Abstract
Legumes and actinorhizal plants are capable of forming root nodules symbiosis with rhizobia and Frankia bacteria. All these nodulating species belong to the nitrogen fixation clade. Most likely, nodulation evolved once in the last common ancestor of this clade. NIN (NODULE INCEPTION) is a transcription factor that is essential for nodulation in all studied species. Therefore, it seems probable that it was recruited at the start when nodulation evolved. NIN is the founding member of the NIN-like protein (NLP) family. It arose by duplication, and this occurred before nodulation evolved. Therefore, several plant species outside the nitrogen fixation clade have NLP(s), which is orthologous to NIN. In this review, we discuss how NIN has diverged from the ancestral NLP, what minimal changes would have been essential for it to become a key transcription controlling nodulation, and which adaptations might have evolved later.
Keywords: NIN (NODULE INCEPTION), NLP (NIN-like Proteins), evolution, root nodule symbiosis, legume, actinorhizal-like plants
1. Introduction
Nitrogen-fixing root nodule-formation is a special property of some plant species. All these species belong to the nitrogen fixation clade (NFC), which is composed of four orders: Fabales, Rosales, Cucurbitales and Fagales [1]. Nodulation occurs abundantly within the Fabales (legumes). Legumes establish nodule symbiosis with Gram-negative bacteria belonging to different genera, which collectively are named rhizobium [2]. In the other three orders, nodulation is more rare. In general, nodulation in these orders is induced by Gram-positive Frankia bacteria [3]. The plants that form nodules with Frankia are named actinorhizal plants. The exception is the genus Parasponia (order Rosales), which forms nodules with rhizobia [4].
Nodule types and modes of infection within the NFC are diverse. Nevertheless, recent studies indicate that a common ancestor of the NFC gained the nodulation ability [5,6,7]. Later during evolution, many species within the NFC lost the nodulation ability. Interestingly, phylogenomic analyses revealed that the transcription factor NIN (NODULE INCEPTION) that is essential for nodulation became a pseudogene, or was lost in most non-nodulating species of the NFC [5,6]. In this review, we will discuss the evolution of NIN and its related NIN-like proteins in relation to nodule symbiosis.
Root nodule-formation involves intracellular infection, nodule organogenesis, and a negative feedback mechanism that controls the number of nodules. Strikingly, the transcription factor NIN has been shown to play an indispensable role in all these processes [8,9,10,11,12,13]. For infection, the most common and advanced way is when bacteria attach to the root hair tip and stimulate the root hair to curl [14]. In this way the bacteria are entrapped in an enclosed cavity. By the deposition of new plant cell wall material, and invagination and growth of the plasma membrane, a tube like structure, the infection thread, is formed, which guides bacteria into the plant [14,15]. Alternatively, the bacteria can enter the roots without forming such infection threads, for example, through intercellular infection or crack entry [16,17].
In several legumes, the role of NIN in forming infection threads has been well studied. Loss of function of NIN leads to excessive root hair curling, and infection thread formation is blocked [8,9,10]. Similarly, NIN also most likely plays a role in infection of the actinorhizal plant, as it has been shown to be required for Frankia-induced root hair deformation in Casuarina glauca (Casuarina) [11]. This suggests that the role of NIN in infection thread formation is conserved in both legumes and actinorhizal plants.
In the model legume Medicago truncatula (Medicago), nodule formation starts with the mitotic activation of pericycle cells, and this is followed by divisions in cortical and endodermal cells [18]. The divisions in pericycle- and endodermis-derived cells stop at an early stage of nodule primordium formation, whereas divisions in the cortical cells persist. The cells derived from the cortex become infected and form the central tissue with infected cells. Cells derived from the cortex also form the peripheral tissue, including nodule vascular bundles [18].
The formation of actinorhizal nodules also starts with mitotic activation of the pericycle and cortical cells [19,20]. During nodule primordium formation, the pericycle-derived cells remain mitotically active. Previously, it has been described that these cells form the (complete) nodule [19,20]. However, it has been shown recently that these pericycle-derived cells only form the nodule vasculature [7]. Furthermore, during actinorhizal nodule primordium formation, the cortex-derived cells form the tissue with infected cells. Nodule formation in Parasponia andersonii (Parasponia) is similar to that in actinorhizal plants (actinorhizal-like) [7]. So, the major difference between legume and actinorhizal(-like) nodule organogenesis is the origin of the vascular bundle. In Medicago, it has been shown that a mutation in NOOT1 causes a homeotic switch in the formation of the nodule vasculature, as it becomes actinorhizal-like since it is formed from pericycle cells that remain mitotically active [7,21].
Legume nodules can be divided in indeterminate and determinate nodules. Indeterminate nodules have a persistent meristem at their apex. This is similar to actinorhizal(-like) nodules. Due to their indeterminate growth, their tissues are of grade age, with the youngest cells near the meristem and the oldest in the part proximal to the root. NIN has been shown to be essential for both determinate [e.g., Lotus japonicus (Lotus)] [8] and indeterminate [e.g., Medicago and Pisum sativum (pea)] [9,10] nodules, as well as actinorhizal(-like) (e.g., Casuarina and Parasponia) [11,12] nodules. This indicates a common role of NIN in the formation of different types of nodules.
Both infection and nodule organogenesis are initiated upon perception of signal molecules from the bacteria [22]. Most rhizobia secrete lipo-chito-oligosaccharides, called Nod factors (NFs), whereas the nature of the signals secreted by Frankia is not known. NFs activate a signaling pathway which is shared with the more ancient arbuscular mycorrhizal symbiosis, and NIN is the first induced transcription factor that distinguishes the rhizobium-activated responses from that of arbuscular mycorrhizae [23]. Although the nature of the Frankia-secreted signal is not clear, this common signaling pathway has also been shown to be required for actinorhizal(-like) nodule formation [24].
To balance costs and benefits during nodule symbiosis, plants developed a mechanism called autoregulation of nodulation (AON) by which nodule number is controlled [25,26,27,28,29,30,31]. It involves a communication between root and shoot. The signals that are sent to the shoot are CLAVATA3/ENDOSPERMSURROUNDING REGION (CLE) peptides [13,28,29,32]. For example, in Lotus, these are CLE-RS1 and CLE-RS2, and NIN directly induces their expression by binding to the NIN-binding sequence (NBS) in their promoters [13]. Upon perception of CLE peptides in the shoot, signals are sent to the root, resulting in reduced NIN expression, and so expression of targets will be reduced [13]. Thus, NIN plays a central role in the feedback loop, which ensures the formation of an optimal number of nodules.
NIN is a founding member of a small gene family called the NIN-like proteins (NLP) [33]. The studies on paralogues of NIN showed that they play an essential role in regulating nitrate-induced responses (reviewed in [34]). Interestingly, studies of Lotus showed that the expression of CLE genes is induced not only by rhizobia, but also by the application of nitrate [29,35]. One NLP (NRSYM1) can directly activate CLE-RS2 expression in response to nitrate [35]. This suggests that the nitrate-induced block of nodulation shares common elements with AON, and NIN is partially functionally equivalent with NLPs. The latter is also supported by Lin et al. [36], which demonstrates that in Medicago NLP1 interacts with NIN to mediate nitrate inhibition of nodulation, and both NIN and NLP1 bind directly to the CYTOKININ RESPONSE 1 (CRE1) promoter. NIN orthologues have also been shown to be present in species outside the NFC, like in Solanum lycopersicum (Tomato) and Arabidopsis thaliana (Arabidopsis) [11]. This indicates that NIN might be recruited in nodulation, based on its original function.
In this review, we will discuss possible evolutionary events underlying the recruitment of NIN in nodule symbiosis, based on comparing the NIN and NLPs of legumes, actinorhizal-like plants and non-nodulating species.
2. Phylogenetic Analysis of NIN
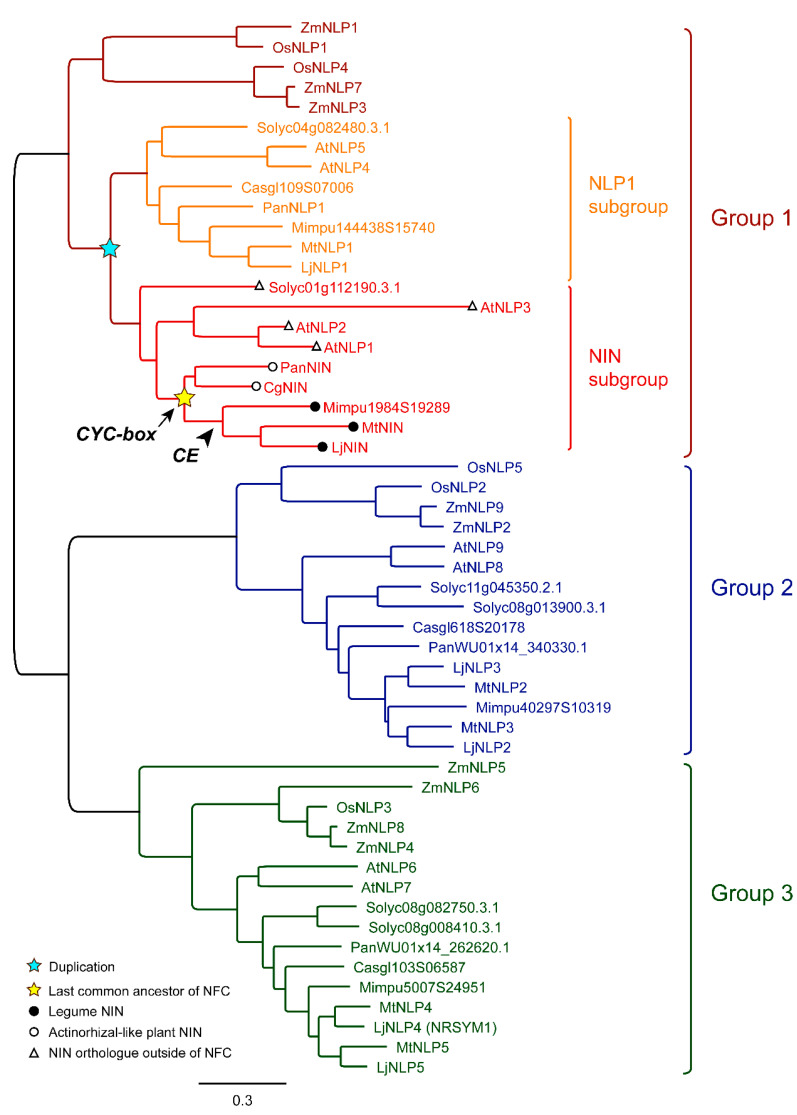
In Figure 1, a phylogenetic tree is shown, including NIN and NLPs from legumes, actinorhizal plant species, and monocot and dicot species outside the NFC. NIN and NLPs are divided into three orthogroups. Group 1 contains the NIN and NLPs of dicots, and is divided into two subgroups. One contains orthologues of NIN, and is named NIN subgroup. The other contains orthologues of MtNLP1 and is named NLP1 subgroup. Most likely these two subgroups are the result of a duplication that occurred before the NFC evolved, but after dicots and monocots separated. As a result, NIN orthologues occur in dicot species that are not within the NFC. Tomato and Arabidopsis NIN orthologues have been included in Figure 1. As these species have maintained a NIN orthologue, it strongly suggests that they have an essential non-symbiotic function. When NIN was recruited in the nodulation process, it most likely lost this non-symbiotic function. This is supported by the fact that most species within NFC that have lost nodulation also lost a functional NIN [5,6].
Figure 1.
Phylogenetic tree of NIN and NLPs. The tree comprises 53 NIN/NLPs from Zea mays (Zm), Oryza sativa (Os), Solanum lycopersicum (Solyc), Arabidopsis thaliana (At), Casuarina glauca (Casgl/Cg), Parasponia andersonii (Pan), Mimosa pudica (Mimpu) Medicago truncatula (Mt) and Lotus japonicus (Lj). These NIN/NLPs are divided into three orthogroups as indicated. Dicots of Group 1 most likely undergo duplication (blue star), which generates NIN and NLP1 subgroups. The NIN subgroup comprises symbiotic NIN from NFC species (yellow star indicates the last common ancestor of NFC), including NIN of actinorhizal-like plant NIN (hollow circle) and legume NIN (filled circle), and putative NIN orthologues in non-nodulating species outside of NFC (hollow triangle). The CYCLOPs binding site (CYC-box) in the NIN promoter was likely gained by the ancestor of NFC (arrow), whereas cytokinin responsive elements (CE) in the NIN promoter specifically evolved in the legume branch (arrow head). Corresponding accession numbers and protein sequences are listed in the Supplementary Material S1.
A comparison of NIN with their orthologues inside and outside the NFC, as well as with NLPs in the NLP1 subgroup, will provide insight into the changes in the ancestral NIN that were essential for it to become a key regulator in nodulation. We will discuss these in the following paragraphs, and will focus on the properties of the proteins as well as the regulation of expression.
3. Evolutionary Adaptations in the NIN Promoter to Serve in Nodule Formation
NIN is a nodule specifically expressed in all studied species [6,8,9,10,11,12,37]. In contrast, most NLPs are constitutively expressed [36,38,39]. For example, in Oryza sativa (Rice), Zea mays (Maize), Arabidopsis and Medicago, NLPs are expressed in almost all organs, although with preferential expression in certain stages and tissues [36,38,39]. In legumes and actinorhizal(-like) plants, nodulation requires the common signaling pathway (see above), and in some species it has been shown that it induces the expression of NIN [6,8,9,10,11,12,37]. Therefore, we hypothesize that when NIN was recruited into root nodule formation, it came under the control of the common signaling pathway in order to express in nodules. Further, at a certain moment, it also lost its constitutive expression.
The spatiotemporal regulation of NIN has been studied in detail in Medicago and Lotus, and it is highly complex. We will first summarize this, and then discuss whether such regulation also occurs in other species. Upon inoculation, NIN is induced in the epidermis, where it is required for infection. This was demonstrated by in situ hybridization, promoter GUS reporter constructs and root hair transcriptome analyses [40,41,42,43]. The expression of NIN in the epidermis could be cell-autonomously regulated upon perception of NFs, which induce the common signaling pathway, resulting in the activation of the transcription factor CYCLOPS by phosphorylation [44]. It was first shown in Lotus that phosphorylated CYCLOPS binds to the NIN promoter in a sequence-specific manner to regulate NIN expression [44]. In Medicago, the CYCLOPS binding site is located about 3 kb upstream of the NIN start codon [41]. A 5-kb long promoter including the CYCLOPS binding site driving NIN expression can restore infection thread formation in a nin knockout mutant [41]. However, it cannot restore nodule organogenesis, showing that additional cis-regulatory elements are required for this (see below). Deletion of the CYCLOPS binding site dramatically reduced the infection thread-forming efficiency [41]; however, this site is not required for tight root hair curling, and bacterial colonies are still formed in these curls. This resembles the Lotus cyclops and Medicago interacting protein of DMI3-2 (ipd3-2) mutant phenotype [45,46]. This indicates that, in addition to the CYCLOPS binding site, other cis-regulatory element(s) must be present, which is sufficient to induce NIN expression that leads to tight root hair curling. It was assumed that the CYCLOPS binding site results in a higher expression level of NIN, and the initiation of infection thread formation requires a higher threshold level than curling [41].
Interestingly, CYCLOPS binding site is conserved in legume NIN promoters [41], and it also occurs in the Parasponia NIN promoter (R. Huisman, personal communication [47]). However, they do not occur in the MtNLP1 promoter. This suggests that the gaining of the CYCLOPS binding site occurred after the duplication that resulted in the NIN and NLP1 subgroups (Figure 1). This CYCLOPS binding site also does not occur in NIN orthologues of Arabidopsis (NLP1/2/3). Taken together, it can be hypothesized that the gain of the CYCLOPS binding site had occurred when NIN was recruited into the nodule formation process, and was present in the NIN of the ancestor of the NFC. This gain of the CYCLOPS binding site seems an essential step for its expression during nodule formation.
At an early stage of Medicago nodule development, when rhizobia have only colonized the epidermis, NIN expression and cell divisions are induced in the pericycle [41]. Subsequently, both extend to the inner cortex and endodermis [41]. Because NFs are immobile molecules [48], most likely a mobile signal was generated upon perception of NFs in the epidermis. This mobile signal was then translocated to the pericycle to activate NIN expression and cell division there [41].
The first insight that other, more remote, promoter elements are involved in the induction of NIN in inner layers came from studies with the nin weak alleles daphne (Lotus) and daphne-like (Medicago), in which the infection process is induced but nodule organogenesis is blocked [40,41]. NIN expression was shown to be induced in the epidermis in daphne-like, but not in pericycle [41]. Both mutants contain a mutation in the promoter region of NIN due to a chromosome translocation [40,41]. In both cases, these are located upstream of the CYCLOPS binding site [41]. This strongly suggested that the regulatory region required for NIN expression in the pericycle and NIN-controlled cell division is located upstream of the insertion. Recently, a remote cis-regulatory region named CE region was identified, which is essential for NIN-controlled nodule organogenesis and the expression of NIN in the pericycle [41]. This CE region contains many putative cytokinin response regulator (RR) binding sites [41]. Studies in which cytokinin is applied exogenously did not induce NIN expression in either daphne or daphne-like [40,41], supporting the conclusion that that this region is important for cytokinin-induced NIN expression. In addition, both the cytokinin receptor CRE1 and B-type cytokinin response regulator RR1 are expressed in the pericycle prior to NIN expression and cell division there [41]. Taken together, it is very likely that cytokinin signaling via the CE region induces NIN expression in the pericycle, and this leads to nodule organogenesis. It has been shown in Medicago that YUCCA genes, involved in auxin biosynthesis, are induced in an inner cell layer of the root (most likely pericycle) in a NIN-dependent manner [49]. Therefore, it is probable that subsequent cell divisions are induced by local auxin production.
The CE region is conserved in legumes and is located far upstream from the NIN start codon [41]. The distance varies between species. For example, in Medicago it is about 18 kb upstream, and in Lotus it is about 45 kb upstream of the NIN start codon [41]. Given that the CE region is conserved in legumes, it is very probable that the regulation of NIN expression in the inner layers by cytokinin signaling is conserved in legumes.
In Actinorhizal(-like) plants, NIN is required for nodule organogenesis, and this involves divisions in the inner root cell layers [12,50]. So is the CE region conserved in actinorhizal NIN genes? So far, only Parasponia NIN (PanNIN) has been analyzed, and the CE region was not identified (L. Rutten, personal communication [51]). Nodule formation in Parasponia is induced by Nod factors, and so, like in legumes, a mobile signal seems essential to trigger cell division in inner layers. Whether or not NIN is expressed in the Parasponia pericycle cells when cell division is induced has not been studied. However, this seems probable, as a putative target of PanNIN, namely PanNF-YA1 (NUCLEAR TRANSCRIPTION FACTOR Y SUBUNIT A-1), is induced there [12]. NF-YA1 has been shown to be a direct target of NIN in Lotus [52]. In Medicago, the expression of NIN and NF-YA1 coincides in the dividing pericycle cells and other nodule primordium cells [41]. Further, PanNF-YA1 expression has been shown to be PanNIN-dependent [12]. As the CE region does not occur in the Parasponia NIN promoter, the mobile signal most likely does not regulate NIN expression through cytokinin signaling. This conclusion is in line with a recent study in which cytokinin is applied to different plant species [53]. It was shown that exogenous application of cytokinin induces nodule-like structures on nodulating legume species, and in Lotus, for example, this depends on NIN [54]. However, nodule-like structures were not induced by cytokinin on either non-nodulating legumes or on actinorhizal species [53]. The non-nodulating legumes most likely have lost the ability to form cytokinin-induced nodule-like structures due to the loss of NIN, whereas in actinorhizal plants this might be due to the absence of a CE region in their NIN promoter. Therefore, the gain of cytokinin responsive elements in the NIN promoter seems specific to the legumes, and might not have already occurred in the ancestor of the NFC.
It cannot be excluded that more changes in the NIN promoter evolved during nodule evolution. For example, NSP1/2, IPN2 and ERN1 have been shown to play a role in Medicago and Lotus NIN expression [55,56,57,58,59]. However, it is not clear that this involved specific evolutionary adaptations in the promoter of NIN, related to its recruitment for nodulation.
Taken together, there seems to be (at least) three main evolutionary changes concerning the regulation of NIN expression during nodule evolution. First, it gained the CYCLOPS binding site, most likely in the ancestor of the NFC. Second, at an early stage of nodulation, the constitutive expression is lost by which, in nodulating plants, NIN became nodule-specific. Third, it developed cytokinin-regulated NIN expression during nodule organogenesis, which most likely occurred in the legume branches.
4. NIN-Controlled Epidermis–Pericycle Communication; A Conserved Module?
It seems probable that the activation of NIN in the inner root layers of legumes and Parasponia requires a mobile signal. What is the probability that this is a general property of nodulating plants? It has been proposed that the last common ancestor of the NFC formed a nodule symbiosis with Frankia [60]. Although it has not been demonstrated that Frankia can produce Nod factors, basal Frankia strains do have genes that are homologous to the rhizobial nod genes [61,62] that are involved in Nod factor biosynthesis. Therefore, it has been hypothesized that Frankia-induced nodulation in the last common ancestor of NFC by secreting Nod factors [60]. If so, as has been argued for legumes and Parasponia, a mobile signal might also be required to induce NIN in the inner root layers in the common ancestor, as well as in current actinorhizal plants that interact with Frankia that secrete Nod factors. It has been shown in legumes that the production of the mobile signal depends on NIN expression in the epidermis [41]. So NIN, expressed in the epidermis might also induce the formation of a mobile signal in the actinorhizal plants that interact with Nod factor-producing Frankia. Subsequently, that mobile signal triggers NIN expression and cell division in the pericycle. Thus, we hypothesize that, in legumes and these actinorhizal plants, a conserved module, involved in communication between the epidermis and pericycle, is active, and this was already present in the common ancestor of the NFC: Nod factor signaling induces NIN expression in the epidermis; NIN activates the production of a mobile signal; this mobile signal induces NIN expression and cell division in the pericycle. Is such a module maintained in all actinorhizal plants? Some Actinorhizal plants interact with Frankia strains that do not produce NFs, such as CcI3, which secretes a hydrophilic symbiotic signal that is resistant to chitinase [50]. Therefore, it is chemically distinct from the amphiphilic and chitin-based NFs. This symbiotic signal is able to induce NIN expression in the Casuarina root epidermis, and so NIN might still be able to induce the production of a mobile signal, which subsequently induces cell division in the pericycle. Alternatively, the mobile signal might not be required in these Actinorhizal plants if the symbiotic signal itself is mobile. However, it has been proposed that an immobile NF may facilitate the detection and localization of the bacteria by which the redirection of root hair growth is induced and a curl is formed that entraps the bacteria [48]. CcI3 infect Casuarina roots through infection threads [50]; therefore, it seems likely that this symbiotic signal is immobile, like NFs.
In case cytokinin is the mobile signal in legumes, then actinorhizal plants will most likely form a different mobile signal. Alternatively, the nature of the mobile signal is conserved, and it induces cytokinin signaling in legumes and a different pathway in actinorhizal(-like) plants. The induction of the formation of a mobile signal might be an ancestral/original function of NIN in the epidermis. As described above, NIN is essential for infection thread formation. Although it has not yet been studied, it seems likely that for more primitive infection modes like crack entry, NIN might not be required. Therefore, the ancestral function of NIN in the epidermis is probably the induction of the production of the mobile signal, whereas its role in infection thread formation evolved later.
5. Function of NIN: Acquired upon Recruitment or Adopted from NLP-Controlled Process?
NIN is essential for nodule initiation, including infection thread formation, nodule organogenesis and AON. We will discuss the functions of NIN in these different processes, including some of its target genes. Further, we will discuss to what extent they are adopted from processes in which NLPs function, or whether they are innovations after its recruitment.
5.1. NF-Ys
NF-Ys are transcription factors, and some of them play a role in the NIN-induced nodulation processes. NF-Y transcription factor complexes are composed of three subunits: NF-YA, NF-YB and NF-YC [63]. NF-YA1 and NF-YB1 have been shown to be direct targets of NIN in Lotus [52]. Knockout mutations in NF-YA1 cause aberrant infection thread formation in Medicago [64] and a block of intracellular infection in Parasponia [12]. In Phaseolus vulgaris (common bean), the knockdown of NF-YC1 also causes aberrant infection thread formation [65]. Further, NF-Ys play a role in nodule organogenesis. In Lotus, Medicago and Parasponia, NF-YA1 mutations lead to the formation of small nodules and/or reduced nodule numbers [12,52,66]. Further, in both Medicago and Parasponia nodule primordia and nodules, NF-YA1 is expressed at sites where NIN has been shown to be expressed [12,41]. This suggests that the involvement of a NIN-NF-Y module in intracellular infection, and nodule organogenesis is conserved within the NFC and it evolved at an early moment during nodule evolution. This raises the question of whether NF-Ys are involved in NLP-controlled processes (in species outside NFC), and whether these processes are related to nodule organogenesis and/or intracellular infection.
In Parasponia [12], a mutation in NF-YA1 also has a non-symbiotic phenotype, namely, reduced lateral root formation. Moreover, in Lotus, overexpression of NIN or NF-YA1 induces extra cell division in the pericycle, and this leads to the formation of lateral roots with a malformed tip [52]. In addition, overexpression of NF-YA1 and NF-YB1 in Lotus increases lateral root densities to twice those of empty vector controls [67]. This suggests that an ancestral function of NF-YA1 could be related to lateral root formation, a process very similar to nodule organogenesis. This hypothesis is further supported by studies on the NF-YA1 orthologues in Arabidopsis, as AtNF-YA2/AtNF-YA10 are both involved in primary and lateral root growth [68]. In addition, they are expressed in pericycle cells, where lateral root as well as nodule organogenesis are initiated.
Interestingly, transcriptome analysis of the Arabidopsis nlp7 (from a different orthogroup than NIN) mutant shows altered expression levels of several genes encoding NF-YA subunits, including AtNF-YA2 and AtNF-YA10 [69,70]. This suggests that (some) NLPs can regulate the expression of NF-Y (A1). So, the NIN-NF-Y module might have been adopted from a NLP-NF-Y module which already occurred before nodulation evolved.
How can NF-Y be involved in both infection and nodule organogenesis? In mammals, NF-Y regulates the expression of cell cycle genes [71]. During root nodule development, not only nodule organogenesis requires expression of the cell cycle genes, but the passage of infection threads through cells also requires entry into the cell cycle [42]. Therefore, NF-Y might regulate infection, as well as organogenesis, through the activation of cell cycle genes. This is supported by the knockdown of NF-YC in the common bean, which results in the reduced expression of cell cycle genes, whereas overexpression of NF-YC1 causes higher expression levels of these genes [65]. Further, in Lotus, ectopic expression of NIN, or NF-YA1 and NF-YB1, results in ectopic expression of a cyclin gene [52].
Accumulation of the phytohormone auxin is correlated with mitotic activity. For example, when lateral root formation is initiated, auxin accumulates in the pericycle cells [72]. In nodulation, auxin signaling is required for both nodule organogenesis and the initiation of infection threads [73,74]. Further, it has been shown that NF-YA1 directly regulates the expression of STY genes, which encode transcription factors that regulate the expression of YUCCA auxin biosynthesis genes in Arabidopsis [75,76,77,78]. So, NF-Y might, by regulating auxin biosynthesis genes, stimulate entry into the cell cycle, which is involved in both infection and organogenesis, and this also holds for NIN, which regulates NF-Y expression.
5.2. LBD16
Recent studies show that NIN directly regulates the expression of LBD16 (lob-domain protein 16), which is known to be essential for lateral root formation [49,67]. In Medicago, an lbd16 mutant shows similar defects in nodule and lateral root initiation [49]. Like NF-YA1, LBD16 promotes auxin biosynthesis via transcriptional induction of STY and YUCCA genes [49]. In Lotus, it was shown that LBD16 and NF-Y in an additive way regulate nodule organogenesis, as a mutation in LBD16 enhances the nodulation phenotypes of nf-y subunit mutants [67]. In addition, the co-expression of LBD16 and NF-Y subunit genes can partially replace NIN, as it can rescue nodule organogenesis in the weak nin allele daphne [67]. In Parasponia, the expression level of LBD16 is about six times increased at the initial stages of nodule formation [6]. This level of induction is comparable to that in Lotus and Medicago [49,67]. In Arabidopsis, LBD16 has been shown to be a direct target of NLP7 [70]. Therefore, NIN-controlled LBD16 expression most likely is adopted from a non-symbiotic module in which its expression is controlled by NLP, and this module evolved before the occurrence of nodulation.
5.3. NPL1
Cell wall remodeling is required during rhizobial infection [15]. In Medicago root hairs, cell wall modification genes are induced upon rhizobial infection, and the expression of many of them depends on NIN [74]. Among these genes, only NODULATION PECTATE LYASE 1 (NPL1) shows nodule-specific expression [74]. In both Lotus and Medicago, NPL1 is essential for infection thread formation [74,79]. Its expression is regulated by NIN, and it is highly induced at infection sites [74,79]. NPL seems to be the result of a tandem gene duplication, and its orthologues occur in Glycine max (Soybean), Lupinus albus (Lupin) and Arachis ipaensis [74]. So, the NIN-NPL module is most likely conserved in the papilionoid legume sub-family.
Parasponia has three putative NPL orthologs, namely PanPLL8, PanPLL9 and PanPLL10 [6]. However, the most closely related PanPLL8 is not nodule-specifically expressed, and PanPLL9 and PanPLL10 are only four-fold induced at the initial stages of colonization. In later developmental stages, the relative fold change is even lower [6]. This is different from legumes, in which NPL1 is highly induced [74,79]. This suggests that the NIN-controlled nodule-specific expression of NPL might have been gained within the legume branch.
5.4. RPG
Rhizobium-directed polar growth (RPG) is a gene of which the expression is controlled by NIN [74]. RPG is a long coiled–coil protein that is nuclear-localized [80]. In Medicago, it has been shown to be essential for normal root hair curling and infection thread formation [80]. RPG is nodule-specifically expressed in Medicago, Lotus and Parasponia [6,80,81], which is consistent with the loss of a functional RPG gene in several non-nodulating NFC species [5,6]. This supports that it only has a symbiotic function in the NFC. In Lotus, it has been shown by ChIP-seq that RPG is a direct target of NIN [13,74]. Together, this suggests that a NIN-controlled RPG expression evolved early in the NFC, or it was already controlled by an NLP before nodulation evolved. However, neither NLP7 CHIP-chip nor the nlp7 transcriptome indicated that the expression of an RPG homologue is controlled [69,82]. To further support that NIN-controlled RPG expression evolved early in NFC, the RPG regulation in other nlp mutants, especially of NIN orthologues, remains to be analyzed.
5.5. CLEs
As described above, AON is essential for the regulation of nodule numbers, to balance the gains and costs of nodulation. In Medicago and Lotus, the CLE genes that are involved in AON are nodule-specifically expressed in a NIN-dependent manner [13,32,74]. In addition, PanCLE9, which is the putative Parasponia orthologue of Medicago CLE12/13, is also expressed at enhanced levels in nodules [6]. This suggest that NIN-controlled AON is conserved in NFC. However, the nodule-specific expression of CLE genes is not only controlled by NIN, but also by NLP whereas in response to nitrate [35]. In Lotus, it has been shown that both NRSYM1 (one of the NLPs belonging to the same orthogroup as AtNLP7) and NIN can bind to the Lotus CLE-RS2 promoter, and NRSYM1 even has a higher affinity [35]. In addition, Arabidopsis nlp7 mutants have altered expressions of several CLE genes, including AtCLE5/6/7, which are putative orthologues of LjCLE-RS1/2 [69]. Therefore, the function of NIN in controlling AON/CLE genes expression is most likely adopted from an ancestral NLP module.
6. Are NIN and NLPs Functionally Equivalent?
Several of the processes and genes regulated by NIN appear to be adopted from those controlled by NLPs. Therefore, it seems probable that NIN and NLPs are to some extent functionally equivalent. To test this, MtNLP1, the closest Medicago NIN paralogue, and AtNLP1, an Arabidopsis NIN orthologue driven by the Medicago NIN promoter, were introduced into the Medicago nin-1 knockout mutant (J. Liu, unpublished data [83]). However, neither the nodules nor infection threads were formed. This shows that NIN and these two NLPs are not functionally equivalent. So their protein sequence has diverged, and most likely NIN obtained amino acid changes that are essential to its function in nodulation.
Most of the NLPs that have been studied are located in the cytoplasm under nitrate starvation, and when high nitrate is sensed, they are transported to the nucleus [35,36,38,82,84]. In contrast, in Medicago nodules, NIN is located in the nucleus of all the cells in which it is expressed, and this became independent of nitrate sensing (Liu et al., unpublished data [85]). So, a major difference between NLPs and NIN is the constitutive nuclear localization of NIN, and this might be the reason that NLPs cannot complement the nin mutant.
In Arabidopsis, it has been shown that, upon nitrate sensing, serine 205 (S205) in AtNLP7 becomes phosphorylated, after which it is translocated from the cytoplasm to nucleus [86]. In addition, S205 in AtNLP7 is located in the region previously predicted as NLP-specific, and it is absent in Medicago and Lotus NIN, whereas MtNLP1 and AtNLP1 have it [39,86,87].
This serine might be the reason that MtNLP1/AtNLP1 fails to complement nin-1, as nodulated plants are grown under low nitrate (0.5 mM) conditions. However, phosphomimic versions of MtNLP1/AtNLP1, serine-modified into aspartate, also did not complement nin-1 (J. Liu, unpublished data [83]). This indicates that in addition to this serine, other changes are introduced in NIN that are required for it to be functional in nodulation. Identifying such critical changes will provide insight into the evolution of NIN.
7. Concluding Remarks
Both functional and phylogenomic analysis underpins the conserved key position of NIN in root nodule symbiosis. Further, the recruitment of NIN in the nodulation process seems to be the key step in the birth of this symbiosis. Therefore, understanding which minimal changes were required will provide major insights into the evolutionary trajectory leading to nodule formation. NIN is closely related to NLPs with whom it shares some downstream targets, pointing to partial functional equivalence. Two major changes are the regulation of expression by the microbe via the common signalling pathway, and the constitutive nuclear localization. Further adaptations will have occurred within the NFC. An example is the regulation of expression by cytokinin in the legume branch. Further studies on NIN orthologues inside and outside of NFC will help to refine the picture. The puzzle in understanding the evolution of NIN comes from its complicated spatiotemporal expression pattern and its multifunctionality. Until now, almost all studies on NIN have focused on its role during nodule initiation. However, NIN is also expressed in the mature nodule [10,88], where it most likely plays a different role than it does during the initial stages. Further studies on NIN in later nodule developmental stages will complete the picture of the evolution of NIN.
Acknowledgments
We would like to thank Michaela Škoríková for transforming nin knockout mutant with NLPs. We also thank Defeng Shen for his kind help with construction of the phylogenetic tree.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/7/777/s1, Supplementary data S1: NIN and NLP sequences used in the phylogenetic analysis.
Author Contributions
J.L. and T.B. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Research Council (2011-AdG-294790) and J.L. was funded by the China Scholarship Council (201506300062).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Soltis D.E., Soltis P.S., Morgan D.R., Swensen S.M., Beth C., Dowd J.M., Martin P.G., Soltis D.E., Soltis P.S., Morgant D.R., et al. Chloroplast Gene Sequence Data Suggest a Single Origin of the Predisposition for Symbiotic Nitrogen Fixation in Angiosperms. Proc. Natl. Acad. Sci. USA. 1995;92:2647–2651. doi: 10.1073/pnas.92.7.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peter J., Young W., Haukka K.E. Diversity and phylogeny of rhizobia. New Phytol. 1996;133:87–94. doi: 10.1111/j.1469-8137.1996.tb04344.x. [DOI] [Google Scholar]
- 3.Santi C., Bogusz D., Franche C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013;111:743–767. doi: 10.1093/aob/mct048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lancelle S.A., Torrey J.G. Early development of Rhizobium-induced root nodules of Parasponia rigida. I. Infection and early nodule initiation. Protoplasma. 1984;123:26–37. doi: 10.1007/BF01283179. [DOI] [Google Scholar]
- 5.Griesmann M., Chang Y., Liu X., Song Y., Haberer G., Crook M.B., Billault-Penneteau B., Lauressergues D., Keller J., Imanishi L., et al. Phylogenomics reveals multiple losses of nitrogen-fixing root nodule symbiosis. Science. 2018;361:1743. doi: 10.1126/science.aat1743. [DOI] [PubMed] [Google Scholar]
- 6.Van Velzen R., Holmer R., Bu F., Rutten L., Van Zeijl A., Liu W., Santuari L., Cao Q., Sharma T., Shen D., et al. Comparative genomics of the nonlegume Parasponia reveals insights into evolution of nitrogen-fixing rhizobium symbioses. Proc. Natl. Acad. Sci. USA. 2018;115:E4700–E4709. doi: 10.1073/pnas.1721395115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen D., Xiao T.T., van Velzen R., Kulikova O., Gong X., Geurts R., Pawlowski K., Bisseling T. A Homeotic Mutation Changes Legume Nodule Ontogeny into Actinorhizal-type Ontogeny. Plant Cell. 2020;32:1868–1885. doi: 10.1105/tpc.19.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schauser L., Roussis A., Stiller J., Stougaard J. A plant regulator controlling development of symbiotic root nodules. Nature. 1999;402:191–195. doi: 10.1038/46058. [DOI] [PubMed] [Google Scholar]
- 9.Marsh J.F., Rakocevic A., Mitra R.M., Brocard L., Sun J., Eschstruth A., Long S.R., Schultze M., Ratet P., Oldroyd G.E.D. Medicago truncatula NIN is Essential for Rhizobial-Independent Nodule Organogenesis Induced by Autoactive Calcium/Calmodulin-Dependent Protein Kinase. Plant Physiol. 2007;144:324–335. doi: 10.1104/pp.106.093021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batagov A.O., Sandal N., Mortensen A., Schauser L., Ellis N., Tikhonovich I.A., Stougaard J. The Sym35 Gene Required for Root Nodule Development in Pea is an Ortholog of Nin from Lotus japonicus. Plant Physiol. 2003;131:1009–1017. doi: 10.1104/pp.102.016071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clavijo F., Diedhiou I., Vaissayre V., Brottier L., Acolatse J., Moukouanga D., Crabos A., Auguy F., Franche C., Gherbi H., et al. The Casuarina NIN gene is transcriptionally activated throughout Frankia root infection as well as in response to bacterial diffusible signals. New Phytol. 2015;208:887–903. doi: 10.1111/nph.13506. [DOI] [PubMed] [Google Scholar]
- 12.Bu F., Rutten L., Roswanjaya Y.P., Kulikova O., Rodriguez-Franco M., Ott T., Bisseling T., van Zeijl A., Geurts R. Mutant analysis in the non-legume Parasponia andersonii identifies NIN and NF-YA1 transcription factors as a core genetic network in nitrogen-fixing nodule symbioses. New Phytol. 2019;226:541–554. doi: 10.1111/nph.16386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soyano T., Hirakawa H., Sato S., Hayashi M., Kawaguchi M. Nodule Inception creates a long-distance negative feedback loop involved in homeostatic regulation of nodule organ production. Proc. Natl. Acad. Sci. USA. 2014;111:14607–14612. doi: 10.1073/pnas.1412716111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oldroyd G.E.D., Murray J.D., Poole P.S., Downie J.A. The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 2011;45:119–144. doi: 10.1146/annurev-genet-110410-132549. [DOI] [PubMed] [Google Scholar]
- 15.Brewin N.J. Plant cell wall remodelling in the rhizobium-legume symbiosis. CRC Crit. Rev. Plant Sci. 2004;23:293–316. doi: 10.1080/07352680490480734. [DOI] [Google Scholar]
- 16.González-Sama A., Lucas M.M., De Felipe M.R., Pueyo J.J. An unusual infection mechanism and nodule morphogenesis in white lupin (Lupinus albus) New Phytol. 2004;163:371–380. doi: 10.1111/j.1469-8137.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- 17.Sprent J.I. Evolving ideas of legume evolution and diversity: A taxonomic perspective on the occurrence of nodulation: Tansley review. New Phytol. 2007;174:11–25. doi: 10.1111/j.1469-8137.2007.02015.x. [DOI] [PubMed] [Google Scholar]
- 18.Xiao T.T., Schilderink S., Moling S., Deinum E.E., Kondorosi E., Franssen H., Kulikova O., Niebel A., Bisseling T. Fate map of Medicago truncatula root nodules. Development. 2014;141:3517–3528. doi: 10.1242/dev.110775. [DOI] [PubMed] [Google Scholar]
- 19.Pawlowski K., Demchenko K.N. The diversity of actinorhizal symbiosis. Protoplasma. 2012;249:967–979. doi: 10.1007/s00709-012-0388-4. [DOI] [PubMed] [Google Scholar]
- 20.Pawlowski K., Bisseling T. Rhizobial and Actinorhizal Symbioses: What are the Shared Features? Plant Cell. 1996;8:1899–1913. doi: 10.2307/3870238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couzigou J.-M., Zhukov V., Mondy S., el Heba G.A., Cosson V., Ellis T.H.N., Ambrose M., Wen J., Tadege M., Tikhonovich I., et al. NODULE ROOT and COCHLEATA maintain nodule development and are legume orthologs of Arabidopsis BLADE-ON-PETIOLE genes. Plant Cell. 2012;24:4498–4510. doi: 10.1105/tpc.112.103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denarie J., Debelle F. Rhizobium Lipo-Chitooligosaccharide Nodulation Factors: Signaling Molecules Mediating Recognition and Morphogenesis. Annu. Rev. Biochem. 1996;65:503–535. doi: 10.1146/annurev.bi.65.070196.002443. [DOI] [PubMed] [Google Scholar]
- 23.Oldroyd G.E.D. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013;11:252–263. doi: 10.1038/nrmicro2990. [DOI] [PubMed] [Google Scholar]
- 24.Svistoonoff S., Hocher V., Gherbi H. Actinorhizal root nodule symbioses: What is signalling telling on the origins of nodulation? Curr. Opin. Plant Biol. 2014;20:11–18. doi: 10.1016/j.pbi.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Kosslak R.M., Bohlool B.B. Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol. 1984;75:125–130. doi: 10.1104/pp.75.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krusell L., Madsen L.H., Sato S., Aubert G., Genua A., Szczyglowski K., Duc G., Kaneko T., Tabata S., De Bruijn F., et al. Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature. 2002;420:422–426. doi: 10.1038/nature01207. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura R., Hayashit M., Wu G.J., Kouchi H., Imaizumi-Anrakull H., Murakami Y., Kawasaki S., Akao S., Ohmori M., Nagasawa M., et al. HAR1 mediates systemic regulation of symbiotic organ development. Nature. 2002;420:426–429. doi: 10.1038/nature01231. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto S., Shinohara H., Mori T., Matsubayashi Y., Kawaguchi M. Root-derived CLE glycopeptides control nodulation by direct binding to HAR1 receptor kinase. Nat. Commun. 2013;4:1–7. doi: 10.1038/ncomms3191. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto S., Ohnishi E., Sato S., Takahashi H., Nakazono M., Tabata S., Kawaguchi M. Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant Cell Physiol. 2009;50:67–77. doi: 10.1093/pcp/pcn194. [DOI] [PubMed] [Google Scholar]
- 30.Lagunas B., Achom M., Bonyadi-Pour R., Pardal A.J., Richmond B.L., Sergaki C., Vázquez S., Schäfer P., Ott S., Hammond J., et al. Regulation of Resource Partitioning Coordinates Nitrogen and Rhizobia Responses and Autoregulation of Nodulation in Medicago truncatula. Mol. Plant. 2019;12:833–846. doi: 10.1016/j.molp.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L., Sun Z., Su C., Wang Y., Yan Q., Chen J., Ott T., Li X. A GmNINa-miR172c-NNC1 Regulatory Network Coordinates the Nodulation and Autoregulation of Nodulation Pathways in Soybean. Mol. Plant. 2019;12:1211–1226. doi: 10.1016/j.molp.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Mortier V., Den Herder G., Whitford R., Van de Velde W., Rombauts S., D’Haeseleer K., Holsters M., Goormachtig S. CLE peptides control Medicago truncatula nodulation locally and systemically. Plant Physiol. 2010;153:222–237. doi: 10.1104/pp.110.153718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schauser L., Wieloch W., Stougaard J. Evolution of NIN-like proteins in Arabidopsis, rice, and Lotus japonicus. J. Mol. Evol. 2005;60:229–237. doi: 10.1007/s00239-004-0144-2. [DOI] [PubMed] [Google Scholar]
- 34.Mu X., Luo J. Evolutionary analyses of NIN-like proteins in plants and their roles in nitrate signaling. Cell. Mol. Life Sci. 2019;76:3753–3764. doi: 10.1007/s00018-019-03164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishida H., Tanaka S., Handa Y., Ito M., Sakamoto Y., Matsunaga S., Betsuyaku S., Miura K., Soyano T., Kawaguchi M., et al. A NIN-LIKE PROTEIN mediates nitrate-induced control of root nodule symbiosis in Lotus japonicus. Nat. Commun. 2018;9:499. doi: 10.1038/s41467-018-02831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin J.S., Li X., Luo Z.L., Mysore K.S., Wen J., Xie F. NIN interacts with NLPs to mediate nitrate inhibition of nodulation in Medicago truncatula. Nat. Plants. 2018;4:942–952. doi: 10.1038/s41477-018-0261-3. [DOI] [PubMed] [Google Scholar]
- 37.Demina I.V., Persson T., Santos P., Plaszczyca M., Pawlowski K. Comparison of the Nodule vs. Root Transcriptome of the Actinorhizal Plant Datisca glomerata: Actinorhizal Nodules Contain a Specific Class of Defensins. PLoS ONE. 2013;8:e72442. doi: 10.1371/journal.pone.0072442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao H., Qi S., Sun M., Li Z., Yang Y., Crawford N.M., Wang Y. Overexpression of the maize ZmNLP6 and ZmNLP8 can complement the arabidopsis nitrate regulatory mutant nlp7 by restoring nitrate signaling and assimilation. Front. Plant Sci. 2017;8:1–14. doi: 10.3389/fpls.2017.01703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chardin C., Girin T., Roudier F., Meyer C., Krapp A. The plant RWP-RK transcription factors: Key regulators of nitrogen responses and of gametophyte development. J. Exp. Bot. 2014;65:5577–5587. doi: 10.1093/jxb/eru261. [DOI] [PubMed] [Google Scholar]
- 40.Yoro E., Suzaki T., Toyokura K., Miyazawa H., Fukaki H., Kawaguchi M. A positive regulator of nodule organogenesis, NODULE INCEPTION, acts as a negative regulator of rhizobial infection in Lotus japonicus. Plant Physiol. 2014;165:747–758. doi: 10.1104/pp.113.233379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J., Rutten L., Limpens E., van der Molen T., van Velzen R., Chen R., Chen Y., Geurts R., Kohlen W., Kulikova O., et al. A Remote cis-Regulatory Region is Required for NIN Expression in the Pericycle to Initiate Nodule Primordium Formation in Medicago truncatula. Plant Cell. 2019;31:68–83. doi: 10.1105/tpc.18.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breakspear A., Liu C., Roy S., Stacey N., Rogers C., Trick M., Morieri G., Mysore K.S., Wen J., Oldroyd G.E.D., et al. The Root Hair “Infectome” of Medicago truncatula Uncovers Changes in Cell Cycle Genes and Reveals a Requirement for Auxin Signaling in Rhizobial Infection. Plant Cell Online. 2014;26:4680–4701. doi: 10.1105/tpc.114.133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vernié T., Kim J., Frances L., Ding Y., Sun J., Guan D., Niebel A., Gifford M.L., de Carvalho-Niebel F., Oldroyd G.E.D. The NIN Transcription Factor Coordinates Diverse Nodulation Programs in Different Tissues of the Medicago truncatula Root. Plant Cell. 2015;27:3410–3424. doi: 10.1105/tpc.15.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh S., Katzer K., Lambert J., Cerri M., Parniske M. CYCLOPS, A DNA-binding transcriptional activator, orchestrates symbiotic root nodule development. Cell Host Microbe. 2014;15:139–152. doi: 10.1016/j.chom.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Horváth B., Yeun L.H., Domonkos Á., Halász G., Gobbato E., Ayaydin F., Miró K., Hirsch S., Sun J., Tadege M., et al. Medicago truncatula IPD3 is a member of the common symbiotic signaling pathway required for rhizobial and mycorrhizal symbioses. Mol. Plant Microbe Interact. 2011;24:1345–1358. doi: 10.1094/MPMI-01-11-0015. [DOI] [PubMed] [Google Scholar]
- 46.Yano K., Yoshida S., Mueller J., Singh S., Banba M., Vickers K., Markmann K., White C., Schuller B., Sato S., et al. CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc. Natl. Acad. Sci. USA. 2008;105:20540–20545. doi: 10.1073/pnas.0806858105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huisman R. (Wageningen University, Wageningen, The Netherlands). Personal communication. 2019.
- 48.Goedhart J., Hink M.A., Visser A.J.W.G., Bisseling T., Gadella T.W.J. In vivo fluorescence correlation microscopy (FCM) reveals accumulation and immobilization of Nod factors in root hair cell walls. Plant J. 2000;21:109–119. doi: 10.1046/j.1365-313x.2000.00656.x. [DOI] [PubMed] [Google Scholar]
- 49.Schiessl K., Lilley J.L.S., Lee T., Tamvakis I., Kohlen W., Bailey P.C., Thomas A., Luptak J., Ramakrishnan K., Carpenter M.D., et al. NODULE INCEPTION Recruits the Lateral Root Developmental Program for Symbiotic Nodule Organogenesis in Medicago truncatula. Curr. Biol. 2019;29:3657–3668. doi: 10.1016/j.cub.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chabaud M., Gherbi H., Pirolles E., Vaissayre V., Fournier J., Moukouanga D., Franche C., Bogusz D., Tisa L.S., Barker D.G., et al. Chitinase-resistant hydrophilic symbiotic factors secreted by Frankia activate both Ca2+ spiking and NIN gene expression in the actinorhizal plant Casuarina glauca. New Phytol. 2016;209:86–93. doi: 10.1111/nph.13732. [DOI] [PubMed] [Google Scholar]
- 51.Rutten L. (Wageningen University, Wageningen, The Netherlands). Personal communication. 2019.
- 52.Soyano T., Kouchi H., Hirota A., Hayashi M. NODULE INCEPTION Directly Targets NF-Y Subunit Genes to Regulate Essential Processes of Root Nodule Development in Lotus japonicus. PLoS Genet. 2013;9:e1003352. doi: 10.1371/journal.pgen.1003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gauthier-Coles C., White R.G., Mathesius U. Nodulating legumes are distinguished by a sensitivity to cytokinin in the root cortex leading to pseudonodule development. Front. Plant Sci. 2019;9:1–14. doi: 10.3389/fpls.2018.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heckmann A.B., Sandal N., Bek A.S., Madsen L.H., Jurkiewicz A., Nielsen M.W., Tirichine L., Stougaard J. Cytokinin Induction of Root Nodule Primordia in Lotus japonicus is Regulated by a Mechanism Operating in the Root Cortex. Mol. Plant Microbe Interact. 2011;24:1385–1395. doi: 10.1094/MPMI-05-11-0142. [DOI] [PubMed] [Google Scholar]
- 55.Liu M., Soyano T., Yano K., Hayashi M., Kawaguchi M. ERN1 and CYCLOPS coordinately activate NIN signaling to promote infection thread formation in Lotus japonicus. J. Plant Res. 2019;132:641–653. doi: 10.1007/s10265-019-01122-w. [DOI] [PubMed] [Google Scholar]
- 56.Kang H., Hong Z., Zhang Z. A MYB Transcription Factor Interacts with NSP2 and is Involved in Nodulation in Lotus japonicus. Biol. Nitrogen Fixat. 2015;2:599–607. doi: 10.1111/nph.12593. [DOI] [PubMed] [Google Scholar]
- 57.Murakami Y., Miwa H., Imaizumi-Anraku H., Kouchi H., Downie J.A., Kawaguchi M., Kawasaki S. Positional cloning identifies Lotus japonicus NSP2, a putative transcription factor of the GRAS family, required for NIN and ENOD40 gene expression in nodule initiation. DNA Res. 2007;13:255–265. doi: 10.1093/dnares/dsl017. [DOI] [PubMed] [Google Scholar]
- 58.Hirsch S., Kim J., Muñoz A., Heckmann A.B., Downie J.A., Oldroyd G.E.D. GRAS proteins form a DNA binding complex to induce gene expression during nodulation signaling in Medicago truncatula. Plant Cell. 2009;21:545–557. doi: 10.1105/tpc.108.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao A., Yu H., Fan Y., Kang H., Ren Y., Huang X., Gao X., Wang C., Zhang Z., Zhu H., et al. Transcriptional regulation of NIN expression by IPN2 is required for root nodule symbiosis in Lotus japonicus. New Phytol. 2020;1:513–528. doi: 10.1111/nph.16553. [DOI] [PubMed] [Google Scholar]
- 60.Van Velzen R., Doyle J.J., Geurts R. A Resurrected Scenario: Single Gain and Massive Loss of Nitrogen-Fixing Nodulation. Trends Plant Sci. 2019;24:49–57. doi: 10.1016/j.tplants.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 61.Van Nguyen T., Wibberg D., Battenberg K., Blom J., Vanden Heuvel B., Berry A.M., Kalinowski J., Pawlowski K. An assemblage of Frankia Cluster II strains from California contains the canonical nod genes and also the sulfotransferase gene nodH. BMC Genomics. 2016;17:796. doi: 10.1186/s12864-016-3140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Persson T., Battenberg K., Demina I.V., Vigil-Stenman T., Vanden Heuvel B., Pujic P., Facciotti M.T., Wilbanks E.G., O’Brien A., Fournier P., et al. Candidatus Frankia datiscae Dg1, the Actinobacterial microsymbiont of datisca glomerata, expresses the canonical nod genes NodABC in symbiosis with its host plant. PLoS ONE. 2015;10:e0127630. doi: 10.1371/journal.pone.0127630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baudin M., Laloum T., Lepage A., Rípodas C., Ariel F., Frances L., Crespi M., Gamas P., Blanco F.A., Zanetti M.E., et al. A phylogenetically conserved group of nuclear factor-Y transcription factors interact to control nodulation in legumes. Plant Physiol. 2015;169:2761–2773. doi: 10.1104/pp.15.01144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laporte P., Lepage A., Fournier J., Catrice O., Moreau S., Jardinaud M.-F., Mun J.-H., Larrainzar E., Cook D.R., Gamas P., et al. The CCAAT box-binding transcription factor NF-YA1 controls rhizobial infection. J. Exp. Bot. 2014;65:481–494. doi: 10.1093/jxb/ert392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zanetti M.E., Blanco F.A., Beker M.P., Battaglia M., Aguilar O.M. AC subunit of the plant nuclear factor NF-Y required for rhizobial infection and nodule development affects partner selection in the common bean-Rhizobium etli Symbiosis. Plant Cell. 2010;22:4142–4157. doi: 10.1105/tpc.110.079137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Combier J., Frugier F., De Billy F., Boualem A., El-yahyaoui F., Moreau S., Vernié T., Ott T., Gamas P., Crespi M., et al. MtHAP2-1 is a key transcriptional regulator of symbiotic nodule development regulated by microRNA169 in Medicago truncatula service MtHAP2-1 is a key transcriptional regulator of symbiotic nodule development regulated by microRNA169 in Medicago truncatula. Res. Commun. 2006;20:3084–3088. doi: 10.1101/gad.402806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soyano T., Shimoda Y., Kawaguchi M., Hayashi M. A shared gene drives lateral root development and root nodule symbiosis pathways in Lotus. Science. 2019;366:1021–1023. doi: 10.1126/science.aax2153. [DOI] [PubMed] [Google Scholar]
- 68.Sorin C., Declerck M., Christ A., Blein T., Ma L., Lelandais-Brière C., Njo M.F., Beeckman T., Crespi M., Hartmann C. A miR169 isoform regulates specific NF-YA targets and root architecture in Arabidopsis. New Phytol. 2014;202:1197–1211. doi: 10.1111/nph.12735. [DOI] [PubMed] [Google Scholar]
- 69.Zhao L., Zhang W., Yang Y., Li Z., Li N., Qi S., Crawford N.M., Wang Y. The Arabidopsis NLP7 gene regulates nitrate signaling via NRT1.1-dependent pathway in the presence of ammonium. Sci. Rep. 2018;8:1–13. doi: 10.1038/s41598-018-20038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alvarez J.M., Schinke A.L., Brooks M.D., Pasquino A., Leonelli L., Varala K., Safi A., Krouk G., Krapp A., Coruzzi G.M. Transient genome-wide interactions of the master transcription factor NLP7 initiate a rapid nitrogen-response cascade. Nat. Commun. 2020;11:1–13. doi: 10.1038/s41467-020-14979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caretti G., Salsi V., Vecchi C., Imbriano C., Mantovani R. Dynamic recruitment of NF-Y and histone acetyltransferases on cell-cycle promoters. J. Biol. Chem. 2003;278:30435–30440. doi: 10.1074/jbc.M304606200. [DOI] [PubMed] [Google Scholar]
- 72.Dubrovsky J.G., Sauer M., Napsucialy-Mendivil S., Ivanchenko M.G., Friml J., Shishkova S., Celenza J., Benková E. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl. Acad. Sci. USA. 2008;105:8790–8794. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mathesius U., Schlaman H.R.M., Spaink H.P., Sautter C., Rolfe B.G., Djordjevic M.A. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J. 1998;14:23–34. doi: 10.1046/j.1365-313X.1998.00090.x. [DOI] [PubMed] [Google Scholar]
- 74.Liu C., Breakspear A., Guan D., Cerri M.R., Jackson K., Jiang S., Robson F., Radhakrishnan G.V., Roy S., Bone C., et al. NIN Acts as a Network Hub Controlling a Growth Module Required for Rhizobial Infection. Plant Physiol. 2019;179:1704–1722. doi: 10.1104/pp.18.01572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hossain M.S., Shrestha A., Zhong S., Miri M., Austin R.S., Sato S., Ross L., Huebert T., Tromas A., Torres-Jerez I., et al. Lotus japonicus NF-YA1 Plays an Essential Role during Nodule Differentiation and Targets Members of the SHI/STY Gene Family. Mol. Plant Microbe Interact. 2016;29:950–964. doi: 10.1094/MPMI-10-16-0206-R. [DOI] [PubMed] [Google Scholar]
- 76.Sohlberg J.J., Myrenås M., Kuusk S., Lagercrantz U., Kowalczyk M., Sandberg G., Sundberg E. STY1 regulates auxin homeostasis and affects apical-basal patterning of the Arabidopsis gynoecium. Plant J. 2006;47:112–123. doi: 10.1111/j.1365-313X.2006.02775.x. [DOI] [PubMed] [Google Scholar]
- 77.Eklund D.M., Thelander M., Landberg K., Staldal V., Nilsson A., Johansson M., Valsecchi I., Pederson E.R.A., Kowalczyk M., Ljung K., et al. Homologues of the Arabidopsis thaliana SHI/STY/LRP1 genes control auxin biosynthesis and affect growth and development in the moss Physcomitrella patens. Development. 2010;137:1275–1284. doi: 10.1242/dev.039594. [DOI] [PubMed] [Google Scholar]
- 78.Eklund D.M., Staldal V., Valsecchi I., Cierlik I., Eriksson C., Hiratsu K., Ohme-Takagi M., Sundstrom J.F., Thelander M., Ezcurra I., et al. The Arabidopsis thaliana STYLISH1 Protein Acts as a Transcriptional Activator Regulating Auxin Biosynthesis. Plant Cell. 2010;22:349–363. doi: 10.1105/tpc.108.064816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xie F., Murray J.D., Kim J., Heckmann A.B., Edwards A., Oldroyd G.E.D., Downie J.A. Legume pectate lyase required for root infection by rhizobia. Proc. Natl. Acad. Sci. USA. 2012;109:633–638. doi: 10.1073/pnas.1113992109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arrighi J.-F., Godfroy O., de Billy F., Saurat O., Jauneau A., Gough C. The RPG gene of Medicago truncatula controls Rhizobium-directed polar growth during infection. Proc. Natl. Acad. Sci. USA. 2008;105:9817–9822. doi: 10.1073/pnas.0710273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mun T., Bachmann A., Gupta V., Stougaard J., Andersen S.U. Lotus Base: An integrated information portal for the model legume Lotus japonicus. Sci. Rep. 2016;6:1–18. doi: 10.1038/srep39447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marchive C., Roudier F., Castaings L., Bréhaut V., Blondet E., Colot V., Meyer C., Krapp A. Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nat. Commun. 2013;4:1–9. doi: 10.1038/ncomms2650. [DOI] [PubMed] [Google Scholar]
- 83.Liu J. Essential differences between NIN and NLPs. Unpublished work.
- 84.Guan P., Ripoll J.-J., Wang R., Vuong L., Bailey-Steinitz L.J., Ye D., Crawford N.M. Interacting TCP and NLP transcription factors control plant responses to nitrate availability. Proc. Natl. Acad. Sci. USA. 2017;114:2419–2424. doi: 10.1073/pnas.1615676114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu J., Rasing M., Zeng T., Kulikova O., Bisseling T. NIN is essential for development of symbiosomes and suppression of defence-related responses in Medicago truncatula nodules. Unpublished work. [DOI] [PMC free article] [PubMed]
- 86.Liu K., Niu Y., Konishi M., Wu Y., Du H., Chung H.S., Li L., Boudsocq M., Mccormack M., Maekawa S., et al. Discovery of nitrate-CPK-NLP signalling in central nutrient—growth networks. Nature. 2017;545:311–316. doi: 10.1038/nature22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suzuki W., Konishi M., Yanagisawa S. The evolutionary events necessary for the emergence of symbiotic nitrogen fixation in legumes may involve a loss of nitrate responsiveness of the NIN transcription factor. Plant Signal. Behav. 2013;8:6–10. doi: 10.4161/psb.25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roux B., Rodde N., Jardinaud M.F., Timmers T., Sauviac L., Cottret L., Carrère S., Sallet E., Courcelle E., Moreau S., et al. An integrated analysis of plant and bacterial gene expression in symbiotic root nodules using laser-capture microdissection coupled to RNA sequencing. Plant J. 2014;77:817–837. doi: 10.1111/tpj.12442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.