Abstract
Background
Current evidence on the benefits of different anastomotic techniques (hand‐sewn (HS), circular stapled (CS), triangulating stapled (TS) or linear stapled/semimechanical (LSSM) techniques) after oesophagectomy is conflicting. The aim of this study was to evaluate the evidence for the techniques for oesophagogastric anastomosis and their impact on perioperative outcomes.
Methods
This was a systematic review and network meta‐analysis. PubMed, EMBASE and Cochrane Library databases were searched systematically for randomized and non‐randomized studies reporting techniques for the oesophagogastric anastomosis. Network meta‐analysis of postoperative anastomotic leaks and strictures was performed.
Results
Of 4192 articles screened, 15 randomized and 22 non‐randomized studies comprising 8618 patients were included. LSSM (odds ratio (OR) 0·50, 95 per cent c.i. 0·33 to 0·74; P = 0·001) and CS (OR 0·68, 0·48 to 0·95; P = 0·027) anastomoses were associated with lower anastomotic leak rates than HS anastomoses. LSSM anastomoses were associated with lower stricture rates than HS anastomoses (OR 0·32, 0·19 to 0·54; P < 0·001).
Conclusion
LSSM anastomoses after oesophagectomy are superior with regard to anastomotic leak and stricture rates.
Stapled anastomoses were associated with lower anastomotic leak rates than hand‐sewn anastomoses following oesophagectomy. Furthermore, linear stapled/semimechanical (LSSM) techniques were associated with lower rates of anastomotic stricture than circular stapled or hand‐sewn anastomoses. Current evidence therefore suggests superiority for the LSSM technique for oesophagogastric anastomosis.

Linear stapler techniques superior
Antecedentes
La evidencia actual sobre los beneficios de diferentes técnicas de anastomosis, incluyen la técnica manual (hand‐sewn, HS), la sutura mecánica circular (circular stapled, CS), la sutura mecánica triangular (triangular stapler, TS) o la sutura mecánica lineal/semi‐mecánica (linear stapler/semi‐mechanical., LSSM) tras una esofaguectomía es contradictoria. El objetivo de este estudio fue evaluar la evidencia referente a las técnicas de anastomosis esofagogástrica (oesophagogastric, OG) y su impacto sobre los resultados perioperatorios.
Métodos
Se efectuó una revisión sistemática y metaanálisis en red, basados en una búsqueda sistemática en las bases de datos PubMed, EMBASE y Cochrane Library de estudios aleatorizados y no aleatorizados que describiese técnicas para la anastomosis OG. Se llevó a cabo un metaanálisis en red para los resultados de fugas anastomóticas y estenosis postoperatorias.
Resultados
De los 4.192 artículos revisados, se incluyeron 15 estudios aleatorizados y 22 no aleatorizados con un total de 8.618 pacientes. Las anastomosis con LSSM (razón de oportunidades, odds ratio, OR 0,49, i.c. del 95%: 0,33‐0,74, P = 0,001) y las anastomosis con CS (OR 0,68, i.c. del 95%: 0.48‐0,95, P = 0,027) se asociaron con tasas de fugas anastomóticas más bajas que las anastomosis con HS. Las anastomosis con LSSM se asociaron con unas tasas más bajas de estenosis (OR 0,15, i.c. del 95%: 0,08‐0,28, P < 0,001), frente a las anastomosis con TS y HS.
Conclusiones
Las anastomosis con LSSM después de esofaguectomía son superiores en relación a las tasas de fugas anastomóticas y estenosis.
Introduction
Despite improvements in perioperative care over recent decades, which have led to improved patient selection, reduced operative morbidity and mortality, and prolonged postoperative survival 1 , 2 , anastomotic leak remains the most serious technical complication after oesophagectomy. Patients who experience anastomotic leakage suffer high morbidity, have a high postoperative mortality rate, ranging between 21 and 35 per cent, incur high hospital costs 3 , 4 , 5 , 6 , 7 . They also suffer long‐term effects, such as an increased risk of anastomotic stricture and poorer long‐term survival, compared with patients who recover uneventfully 8 . Many perioperative factors are thought to be responsible for anastomotic integrity after oesophagectomy, such as surgical approach, tumour location (cervical or thoracic) and technique of oesophagogastric anastomosis 9 .
Several meta‐analyses 10 , 11 , 12 , 13 , 14 have compared stapled and hand‐sewn anastomotic techniques. These studies have included both randomized and non‐randomized trials, and have found no significant differences in anastomotic leak rates between the two anastomotic techniques. Most individual comparative studies, however, chose either to look at two types of stapled anastomosis or to group all stapled anastomoses together. There is a paucity of literature comparing all anastomotic techniques described in this study. Anastomotic techniques can include hand‐sewn (HS), circular stapled (CS), linear stapled/semimechanical (LSSM) 15 , 16 and triangulating stapled (TS) 17 , 18 . There are encouraging reports of low anastomotic leak rates when linear stapled techniques are employed 19 . The strength of performing a network meta‐analysis is that it allows the evaluation of treatments that have not been compared directly (for example, comparison of B versus C, using data from studies comparing A versus B and A versus C). Network meta‐analysis ranks multiple treatments based on their efficacy, and pools together direct and indirect evidence within mixed comparisons, improving the precision of estimates 20 , 21 .
The aim of this systematic review was to evaluate current evidence and perform a network meta‐analysis to identify techniques associated with superior perioperative outcomes in patients undergoing oesophagectomy for oesophageal cancer.
Methods
This was a systematic review and network meta‐analysis. The study was registered with the PROSPERO database (Registration CRD42018106086) and reported according to the PRISMA guidelines 22 .
Search strategy
A systematic search of PubMed, EMBASE and Cochrane Library databases was conducted by two independent investigators on 22 April 2019, to include studies up to 31 March 2019. Search terms included ‘oesophageal cancer’ or ‘esophageal cancer’ or ‘gastro‐oesophageal cancer’, and ‘anastomosis’ or ‘hand‐sewn’ or ‘linear stapler’ or ‘circular stapler’ individually or in combination (Table S1 , supporting information). The ‘related articles’ function was used to broaden the search, and all citations were considered for relevance. A manual search of reference lists in recent reviews was also undertaken. After excluding duplicates, two researchers independently reviewed the titles and abstracts of studies identified by the literature search. If a study was considered to be potentially relevant to the research question, the full publication was reviewed. Reference lists of all included studies were hand‐searched to identify other potentially relevant studies. Any areas of disagreement between the two primary researchers were resolved through discussion with all authors.
Inclusion and exclusion criteria
Inclusion criteria included studies reporting the comparison of anastomotic technique (by any method) in patients with oesophageal cancer who underwent oesophagectomy, published in the English language. Exclusion criteria included conference abstracts, review articles, case reports (fewer than 5 patients), and publications with mixed populations, in which the outcomes of patients with either benign disease or cancer at another site could not be separated from those of patients with oesophageal cancer.
Study outcomes
The primary outcome measures were anastomotic complications, including anastomotic leak or stricture 23 . Secondary outcome measures were surgery‐specific complications (pulmonary, cardiac) and death (30‐day and in‐hospital mortality).
Data extraction
One researcher extracted data on study characteristics (author, year of publication, country of origin, study design, patient number), patient demographics (age, sex), tumour stage (AJCC T category and AJCC stage), method and details of anastomotic technique, and reported clinical outcomes.
Definitions
Oesophageal cancer was defined as a malignancy of any portion of the oesophagus. Anastomotic technique was defined as any method of oesophagogastric anastomosis including HS, CS, LSSM 15 , 16 and TS 17 , 18 anastomoses. These anastomotic techniques may be employed in either the thoracic or the cervical phase of the operation. Subtle variations of the LSSM technique were described. The nomenclature includes a (modified) Collard technique 24 and a side‐to‐side semimechanical technique, which both refer to a combination of a linear stapled and hand‐sewn technique.
Assessment of methodological quality
Methodological quality and standard of outcome reporting within included studies were assessed by two independent researchers. Methodological quality was assessed formally using the Newcastle–Ottawa Scale (NOS) 25 , 26 for cohort studies and the Cochrane risk‐of‐bias tool for RCTs.
Statistical analysis
Dichotomous outcomes were compared between anastomotic formation techniques using odds ratios (ORs), produced using random‐effects DerSimonian–Laird meta‐analytical models. Both randomized and non‐randomized studies were pooled into a network meta‐analysis comparing the above anastomotic formation techniques. Sensitivity analyses were performed for type of study (RCTs only, RCTs and prospective cohort studies (PCSs) and all RCT and cohort studies with a NOS score of 8 or above), study year (2005–2018), and level of anastomosis (cervical versus thoracic). For each outcome, graphical representations of treatments (nodes) and comparisons (lines) were mapped. Network maps were then analysed for closed loops to be entered into network analyses.
Networks were then examined for the presence of inconsistency, allowing for comparisons between direct and indirect treatment effects. Initially, this was assessed by checking for overall inconsistency throughout the entire network. A further check was then performed by fitting node side‐splitting models, to identify loop inconsistency, within all three‐way treatment comparison loops, as described by Dias and colleagues 27 . When P values were greater than 0·050, representing acceptance of the null hypothesis, consistency was assumed and networks were entered into consistency modelling. Consistency modelling utilized a restricted maximum likelihood model, generating network forest plots. Heterogeneity was examined by calculating the value of τ 2 . Hand‐sewn anastomosis was used as the common reference treatment for all comparisons. These were supplemented with interval plots of pooled effect estimates.
Anastomotic techniques were then ranked using the P‐score provided by the netmeta package (RStudio® 3.2.1, Boston, Massachusetts, USA; https://CRAN.R‐project.org/package=netmeta). The surface under the cumulative ranking areas for all outcomes assessed the probability of the superiority of each treatment 28 , 29 , 30 . The probability of ranking of a treatment (that a treatment ranks as the best treatment, second best treatment, third best treatment) for each outcome of interest was calculated. A probability of ranking below 90 per cent was not considered to be high enough to be confidently reported as the correct ranking position of a surgical technique for that outcome of interest 27 , 31 . Statistical significance was considered when P < 0·050. Statistical analyses were performed using R Foundation statistical software (RStudio® 3.2.1).
Results
Of 4192 studies screened, 40 studies comparing different anastomotic techniques were eligible (Fig. 1 ). Details of these studies 15 , 16 , 17 , 18 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 are shown in Table 1 . The majority of included studies reported on open oesophagectomy. Three studies 57 , 60 , 68 compared different types of hand‐sewn layers and were therefore excluded from meta‐analysis. The remaining 37 studies comprised 8571 patients. Fifteen RCTs, five non‐randomized prospective and 17 retrospective studies were included. Within the non‐randomized studies, the mean NOS score was 7 (range 5–9) (Table 2 ). In RCTs, reporting of the blinding of participants and outcomes was unclear, but the risk of bias was mainly low for other domains.
Figure 1.

PRISMA diagram for the review
Table 1.
Characteristics of included studies
| Reference | Study design | Intervention | No. of patients | % of men | Mean age (years) | Neoadjuvant therapy (%) | Tumour location | Pathology |
|---|---|---|---|---|---|---|---|---|
| Perrachia et al. 32 | PCS | CS versus HS | 214 versus 28 | 86 | 60 | n.r. | n.r. | Mixed |
| Rostas et al. 33 | PCS | CS versus HS | 60 versus 82 | 82 | n.r. | 48 | Upper 1, middle 24, lower 117 | AC 110, SCC 31, other 1 |
| McManus et al. 34 | RCS | CS versus HS | 99 versus 122 | n.r. | n.r. | n.r. | n.r. | n.r. |
| Lee et al. 35 | RCS | CS versus HS | 141 versus 211 | 85 | 63 | n.r. | n.r. | SCC |
| Honkoop et al. 36 | RCS | CS versus HS | 154 versus 114 | 75 | 61 | n.r. | Any | AC 161, SCC 92 |
| Klink et al. 37 | RCS | CS versus HS | 36 versus 36 | 89 | 62 | 51 | n.r. | AC, SCC |
| West of Scotland and Highland Anastomosis Study Group 38 | RCT | CS versus HS | 27 versus 25 | n.r. | 64 | n.r. | n.r. | n.r. |
| Craig et al. 39 | RCT | CS versus HS | 50 versus 50 | 61 | 65 | n.r. | Lower 100 | AC, SCC |
| Valverde et al. 40 | RCT | CS versus HS | 78 versus 74 | 91 | 50 | n.r. | Middle 81, lower 71 | AC, SCC |
| Law et al. 41 | RCT | CS versus HS | 61 versus 61 | 88 | 64 | n.r. | Middle 99, lower 21, double 2 | SCC |
| Hsu et al. 42 | RCT | CS versus HS | 31 versus 32 | 90 | 62 | 52 | Upper 16, middle 26, lower 21 | SCC |
| Okuyama et al. 43 | RCT | CS versus HS | 14 versus 18 | 91 | 64 | 0 | Middle 23, lower 9 | SCC 30, undifferentiated 2 |
| Luechakiettisak et al. 44 | RCT | CS versus HS | 58 versus 59 | 84 | 63 | n.r. | Middle 57, lower 60 | SCC |
| Zhang et al. 45 | RCT | CS versus HS | 272 versus 244 | 58 | 60 | 0 | n.r. | n.r. |
| Cayi et al. 46 | RCT | CS versus HS | 102 versus 125 | 75 | 58 | 0 | Upper/middle | n.r. |
| Liu et al. 47 | RCT | CS versus HS | 241 versus 237 | 75 | 62 | 13 | Upper 82, middle 283, lower 113 | n.r. |
| Zhu et al. 48 | RCS | CS versus HS versus LHS | 170 versus 69 versus 1024 | 80 | 64 | NR | n.r. | Mixed |
| Xu et al. 49 | PCS | CS versus LSSM versus HS | 68 versus 166 versus 59 | 86 | 60 | 0 | Upper 5, middle 198, lower 19 | AC, SCC |
| Blackmon et al. 50 | RCS | CS versus LSSM versus HS | 147 versus 44 versus 23 | n.r. | n.r. | n.r. | n.r. | AC, SCC |
| Liu et al. 51 | RCS | CS versus LSSM versus HS | 233 versus 147 versus 78 | 81 | 63 | 52 | Lower/GOJ | AC 345, SCC 105 |
| Wang et al. 15 | RCT | CS versus LSSM versus HS | 47 versus 45 versus 52 | 56 | 60 | 0 | Middle 81, lower 18 | AC 12, SCC 131, undifferentiated 1 |
| Price et al. 52 | PCS | CS versus LSSM versus HS versus MC* | 48 versus 260 versus 57 | 83 | 64 | 57 | n.r. | AC, SCC |
| Li et al. 17 | RCS | CS versus TS | 51 versus 33 | 81 | 61 | 10 | Upper 9, middle 57, lower 18 | AC, SCC |
| Hayata et al. 18 | RCT | CS versus TS | 49 versus 51 | 77 | 67 | 57 | Upper 6, middle 60, lower 34 | AC, SCC |
| Furukawa et al. 53 | PCS | CS versus TS versus HS | 8 versus 11 versus 12 | n.r. | n.r. | n.r. | n.r. | n.r. |
| Wang et al. 54 | RCS | CS versus TS versus HS | 164 versus 34 versus 192 | 56 | NR | 7 | Upper 84, middle 215, lower 91 | n.r. |
| Zieren et al. 55 | RCT | OLHS versus DLHS | 107† | 79 | 58 | 34 | n.r. | SCC |
| Casson et al. 56 | RCS | LSSM versus HS | 38 versus 53 | 80 | 63 | n.r. | n.r. | AC |
| Behzadi et al. 57 | RCS | LSSM versus HS | 75 versus 205 | 84 | 65 | n.r. | n.r. | n.r. |
| Ercan et al. 58 | RCS | LSSM versus HS | 85 versus 85 | 90 | NR | 41 | Upper 5, middle 4, lower 161 | AC, SCC |
| Kondra et al. 59 | RCS | LSSM versus HS | 79 versus 89 | 85 | 64 | 24 | Middle 15, lower 68, GOJ 85 | AC, SCC |
| Harustiak et al. 60 | RCS | LSSM versus HS | 281 versus 134 | 88 | 60 | 56 | n.r. | AC, SCC |
| Mishra et al. 61 | RCS | LSSM versus HS | 74 versus 66 | 56 | 53 | 0 | Upper 2, middle 61, lower 62, GOJ 15 | AC, SCC |
| Sugimura et al. 62 | RCS | LSSM versus HS | 225 versus 173 | 80 | n.r. | 74 | Upper 41, middle 229, lower 128 | AC 13, SCC 381 |
| Laterza et al. 63 | RCT | LSSM versus HS | 20 versus 21 | 17 | 51 | n.r. | Upper 10, middle 24, lower 5 | AC, SCC |
| Walther et al. 64 | RCT | LSSM versus HS | 42 versus 41 | 69 | 67 | 0 | Upper 4, middle 29, lower 40 | AC, SCC |
| Saluja et al. 16 | RCT | LSSM versus HS | 87 versus 87 | 66 | 51 | 61 | Middle 84, lower 80, unknown 10 | AC, SCC |
| Singh et al. 65 | RCS | LSSM versus TS versus HS | 16 versus 43 versus 34 | n.r. | n.r. | n.r. | n.r. | Mixed |
| Sokouti et al. 66 | RCS | TLHS versus OLHS | 228† | 59 | 60 | n.r. | Upper 13, middle 100, lower 97 | Mixed |
| Sun et al. 67 | RCS | TLHS versus DLHS | 339† | 61 | 61 | n.r. | Upper 98, middle 114, lower 127 | Mixed |
Combined longitudinal and transverse anastomosis.
Comparison of two hand‐sewn anastomosis techniques. PCS, prospective cohort study; CS, circular stapled; HS, hand‐sewn; n.r., not reported; AC, adenocarcinoma; SCC, squamous cell carcinoma; RCS, retrospective cohort study; LHS, layered hand‐sewn; LSSM, linear stapled/semimechanical; GOJ, gastro‐oesophageal junction; MC, modified Collard; TS, triangulating stapled; OLHS, one layer hand‐sewn; DLHS, double layer hand‐sewn; TLHS, triple layer hand‐sewn.
Table 2.
Assessment of risk of bias in RCTs and cohort studies
| Reference | Study design | Adequate sequence generation | Allocation concealment | Blinding of participants | Blinding of outcomes | Incomplete outcome data | Selective outcome reporting | Free from other bias | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| RCTs | |||||||||
| West of Scotland and Highland Anastomosis Study Group 38 | RCT | Low | Low | Unclear | Unclear | Unclear | Low | Low | – |
| Craig et al. 39 | RCT | High | High | Unclear | Unclear | Unclear | Low | Low | – |
| Valverde et al. 40 | RCT | Low | Unclear | Unclear | Unclear | Unclear | Low | Low | – |
| Law et al. 41 | RCT | Low | Low | Unclear | Unclear | Low | Low | Low | – |
| Hsu et al. 42 | RCT | High | High | Unclear | Unclear | Low | Low | Low | – |
| Okuyama et al. 43 | RCT | Low | Unclear | Unclear | Unclear | Unclear | Low | High | – |
| Luechakiettisak et al. 44 | RCT | High | High | Unclear | Unclear | Low | Low | Low | – |
| Zhang et al. 45 | RCT | High | High | Unclear | Unclear | Unclear | Low | Low | – |
| Cayi et al. 46 | RCT | Uncertain | Unclear | Unclear | Unclear | Unclear | Low | High | – |
| Liu et al. 47 | RCT | Low | Low | Unclear | Low | Unclear | Low | Low | – |
| Wang et al. 15 | RCT | High | High | Unclear | Unclear | Low | Low | Low | – |
| Hayata et al. 18 | RCT | Uncertain | Low | Unclear | Unclear | Low | Low | High | – |
| Zieren et al. 55 | RCT | Low | Low | Unclear | Unclear | Unclear | Unclear | Low | – |
| Laterza et al. 63 | RCT | Low | Unclear | Unclear | Unclear | Low | Low | Low | – |
| Walther et al. 64 | RCT | Low | Unclear | Unclear | Unclear | Low | Low | High | – |
| Saluja et al. 16 | RCT | Low | Unclear | Unclear | Unclear | Low | Low | Low | – |
| Cohort studies | |||||||||
| Perrachia et al. 32 | PCS | – | – | – | – | – | – | – | 7 |
| Rostas et al. 33 | PCS | – | – | – | – | – | – | – | 7 |
| McManus et al. 34 | RCS | – | – | – | – | – | – | – | 6 |
| Lee et al. 35 | RCS | – | – | – | – | – | – | – | 6 |
| Honkoop et al. 36 | RCS | – | – | – | – | – | – | – | 9 |
| Klink et al. 37 | RCS | – | – | – | – | – | – | – | 7 |
| Zhu et al. 48 | RCS | – | – | – | – | – | – | – | 6 |
| Xu et al. 49 | PCS | – | – | – | – | – | – | – | 9 |
| Blackmon et al. 50 | RCS | – | – | – | – | – | – | – | 7 |
| Liu et al. 51 | RCS | – | – | – | – | – | – | – | 7 |
| Price et al. 52 | PCS | – | – | – | – | – | – | – | 8 |
| Li et al. 17 | RCS | – | – | – | – | – | – | – | 6 |
| Furukawa et al. 53 | PCS | – | – | – | – | – | – | – | 8 |
| Wang et al. 54 | RCS | – | – | – | – | – | – | – | 7 |
| Casson et al. 56 | RCS | – | – | – | – | – | – | – | 7 |
| Behzadi et al. 57 | RCS | – | – | – | – | – | – | – | 6 |
| Ercan et al. 58 | RCS | – | – | – | – | – | – | – | n.a. |
| Kondra et al. 59 | RCS | – | – | – | – | – | – | – | 8 |
| Harustiak et al. 60 | RCS | – | – | – | – | – | – | – | 9 |
| Mishra et al. 61 | RCS | – | – | – | – | – | – | – | 8 |
| Sugimura et al. 62 | RCS | – | – | – | – | – | – | – | 8 |
| Singh et al. 65 | RCS | – | – | – | – | – | – | – | 5 |
| Sokouti et al. 66 | RCS | – | – | – | – | – | – | – | 7 |
| Sun et al. 67 | RCS | – | – | – | – | – | – | – | 7 |
Level of bias was determined as: low, indicating a low risk of bias; unclear, indicating an uncertain risk of bias, and high, indicating a high risk of bias. NOS, Newcastle–Ottawa Scale; PCS, prospective cohort study; RCS, retrospective cohort study; n.a., not applicable.
The anastomotic techniques analysed most commonly were HS (35 studies) and CS (26), with these two techniques being directly compared in 24 studies. LSSM was analysed in 16 studies, of which ten included comparisons with HS, and a further five studies reported comparisons with both HS and CS. In addition, five studies analysed TS anastomoses. One study 52 described a linear stapled technique without the use of a hand‐sewn component. A summary of technical details of anastomosis is presented in Table S2 (supporting information).
Network meta‐analysis
Network meta‐analyses for the two primary outcomes, anastomotic leak and stricture, were conducted comparing all anastomotic techniques described in two or more studies including HS, TS, LSSM and CS. Initially, visual representations of the network of studies used for each outcome were generated (Figs 2a and 3a ). For anastomotic leak, 36 studies were included, consisting of 2623 patients with a CS anastomosis, 1876 with a LSSM anastomosis, 3922 receiving a HS anastomosis, and 197 who had a TS anastomosis. For anastomotic stricture formation, 27 studies were included, comprising 1775 patients with a CS anastomosis, 1602 with a LSSM anastomosis, 3224 with a HS anastomosis, and 197 receiving a TS anastomosis.
Figure 2.

Network map and forest plot for anastomotic leak a Network map. b Forest plot. A DerSimonian–Laird random‐effects model was used for meta‐analysis. Odds ratios are shown with 95 per cent confidence intervals. CS, circular stapled; HS, hand‐sewn; TS, triangulating stapled; LSSM, linear stapled/semimechanical.
Figure 3.
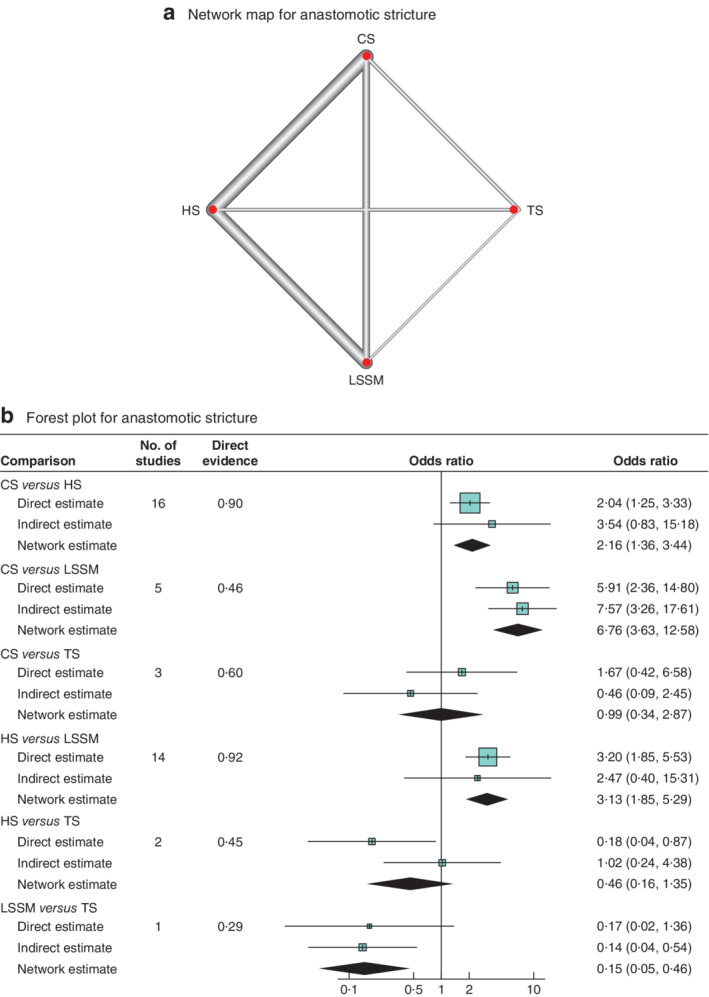
Network map and forest plot for anastomotic stricture a Network map. b Forest plot. A DerSimonian–Laird random‐effects model was used for meta‐analysis. Odds ratios are shown with 95 per cent confidence intervals. CS, circular stapled; HS, hand‐sewn; TS, triangulating stapled; LSSM, linear stapled/semimechanical.
Anastomotic leak
After overall inconsistency testing (P = 0·958) and fitting of node side‐splitting models (P values: 0·565, 0·972, 0·916, 0·715, 0·743, 0·617), overall and local consistency was assumed. Upon generation of network forest and interval plots (Fig. 2b and Table 3 ), CS (OR 0·68; P = 0·027) and LSSM (OR 0·50; P = 0·001) anastomoses were associated with lower anastomotic leak rates than HS anastomosis. Anastomotic leak rates were no different for CS and LSSM anastomoses (OR 1·37; P = 0·198). There were no significant differences in leak rates between TS and HS. Overall, LSSM was ranked the best technique regarding anastomotic leakage with high probability, followed by CS and TS ( Table S3 , supporting information).
Table 3.
Results of network meta‐analysis of all techniques for anastomotic leak and benign anastomotic stricture formation, for overall studies and subgroup analyses
| Anastomotic leak | Anastomotic stricture | |||||
|---|---|---|---|---|---|---|
| No. of studies | Odds ratio | P | No. of studies | Odds ratio | P | |
| All studies | ||||||
| CS versus HS | 24 | 0·68 (0·48, 0·95) | 0·027 | 16 | 2·16 (1·36, 3·44) | 0·001 |
| LSSM versus CS | 6 | 0·73 (0·45, 1·18) | 0·198 | 5 | 0·15 (0·08, 0·28) | < 0·001 |
| CS versus TS | 3 | 0·78 (0·26, 2·30) | 0·668 | 3 | 0·99 (0·34, 2·87) | 0·987 |
| LSSM versus HS | 16 | 0·50 (0·33, 0·74) | 0·001 | 14 | 0·32 (0·19, 0·54) | < 0·001 |
| HS versus TS | 2 | 1·14 (0·38, 3·43) | 0·827 | 2 | 0·46 (0·16, 1·35) | 0·154 |
| LSSM versus TS | 1 | 0·57 (0·18, 1·76) | 0·339 | 1 | 0·15 (0·05, 0·46) | 0·001 |
| Level of anastomosis | ||||||
| Cervical | ||||||
| CS versus HS | 6 | 1·04 (0·49, 2·20) | 0·925 | 4 | 2·86 (1·11, 7·37) | 0·029 |
| LSSM versus CS | 1 | 0·50 (0·19, 1·30) | 0·153 | 1 | 0·10 (0·03, 0·32) | < 0·001 |
| CS versus TS | 2 | 1·18 (0·29, 4·83) | 0·829 | 2 | 1·19 (0·31, 4·60) | 0·812 |
| LSSM versus HS | 8 | 0·52 (0·26, 1·02) | 0·058 | 8 | 0·30 (0·13, 0·65) | 0·002 |
| HS versus TS | 1 | 1·13 (0·27, 4·78) | 0·877 | 1 | 0·42 (0·10, 1·66) | 0·228 |
| LSSM versus TS | 1 | 0·59 (0·13, 2·64) | 0·502 | 1 | 0·12 (0·03, 0·53) | 0·004 |
| Thoracic | ||||||
| CS versus HS | 7 | 0·56 (0·38, 0·83) | 0·004 | 6 | 1·95 (0·66, 5·78) | 0·23 |
| LSSM versus CS | 3 | 0·75 (0·42, 1·37) | 0·353 | 3 | 0·14 (0·03, 0·63) | 0·01 |
| CS versus TS | 1 | 0·44 (0·07, 2·73) | 0·386 | 1 | 0·97 (0·06, 15·85) | 0·985 |
| LSSM versus HS | 4 | 0·42 (0·26, 0·69) | 0·001 | 4 | 0·28 (0·07, 1·12) | 0·072 |
| HS versus TS | 1 | 0·78 (0·12, 4·93) | 0·805 | 1 | 0·50 (0·03, 8·42) | 0·643 |
| LSSM versus TS | 0 | 0·33 (0·05, 2·21) | 0·254 | 0 | 0·14 (0·01, 2·99) | 0·177 |
| Study type | ||||||
| RCT only | ||||||
| CS versus HS | 11 | 0·72 (0·42, 1·24) | 0·237 | 7 | 1·92 (0·99, 3·72) | 0·053 |
| LSSM versus CS | 1 | 1·52 (0·49, 4·76) | 0·484 | 1 | 0·20 (0·04, 1·02) | 0·053 |
| CS versus TS | 1 | 5·68 (0·52, 61·94] | 0·155 | 1 | 0·91 (0·16, 5·04) | 0·922 |
| LSSM versus HS | 4 | 1·09 (0·40, 2·94) | 0·880 | 3 | 0·39 (0·09, 1·75) | 0·224 |
| HS versus TS | 0 | 7·92 (0·68, 91·83) | 0·098 | 0 | 0·47 (0·08, 2·97) | 0·421 |
| LSSM versus TS | 0 | 8·59 (0·61, 120·59) | 0·111 | 0 | 0·19 (0·02, 1·95) | 0·156 |
| RCT + cohort studies (NOS score ≥ 8) | ||||||
| CS versus HS | 15 | 0·68 (0·46, 1·01) | 0·054 | 11 | 1·91 (1·18, 3·10) | 0·009 |
| LSSM versus CS | 3 | 0·81 (0·48, 1·39) | 0·453 | 3 | 0·14 (0·07, 0·28) | < 0·001 |
| CS versus TS | 2 | 1·31 (0·30, 5·70) | 0·732 | 2 | 0·95 (0·26, 3·42) | 0·943 |
| LSSM versus HS | 10 | 0·56 (0·38, 0·82) | 0·003 | 9 | 0·28 (0·16, 0·48) | < 0·001 |
| HS versus TS | 1 | 1·92 (0·43, 8·60) | 0·400 | 1 | 0·50 (0·13, 1·91) | 0·317 |
| LSSM versus TS | 0 | 1·07 (0·23, 4·99) | 0·937 | 0 | 0·14 (0·03, 0·57) | 0·009 |
| Study year (2005–2018) | ||||||
| CS versus HS | 15 | 0·70 (0·45, 1·10) | 0·118 | 12 | 1·77 (1·01, 3·11) | 0·046 |
| LSSM versus CS | 6 | 0·70 (0·41, 1·20) | 0·197 | 5 | 0·15 (0·07, 0·30) | < 0·001 |
| CS versus TS | 3 | 1·86 (0·49, 7·02) | 0·367 | 3 | 1·41 (0·36, 5·46) | 0·633 |
| LSSM versus HS | 12 | 0·50 (0·32, 0·76) | 0·002 | 11 | 0·26 (0·14, 0·47) | < 0·001 |
| HS versus TS | 1 | 2·65 (0·67, 10·47) | 0·165 | 1 | 0·80 (0·19, 3·35) | 0·773 |
| LSSM versus TS | 0 | 1·31 (0·32, 5·38) | 0·721 | 0 | 0·21 (0·05, 0·93) | 0·036 |
Values in parentheses are percentages. CS, circular stapled; HS, hand‐sewn; LSSM, linear stapled/semimechanical; TS, triangulating stapled; NOS, Newcastle–Ottawa Scale.
Sensitivity analysis of anastomotic leak
For cervical anastomosis, no technique was superior with regard to anastomotic leakage (Table 3 ). For thoracic anastomosis, LSSM (OR 0·42) and CS (OR 0·56) anastomoses were superior to HS with regard to anastomotic leakage. There were no differences in anastomotic leaks between CS and LSSM (OR 1·33). In the analyses split by study type, only LSSM anastomoses (OR 0·56) had a lower anastomotic leak rate than HS in the ‘RCT and cohort studies with a NOS score of 8 or above’ subgroup only. In the subgroup analysis of RCTs there were no statistically significant differences. For studies published in 2005–2018, only LSSM (OR 0·50) was superior to HS anastomosis.
Anastomotic stricture
After overall inconsistency testing (P = 0·425) and fitting of node side‐splitting models (P values: 0·995, 0·124, 0·516, 0·413, 0·782), overall and local consistency was assumed. Upon generation of network forest and interval plots (Fig. 3b and Table 3 ), LSSM anastomosis was found to be superior to CS (OR 0·15; P < 0·001), HS (OR 0·32; P < 0·001) and TS (OR 0·15; P = 0·001) anastomoses respectively. CS was inferior to HS (OR 2·16; P = 0·001). LSSM was ranked the best technique with high probability followed by HS, TS and CS anastomoses respectively.
Sensitivity analysis in anastomotic stricture
For cervical anastomosis, LSSM had lower rates of anastomotic stricture than CS (OR 0·10; P < 0·001), HS (OR 0·30; P = 0·002) and TS (OR 0·12; P = 0·004) anastomoses (Table 3 ). CS had higher rates of anastomotic stricture than HS (OR 2·86; P = 0·029). For thoracic anastomosis, LSSM had lower rates of anastomotic stricture than CS anastomosis (OR 0·14; P = 0·010). There were no significant differences in anastomotic stricture between CS and HS.
By study type, no significant differences were noted in the RCT‐only sensitivity analysis. LSSM was superior to TS, CS and HS for anastomotic strictures in the ‘RCT and cohort studies with a NOS score of 8 or above’ subgroup only. CS had significantly higher rates of stricture than LSSM and HS. For studies published in 2005–2018, LSSM was superior to CS (OR 0·15; P < 0·001), HS (OR 0·26; P < 0·001) and TS (OR 0·21; P = 0·036) anastomoses (Table 3 ).
Intraoperative outcomes
Duration of surgery was reported in 16 studies. There were no differences in operating times between techniques ( Table S4 , supporting information). LSSM was ranked first for the entire cohort and for cervical anastomosis only. Blood loss was reported in 11 studies. LSSM had significantly lower blood loss than HS (mean difference 24 ml; P = 0·024). LSSM was ranked first for the entire cohort and for cervical anastomosis only. There were insufficient studies in the thoracic anastomosis subgroup only for analysis.
Other postoperative complications
Cardiac complications rates were reported in ten studies (CS versus HS, 7 studies; LSSM versus HS, 2; CS versus TS, 1) ( Table S4 , supporting information). There were no significant differences in cardiac complications between the different techniques. LSSM was ranked first for the overall and cervical anastomosis only subgroup. There were not enough studies in the thoracic anastomosis only subgroup for analysis.
Pulmonary complications were reported in 12 studies (CS versus HS, 8 studies; LSSM versus HS, 2; CS versus TS, 2). There were no significant differences in pulmonary complications between the different techniques. TS was ranked first in the overall group. LSSM was ranked first in the cervical anastomosis only subgroup. There were not enough studies in the thoracic anastomosis only subgroup for analysis.
Thirty‐day mortality was reported in 11 studies (CS versus HS, 7 studies; LSSM versus HS, 3; CS versus TS, 1). LSSM was associated with lower rates of 30‐day mortality than HS (OR 0·33; P = 0·016) and CS (OR 0·18; P = 0·002) anastomoses. CS was not associated with higher mortality rates than HS anastomosis. LSSM was ranked the best technique with high probability, followed by TS. For cervical anastomosis, LSSM was ranked first. There were not enough studies in the thoracic anastomosis only subgroup for analysis.
In‐hospital mortality was reported in several pairwise comparisons: CS versus HS, 16 studies; LSSM versus HS, five; CS versus TS, three; LSSM versus HS, four studies. LSSM was associated with lower rates of in‐hospital mortality than HS (OR 0·32; P < 0·001), CS (OR 0·15; P < 0·001) and TS (OR 0·15; P = 0·001) anastomoses. CS was associated with higher in‐hospital mortality rates than HS anastomosis. LSSM was ranked the best technique with high probability, followed by HS. For cervical anastomosis, HS was ranked first. There were not enough studies in the thoracic anastomosis only subgroup for analysis.
Discussion
This study demonstrates that stapled anastomoses, specifically using an LSSM technique, are associated with lower anastomotic leak rates than HS anastomoses following oesophagectomy. The LSSM technique was associated with a lower rate of anastomotic stricture than CS, TS and HS anastomoses. This effect was consistent across the majority of subgroups in sensitivity analyses. LSSM anastomoses were associated with lower rates of 30‐day mortality. Overall, the results indicate superiority of the LSSM technique for oesophagogastric anastomosis following oesophagectomy.
Previous systematic reviews and meta‐analyses 10 , 11 , 12 , 13 , 14 have examined the impact of stapled versus HS anastomoses following oesophagectomy (Table S5, supporting information). These, however, did not distinguish between CS and LSSM stapling techniques. Honda and colleagues 10 did not include LSSM anastomoses, looking only at the differences between HS and CS anastomoses. They reported no differences in anastomotic leak rates but an increased risk of anastomotic stricture with CS. Wang and co‐workers 13 only compared HS with CS anastomoses. All anastomoses were performed in the neck, and no significant differences were demonstrated with regard to anastomotic leak, stricture or mortality. Markar et al. 14 compared HS with stapled oesophagogastric anastomoses, but did not separate the types of stapled anastomosis further. They did not observe significant differences in anastomotic leakage or 30‐day mortality. Anastomotic stricture occurred more frequently with stapled than with HS anastomoses. Another systematic review 11 examined eight RCTs, all comparing HS with CS anastomoses. No meta‐analysis was performed, and the authors concluded that there was insufficient evidence to recommend either technique. Liu and colleagues 12 grouped all stapled anastomoses together and compared them with HS anastomoses. Although a number of subgroup analyses were performed, the overall results demonstrated no significant differences in anastomotic leak rates or 30‐day mortality between HS and stapled anastomoses.
Although the data from the present study are interesting, it is not known precisely why LSSM anastomoses may have a reduced anastomotic leak rate. Theories include: the wider anastomosis, hence also reducing the risk of anastomotic stricture; anastomosis performed near the greater curve arcade on the best perfused part of the stomach allows for improved healing; and the side‐to‐side orientation reduces traction‐related tension24,58.
The present study has some limitations. The studies included in the review span a large time scale of over 28 years, and used slightly different definitions of anastomotic leakage. Future trials and studies in this area should adhere to the Esophagectomy Complications Consensus Group definitions of anastomotic leakage 68 , which classify leaks into three types in relation to severity and treatment needs. There is only limited published evidence on the use of the LSSM anastomoses, and this tends to be from more recently conducted studies. The included studies are heterogeneous in that they included different levels of anastomosis, suture material used, stapling device types and sizes, and approaches employed for oesophagectomy (open versus minimal access).
In the absence of large, high‐quality, randomized trial data, this network meta‐analysis provides the most up‐to‐date evidence base for comparing HS versus LSSM and CS techniques. Although triangular stapling showed promising results in the overall network, the encouraging results were not consistent in the sensitivity analysis. There is limited literature on the TS method, with only two papers describing outcomes for this technique; therefore it is difficult to give recommendations. The TS technique may be better viewed as a variation of the LSSM technique. The current multicentre international Oesophago‐Gastric Anastomosis Audit 69 (https://www.ogaa.org.uk) is collecting data on outcomes after oesophagectomy, and includes intraoperative details regarding anastomotic techniques. Data from this large database will further inform surgical teams about the benefits of different anastomotic techniques.
Supporting information
Table S1 Search terms
Table S2 Technical details of anastomoses reported
Table S3 Summary of studies reporting outcomes included in meta‐analysis
Table S4 Summary of other intraoperative and postoperative outcomes included in network meta‐analysis
Table S5 Summary of outcomes in previously published meta‐analyses
Acknowledgements
S.K.K. and J.R.B. contributed equally to this work.
The authors thank the staff of the Medical Library, Queen Elizabeth Hospital, Birmingham, for obtaining some of the papers used in this meta‐analysis. They also thank all members of the Oesophago‐Gastric Anastomotic Audit, West Midlands Research Collaborative, for discussions that helped improve this paper, including J. Hodson, Statistician, Institute of Translational Medicine, Queen Elizabeth Hospital, for checking the statistical methods.
Disclosure: The authors declare no conflict of interest.
Funding information
No funding
References
- 1. Muller JM, Erasmi H, Stelzner M, Zieren U, Pichlmaier H. Surgical therapy of oesophageal carcinoma. Br J Surg 1990; 77: 845–857. [DOI] [PubMed] [Google Scholar]
- 2. Sanghera SS, Nurkin SJ, Demmy TL. Quality of life after an esophagectomy. Surg Clin North Am 2012; 92: 1315–1335. [DOI] [PubMed] [Google Scholar]
- 3. Goense L, van Dijk WA, Govaert JA, van Rossum PS, Ruurda JP, van Hillegersberg R. Hospital costs of complications after esophagectomy for cancer. Eur J Surg Oncol 2017; 43: 696–702. [DOI] [PubMed] [Google Scholar]
- 4. Rutegard M, Lagergren P, Rouvelas I, Lagergren J. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population‐based study. Ann Surg Oncol 2012; 19: 99–103. [DOI] [PubMed] [Google Scholar]
- 5. Junemann‐Ramirez M, Awan MY, Khan ZM, Rahamim JS. Anastomotic leakage post‐esophagogastrectomy for esophageal carcinoma: retrospective analysis of predictive factors, management and influence on longterm survival in a high volume centre. Eur J Cardiothorac Surg 2005; 27: 3–7. [DOI] [PubMed] [Google Scholar]
- 6. Sauvanet A, Mariette C, Thomas P, Lozac'h P, Segol P, Tiret E et al Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg 2005; 201: 253–262. [DOI] [PubMed] [Google Scholar]
- 7. Griffin SM, Shaw IH, Dresner SM. Early complications after Ivor Lewis subtotal esophagectomy with two‐field lymphadenectomy: risk factors and management. J Am Coll Surg 2002; 194: 285–297. [DOI] [PubMed] [Google Scholar]
- 8. Markar S, Gronnier C, Duhamel A, Mabrut JY, Bail JP, Carrere N et al; FREGAT (French Eso‐Gastric Tumors) working group, FRENCH (Fédération de Recherche EN CHirurgie), and AFC (Association Française de Chirurgie) . The impact of severe anastomotic leak on long‐term survival and cancer recurrence after surgical resection for esophageal malignancy. Ann Surg 2015; 262: 972–980. [DOI] [PubMed] [Google Scholar]
- 9. Markar SR, Arya S, Karthikesalingam A, Hanna GB. Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta‐analysis. Ann Surg Oncol 2013; 20: 4274–4281. [DOI] [PubMed] [Google Scholar]
- 10. Honda M, Kuriyama A, Noma H, Nunobe S, Furukawa TA. Hand‐sewn versus mechanical esophagogastric anastomosis after esophagectomy: a systematic review and meta‐analysis. Ann Surg 2013; 257: 238–248. [DOI] [PubMed] [Google Scholar]
- 11. Kim RH, Takabe K. Methods of esophagogastric anastomoses following esophagectomy for cancer: a systematic review. J Surg Oncol 2010; 101: 527–533. [DOI] [PubMed] [Google Scholar]
- 12. Liu QX, Min JX, Deng XF, Dai JG. Is hand sewing comparable with stapling for anastomotic leakage after esophagectomy? A meta‐analysis. World J Gastroenterol 2014; 20: 17218–17226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Q, He XR, Shi CH, Tian JH, Jiang L, He SL et al Hand‐sewn versus stapled esophagogastric anastomosis in the neck: a systematic review and meta‐analysis of randomized controlled trials. Indian J Surg 2015; 77: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Markar SR, Karthikesalingam A, Vyas S, Hashemi M, Winslet M. Hand‐sewn versus stapled oesophago‐gastric anastomosis: systematic review and meta‐analysis. J Gastrointest Surg 2011; 15: 876–884. [DOI] [PubMed] [Google Scholar]
- 15. Wang WP, Gao Q, Wang KN, Shi H, Chen LQ. A prospective randomized controlled trial of semi‐mechanical versus hand‐sewn or circular stapled esophagogastrostomy for prevention of anastomotic stricture. World J Surg 2013; 37: 1043–1050. [DOI] [PubMed] [Google Scholar]
- 16. Saluja SS, Ray S, Pal S, Sanyal S, Agrawal N, Dash NR et al Randomized trial comparing side‐to‐side stapled and hand‐sewn esophagogastric anastomosis in neck. J Gastrointest Surg 2012; 16: 1287–1295. [DOI] [PubMed] [Google Scholar]
- 17. Li J, Shen Y, Tan L, Feng M, Wang H, Xi Y et al Cervical triangulating stapled anastomosis: technique and initial experience. J Thorac Dis 2014; 6(Suppl 3): S350–S354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayata K, Nakamori M, Nakamura M, Nakamura M, Katsuda M, Hayata K et al Circular stapling versus triangulating stapling for the cervical esophagogastric anastomosis after esophagectomy in patients with thoracic esophageal cancer: a prospective, randomized, controlled trial. Surgery 2017; 162: 131–138. [DOI] [PubMed] [Google Scholar]
- 19. Kesler KA, Ramchandani NK, Jalal SI, Stokes SM, Mankins MR, Ceppa D et al Outcomes of a novel intrathoracic esophagogastric anastomotic technique. J Thorac Cardiovasc Surg 2018; 156: 1739–1745.e1. [DOI] [PubMed] [Google Scholar]
- 20. Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta‐analysis. Ann Intern Med 2013; 159: 130–137. [DOI] [PubMed] [Google Scholar]
- 21. Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods 2012; 3: 80–97. [DOI] [PubMed] [Google Scholar]
- 22. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Low DE, Kuppusamy MK, Alderson D, Cecconello I, Chang AC, Darling G et al Benchmarking complications associated with esophagectomy. Ann Surg 2017; 269: 291–298. [DOI] [PubMed] [Google Scholar]
- 24. Collard JM, Romagnoli R, Goncette L, Otte JB, Kestens PJ. Terminalized semimechanical side‐to‐side suture technique for cervical esophagogastrostomy. Ann Thorac Surg 1998; 65: 814–817. [DOI] [PubMed] [Google Scholar]
- 25. Lo CK, Mertz D, Loeb M. Newcastle–Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol 2014; 14: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 27. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Stat Med 2010; 29: 932–944. [DOI] [PubMed] [Google Scholar]
- 28. Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta‐analysis using R: a review of currently available automated packages. PLoS One 2014; 9: e115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rucker G, Schwarzer G. Ranking treatments in frequentist network meta‐analysis works without resampling methods. BMC Med Res Methodol 2015; 15: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simillis C, Lal N, Thoukididou SN, Kontovounisios C, Smith JJ, Hompes R et al Open versus laparoscopic versus robotic versus transanal mesorectal excision for rectal cancer: a systematic review and network meta‐analysis. Ann Surg 2019; 270: 59–68. [DOI] [PubMed] [Google Scholar]
- 31. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Inconsistency in Networks of Evidence Based on Randomised Controlled Trials. National Institute for Health and Care Excellence: London, 2014. [PubMed] [Google Scholar]
- 32. Peracchia A, Bardini R, Ruol A, Asolati M, Scibetta D. Esophagovisceral anastomotic leak. A prospective statistical study of predisposing factors. J Thorac Cardiovasc Surg 1988; 95: 685–691. [PubMed] [Google Scholar]
- 33. Rostas JW, Graffree BD, Scoggins CR, McMasters KM, Martin RCG. Long‐term outcomes after hand‐sewn versus circular‐stapled (25 and 29 mm) anastomotic technique after esophagogastrectomy for esophageal cancer. J Surg Oncol 2018; 117: 469–472. [DOI] [PubMed] [Google Scholar]
- 34. McManus KG, Ritchie AJ, McGuigan J, Stevenson HM, Gibbons JR. Sutures, staplers, leaks and strictures. A review of anastomoses in oesophageal resection at Royal Victoria Hospital, Belfast 1977–1986. Eur J Cardiothorac Surg 1990; 4: 97–100. [DOI] [PubMed] [Google Scholar]
- 35. Lee Y, Fujita H, Yamana H, Kakegawa T. Factors affecting leakage following esophageal anastomosis. Surg Today 1994; 24: 24–29. [DOI] [PubMed] [Google Scholar]
- 36. Honkoop P, Siersema PD, Tilanus HW, Stassen LP, Hop WC, van Blankenstein M. Benign anastomotic strictures after transhiatal esophagectomy and cervical esophagogastrostomy: risk factors and management. J Thorac Cardiovasc Surg 1996; 111: 1141–1148. [DOI] [PubMed] [Google Scholar]
- 37. Klink CD, Binnebosel M, Otto J, Boehm G, von Trotha KT, Hilgers RD et al Intrathoracic versus cervical anastomosis after resection of esophageal cancer: a matched pair analysis of 72 patients in a single center study. World J Surg Oncol 2012; 10: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. West of Scotland and Highland Anastomosis Study Group . Suturing or stapling in gastrointestinal surgery: a prospective randomized study. Br J Surg 1991; 78: 337–341. [DOI] [PubMed] [Google Scholar]
- 39. Craig SR, Walker WS, Cameron EW, Wightman AJ. A prospective randomized study comparing stapled with handsewn oesophagogastric anastomoses. J R Coll Surg Edinb 1996; 41: 17–19. [PubMed] [Google Scholar]
- 40. Valverde A, Hay JM, Fingerhut A, Elhadad A. Manual versus mechanical esophagogastric anastomosis after resection for carcinoma: a controlled trial. French Associations for Surgical Research. Surgery 1996; 120: 476–483. [DOI] [PubMed] [Google Scholar]
- 41. Law S, Fok M, Chu KM, Wong J. Comparison of hand‐sewn and stapled esophagogastric anastomosis after esophageal resection for cancer: a prospective randomized controlled trial. Ann Surg 1997; 226: 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hsu HH, Chen JS, Huang PM, Lee JM, Lee YC. Comparison of manual and mechanical cervical esophagogastric anastomosis after esophageal resection for squamous cell carcinoma: a prospective randomized controlled trial. Eur J Cardiothorac Surg 2004; 25: 1097–1101. [DOI] [PubMed] [Google Scholar]
- 43. Okuyama M, Motoyama S, Suzuki H, Saito R, Maruyama K, Ogawa J. Hand‐sewn cervical anastomosis versus stapled intrathoracic anastomosis after esophagectomy for middle or lower thoracic esophageal cancer: a prospective randomized controlled study. Surg Today 2007; 37: 947–952. [DOI] [PubMed] [Google Scholar]
- 44. Luechakiettisak P, Kasetsunthorn S. Comparison of hand‐sewn and stapled in esophagogastric anastomosis after esophageal cancer resection: a prospective randomized study. J Med Assoc Thai 2008; 91: 681–685. [PubMed] [Google Scholar]
- 45. Zhang YS, Gao BR, Wang HJ, Su YF, Yang YZ, Zhang JH et al Comparison of anastomotic leakage and stricture formation following layered and stapler oesophagogastric anastomosis for cancer: a prospective randomized controlled trial. J Int Med Res 2010; 38: 227–233. [DOI] [PubMed] [Google Scholar]
- 46. Cayi R, Li M, Xiong G, Cai K, Wang W. Comparative analysis of mechanical and manual cervical esophagogastric anastomosis following esophagectomy for esophageal cancer. Nan Fang Yi Ke Da Xue Xue Bao 2012; 32: 908–909. [PubMed] [Google Scholar]
- 47. Liu QX, Qiu Y, Deng XF, Min JX, Dai JG. Comparison of outcomes following end‐to‐end hand‐sewn and mechanical oesophagogastric anastomosis after oesophagectomy for carcinoma: a prospective randomized controlled trial. Eur J Cardiothorac Surg 2015; 47: e118–e123. [DOI] [PubMed] [Google Scholar]
- 48. Zhu ZJ, Zhao YF, Chen LQ, Hu Y, Liu LX, Wang Y et al Clinical application of layered anastomosis during esophagogastrostomy. World J Surg 2008; 32: 583–588. [DOI] [PubMed] [Google Scholar]
- 49. Xu QR, Wang KN, Wang WP, Zhang K, Chen LQ. Linear stapled esophagogastrostomy is more effective than hand‐sewn or circular stapler in prevention of anastomotic stricture: a comparative clinical study. J Gastrointest Surg 2011; 15: 915–921. [DOI] [PubMed] [Google Scholar]
- 50. Blackmon SH, Correa AM, Wynn B, Hofstetter WL, Martin LW, Mehran RJ et al Propensity‐matched analysis of three techniques for intrathoracic esophagogastric anastomosis. Ann Thorac Surg 2007; 83: 1805–1813. [DOI] [PubMed] [Google Scholar]
- 51. Liu YJ, Fan J, He HH, Zhu SS, Chen QL, Cao RH. Anastomotic leakage after intrathoracic versus cervical oesophagogastric anastomosis for oesophageal carcinoma in Chinese population: a retrospective cohort study. BMJ Open 2018; 8: e021025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Price TN, Nichols FC, Harmsen WS, Allen MS, Cassivi SD, Wigle DA et al A comprehensive review of anastomotic technique in 432 esophagectomies. Ann Thorac Surg 2013; 95: 1154–1161. [DOI] [PubMed] [Google Scholar]
- 53. Furukawa Y, Hanyu N, Hirai K, Ushigome T, Kawasaki N, Toyama Y et al Usefulness of automatic triangular anastomosis for esophageal cancer surgery using a linear stapler (TA‐30). Ann Thorac Cardiovasc Surg 2005; 11: 80–86. [PubMed] [Google Scholar]
- 54. Wang ZQ, Jiang YQ, Xu W, Cai HR, Zhang Z, Yin Z et al A novel technique for cervical gastro‐oesophageal anastomosis during minimally invasive oesophagectomy. Int J Surg 2018; 53: 221–229. [DOI] [PubMed] [Google Scholar]
- 55. Zieren HU, Muller JM, Pichlmaier H. Prospective randomized study of one‐ or two‐layer anastomosis following oesophageal resection and cervical oesophagogastrostomy. Br J Surg 1993; 80: 608–611. [DOI] [PubMed] [Google Scholar]
- 56. Casson AG, Porter GA, Veugelers PJ. Evolution and critical appraisal of anastomotic technique following resection of esophageal adenocarcinoma. Dis Esophagus 2002; 15: 296–302. [DOI] [PubMed] [Google Scholar]
- 57. Behzadi A, Nichols FC, Cassivi SD, Deschamps C, Allen MS, Pairolero PC. Esophagogastrectomy: the influence of stapled versus hand‐sewn anastomosis on outcome. J Gastrointest Surg 2005; 9: 1031–1042. [DOI] [PubMed] [Google Scholar]
- 58. Ercan S, Rice TW, Murthy SC, Rybicki LA, Blackstone EH. Does esophagogastric anastomotic technique influence the outcome of patients with esophageal cancer? J Thorac Cardiovasc Surg 2015; 129: 623–631. [DOI] [PubMed] [Google Scholar]
- 59. Kondra J, Ong SR, Clifton J, Evans K, Finley RJ, Yee J. A change in clinical practice: a partially stapled cervical esophagogastric anastomosis reduces morbidity and improves functional outcome after esophagectomy for cancer. Dis Esophagus 2008; 21: 422–429. [DOI] [PubMed] [Google Scholar]
- 60. Harustiak T, Pazdro A, Snajdauf M, Stolz A, Lischke R. Anastomotic leak and stricture after hand‐sewn versus linear‐stapled intrathoracic oesophagogastric anastomosis: single‐centre analysis of 415 oesophagectomies. Eur J Cardiothorac Surg 2016; 49: 1650–1659. [DOI] [PubMed] [Google Scholar]
- 61. Mishra PK, Shah H, Gupta N, Varshney V, Patil NS, Jain A et al Stapled versus hand‐sewn cervical esophagogastric anastomosis in patients undergoing esophagectomy: a retrospective cohort study. Ann Med Surg (Lond) 2016; 5: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sugimura K, Miyata H, Matsunaga T, Asukai K, Yanagimoto Y, Takahashi Y et al Comparison of the modified Collard and hand‐sewn anastomosis for cervical esophagogastric anastomosis after esophagectomy in esophageal cancer patients: a propensity score‐matched analysis. Ann Gastroenterol Surg 2019; 3: 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Laterza E, de' Manzoni G, Veraldi GF, Guglielmi A, Tedesco P, Cordiano C. Manual compared with mechanical cervical oesophagogastric anastomosis: a randomised trial. Eur J Surg 1999; 165: 1051–1054. [DOI] [PubMed] [Google Scholar]
- 64. Walther B, Johansson J, Johnsson F, Von Holstein CS, Zilling T. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg 2003; 238: 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Singh D, Maley RH, Santucci T, Macherey RS, Bartley S, Weyant RJ et al Experience and technique of stapled mechanical cervical esophagogastric anastomosis. Ann Thorac Surg 2001; 71: 419–424. [DOI] [PubMed] [Google Scholar]
- 66. Sokouti M, Golzari SE, Pezeshkian M, Farahnak MR. The role of esophagogastric anastomotic technique in decreasing benign stricture formation in the surgery of esophageal carcinoma. J Cardiovasc Thorac Res 2013; 5: 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sun HB, Li Y, Liu XB, Zhang RX, Wang ZF, Zheng Y et al Embedded three‐layer esophagogastric anastomosis reduces morbidity and improves short‐term outcomes after esophagectomy for cancer. Ann Thorac Surg 2016; 101: 1131–1138. [DOI] [PubMed] [Google Scholar]
- 68. Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, XB D'Journo et al International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015; 262: 286–294. [DOI] [PubMed] [Google Scholar]
- 69. Kamarajah SKSP, Bundred J, Siaw‐Acheampong K, Jefferies B, Nepogodiev D, Evans R et al International variation in surgical practices in units performing oesophagectomy for oesophageal cancer: a unit survey of the participants of the Oesophago‐Gastric Anastomosis Audit (OGAA). Meeting of the Association of Upper Gastrointestinal Surgery, Edinburgh 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Search terms
Table S2 Technical details of anastomoses reported
Table S3 Summary of studies reporting outcomes included in meta‐analysis
Table S4 Summary of other intraoperative and postoperative outcomes included in network meta‐analysis
Table S5 Summary of outcomes in previously published meta‐analyses


