Abstract
Bacterial glycosphingolipids such as glucuronosylceramide and galactosylceramide have been identified as ligands for invariant natural killer T cells and play important roles in host defense. However, the glycosphingolipid synthases required for production of these ceramides have not been well-characterized. Here, we report the identification and characterization of glucuronosylceramide synthase (ceramide UDP-glucuronosyltransferase [Cer-GlcAT]) in Zymomonas mobilis, a Gram-negative bacterium whose cellular membranes contain glucuronosylceramide. On comparing the gene sequences that encode the diacylglycerol GlcAT in bacteria and plants, we found a homologous gene that is widely distributed in the order Sphingomonadales in the Z. mobilis genome. We first cloned the gene and expressed it in Escherichia coli, followed by protein purification using nickel–Sepharose affinity and gel filtration chromatography. Using the highly enriched enzyme, we observed that it has high glycosyltransferase activity with UDP-glucuronic acid and ceramide as sugar donor and acceptor substrate, respectively. Cer-GlcAT deletion resulted in a loss of glucuronosylceramide and increased the levels of ceramide phosphoglycerol, which was expressed in WT cells only at very low levels. Furthermore, we found sequences homologous to Cer-GlcAT in Sphingobium yanoikuyae and Bacteroides fragilis, which have been reported to produce glucuronosylceramide and α-galactosylceramide, respectively. We expressed the two homologs of the cer-glcat gene in E. coli and found that each gene encodes Cer-GlcAT and Cer-galactosyltransferase, respectively. These results contribute to the understanding of the roles of bacterial glycosphingolipids in host–bacteria interactions and the function of bacterial glycosphingolipids in bacterial physiology.
Keywords: glycolipid, glycosyltransferase, bacteria, sphingolipid, ceramide, bacterial metabolism, innate immunity, α-galactosylceramide, glucuronosylceramide, invariant natural killer T cells
Glycosphingolipids (GSLs) are amphipathic compounds, consisting of a hydrophobic ceramide (Cer) portion and hydrophilic sugar moiety, mainly distributed in the plasma membrane of eukaryotic cells (1, 2). They cluster with cholesterols to form membrane microdomains, named lipid rafts, and play important roles in mediating signal transduction pathways through modulating receptor proteins (3). Several lines of evidence suggest that cell-surface GSLs also function as receptors for many pathogens and their toxins (4, 5). In general, GSLs are ubiquitously found in eukaryotes but are also found in some bacteria mainly belonging to the order Sphingomonadales of α-proteobacteria (6, 7). Although the eukaryotic genes involved in GSL biosynthesis have been identified, GSL synthesis in bacteria is largely unknown except for the role of serine palmitoyltransferase, the first-step enzyme of Cer synthesis, which catalyzes the condensation of l-serine with palmitoyl-CoA to form 3-ketodihydrosphingosine (8, 9). Because some sphingolipid (SL)–possessing bacteria lack the lipopolysaccharide and GSLs are located in the outer membrane of bacteria, bacterial GSLs may be a functional replacement of lipopolysaccharide, but the physiological functions of bacterial GSLs are largely unknown (10).
In the human immune system, invariant natural killer T (iNKT) cells are a heterogeneous group of T cells that share properties of both T cells and NK cells (11). On the cell surface, they express an invariant T-cell antigen receptor, which can recognize some GSLs presented by CD1d. After that, iNKT cells can be activated by these GSL–CD1d complexes and secrete several types of cytokines, such as interleukin (IL)–2, IL-4, and interferon-γ, and then they play an important role as effectors in the subsequent immune reaction for sending signals to other immune cells (12). In 1993, agelasphin was first discovered as an antitumor agent in the marine sponge Agelas mauritianus, and its structure was determined as α-galactosylceramide (α-GalCer) (13, 14). On the basis of biological activity (lymphocyte-proliferation stimulating effect and anti-tumor activity against mice), the Cer portion of α-GalCer was optimized, and α-GalCer with C18-phytosphingosine and cerotic acid (t18:0/26:0), named KRN7000, was selected as a candidate for clinical application (15). Despite the importance of the biological activity of this lipid, the reaction mechanism had not been elucidated. Later, α-GalCer was identified as the first CD1d-presented lipid antigen for iNKT cells (16), and this lipid has contributed to our understanding of iNKT cell biology (17–19).
It has been reported that α-GlcACer and α-galacturonosylceramide from Sphingomonas, a Gram-negative bacterium, can stimulate iNKT cells and lead to the release of interferon-γ through CD1d-mediated signaling (20, 21). In addition, α-GalCer was isolated from an intestinal symbiotic bacterium, Bacteroides fragilis, and was found to be a specific molecule that can regulate the homeostasis of the host's intestinal immune system through iNKT cells (22, 23). Even bacterial GSLs are recognized as exogenous ligands of CD1d and play important roles in the host's immune system, but their synthases (Cer-GlcAT and/or Cer-GalT) have not been well-characterized. Furthermore, α-GalCer synthase in the marine sponge remains unknown, and the possibility that α-GalCer is produced by symbiotic bacteria has been pointed out (12).
To investigate bacterial GSL synthase, we selected Zymomonas mobilis, a Gram-negative, facultative anaerobic bacterium belonging to the family Sphingomonadaceae (24, 25) and known for the production of GlcACer (26). Furthermore, the genome size of this bacterium (∼2 Mb with 1,988 ORFs) is small (27) when compared with that of other bacteria belonging to the same family such as Sphingobium yanoikuyae TJ (∼5.1 Mb with 4,606 ORFs) (28) and Sphingomonas wittichii (∼5.9 Mb with 5,345 ORFs) (29), and the gene manipulation system has been well-established. In this study, we report the molecular cloning, biochemical analysis, and functional analysis of Cer-GlcAT in Z. mobilis. Recombinant Cer-GlcAT showed strict specificity for Cer and UDP-glucuronic acid (GlcA) as a sugar acceptor and sugar donor, respectively. The disruption of Cer-GlcAT resulted in the lack of GlcACer and production of the unknown sphingophospholipid, which was subsequently identified as ceramide phosphoglycerol (CPG). These results indicate that Cer-GlcAT is the sole enzyme responsible for the production of GlcACer in Z. mobilis. We also report the identification and biochemical characterization of Cer-GlcAT and Cer-GalT from S. yanoikuyae and B. fragilis, which were found to produce GlcACer and α-GalCer, respectively.
Results
Occurrence of GlcACer in Z. mobilis
Because Tahara and Kawazu (26) reported the presence of GlcACer in Z. mobilis, total lipids from Z. mobilis were saponified and analyzed by TLC. As shown in Fig. 1A, one major band was stained by orcinol sulfuric acid reagent, indicating the existence of GSLs in this preparation. To confirm the structure of this GSL, we first analyzed the saponified lipid fraction by LC–electrospray ionization (ESI)–MS in positive Q1 scan mode between m/z 600 and 700 and obtained three main peaks at m/z: 660.5, 682.5, and 688.5 (Fig. 1B, upper panel). Furthermore, neutral loss scan analysis at 194, which is derived from hexuronic acid, was also performed by LC–ESI–MS/MS, and it showed the two major peaks: m/z 660.5 and 688.5 (Fig. 1B, lower panel). The results indicate that these two peaks were hexuronosyl Cer (HexACer) and probably GlcACer. Next, we determined the structure of the Cer portion of HexACer (GlcACer) by LC–ESI–MS/MS in positive ion mode. The MS/MS spectra of m/z 660.5 and 688.5 showed fragment ions at m/z 226.2, 228.2, 238.2, and 256.2 and m/z 228.2, 254.3, 266.3, and 284.3, respectively (Fig. 1C). Product ions m/z 238.2 ([d16:0 LCB+H-2H2O]+) (Fig. 1C, upper panel) and 266.3 ([d18:0 LCB + H-2H2O]+) (Fig. 1C, lower panel) were characteristic fragment ions of the d16:0 and d18:0 long-chain base (LCB), respectively (30). In addition, as shown in Fig. 1C, product ions m/z 226.2 and 256.2 (Fig. 1C, upper panel) and m/z 254.3 and 284.3 (Fig. 1C, lower panel) were considered to be [d16:0 LCB+H-H2O-CH2O]+, [d16:0 LCB+H-H2O]+, [d18:0 LCB+H-H2O-CH2O]+, and [d18:0 LCB+H-H2O]+, respectively (31, 32). Furthermore, the product ion m/z 228.2 may be derived from the N-acyl chain, in this case, myristic acid (33). Judging from the previous report by Tahara et al. (26) and the results obtained in this experiment, these two peaks were assigned to GlcACer, composed of a d16:0 or d18:0 dihydrosphingosine skeleton with myristic acid as a fatty acid moiety (Fig. 1D).
Figure 1.

Analysis of GSLs from Z. mobilis. A, TLC showing the GSLs from Z. mobilis. GSLs were extracted from Z. mobilis and analyzed by the method described under “Experimental procedures.” Lane 1, GalCer (marker); lane 2, Cer (marker); lane 3, GSLs from Z. mobilis. The arrow indicates the GSLs from Z. mobilis. B, MS spectrum of the extracted GSLs in positive ion mode. The upper panel shows the MS in the positive Q1 scan mode of the GSLs fraction. The lower panel shows a neutral loss scan on m/z 194 (derived from hexuronic acid) analysis of GlcA-containing GSLs. C, MS/MS analysis of the GSLs. MS/MS was conducted using the peak at m/z 660.5 and 688.5 as a precursor ion. The upper and lower panels indicate the MS/MS in the positive ion mode of the peak at m/z 660.5 and 688.5, respectively. HexA, hexuronic acid. Nomenclature for sphingoid bases is as follows: aX:Y, in which a represents number of hydroxyl groups (m = 1, d = 2, t = 3), X represents number of carbon atoms, and Y represents number of double bonds. For example, d16:0 represents dihydroxy base with a 16-carbon chain length and no double bonds. D, putative structure of GlcACer from Z. mobilis.
Identification of putative GlcACer synthase (Cer-GlcAT)
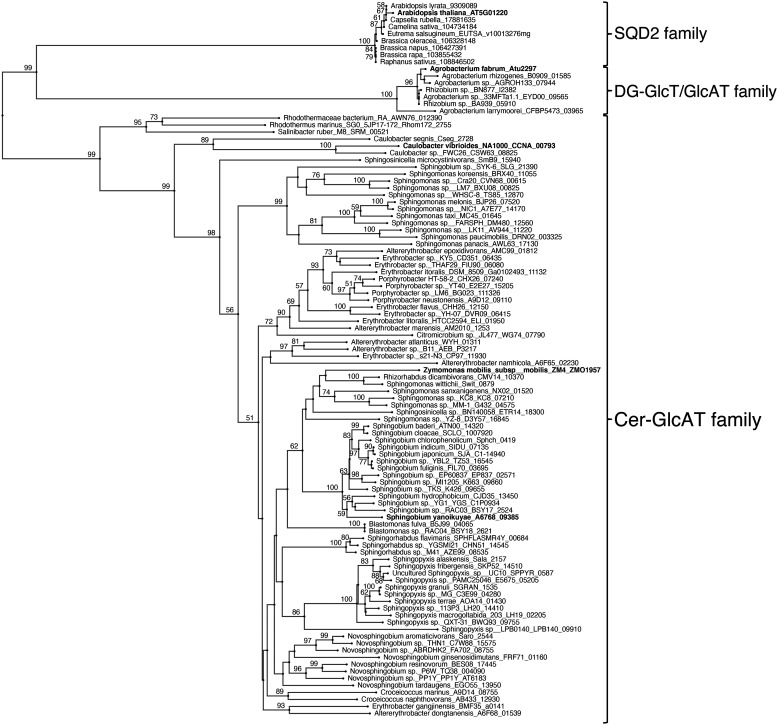
A putative sequence of Z. mobilis Cer-GlcAT (ZMO1957) was identified as a homolog of diacylglycerol sulfoquinovosyltransferase/glucuronosyltransferase (SQD2) of Arabidopsis (AtSQD2) (34) and a bifunctional glycosyltransferase (diacylglycerol glucosyltransferase/glucuronosyltransferase, DG-GlcT/GlcAT) from Agrobacterium tumefaciens (atu2297) (35), which synthesizes glucosyldiacylglycerol and glucuronosyl diacylglycerol (GlcADG) in the Kyoto Encyclopedia of Genes and Genomes (KEGG) Database of Z. mobilis subsp. mobilis ZM4 (ATCC 31821), named ZmoGlcAT. To obtain insight into glycosyltransferases in Z. mobilis, we also searched for glycosyltransferases using the CAZy Database (36) and found 20 glycosyltransferases including ZMO1957 (ZmoGlcAT). ZmoGlcAT consisted of 1,179 base pairs, coding 393 amino acids and sharing 23.3 and 30.0% identity with AtSQD2 and atu2297, respectively (Fig. 2). To investigate the distribution of ZmoGlcAT homologs in bacteria, we performed a homolog search using the KEGG database. As shown in Fig. 3, ZmoGlcAT homologs were widely distributed in the order Sphingomonadales and formed a new gene family, which was independent of SQD2 in plants and DG-GlcT/GlcAT in bacteria.
Figure 2.
Alignment of ZMO1957 with atu2297 and AtSQD2. The amino acid sequences of Cer-GlcAT from Z. mobilis (ZMO1957), bifunctional glycosyltransferase from A. tumefaciens (atu2297), and SQDG/GlcADG synthase from A. thaliana (AtSQD2) were aligned by the multiple alignment algorithm using GENETYX-MAC version 19. Identical amino acids in two or three sequences are indicated by black letters in gray boxes and white letters in black boxes, respectively.
Figure 3.
Phylogenetic tree of Cer-GlcAT, DG-GlcT/GlcAT, SQD2, and their homologs. We conducted multiple alignments using MAFFT version 7 and analyzed the relationship of each protein with the neighbor-joining method. The details are presented under “Experimental procedures.” Confidence values from 1,000 resamplings are shown. The numbers indicate bootstrap values (only values of >50 are shown). The proteins identified as glycosyltransferases are indicated in bold type.
Purification of recombinant ZmoGlcAT
To characterize ZmoGlcAT, the ORF of ZmoGlcAT was cloned, inserted into pET23a, and expressed in Escherichia coli BL21(λDE3)pLysS as a His tag–fused protein. Recombinant ZmoGlcAT was purified by nickel–Sepharose and Superdex 200 chromatography. The purified enzyme showed a single protein band possessing a molecular mass of 45,800 on SDS-PAGE after staining with Coomassie Brilliant Blue (Fig. 4A). This value agreed well with the molecular weight deduced from the nucleotide sequence (Mr = 44,279). Furthermore, we analyzed the Cer-GlcAT activity of the purified enzyme and revealed that the enzyme catalyzed the transfer reaction when C6-NBD-Cer and UDP-GlcA were used as a sugar acceptor and a sugar donor, respectively (Fig. 4B, lane 2). This reaction was considered to be due to the action of the enzyme, because treatment of the enzyme at 100 °C for 10 min completely abolished formation of the reaction product (Fig. 4B, lane 3).
Figure 4.
Purification and characterization of recombinant Cer-GlcAT of Z. mobilis. A, final preparation of rCer-GlcAT on 12.5% SDS-PAGE. The protein eluted from the nickel–Sepharose column was purified using Superdex 200 10/300 GL column. Lane 1, protein marker; lane 2, final preparation. The arrow indicates the rCer-GlcAT of Z. mobilis. B, TLC showing the synthesis of GlcACer by rCer-GlcAT. Lane 1, C6-NBC-Cer standard; lane 2, C6-NBD-Cer + UDP-GlcA + rCer-GlcAT; lane 3, C6-NBD-Cer + UDP-GlcA + boiled enzyme. The arrow indicates the C6-NBD-GlcACer. C, specificity for the sugar acceptor. D, specificity for the sugar donor. GlcACer synthase activity was determined by the method described under “Experimental procedures.” Open bar, 1-h incubation; black bar, 24-h incubation. The values are expressed as the means ± S.D. (n = 3). E, MS spectra of the synthesized C6-NBD-GlcACer in positive ion mode. A precursor ion scan at m/z 264.4 (derived from d18:1 sphingosine) was used to identify C6-NBD-GlcACer. The inset shows the structure of C6-NBD-GlcACer.
Properties of ZmoGlcAT
Substrate specificity of ZmoGlcAT was analyzed using various UDP sugars as a sugar donor and NBD-Cer and NBD-DG as a sugar acceptor. As shown in Fig. 4C, when UDP-GlcA was used as a sugar donor, this enzyme utilized NBD-Cer but not NBD-DG as a sugar acceptor, and C12-NBD-Cer was preferable to C6-NBD-Cer. Among UDP sugars used in this experiment, only UDP-GlcA was utilized as a sugar donor, and this specificity did not change even with a longer incubation time (Fig. 4D). These results indicate that ZmoGlcAT is a Cer-GlcAT, which synthesizes GlcACer from UDP-GlcA and Cer, and thus, ZmoGlcAT was renamed Cer-GlcAT. In addition, the substrate specificity of Cer-GlcAT is different from bifunctional glycosyltransferase from Agrobacterium (atu2297), which synthesizes glucosyldiacylglycerol and GlcADG from DG and UDP-Glc or UDP-GlcA (35).
To clarify the structure of the reaction product produced by Cer-GlcAT of Z. mobilis, C6-NBD-GlcACer was synthesized by providing C6-NBD-Cer and UDP-GlcA as substrates. After purification of the reaction product, the product was analyzed by LC–ESI–MS in positive ion mode (precursor ion scan at m/z 264.4, which is derived from d18:1 sphingosine), and the three main peaks of m/z 734.5, 752.6, and 769.5 were obtained. Judging from the molecular mass of C6-NBD-GlcACer (751.4), these peaks were assigned as [M + H-H2O]+, [M + H]+, and [M+NH4]+, respectively (Fig. 4E).
The maximal activity of Cer-GlcAT was observed at pH 5.5 when C6-NBD-Cer and UDP-GlcA was used as substrates (Fig. 5A). The optimal temperature of Cer-GlcAT was between 37 and 50 °C (Fig. 5B). The activity of Cer-GlcAT was enhanced by the addition of Mg2+, Mn2+, and Ca2+ at a concentration of 5 mm and was not influenced by the addition of 5 mm EDTA (Fig. 5C). The maximum activity was observed by adding Mg2+ to more than 20 mm (Fig. 5D). The divalent cation requirement is similar to DG-GlcT from Acholeplasma laidlawii, which requires Mg2+ for full activity (37). Furthermore, the detailed metal ion requirement of DG-GlcT/GlcAT of A. tumefaciens is unknown, but Mg2+ was included in the reaction mixture (35).
Figure 5.
General properties of recombinant Cer-GlcAT of Z. mobilis. A, pH dependence of rCer-GlcAT. A total of 150 mm of GTA buffer (50 mm 3,3-dimethylglutaric acid, 50 mm Tris(hydroxymethyl) aminomethane, and 50 mm 2-amino-2-methyl-1,3-propanediol) at the indicated pH was used for the assay. B, effects of temperature on rCer-GlcAT activity. C, effects of metal ions on rCer-GlcAT activity. 5 mm of each cation was included in the reaction mixture. Left lane (-), control experiment without cations, D, effect of magnesium ions on rCer-GlcAT activity. The data represent the means ± S.D. (error bars) of three experiments. The assay was conducted using C6-NBD-Cer as a sugar acceptor and UDP-GlcA as a sugar donor. GlcACer synthase activity was determined by the method described under “Experimental procedures.”
Generation of Cer-GlcAT knockout (KO) strain
To clarify the functions of Cer-GlcAT in Z. mobilis, a Cer-GlcAT KO strain was generated by homologous recombination using the chloramphenicol acetyltransferase gene (Cmr) as a selection marker (Fig. 6A). The homologous recombination was confirmed by PCR and lipid analysis. From the electrophoresis gel, a ∼400-bp difference in the total gene length was detected between the WT and KO strains (Fig. 6B, PCR1). This difference was consistent with the calculated theoretical results (Fig. 6A). On the other hand, the one-side confirmation experiments indicated that the cer-glcat band was not present in the KO strain (Fig. 6B, PCR2). Furthermore, as shown in Fig. 6C, lipid analysis of the WT and KO strain showed the disappearance of GlcACer in the KO strain. These results suggest that the cer-glcat gene was successfully deleted in KO strain and that Cer-GlcAT is the sole enzyme for the production of GlcACer in Z. mobilis.
Figure 6.
Disruption of the cer-glcat gene in Z. mobilis. A, strategy for the disruption of Z. mobilis cer-glcat gene by homologous recombination using a chloramphenicol acetyltransferase gene (Cmr) as a marker. The primers used and the PCR product size are indicated. The dotted lines indicate the recombination site. B, PCR analysis of cer-glcat-disrupted mutants (KO) and the WT of Z. mobilis. In PCR1, a 3,421-bp amplicon was observed in WT and a 3,002-bp amplicon was observed in Cer-GlcAT KO. In PCR2, a 961-bp amplicon was observed in WT. Lanes 1, OneSTEP Ladder 1 kb (marker); lanes 2, WT; lanes 3, Cer-GlcAT KO. C, TLC analysis of GSLs in WT and Cer-GlcAT KO strain. Lane 1, GalCer (marker); lane 2, Cer-GlcAT KO; lane 3, WT. The arrow indicates the GlcACer from Z. mobilis.
Identification of lipid that accumulated in Cer-GlcAT KO strain
Because some GSL-producing bacteria also synthesize sphingophospholipids such as ceramide phosphoethanolamine (CPE), we saponified and separated the lipids of the Cer-GlcAT KO strain and tested them with several chromogenic agents. After staining with Dittmer–Lester reagent, a weak blue signal band and a strong blue signal band were observed in the WT and KO strains, respectively (Fig. 7A). However, this lipid was not stained with ninhydrin reagent, indicating that this was not CPE, found in some bacteria (Fig. 7B). Therefore, this alkaline-resistant lipid was considered as a sphingophospholipid other than CPE. To clarify its structure, the lipid was extracted from the Cer-GlcAT KO strain and purified as described under “Experimental procedures.” The purified lipid was first analyzed by LC–ESI–MS in negative Q1 scan mode. Two main peaks were observed at m/z 636.7 and 664.7, and the difference between the two peaks (28 m/z units) corresponded to two carbons plus four hydrogens. The MS/MS spectra of m/z 636.7 and 664.7 showed a common fragment ion at m/z 171, which corresponds to a phosphoglycerol (Fig. 7C). Furthermore, m/z 153 and 79 are dehydration products of phosphoglycerol and phosphate groups, respectively. Collectively, the sphingophospholipid that accumulated in the Cer-GlcAT KO strain was CPG (Fig. 7D); however, the content of this lipid was low in WT (Fig. 7A). These results indicate that CPG was synthesized in the Cer-GlcAT KO strain in response to a lack of GlcACer.
Figure 7.

Analysis of SLs in WT and Cer-GlcAT KO strain. The cells were cultured in 3 ml of RM medium at 30 °C for 24 h, and SLs were extracted by the method described under “Experimental procedures.” A and B, extracted lipids were separated by TLC and visualized by Dittmer–Lester reagent (A) and ninhydrin reagent (B). Lanes 1, CPE; lanes 2, WT; lanes 3, Cer-GlcAT KO. The arrow in A indicates the accumulated lipid. C, identification of the sphingophospholipid that accumulated in Cer-GlcAT KO. The upper panel shows the MS in the negative Q1 scan mode of the accumulated lipid. MS/MS was conducted using the peak at m/z 636.7 and 664.7 as a precursor ion. The middle and lower panels indicate the MS/MS in the negative ion mode of the peak at m/z 636.7 and 664.7, respectively. D, the proposed structure of sphingophospholipid that accumulated in Cer-GlcAT KO.
Cell proliferation of WT and Cer-GlcAT KO strain
To clarify the physiological significance of GlcACer in Z. mobilis, cell growth of the Cer-GlcAT KO strain was compared with that of WT under aerobic (shaking culture) and microaerobic (static culture) conditions. Because Z. mobilis is a facultative anaerobic bacterium, growth of this bacterium was much better under lower oxygen conditions (38). Under microaerobic conditions, the KO strain showed slower proliferation in the logarithmic phase than WT (Fig. 8). On the other hand, under aerobic conditions, cell growth of these strains was almost at the same level (Fig. 8). Although the KO strain showed slow proliferation under microaerobic conditions, the KO strain did not show a strong growth phenotype compared with the WT strain under the culture conditions used in this study. As shown in Fig. 7A, CPG expression was up-regulated in the KO strain, and increased CPG may compensate for the function of GlcACer in the KO strain.
Figure 8.
Growth curves of WT and Cer-GlcAT KO strain. The cells were cultured in 30 ml of RM medium for 24 h with or without shaking at 120 rpm. The samples were taken at different times, and the A550 was determined. The values are expressed as the means ± S.D. (n = 3). Closed symbols are without shaking, and open symbols are with shaking. Circles, WT; squares, Cer-GlcAT KO1; triangles, Cer-GlcAT KO2. Significance is shown as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001 significantly different from WT. Statistical analyzes were performed by Welch's t test.
Glycosyltransferase activity of Cer-GlcAT homolog from S. yanoikuyae and B. fragilis
To further knowledge of the distribution of Cer-GlcAT, we searched for the Cer-GlcAT homolog in the genome of bacteria reported to synthesize GlcACer. We found a sequence (A6768_09385) that shared 64.6% identity with Z. mobilis Cer-GlcAT in the genome of S. yanoikuyae (Fig. 9). We synthesized the gene, expressed it in E. coli, and purified the recombinant enzyme. As shown in Fig. 10A, recombinant A6768_09385 synthesized GlcACer when UDP-GlcA and C6-NBD-Cer were used as sugar donor and acceptor substrates, respectively (Fig. 10A, lanes 5 and 6). Furthermore, the enzyme showed high specificity for UDP-GlcA and NBD-Cer as a sugar donor and sugar acceptor, respectively (Fig. 10, B and D). These results clearly indicate that A6768_09385 encoded Cer-GlcAT.
Figure 9.
Alignment of Z. mobilis Cer-GlcAT with A6768_09385 and BF9343_3149. The amino acid sequences of Cer-GlcAT from Z. mobilis (ZMO1957), Cer-GlcAT homolog from S. yanoikuyae (A6768_09385), and Cer-GlcAT homolog from B. fragilis (BF9343_3149) were aligned by the multiple alignment algorithm using GENETYX-MAC version 19. Identical amino acids in two or three sequences are indicated by black letters in gray boxes and white letters in black boxes, respectively.
Figure 10.
Glycosyltransferase activity of Cer-GlcAT homologs. A, TLC showing the synthesis of GSLs by rCer-GlcAT from Z. mobilis and its homologs. Glycosyltransferase activities were analyzed by incubating C6-NBC-Cer and each sugar donor in the presence of each enzyme at 37 °C for 2 h. Lane 1, C6-NBC-Cer standard; lane 2, C6-NBD-GalCer standard; lane 3, C6-NBD-Cer + UDP-GlcA + rCer-GlcAT from Z. mobilis; lane 4, C6-NBD-Cer + UDP-GlcA + boiled rCer-GlcAT from Z. mobilis; lane 5, C6-NBD-Cer + UDP-GlcA + recombinant A6768_09385; lane 6, C6-NBD-Cer + UDP-GlcA + boiled recombinant A6768_09385; lane 7, C6-NBD-Cer + UDP-Gal + recombinant BF9343_3149; lane 8, C6-NBD-Cer + UDP-Gal + boiled recombinant BF9343_3149. B, sugar donor specificity of recombinant A6768_09385. Glycosyltransferase activity was determined using C6-NBD-Cer and various nucleotide sugars at 37 °C for 2 h by the method described under “Experimental procedures.” C, sugar donor specificity of recombinant BF9343_3149. Glycosyltransferase activity was determined using C6-NBD-Cer and various nucleotide sugars at 37 °C for 2 h by the method described under “Experimental procedures.” D, sugar acceptor specificity of recombinant A6768_09385. Glycosyltransferase activity was determined using UDP-GlcA and NBD-Cer or NBD-DG at 37 °C for 2 h by the method described under “Experimental procedures.” E, sugar acceptor specificity of recombinant BF9343_3149. Glycosyltransferase activity was determined using UDP-Gal and NBD-Cer or NBD-DG at 37 °C for 2 h by the method described under “Experimental procedures.” The values are expressed as the means ± S.D. (n = 3). F, TLC showing the hydrolysis of C6-NBD-GalCer by EGALC. C6-NBD-βGalCer and C6-NBD-GalCer synthesized by BF9343_3149 were incubated in 20 μl of 50 mm sodium acetate buffer, pH 5.5, containing 0.1% (w/v) Triton X-100, with EGALC at 37 °C for 16 h. The samples were loaded onto a TLC plate, which was developed with chloroform/methanol/water (65:25:4, v/v/v). Lane 1, C6-NBC-Cer standard; lane 2, C6-NBD-βGalCer + water; lane 3, C6-NBD-βGalCer + EGALC; lane 4, C6-NBD-GalCer synthesized by BF9343_3149 + water; lane 5, C6-NBD-GalCer synthesized by BF9343_3149 + EGALC. G, LC–ESI–MS analysis of GalCer synthesized by Cer-GalT from B. fragilis. For preparation of C18-GalCer, C18-Cer liposome (3 nmol of C18-Cer (d18:1/C18:0) and 19.5 nmol of lecithin) and 150 nmol of UDP-Gal were incubated at 37 °C for 16 h with recombinant BF9343_3149 in 150 μl of 100 mm MES, pH 6.0, and the reaction product obtained was extracted and analyzed by HILIC-phase LC–ESI–MS/MS. For negative control, the reaction was performed in the absence of the enzyme. H, the structure of C18-α-GalCer.
We also searched for the Cer-GlcAT homolog in the genome of B. fragilis, which is a well-known intestinal microbe and known for the production of α-GalCer (22), and found a sequence (BF9343_3149), sharing 23.8% identity with Z. mobilis Cer-GlcAT (Fig. 9). To evaluate the glycosyltransferase activity, we synthesized the gene and expressed it in E. coli. As shown in Fig. 10A, recombinant BF9343_3149 synthesized C6-NBD-GalCer when UDP-Gal and C6-NBD-Cer were used as sugar donor and acceptor substrates, respectively (Fig. 10A, lanes 7 and 8). We next analyzed sugar donor specificity of recombinant BF9343_3149 using C6-NBD-Cer and various nucleotide sugars. Among the nucleotide sugars tested, UDP-Gal was efficiently utilized as a sugar donor (Fig. 10C). UDP-Glc was utilized to a limited extent, whereas GDP-Man, UDP-GlcA, UDP-GalNAc, and UDP-GlcNAc were not utilized at all. We next evaluated sugar acceptor specificity of the enzyme using UDP-Gal and NBD-Cer or NBD-DG. As shown in Fig. 10E, the enzyme utilized NBD-Cer but not NBD-DG as a sugar acceptor. Judging from these results, we demonstrated that the Cer-GlcAT homolog BF9343_3149 encoded the Cer-GalT.
To obtain insight into the anomeric conformation of the synthesized C6-NBD-GalCer, we treated C6-NBD-GalCer with endogalactosylceramidase (EGALC), which can hydrolyze β-GalCer (39), and compared it with C6-NBD-βGalCer. As shown in Fig. 10F, EGALC completely hydrolyzed C6-NBD-β-GalCer but not C6-NBD-GalCer synthesized by recombinant BF9343_3149. This result suggests that the anomeric conformation of the synthesized C6-NBD-GalCer is the α type. We also analyzed the anomeric conformation of GalCer synthesized in this study using HILIC-phase LC–ESI–MS/MS (40). Using this LC–ESI–MS/MS system, we were able to separate C18-α-GalCer (d18:1/C18:0) and C18-β-GalCer (d18:1/C18:0). Next, we reacted UDP-Gal and C18-Cer (d18:1/C18:0) in the presence of recombinant BF9343_3149, and the reaction product obtained was analyzed by HILIC-phase LC–ESI–MS/MS. As shown in Fig. 10G, the retention time of the reaction product well-matched that of C18-α-GalCer (Fig. 10H). Judging from these data, we conclude that recombinant BF9343_3149 is an α-GalCer synthase (Cer-GalT).
Discussion
Production of SLs such as GSLs and/or sphingophospholipids is one of the typical features of the order Sphingomonadales (7), and bacterial GSLs are considered to be localized in the outer membrane of bacteria (10). Furthermore, the glycosidic linkage between carbohydrate and Cer of bacterial GSLs is α-linkage, unlike eukaryotes, which is β-linkage. Because bacterial GSLs were recognized as exogenous glycolipid antigens for iNKT cells, they emerged as an important molecule in the mammalian host defense system (18). Despite the importance of bacterial GSLs, their synthases (glycosyltransferases) have not been well-characterized. In the present study, we reported the identification, biochemical characterization, and functional analysis of GlcACer synthase (Cer-GlcAT) in Z. mobilis. Furthermore, we succeeded in identifying the α-GalCer synthase (Cer-GalT) of B. fragilis as a homolog of Z. mobilis Cer-GlcAT. This study provides important insight into the primary sequence of bacterial GSL synthases.
To investigate the distribution of Cer-GlcAT in bacteria, we performed a homology search using the amino acid sequence of Z. mobilis Cer-GlcAT (ZMO1957). As shown in Fig. 3, the Cer-GlcAT sequence was found in many bacteria belonging to the order Sphingomonadales, and the homologous proteins may belong to a different protein family with sulfoquinovosyldiacylglycerol (SQDG)/GlcADG synthase, SQD2 in plants and DG-GlcT/GlcAT in bacteria. Because the existence of GlcACer has been reported in a limited number of bacteria, the Cer-GlcAT sequence obtained in this study further suggests the existence of GlcACer in bacteria. To confirm the distribution of Cer-GlcAT in the family Sphingomonadaceae, we analyzed the Cer-GlcAT homolog of S. yanoikuyae (A6768_09385) and revealed that A6768_09385 showed Cer-GlcAT activity. This result supports our hypothesis that Cer-GlcAT is widely distributed in the family Sphingomonadaceae. Among the bacteria with the Cer-GlcAT sequence, Sphingomonas paucimobilis (41), Sphingomonas koreensis (42), and Novosphingobium aromaticivorans (43) are considered to be pathogenic bacteria, and thus, they could infect humans and lead to inflammatory reactions through GlcACer. Because Kinjo et al. (20) and Mattner et al. (21) reported the in vivo activation of iNKT cells after exposure to Sphingomonas, the KO strain of Cer-GlcAT of Sphingomonas might explain the function of GlcACer in bacterial infection more clearly.
Very recently, Stankeviciute et al. (44) analyzed the lipid composition of Caulobacter crescentus, an oligotrophic Gram-negative bacterium, under phosphate starvation conditions and revealed that this bacterium synthesized not only glycoglycerolipids but also a novel GSL (GSL-2, HexHexACer) to adapt to phosphate starvation. They also identified the glycosyltransferases responsible for synthesis of a novel GSL, GSL-2. One is sphingolipid glycosyltransferase 1, Sgt1 (ccna_00793), which transfers hexuronic acid to Cer to form HexACer, and the other is Sgt2 (ccna_00792), which transfers hexose to HexACer to form GSL-2. Although the substrate specificities of these enzymes have not been characterized, HexACer synthase Sgt1 (ccna_00793) may be GlcACer synthase, because it shows 39.9% amino acid identity with Cer-GlcAT of Z. mobilis.
α-GalCer (agelasphin) was first obtained from the marine sponge as an antitumor compound and could activate iNKT cells more strongly than GlcACer, but its synthetic enzyme has not been identified, and the symbiont of the sponge is considered as the potential producer (12). α-GalCer was also found in B. fragilis, a well-known human gut microbe (22), and recently, this lipid was detected in the other gut microbes Bacteroides vulgatus and Prevotella copri (40). Because human gut microbiota produces many molecules that affect host biology, α-GalCer produced from Bacteroides is thought to be one of the critical signaling molecules in host gut physiology (22, 23). Very recently, von Gerichten et al. (45) reported the existence of α-GalCer containing a Cer with a β-hydroxylated palmitic acid and C18-sphinganine (α-GalCerMLI), which differed from the structure reported in B. fragilis, in the cecum and colon of mice. The detail synthetic pathway of this unique α-GalCer is unknown, but B. fragilis may be involved in the production of this glycolipid, because it was not detected in germ-free mice. They also found that the production of this unique α-GalCer depended on diet and inflammation and suggested that the α-GalCer–mediated immune response through iNKT cells is important in gut energy metabolism and immunity. In the present study, we succeeded in identifying Cer-GalT of B. fragilis as a homolog of Cer-GlcAT of Z. mobilis, and thus, Cer-GalT of B. fragilis identified in this study will contribute to elucidating the function of α-GalCer in B. fragilis–related immune response in the human intestine.
Total cellular lipid analysis was previously performed in Z. mobilis, revealing that phospholipids and hopanoids comprised ∼60 and 30% of cellular lipids, respectively (46). Among the phospholipids, the predominant lipid was phosphatidylethanolamine (∼50%), and phosphatidylcholine, phosphatidylglycerol, and cardiolipin were present to some extent. Hopanoids are widespread in bacteria and are considered as bacterial sterol analogs (9). In Z. mobilis, hopanoids are considered to play important roles in growth and survival in the presence of ethanol (47). In the case of sphingolipids, the content of GlcACer was estimated to be 4.6% of the total extractable lipids in Z. mobilis (26). To understand the physiological functions of GlcACer in Z. mobilis, we established the Cer-GlcAT KO strain in Z. mobilis and revealed that the loss of GlcACer caused the accumulation of CPG, which is expressed at a low level in WT. CPG was found in Z. mobilis for the first time, but the existence of this lipid has been reported in other Sphingobacteria (6). In Sphingobacteria species, it is well-known that they express many types of SLs, such as CPG, CPE, ceramide phosphoinositol, and ceramide phosphomannose (48). Nichols et al. (49) identified CPG in Porphyromonas gingivalis, a well-known periodontal pathogen, and demonstrated that CPG but not CPE from P. gingivalis caused the production of prostaglandin E2 from IL-1β–treated gingival fibroblasts and induced a change in the fibroblast morphology. Because P. gingivalis is considered a major periodontal pathogen, CPG of P. gingivalis might play important roles in inflammation processes associated with periodontal diseases. Although elucidation of the CPG synthetic pathway is indispensable for further analysis of the function of CPG in P. gingivalis–induced periodontal diseases, its synthetic enzymes have not been identified in bacteria. Because we noted up-regulation of the CPG level in the Cer-GlcAT KO strain of Z. mobilis, analysis of gene expression between WT and the KO strain might provide important information to elucidate the CPG synthetic pathway. An et al. (50) reported that treatment with myriosin, a specific inhibitor of serine palmitoyltransferase, of the SL-producing intestinal microbe B. fragilis caused the reduction of SLs and resulted in marked growth defects under stress but not under normal conditions. Their report suggests the existence of an SL-mediated response system in Bacteroides and the importance of SLs in Bacteroides survival in the intestine. In the present study, we found that under reduced oxygen conditions, the proliferation rate of the Cer-GlcAT KO strain is slower than that of the WT in the logarithmic phase, but with more oxygen, the KO strain did not show any differences (Fig. 8). This result may explain the importance of GlcACer in the cell proliferation of Z. mobilis, but both GlcACer and CPG are anionic under physiological conditions; the function of GlcACer may be partly compensated for by CPG. Furthermore, the reason for up-regulation of the CPG level in the KO strain is unknown, but the replacement of GlcACer by CPG in the KO strain indicates the importance of maintaining the biophysical properties of the membrane, such as the membrane's negative charge in Z. mobilis.
In plants and some bacteria, membrane phospholipids function as a phosphate reservoir and are degraded to obtain the phosphate in response to phosphate deficiency (9, 51, 52). Each membrane phospholipid has its own function in the membrane, and their functions may be effectively substituted by nonphosphorous lipids under phosphate-deficient conditions. It has been reported that concomitant with the reduction of membrane glycerophospholipids, phosphorus free lipids, such as SQDG, ornithine-containing lipid, diacylglycerol trimethylhomoserine, monoglycosyl diacylglycerol, and GlcADG, have been synthesized (9). In this situation, the functional counterpart of phospholipid phosphoglycerol is considered to be GlcADG. In the Cer-GlcAT KO strain, loss of GlcACer is substituted by CPG, which also has a negative charge in its structure. This replacement suggests the existence of compensatory mechanisms to maintain the membrane charge in Z. mobilis, like the synthesis of phosphorus free lipids under the phosphate starvation conditions in plants and bacteria.
Collectively, this study identified the GlcACer synthase in Z. mobilis and revealed that the lack of GlcACer was compensated for by the synthesis of CPG in this bacterium. Furthermore, we succeeded in identifying the α-GalCer synthase of B. fragilis as a homolog of GlcACer synthase of Z. mobilis. The importance of GSLs in eukaryotes has been extensively studied; however, in bacteria, their physiological functions are not fully understood. Therefore, this study will facilitate understanding of bacterial GSL metabolism and its physiological significance and the utilization of bacterial GSLs in the future.
Experimental procedures
Cell culture
A strain of Z. mobilis (NBRC 13756) was provided by the Biological Resource Center of the National Institute of Technology and Evaluation (Chiba, Japan). Z. mobilis was cultured in RM medium (50 g/liter of glucose, 10 g/liter of yeast extract, 1 g/liter of MgSO4, 1 g/liter of (NH4)2SO4, and 2 g/liter of KH2PO4) at 30 °C with or without shaking. An RM plate (RM medium containing 1.5% agarose) containing 75 µg/ml chloramphenicol was used for KO strain selection.
Extraction of SLs from Z. mobilis
Total lipids were extracted by incubating with chloroform/methanol/water (1/2/0.8, v/v/v) at 37 °C for 2 h followed by adding chloroform and distilled water to adjust the proportion to chloroform/methanol/water = 1:1:1, and centrifuged at 4 °C and 10,000 × g, for 5 min. The lower phase was transferred to a new tube and evaporated by the SpeedVac concentrator. Extracted lipids were dissolved in methanol, saponified by NaOH at 37 °C for 2 h, and then neutralized by acetic acid. After the addition of water to adjust the proportion of methanol to 50%, the samples were applied to an OASIS HLB cartridge (1 cc/30 mg, Waters), washed with methanol/water (1/1, v/v) and water successively, and then eluted by methanol.
TLC analysis of lipids
The lipids were analyzed using a Silica Gel 60 TLC plate, which was developed with chloroform/methanol/water (65/25/4, v/v/v). Glycolipids, phospholipids, and lipids containing the amino group were stained by orcinol sulfuric acid reagent, Dittmer–Lester reagent, and ninhydrin reagent, respectively.
LC–ESI–MS analysis
Extracted lipids were dissolved in isopropanol and centrifuged at room temperature at 1,000 × g for 5 min. The supernatant obtained was transferred to a glass vial and applied to LC–ESI–MS/MS using a HPLC system (1200 series, Agilent Technologies) equipped with a mass spectrometer (3200 QTRAP LC–MS/MS system, SCIEX). The glycerolipids and SLs were separated by reverse-phase chromatography in a binary solvent gradient with a 200 μl/min flow rate passing through an InertSustain C18 column (2.1 × 150 mm, 5 μm; GL Sciences). The gradient was started with 3% buffer B (2-propanol with 0.1% formic acid and 0.028% ammonia) in buffer A (acetonitrile/methanol/distilled water, 19:19:2, v/v/v containing 0.1% formic acid and 0.028% ammonia) and was held for 3 min. The gradient was then converted to 40% B for 21 min, 70% B for 1 min, and held for 7 min. The gradient was turned back to the initial conditions for 1 min and was held for 7 min for re-equilibration. Single MS analysis was performed with a positive or negative Q1 scan to determine the m/z of the precursor ion, and then the precursor ion was applied to MS/MS analysis to generate product ions. A precursor ion scan at 264.4 and neutral loss scan at 194 were carried out to identify the structure of GlcA-containing GSLs.
Molecular cloning of ZmoGlcAT
By comparing with diacylglycerol glucuronosyltransferases (DG-GlcATs) in bacteria and plants, a homologous sequence (ZMO1957) was found in the Z. mobilis subsp. mobilis ZM4 genome. The ZMO1957 gene was amplified by PCR using Z. mobilis genomic DNA as a template and the primers Zmo1957_InfuF and Zmo1957_InfuR (Table S1). The amplified product was inserted into BamHI-digested pColdI (Takara Bio, Kusatsu, Japan) to generate pCold-Zmo1957. The insert was then sequenced using the BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems) and a 3130 Genetic Analyzer (Applied Biosystems).
Expression and purification of ZmoGlcAT
The expression plasmid carrying the ZMO1957 gene was constructed by PCR using pCold-Zmo1957 as a template and the primers pET23_Zmo1957_InfuF and pET23_Zmo1957_InfuR (Table S1). The amplified product was inserted into the pET23a vector (Novagen), which was linearized by PCR using pET23a as a template and the primers pET23a_F and pET23a_R. The resulting plasmid was named pET23_ZmoGlcAT.
BL21(λDE3)pLysS was transformed with the pET23_ZmoGlcAT plasmid and cultured at 37 °C overnight with shaking in 3 ml of LB medium supplemented with 100 μg/ml of carbenicillin and 35 μg/ml of chloramphenicol. The culture was then transferred to a 500-ml flask containing 200 ml of LB medium under the same antibiotic conditions and incubated for 24 h at 25 °C with shaking. The cells were harvested at 4 °C at 10,000 × g for 10 min and suspended in lysis buffer (50 mm HEPES, pH 7.0, 0.5% Triton X-100, 5 mm Tris (2-carboxyethyl) phosphine hydrochloride (TCEP-HCl), protease inhibitor, 20 mm MgCl2, 500 mm NaCl, and 20% glycerol). After sonication for 5 min, cell debris was removed by centrifugation (10,000 × g for 20 min). The supernatant was loaded onto a column of nickel–Sepharose 6 Fast Flow (GE Healthcare), and the column was washed with buffer A (50 mm HEPES, pH 7.0, 20 mm MgCl2, 20% glycerol, 500 mm NaCl, 0.1 mm n-dodecyl-β-d-maltoside, 1 mm TCEP-HCl) containing 20 or 40 mm imidazole. The enzyme was eluted from the column with buffer A containing 200 mm imidazole. The fractions containing the enzyme were concentrated with Amicon Ultra-4 centrifugal filter unit (10,000 NMWL) at 4 °C, 10,000 × g. The sample was filtered with Cosmospin filter G (0.45 μm) at 4 °C at 10,000 × g for 3 min, and then it was injected into the Superdex 200 10/300 GL column (10 × 300 mm, GE Healthcare) equilibrated with 50 mm HEPES, pH 7.0, 10 mm MgCl2, 10% glycerol, 500 mm NaCl, 0.1 mm n-dodecyl-β-d-maltoside, and 1 mm TCEP-HCl. Corresponding tubes of the highest peak were analyzed by SDS-PAGE. The authentic enzyme was concentrated by an Amicon Ultra-4 centrifugal filter unit (10,000 NMWL) at 4 °C at 10,000 × g. The protein concentration was measured by PierceTM 660 nm protein assay (Thermo Fisher Scientific).
Cer-GlcAT assay
The activity of GlcACer synthase was measured using C6-NBD-Cer and UDP-GlcA as substrates, as described below. The reaction mixture contained Cer liposome (50 pmol of C6-NBD-Cer and 6.5 nmol of lecithin), 200 μm UDP-GlcA, and an appropriate amount of the enzyme in 50 μl of 100 mm MES, pH 6.0, containing 20 mm MgCl2. Following incubation at 37 °C for an appropriate time, the reaction was terminated by adding 125 μl of chloroform/methanol (2:1, v/v), mixed, and then centrifuged at 10,000 × g for 2 min. After the organic phase had dried, the lipids were dissolved in 15 μl of chloroform/methanol (2:1, v/v) and then applied to a TLC plate, which was developed with chloroform/methanol/water (65:25:4, v/v/v) as a developing solvent. C6-NBD-Cer and C6-NBD-GlcACer were quantified using a Shimadzu CS-9300 chromatoscanner (excitation, 475 nm; emission, 525 nm). The extent of the synthesis of NBD-labeled GlcACer (reaction efficiency) was calculated as follows: reaction efficiency (%) = (peak area for the NBD-GlcACer synthesized) × 100/(peak area for the NBD-GlcACer synthesized + peak area for the remaining NBD-Cer).
Construction of cer-glcat gene KO vector
The up- and downstream sequence fragments (1,000 bp) flanking the cer-glcat gene were amplified by PCR using Z. mobilis genome DNA as a template and the primers Zmo1957KO_pUC19_Infu1 and Zmo1957KO_pUC19_Infu2. The amplified product was cloned into the linearized pUC19 vector (Takara Bio), and the resulting plasmid was named pUC19-Zmo1957. To replace the cer-glcat gene with the Cmr cassette, the cer-glcat gene was removed by PCR using pUC19-Zmo1957 as a template and the primers Zmo1957KO_VecF and Zmo1957KO_VecR. The fragment obtained was ligated with the Cmr cassette amplified from pSTV29 (Takara Bio) using the primers Zmo1957KO_CmR_InfuF and Zmo1957KO_CmR_InfuR. The primers used are summarized in Table S1. The resulting plasmid was named pUC19-Zmo1957KO. The plasmid containing a mutant cer-glcat gene disrupted with the Cmr gene was introduced into Z. mobilis by the methods described below.
Generation of Cer-GlcAT KO mutant
Z. mobilis was cultured at 30 °C overnight in 10 ml of RM medium without shaking. The culture was transferred into 100 ml of RM medium to adjust the initial A550 to 0.1 and cultured at 30 °C for several hours without shaking until A550 reached 0.3–0.4. The cells were harvested at 4 °C at 5,000 rpm for 5 min and then suspended in 10 ml of 10% glycerol solution containing sterilized 0.85% NaCl. They were centrifuged again under the same conditions, and all supernatant was removed. Finally, the cells were suspended in 1 ml of sterilized 10% glycerol solution. To obtain the KO strain, a 100-μl aliquot of electrocompetent cells was mixed with 250 ng of pUC19-Zmo1957KO plasmid, and electroporation was then performed with a Gene Pulser Xcell electroporator (Bio-Rad Laboratories) in 0.1-cm cuvettes at 1.5 kV, 25 microfarads, and 200 Ω. The cells were transferred to a 1.5-ml tube containing 1 ml of RM medium and incubated at 30 °C for 3 h. Transformants were selected on RM agar containing 75 μg/ml of chloramphenicol at 30 °C for 2 days. Over 100 chloramphenicol-resistant colonies were randomly selected from RM agar plates and streaked on a new RM agar plate (eight colonies on one plate) containing 75 μg/ml of chloramphenicol and incubated at 30 °C for another 2 days. Transformants were cultured in 3 ml of RM medium containing 75 μg/ml of chloramphenicol at 30 °C for 2 days and harvested at 4 °C, 13,500 rpm for 2 min, and the cells were washed with 500 μl of Tris-buffered saline. Genome DNA was prepared using the Gentra Puregene yeast/Bact. kit (Qiagen) according to the manufacturer's instructions. Disruption of the cer-glcat gene was examined by PCR1 using the primer set ZmoG1500_397F and ZmoG1500_3799R and PCR2 using the primer set ZMO1957_13F and ZMO1957_973R (Fig. 6A and Table S1).
Structural analysis of SL accumulated in Cer-GlcAT KO strain
Z. mobilis Cer-GlcAT KO strain was cultured in 800 ml of RM medium (A550 = 0.1) at 30 °C for 2 days without shaking. The cells were harvested and centrifuged at 4 °C, 7,500 rpm for 20 min. After freeze-drying overnight, total lipids were extracted crudely by 15 ml of chloroform/methanol (2:1, v/v) twice. Extracted lipids were evaporated, dissolved in 7.6 ml of chloroform/methanol/water (1:2:0.8, v/v/v) and then subjected to mild alkaline hydrolysis. After a 120-min incubation, the sample was neutralized by acetic acid and partitioned with Folch's method. The dried lipid was dissolved in 5 ml of chloroform/methanol (95:5, v/v) and loaded onto a Sep-Pak plus silica column cartridge equilibrated with chloroform/methanol (95:5, v/v). The column was eluted with 4 ml of chloroform/methanol (95:5, v/v), chloroform/methanol (80:20, v/v), chloroform/methanol (75:25, v/v), chloroform/methanol (70:30, v/v), chloroform/methanol (65:35, v/v), chloroform/methanol (60:40, v/v), chloroform/methanol (55:45, v/v), chloroform/methanol (50:50, v/v), and finally with methanol. An aliquot of each fraction was applied to a TLC plate that was developed with chloroform, methanol, and water (65/25/4, v/v/v). The TLC plate was stained with 3% copper sulfate in 15% phosphoric acid reagent and Dittmer–Lester reagent. The SL was mainly recovered in the chloroform/methanol (75:25, v/v), chloroform/methanol (70:30, v/v), and chloroform/methanol (65:35, v/v) fractions. The SL-containing fractions were evaporated, redissolved in 3 ml of chloroform, methanol, and water (1/2/0.2 v/v/v) and loaded onto OASIS® MAX 3-cc (60 mg) extraction cartridges (Waters) equilibrated with the same solvent. The cartridge was washed with 3 ml of chloroform, methanol, and water (1/2/0.2, v/v/v) and 3 ml of methanol and eluted with 3 ml of methanol containing 2% formic acid. The SL-containing fractions were combined and evaporated by SpeedVac and then used as the purified SL.
Measurement of cell proliferation
Both the WT and Cer-GlcAT KO strain of Z. mobilis were precultured in RM medium at 30 °C, and the main cultures were started in 30 ml of RM medium with an A550 value equal to 0.1. The cells were cultured at 30 °C under microaerobic (static) and aerobic (shaking at 120 rpm) conditions. The samples were taken at different times, and cell proliferation was analyzed by measuring A550 using a spectrophotometer (GENESYS 10S UV-visible; Thermo Fisher Scientific) after dilution by RM medium.
Construction of phylogenetic tree of Cer-GlcAT and DG-GlcAT
Homologous sequences of Cer-GlcAT and DG-GlcAT were searched for in the KEGG database using Cer-GlcAT from Z. mobilis (ZMO1957), bifunctional glycosyltransferase (DG-GlcT/GlcAT) from A. tumefaciens (atu2297), and SQDG/GlcADG synthase (SQD2) from Arabidopsis thaliana (AtSQD2) as query sequences. Multiple alignment of those sequences and Cer-GlcAT from Z. mobilis was calculated by MAFFT version 7 (53, 54) with the L-INS-i strategy and homologs with identity values > 0.8 (atu2297 and AtSQD2) and identity values > 0.4 (ZMO1957) in the KEGG database. A phylogenetic tree was constructed using the neighbor-joining method under the JTT model with all of the gap-free sites of multiple alignment, and it was visualized by Phylo.io (55).
Expression of putative glycosyltransferase from S. yanoikuyae
The putative glycosyltransferase gene from S. yanoikuyae (A6768_09385) was synthesized with optimized codon usage to reflect the codon bias of E. coli. Expression plasmids carrying the A6768_09385 gene were constructed by PCR using pMA_SYA09385 as a template and the primers pCold_SYA09385_InfuF and pCold_SYA09385_InfuR (Table S1). The amplified product was inserted into pCold I vector (Takara Bio), which was linearized by PCR using pCold I as a template and the primers pCold_InfuF and pCold_InfuR. The resulting plasmid was named pCold_SYA09385.
pCold_SYA09385 was introduced into E. coli HST08 (Takara Bio), and the transformants were cultured at 37 °C in LB medium containing 100 µg/ml carbenicillin to A600 = 0.5. Protein expression was induced by the addition of 0.25 mm isopropyl β-d-thiogalactopyranoside for 24 h at 15 °C. The cells were harvested at 4 °C at 10,000 × g for 10 min and suspended in lysis buffer. After sonication for 5 min, cell debris was removed by centrifugation (10,000 × g for 20 min). The supernatant was loaded onto a column of TALON metal affinity resin (Takara Bio), and the column was washed with buffer A containing 20 or 40 mm imidazole. The enzyme was eluted from the column with buffer A containing 200 mm imidazole.
Expression of putative glycosyltransferase from B. fragilis
The putative glycosyltransferase gene from B. fragilis (BF9343_3149) was synthesized with optimized codon usage to reflect the codon bias of E. coli. Expression plasmids carrying the BF9343_3149 gene were constructed by PCR using pMA_BF3149 as a template and the primers pMAL_BF3149_InfuF and pMAL_BF3149_InfuR (Table S1). The amplified product was inserted into the pMAL-c5X vector (NEB), which was linearized by PCR using pMAL-c5X as a template and the primers pMAL_InfuF and pMAL_InfuR. The resulting plasmid was named pMAL_BF3149.
pMAL_BF3149 was introduced into E. coli BL21, and the transformants were cultured at 37 °C in 3 ml of LB medium containing 100 µg/ml carbenicillin. The culture was then transferred to a 500-ml flask containing 200 ml of LB medium supplemented with 0.2% glucose and 100 μg/ml of carbenicillin and incubated at 37 °C with shaking to A600 = 0.5. Protein expression was induced by the addition of 0.25 mm isopropyl β-d-thiogalactopyranoside for 4 h at 30 °C. The cells were harvested by centrifugation and suspended in 5 ml of buffer B (20 mm Tris-HCl, pH 7.5, 200 mm NaCl, 1 mm EDTA, and 1 mm DTT). After sonication for 3 min, cell debris was removed by centrifugation (15,000 × g for 20 min at 4 °C). The supernatant was loaded onto a column of amylose resin (NEB), and the column was washed with buffer B. The enzyme was eluted from the column with buffer B containing 10 mm maltose.
Hydrophilic interaction liquid chromatography (HILIC)–phase LC–MS analysis of GalCer
α- and β-GalCer were analyzed by LC–ESI–MS according to the method described by von Gerichten et al. (40). A binary solvent gradient with a flow rate of 250 μl/min was used to separate α- and β-GalCer by HILIC using a CORTECS HILIC column (2.1 × 150 mm, 2.7 μm; Waters). The gradient was started with 100% A (97% propionitrile, 2% 2-butanol, and 1% water, containing 0.1% formic acid) and was maintained for 1 min. The gradient reached 44% B (97% methanol, 2% 2-butanol, and 10 mm ammonium formate, containing 0.1% formic acid) for 5 min and then 100% B for 1 min and was maintained for 1 min. The gradient was returned to the starting conditions for 2 min, and the column was equilibrated for 20 min before the next run. α- and β-GalCer were detected by multiple reaction monitoring (MRM, C18-GalCer, d18:1/C18:0, Q1/Q3 = 728.6/264.2). C18-α-GalCer (d18:1/C18:0) and C18-β-GalCer (d18:1/C18:0) were purchased from Toronto Research Chemicals (North York, Canada) and Avanti Polar Lipids (Alabaster, Alabama, USA) and used as standards to identify α- and β-anomers of GalCer.
Statistical analysis
All results are expressed as mean values and S.D. The data are representative of three or more independent experiments. Significance was assessed using Welch's t test. Probability values of less than 0.05 were considered significant.
Data availability
All data not present in the article are available upon request (correspondence to N. O., nokino@agr.kyushu-u.ac.jp).
Supplementary Material
Acknowledgments
We thank Dr. Hiroko Ikushiro (Osaka Medical University) for valuable suggestions.
This article contains supporting information.
Author contributions—N. O., M. M., and M. I. conceptualization; N. O., M. L., and Y. I. data curation; N. O. and M. L. formal analysis; N. O., M. M., Y. I., and M. I. supervision; N. O. funding acquisition; N. O., Y. I., and M. I. validation; N. O., M. L., Q. Q., T. N., and Y. H. investigation; N. O. and M. L. visualization; N. O., T. N., Y. H., and Y. I. methodology; N. O. and M. L. writing-original draft; N. O. and M. I. project administration; N. O., Y. H., Y. I., and M. I. writing-review and editing.
Funding and additional information—This work was supported by Japan Society for the Promotion of Science KAKENHI Grants, JP15K06976 and JP19H02888 (to N. O.).
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- Cer
- ceramide
- GlcAT
- UDP-glucuronosyltransferase
- DG
- diacylglycerol
- GlcT/GlcAT
- glucosyltransferase/glucuronosyltransferase
- CPE
- ceramide phosphoethanolamine
- CPG
- ceramide phosphoglycerol
- EGALC
- endogalactosylceramidase
- GalCer
- galactosylceramide
- GlcA
- glucuronic acid
- GlcACer
- glucuronosylceramide
- GlcADG
- glucuronosyldiacylglycerol
- GSL
- glycosphingolipid
- HexACer
- hexuronosyl ceramide
- HILIC
- hydrophilic interaction liquid chromatography
- IL
- interleukin
- iNKT
- invariant natural killer T
- KO
- knockout
- LCB
- long-chain base
- ESI
- electrospray ionization
- NBD
- 7-nitrobenz-2-oxal-1,3-diazole
- SL
- sphingolipid
- SQDG
- sulfoquinovosyldiacylglycerol.
References
- 1. Degroote S., Wolthoorn J., and van Meer G. (2004) The cell biology of glycosphingolipids. Semin. Cell Dev. Biol. 15, 375–387 10.1016/j.semcdb.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 2. Lingwood C. A. (2011) Glycosphingolipid functions. Cold Spring Harb. Perspect. Biol. 3, a004788–a004788 10.1101/cshperspect.a004788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simons K., and Toomre D. (2000) Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39 10.1038/35036052 [DOI] [PubMed] [Google Scholar]
- 4. Beachey E. H. (1981) Bacterial adherence: adhesin–receptor interactions mediating the attachment of bacteria to mucosal surface. J. Infect. Dis. 143, 325–345 10.1093/infdis/143.3.325 [DOI] [PubMed] [Google Scholar]
- 5. Karlsson K.-A. (1989) Animal glycosphingolipids as membrane attachment sites for bacteria. Annu. Rev. Biochem. 58, 309–350 10.1146/annurev.bi.58.070189.001521 [DOI] [PubMed] [Google Scholar]
- 6. Olsen I., and Jantzen E. (2001) Sphingolipids in bacteria and fungi. Anaerobe 7, 103–112 10.1006/anae.2001.0376 [DOI] [Google Scholar]
- 7. Glaeser S. P., and Kämpfer P. (2014) The family Sphingomonadaceae. In The Prokaryotes: Alphaproteobacteria and Betaproteobacteria (Rosenberg E., DeLong E. F., Lory S., Stackebrandt E., and Thompson F., eds) pp. 641–707, Springer, Berlin [Google Scholar]
- 8. Ikushiro H., Hayashi H., and Kagamiyama H. (2001) A water-soluble homodimeric serine palmitoyltransferase from Sphingomonas paucimobilis EY2395T strain: purification, characterization, cloning, and overproduction. J. Biol. Chem. 276, 18249–18256 10.1074/jbc.M101550200 [DOI] [PubMed] [Google Scholar]
- 9. Sohlenkamp C., and Geiger O. (2016) Bacterial membrane lipids: diversity in structures and pathways. FEMS Microbiol. Rev. 40, 133–159 10.1093/femsre/fuv008 [DOI] [PubMed] [Google Scholar]
- 10. Kawasaki S., Moriguchi R., Sekiya K., Nakai T., Ono E., Kume K., and Kawahara K. (1994) The cell envelope structure of the lipopolysaccharide-lacking Gram-negative bacterium Sphingomonas paucimobilis. J. Bacteriol. 176, 284–290 10.1128/jb.176.2.284-290.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Godfrey D. I., MacDonald H. R., Kronenberg M., Smyth M. J., and Kaer L. V. (2004) NKT cells: what's in a name? Nat. Rev. Immunol. 4, 231–237 10.1038/nri1309 [DOI] [PubMed] [Google Scholar]
- 12. Bendelac A., Savage P. B., and Teyton L. (2007) The biology of NKT cells. Annu. Rev. Immunol. 25, 297–336 10.1146/annurev.immunol.25.022106.141711 [DOI] [PubMed] [Google Scholar]
- 13. Natori T., Koezuka Y., and Higa T. (1993) Agelasphins, novel α-galactosylceramides from the marine sponge Agelas mauritianus. Tetrahedron Lett. 34, 5591–5592 10.1016/S0040-4039(00)73889-5 [DOI] [Google Scholar]
- 14. Natori T., Morita M., Akimoto K., and Koezuka Y. (1994) Agelasphins, novel antitumor and immunostimulatory cerebrosides from the marine sponge Agelas mauritianus. Tetrahedron 50, 2771–2784 10.1016/S0040-4020(01)86991-X [DOI] [Google Scholar]
- 15. Morita M., Motoki K., Akimoto K., Natori T., Sakai T., Sawa E., Yamaji K., Koezuka Y., Kobayashi E., and Fukushima H. (1995) Structure–activity relationship of α-galactosylceramides against B16-bearing mice. J. Med. Chem. 38, 2176–2187 10.1021/jm00012a018 [DOI] [PubMed] [Google Scholar]
- 16. Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K., Ueno H., Nakagawa R., Sato H., Kondo E., Koseki H., and Taniguchi M. (1997) CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 10.1126/science.278.5343.1626 [DOI] [PubMed] [Google Scholar]
- 17. Brennan P. J., Brigl M., and Brenner M. B. (2013) Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 13, 101–117 10.1038/nri3369 [DOI] [PubMed] [Google Scholar]
- 18. McEwen-Smith R. M., Salio M., and Cerundolo V. (2015) CD1d-dependent endogenous and exogenous lipid antigen presentation. Curr. Opin. Immunol. 34, 116–125 10.1016/j.coi.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 19. Kohlgruber A. C., Donado C. A., LaMarche N. M., Brenner M. B., and Brennan P. J. (2016) Activation strategies for invariant natural killer T cells. Immunogenetics 68, 649–663 10.1007/s00251-016-0944-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kinjo Y., Wu D., Kim G., Xing G.-W., Poles M. A., Ho D. D., Tsuji M., Kawahara K., Wong C.-H., and Kronenberg M. (2005) Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434, 520–525 10.1038/nature03407 [DOI] [PubMed] [Google Scholar]
- 21. Mattner J., DeBord K. L., Ismail N., Goff R. D., Cantu C., Zhou D., Saint-Mezard P., Wang V., Gao Y., Yin N., Hoebe K., Schneewind O., Walker D., Beutler B., Teyton L., et al. (2005) Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 10.1038/nature03408 [DOI] [PubMed] [Google Scholar]
- 22. Wieland Brown L. C., Penaranda C., Kashyap P. C., Williams B. B., Clardy J., Kronenberg M., Sonnenburg J. L., Comstock L. E., Bluestone J. A., and Fischbach M. A. (2013) Production of α-galactosylceramide by a prominent member of the human gut microbiota. PLoS Biol. 11, e1001610 10.1371/journal.pbio.1001610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. An D., Oh S. F., Olszak T., Neves J. F., Avci F. Y., Erturk-Hasdemir D., Lu X., Zeissig S., Blumberg R. S., and Kasper D. L. (2014) Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 156, 123–133 10.1016/j.cell.2013.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swings J., and De Ley J. (1977) The biology of Zymomonas. Bacteriol. Rev. 41, 1–46 10.1128/MMBR.41.1.1-46.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baratti J. C., and Bu'lock J. D. (1986) Zymomonas mobilis: a bacterium for ethanol production. Biotechnol. Adv. 4, 95–115 10.1016/0734-9750(86)90006-6 [DOI] [PubMed] [Google Scholar]
- 26. Tahara Y., and Kawazu M. (1994) Isolation of glucuronic acid–containing glycosphingolipid from Zymomonas mobilis. Biosci. Biotechnol. Biochem. 58, 586–587 10.1271/bbb.58.586 [DOI] [Google Scholar]
- 27. Seo J.-S., Chong H., Park H. S., Yoon K.-O., Jung C., Kim J. J., Hong J. H., Kim H., Kim J.-H., Kil J.-I., Park C. J., Oh H.-M., Lee J.-S., Jin S.-J., Um H.-W., et al. (2005) The genome sequence of the ethanologenic bacterium Zymomonas mobilis ZM4. Nat. Biotechnol. 23, 63–68 10.1038/nbt1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jin D., Zhu Y., Wang X., Kong X., Liu H., Wang Y., Deng Y., and Jia M. (2016) Draft genome sequence of Sphingobium yanoikuyae TJ, a halotolerant di-n-butyl-phthalate-degrading bacterium. Genome Announc. 4, e00569–16 10.1128/genomeA.00569-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miller T. R., Delcher A. L., Salzberg S. L., Saunders E., Detter J. C., and Halden R. U. (2010) Genome sequence of the dioxin-mineralizing bacterium Sphingomonas wittichii RW1. J. Bacteriol. 192, 6101–6102 10.1128/JB.01030-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Merrill A. H. Jr., Sullards M. C., Allegood J. C., Kelly S., and Wang E. (2005) Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 36, 207–224 10.1016/j.ymeth.2005.01.009 [DOI] [PubMed] [Google Scholar]
- 31. Shaner R. L., Allegood J. C., Park H., Wang E., Kelly S., Haynes C. A., Sullards M. C., and Merrill A. H. Jr. (2009) Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J. Lipid Res. 50, 1692–1707 10.1194/jlr.D800051-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y., Lou Y., Mu T., Ke A., Ran Z., Xu J., Chen J., Zhou C., Yan X., Xu Q., and Tan Y. (2017) Sphingolipids in marine microalgae: development and application of a mass spectrometric method for global structural characterization of ceramides and glycosphingolipids in three major phyla. Anal. Chim. Acta 986, 82–94 10.1016/j.aca.2017.07.039 [DOI] [PubMed] [Google Scholar]
- 33. Costello C. E., and Vath J. E. (1990) Tandem mass spectrometry of glycolipids. Methods Enzymol. 193, 738–768 10.1016/0076-6879(90)93448-T [DOI] [PubMed] [Google Scholar]
- 34. Okazaki Y., Otsuki H., Narisawa T., Kobayashi M., Sawai S., Kamide Y., Kusano M., Aoki T., Hirai M. Y., and Saito K. (2013) A new class of plant lipid is essential for protection against phosphorus depletion. Nat. Commun. 4, 1510 10.1038/ncomms2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Semeniuk A., Sohlenkamp C., Duda K., and Hölzl G. (2014) A bifunctional glycosyltransferase from Agrobacterium tumefaciens synthesizes monoglucosyl and glucuronosyl diacylglycerol under phosphate deprivation. J. Biol. Chem. 289, 10104–10114 10.1074/jbc.M113.519298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cantarel B. L., Coutinho P. M., Rancurel C., Bernard T., Lombard V., and Henrissat B. (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 37, D233–D238 10.1093/nar/gkn663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karlsson O. P., Dahlqvist A., Vikström S., and Wieslander A. K. (1997) Lipid dependence and basic kinetics of the purified 1,2-diacylglycerol 3-glucosyltransferase from membranes of Acholeplasma laidlawii. J. Biol. Chem. 272, 929–936 10.1074/jbc.272.2.929 [DOI] [PubMed] [Google Scholar]
- 38. Bringer S., Finn R. K., and Sahm H. (1984) Effect of oxygen on the metabolism of Zymomonas mobilis. Arch. Microbiol. 139, 376–381 10.1007/BF00408383 [DOI] [Google Scholar]
- 39. Ishibashi Y., Nakasone T., Kiyohara M., Horibata Y., Sakaguchi K., Hijikata A., Ichinose S., Omori A., Yasui Y., Imamura A., Ishida H., Kiso M., Okino N., and Ito M. (2007) A novel endoglycoceramidase hydrolyzes oligogalactosylceramides to produce galactooligosaccharides and ceramides. J. Biol. Chem. 282, 11386–11396 10.1074/jbc.M608445200 [DOI] [PubMed] [Google Scholar]
- 40. von Gerichten J., Schlosser K., Lamprecht D., Morace I., Eckhardt M., Wachten D., Jennemann R., Gröne H.-J., Mack M., and Sandhoff R. (2017) Diastereomer-specific quantification of bioactive hexosylceramides from bacteria and mammals. J. Lipid Res. 58, 1247–1258 10.1194/jlr.D076190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin J.-N., Lai C.-H., Chen Y.-H., Lin H.-L., Huang C.-K., Chen W.-F., Wang J.-L., Chung H.-C., Liang S.-H., and Lin H.-H. (2010) Sphingomonas paucimobilis bacteremia in humans: 16 case reports and a literature review. J. Microbiol. Immunol. Infect. 43, 35–42 10.1016/S1684-1182(10)60005-9 [DOI] [PubMed] [Google Scholar]
- 42. Marbjerg L. H., Gaini S., and Justesen U. S. (2015) First report of Sphingomonas koreensis as a human pathogen in a patient with meningitis. J. Clin. Microbiol. 53, 1028–1030 10.1128/JCM.03069-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mattner J., Savage P. B., Leung P., Oertelt S. S., Wang V., Trivedi O., Scanlon S. T., Pendem K., Teyton L., Hart J., Ridgway W. M., Wicker L. S., Gershwin M. E., and Bendelac A. (2008) Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe 3, 304–315 10.1016/j.chom.2008.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stankeviciute G., Guan Z., Goldfine H., and Klein E. A. (2019) Caulobacter crescentus adapts to phosphate starvation by synthesizing anionic glycoglycerolipids and a novel glycosphingolipid. mBio 10, e00107–19 10.1128/mBio.00107-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. von Gerichten J., Lamprecht D., Opálka L., Soulard D., Marsching C., Pilz R., Sencio V., Herzer S., Galy B., Nordström V., Hopf C., Gröne H.-J., Trottein F., and Sandhoff R. (2019) Bacterial immunogenic α-galactosylceramide identified in the murine large intestine: dependency on diet and inflammation. J. Lipid Res. 60, 1892–1904 10.1194/jlr.RA119000236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moreau R. A., Powell M. J., Fett W. F., and Whitaker B. D. (1997) The effect of ethanol and oxygen on the growth of Zymomonas mobilis and the levels of hopanoids and other membrane lipids. Curr. Microbiol. 35, 124–128 10.1007/s002849900224 [DOI] [PubMed] [Google Scholar]
- 47. Brenac L., Baidoo E. E. K., Keasling J. D., and Budin I. (2019) Distinct functional roles for hopanoid composition in the chemical tolerance of Zymomonas mobilis. Mol. Microbiol. 112, 1564–1575 10.1111/mmi.14380 [DOI] [PubMed] [Google Scholar]
- 48. Naka T., Fujiwara N., Yano I., Maeda S., Doe M., Minamino M., Ikeda N., Kato Y., Watabe K., Kumazawa Y., Tomiyasu I., and Kobayashi K. (2003) Structural analysis of sphingophospholipids derived from Sphingobacterium spiritivorum, the type species of genus Sphingobacterium. Biochim. Biophys. Acta 1635, 83–92 10.1016/j.bbalip.2003.10.010 [DOI] [PubMed] [Google Scholar]
- 49. Nichols F. C., Riep B., Mun J., Morton M. D., Bojarski M. T., Dewhirst F. E., and Smith M. B. (2004) Structures and biological activity of phosphorylated dihydroceramides of Porphyromonas gingivalis. J. Lipid Res. 45, 2317–2330 10.1194/jlr.M400278-JLR200 [DOI] [PubMed] [Google Scholar]
- 50. An D., Na C., Bielawski J., Hannun Y. A., and Kasper D. L. (2011) Membrane sphingolipids as essential molecular signals for Bacteroides survival in the intestine. Proc. Natl. Acad. Sci. U.S.A. 108, 4666–4671 10.1073/pnas.1001501107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hölzl G., and Dörmann P. (2007) Structure and function of glycoglycerolipids in plants and bacteria. Prog. Lipid Res. 46, 225–243 10.1016/j.plipres.2007.05.001 [DOI] [PubMed] [Google Scholar]
- 52. Geiger O., González-Silva N., López-Lara I. M., and Sohlenkamp C. (2010) Amino acid-containing membrane lipids in bacteria. Prog. Lipid Res. 49, 46–60 10.1016/j.plipres.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 53. Kuraku S., Zmasek C. M., Nishimura O., and Katoh K. (2013) aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res. 41, W22–W28 10.1093/nar/gkt389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Katoh K., Rozewicki J., and Yamada K. D. (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinformatics 20, 1160–1166 10.1093/bib/bbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Robinson O., Dylus D., and Dessimoz C. (2016) Phylo.io: interactive viewing and comparison of large phylogenetic trees on the web. Mol. Biol. Evol 33, 2163–2166 10.1093/molbev/msw080 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data not present in the article are available upon request (correspondence to N. O., nokino@agr.kyushu-u.ac.jp).