To the Editor: A recent article suggested the rapid decay of anti–SARS-CoV-2 IgG in early infection,1 but the rate was not described in detail. We evaluated persons who had recovered from Covid-19 and referred themselves to our institution for observational research. Written informed consent was obtained from all the participants, with approval by the institutional review board. Blood samples were analyzed by enzyme-linked immunosorbent assay (ELISA) to detect anti–SARS-CoV-2 spike receptor-binding domain IgG.2 The ELISA was further modified to precisely quantify serum anti–receptor-binding domain activity in terms of equivalence to the concentration of a control anti–receptor-binding domain monoclonal IgG (CR3022, Creative Biolabs).
Infection had been confirmed by polymerase-chain-reaction assay in 30 of the 34 participants. The other 4 participants had had symptoms compatible with Covid-19 and had cohabitated with persons who were known to have Covid-19 but were not tested because of mild illness and the limited availability of testing. Most of the participants had mild illness; 2 received low-flow supplemental oxygen and leronlimab (a CCR5 antagonist), but they did not receive remdesivir. There were 20 women and 14 men. The mean age was 43 years (range, 21 to 68) (see the Supplementary Appendix, available with the full text of this letter at NEJM.org).
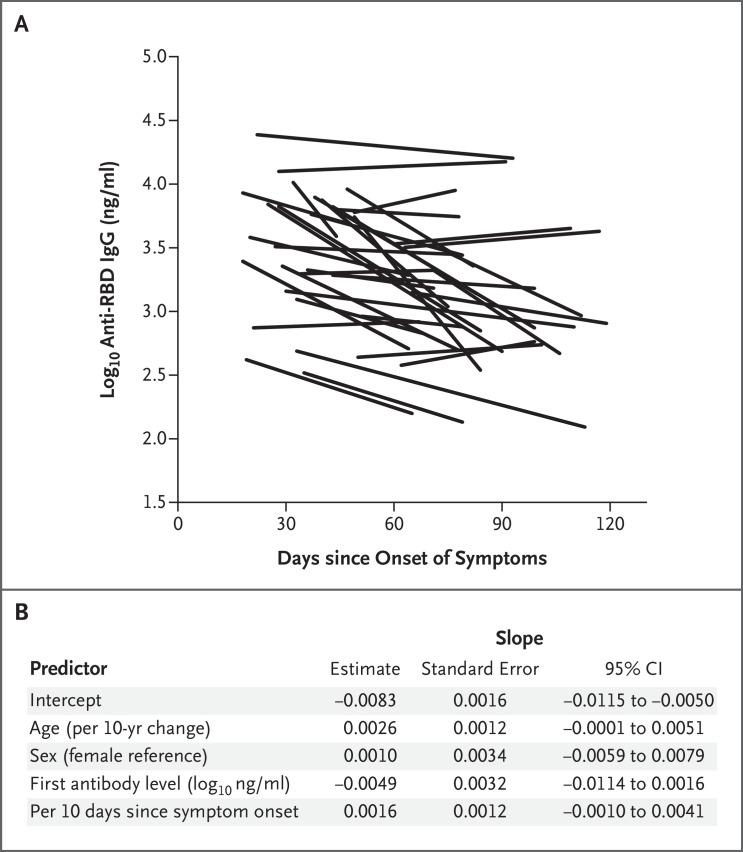
A total of 31 of the 34 participants had two serial measurements of IgG levels, and the remaining 3 participants had three serial measurements. The first measurement was obtained at a mean of 37 days after the onset of symptoms (range, 18 to 65), and the last measurement was obtained at a mean of 86 days after the onset of symptoms (range, 44 to 119).
The initial mean IgG level was 3.48 log10 ng per milliliter (range, 2.52 to 4.41). On the basis of a linear regression model that included the participants’ age and sex, the days from symptom onset to the first measurement, and the first log10 antibody level, the estimated mean change (slope) was −0.0083 log10 ng per milliliter per day (range, −0.0352 to 0.0062), which corresponds to a half-life of approximately 36 days over the observation period (Figure 1A). The 95% confidence interval for the slope was −0.0115 to −0.0050 log10 ng per milliliter per day (half-life, 26 to 60 days) (Figure 1B).
Figure 1. Longitudinal Assessment of Anti–SARS-CoV-2 Receptor-Binding Domain IgG in Persons Who Recovered from Covid-19.
Approximately 80 persons who recovered from Covid-19 referred themselves to our institution to inquire about observational research. Of 68 persons who volunteered to provide initial blood samples, 41 returned to provide repeat samples. Of those persons, 3 were excluded from this analysis because of unclear timing of infection and 4 were excluded because of initial and repeat serum antibody measurements below the limit of reliable quantitative detection. For the 34 participants in our analysis, anti–SARS-CoV-2 receptor-binding domain (RBD) serum IgG concentrations were quantified by enzyme-linked immunosorbent assay as equivalent binding activity to a concentration of a control monoclonal IgG for at least two time points (31 of the 34 participants had two measurements, and the remaining 3 participants had three measurements). Panel A shows log-transformed IgG concentrations plotted against the time since the onset of symptoms in each participant. Panel B shows a linear regression model that was created to estimate the effects of the participants’ age and sex, the days from symptom onset to the first measurement, and the first measured log10 antibody level on the slope reflecting the change in anti-RBD antibody levels (in log10 ng per milliliters per day). The values for age and antibody level were centered at the mean. The time since symptom onset was centered at day 18 and adjusted per 100 days. Thus, the intercept of the model can be interpreted as the average slope adjusted for age, sex, and time and value of the first measurement. CI denotes confidence interval.
The protective role of antibodies against SARS-CoV-2 is unknown, but these antibodies are usually a reasonable correlate of antiviral immunity, and anti–receptor-binding domain antibody levels correspond to plasma viral neutralizing activity. Given that early antibody decay after acute viral antigenic exposure is approximately exponential,3 we found antibody loss that was quicker than that reported for SARS-CoV-1,4,5 and our findings were more consistent with those of Long et al.1 Our findings raise concern that humoral immunity against SARS-CoV-2 may not be long lasting in persons with mild illness, who compose the majority of persons with Covid-19. It is difficult to extrapolate beyond our observation period of approximately 90 days because it is likely that the decay will decelerate.3 Still, the results call for caution regarding antibody-based “immunity passports,” herd immunity, and perhaps vaccine durability, especially in light of short-lived immunity against common human coronaviruses. Further studies will be needed to define a quantitative protection threshold and rate of decline of antiviral antibodies beyond 90 days.
Supplementary Appendix
Disclosure Forms
This letter was published on July 21, 2020, and last updated on July 24, 2020, at NEJM.org.
Footnotes
Supported by grants from the AIDS Healthcare Foundation, the Doris Duke Charitable Foundation (2019086), the National Institutes of Health (AI124979, AI131879, AI140775, AI068616, AI068632, and AI106716), the James B. Pendleton Charitable Trust, and the McCarthy Family Foundation.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020. June 18 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 2.Stadlbauer D, Amanat F, Chromikova V, et al. SARS-CoV-2 Seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol 2020;57(1):e100-e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andraud M, Lejeune O, Musoro JZ, Ogunjimi B, Beutels P, Hens N. Living on three time scales: the dynamics of plasma cell and antibody populations illustrated for hepatitis a virus. PLoS Comput Biol 2012;8(3):e1002418-e1002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao W-C, Liu W, Zhang P-H, Zhang F, Richardus JH. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med 2007;357:1162-1163. [DOI] [PubMed] [Google Scholar]
- 5.Chang S-C, Wang J-T, Huang L-M, et al. Longitudinal analysis of severe acute respiratory syndrome (SARS) coronavirus-specific antibody in SARS patients. Clin Diagn Lab Immunol 2005;12:1455-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.