: 10922–34. [Google Scholar]
- Wang L, Hong X, Yao Z, et al. (2018). Glucuronidation of icaritin by human liver microsomes, human intestine microsomes and expressed UDP-glucuronosyltransferase enzymes: identification of UGT1A3, 1A9 and 2B7 as the main contributing enzymes. Xenobiotica 48:357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PL, Yao ZH, Zhang FX, Shen XY, et al. (2015). Identification of metabolites of PSORALEAE FRUCTUS in rats by ultra performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry analysis. J Pharm Biomed Anal 112:23–35. [DOI] [PubMed] [Google Scholar]
- Wang XX, Lv X, Li SY, et al. (2015). Identification and characterization of naturally occurring inhibitors against UDP-glucuronosyltransferase 1A1 in Fructus Psoraleae (Bu-gu-zhi). Toxicol Appl Pharmacol 289:70–8. [DOI] [PubMed] [Google Scholar]
- Wu B, Kulkarni K, Basu S, et al. (2011). First-pass metabolism via UDP-glucuronosyltransferase: a barrier to oral bioavailability of phenolics. J Pharm Sci 100:3655–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Dong D, Hu M, Zhang S. (2013). Quantitative prediction of glucuronidation in humans using the in vitro–in vivo extrapolation approach. Curr Top Med Chem 13:1343–52. [DOI] [PubMed] [Google Scholar]
- Xu J, Li M, Yao Z, et al. (2018). In vitro metabolic mapping of neobavaisoflavone in human cytochromes P450 and UDP-glucuronosyltransferase enzymes by ultra high-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. J Pharm Biomed Anal 158:351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang B, Qin Z, et al. (2018). Efflux excretion of bisdemethoxycurcumin-O-glucuronide in UGT1A1-overexpressing HeLa cells: identification of breast cancer resistance protein (BCRP) and multidrug resistance-associated proteins 1 (MRP1) as the glucuronide transporters. BioFactors 44:558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YF, Zhang YB, Chen ZJ, et al. (2018). Plasma pharmacokinetics and cerebral nuclei distribution of major constituents of Psoraleae fructus in rats after oral administration. Phytomedicine 38:166–74. [DOI] [PubMed] [Google Scholar]
- Zhang B, Yang J, Qin Z, et al. (2019). Mechanism of the efflux transport of demethoxycurcumin-O-glucuronides in HeLa cells stably transfected with UDP-glucuronosyltransferase 1A1. PLoS One 14:e0217695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Dong D, Wang H, et al. (2015). Stable knock-down of efflux transporters leads to reduced glucuronidation in UGT1A1-overexpressing HeLa cells: the evidence for glucuronidation-transport interplay. Mol Pharm 12:1268–78. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen Z, Xu X, et al. (2017). Rapid separation and simultaneous quantitative determination of 13 constituents in Psoraleae Fructus by a single marker using high-performance liquid chromatography with diode array detection. J Sep Sci 40:4191–202. [DOI] [PubMed] [Google Scholar]
- Zhao F, Wang J, Yang Y, et al. (2008). Effect of CYP2C19 genetic polymorphisms on the efficacy of H. pylori eradication proton pump inhibitor-based triple therapy for Helicobacter pylori eradication: a meta-analysis. Helicobacter 13:532–41. [DOI] [PubMed] [Google Scholar]
- Zhou QH, Zhu Y-D, Zhang F, et al. (2019). Interactions of drug-metabolizing enzymes with the Chinese herb Psoraleae Fructus. Chin J Nat Med 17:858–70. [DOI] [PubMed] [Google Scholar]
- Zhu L, Ge G, Liu Y, et al. (2012). Characterization of UDP-glucuronosyltransferases involved in glucuronidation of diethylstilbestrol in human liver and intestine. Chem Res Toxicol 25:2663–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.