Abstract
Ergosterol is an essential component of fungal cell membranes that determines the fluidity, permeability and activity of membrane-associated proteins. Ergosterol biosynthesis is a complex and highly energy-consuming pathway that involves the participation of many enzymes. Deficiencies in sterol biosynthesis cause pleiotropic defects that limit cellular proliferation and adaptation to stress. Thereby, fungal ergosterol levels are tightly controlled by the bioavailability of particular metabolites (e.g., sterols, oxygen and iron) and environmental conditions. The regulation of ergosterol synthesis is achieved by overlapping mechanisms that include transcriptional expression, feedback inhibition of enzymes and changes in their subcellular localization. In the budding yeast Saccharomyces cerevisiae, the sterol regulatory element (SRE)-binding proteins Upc2 and Ecm22, the heme-binding protein Hap1 and the repressor factors Rox1 and Mot3 coordinate ergosterol biosynthesis (ERG) gene expression. Here, we summarize the sterol biosynthesis, transport and detoxification systems of S. cerevisiae, as well as its adaptive response to sterol depletion, low oxygen, hyperosmotic stress and iron deficiency. Because of the large number of ERG genes and the crosstalk between different environmental signals and pathways, many aspects of ergosterol regulation are still unknown. The study of sterol metabolism and its regulation is highly relevant due to its wide applications in antifungal treatments, as well as in food and pharmaceutical industries.
Keywords: ergosterol, sterol biosynthesis, sterol regulation, yeast, Saccharomyces cerevisiae, oxygen, iron
1. Introduction
Sterols are essential components of eukaryotic cellular membranes that maintain membrane structural integrity, fluidity and permeability. They have further functions in regulating membrane-bound enzyme activity, lipid raft formation and function, substance transportation and cell cycle. The most important sterol in animals is cholesterol, which is additionally necessary as a precursor for the synthesis of vitamin D, bile acids and steroid hormones. Disturbed cholesterol homeostasis can lead to several human diseases, such as diabetes, atherosclerosis and neurodegeneration [1]. Plant sterols are called phytosterols, and the most notable are stigmasterol, sitosterol and campesterol. They are essential for plant growth and development, and play an important role in stress adaptation [2]. The main fungal sterol is ergosterol, which is regarded as a “fungal hormone” that can stimulate growth and proliferation. Ergosterol has recently been identified as an immunoactive lipid that induces host cells pyroptosis, a necrotic and inflammatory programmed cell death [3]. Significantly, recent studies have shown that ergosterol is essential for mitochondrial DNA maintenance in fungi, as cholesterol does in humans [4,5]. In fact, the pharmacological or genetic inhibition of ergosterol biosynthesis leads to the loss of mitochondrial DNA in S. cerevisiae unless exogenous ergosterol is added, highlighting the crosstalk between mitochondria and ergosterol biosynthesis [4]. Thus, ergosterol abundance is critical for yeast stress adaptation. For example, increased ergosterol levels have been associated with higher resistance to low temperature, freezing, low sugar, alcohol and oxidative stress. Conversely, hyperosmotic stress adaptation (such as salt or sorbitol treatment) leads to the decrease in ergosterol abundance [6,7]. Ergosterol levels are also important for the hypoxic and iron deficiency responses in S. cerevisiae, conditions in which the rate of ergosterol biosynthesis is limited by defects in specific enzymes that depend on oxygen and/or iron as an essential substrate or cofactor [8,9].
As a result of their versatile properties, sterols show wide applications in food and pharmaceutical industries [8]. The industrial production of ergosterol is achieved by yeast fermentation or extraction from fungal mycelia. Ergosterol, which is structurally analogous to cholesterol, has been used as a precursor of vitamin D2 and steroid hormone drugs. Intermediates of the ergosterol biosynthesis pathway, such as farnesyl diphosphate and squalene, are also economically interesting due to their use in terpenoid production, and for applications such as perfume ingredients, pharmaceuticals and advanced biofuels [10]. Moreover, ergosterol derivatives have significant antitumor and anti-HIV activities [11,12]. Remarkably, the ergosterol biosynthetic pathway represents the main target for the development of many antifungal agents because it is essential for fungal growth and viability, and its synthesis differs in particular steps from the major sterol-producing pathway in humans.
2. Ergosterol Synthesis, Uptake and Detoxification in S. cerevisiae
2.1. Ergosterol Biosynthesis in S. cerevisiae
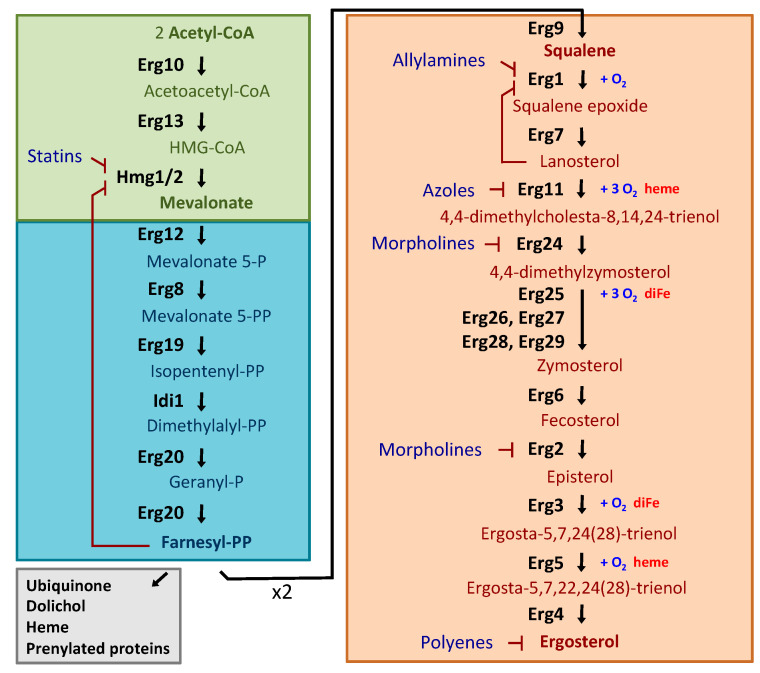
The budding yeast S. cerevisiae is a popular model organism to study intracellular sterol homoeostasis because the basic steps of yeast sterol metabolism and trafficking are conserved in other eukaryotic organisms. Under aerobic conditions, yeast cells do not incorporate exogenous sterols. Instead, they satisfy their sterol requirements by synthesizing their own ergosterol. Yeast ergosterol is synthesized through a highly conserved and complex pathway that can be divided into three modules (Figure 1) (reviewed in [6]). The first module is conserved across all eukaryotes and results in the formation of mevalonate from acetyl-coenzyme A (acetyl-CoA). The rate-limiting step of this stage is the reduction to mevalonate by the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductases (HMGR) Hmg1 and Hmg2 (Hmg1/2), which are derived from a single ancestral HMGR gene by gene duplication. The second module is carried out in the vacuole and involves the formation of farnesyl pyrophosphate (farnesyl-PP), which is also an important intermediate in the biosynthesis of ubiquinone, dolichol, heme and prenylated proteins. The third module or late pathway involves the ergosterol synthesis itself through consecutive reactions that mainly occur in the endoplasmic reticulum (ER) membrane. Firstly, two molecules of farnesyl-PP are used by the squalene synthase Erg9 enzyme to form squalene, which is the precursor of all steroids. Secondly, squalene is converted into lanosterol by the consecutive action of the squalene epoxidase Erg1 and the lanosterol synthase Erg7 enzymes. In the next steps, lanosterol is transformed to zymosterol through a complex process involving various demethylation, reduction and desaturation reactions catalyzed by the lanosterol 14-α-demethylase Erg11 (also known as Cyp51), the C-14 reductase Erg24 and the C-4 demethylation complex Erg25-Erg26-Erg27. Erg28 and Erg29 are likely to function in the C-4 demethylation complex reaction [8,13]. Zymosterol is the first intermediate of the biosynthesis pathway that can be incorporated into cellular membranes [14]. Then, Erg6 converts zymosterol into fecosterol, followed by the formation of episterol by Erg2, which is finally desaturated and reduced by Erg3, Erg5 and Erg4 to ergosterol. Ergosterol is synthesized in the ER, but is mostly transported to the plasma membrane (PM). Erg1 and Erg11 represent two rate-limiting steps in this part of the pathway. Ergosterol biosynthesis depends on both oxygen and iron in multiple enzymatic steps (Figure 1). Molecular oxygen is the electron acceptor in the enzymatic steps catalyzed by Erg1, Erg11, Erg25, Erg3 and Erg5; and heme, whose biosynthesis requires oxygen and iron, directly associates to Erg11 and Erg5, as well as to Erg25 and Erg3 as a cytochrome b5 cofactor (Figure 1). Moreover, Erg11 and Erg5 belong to the cytochrome P450 family of enzymes that function in association with regulators such as the P450 reductase Ncp1 or the heme-binding protein Dap1. Meanwhile, Erg25 and Erg3 are oxo-diiron enzymes of the fatty acid hydroxylase/sterol desaturase family (Figure 1). Thus, oxygen and iron depletion are associated with reduced activity of these enzymes and changes in sterol production.
Figure 1.
Ergosterol biosynthetic pathway in S. cerevisiae. The different color boxes represent the three modules into which the pathway can be divided: the green box is the mevalonate pathway, which occurs in the vacuole and mitochondria; the blue box consists of farnesyl pyrophosphate (farnesyl-PP) biosynthesis and is carried out in the vacuole; and the orange box contains the late pathway, which ends with ergosterol biosynthesis, and mainly takes place in the ER. In addition, farnesyl-PP is used in the synthesis of ubiquinone, dolichol, heme and prenylated proteins (gray box). Enzymes, intermediates, inhibitors and requirements of oxygen, heme and iron are indicated. Enzymes: Erg10, acetyl-CoA C-acetyltransferase; Erg13, HMG-CoA synthase; Hmg1/2, HMG-CoA reductase; Erg12, mevalonate kinase; Erg8, phosphomevalonate kinase; Mvd1/Erg19, mevalonate pyrophosphate decarboxylase; Idi1, Isopentenyl diphosphate Isomerase; Erg20, farnesyl pyrophosphate synthetase; Erg9, squalene synthase; Erg1, squalene epoxidase; Erg7, lanosterol synthase; Erg11 (Cyp51), lanosterol C-14 demethylase; Erg24, sterol C-14 reductase; Erg25, sterol C-4 methyloxydase; Erg26, sterol C-3 dehydrogenase (C4-decarboxylase); Erg27, sterol C-3 ketoreductase; Erg6, sterol C-24 methyltransferase; Erg2, sterol C-8 isomerase; Erg3, sterol C-5 desaturase; Erg5, sterol C-22 desaturase; Erg4, sterol C-24 reductase. Inhibitors: statins target Hmg1/2; allylamines inhibit Erg1; azoles inhibit Erg11; morpholines target Erg2 and, mainly, Erg24; polyenes bind ergosterol.
Defects in ergosterol biosynthesis lead to impaired endocytosis, cell polarization, cell fusion and cell wall assembly (reviewed in [15]). The single deletion of the majority of ergosterol biosynthetic (ERG) genes within the late pathway is lethal under standard growth conditions without the addition of ergosterol. The only exceptions are the last five enzymes, encoded by the ERG2 to ERG6 genes, probably due to the similar physicochemical properties that the intermediates accumulated in these mutants exhibit with respect to ergosterol, and ERG28. Curiously, sterol profiling suggests that Erg2 to Erg6 enzymes display low substrate specificity and can accept a broad range of similar sterol structures. Thereby, their deletion leads to the accumulation of sterol mixtures instead of only their substrate in the pathway (reviewed in [16]). For instance, the erg6 mutant accumulates its substrate and several sterols resulting from the catalytic activity of Erg2, Erg3 and Erg5 on zymosterol [17]. These erg mutants are defective in different cellular processes and show alterations in the resistance to certain stresses (reviewed in [16,18]). The deletion of one of the two isozymes encoded by HMGR (HMG1 or HMG2) has only a little effect on cell growth, but the double mutant is inviable. Significantly, the overexpression of each ERG gene leads to a great variability in the tolerance to stressors and antifungal drugs, which reflects the specialization of each enzyme and the pleiotropic nature of ergosterol biosynthesis disruption [19].
The main targets for antifungal drugs are the enzymes that constitute the last ergosterol biosynthesis module (Figure 1). Allylamines are noncompetitive inhibitors of fungal Erg1 and are effective against dermatophyte infections [20]. Azoles, the most common drug used to treat fungal infections, target Erg11 by directly binding to the iron atom within the heme group of the enzyme [21]. When Erg11 is inhibited, an alternate pathway catalyzed by Erg6, Erg25-Erg26-Erg27 and Erg3 is activated leading to the formation of the fungistatic 14α methylergosta 8-24 (28) dienol [19,22]. Thus, mutations in Candida albicans ERG6 and ERG3 genes lead to the development of azole resistance [19,23]. Morpholines are used as agricultural fungicides via inhibition of two separate steps, Erg2 and, particularly, Erg24 [24]. Polyenes, including nystatin and amphotericin B, which are frequently used in the medical treatment of systemic infections, directly interact with cell surface ergosterol [25]. In addition, statins competitively inhibit human and fungal Hmg1, and are commonly used in humans to lower cholesterol levels. The only Erg enzymes that are not conserved in mammals are Erg6, Erg2, Erg5 and Erg4 (reviewed in [26]). Of them, both the deletion and the overexpression of ERG6 provoke the most compromised phenotypes suggesting that it could be a suitable target for a new generation of antifungal agents [19,27].
2.2. Sterol Acquisition and Transport in S. cerevisiae
Ergosterol biosynthesis is a highly energy-consuming process that requires 24 molecules of ATP and 16 molecules of NADPH to obtain a single ergosterol compound (reviewed in [6]). Despite this, S. cerevisiae cells do not import significant amounts of extracellular sterols in the presence of oxygen, a phenomenon referred to as “aerobic sterol exclusion” [28]. It has been proposed that aerobic sterol exclusion could be a strategy to ensure that only the most adequate sterols are incorporated to membranes. The decreased ability to synthesize sterols under hypoxic or anaerobic conditions is compensated by the import of sterols from the medium. Different sterol uptake responses have been observed for fungi like Schizosaccharomyces pombe, Candida glabrata and C. albicans [29,30]. Due to their hydrophobicity, sterols are transported inside cells either in a vesicular manner or via lipid binding/transfer proteins. The last mechanism seems to be the prevailing one in S. cerevisiae, since the inhibition of vesicular transport through the secretory pathway does not significantly affect sterol transport to the PM (reviewed in [18]). In S. cerevisiae, sterol uptake initiates with the interaction between the external sterol and the cell wall, followed by its incorporation into the PM in an ATP-dependent manner by the PM ATP-binding cassette (ABC) transporters Aus1 and Pdr11 [31]. Then, sterol sphingolipid-enriched microdomains, called rafts, are formed, and sterols are progressively transported from the cell surface to the ER through a non-vesicular process also mediated by Aus1 and Pdr11 [32,33]. In the ER, sterols are partly esterified to be stored in lipid particles (LPs), and the non-esterified sterols are returned to the PM [32]. Sterol esterification is essential for efficient sterol uptake [32]. After shifting to aerobic conditions, newly synthesized ergosterol is likely to displace the exogenous sterols at the PM [34].
Despite the ER being the site of sterol biosynthesis, it contains very little ergosterol, which is mostly transported to other cellular membranes, especially the PM. Most intracellular sterol trafficking seems to take place through non-vesicular transport. Two families of evolutionary conserved sterol-binding proteins mediate intracellular sterol distribution: oxysterol-binding protein homologs (Osh1 to Osh7) and lipid transfer proteins anchored at membrane contact sites (Lam1 to Lam6) [35,36,37,38]. Another protein that is involved in sterol transport and exogenous sterol uptake is Arv1, which seems to catalyze the insertion of tail-anchored proteins with a single carboxy-terminal transmembrane domain into membranes [39,40,41].
2.3. Sterol Detoxification
Yeast cells can overproduce ergosterol or sterol intermediates, which can be incorporated into cellular membranes to some extent, to modulate their physicochemical properties. However, the accumulation of excess free sterol intermediates may become toxic for cells since it alters mitochondrial respiration, resulting in the generation of toxic methyl sterol intermediates, which raise mitochondrial oxidants and decrease the ability to synthesize iron–sulfur (Fe-S) clusters [13,42,43]. To prevent these harmful effects, yeast cells utilize multiple detoxification mechanisms. Sterols cannot be degraded, but are either stored in the form of steryl esters (SE) in LPs or secreted into the medium as sterol acetates (reviewed in [6,18]). Under aerobic conditions, SE formation in the ER is catalyzed mainly by Are2, which has a significant preference for ergosterol as a substrate, whereas Are1 performs this function in hypoxia [44]. Conversely, Yeh1 and Tgl1 catalyze SE hydrolysis in LPs, and Yeh2 in the PM [45,46,47]. Esterified sterol reserves can be interconverted into free sterols and mobilized when ergosterol levels decrease. Interestingly, the double mutant are1∆are2∆ does not display any altered growth under normal conditions, despite overall sterol biosynthesis drops and the level of free sterols increases [44,48]. These findings suggest that sterol biosynthesis and SE formation are interconnected through a regulatory mechanism. In fact, it has been reported that the expression of ERG3 is down-regulated and Erg1 protein is destabilized in the are1∆are2∆ double mutant [48,49,50]. In another detoxification mechanism, sterols are acetylated by Atf2 in the ER and then transported to the PM, where they are secreted via Pry1 and Pry2 (pathogen-related yeast proteins) [51,52]. Under normal conditions, newly synthesized ergosterol is acetylated by Atf2 and then rapidly deacetylated by Say1 in the ER [51]. Besides detoxification, sterol acetylation also contributes to the elimination of damaged lipids.
3. Regulation of Ergosterol Biosynthesis
To alter the ergosterol composition of lipid bilayers and, consequently, to be able to properly adapt to particular environmental stresses, yeast cells have evolved different regulatory mechanisms that tightly control sterol levels. The maintenance of suitable ergosterol concentrations is achieved by feedback mechanisms at the transcriptional and post-translational levels.
3.1. Subcellular Localization of Ergosterol Biosynthesis Enzymes
Multiple enzymes that participate in the late stage of ergosterol biosynthesis are transmembrane-containing proteins that associate into a functional complex, denoted the ergosome, to facilitate catalysis [53]. Erg11, Erg25, Erg27 and Erg28 represent the core center of the complex that interacts with other enzymes of the pathway [53]. Although sterol synthesis takes place in the ER, various Erg enzymes (Erg1, Erg7, Erg27 and Erg6) can also localize to LPs [54,55], which are not only universal storage organelles for neutral lipids. The dual localization of sterol enzymes in both subcellular organelles is also observed in cholesterol biosynthesis [56]. The association and subcellular localization of Erg enzymes seems to determine their functionality, although its regulation is poorly characterized. Erg1 displays a dual localization at the ER and LPs, being enzymatically inactive in the latter [57]. When ergosterol synthesis decreases due to iron deficiency, Erg1 exclusively localizes to the ER, where it is active, probably to enhance the iron-limited production of ergosterol [9]. Erg7 also displays a dual ER and LP localization, whereas Erg11 and Erg24 localize to the ER membrane. Erg28 functions as a scaffold of the C-4 demethylation enzyme complex Erg25–Erg26–Erg27 tethering it to the ER, and acting as a bridge to the Erg6 enzyme required for the next biosynthetic step [58,59]. As indicated above, Erg27 can also localize to LPs, where it physically binds to Erg7, promoting its association with LPs and preventing its degradation [60]. Upon a block in mitochondrial respiration, Erg27 migrates rapidly from LPs to the ER, although this does not imply the relocation of Erg7 [4]. Interestingly, a fusion between Erg7 and the ER-resident protein Erg28 restores ergosterol biosynthesis to erg27 mutants, suggesting that the retention of Erg7 in the ER increases its activity [61]. Interestingly, Erg6 is mostly located in LPs [57], but can also be found in the ER, the cytoplasm and mitochondria. Finally, Erg2, Erg3, Erg5 and Erg4 are located primarily in the ER, from where ergosterol is distributed to its final membranous destinations [44,55].
3.2. Post-Translational Feedback Regulation
A key checkpoint for ergosterol biosynthesis involves the synthesis of HMG-CoA, catalyzed by HMGR (reviewed in [62]). Given that HMGR acts at the initial steps of the pathway, its regulation alters the synthesis of all sterols but also of other essential mevalonate-derived metabolites, such as ubiquinone, dolichol or heme. In S. cerevisiae, excess of oxysterols promotes the degradation of the Hmg2 enzyme by the ER-related degradation (ERAD) pathway. This process does not require the typical ERAD factors; instead, Hmg2 ubiquitination is mediated by the membrane-spanning E3 ligase Hrd1 and the E2 ubiquitin conjugating enzyme Ubc7, and it is subsequently released from the ER to the cytoplasm, where it is degraded by the proteasome [63,64]. The yeast squalene monooxygenase Erg1 is also degraded through the ERAD pathway via the ubiquitin ligase Doa10 when lanosterol concentrations increase, in order to prevent the accumulation of toxic sterol intermediates (Figure 1) [65]. Both ERAD-dependent mechanisms seem to be conserved in mammalian cells.
3.3. Transcriptional Regulation
3.3.1. Transcriptional Regulation by Sterols
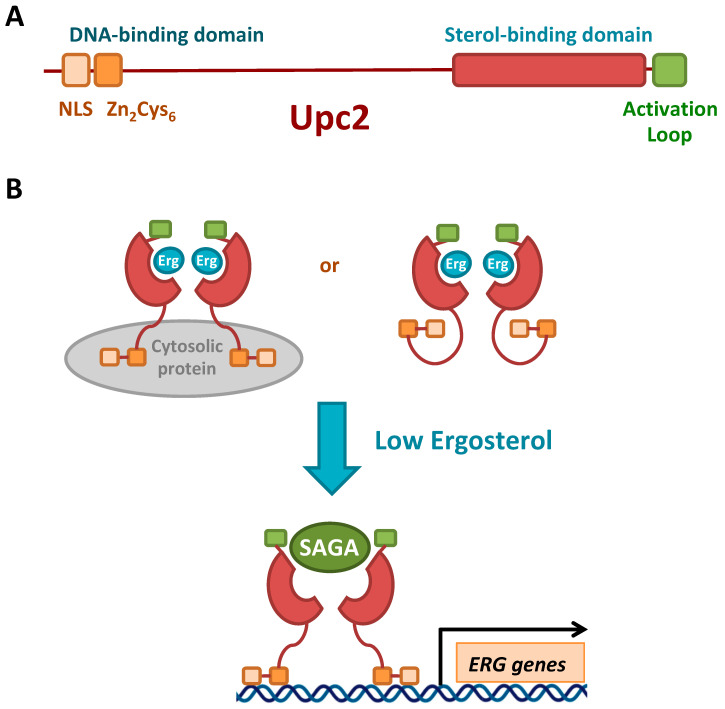
The expression of many enzymes within the ergosterol biosynthesis pathway is regulated at the transcriptional level by sterol abundance through the action of transcriptional factors that bind to 7-base pair DNA motifs, known as sterol regulatory elements (SREs), located in the promoter of their corresponding genes (reviewed in [26]). In mammals, the membrane-bound basic helix-loop-helix transcription factor sterol regulatory element binding protein (SREBP) is cleaved from the ER membrane by the SREBP cleavage activating protein (SCAP) and activated in response to sterol depletion. Most fungi, including the fission yeast S. pombe and the opportunistic pathogen Cryptococcus neoformans contain homologs of both SREBP and SCAP, known as Sre1 and Scp1, respectively [29,66]. SCAP and Scp1 constitute the sterol sensors in mammals and S. pombe, respectively [29,67]. Remarkably, the SREBPs from S. cerevisiae and the fungal commensal-pathogen C. albicans do not regulate sterol synthesis; instead they control filamentous growth. During Saccharomycotina evolution, a member of the fungus specific Zn2-Cys6 binuclear cluster family of transcription factors called Upc2 displaced SREBP proteins as the major sterol regulator [68,69,70]. Due to the whole genome duplication, S. cerevisiae also expresses a Upc2 paralog, known as Ecm22. Both transcription factors are able to bind, through their amino-terminal Zn2-Cys6 DNA-binding domain, to TATACGA SREs, which are identical to the anaerobic response elements AR1c (Figure 2A) [68]. The recent elucidation of the structure of the carboxy-terminal domain (CTD) of yeast Upc2, which displays a novel α-helical fold, has demonstrated that it serves as an ergosterol-binding and sensing domain (Figure 2A) [71]. Under normal conditions, the binding of ergosterol to a deep hydrophobic pocket within Upc2 CTD retains the transcription factor in the cytosol in its repressed form, probably because sterol binding masks its bipartite amino-terminal nuclear localization signal (NLS) or because of its interaction with a hypothetical cytosolic protein (Figure 2B) [71,72]. Upon ergosterol depletion, the dissociation of the sterol ligand causes a conformational change that exposes Upc2 NLS, causing its translocation to the nucleus, where it activates the transcription of SRE/AR1c-containing genes including most ERG genes, sterol uptake genes (AUS1, PDR11), the DAN/TIR genes, which encode nine cell wall mannoproteins, and members of the seripauperin (PAU) gene family (Figure 2B) [68,71,72,73,74,75]. The conformational flexibility of the carboxy-terminal 30 amino acids of Upc2 constitute an activation loop that, in addition to sterol binding and the regulation of subcellular localization, contributes to transcriptional activation [71]. The dissociation of ergosterol from the Upc2 ligand-binding domain could also promote conformational changes that allow the activation loop to recruit downstream transcriptional Upc2 co-activators (Figure 2B). Recent data using an erg3 mutant strain indicate that the SAGA co-activator complex is recruited to the promoter of ERG genes to promote their transcriptional activation [76]. Therefore, it has been proposed that the SAGA complex could be the co-activator that Upc2 recruits to enhance the expression of ERG genes when sterols abundance decreases, although no direct evidence exists (Figure 2B). Moreover, size-exclusion chromatography analyses strongly suggest that the biologically relevant form of Upc2 is a constitutive homodimer, which is assembled through its carboxy-terminal sterol-binding domain (Figure 2B) [71]. Dimerization is essential for Upc2 regulatory function, as its disruption traps the transcription factor in the cytosol [71]. In fact, this dimeric nature seems to be conserved because C. albicans Upc2 and S. cerevisiae Ecm22 ligand-binding domains also form dimers in solution [71]. Multiple gain-of-function mutations in C. albicans Upc2 CTD result in the constitutive activation of the ergosterol synthesis pathway and sterol uptake, which diminishes the strain susceptibility to azole drugs. Moreover, UPC2 deletion increases C. albicans sensitivity to azole treatments, even in highly azole resistant clinical isolates containing multiple resistance mechanisms [70,77]. Therefore, Upc2 represents an important target to improve antifungal azole therapies.
Figure 2.
Structure and regulation of the S. cerevisiae Upc2 transcriptional factor. (A) Schematic representation of the primary structure of yeast Upc2 protein. NLS, nuclear localization signal. (B) Proposed model for the transcriptional regulation of Upc2. Under high-sterol conditions, Upc2 associates to ergosterol (Erg) and localizes to the cytosol. Although the mechanism is currently unknown, it has been proposed that Upc2 stays in the cytosol either because it interacts with a cytosolic protein or because its NLS is not available for nuclear import. Upon sterol depletion, ergosterol-free Upc2 undergoes a conformational change that allows its import to the nucleus, where it binds to ERG gene promoters to activate their transcription through the recruitment of a co-activator, probably the SAGA complex.
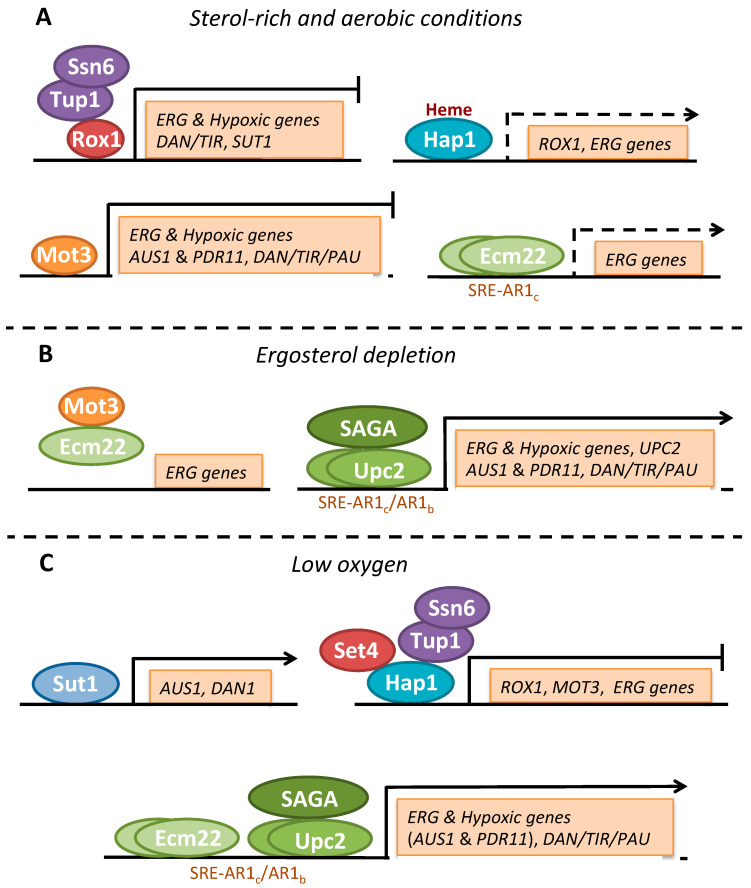
Under normal laboratory growth conditions, Ecm22 and, to a lesser extent, Upc2 bind to ERG gene promoters to maintain a basal expression level (Figure 3A) [68]. Upon low sterol levels, the abundance of Ecm22 protein, and consequently its binding to ERG promoters, decreases in a process that involves its direct physical interaction with the repressor Mot3 (Figure 3B) [74,75]. At the same time, the levels of Upc2, and their association to ERG promoters, strongly increase, which finally results in the transcriptional induction of ERG genes [68,74]. Interestingly, an auto-induction of UPC2 transcription, exclusively mediated by the direct binding of Upc2 to two TAAACGA anaerobic response (AR1b) elements within its own promoter, is required not only for the increase in Upc2 protein abundance, but also for the global activation of ERG genes and the resistance to antifungal drug treatments (Figure 3B) [78]. In addition to UPC2, ERG genes including ERG3 and ERG25 contain AR1b motifs essential for their correct antifungal-induced expression through Upc2 [78]. The specific in vitro and in vivo binding of Upc2 proteins from S. cerevisiae, but also from the pathogenic fungi C. albicans and C. glabrata, to SRE/AR1c and AR1b elements with similar affinities reinforces this regulatory mechanism [78]. However, not all SRE/AR1c or AR1b consensus sequences seem to be functional in vivo [78]. In mammals, SREBPs have also been shown to bind promoters containing sequences other that the SRE consensus.
Figure 3.
Transcriptional regulation of S. cerevisiae genes involved in ergosterol biosynthesis and uptake in response to low sterols or oxygen. (A) Under normal growth conditions, basal ERG gene expression is mostly maintained by Ecm22 and Hap1. Moreover, Mot3 and Rox1 (in a Tup1-Ssn6-dependent manner) inhibit the expression of hypoxic and ergosterol uptake genes. (B) Upon sterol depletion, Upc2 activates the transcription of ERG genes probably by recruiting the SAGA complex. (C) A drop in oxygen availability decreases heme levels, which lead to the Tup1-Ssn6 and Set4 recruitment by Hap1 to repress the expression of some ERG genes, ROX1 and MOT3. The decrease in Rox1 and Mot3 levels increases the expression of ERG, hypoxic and sterol uptake genes in a Upc2-Ecm22-dependent manner. Finally, Sut1 activates the transcription of AUS1 and DAN1, but not PDR11.
Other factors beyond Upc2 and Ecm2 contribute to the activation of ERG genes. The heme-dependent transcription factor Hap1 is also necessary for the expression of genes involved in ergosterol biosynthesis (Figure 3A) [75,79,80,81,82]. Genetic data suggest that Hap1 is required for the basal expression of some ERG genes and for their Ecm22-dependent activation by sterol depletion [75]. In fact, the deletion of both UPC2 and ECM22 is synthetically lethal in the S288C yeast genetic background due to a Ty1 insertion mutation into HAP1 coding sequence that partially compromises its function, while it is viable in the W303 background with a wild-type HAP1 gene [75,83]. Consistent with these observations, yeast strains with a HAP1-inserted transposon have less cellular ergosterol content [80]. Finally, Mga2, the transcription factor that controls the expression of the ∆9 fatty acid desaturase Ole1, which is responsible for the synthesis of unsaturated fatty acids, is required for the full basal expression of ERG1 [84]. As a matter of fact, mga2∆ cells accumulate higher relative levels of squalene than wild-type cells, suggesting a coordinated regulation between fatty acid and sterol biosynthesis [84]. In S. pombe, SREBP Sre1 activation requires unsaturated fatty acid synthesis, and sterol biosynthesis is necessary for Mga2 transcriptional activity [85]. These observations highlight the connections between ergosterol and fatty acids metabolisms to modulate membrane properties.
3.3.2. Transcriptional Regulation by Oxygen
As mentioned above, ergosterol biosynthesis depends on oxygen and heme as cofactors for critical enzymes of the pathway (Figure 1). During aerobic growth, heme is properly synthesized and available for binding to Hap1 protein. Under these conditions, heme-bound Hap1 not only activates the expression of ERG genes, but also of the gene coding for the DNA-binding protein Rox1, which represses, in a Tup1-Ssn6-dependent manner, the transcription of a group of genes referred to as “hypoxic genes”, which encode intracellular proteins that allow an efficient utilization of oxygen (e.g., ANB1 and HEM13), as well as the DAN/TIR genes and UPC2 [86,87,88,89,90] (Figure 3A). In addition, to limit Ecm22 function [75], the transcriptional factor Mot3 also represses the expression of hypoxic, DAN/TIR/PAU and ERG genes including ERG2, ERG6 and ERG9 (Figure 3A) [86,89,90,91,92,93]. Rox1 and Mot3 regulatory factors act synergistically to achieve stringent repression of target genes [89,90]. As a general result, under aerobic conditions, ERG genes display basal levels necessary for ergosterol biosynthesis, whereas the hypoxic-responsive genes are fully repressed. Conversely, a decrease in oxygen bioavailability causes a drop in heme and ergosterol levels that triggers the activation of signaling pathways that induce the expression of both hypoxic and ERG genes [94]. The up-regulation of oxygen-dependent enzymes within the ergosterol biosynthesis pathway upon oxygen limitation could be considered as a cellular strategy to compensate for their decrease in activity. Although both pathways are interdependent, heme depletion mainly signals the Hap1 pathway, while sterol depletion directly activates the expression of ERG and DAN/TIR/PAU genes via Upc2-Ecm22 (Figure 3C) [75,86,95]. In addition to Upc2, the mitogen-activated protein kinase (MAPK) Hog1 and specific components of the SAGA co-activator complex also contribute to the induction of PAU genes in response to oxygen limitation [96]. Oxygen depletion also enhances the expression of the oxygen-dependent fatty acid desaturase Ole1 through the Mga2 transcriptional factor [97]. Importantly, Hap1 seems to shift from an activator to a transcriptional repressor when oxygen availability decreases [98]. Thus, under low oxygen conditions, heme-free Hap1 recruits the Tup1-Ssn6 general co-repressor complex and the SET domain-containing epigenetic factor Set4 to repress many ERG genes (including ERG2, ERG3, ERG5 and ERG11), ROX1 and MOT3 [89,98,99], which in turn facilitates Upc2 and Ecm22 activation and the derepression of the hypoxic genes (Figure 3C) [75,87]. However, conflicting results have been observed for the effect of Hap1 on the expression of ERG genes during anaerobic conditions [75,96,99,100]. These discrepancies could be due to the use of yeast strains expressing different Hap1 factors, or being cultivated in media with different oxygen, ergosterol or fatty acid availability [29,96,100].
Many aspects of their regulation are still unknown due to the crosstalk between different environmental signals and the opposite regulatory effects triggered by Hap1, Rox1, Mot3, Upc2 and Ecm22 regulatory factors on a large number of ERG genes. Several genome-wide expression studies based on microarray and RNA-Seq analyses under low oxygen conditions have reflected that the pattern of expression differs among ERG genes, although some general trends can be extracted [94,96,98,100,101,102,103]. ERG genes involved in the first two modules of the ergosterol biosynthesis pathway seem to be down-regulated (e.g., ERG8, ERG13 and ERG19) or constitutively expressed (e.g., ERG10 and IDI1) when oxygen availability decreases, whereas most genes involved in the latter module are induced (e.g., NCP1, DAP1, ERG25, ERG26, ERG28) and only few of them are down-regulated (e.g., ERG5). As expected, the number of ERG genes with altered expression increases with the severity of oxygen restriction, and genes encoding for oxygen-dependent enzymes are up-regulated (e.g., ERG1, ERG11 and ERG3) upon acute oxygen starvation conditions [94,102,103]. Different hypotheses have been proposed to explain these observations. The increase in the expression of oxygen-using enzymes may be taking place to maintain the flux of ergosterol formation. However, the pattern of ERG5 expression contradicts this hypothesis, since it is down-regulated. Another possibility is that only the expression of particular ERG genes is enhanced to prevent the accumulation of toxic sterol intermediates. The up-regulation of the latter module of the ergosterol biosynthesis pathway under severe low oxygen conditions could favor the rapid production of ergosterol upon reoxygenation [94,102,103]. In accordance with this hypothesis, some of the genes that are most strongly induced upon reoxygenation are involved in the two first modules of ergosterol synthesis [103]. This could be important during anaerobic-to-aerobic transitions, since de novo sterol synthesis is required for the induction of respiratory genes [104]. Further studies are necessary to fully elucidate the regulation of ERG genes upon oxygen limitation.
As already mentioned, yeast cells activate sterol import under anaerobic conditions. By using a hyperactive upc2-1 allele, the cell wall mannoprotein Dan1 was identified as a facilitator of sterol influx, in addition to Aus1 and Pdr11 [73]. In fact, the overexpression of DAN1 and AUS1 is sufficient to promote the uptake of sterol in aerobiosis [105]. When the levels of oxygen are appropriate (normoxia), Mot3 represses AUS1 and PDR11 expression [106], whereas Upc2 allows their up-regulation upon oxygen depletion [73]. Additionally, the transcription factor Sut1, which is up-regulated under anaerobiosis due to loss of Rox1 repression [94], stimulates the expression of AUS1 and DAN1, but not PDR11 (Figure 3C) [105]. As well as the upc2-1 mutation, the constitutively active mutant of Ecm22 imports sterols under normoxia, but the underlying mechanisms are poorly characterized [107]. Under oxygen limitation, DAN/TIR/PAU genes replace the major aerobic cell wall mannoproteins encoded by CWP1 and CWP2. As mentioned above, the hypoxic expression of DAN/TIR/PAU genes depends on both sterol and heme levels due to Rox1-Mot3 derepression and Upc2-Ecm22-mediated activation (Figure 3C) [75,89,91,93,95]. However, the relative importance of each factor differs and depends on each particular DAN/TIR gene. Thus, DAN2 and DAN4 are not regulated by Rox1 and seem to be primarily induced by low sterols [86], while DAN1 and TIR1 respond to the depletion of both metabolites [75,95].
3.3.3. Transcriptional Regulation by Osmotic Stress
The response and adaptation to hyperosmotic stress is mostly governed by the high osmolarity glycerol (HOG) pathway and its terminal signaling MAPK Hog1 (reviewed in [108]). Upon osmostresses such as high extracellular salt concentrations, Hog1 induces the transcription of MOT3 [109], leading to a transient increase in Mot3 protein abundance that facilitates its association to particular ERG promoters [7]. As a result, there is a rapid and transient down-regulation of genes related to sterol biosynthesis (ECM22, ERG2 and ERG11), sterol uptake (SUT1 and AUS1) and cell wall components (several DAN/TIR and PAU genes) that causes a decrease in cellular ergosterol content that is physiologically relevant for osmostress adaptation [7,110]. Consistent with this, the expression of the hyperactive upc2-1 allele increases ergosterol content and renders cells highly salt sensitive, whereas inhibition of ergosterol synthesis with azole drugs is beneficial for salt-stress tolerance [7]. Both mot3∆ and rox1∆ mutants accumulate Na+ upon salt stress [7], suggesting that ergosterol down-regulation may be important for the appropriate regulation of plasma membrane ion transporters such as the Na+-ATPase Ena1 or the H+-ATPase Pma1, although further studies are required to decipher their involvement.
3.4. Regulation by Iron Bioavailability
As already indicated, ergosterol biosynthesis depends on iron in four steps, which are catalyzed by enzymes that contain a cofactor in the form of heme (Erg5, Erg11 and its regulator Dap1) or oxo-diiron (Erg25 and Erg3) centers (Figure 1). Consequently, iron deficiency reduces the metabolic flux through the sterol pathway, leading to a decrease in ergosterol and zymosterol levels, and the accumulation of squalene and lanosterol [9], which are the substrates of Erg1 and Erg11, respectively. Erg11 function is likely to decrease due to the drop in heme levels that occurs when iron is scarce, and the subsequent lanosterol accumulation may inhibit Erg1 [9,65]. Further studies have indicated that the heme-binding domain of the cytochrome b5 related protein Dap1 is required for the activity of the cytochrome P450 enzyme Erg11 as well as for growth in iron-deficient conditions [111]. Although it has been proposed that Dap1 increases Erg11 protein abundance in a heme-dependent manner, the regulation of Erg11 by Dap1 has not been fully deciphered [112,113]. In any case, under iron deprivation, the loss of Dap1 is rescued by ERG11 overexpression but not by increasing heme biosynthesis [111]. In addition to Dap1, mutants in other components of ergosterol biosynthesis, such as in the essential genes ERG25 and ERG29, also lead to growth defects in low iron and respiratory conditions, respectively, due to impaired ergosterol production [114,115]. A recent study has revealed that, similarly to Erg25, Erg29 participates in the methyl sterol oxidase step of ergosterol biosynthesis [13]. Defects in this step lead to the accumulation of toxic intermediates of the methyl sterol oxidase reaction that increase mitochondrial oxidation and affect the stability of the yeast frataxin homolog Ffh1, which is implicated in mitochondrial iron metabolism [13]. As a consequence, erg29 mutants exhibit defects in iron-sulfur cluster assembly and mitochondrial iron accumulation [13,115]. These results emphasize the multiple connections between iron metabolism and ergosterol biosynthesis.
Genome-wide expression studies have shown that the expression of ERG genes is altered in response to iron deficiency [116,117]. Under iron limitation, yeast cells lacking the iron-regulated RNA-binding protein Cth2 display high mRNA levels of multiple ERG genes including those of the first steps of the late stage of synthesis (ERG1, ERG7, ERG11 and DAP1) [116,118]. Upon iron starvation, Cth2 protein binds to multiple mRNAs containing AU-rich elements (AREs) within their 3′ untranslated region (3′UTR), and promotes their degradation and translation inhibition [116,119,120]. The presence of multiple AREs on the 3′UTR of this subset of ERG genes suggests that Cth2 may trigger their down-regulation when iron is scarce. Consistent with this, the protein levels of both Erg1 and Erg11 decrease in response to iron depletion [9]. However, the abundance of other Erg proteins, such as Erg6 and Erg25, is kept roughly constant or even increases, which is the case of Erg3 [9]. In this sense, a recent global kinetic study of gene expression during the progress of iron deficiency has shown that the pattern of expression differs among ERG genes and depends on the severity of the depletion [121]. Therefore, we hypothesize that additional regulatory factors, including Hap1, Upc2, Ecm22 and others may directly or indirectly respond to iron limitation to control ERG gene expression and ergosterol synthesis.
4. Conclusions
The yeast S. cerevisiae is used as a reliable model to study multiple aspects of lipid biology due to its well characterized genome, the relative simplicity of its lipid metabolism and homeostasis, and the conservation of genes, routes and networks in other eukaryotes (reviewed in [122]). In recent years, ergosterol biosynthesis has been greatly studied in S. cerevisiae, leading to the identification of many conserved but also fungal-specific steps.
The balanced regulation of all the enzymes in the ergosterol biosynthesis pathway is an essential determinant of the efficiency of sterol synthesis and, therefore, of optimal growth and adaptation to environmental cues. Moreover, one of the main approaches to overproduce sterols of high-value for food and pharmaceutical industries includes yeast genetic modification. However, changes in ergosterol biosynthesis lead to pleiotropic defects that limit cellular proliferation and adaptation to stresses. Because of these reasons, the study of ergosterol biosynthesis regulation may provide new ideas for enhancing sterol production and the adaptation of yeast cell factories to the environment. Furthermore, chemicals could be developed to increase the production of specific sterols during yeast fermentation. For instance, the treatment with terbinafine, which targets the squalene epoxidase Erg1, results in the accumulation of squalene, which can be used for the synthesis of terpenes. In this sense, the genetic alteration of sterol metabolism could reduce the concentration of these expensive chemicals, drastically lowering production costs.
The ergosterol biosynthetic pathway constitutes one of the main targets for antifungal agents in health and agriculture. Nevertheless, the currently available drugs have been related to emerging resistance by fungal pathogens, significant side effects and toxicity (reviewed in [123]). The detailed study of the molecular mechanisms that contribute to the regulation, synthesis and transport of sterols in S. cerevisiae and fungal pathogens is crucial for the design of new antifungal strategies. Ergosterol regulation by particular metabolites and environmental cues is still far from being understood in all its complexity. Future lines of investigation should include the identification of factors that regulate the subcellular localization and function of Erg proteins and the structural and functional characterization of the fungal-specific zinc-finger regulatory proteins that control the expression of ERG genes.
Acknowledgments
We are grateful to María Teresa Martínez-Pastor and Antonia María Romero for critically reading the manuscript, and to the members of the Iron Homeostasis laboratory for technical and scientific assistance.
Author Contributions
Conceptualization, S.P.; writing—original draft preparation, T.J.; writing—review and editing, S.P. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a predoctoral contract from the Regional Government of Valencia “Generalitat Valenciana” to T.J., and the Spanish Ministry of Science, Innovation and Universities grant BIO2017-87828-C2-1-P and FEDER (Fondo Europeo de Desarrollo Regional) funds to S.P.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Maxfield F.R., Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 2.Tarkowska D., Strnad M. Plant ecdysteroids: Plant sterols with intriguing distributions, biological effects and relations to plant hormones. Planta. 2016;244:545–555. doi: 10.1007/s00425-016-2561-z. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues M.L. The Multifunctional Fungal Ergosterol. mBio. 2018;9 doi: 10.1128/mBio.01755-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cirigliano A., Macone A., Bianchi M.M., Oliaro-Bosso S., Balliano G., Negri R., Rinaldi T. Ergosterol reduction impairs mitochondrial DNA maintenance in S. cerevisiae. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1864:290–303. doi: 10.1016/j.bbalip.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Gerhold J.M., Cansiz-Arda S., Lohmus M., Engberg O., Reyes A., van Rennes H., Sanz A., Holt I.J., Cooper H.M., Spelbrink J.N. Human Mitochondrial DNA-Protein Complexes Attach to a Cholesterol-Rich Membrane Structure. Sci. Rep. 2015;5:15292. doi: 10.1038/srep15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Z., He B., Ma L., Sun Y., Niu Y., Zeng B. Recent Advances in Ergosterol Biosynthesis and Regulation Mechanisms in Saccharomyces cerevisiae. Indian J. Microbiol. 2017;57:270–277. doi: 10.1007/s12088-017-0657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montanes F.M., Pascual-Ahuir A., Proft M. Repression of ergosterol biosynthesis is essential for stress resistance and is mediated by the Hog1 MAP kinase and the Mot3 and Rox1 transcription factors. Mol. Microbiol. 2011;79:1008–1023. doi: 10.1111/j.1365-2958.2010.07502.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu J.F., Xia J.J., Nie K.L., Wang F., Deng L. Outline of the biosynthesis and regulation of ergosterol in yeast. World J. Microbiol. Biotechnol. 2019;35:98. doi: 10.1007/s11274-019-2673-2. [DOI] [PubMed] [Google Scholar]
- 9.Shakoury-Elizeh M., Protchenko O., Berger A., Cox J., Gable K., Dunn T.M., Prinz W.A., Bard M., Philpott C.C. Metabolic response to iron deficiency in Saccharomyces cerevisiae. J. Biol. Chem. 2010;285:14823–14833. doi: 10.1074/jbc.M109.091710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Nielsen J., Liu Z. Engineering yeast metabolism for production of terpenoids for use as perfume ingredients, pharmaceuticals and biofuels. FEMS Yeast Res. 2017;17 doi: 10.1093/femsyr/fox080. [DOI] [PubMed] [Google Scholar]
- 11.Vil V.A., Gloriozova T.A., Poroikov V.V., Terent’ev A.O., Savidov N., Dembitsky V.M. Peroxy steroids derived from plant and fungi and their biological activities. Appl. Microbiol. Biotechnol. 2018;102:7657–7667. doi: 10.1007/s00253-018-9211-2. [DOI] [PubMed] [Google Scholar]
- 12.Tan W., Pan M., Liu H., Tian H., Ye Q., Liu H. Ergosterol peroxide inhibits ovarian cancer cell growth through multiple pathways. Onco Targets Ther. 2017;10:3467–3474. doi: 10.2147/OTT.S139009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward D.M., Chen O.S., Li L., Kaplan J., Bhuiyan S.A., Natarajan S.K., Bard M., Cox J.E. Altered sterol metabolism in budding yeast affects mitochondrial iron-sulfur (Fe-S) cluster synthesis. J. Biol. Chem. 2018;293:10782–10795. doi: 10.1074/jbc.RA118.001781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zinser E., Paltauf F., Daum G. Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J. Bacteriol. 1993;175:2853–2858. doi: 10.1128/JB.175.10.2853-2858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshua I.M., Hofken T. From Lipid Homeostasis to Differentiation: Old and New Functions of the Zinc Cluster Proteins Ecm22, Upc2, Sut1 and Sut2. Int. J. Mol. Sci. 2017;18:772. doi: 10.3390/ijms18040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston E.J., Moses T., Rosser S.J. The wide-Ranging phenotypes of ergosterol biosynthesis mutants, and implications for microbial cell factories. Yeast. 2020;37:27–44. doi: 10.1002/yea.3452. [DOI] [PubMed] [Google Scholar]
- 17.Heese-Peck A., Pichler H., Zanolari B., Watanabe R., Daum G., Riezman H. Multiple functions of sterols in yeast endocytosis. Mol. Biol. Cell. 2002;13:2664–2680. doi: 10.1091/mbc.e02-04-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sokolov S.S., Trushina N.I., Severin F.F., Knorre D.A. Ergosterol Turnover in Yeast: An Interplay between Biosynthesis and Transport. Biochemistry (Mosc) 2019;84:346–357. doi: 10.1134/S0006297919040023. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharya S., Esquivel B.D., White T.C. Overexpression or Deletion of Ergosterol Biosynthesis Genes Alters Doubling Time, Response to Stress Agents, and Drug Susceptibility in Saccharomyces cerevisiae. mBio. 2018;9 doi: 10.1128/mBio.01291-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruckenstuhl C., Lang S., Poschenel A., Eidenberger A., Baral P.K., Kohut P., Hapala I., Gruber K., Turnowsky F. Characterization of squalene epoxidase of Saccharomyces cerevisiae by applying terbinafine-sensitive variants. Antimicrob. Agents Chemother. 2007;51:275–284. doi: 10.1128/AAC.00988-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkow E.L., Lockhart S.R. Fluconazole resistance in Candida species: A current perspective. Infect Drug Resist. 2017;10:237–245. doi: 10.2147/IDR.S118892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly S.L., Lamb D.C., Kelly D.E., Manning N.J., Loeffler J., Hebart H., Schumacher U., Einsele H. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta5,6-desaturation. FEBS Lett. 1997;400:80–82. doi: 10.1016/S0014-5793(96)01360-9. [DOI] [PubMed] [Google Scholar]
- 23.Sanglard D., Ischer F., Parkinson T., Falconer D., Bille J. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob. Agents Chemother. 2003;47:2404–2412. doi: 10.1128/AAC.47.8.2404-2412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White T.C., Marr K.A., Bowden R.A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 1998;11:382–402. doi: 10.1128/CMR.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serhan G., Stack C.M., Perrone G.G., Morton C.O. The polyene antifungals, amphotericin B and nystatin, cause cell death in Saccharomyces cerevisiae by a distinct mechanism to amphibian-derived antimicrobial peptides. Ann. Clin. Microbiol. Antimicrob. 2014;13:18. doi: 10.1186/1476-0711-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Espenshade P.J., Hughes A.L. Regulation of sterol synthesis in eukaryotes. Annu. Rev. Genet. 2007;41:401–427. doi: 10.1146/annurev.genet.41.110306.130315. [DOI] [PubMed] [Google Scholar]
- 27.Kodedova M., Sychrova H. Changes in the Sterol Composition of the Plasma Membrane Affect Membrane Potential, Salt Tolerance and the Activity of Multidrug Resistance Pumps in Saccharomyces cerevisiae. PLoS ONE. 2015;10:e0139306. doi: 10.1371/journal.pone.0139306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorenz R.T., Parks L.W. Regulation of ergosterol biosynthesis and sterol uptake in a sterol-auxotrophic yeast. J. Bacteriol. 1987;169:3707–3711. doi: 10.1128/JB.169.8.3707-3711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes A.L., Todd B.L., Espenshade P.J. SREBP pathway responds to sterols and functions as an oxygen sensor in fission yeast. Cell. 2005;120:831–842. doi: 10.1016/j.cell.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Zavrel M., Hoot S.J., White T.C. Comparison of sterol import under aerobic and anaerobic conditions in three fungal species, Candida albicans, Candida glabrata, and Saccharomyces cerevisiae. Eukaryot Cell. 2013;12:725–738. doi: 10.1128/EC.00345-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohut P., Wustner D., Hronska L., Kuchler K., Hapala I., Valachovic M. The role of ABC proteins Aus1p and Pdr11p in the uptake of external sterols in yeast: Dehydroergosterol fluorescence study. Biochem. Biophys. Res. Commun. 2011;404:233–238. doi: 10.1016/j.bbrc.2010.11.099. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Prinz W.A. ATP-Binding cassette (ABC) transporters mediate nonvesicular, raft-Modulated sterol movement from the plasma membrane to the endoplasmic reticulum. J. Biol. Chem. 2004;279:45226–45234. doi: 10.1074/jbc.M407600200. [DOI] [PubMed] [Google Scholar]
- 33.Gulati S., Balderes D., Kim C., Guo Z.A., Wilcox L., Area-Gomez E., Snider J., Wolinski H., Stagljar I., Granato J.T., et al. ATP-Binding cassette transporters and sterol O-acyltransferases interact at membrane microdomains to modulate sterol uptake and esterification. FASEB J. 2015;29:4682–4694. doi: 10.1096/fj.14-264796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Georgiev A.G., Sullivan D.P., Kersting M.C., Dittman J.S., Beh C.T., Menon A.K. Osh proteins regulate membrane sterol organization but are not required for sterol movement between the ER and PM. Traffic. 2011;12:1341–1355. doi: 10.1111/j.1600-0854.2011.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kentala H., Weber-Boyvat M., Olkkonen V.M. OSBP-Related Protein Family: Mediators of Lipid Transport and Signaling at Membrane Contact Sites. Int. Rev. Cell Mol. Biol. 2016;321:299–340. doi: 10.1016/bs.ircmb.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Tian S., Ohta A., Horiuchi H., Fukuda R. Oxysterol-Binding protein homologs mediate sterol transport from the endoplasmic reticulum to mitochondria in yeast. J. Biol. Chem. 2018;293:5636–5648. doi: 10.1074/jbc.RA117.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gatta A.T., Wong L.H., Sere Y.Y., Calderon-Norena D.M., Cockcroft S., Menon A.K., Levine T.P. A new family of StART domain proteins at membrane contact sites has a role in ER-PM sterol transport. Elife. 2015;4 doi: 10.7554/eLife.07253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong J., Manik M.K., Im Y.J. Structural basis of sterol recognition and nonvesicular transport by lipid transfer proteins anchored at membrane contact sites. Proc. Natl. Acad. Sci. USA. 2018;115:E856–E865. doi: 10.1073/pnas.1719709115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tinkelenberg A.H., Liu Y., Alcantara F., Khan S., Guo Z., Bard M., Sturley S.L. Mutations in yeast ARV1 alter intracellular sterol distribution and are complemented by human ARV1. J. Biol. Chem. 2000;275:40667–40670. doi: 10.1074/jbc.C000710200. [DOI] [PubMed] [Google Scholar]
- 40.Kajiwara K., Watanabe R., Pichler H., Ihara K., Murakami S., Riezman H., Funato K. Yeast ARV1 is required for efficient delivery of an early GPI intermediate to the first mannosyltransferase during GPI assembly and controls lipid flow from the endoplasmic reticulum. Mol. Biol. Cell. 2008;19:2069–2082. doi: 10.1091/mbc.e07-08-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Georgiev A.G., Johansen J., Ramanathan V.D., Sere Y.Y., Beh C.T., Menon A.K. Arv1 regulates PM and ER membrane structure and homeostasis but is dispensable for intracellular sterol transport. Traffic. 2013;14:912–921. doi: 10.1111/tra.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demuyser L., Swinnen E., Fiori A., Herrera-Malaver B., Vestrepen K., Van Dijck P. Mitochondrial Cochaperone Mge1 Is Involved in Regulating Susceptibility to Fluconazole in Saccharomyces cerevisiae and Candida Species. mBio. 2017;8 doi: 10.1128/mBio.00201-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrillo-Munoz A.J., Giusiano G., Ezkurra P.A., Quindos G. Antifungal agents: Mode of action in yeast cells. Rev. Esp. Quimioter. 2006;19:130–139. [PubMed] [Google Scholar]
- 44.Zweytick D., Leitner E., Kohlwein S.D., Yu C., Rothblatt J., Daum G. Contribution of Are1p and Are2p to steryl ester synthesis in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 2000;267:1075–1082. doi: 10.1046/j.1432-1327.2000.01103.x. [DOI] [PubMed] [Google Scholar]
- 45.Koffel R., Tiwari R., Falquet L., Schneiter R. The Saccharomyces cerevisiae YLL012/YEH1, YLR020/YEH2, and TGL1 genes encode a novel family of membrane-anchored lipases that are required for steryl ester hydrolysis. Mol. Cell Biol. 2005;25:1655–1668. doi: 10.1128/MCB.25.5.1655-1668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koffel R., Schneiter R. Yeh1 constitutes the major steryl ester hydrolase under heme-deficient conditions in Saccharomyces cerevisiae. Eukaryot. Cell. 2006;5:1018–1025. doi: 10.1128/EC.00002-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jandrositz A., Petschnigg J., Zimmermann R., Natter K., Scholze H., Hermetter A., Kohlwein S.D., Leber R. The lipid droplet enzyme Tgl1p hydrolyzes both steryl esters and triglycerides in the yeast, Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2005;1735:50–58. doi: 10.1016/j.bbalip.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Sorger D., Athenstaedt K., Hrastnik C., Daum G. A yeast strain lacking lipid particles bears a defect in ergosterol formation. J. Biol. Chem. 2004;279:31190–31196. doi: 10.1074/jbc.M403251200. [DOI] [PubMed] [Google Scholar]
- 49.Arthington-Skaggs B.A., Crowell D.N., Yang H., Sturley S.L., Bard M. Positive and negative regulation of a sterol biosynthetic gene (ERG3) in the post-squalene portion of the yeast ergosterol pathway. FEBS Lett. 1996;392:161–165. doi: 10.1016/0014-5793(96)00807-1. [DOI] [PubMed] [Google Scholar]
- 50.Ploier B., Korber M., Schmidt C., Koch B., Leitner E., Daum G. Regulatory link between steryl ester formation and hydrolysis in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2015;1851:977–986. doi: 10.1016/j.bbalip.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Tiwari R., Koffel R., Schneiter R. An acetylation/deacetylation cycle controls the export of sterols and steroids from S. cerevisiae. EMBO J. 2007;26:5109–5119. doi: 10.1038/sj.emboj.7601924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choudhary V., Schneiter R. Pathogen-Related Yeast (PRY) proteins and members of the CAP superfamily are secreted sterol-binding proteins. Proc. Natl. Acad. Sci. USA. 2012;109:16882–16887. doi: 10.1073/pnas.1209086109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mo C., Bard M. A systematic study of yeast sterol biosynthetic protein-protein interactions using the split-Ubiquitin system. Biochim. Biophys. Acta. 2005;1737:152–160. doi: 10.1016/j.bbalip.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Mullner H., Zweytick D., Leber R., Turnowsky F., Daum G. Targeting of proteins involved in sterol biosynthesis to lipid particles of the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2004;1663:9–13. doi: 10.1016/j.bbamem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Kristan K., Rizner T.L. Steroid-Transforming enzymes in fungi. J. Steroid Biochem. Mol. Biol. 2012;129:79–91. doi: 10.1016/j.jsbmb.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Caldas H., Herman G.E. NSDHL, an enzyme involved in cholesterol biosynthesis, traffics through the Golgi and accumulates on ER membranes and on the surface of lipid droplets. Hum. Mol. Genet. 2003;12:2981–2991. doi: 10.1093/hmg/ddg321. [DOI] [PubMed] [Google Scholar]
- 57.Leber R., Landl K., Zinser E., Ahorn H., Spok A., Kohlwein S.D., Turnowsky F., Daum G. Dual localization of squalene epoxidase, Erg1p, in yeast reflects a relationship between the endoplasmic reticulum and lipid particles. Mol. Biol. Cell. 1998;9:375–386. doi: 10.1091/mbc.9.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukherjee D., Gao M., O’Connor J.P., Raijmakers R., Pruijn G., Lutz C.S., Wilusz J. The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J. 2002;21:165–174. doi: 10.1093/emboj/21.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mo C., Valachovic M., Bard M. The ERG28-Encoded protein, Erg28p, interacts with both the sterol C-4 demethylation enzyme complex as well as the late biosynthetic protein, the C-24 sterol methyltransferase (Erg6p) Biochim. Biophys. Acta. 2004;1686:30–36. doi: 10.1016/j.bbalip.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Mo C., Milla P., Athenstaedt K., Ott R., Balliano G., Daum G., Bard M. In yeast sterol biosynthesis the 3-keto reductase protein (Erg27p) is required for oxidosqualene cyclase (Erg7p) activity. Biochim. Biophys. Acta. 2003;1633:68–74. doi: 10.1016/S1388-1981(03)00088-X. [DOI] [PubMed] [Google Scholar]
- 61.Layer J.V., Barnes B.M., Yamasaki Y., Barbuch R., Li L., Taramino S., Balliano G., Bard M. Characterization of a mutation that results in independence of oxidosqualene cyclase (Erg7) activity from the downstream 3-ketoreductase (Erg27) in the yeast ergosterol biosynthetic pathway. Biochim. Biophys. Acta. 2013;1831:361–369. doi: 10.1016/j.bbalip.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 62.Burg J.S., Espenshade P.J. Regulation of HMG-CoA reductase in mammals and yeast. Prog. Lipid Res. 2011;50:403–410. doi: 10.1016/j.plipres.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hampton R.Y., Gardner R.G., Rine J. Role of 26S proteasome and HRD genes in the degradation of 3-hydroxy-3-methylglutaryl-CoA reductase, an integral endoplasmic reticulum membrane protein. Mol. Biol. Cell. 1996;7:2029–2044. doi: 10.1091/mbc.7.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hampton R.Y., Bhakta H. Ubiquitin-mediated regulation of 3-hydroxy-3-methylglutaryl-CoA reductase. Proc. Natl. Acad. Sci. USA. 1997;94:12944–12948. doi: 10.1073/pnas.94.24.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foresti O., Ruggiano A., Hannibal-Bach H.K., Ejsing C.S., Carvalho P. Sterol homeostasis requires regulated degradation of squalene monooxygenase by the ubiquitin ligase Doa10/Teb4. Elife. 2013;2:e00953. doi: 10.7554/eLife.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang Y.C., Bien C.M., Lee H., Espenshade P.J., Kwon-Chung K.J. Sre1p, a regulator of oxygen sensing and sterol homeostasis, is required for virulence in Cryptococcus neoformans. Mol. Microbiol. 2007;64:614–629. doi: 10.1111/j.1365-2958.2007.05676.x. [DOI] [PubMed] [Google Scholar]
- 67.Radhakrishnan A., Sun L.P., Kwon H.J., Brown M.S., Goldstein J.L. Direct binding of cholesterol to the purified membrane region of SCAP: Mechanism for a sterol-Sensing domain. Mol. Cell. 2004;15:259–268. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 68.Vik A., Rine J. Upc2p and Ecm22p, dual regulators of sterol biosynthesis in Saccharomyces cerevisiae. Mol. Cell Biol. 2001;21:6395–6405. doi: 10.1128/MCB.21.19.6395-6405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maguire S.L., Wang C., Holland L.M., Brunel F., Neuveglise C., Nicaud J.M., Zavrel M., White T.C., Wolfe K.H., Butler G. Zinc finger transcription factors displaced SREBP proteins as the major Sterol regulators during Saccharomycotina evolution. PLoS Genet. 2014;10:e1004076. doi: 10.1371/journal.pgen.1004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacPherson S., Akache B., Weber S., De Deken X., Raymond M., Turcotte B. Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes. Antimicrob. Agents Chemother. 2005;49:1745–1752. doi: 10.1128/AAC.49.5.1745-1752.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang H., Tong J., Lee C.W., Ha S., Eom S.H., Im Y.J. Structural mechanism of ergosterol regulation by fungal sterol transcription factor Upc2. Nat. Commun. 2015;6:6129. doi: 10.1038/ncomms7129. [DOI] [PubMed] [Google Scholar]
- 72.Marie C., Leyde S., White T.C. Cytoplasmic localization of sterol transcription factors Upc2p and Ecm22p in S. cerevisiae. Fungal Genet Biol. 2008;45:1430–1438. doi: 10.1016/j.fgb.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilcox L.J., Balderes D.A., Wharton B., Tinkelenberg A.H., Rao G., Sturley S.L. Transcriptional profiling identifies two members of the ATP-Binding cassette transporter superfamily required for sterol uptake in yeast. J. Biol. Chem. 2002;277:32466–32472. doi: 10.1074/jbc.M204707200. [DOI] [PubMed] [Google Scholar]
- 74.Davies B.S., Wang H.S., Rine J. Dual activators of the sterol biosynthetic pathway of Saccharomyces cerevisiae: Similar activation/regulatory domains but different response mechanisms. Mol. Cell Biol. 2005;25:7375–7385. doi: 10.1128/MCB.25.16.7375-7385.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davies B.S., Rine J. A role for sterol levels in oxygen sensing in Saccharomyces cerevisiae. Genetics. 2006;174:191–201. doi: 10.1534/genetics.106.059964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dewhurst-Maridor G., Abegg D., David F.P.A., Rougemont J., Scott C.C., Adibekian A., Riezman H. The SAGA complex, together with transcription factors and the endocytic protein Rvs167p, coordinates the reprofiling of gene expression in response to changes in sterol composition in Saccharomyces cerevisiae. Mol. Biol. Cell. 2017;28:2637–2649. doi: 10.1091/mbc.e17-03-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vasicek E.M., Berkow E.L., Flowers S.A., Barker K.S., Rogers P.D. UPC2 is universally essential for azole antifungal resistance in Candida albicans. Eukaryot Cell. 2014;13:933–946. doi: 10.1128/EC.00221-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gallo-Ebert C., Donigan M., Liu H.Y., Pascual F., Manners M., Pandya D., Swanson R., Gallagher D., Chen W., Carman G.M., et al. The yeast anaerobic response element AR1b regulates aerobic antifungal drug-dependent sterol gene expression. J. Biol. Chem. 2013;288:35466–35477. doi: 10.1074/jbc.M113.526087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kennedy M.A., Barbuch R., Bard M. Transcriptional regulation of the squalene synthase gene (ERG9) in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1999;1445:110–122. doi: 10.1016/S0167-4781(99)00035-4. [DOI] [PubMed] [Google Scholar]
- 80.Tamura K., Gu Y., Wang Q., Yamada T., Ito K., Shimoi H. A hap1 mutation in a laboratory strain of Saccharomyces cerevisiae results in decreased expression of ergosterol-related genes and cellular ergosterol content compared to sake yeast. J. Biosci. Bioeng. 2004;98:159–166. doi: 10.1016/S1389-1723(04)00260-9. [DOI] [PubMed] [Google Scholar]
- 81.MacIsaac K.D., Wang T., Gordon D.B., Gifford D.K., Stormo G.D., Fraenkel E. An improved map of conserved regulatory sites for Saccharomyces cerevisiae. BMC Bioinform. 2006;7:113. doi: 10.1186/1471-2105-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bergenholm D., Liu G., Holland P., Nielsen J. Reconstruction of a Global Transcriptional Regulatory Network for Control of Lipid Metabolism in Yeast by Using Chromatin Immunoprecipitation with Lambda Exonuclease Digestion. mSystems. 2018;3 doi: 10.1128/mSystems.00215-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valachovic M., Bareither B.M., Shah Alam Bhuiyan M., Eckstein J., Barbuch R., Balderes D., Wilcox L., Sturley S.L., Dickson R.C., Bard M. Cumulative mutations affecting sterol biosynthesis in the yeast Saccharomyces cerevisiae result in synthetic lethality that is suppressed by alterations in sphingolipid profiles. Genetics. 2006;173:1893–1908. doi: 10.1534/genetics.105.053025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rice C., Cooke M., Treloar N., Vollbrecht P., Stukey J., McDonough V. A role for MGA2, but not SPT23, in activation of transcription of ERG1 in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2010;403:293–297. doi: 10.1016/j.bbrc.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 85.Burr R., Stewart E.V., Espenshade P.J. Coordinate Regulation of Yeast Sterol Regulatory Element-binding Protein (SREBP) and Mga2 Transcription Factors. J. Biol. Chem. 2017;292:5311–5324. doi: 10.1074/jbc.M117.778209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abramova N.E., Cohen B.D., Sertil O., Kapoor R., Davies K.J., Lowry C.V. Regulatory mechanisms controlling expression of the DAN/TIR mannoprotein genes during anaerobic remodeling of the cell wall in Saccharomyces cerevisiae. Genetics. 2001;157:1169–1177. doi: 10.1093/genetics/157.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kwast K.E., Lai L.C., Menda N., James D.T., Aref S., Burke P.V. Genomic analyses of anaerobically induced genes in Saccharomyces cerevisiae: Functional roles of Rox1 and other factors in mediating the anoxic response. J. Bacteriol. 2002;184:250–265. doi: 10.1128/JB.184.1.250-265.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ter Linde J.J., Steensma H.Y. A microarray-Assisted screen for potential Hap1 and Rox1 target genes in Saccharomyces cerevisiae. Yeast. 2002;19:825–840. doi: 10.1002/yea.879. [DOI] [PubMed] [Google Scholar]
- 89.Sertil O., Kapoor R., Cohen B.D., Abramova N., Lowry C.V. Synergistic repression of anaerobic genes by Mot3 and Rox1 in Saccharomyces cerevisiae. Nucleic Acids Res. 2003;31:5831–5837. doi: 10.1093/nar/gkg792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klinkenberg L.G., Mennella T.A., Luetkenhaus K., Zitomer R.S. Combinatorial repression of the hypoxic genes of Saccharomyces cerevisiae by DNA binding proteins Rox1 and Mot3. Eukaryot Cell. 2005;4:649–660. doi: 10.1128/EC.4.4.649-660.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abramova N., Sertil O., Mehta S., Lowry C.V. Reciprocal regulation of anaerobic and aerobic cell wall mannoprotein gene expression in Saccharomyces cerevisiae. J. Bacteriol. 2001;183:2881–2887. doi: 10.1128/JB.183.9.2881-2887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hongay C., Jia N., Bard M., Winston F. Mot3 is a transcriptional repressor of ergosterol biosynthetic genes and is required for normal vacuolar function in Saccharomyces cerevisiae. EMBO J. 2002;21:4114–4124. doi: 10.1093/emboj/cdf415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luo Z., van Vuuren H.J.J. Functional analyses of PAU genes in Saccharomyces cerevisiae. Microbiology. 2009;155:4036–4049. doi: 10.1099/mic.0.030726-0. [DOI] [PubMed] [Google Scholar]
- 94.Kwast K.E., Burke P.V., Staahl B.T., Poyton R.O. Oxygen sensing in yeast: Evidence for the involvement of the respiratory chain in regulating the transcription of a subset of hypoxic genes. Proc. Natl. Acad. Sci. USA. 1999;96:5446–5451. doi: 10.1073/pnas.96.10.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cohen B.D., Sertil O., Abramova N.E., Davies K.J., Lowry C.V. Induction and repression of DAN1 and the family of anaerobic mannoprotein genes in Saccharomyces cerevisiae occurs through a complex array of regulatory sites. Nucleic Acids Res. 2001;29:799–808. doi: 10.1093/nar/29.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hickman M.J., Spatt D., Winston F. The Hog1 mitogen-activated protein kinase mediates a hypoxic response in Saccharomyces cerevisiae. Genetics. 2011;188:325–338. doi: 10.1534/genetics.111.128322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang Y., Vasconcelles M.J., Wretzel S., Light A., Martin C.E., Goldberg M.A. MGA2 is involved in the low-oxygen response element-dependent hypoxic induction of genes in Saccharomyces cerevisiae. Mol. Cell Biol. 2001;21:6161–6169. doi: 10.1128/MCB.21.18.6161-6169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hickman M.J., Winston F. Heme levels switch the function of Hap1 of Saccharomyces cerevisiae between transcriptional activator and transcriptional repressor. Mol. Cell Biol. 2007;27:7414–7424. doi: 10.1128/MCB.00887-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Serratore N.D., Baker K.M., Macadlo L.A., Gress A.R., Powers B.L., Atallah N., Westerhouse K.M., Hall M.C., Weake V.M., Briggs S.D. A Novel Sterol-Signaling Pathway Governs Azole Antifungal Drug Resistance and Hypoxic Gene Repression in Saccharomyces cerevisiae. Genetics. 2018;208:1037–1055. doi: 10.1534/genetics.117.300554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bendjilali N., MacLeon S., Kalra G., Willis S.D., Hossian A.K., Avery E., Wojtowicz O., Hickman M.J. Time-Course Analysis of Gene Expression During the Saccharomyces cerevisiae Hypoxic Response. G3 (Bethesda) 2017;7:221–231. doi: 10.1534/g3.116.034991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Becerra M., Lombardia-Ferreira L.J., Hauser N.C., Hoheisel J.D., Tizon B., Cerdan M.E. The yeast transcriptome in aerobic and hypoxic conditions: Effects of hap1, rox1, rox3 and srb10 deletions. Mol. Microbiol. 2002;43:545–555. doi: 10.1046/j.1365-2958.2002.02724.x. [DOI] [PubMed] [Google Scholar]
- 102.Lai L.C., Kosorukoff A.L., Burke P.V., Kwast K.E. Dynamical remodeling of the transcriptome during short-term anaerobiosis in Saccharomyces cerevisiae: Differential response and role of Msn2 and/or Msn4 and other factors in galactose and glucose media. Mol. Cell Biol. 2005;25:4075–4091. doi: 10.1128/MCB.25.10.4075-4091.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lai L.C., Kosorukoff A.L., Burke P.V., Kwast K.E. Metabolic-state-dependent remodeling of the transcriptome in response to anoxia and subsequent reoxygenation in Saccharomyces cerevisiae. Eukaryot Cell. 2006;5:1468–1489. doi: 10.1128/EC.00107-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosenfeld E., Beauvoit B. Role of the non-Respiratory pathways in the utilization of molecular oxygen by Saccharomyces cerevisiae. Yeast. 2003;20:1115–1144. doi: 10.1002/yea.1026. [DOI] [PubMed] [Google Scholar]
- 105.Alimardani P., Regnacq M., Moreau-Vauzelle C., Ferreira T., Rossignol T., Blondin B., Berges T. SUT1-promoted sterol uptake involves the ABC transporter Aus1 and the mannoprotein Dan1 whose synergistic action is sufficient for this process. Biochem. J. 2004;381:195–202. doi: 10.1042/BJ20040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sullivan D.P., Georgiev A., Menon A.K. Tritium suicide selection identifies proteins involved in the uptake and intracellular transport of sterols in Saccharomyces cerevisiae. Eukaryot Cell. 2009;8:161–169. doi: 10.1128/EC.00135-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shianna K.V., Dotson W.D., Tove S., Parks L.W. Identification of a UPC2 homolog in Saccharomyces cerevisiae and its involvement in aerobic sterol uptake. J. Bacteriol. 2001;183:830–834. doi: 10.1128/JB.183.3.830-834.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saito H., Posas F. Response to hyperosmotic stress. Genetics. 2012;192:289–318. doi: 10.1534/genetics.112.140863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Proft M., Gibbons F.D., Copeland M., Roth F.P., Struhl K. Genomewide identification of Sko1 target promoters reveals a regulatory network that operates in response to osmotic stress in Saccharomyces cerevisiae. Eukaryot Cell. 2005;4:1343–1352. doi: 10.1128/EC.4.8.1343-1352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martinez-Montanes F., Rienzo A., Poveda-Huertes D., Pascual-Ahuir A., Proft M. Activator and repressor functions of the Mot3 transcription factor in the osmostress response of Saccharomyces cerevisiae. Eukaryot Cell. 2013;12:636–647. doi: 10.1128/EC.00037-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Craven R.J., Mallory J.C., Hand R.A. Regulation of iron homeostasis mediated by the heme-binding protein Dap1 (damage resistance protein 1) via the P450 protein Erg11/Cyp51. J. Biol. Chem. 2007;282:36543–36551. doi: 10.1074/jbc.M706770200. [DOI] [PubMed] [Google Scholar]
- 112.Mallory J.C., Crudden G., Johnson B.L., Mo C., Pierson C.A., Bard M., Craven R.J. Dap1p, a heme-Binding protein that regulates the cytochrome P450 protein Erg11p/Cyp51p in Saccharomyces cerevisiae. Mol Cell Biol. 2005;25:1669–1679. doi: 10.1128/MCB.25.5.1669-1679.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thompson A.M., Reddi A.R., Shi X., Goldbeck R.A., Moenne-Loccoz P., Gibney B.R., Holman T.R. Measurement of the heme affinity for yeast dap1p, and its importance in cellular function. Biochemistry. 2007;46:14629–14637. doi: 10.1021/bi7013739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li L., Kaplan J. Characterization of yeast methyl sterol oxidase (ERG25) and identification of a human homologue. J. Biol. Chem. 1996;271:16927–16933. doi: 10.1074/jbc.271.28.16927. [DOI] [PubMed] [Google Scholar]
- 115.Moretti-Almeida G., Netto L.E., Monteiro G. The essential gene YMR134W from Saccharomyces cerevisiae is important for appropriate mitochondrial iron utilization and the ergosterol biosynthetic pathway. FEBS Lett. 2013;587:3008–3013. doi: 10.1016/j.febslet.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 116.Puig S., Askeland E., Thiele D.J. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell. 2005;120:99–110. doi: 10.1016/j.cell.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 117.Hausmann A., Samans B., Lill R., Muhlenhoff U. Cellular and mitochondrial remodeling upon defects in iron-sulfur protein biogenesis. J. Biol. Chem. 2008;283:8318–8330. doi: 10.1074/jbc.M705570200. [DOI] [PubMed] [Google Scholar]
- 118.Puig S., Vergara S.V., Thiele D.J. Cooperation of two mRNA-Binding proteins drives metabolic adaptation to iron deficiency. Cell Metab. 2008;7:555–564. doi: 10.1016/j.cmet.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pedro-Segura E., Vergara S.V., Rodriguez-Navarro S., Parker R., Thiele D.J., Puig S. The Cth2 ARE-binding protein recruits the Dhh1 helicase to promote the decay of succinate dehydrogenase SDH4 mRNA in response to iron deficiency. J. Biol. Chem. 2008;283:28527–28535. doi: 10.1074/jbc.M804910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ramos-Alonso L., Romero A.M., Soler M.A., Perea-Garcia A., Alepuz P., Puig S., Martinez-Pastor M.T. Yeast Cth2 protein represses the translation of ARE-containing mRNAs in response to iron deficiency. PLoS Genet. 2018;14:e1007476. doi: 10.1371/journal.pgen.1007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Romero A.M., Ramos-Alonso L., Montella-Manuel S., Garcia-Martinez J., de la Torre-Ruiz M.A., Perez-Ortin J.E., Martinez-Pastor M.T., Puig S. A genome-Wide transcriptional study reveals that iron deficiency inhibits the yeast TORC1 pathway. Biochim. Biophys. Acta Gene Regul. Mech. 2019;1862:194414. doi: 10.1016/j.bbagrm.2019.194414. [DOI] [PubMed] [Google Scholar]
- 122.Singh P. Budding Yeast: An Ideal Backdrop for In vivo Lipid Biochemistry. Front Cell Dev. Biol. 2017;4:156. doi: 10.3389/fcell.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sanglard D. Emerging Threats in Antifungal-Resistant Fungal Pathogens. Front Med (Lausanne) 2016;3:11. doi: 10.3389/fmed.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]