Abstract
Background
Hydroxychloroquine and azithromycin have been used to treat patients with coronavirus disease 2019 (Covid-19). However, evidence on the safety and efficacy of these therapies is limited.
Methods
We conducted a multicenter, randomized, open-label, three-group, controlled trial involving hospitalized patients with suspected or confirmed Covid-19 who were receiving either no supplemental oxygen or a maximum of 4 liters per minute of supplemental oxygen. Patients were randomly assigned in a 1:1:1 ratio to receive standard care, standard care plus hydroxychloroquine at a dose of 400 mg twice daily, or standard care plus hydroxychloroquine at a dose of 400 mg twice daily plus azithromycin at a dose of 500 mg once daily for 7 days. The primary outcome was clinical status at 15 days as assessed with the use of a seven-level ordinal scale (with levels ranging from one to seven and higher scores indicating a worse condition) in the modified intention-to-treat population (patients with a confirmed diagnosis of Covid-19). Safety was also assessed.
Results
A total of 667 patients underwent randomization; 504 patients had confirmed Covid-19 and were included in the modified intention-to-treat analysis. As compared with standard care, the proportional odds of having a higher score on the seven-point ordinal scale at 15 days was not affected by either hydroxychloroquine alone (odds ratio, 1.21; 95% confidence interval [CI], 0.69 to 2.11; P=1.00) or hydroxychloroquine plus azithromycin (odds ratio, 0.99; 95% CI, 0.57 to 1.73; P=1.00). Prolongation of the corrected QT interval and elevation of liver-enzyme levels were more frequent in patients receiving hydroxychloroquine, alone or with azithromycin, than in those who were not receiving either agent.
Conclusions
Among patients hospitalized with mild-to-moderate Covid-19, the use of hydroxychloroquine, alone or with azithromycin, did not improve clinical status at 15 days as compared with standard care. (Funded by the Coalition Covid-19 Brazil and EMS Pharma; ClinicalTrials.gov number, NCT04322123.)
Coronavirus disease 2019 (Covid-19), the disease caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), is associated with considerable morbidity and mortality.1,2 Hydroxychloroquine has antiviral effects in vitro, and, in association with azithromycin, was suggested to decrease SARS-CoV-2 viral load in a small, nonrandomized study.3,4 On the basis of this evidence, hydroxychloroquine plus azithromycin has been used by some practitioners to treat patients with Covid-19. Furthermore, some national regulatory agencies have authorized the use of hydroxychloroquine in hospitalized patients with this disease.5,6 However, observational studies have suggested no beneficial effect of chloroquine or hydroxychloroquine in hospitalized patients with Covid-19.7,8 Previous randomized, controlled trials have shown no benefit of hydroxychloroquine for either postexposure prophylaxis or treatment of Covid-19.9-11 We performed a multicenter, randomized, open-label, controlled trial (Coalition Covid-19 Brazil I) to assess whether hydroxychloroquine, either alone or in combination with azithromycin, would be effective in improving clinical status at 15 days after hospital admission due to mild-to-moderate Covid-19.
Methods
Trial Design and Oversight
We conducted this three-group trial at 55 hospitals in Brazil. The trial was designed by the executive committee (see the Supplementary Appendix, available with the full text of this article at NEJM.org) and approved by the Brazilian National Commission for Research Ethics, the Brazilian Health Regulatory Agency (ANVISA), and ethics committees at the participating sites. The trial was funded by the hospitals and research institutes participating in Coalition Covid-19 Brazil (see the Supplementary Appendix). EMS Pharma provided additional funding and logistic support for the trial and also donated and supplied the trial drugs. EMS Pharma had no role in the conduct of the trial, the analysis, or the decision to submit the manuscript for publication. The trial was overseen by an independent international data and safety monitoring committee. The executive committee vouches for the completeness and accuracy of the data and for the fidelity of the trial to the protocol (available at NEJM.org).
Participants
The trial included consecutive patients who were 18 years of age or older and who had been hospitalized with suspected or confirmed Covid-19 with 14 or fewer days since symptom onset. Among the reasons for exclusion from the trial were the use of supplemental oxygen at a rate of more than 4 liters per minute as administered by a nasal cannula or at a level of at least 40% as administered by a Venturi mask; the use of supplemental oxygen administered by a high-flow nasal cannula or invasive or noninvasive ventilation; previous use of chloroquine, hydroxychloroquine, azithromycin, or any other macrolide for more than 24 hours before enrollment (and since the onset of symptoms); and a history of severe ventricular tachycardia or electrocardiographic findings with a corrected QT interval (QTc) of at least 480 msec. Complete information on the inclusion and exclusion criteria is provided in the Supplementary Appendix. All the patients provided written or electronic informed consent before randomization.
Randomization, Interventions, and Follow-up
Patients were randomly assigned in a 1:1:1 ratio to receive standard care (control group), standard care plus hydroxychloroquine at a dose of 400 mg twice daily for 7 days (hydroxychloroquine-alone group), or standard care plus hydroxychloroquine at a dose of 400 mg twice daily plus azithromycin at a dose of 500 mg once a day for 7 days. Randomization was performed in blocks of six and was stratified according to the use or nonuse of supplemental oxygen at the time of randomization. Randomization was performed centrally by means of an electronic case-report form system (RedCap) as described in the Supplementary Appendix.12
The current standard care for Covid-19 was at the discretion of the treating physicians. The use of glucocorticoids, other immunomodulators, antibiotic agents, and antiviral agents was allowed (see the Supplementary Appendix). The administration of hydroxychloroquine or chloroquine was not allowed in the control group, and the use of macrolides was not allowed in the control group or the hydroxychloroquine-alone group. Guidance was provided to the investigators about how to adjust or interrupt treatment according to side effects and laboratory abnormalities.
Data were collected daily, from randomization until day 15, in the electronic case-report form. For patients who were discharged before day 15, a structured telephone call to the patient or the patient’s family was conducted on or after day 15 by an interviewer who was unaware of the assigned trial group in order to assess vital status and return to routine activities.
Outcomes
The primary outcome was clinical status at 15 days, evaluated with the use of a seven-level ordinal scale. Scores on the scale were defined as follows: a score of 1 indicated not hospitalized with no limitations on activities; 2, not hospitalized but with limitations on activities; 3, hospitalized and not receiving supplemental oxygen; 4, hospitalized and receiving supplemental oxygen; 5, hospitalized and receiving oxygen supplementation administered by a high-flow nasal cannula or noninvasive ventilation; 6, hospitalized and receiving mechanical ventilation; and 7, death.
Secondary outcomes included clinical status at 7 days, evaluated with the use of a six-level ordinal scale (see below and see the Supplementary Appendix); an indication for intubation within 15 days; the receipt of supplemental oxygen administered by a high-flow nasal cannula or noninvasive ventilation between randomization and 15 days; duration of hospital stay; in-hospital death; thromboembolic complications; acute kidney injury; and the number of days alive and free from respiratory support up to 15 days. A day alive and free from respiratory support was defined as any day in which the patient did not receive supplemental oxygen or invasive or noninvasive mechanical ventilation, from randomization to day 15. Patients who died during the 15-day window were assigned a value of 0 days alive and free from respiratory support in this assessment. Safety outcomes are listed in the Supplementary Appendix. All the trial outcomes were assessed by the site investigators, who were aware of the trial-group assignments (except as noted above for patients who had been discharged before day 15 and who were assessed for the primary outcome by means of a blinded telephone interview). No formal adjudication of trial outcomes was performed.
Sample-Size Calculation and Protocol Changes
We had originally planned for the trial to include 630 patients, using the intention-to-treat analysis population, with a six-level ordinal outcome as the primary outcome, as described in the Supplementary Appendix. However, before the first interim analysis was conducted, we changed the primary-outcome assessment to the seven-level ordinal scale and the main analysis population from the intention-to-treat population to a modified intention-to-treat population that included only patients with a diagnosis of Covid-19 that had been confirmed by reverse-transcriptase–polymerase-chain-reaction (RT-PCR) testing (using the test available at each site).
The change to the use of the seven-level ordinal scale was adopted because on April 10, 2020 (before the first enrolled patient had reached 15 days of follow-up), we established the capability to obtain 15-day information on limitations on activities with the use of blinded telephone interviews. We therefore added another level to the six-level ordinal outcome, dividing the first level (not hospitalized) into two levels (level 1, not hospitalized and with no limitations on activities; and level 2, not hospitalized but with limitations on activities). The change to the modified intention-to-treat population was adopted because, under the hypothesis that treatment would have beneficial effects on the primary outcome only for patients who had a confirmed diagnosis, the inclusion of unconfirmed cases would decrease the estimated effect size and power. As a related change, we added external adjudication of unconfirmed cases, which were classified as probable, possible, or probably not Covid-19 (see the Supplementary Appendix).
The sample size was revised with the use of the overall distribution of the seven-level ordinal outcome at day 15 observed among the first 120 patients, with the levels 1 through 7 having the following proportions of patients: 60%, 19%, 7%, 1%, 1%, 5%, and 7%, respectively. With 630 patients who had undergone randomization and 510 patients included in the modified intention-to-treat analysis, we calculated that the trial would have 80% power to detect an odds ratio of 0.5 between groups (two-by-two comparisons), at a significance level of 5% and with Bonferroni adjustment for multiple comparisons (α=5%, divided by 3 for each comparison).13
Statistical Analysis
The primary outcome was analyzed by mixed ordinal logistic regression with random intercept according to site, assuming proportional odds. We report all two-by-two comparisons. Binary outcomes were assessed with the use of a mixed logistic-regression model, except for in-hospital mortality, which was assessed with a Cox proportional-hazards model. Continuous outcomes were evaluated by means of generalized linear regression or mixed models for repeated variables, as appropriate. All models were adjusted for age and the use of supplemental oxygen at admission.
We also performed sensitivity analyses that included all the patients who had undergone randomization (intention-to-treat population) and sensitivity analyses for the primary outcome for the following groups: patients with definitive, probable, or possible Covid-19; and patients with definitive or probable Covid-19. Two additional populations were considered. An efficacy population included patients with a confirmed diagnosis who received at least one dose of the assigned trial drug. The safety population included patients according to the medications received, regardless of the assigned trial group or the result of Covid-19 testing.
We planned three interim analyses, to be conducted when 120 patients, 315 patients, and 504 patients had completed 15 days of follow-up. However, only the first interim analysis was conducted. Owing to faster-than-expected enrollment, primary-outcome data for the second and third interim analyses were available only after trial recruitment was finished. After discussion with the data and safety monitoring committee, the second and third interim analyses were cancelled. The data and safety monitoring committee used Haybittle–Peto14 stopping boundaries, with a P-value threshold of less than 0.001 to interrupt the trial for safety and a P-value threshold of less than 0.0001 to interrupt the trial for efficacy. We did not adjust the final values of the hypothesis test for sequential analyses.
Analyses were performed with the use of R software (R Core Team).15 P values for the primary outcome were adjusted with the use of Bonferroni correction. No P values are reported for secondary outcomes; the widths of the confidence intervals for the secondary outcomes have not been adjusted for multiple comparisons, so the intervals should not be used to infer definitive treatment effects. P values for the safety analyses were not adjusted given the importance of identifying potential signals of harm. Additional details about the statistical analyses are provided in the Supplementary Appendix.
Results
Characteristics of the Patients
We recruited 667 patients, including 504 patients with confirmed Covid-19. The numbers of enrolled patients according to site are presented in Table S1 in the Supplementary Appendix. A total of 217 patients were randomly assigned to receive hydroxychloroquine plus azithromycin, 221 to receive hydroxychloroquine, and 229 to receive standard care (control group) (Fig. S1). The first patient underwent randomization on March 29, 2020; the last patient underwent randomization on May 17, 2020; and follow-up was completed on June 2, 2020. Two patients were excluded after randomization (1 withdrew consent after randomization and 1 had been enrolled twice). The 15-day follow-up was completed for all the remaining 665 patients.
The characteristics of the patients are shown in Table 1 and Table S2. Most patients (584 patients [87.8%]) underwent randomization within 10 days after symptom onset. The mean age of the patients was 50 years, and 58% of all the included patients were men. A total of 42% of the patients were receiving supplemental oxygen at baseline. The baseline data for the modified intention-to-treat population are shown in Table S3. A comparison between the patients in the modified intention-to-treat population and those without a confirmed diagnosis of Covid-19 (161 patients) is shown in Table S4. Patients without a confirmed diagnosis of Covid-19 had a higher prevalence of chronic pulmonary obstructive disease or smoking, a lower prevalence of diabetes, and were less frequently receiving supplemental oxygen than patients who had a confirmed diagnosis. Details concerning adherence to the trial regimen and the use of other medications are presented in Tables S5 and S6, respectively.
Table 1. Characteristics of the Patients at Baseline (Intention-to-Treat Population).*.
| Characteristic | Hydroxychloroquine plus Azithromycin (N=217) |
Hydroxychloroquine (N=221) |
Control (N=227) |
Total (N=665) |
|---|---|---|---|---|
| Age — yr | 49.6±14.2 | 51.3±14.5 | 49.9±15.1 | 50.3±14.6 |
| Male sex — no. (%) | 123 (56.7) | 142 (64.3) | 123 (54.2) | 388 (58.3) |
| Coexisting condition — no. (%) | ||||
| Hypertension | 81 (37.3) | 94 (42.5) | 83 (36.6) | 258 (38.8) |
| Diabetes | 40 (18.4) | 47 (21.3) | 40 (17.6) | 127 (19.1) |
| Current or former smoking | 17 (7.8) | 12 (5.4) | 15 (6.6) | 44 (6.6) |
| Obesity | 29 (13.4) | 37 (16.7) | 37 (16.3) | 103 (15.5) |
| Cancer | 7 (3.2) | 4 (1.8) | 8 (3.5) | 19 (2.9) |
| Heart failure | 4 (1.8) | 3 (1.4) | 3 (1.3) | 10 (1.5) |
| COPD | 4 (1.8) | 4 (1.8) | 4 (1.8) | 12 (1.8) |
| AIDS | 1 (0.5) | 0 | 3 (1.3) | 4 (0.6) |
| Chronic renal disease | 2 (0.9) | 1 (0.5) | 2 (0.9) | 5 (0.8) |
| Asthma | 16 (7.4) | 9 (4.1) | 15 (6.6) | 40 (6.0) |
| Previous medication use — no. (%) | ||||
| Glucocorticoid | 4 (1.8) | 1 (0.5) | 3 (1.3) | 8 (1.2) |
| ACE inhibitor | 16 (7.4) | 19 (8.6) | 13 (5.7) | 48 (7.2) |
| Angiotensin II–receptor antagonist | 39 (18.0) | 36 (16.3) | 41 (18.1) | 116 (17.4) |
| NSAID | 8 (3.7) | 12 (5.4) | 9 (4.0) | 29 (4.4) |
| Randomization location — no. (%) | ||||
| Emergency department or ward | 187 (86.2) | 189 (85.5) | 197 (86.8) | 573 (86.2) |
| ICU | 30 (13.8) | 32 (14.5) | 30 (13.2) | 92 (13.8) |
| Testing for Covid-19 — no. (%) | ||||
| Positive on RT-PCR | 172 (79.3) | 159 (71.9) | 173 (76.2) | 504 (75.8) |
| Negative on RT-PCR or unavailable | 45 (20.7) | 62 (28.1) | 54 (23.8) | 161 (24.2) |
| Score on seven-level ordinal scale — no. (%)† | ||||
| 3: Hospitalized and not receiving supplemental oxygen | 125 (57.6) | 132 (59.7) | 130 (57.3) | 387 (58.2) |
| 4: Hospitalized and receiving supplemental oxygen | 92 (42.4) | 89 (40.3) | 97 (42.7) | 278 (41.8) |
| Use of trial medication‡ | ||||
| Hydroxychloroquine — no. (%) | 23 (10.6) | 20 (9.0) | 19 (8.4) | 62 (9.3) |
| Azithromycin — no./total no. (%) | 74/217 (34.1) | 76/221 (34.4) | 90/226 (39.8) | 240/664 (36.1) |
| Median time from admission to randomization (IQR) — days | 1 (0–1) | 1 (0–1) | 1 (0–1) | 1 (0–1) |
| Median time from symptom onset to randomization (IQR) — days | 7 (5–9) | 7 (5–8) | 7 (4–9) | 7 (5–9) |
Plus-minus values are means ±SD. The intention-to-treat population included all the patients who had undergone randomization. Information on coexisting conditions was obtained from the medical records. The values shown are based on available data. Percentages may not total 100 because of rounding. ACE denotes angiotensin-converting enzyme, AIDS acquired immunodeficiency syndrome, COPD chronic obstructive pulmonary disease, ICU intensive care unit, IQR interquartile range, NSAID nonsteroidal antiinflammatory drug, and RT-PCR reverse transcriptase–polymerase chain reaction.
Only hospitalized patients who were not receiving supplemental oxygen or who were receiving up to 4 liters per minute of supplemental oxygen were eligible for the trial. Patients who had scores on other levels of the seven-level ordinal scale were not eligible.
Use of trial medication was defined as the use of hydroxychloroquine or azithromycin during the 24-hour period before randomization.
Primary Outcome
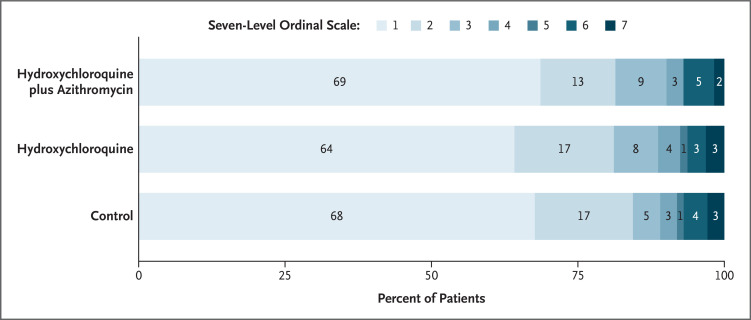
The primary outcome (status on the seven-point ordinal scale at day 15) was assessed in all patients who were still in the hospital on day 15 exactly and in outpatients (by means of telephone interview) as close to day 15 as possible (see the Supplementary Appendix and Fig. S2). Among patients with confirmed Covid-19, there were no significant between-group differences in the proportional odds of having a higher (worse) score on the seven-point ordinal scale at 15 days (hydroxychloroquine plus azithromycin vs. control: odds ratio, 0.99; 95% confidence interval [CI], 0.57 to 1.73; P=1.00; hydroxychloroquine alone vs. control: odds ratio, 1.21; 95% CI, 0.69 to 2.11; P=1.00; and hydroxychloroquine plus azithromycin vs. hydroxychloroquine alone: odds ratio, 0.82; 95% CI, 0.47 to 1.43; P=1.00) (Table 2). The distribution of patients’ scores on the seven-level ordinal scale at 15 days is shown in Figure 1.
Table 2. Primary and Secondary Outcomes (Modified Intention-to-Treat Population).*.
| Outcome | Hydroxychloroquine plus Azithromycin (N=172) |
Hydroxychloroquine (N=159) |
Control (N=173) |
Effect Estimate (95% CI) | ||
|---|---|---|---|---|---|---|
| Hydroxychloroquine plus Azithromycin vs. Control |
Hydroxychloroquine vs. Control |
Hydroxychloroquine plus Azithromycin vs. Hydroxychloroquine |
||||
| Primary outcome: seven-level ordinal outcome at 15 days† | ||||||
| Median score (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.99 (0.57 to 1.73)‡ | 1.21 (0.69 to 2.11)‡ | 0.82 (0.47 to 1.43)‡ |
| Distribution — no. (%) | ||||||
| 1: Not hospitalized with no limitations on activities | 118 (68.6) | 102 (64.2) | 117 (67.6) | |||
| 2: Not hospitalized but with limitations on activities | 22 (12.8) | 27 (17.0) | 29 (16.8) | |||
| 3: Hospitalized, not receiving supplemental oxygen | 15 (8.7) | 12 (7.5) | 8 (4.6) | |||
| 4: Hospitalized, receiving supplemental oxygen | 5 (2.9) | 6 (3.8) | 5 (2.9) | |||
| 5: Hospitalized, receiving noninvasive ventilation or high-flow nasal cannula | 0 | 2 (1.3) | 2 (1.2) | |||
| 6: Hospitalized, receiving mechanical ventilation | 9 (5.2) | 5 (3.1) | 7 (4.0) | |||
| 7: Death | 3 (1.7) | 5 (3.1) | 5 (2.9) | |||
| Secondary outcomes | ||||||
| Six-level ordinal outcome at 7 days§ | ||||||
| Median score (IQR) | 2 (1–3) | 2 (1–2) | 2 (1–3) | 0.81 (0.54 to 1.22) | 0.92 (0.61 to 1.38) | 0.89 (0.58 to 1.34) |
| Distribution — no./total no. (%) | ||||||
| 1: Not hospitalized | 84 (48.8) | 67 (42.1) | 75 (43.4) | |||
| 2: Hospitalized, not receiving supplemental oxygen | 38 (22.1) | 53 (33.3) | 45 (26.0) | |||
| 3: Hospitalized, receiving supplemental oxygen | 31 (18.0) | 25 (15.7) | 38 (22.0) | |||
| 4: Hospitalized, receiving noninvasive ventilation or high-flow nasal cannula | 3 (1.7) | 2 (1.3) | 4 (2.3) | |||
| 5: Hospitalized, receiving mechanical ventilation | 15 (8.7) | 10 (6.3) | 9 (5.2) | |||
| 6: Death | 1 (0.6) | 2 (1.3) | 2 (1.2) | |||
| No. of days free from respiratory support within 15 days¶ | 11.1±4.9 | 11.2±4.9 | 11.1±4.9 | 0.1 (−0.7– to 0.9) | −0.2 (−1.1 to 0.6) | 0.3 (−0.6 to 1.1) |
| Use of high-flow nasal cannula or noninvasive ventilation within 15 days — no. (%) | 16 (9.3) | 17 (10.7) | 16 (9.2) | 1.10 (0.60 to 2.03) | 1.19 (0.65 to 2.21) | 0.92 (0.50 to 1.70) |
| Use of mechanical ventilation within 15 days — no. (%) | 19 (11.0) | 12 (7.5) | 12 (6.9) | 1.77 (0.81 to 3.87) | 1.15 (0.49 to 2.70) | 1.54 (0.71 to 3.35) |
| Duration of hospital stay — days‖ | 10.3±8.4 | 9.6±6.5 | 9.5±7.2 | 0.9 (−0.3 to 2.1) | 0.2 (−1.0 to 1.3) | 0.7 (−0.6 to 1.9) |
| In-hospital death — no. (%)‖** | 5 (2.9) | 7 (4.4) | 6 (3.5) | 0.64 (0.18 to 2.21) | 1.47 (0.48 to 4.53) | 0.43 (0.13 to 1.45) |
| Thromboembolic complications within 15 days — no. (%) | 2 (1.2) | 3 (1.9) | 2 (1.2) | 0.89 (0.31 to 2.54) | 1.39 (0.53 to 3.65) | 0.64 (0.24 to 1.68) |
| Acute kidney injury within 15 days — no. (%) | 6 (3.5) | 4 (2.5) | 5 (2.9) | 1.18 (0.44 to 3.20) | 0.88 (0.29 to 2.63) | 1.35 (0.47 to 3.84) |
Plus–minus values are means ±SD. The modified intention-to-treat population included patients who had undergone randomization and who had a confirmed diagnosis of coronavirus disease 2019 (Covid-19). Effect estimates are mean differences (for the outcomes of the number of days free from respiratory support within 15 days and the duration of hospital stay), hazard ratios (for the outcome of in-hospital death), or odds ratios (for all other outcomes). Odds ratios for the ordinal outcome at 7 days and at 15 days that are lower than 1 indicate a benefit for treatment groups as compared with control. We used the Lipsitz method to test proportionality of odds with a P value of 0.15 (see the Additional Details on Statistical Analyses section in the Supplementary Appendix). The widths of the confidence intervals for the secondary outcomes were not adjusted for multiple comparisons, so the intervals should not be used to infer definitive treatment effects.
The ordinal outcome as assessed at 15 days was evaluated on the seven-level scale. Odds ratios lower than 1 indicate treatment benefit.
P=1.00 for all comparisons. P values for the three two-by-two comparisons for the primary outcome were adjusted with the use of Bonferroni correction for multiple comparisons.
The ordinal outcome as assessed at 7 days was evaluated on a six-level scale, in which levels 1 and 2 from the seven-level scale were combined as level 1 (no hospitalization). Data were available for 171 patients assigned to receive hydroxychloroquine plus azithromycin, for 157 in the hydroxychloroquine-alone group, and for 173 in the control group. Effects were calculated after multiple imputation. Odds ratio values lower than 1 indicate treatment benefit.
Data were available for 169 patients assigned to receive hydroxychloroquine plus azithromycin, 157 patients in the hydroxychloroquine-alone group, and 171 patients in the control group. Effects were calculated after multiple imputation.
As of June 4, 2020, a total of 21 patients were still in the hospital (range of duration of follow-up, 22 to 49 days): 8 patients assigned to receive hydroxychloroquine plus azithromycin, 5 patients in the hydroxychloroquine-alone group, and 8 in the control group. These patients were considered to be discharged alive.
A total of 18 patients died in the hospital. Hazard ratios are shown for this analysis. The causes of death were as follows: among patients assigned to receive hydroxychloroquine plus azithromycin, 5 patients died (the cause of death was Covid-19–related acute respiratory failure or septic shock in all patients); among patients assigned to receive hydroxychloroquine, 7 patients died (the cause of death was Covid-19–related acute respiratory failure or septic shock in 6 patients and abdominal-wall hemorrhage with shock in 1); and among patients assigned to the control group, 6 patients died (the cause of death was Covid-19–related acute respiratory failure or septic shock in 5 and myocardial infarction in 1).
Figure 1. Status of Patients on Day 15.
The primary outcome was clinical status evaluated at 15 days according to a seven-level ordinal scale. The scores on the scale were defined as follows: a score of 1 indicated not hospitalized with no limitations on activities; 2, not hospitalized but with limitations on activities; 3, hospitalized and not receiving supplemental oxygen; 4, hospitalized and receiving supplemental oxygen; 5, hospitalized and receiving oxygen supplementation administered by a high-flow nasal cannula or noninvasive ventilation; 6, hospitalized and receiving mechanical ventilation; and 7, death. The percentages shown have been rounded to whole numbers.
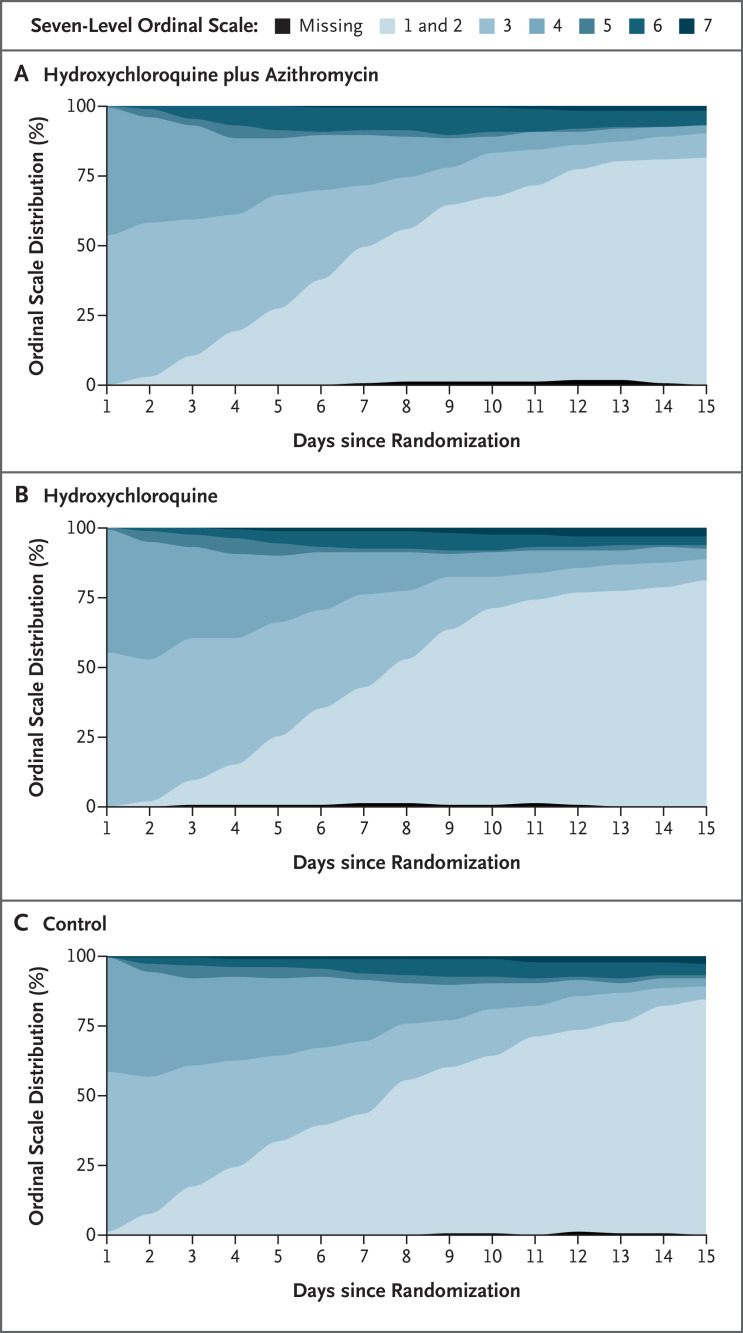
The estimates and standard errors for the mixed-model analysis of the primary outcome are shown in Table S7. The distribution of patients in the ordinal-scale categories over time is shown in Figure 2. In this figure, levels 1 and 2 of the seven-level scale are combined (equivalent to the six-level scale previously described) because data on activity limitation were not available on a daily basis for outpatients.
Figure 2. Distribution of the Ordinal-Scale Results over Time.
Shown is the course of ordinal-scale results as assessed over the time since randomization. However, not all levels of the seven-level scale are shown. Because data on activity limitation were not available on a daily basis for outpatients, levels 1 and 2 (i.e., the levels for patients who were not hospitalized and had no limitations on activities and for those who were not hospitalized but who had limitations on activities, respectively) were combined (equivalent to the six-level scale described in the Methods section). Thus, in this figure, levels 1 and 2 indicate not hospitalized. A total of 36 patients were discharged after a 1-day hospital stay (7 patients who had been assigned to receive hydroxychloroquine plus azithromycin, 8 in the hydroxychloroquine-alone group, and 21 in the control group). Missing data are shown at the bottom of the graphs.
In the intention-to-treat analysis, there was also no significant effect of treatment with either hydroxychloroquine plus azithromycin or hydroxychloroquine alone as compared with the control group (Table S8). Other sensitivity analyses showed similar results (Table S9). The results for the primary outcome were not different across the three prespecified subgroups (Table S10).
Secondary Outcomes
There were no significant differences in any of the secondary outcomes (Table 2). A total of 43 patients received mechanical ventilation during the first 15 days (11.0% of the patients assigned to receive hydroxychloroquine plus azithromycin, 7.5% of those in the hydroxychloroquine-alone group, and 6.9% of those in the control group). The mean (±SD) numbers of days alive and free from respiratory support were 11.1±4.9 in the group assigned to receive hydroxychloroquine plus azithromycin, 11.2±4.9 in the hydroxychloroquine-alone group, and 11.1±4.9 in the control group.
A total of 18 patients died in the hospital during the trial (5 patients assigned to receive hydroxychloroquine plus azithromycin, 7 in the hydroxychloroquine-alone group, and 6 in the control group). There were no significant between-group differences with regard to the secondary outcomes of thromboembolic complications or acute kidney injury within 15 days, either in the prespecified analyses (Table 2) or in post hoc analyses that accounted for the competing risk of death (Table S11). Marginal estimates of effects for the primary and secondary outcomes are shown in Table S12.
Safety
Adverse events in the safety population are reported in Table 3. More adverse events were reported in patients who received hydroxychloroquine plus azithromycin (39.3%) or hydroxychloroquine alone (33.7%) than in those who received azithromycin alone (18.0%) or none of the trial drugs (22.6%). Serious adverse events occurred in nine patients (Table 3). Prolongation of the QTc interval was more common in patients receiving hydroxychloroquine plus azithromycin or hydroxychloroquine alone than in patients in the control group; however, fewer patients in the control group had serial electrocardiographic studies performed during follow-up than did patients in the other two groups. Elevation in liver-enzyme levels was more common in patients receiving hydroxychloroquine plus azithromycin than in the control group. Adverse events in the intention-to-treat population and in the modified intention-to-treat population are shown in Tables S13 and S14, respectively.
Table 3. Adverse Events (Safety Population).*.
| Event | Hydroxychloroquine plus Azithromycin (N=239) |
Hydroxychloroquine (N=199) |
Azithromycin (N=50) |
Neither Hydroxychloroquine nor Azithromycin (N=177) |
Total (N=665) |
|---|---|---|---|---|---|
| Reported serious adverse event, according to classification† | 5 (2.1) | 2 (1.0) | 0 | 2 (1.1) | 9 (1.4) |
| Risk to life — no. (%) | 1 (0.4) | 1 (0.5) | 0 | 0 | 2 (0.3) |
| Extension of hospitalization — no. (%) | 2 (0.8) | 0 | 0 | 1 (0.6) | 3 (0.5) |
| Clinically significant event — no. (%) | 0 | 1 (0.5) | 0 | 1 (0.6) | 2 (0.3) |
| Death — no. (%) | 2 (0.8) | 0 | 0 | 0 | 2 (0.3) |
| Other adverse events | |||||
| Any adverse event — no. (%) | 94 (39.3) | 67 (33.7) | 9 (18.0) | 40 (22.6) | 210 (31.6) |
| QTc interval >480 msec within 7 days — no./total no. (%) | 17/116 (14.7) | 13/89 (14.6) | 0/6 | 1/58 (1.7) | 31/269 (11.5) |
| Arrhythmia — no. (%) | 3 (1.3) | 3 (1.5) | 0 | 1 (0.6) | 7 (1.1) |
| Bradycardia — no. (%) | 2 (0.8) | 1 (0.5) | 0 | 1 (0.6) | 4 (0.6) |
| Supraventricular tachycardia — no. (%) | 1 (0.4) | 2 (1.0) | 0 | 0 | 3 (0.5) |
| Ventricular tachycardia — no. (%) | 0 | 0 | 0 | 0 | 0 |
| Myocardial infarction — no. (%) | 1 (0.4) | 0 | 0 | 0 | 1 (0.2) |
| Abdominal-wall hemorrhage — no. (%) | 1 (0.4) | 0 | 0 | 0 | 1 (0.2) |
| Pulmonary embolism — no. (%) | 2 (0.8) | 0 | 0 | 0 | 2 (0.3) |
| Pneumothorax — no. (%) | 0 | 1 (0.5) | 0 | 0 | 1 (0.2) |
| Bronchospasm — no. (%) | 0 | 0 | 0 | 1 (0.6) | 1 (0.2) |
| Epistaxis — no. (%) | 2 (0.8) | 0 | 0 | 0 | 2 (0.3) |
| Bloodstream infection — no. (%) | 0 | 1 (0.5) | 0 | 0 | 1 (0.2) |
| Itching — no. (%) | 0 | 1 (0.5) | 0 | 0 | 1 (0.2) |
| Nausea — no. (%) | 6 (2.5) | 9 (4.5) | 0 | 2 (1.1) | 17 (2.6) |
| Vomiting — no. (%) | 0 | 0 | 0 | 1 (0.6) | 1 (0.2) |
| Anemia — no. (%)‡ | 23 (9.6) | 14 (7.0) | 5 (10.0) | 11 (6.2) | 53 (8.0) |
| Elevated ALT or AST level — no. (%)§ | 26 (10.9) | 17 (8.5) | 2 (4.0) | 6 (3.4) | 51 (7.7) |
| Hypoglycemia — no. (%)¶ | 0 | 1 (0.5) | 0 | 0 | 1 (0.2) |
| Elevated bilirubin level — no. (%) | 1 (0.4) | 5 (2.5) | 0 | 2 (1.1) | 8 (1.2) |
| Leukopenia — no. (%)‖ | 6 (2.5) | 3 (1.5) | 2 (4.0) | 3 (1.7) | 14 (2.1) |
| Low lymphocyte level — no. (%)** | 29 (12.1) | 17 (8.5) | 2 (4.0) | 16 (9.0) | 64 (9.6) |
| Thrombocytopenia — no. (%)†† | 17 (7.1) | 14 (7.0) | 1 (2.0) | 18 (10.2) | 50 (7.5) |
| Hypoacusis — no. (%) | 0 | 0 | 0 | 0 | 0 |
The safety population included patients according to the medications received (as treated). P<0.001 for the occurrence of any adverse events (pair-wise comparisons: P<0.001 for hydroxychloroquine plus azithromycin vs. control; P=0.01 for hydroxychloroquine alone vs. control). P=0.02 for the occurrence of a corrected QT (QTc) interval of more than 480 msec within 7 days (pair-wise comparisons: P=0.04 for hydroxychloroquine plus azithromycin vs. control; P=0.01 for hydroxychloroquine alone vs. control). P=0.02 for the occurrence of an elevated alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level (pair-wise comparisons: P=0.01 for hydroxychloroquine plus azithromycin vs. control; P=0.09 for hydroxychloroquine alone vs. control).
Among patients who received hydroxychloroquine plus azithromycin, the serious adverse events were pulmonary embolism (in 2 patients), QTc prolongation (in 1; considered to be clinically important), myocardial infarction (in 1), and abdominal-wall hemorrhage (in 1); among patients who received hydroxychloroquine only, the serious adverse events were bradycardia (in 1) and pneumothorax (in 1); and among patients who received neither hydroxychloroquine nor azithromycin, the serious adverse events were bradycardia (in 1) and severe vomiting (in 1). The two serious adverse events leading to death were myocardial infarction and abdominal-wall hemorrhage with shock, both of which occurred in patients receiving hydroxychloroquine and azithromycin.
Anemia was defined as a hemoglobin level lower than 11 g per deciliter for men and lower than 10.5 g per deciliter for women.
An elevated ALT or AST level was defined as a level that was more than 3 times the upper limit of the normal range.
Hypoglycemia was defined as a glucose level below 40 mg per deciliter (2.2 mmol per liter).
Leukopenia was defined as less than 2500 leukocytes per cubic millimeter.
A low lymphocyte level was defined as less than 650 lymphocytes per cubic millimeter.
Thrombocytopenia was defined as a platelet count below 125,000 per cubic millimeter.
Discussion
In this open-label, multicenter, randomized, controlled trial involving hospitalized patients with confirmed mild-to-moderate Covid-19, a 7-day course of hydroxychloroquine either with azithromycin or alone did not result in better clinical outcomes as measured by a seven-level ordinal scale at 15 days. There was also no effect on any of the secondary outcomes. Occurrence of any adverse event, elevation of liver-enzyme levels, and prolongation of the QTc interval was more frequent in patients receiving hydroxychloroquine with azithromycin or hydroxychloroquine alone than in those receiving neither agent.
The prescription of short courses (<28 days) of hydroxychloroquine or chloroquine in the United States increased almost 2000% between March 21, 2019, and March 21, 2020, with a subsequent decrease.16 In Brazil, hydroxychloroquine has been formally recommended for the treatment of Covid-19 by the Ministry of Health since March 25, 2020, for severe cases and since May 20, 2020, for mild cases.5,6 However, no clinical benefit has been observed to date in randomized, controlled trials evaluating hydroxychloroquine for the treatment of Covid-19.9-11 In addition, higher doses of chloroquine (600 mg twice daily for 10 days) were possibly associated with higher mortality.17 Our trial enrolled patients with mild-to-moderate Covid-19 who were receiving no more than 4 liters per minute of supplemental oxygen. Hydroxychloroquine was administered relatively early after symptom onset (median, 7 days), which is earlier than the median time from symptom onset to treatment in a trial of remdesivir treatment for Covid-19.18 Furthermore, the addition of azithromycin did not improve outcomes as had been suggested by observational case series.4
Our trial has several limitations. First, although the point estimate of effect suggests no major difference between the groups with respect to the primary outcome, the trial cannot definitively rule out either a substantial benefit of the trial drugs or a substantial harm. For the comparison between hydroxychloroquine and control, for example, our data are compatible with odds ratios as low as 0.69 and as high as 2.11. Second, the trial was not blinded. Third, despite intense efforts to maintain adherence to the assigned treatments, a lack of medications that were perceived as beneficial by clinicians and patients led to some protocol deviations. Fourth, the use of hydroxychloroquine plus azithromycin was widespread among patients hospitalized with Covid-19 in participating hospitals. The enrollment of patients with no previous use of these medications was challenging, so we decided to enroll patients provided that their previous use since the onset of symptoms was limited to 24 hours. Finally, although the median time from symptom onset to randomization was 7 days, we included patients up to 14 days after the beginning of symptoms; it is conceivable that interventions that may limit viral replication (e.g., hydroxychloroquine) may be more effective earlier in the course of the disease.
In this trial involving hospitalized patients with mild-to-moderate Covid-19, we did not find a significant difference in a 15-day ordinal clinical-status outcome among groups that received standard care, hydroxychloroquine alone, or hydroxychloroquine plus azithromycin. Patients who received hydroxychloroquine, either with azithromycin or alone, had more frequent events of QTc interval prolongation and elevation of liver-enzyme levels than patients who did not receive either agent.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on July 23, 2020, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by institutions participating in the Coalition Covid-19 Brazil and by EMS Pharma, which provided partial funding, the trial drugs, and logistic support.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;323:1574-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO coronavirus disease (COVID-19) dashboard. Geneva: World Health Organization (https://covid19.who.int/).
- 3.Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020;6:16-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020. March 20 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Cloroquina poderá ser usada em casos graves do coronavírus. Brazil: Ministério da Saúde, May 25, 2020 (https://www.saude.gov.br/noticias/agencia-saude/46601-cloroquina-podera-ser-usada-em-casos-graves-do-coronavirus).
- 6.Ministério da Saúde divulga diretrizes para tratamento medicamentoso de pacientes. Brazil: Ministério da Saúde, May 20, 2020 (https://www.saude.gov.br/noticias/agencia-saude/46919-ministerio-da-saude-divulga-diretrizes-para-tratamento-medicamentoso-de-pacientes).
- 7.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 2020;382:2411-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA 2020;323:2493-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. DOI: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19. Press release from the chief investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial. June 5, 2020 (https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19).
- 11.Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 2020;369:m1849-m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208-103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020;382:1787-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol 1971;44:793-797. [DOI] [PubMed] [Google Scholar]
- 15.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing (https://www.R-project.org/).
- 16.Vaduganathan M, van Meijgaard J, Mehra MR, Joseph J, O’Donnell CJ, Warraich HJ. Prescription fill patterns for commonly used drugs during the COVID-19 pandemic in the United States. JAMA 2020;323:2524-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 2020;3(4):e208857-e208857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — preliminary report. N Engl J Med. DOI: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.