Abstract
Background
This study aimed to evaluate the effect of perioperative supplementation with omega‐3 fatty acids (n‐3 FA) on perioperative outcomes and survival in patients undergoing colorectal cancer surgery.
Methods
Patients scheduled for elective resection of colorectal cancer between 2007 and 2010 were randomized to either an n‐3 FA‐enriched oral nutrition supplement (ONS) twice daily or a standard ONS (control) for 7 days before and after surgery. Outcome measures, including postoperative complications, 3‐year cumulative incidence of local or metastatic colorectal cancer recurrence and 5‐year overall survival, were compared between the groups.
Results
Of 148 patients enrolled in the study, 125 (65 patients receiving n‐3 FA‐enriched ONS and 60 receiving standard ONS) were analysed. There were no differences in postoperative complications after surgery (P = 0·544). The risk of disease recurrence at 3 years was similar (relative risk 1·66, 95 per cent c.i. 0·65 to 4·26).The 5‐year survival rate of patients treated with n‐3 FA was 69·2 (95 per cent c.i. 56·5 to 78·9) per cent, compared with 81·7 (69·3 to 89·4) per cent in the control group (P = 0·193). After adjustment for age, stage of disease and adjuvant chemotherapy, n‐3 FA was associated with higher mortality compared with controls (hazard ratio 1·73, 95 per cent c.i. 1·06 to 2·83; P = 0·029). The interaction between n‐3 FA and adjuvant chemotherapy was not statistically significant.
Conclusion
Perioperative supplementation with n‐3 FA did not confer a survival benefit in patients undergoing colorectal cancer surgery. n‐3 FA did not benefit the subgroup of patients treated with adjuvant chemotherapy or decrease the risk of disease recurrence.
Perioperative treatment with omega‐3 fatty acids (n‐3 FA) does not confer an overall survival benefit in patients undergoing colorectal cancer surgery, and neither is it associated with an increased efficacy of adjuvant chemotherapy. Clinicians should be careful when making any recommendations regarding the use of n‐3 FA oral nutritional supplements, as the effects are not thoroughly established.
Fatty acids supplements and colon cancer
Antecedentes
Este estudio tuvo como objetivo evaluar el efecto de la suplementación perioperatoria con ácidos grasos omega‐3 (omega‐3 fatty acids, n‐3 FA) sobre los resultados perioperatorios y la supervivencia en pacientes sometidos a cirugía de cáncer colorrectal (colorectal cáncer, CRC).
Métodos
Los pacientes programados para una resección electiva de CRC entre 2007 y 2010 fueron asignados al azar a recibir dos veces al día un suplemento nutricional oral (oral nutrition supplement, ONS) enriquecido con n‐3 FA o un ONS estándar (control) durante siete días antes y después de la cirugía de CRC. Los grupos se compararon mediante análisis estadísticos. Las medidas de resultado incluyeron las complicaciones postoperatorias, la incidencia acumulada de recidivas locales o metastásicas de CCR a los 3 años y la supervivencia global a los 5 años.
Resultados
De 148 pacientes reclutados, se analizaron 125 pacientes (65 que recibieron el ONS enriquecido con n‐3 FA y 60 que recibieron el ONS estándar). No hubo diferencias en las complicaciones postoperatorias después de la cirugía (P = 0,544). El riesgo de recidiva de la enfermedad a los 3 años no fue diferente entre los grupos (riesgo relativo, RR = 1,66; i.c. del 95% (0,65; 4,26)). La supervivencia a los 5 años para los pacientes tratados con n‐3 FA fue del 69,2% (i.c. del 95% (56,5; 78,9)) en comparación con el 81,7% (i.c. del 95% (69,4; 89,4)) en el grupo control (P = 0,193). Después del ajuste por edad, estadio de la enfermedad y quimioterapia adyuvante, n‐3 FA se asoció con una mayor mortalidad (cociente de riesgos instantáneos, hazard ratio, HR = 1,73; i.c. del 95% (1,05; 2,83); P = 0,029) en comparación con los controles. Sin embargo, la interacción entre n‐3 FA y la quimioterapia adyuvante no fue estadísticamente significativa.
Conclusión
La suplementación perioperatoria con n‐3 FA no confirió un beneficio de supervivencia en pacientes sometidos a cirugía de CRC. El n‐3 FA tampoco benefició al subgrupo de pacientes tratados con quimioterapia adyuvante, ni disminuyó el riesgo de recidiva de la enfermedad.
Introduction
Colorectal cancer surgery has been associated with a phase of hyperinflammation followed by a relative immune incompetence1, 2. Patients' nutritional status and, in particular, the availability of specific biologically active nutrients, such as the marine omega‐3 fatty acids (n‐3 FA) (eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA)) and docosapentaenoic acid, have been investigated in the literature3, 4, 5, 6, 7 as factors influencing the postoperative course. In particular, n‐3 FA have been associated with an improved immunoproliferative response8.
Several colorectal cancer cell line studies have investigated also whether n‐3 FA could improve the response to chemotherapeutic agents. A previous study9 demonstrated a synergistic anticancer effect between EPA and a regimen of 5‐fluorouracil (5‐FU) and oxaliplatin in vitro and in vivo against the human colonic cancer cells. In line with this, other researchers10 hypothesized that EPA could have a role in adjuvant therapy for the prevention of metastatic colorectal cancer. Oral administration of EPA reduced the growth of experimental colorectal cancer liver metastases and, importantly, preoperative treatment with EPA resulted in improved disease‐free survival10. Finally, it has been proposed11 that n‐3 FA might increase the susceptibility of tumour cells to chemotherapeutic regimens: in an experimental study conducted on mice with colonic cancer, the administration of 5‐FU and n‐3 FA increased both DNA damage and the apoptotic index by activation of extrinsic and intrinsic apoptotic pathways. The increased proapoptotic effect by the synergism of 5‐FU and n‐3 FA could be attributed to the incorporation of n‐3 FA into the cancer cell membranes, altering membrane fluidity and thereby chemosensitizing the tumour cells.
The aim of the present study was to investigate whether oral supplementation with n‐3 FA in the perioperative period might be associated with improved postoperative outcomes (decreased number of complications), decreased risk of disease recurrence, and improved survival in patients undergoing colorectal cancer surgery.
Methods
A randomized, double‐blind, placebo‐controlled, single‐centre trial was conducted to study the effect of an n‐3 FA‐enriched oral nutrition supplement (ONS) on long‐term outcomes in patients scheduled for colorectal cancer surgery.
Patients scheduled for colorectal cancer surgery at the Department of Gastrointestinal Surgery, Aalborg University Hospital, between July 2007 and January 2010 were screened for eligibility. Aalborg University Hospital treats some 260–350 patients with colorectal cancer each year. Each patient was referred from the surgical outpatient clinic and managed according to the current Danish guidelines. The inclusion and exclusion criteria have been described previously12 (ClinicalTrials identifier NCT00488904).
Briefly, patients in both the n‐3 FA and control group received the ONS as a sip feed (200 ml twice daily, once in the morning and once in the afternoon) for 7 days before and 7 days after surgery. The feeds were isocaloric (1·5 kcal/ml) and isonitrogenous (Supportan®; Fresenius Kabi, Bad Homburg, Germany). Both feeds contained the same amounts of carbohydrate, protein, total fat and n‐6 FA, as well as vitamins and minerals. However, the fatty acid composition differed between the two feeds: both contained medium‐chain triglycerides, sunflower oil and safflower oil, but the n‐3 FA ONS also contained fish oil. Thus, patients in the n‐3 FA group received 2·0 g EPA and 1·0 g DHA per day from the ONS, whereas no n‐3 FA was provided to controls.
Outcome measures
Outcomes included perioperative results (pneumonia, wound infection, urinary tract infection, peritonitis (including anastomotic leakage) and sepsis) and survival. Five‐year overall survival (defined as being alive 5 years after colorectal cancer surgery) and the risk of disease recurrence (defined as the cumulative incidence of local or metastatic colorectal cancer recurrence) at 3 years according to treatment allocation were evaluated. According to the Danish national guidelines for colorectal cancer treatment, each patient was followed with CT of the thorax and abdomen 1 and 3 years after treatment. If the patient did not have full colonoscopy before surgery, a new colonoscopy was planned for 3 months after surgery; otherwise, all patients had colonoscopy 5 years after treatment. Data on patient survival and disease recurrence were collected from patient records and the Danish National Patient Registry.
After evaluation of the surgical resection specimen, the patients were restaged and evaluated at a new multidisciplinary team conference, after which those with high‐risk stage II and III disease were referred for adjuvant chemotherapy. Patients with stage IV disease with no surgically treatable liver metastasis received chemotherapy with palliative intent only. A subgroup analysis of all patients receiving adjuvant chemotherapy with curative intent was also performed, and therefore excluded patients who had either neoadjuvant radiochemotherapy or postoperative palliative chemotherapy (patients with stage IV disease).
Post‐trial analysis of survival was approved by the North Denmark Committee on Health Research Ethics Committee (N‐20140064) and the Danish Data Protection Agency.
Statistical analysis
The study was designed to detect a 20 per cent difference in postoperative complication rates between groups (a reduction from 30 to 10 per cent was hypothesized). The required sample size was 72 patients in each group (80 per cent power, α = 0·05). Baseline characteristics were compared using Student's t test or Fisher's exact test, as appropriate. The median length of hospital stay was compared using the Wilcoxon–Mann–Whitney rank test. The Kaplan–Meier estimator was used to depict the association between treatment with the n‐3 FA ONS and overall survival. Overall 5‐year survival was calculated from Kaplan–Meier estimates and compared with the log rank test. To evaluate the effects of a supplement with n‐3 FA along with interaction with adjuvant chemotherapy, a generalized linear model was constructed using the pseudo‐observation method13. Estimates were presented as hazard ratios (HRs) with 95 per cent confidence intervals. The risk of disease recurrence was estimated using competing risk regression. The Aalen–Johansen estimator was used to produce the cumulative incidence plot, and the pseudo‐observation method was employed to determine the relative risk (RR) of disease recurrence 3 years after surgery. All analyses were conducted using STATA® V.15.1 (StataCorp, College Station, Texas, USA).
Results
Of 610 patients who had colorectal cancer surgery during the study period, 201 did not meet the inclusion criteria, 31 declined to participate, and 230 could not be included because they had surgery within 4–5 days of diagnosis and therefore could not receive the allocated 7‐day intervention before surgery (Fig. 1). The remaining 148 patients were randomized (in line with the power calculation); however, 23 patients were subsequently excluded owing to patient refusal, postoperative death, logistical reasons, or because they did not have a standard colorectal cancer resection. Accordingly, 65 patients were enrolled in the n‐3 FA treatment arm and 60 patients in the control arm.
Figure 1.
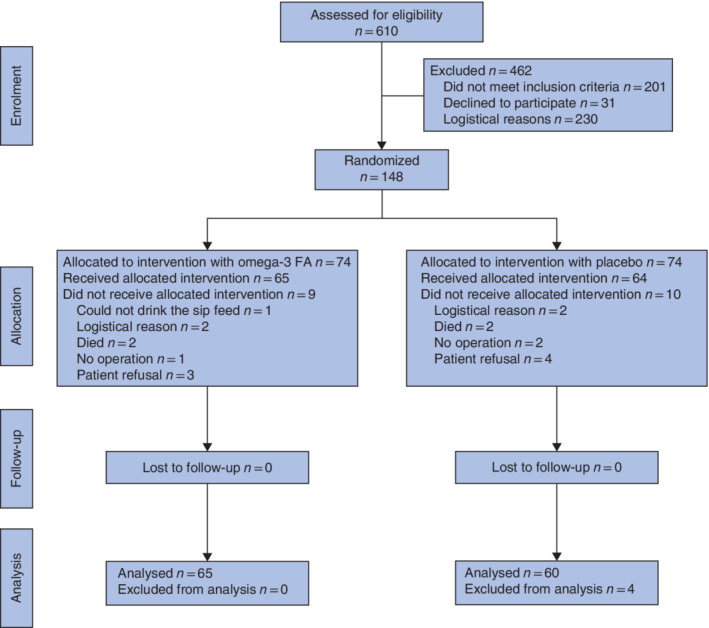
CONSORT diagram for the study FA, fatty acids.
Baseline characteristics of the patients are shown in Table 1. Twenty‐two patients received adjuvant chemotherapy, either as monotherapy with 5‐FU (5 patients) or as combined treatment (17) with folinic acid, 5‐FU and oxaliplatin (FOLFOX).
Table 1.
Study population
| Enriched ONS (n = 65) | Control ONS (n = 60) | P† | |
|---|---|---|---|
| Age (years)* | 68·3(11·3) | 70·6(10·1) | 0·246‡ |
| Sex ratio (M : F) | 27 : 38 | 30 : 30 | 0·373 |
| Smoking status | |||
| Non‐smoker | 47 (72) | 51 (85) | |
| Smoker | 17 (26) | 8 (13) | |
| Unknown | 1 (1·5) | 1 (2) | 0·148 |
| Cancer location | |||
| Colon | 36 (55) | 34 (57) | |
| Rectum | 29 (45) | 26 (43) | 1·000 |
| UICC stage | |||
| I | 14 (22) | 10 (17) | |
| II | 26 (40) | 29 (48) | |
| III | 18 (28) | 19 (32) | |
| IV | 7 (11) | 2 (3) | 0·348 |
| Adjuvant therapy | 11 (17) | 11 (18) | 1·000 |
Values in parentheses are percentages unless indicated otherwise; *values are mean(s.d.). ONS, oral nutrition supplement. †Fisher's exact test, except ‡Student's t test.
Perioperative outcomes
Data on perioperative outcomes have been reported elsewhere12. In brief, there was no significant difference in the number of infectious (35·4 per cent in patients treated with n‐3 FA versus 38·3 per cent in the control group; P = 0·853) or non‐infectious (29·2 and 23·3 per cent respectively; P = 0·544) complications, 30‐day mortality rate (0 per cent in both groups; P = 1·000) or median length of hospital stay (9 versus 10 days respectively; P = 0·807).
Survival
The 5‐year overall survival rate was 69·2 (95 per cent c.i. 56·5 to 78·9) per cent in patients treated with n‐3 FA, compared with 81·7 (69·3 to 89·4) per cent in the control group (P = 0·193). The Kaplan–Meier survival curves (Fig. 2) indicate that the assumption of proportional hazards was violated over the recorded time period (which was also evident from the log–log plot). Therefore, the pseudo‐observation method was used to construct a generalized linear model to evaluate the effect of treatment with n‐3 FA and adjuvant chemotherapy on 5‐year survival. The model was adjusted for age and stage of disease at the time of diagnosis. The unadjusted HR 5 years after surgery was 1·68 (95 per cent c.i. 0·88 to 3·21), suggesting no effect of n‐3 FA treatment on survival of surgically treated patients with colorectal cancer (Table 2). After adjustment for adjuvant chemotherapy, age and stage at time of diagnosis, supplementation with n‐3 FA was associated with a higher overall mortality (HR 1·73 (1·06 to 2·83); P = 0·029).
Figure 2.
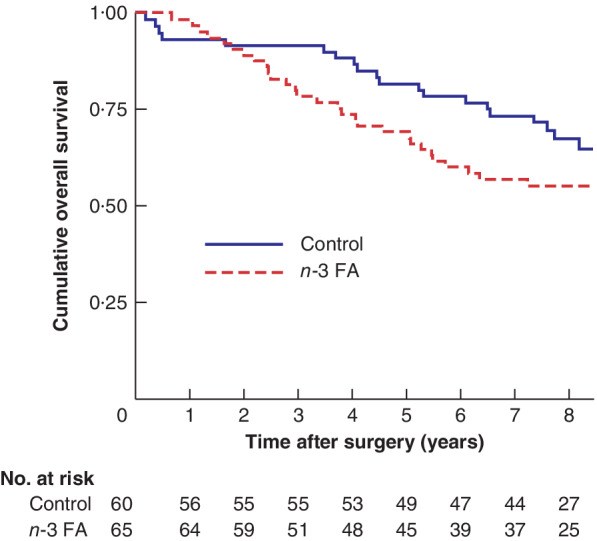
Kaplan–Meier analysis of overall survival according to treatment allocation n‐3 FA, omega‐3 fatty acids. P =0·193 (log rank test).
Table 2.
Hazard ratios for mortality at 5 years after surgery
| Univariable estimates | Multivariable estimates | |||
|---|---|---|---|---|
| Hazard ratio | P | Hazard ratio | P | |
| Age | 1·05 (1·01, 1·09) | 0·011 | 1·07 (1·03, 1·12) | 0·001 |
| n‐3 fatty acids | 1·68 (0·88, 3·21) | 0·118 | 1·73 (1·06, 2·83) | 0·029 |
| Adjuvant chemotherapy | 2·23 (1·23, 4·06) | 0·009 | 1·52 (0·87, 2·65) | 0·138 |
| UICC stage | ||||
| II | 1·75 (0·40, 7·66) | 0·461 | 3·89 (0·43, 34·71) | 0·223 |
| III | 5·19 (1·30, 20·69) | 0·020 | 12·57 (1·66, 95·37) | 0·014 |
| IV | 6·67 (1·55, 28·58) | 0·011 | 7·87 (1·03, 60·82) | 0·047 |
Values in parentheses are 95 per cent confidence intervals. n‐3 FA, omega‐3 fatty acids.
The interaction between treatment with n‐3 FA and adjuvant chemotherapy was, however, not statistically significant, suggesting a spurious association. Of patients treated with adjuvant chemotherapy, the stratified HR for patients in the n‐3 FA group was 2·09 (95 per cent c.i. 0·92 to 4·75) compared with 1·29 (0·51 to 3·25) in the control group (P = 0·138).
Disease recurrence
The effect of treatment with n‐3 FA on the risk of disease recurrence, using death as a competing risk, was calculated for the 110 patients who had an R0 resection (55 in each group) (Fig. 3). The risk of disease recurrence did not differ between groups (RR 1·66, 95 per cent c.i. 0·65 to 4·26), and adjustment for stage and adjuvant chemotherapy did not alter the estimate.
Figure 3.
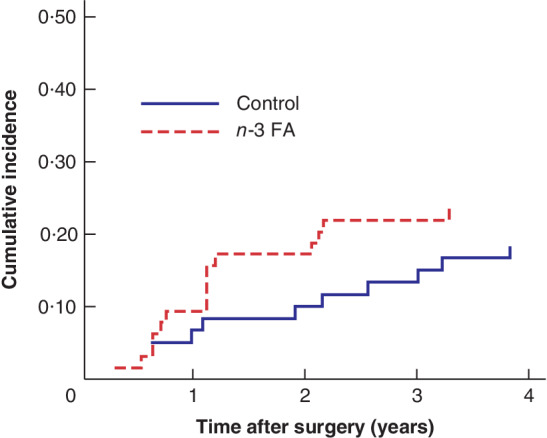
Risk of disease recurrence in patients who had colorectal surgery with curative intent, according to treatment allocation There were 55 patients in the omega‐3 fatty acids (n‐3 FA) group and 55 in the control group. Relative risk 1·66 (95 per cent c.i. 0·65 to 4·26).
Discussion
This study aimed to evaluate perioperative treatment with n‐3 FA on the outcomes of patients undergoing colorectal cancer surgery in a randomized clinical setting. Of note, the investigation was designed as an RCT, enrolling blinded participants; the sip feeds were nutritionally similar and had an identical appearance and taste, apart from content of marine n‐3 FA. Compliance with the allocated intervention (n‐3 FA versus control) was acceptable, and the study population was relatively homogeneous12.
However, the study was powered to show a difference in postoperative complications and not a difference in survival in patients receiving chemotherapy and n‐3 FA; thus, the number of patients might have been too low for this outcome. The low number of patients receiving adjuvant chemotherapy (11 in each group) also limited the study power, and any association with reduced survival or other events should be interpreted with caution. Follow‐up on postoperative dietary habits, other disease states and medications was not conducted, and this should also be regarded as a limitation. The intake of long‐chain n‐3 FA in Western countries is generally low, but is traditionally higher in Scandinavian countries, and therefore the results may not be directly comparable for other populations. Finally, other n‐3 FA dosages and different lengths of treatment duration were not considered. Prolonged treatment might be needed to ensure an effect on postoperative outcomes.
Some meta‐analyses14, 15 identified that so‐called immunonutrition, containing arginine, glutamine, nucleotides and n‐3 FA, could reduce postoperative infections in selected patient groups. However, it is unclear which of the components is responsible for the clinical effect. In the present study, daily supplementation with 3 g EPA plus DHA for 7 days before and 7 days after colorectal cancer surgery did lead to increased incorporation of these n‐3 FA in the cell membranes, as well as a significant increase in the production of leukotriene B5 from EPA and a significant decrease in the production of leukotriene B4, but had no effect on postoperative complications12, 16, 17.
It has been suggested18 that supplementation with n‐3 FA could increase chemotherapeutic efficacy and thereby contribute to increased survival. In a combined analysis of the Nurses' Health Study and the Health Professionals Follow‐up Study, it was reported18 that a high intake of n‐3 FA after colorectal cancer diagnosis was associated with a lower risk of mortality from the disease. Thus, patients consuming at least 0·3 g n‐3 FA daily had an adjusted HR for colorectal cancer‐specific mortality of 0·59 (95 per cent c.i. 0·35 to 1·01). Patients who increased their marine n‐3 FA intake by at least 0·15 g/day after diagnosis had a HR of 0·30 for colorectal cancer death, compared with those who did not change their intake of marine n‐3 FA.
A previous phase II double‐blind placebo‐controlled RCT enrolled patients with colorectal cancer and liver metastases who received 2 g EPA daily or placebo twice daily from randomization to the day of liver resection10. The n‐3 FA EPA was successfully incorporated into liver metastases removed at operation, and a trend towards reduced tumour vascularization in n‐3 FA‐treated colorectal liver metastases was recorded. The authors report a non‐significant trend towards improved overall survival from n‐3 FA in the first 18 months after surgery, and early colorectal cancer recurrence was similar in the groups10.
A meta‐analysis19 compared RCTs using pharmaconutrition with those using standard nutrition in elective adult surgical patients operated on between 1980 and 2011. No differences were reported in postoperative mortality, but there was a significant reduction in infectious complications and length of hospital stay. Regarding perioperative administration, there was a reduction in anastomotic dehiscence. Furthermore, a reduction in non‐infectious complications was detected with postoperative administration. Pharmaconutrition given before surgery did not demonstrate a notable advantage compared with standard nutrition for any of the assessed clinical outcomes19. The importance of timing was also shown in another review20 of published meta‐analyses, implying improved outcome after oncological gastrointestinal surgery, but before issuing recommendations further studies are warranted. Additionally, two meta‐analyses19, 21 showed that preoperative pharmaconutrition did not confer any benefit over standard formulations. The earlier seen benefits of pharmaconutrition (reduction in infectious complications) were reported only with perioperative or postoperative administration.
Acknowledgements
The authors thank study nurse A. Madsen and the staff at the Lipid Research Clinic at Aalborg University Hospital for invaluable assistance in completing the study, and H. C. Brix Noergaard for helping with the initial phase of the study. Fresenius Kabi (Bad Homburg, Germany) supplied sip feeds.
This study was funded by the Obel Family Foundation, Aase and Ejnar Danielsens Foundation, Augustinus Foundation and L. F. Foghts Foundation.
Disclosure: The authors declare no conflict of interest.
Funding information
Obel Family Foundation
Aase and Ejnar Danielsens Foundation
Augustinus Foundation
L. F. Foghts Foundation
References
- 1. Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003; 362: 1921–1928. [DOI] [PubMed] [Google Scholar]
- 2. Calder PC. n‐3 fatty acids, inflammation, and immunity – Relevance to postsurgical and critically ill patients. Lipids 2004; 39: 1147–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet 2001; 358: 1487–1492. [DOI] [PubMed] [Google Scholar]
- 4. Braga M, Gianotti L, Vignali A, Di Carlo V. Preoperative oral arginine and n‐3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery 2002; 132: 805–814. [DOI] [PubMed] [Google Scholar]
- 5. Zhu MW, Tang DN, Hou J, Wei JM, Hua B, Sun JH et al Impact of fish oil enriched total parenteral nutrition on elderly patients after colorectal cancer surgery. Chin Med J (Engl) 2012; 125: 178–181. [PubMed] [Google Scholar]
- 6. Jie B, Jiang ZM, Nolan MT, Zhu SN, Yu K, Kondrup J. Impact of preoperative nutritional support on clinical outcome in abdominal surgical patients at nutritional risk. Nutrition 2012; 28: 1022–1027. [DOI] [PubMed] [Google Scholar]
- 7. Ryan AM, Reynolds JV, Healy L, Byrne M, Moore J, Brannelly N et al Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double‐blinded randomized controlled trial. Ann Surg 2009; 249: 355–363. [DOI] [PubMed] [Google Scholar]
- 8. Marimuthu K, Varadhan KK, Ljungqvist O, Lobo DN. A meta‐analysis of the effect of combinations of immune modulating nutrients on outcome in patients undergoing major open gastrointestinal surgery. Ann Surg 2012; 255: 1060–1068. [DOI] [PubMed] [Google Scholar]
- 9. Vasudevan A, Yu Y, Banerjee S, Woods J, Farhana L, Rajendra SG et al Omega‐3 fatty acid is a potential preventive agent for recurrent colon cancer. Cancer Prev Res 2014; 7: 1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cockbain AJ, Volpato M, Race AD, Munarini A, Fazio C, Belluzzi A et al Anticolorectal cancer activity of the omega‐3 polyunsaturated fatty acid eicosapentaenoic acid. Gut 2014; 63: 1760–1768. [DOI] [PubMed] [Google Scholar]
- 11. Rani I, Sharma B, Kumar S, Kaur S, Agnihotri N. Apoptosis mediated chemosensitization of tumor cells to 5‐fluorouracil on supplementation of fish oil in experimental colon carcinoma. Tumor Biol 2017; 39: 101042831769501. [DOI] [PubMed] [Google Scholar]
- 12. Sorensen LS, Thorlacius‐Ussing O, Schmidt EB, Rasmussen HH, Lundbye‐Christensen S, Calder PC et al Randomized clinical trial of perioperative omega‐3 fatty acid supplements in elective colorectal cancer surgery. Br J Surg 2014; 101: 33–42. [DOI] [PubMed] [Google Scholar]
- 13. Hansen SN, Andersen PK, Parner ET. Events per variable for risk differences and relative risks using pseudo‐observations. Lifetime Data Anal 2014; 20: 584–598. [DOI] [PubMed] [Google Scholar]
- 14. Cerantola Y, Hübner M, Grass F, Demartines N, Schäfer M. Immunonutrition in gastrointestinal surgery. Br J Surg 2011; 98: 37–48. [DOI] [PubMed] [Google Scholar]
- 15. Heys SD, Walker LG, Smith I, Eremin O. Enteral nutritional supplementation with key nutrients in patients with critical illness and cancer: a meta‐analysis of randomized controlled clinical trials. Ann Surg 1999; 229: 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorensen LS, Rasmussen HH, Aardestrup IV, Thorlacius‐Ussing O, Lindorff‐Larsen K, Schmidt EB et al Rapid incorporation of ω‐3 fatty acids into colonic tissue after oral supplementation in patients with colorectal cancer: a randomized, placebo‐controlled intervention trial. J Parenter Enteral Nutr 2014; 38: 617–624. [DOI] [PubMed] [Google Scholar]
- 17. Sorensen L, Thorlacius‐Ussing O, Rasmussen HH, Lundbye‐Christensen S, Calder PC, Lindorff‐Larsen K et al Effects of perioperative supplementation with omega‐3 fatty acids on leukotriene B4 and leukotriene B5 production by stimulated neutrophils in patients with colorectal cancer: a randomized, placebo‐controlled intervention trial. Nutrients 2014; 6: 4043–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song M, Zhang X, Meyerhardt JA, Giovannucci EL, Ogino S, Fuchs CS et al Marine ω‐3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut 2017; 66: 1790–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Osland E, Hossain MB, Khan S, Memon MA. Effect of timing of pharmaconutrition (immunonutrition) administration on outcomes of elective surgery for gastrointestinal malignancies: a systematic review and meta‐analysis. J Parenter Enteral Nutr 2014; 38: 53–69. [DOI] [PubMed] [Google Scholar]
- 20. Osland E, Memon B, Memon MA. Pharmaconutrition administration on outcomes of elective oncological surgery for gastrointestinal malignancies: is timing everything? – a review of published meta‐analyses until the end of 2016. Transl Gastroenterol Hepatol 2018; 3: 52–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song G‐M, Tian X, Zhang L, Ou Y‐X, Yi L‐J, Shuai T et al Immunonutrition support for patients undergoing surgery for gastrointestinal malignancy. Medicine (Baltimore) 2015; 94: e1225. [DOI] [PMC free article] [PubMed] [Google Scholar]


