Abstract
Background
The incidence of colorectal cancer in patients aged less than 50 years is increasing in Western countries. This population‐based study investigated the age‐ and sex‐specific incidence of colorectal cancer over time in Sweden, and characterized trends in tumour localization and stage at diagnosis.
Methods
Patients diagnosed with colorectal cancer between 1970 and 2016 were identified from the Swedish Cancer Registry, and categorized by sex, age and tumour location. The incidence and average annual percentage change (AAPC) were estimated and compared between age groups.
Results
There was an overall increase in the incidence of colorectal cancer between 1970 and 2006, but a decrease in 2006–2016 (AAPC −0·55 (95 per cent c.i. −1·02 to −0·07) per cent). The largest increase in colonic cancer was in 1995–2005 in women aged less than 50 years (AAPC 2·30 (0·09 to 4·56) per cent versus 0·04 (−1·35 to 1·44) and − 0·67 (−1·62 to 0·28) per cent in women aged 50–74 and 75 years or more respectively). Since 1990, rectal cancer increased in patients of both sexes aged below 50 years, with higher AAPC values in women (2006–2016: 2·01 (−1·46 to 5·61) per cent versus 0·20 (−2·25 to 2·71) per cent in men). Younger patients were more likely than those aged 50–74 and 75 years or more to present with stage III–IV colonic (66·2, 57·6 and 49·6 per cent respectively) and rectal (61·2, 54·3 and 51·3 per cent) cancer. From the mid 1990s, rates of proximal and distal colorectal cancer, and rectal cancer were increased in patients aged less than 50 years.
Conclusion
The overall incidence of colorectal cancer in Sweden decreased in the past decade. However, in patients under 50 years of age the incidence of colorectal cancer – proximal, distal and rectal – continued to increase over time.
The overall incidence of colorectal cancer in Sweden increased between 1970 and 2006, with a decrease observed only in the last decade, 2006–2016. However, in patients younger than 50 years of age there was an ongoing increase, more marked in women.

Early onset colorectal cancer in sweden
Antecedentes
La incidencia del cáncer colorrectal (CCR) en pacientes < 50 años está aumentando en los países occidentales. El objetivo de este estudio de base poblacional fue investigar las tendencias y la incidencia específica por edad y sexo del CCR a lo largo del tiempo en Suecia, así como caracterizar las tendencias en la localización tumoral y en el estadio del CCR en el momento del diagnóstico.
Métodos
Los pacientes diagnosticados con CCR entre 1970 y 2016 fueron identificados a partir del Registro de Cáncer de Suecia. Se clasificaron por sexo, edad y localización del tumor. Se calcularon la incidencia media y el promedio del cambio porcentual anual (average annual percentage change, AAPC), comparándose entre los grupos de edad.
Resultados
Globalmente, la incidencia de CCR aumentó entre 1970‐2006, pero se observó una disminución de 0,6% (i.c. del 95% ‐1,02 a 0,07) entre 2006‐2016. El AAPC del cáncer de colon aumentó con el tiempo tanto en mujeres como en varones. En particular, el mayor aumento se observó entre 1995‐2005 en mujeres de < 50 años, que presentaron un AAPC de cáncer de colon de 2,3% (i.c. del 95% 0,09 a 4,56), mayor en comparación con los grupos de edad más avanzada (50‐74 años: 0,04%; i.c. del 95% ‐1,35 a 1,44; grupo de edad 75+: ‐0,67%; i.c. del 95% ‐1,62 a 0,28), aunque el análisis de datos proporcionó valores limitados de i.c del 95%. En los varones de < 50 años, el AAPC del cáncer de colon aumentó en un 1,2% (i.c. del 95% ‐0,80 a 3,21) entre 2006‐2016, pero la diferencia no fue significativa en comparación con otros grupos de edad. Desde 1990, los cánceres rectales aumentaron en pacientes de < 50 años, en ambos sexos y en particular en mujeres más que en varones (2006‐2016: mujeres 2,0%, i.c. del 95% −1,46 a 5,61 versus varones 0,2%, i.c. del 95% ‐2,25 a 2,71). En comparación con los grupos de mayor edad, los pacientes de < 50 años tenían más probabilidades de presentar cáncer de colon en estadio III/IV (66%, 58% y 50% en los grupos de edad de < 50, 50‐74 y mayores de 75 años, respectivamente) y cáncer de recto (61%, 54% y 51% en los grupos de edad de < 50, 50‐74 y mayores de 75 años, respectivamente). Desde mediados de los 90 se observaron tasas cada vez mayores de CCR proximal, distal y de cáncer de recto en pacientes de < 50 años.
Conclusión
La incidencia global de CCR en Suecia disminuyó en la última década. Sin embargo, en pacientes menores de 50 años, la incidencia del cáncer colorrectal, proximal, distal y rectal ha continuado aumentando a lo largo del tiempo.
Introduction
Colorectal cancer is the third most common cancer, with 1·36 million new cases estimated every year1. The annual incidence of colonic cancer in 2007–2011 in Sweden was 44 per 100 000 population in both sexes, and that of rectal cancer in the same period was 25 per 100 000 in men and 17 per 100 000 in women2.
A decreased trend in the overall incidence of colorectal cancer has usually been attributed to the introduction of screening programmes3, 4. Sweden has not yet introduced a national screening programme; however, a regional population‐based screening programme began in 2008 in the capital region of Stockholm. On average, 110 000 individuals aged 60–69 years were screened annually using faecal occult blood testing at 2‐year intervals.
Colorectal cancer usually affects the elderly, and only 4–5 per cent of cases are diagnosed in patients younger than 50 years2. An increased incidence of colorectal cancer in these younger patients has been reported in recent literature5, 6, 7, 8, although few of these were population‐based studies. Interestingly, patients under the age of 50 years are also more likely to present with more advanced tumour stages9, 10.
With respect to localization, the number of proximal colonic cancers has been reported as increasing in the older population, whereas distal tumours are decreasing, with a so‐called left‐to‐right shift11, 12, 13.
The aim of this study was to investigate the incidence of colorectal cancer in Sweden over time, including overall, age‐ and sex‐specific trends. Additional analyses were performed to examine the trends in localization and stage of disease at the time of diagnosis.
Methods
Data were extracted from Statistics Sweden, a national registry maintained by a government agency. The registry was searched for patients diagnosed with colorectal cancer and recorded in the Swedish Cancer Registry between 1970 and 2016. The reporting of all malignant tumours diagnosed at clinical, morphological and laboratory examination and/or autopsy to the Swedish Cancer Registry has been compulsory since its inception in 1958.
Tumour stage has been recorded in the Swedish Cancer Registry since 2004. The completeness of the registry has been benchmarked, with an underreporting rate estimated as about 4 per cent14. Although colorectal cancers have been registered since 1958, estimation of the incidence of rectal cancer before 1970 was deemed unreliable owing to the fact that anal cancers could not be excluded reliably. Thus, the study period was restricted to the years 1970–2016.
For the purpose of this study, inclusion criteria were: tumours of the colon and rectum diagnosed histologically as adenocarcinoma. Patients with tumour locations in the appendix, anus or unspecified were excluded. Individuals with synchronous primary colorectal tumours were excluded to avoid overestimating the incidence when performing subgroup analysis on localization. In patients with metachronous tumours, the first tumour was recorded and subsequent tumours were excluded.
Cancer localization was subgrouped using ICD‐7 codes as proximal (153·0 and 153·1, from caecum including splenic flexure), distal (153·2 and 153·3, from descending including sigmoid) and rectal (154·0, from rectosigmoid including rectum).
Patients included in the study were divided into the following age groups: 0–49, 50–74 and 75 years or more, based on the proposed lower age cut‐offs recommended in the USA15 and EU16 for colorectal cancer screening,.
The sixth edition of the TNM classification of malignant tumours was used for tumour staging. The TNM stage was based either on evidence acquired before treatment (cTNM) or on histopathology findings (pTNM). Data on tumour stage were combined with data retrieved from the Swedish Colorectal Cancer Registry based on the unique Swedish identification number. By combining the two registers, missing values were aimed to be kept to a minimum (0–11·7 per cent); analyses were possible starting from 2007. Accordingly, tumour stage was analysed for 2007–2016.
Endpoints
The primary endpoint was the overall age‐ and sex‐specific incidence of colorectal cancer (colon and rectum), including variation over time, between 1970 and 2016. Additional endpoints included tumour localization and stage at the time of diagnosis by age and sex over time.
Ethical approval for the study was obtained from the regional ethics committee (Stockholm; dnr 2016/1145‐31/2).
Statistical analysis
Incidence was estimated as the number of cases divided by the population on 31 December the previous year (cumulative incidence). It was reported as number of cases per 100 000 and age‐adjusted to the European Standard Population (1976). The cumulative incidence approach was considered reasonable because colorectal cancer is relatively rare and follow‐up is short (12 months).
The average incidence was characterized using an additive model for the logarithm of the rates in which the temporal non‐linearities were characterized by specifying the conditional mean using restricted cubic splines. Age group and sex, and their interactions with the spline effect, were added as effects to the model to enable curve estimation for each age group and sex17. For colonic cancer, localization (proximal and distal) was included in the same manner. The curves were presented graphically on the original scale by calculating the exponential of the mean predictions and the 95 per cent confidence intervals. The average annual percentage change (AAPC) was estimated separately for each period, 1970–1983, 1984–1994, 1995–2005 and 2006–2016, using linear least‐squares regression of log AAPC, in which time, age group and sex were included as co‐variables. Interactions between time, age group and sex were included to enable comparison between age groups. The AAPC, its group comparisons and 95 per cent c.i. were derived from the estimated slopes by the same back‐transformation as for the analysis of incidence.
Statistical analyses were performed using SPSS® (IBM, Armonk, New York, USA), SAS® (SAS Institute, Cary, North Carolina, USA), and R (R Foundation for Statistical Computing, Vienna, Austria).
Results
Some 212 451 colorectal cancers that met the inclusion criteria were identified from the Swedish Cancer Registry between 1970 and 2016. After the exclusion of 9468 patients with synchronous tumours and 33 859 reported as having subsequent metachronous tumours, 169 124 patients were left for analysis, with 104 816 colonic and 64 308 rectal cancers. With respect to tumour localization, there were no patients with missing information in 1970–1992, but in 1992–2016 there were missing records for 6 per cent, in both female and male populations, but with a slightly higher proportion in the youngest age group.
For the additional analysis by tumour stage (2007–2016), 26 484 patients with colonic cancer and complete TNM stage were identified, 76·0 per cent based on pTNM and 24·0 per cent on cTNM. Some 14 133 patients with rectal cancer and complete TNM stage were also identified, with the majority of TNM stages based on pTNM rather than cTNM (69·0 and 30·9 per cent respectively).
Colorectal cancer
The overall age‐standardized incidence of colorectal cancer in Sweden increased until 2006; a significant decrease was observed in the last decade (2006–2016), with an average annual decrease of 0·55 (95 per cent c.i. −1·02 to −0·07) per cent (Table 1 and Fig. 1). This decrease was observed for both colonic and rectal cancer (Fig. 2). However, incidence rates varied considerably over time, with a decrease in colorectal cancer around 1990, explained by a concurrent decrease in colonic cancer.
Table 1.
Average annual percentage change, overall and by age, sex and tumour location
| AAPC (%) | ||||
|---|---|---|---|---|
| 1970–1983 | 1984–1994 | 1995–2005 | 2006–2016 | |
| Overall | ||||
| Colorectal cancer | 0·55 (0·34, 0·77) | 0·28 (−0·96, 1·54) | 0·18 (−0·22, 0·58) | −0·55 (−1·02, −0·07) |
| Colonic cancer | 0·30 (−0·03, 0·63) | 0·46 (−1·41, 2·37) | 0·25 (−0·48, 0·98) | −0·39 (−0·99, 0·22) |
| Rectal cancer | 0·93 (0·52, 1·34) | 0·00 (−0·53, 0·54) | 0·07 (−0·56, 0·72) | −0·82 (−1·51, −0·13) |
| Colonic cancer | ||||
| Age < 50 years | ||||
| Women | −1·29 (−3·34, 0·80) | 0·84 (−2·83, 4·65) | 2·30 (0·09, 4·56) | 2·13 (−1·15, 5·52) |
| Men | −2·85 (−5·35, −0·29) | 0·00 (−3·99, 4·15) | 0·51 (−2·32, 3·41) | 1·19 (−0·80, 3·21) |
| Age 50–74 years | ||||
| Women | 0·54 (0·09, 0·99) | 0·27 (−1·61, 2·18) | 0·04 (−1·35, 1·44) | −1·07 (−2·03, −0·10) |
| Men | 0·57 (0·13, 1·01) | 1·39 (−0·79, 3·62) | 0·43 (−0·63, 1·51) | −0·73 (−1·78, 0·33) |
| Age ≥ 75 years | ||||
| Women | −0·08 (−1·18, 1·03) | −1·36 (−3·04, 0·34) | −0·67 (−1·62, 0·28) | 0·36 (−0·64, 1·37) |
| Men | 0·94 (0·11, 1·77) | 0·82 (−1·33, 3·01) | 0·79 (−0·37, 1·97) | 0·04 (−1·03, 1·13) |
| Rectal cancer | ||||
| Age < 50 years | ||||
| Women | 1·70 (−2·73, 6·34) | −1·20 (−4·59, 2·32) | 3·14 (0·51, 5·84) | 2·01 (−1·46, 5·61) |
| Men | −1·71 (−4·37, 1·01) | −1·86 (−4·35, 0·68) | −1·97 (−5·44, 1·62) | 0·20 (−2·25, 2·71) |
| Age 50–74 years | ||||
| Women | 0·72 (0·02, 1·42) | −1·73 (−2·71, −0·75) | −0·36 (−1·91, 1·22) | −1·80 (−2·58, −1·02) |
| Men | 1·69 (0·79, 2·59) | 1·98 (1·58, 2·39) | 1·25 (0·51, 2·01) | −0·02 (−1·05, 1·02) |
| Age ≥ 75 years | ||||
| Women | −0·18 (−1·43, 1·09) | −2·40 (−3·94, −0·84) | −1·54 (−2·74, −0·32) | −2·22 (−3·22, −1·21) |
| Men | 1·56 (0·72, 2·41) | 1·66 (0·41, 2·92) | −0·15 (−1·13, 0·83) | 0·04 (−1·40, 1·50) |
Values in parentheses are 95 per cent confidence intervals. AAPC, average annual percentage change.
Figure 1.
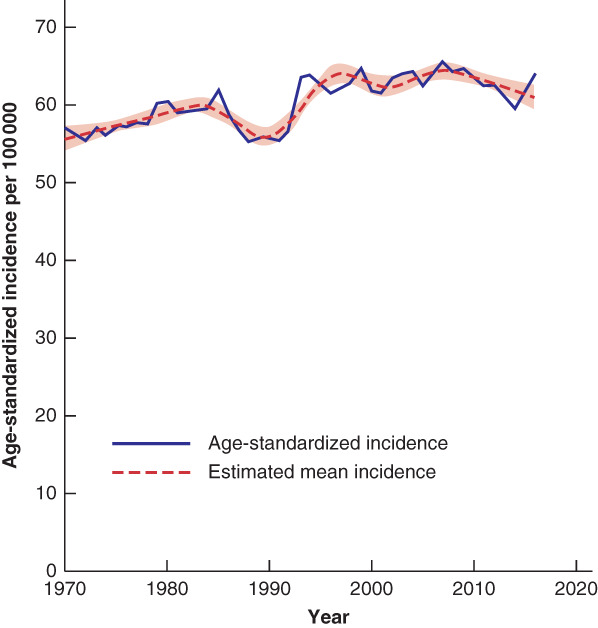
Overall age‐standardized incidence of colorectal cancer in Sweden, 1970–2016 The estimated mean incidence is also shown, with 95 per cent confidence intervals.
Figure 2.
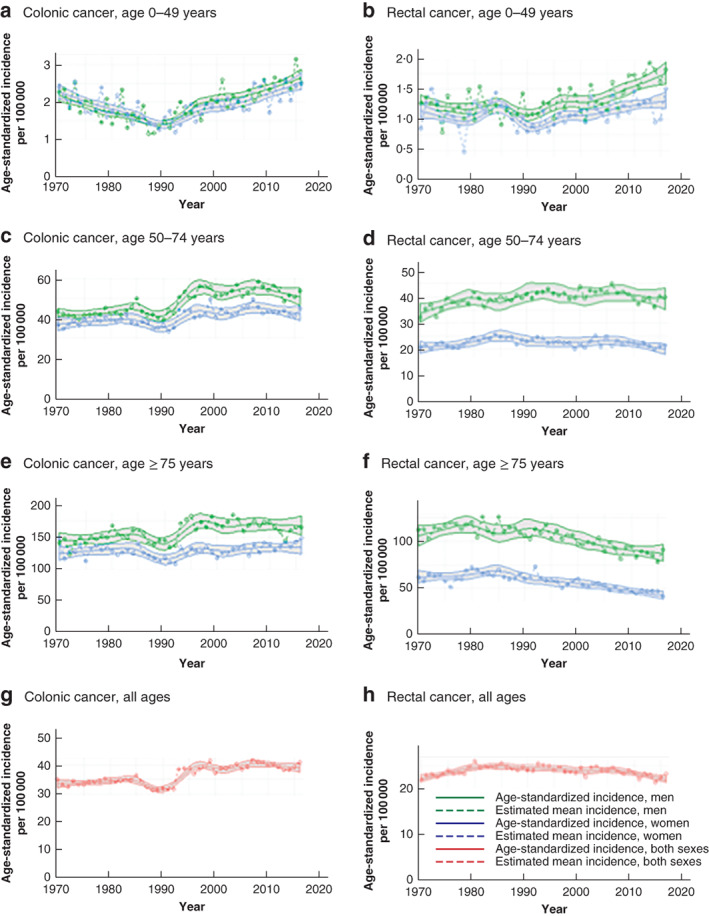
Age‐standardized incidence of colonic and rectal cancer in Sweden by age and sex, 1970–2016 Colonic cancer: a 0–49 years; c 50–74 years; e 75 years and above; g all patients. Rectal cancer: b 0–49 years; d 50–74 years; f 75 years and above; h all patients. The estimated mean incidence is also shown, with 95 per cent confidence intervals.
Colonic cancer
The age‐standardized overall incidence of colonic cancer from 1970 to 2016 is shown in Fig. 2. An overall increase was observed over time, although the incidence stabilized over the last two decades and there was a significant decrease in 2006–2016, with an average annual decrease of 0·39 (95 per cent c.i. −0·99 to 0·22) per cent (Table 1). The age‐standardized incidence showed substantial variation over time and between the different age groups.
The age‐specific incidence of colonic cancer increased steadily in the 0–49 years age group from the 1990s, whereas in the age groups 50–74 and 75 years or above it increased in the early 1990s, but then declined and stabilized (Fig. 2).
The greatest increase was seen in women under 50 years of age between 1995 and 2016, with an AAPC of 2·30 (95 per cent c.i. 0·09 to 4·56) per cent in 1995–2005; the increase continued in 2006–2016. This increase in younger women was higher in comparison to that in older age groups in 1995–2005 (0·04 per cent in women aged 50–74 years and − 0·67 per cent in those aged 75 years or more), but was not significantly different from that in the oldest age group in 2006–2016, and data analysis disclosed limited/borderline values for 95 per cent c.i. (Tables 1 and 2).
Table 2.
Ratio of average annual percentage change: comparison with patients aged under 50 years and by age, sex and tumour location
| AAPC ratio | ||
|---|---|---|
| Sex and age group compared | Colonic cancer | Rectal cancer |
| 2006–2016 | ||
| F, 0–49 versus ≥ 75 years | 1·02 (0·99, 1·04) | 1·04 (1·02, 1·07) |
| M, 0–49 versus ≥ 75 years | 1·01 (0·99, 1·03) | 1·00 (0·98, 1·03) |
| F, 0–49 versus 50–74 years | 1·03 (1·01, 1·05) | 1·04 (1·01, 1·06) |
| F, 50–74 versus ≥ 75 years | 0·99 (0·97, 1·00) | 1·00 (0·98, 1·03) |
| M, 0–49 versus 50–74 years | 1·02 (1·00, 1·04) | 1·00 (0·98, 1·03) |
| M, 50–74 versus ≥ 75 years | 0·99 (0·97, 1·01) | 1·00 (0·98, 1·02) |
| 1995–2005 | ||
| F, 0–49 versus ≥ 75 years | 1·03 (1·01, 1·05) | 1·05 (1·02, 1·07) |
| M, 0–49 versus ≥ 75 years | 1·00 (0·98, 1·02) | 0·98 (0·96, 1·00) |
| F, 0–49 versus 50–74 years | 1·02 (1·00, 1·04) | 1·04 (1·01, 1·06) |
| F, 50–74 versus ≥ 75 years | 1·01 (0·99, 1·03) | 1·01 (0·99, 1·04) |
| M, 0–49 versus 50–74 years | 1·00 (0·98, 1·02) | 0·97 (0·95, 0·99) |
| M, 50–74 versus ≥ 75 years | 1·00 (0·98, 1·01) | 1·01 (0·99, 1·04) |
| 1984–1994 | ||
| F, 0–49 versus ≥ 75 years | 1·02 (0·99, 1·05) | 1·01 (0·99, 1·04) |
| M, 0–49 versus ≥ 75 years | 0·99 (0·96, 1·02) | 0·97 (0·94, 0·99) |
| F, 0–49 versus 50–74 years | 1·01 (0·98, 1·04) | 1·01 (0·98, 1·03) |
| F, 50–74 versus ≥ 75 years | 1·02 (0·99, 1·05) | 1·01 (0·98, 1·04) |
| M, 0–49 versus 50–74 years | 0·99 (0·96, 1·02) | 0·96 (0·94, 0·99) |
| M, 50–74 versus ≥ 75 years | 1·01 (0·98, 1·04) | 1·00 (0·98, 1·03) |
| 1970–1983 | ||
| F, 0–49 versus ≥ 75 years | 0·99 (0·97, 1·00) | 1·02 (0·99, 1·04) |
| M, 0–49 versus ≥ 75 years | 0·96 (0·95, 0·98) | 0·97 (0·95, 0·99) |
| F, 0–49 versus 50–74 years | 0·98 (0·97, 1·00) | 1·01 (0·99, 1·04) |
| F, 50–74 versus ≥ 75 years | 1·01 (0·99, 1·02) | 1·01 (0·99, 1·03) |
| M, 0–49 versus 50–74 years | 0·97 (0·95, 0·98) | 0·97 (0·94, 0·99) |
| M, 50–74 versus ≥ 75 years | 1·00 (0·98, 1·01) | 1·00 (0·98, 1·03) |
Values in parentheses are 95 per cent confidence intervals. AAPC, average annual percentage change.
In men aged under 50 years the AAPC increased by 1·19 (95 per cent c.i. −0·80 to 3·21) per cent in 2006–2016, higher than that in men aged 50–74 years (–0·73 per cent), but not significantly different compared with that in men aged 75 years or above (0·04 per cent) (Tables 1 and 2).
In the 50–74 years age group, there was a negative AAPC in overall incidence among both men and women over the years 2006–2016 (−0·73 (95 per cent c.i. −1·78 to 0·33) versus −1·07 (−2·03 to −0·10) per cent respectively) (Table 1).
Incidence by age and localization revealed an increase in proximal and distal colonic cancer in both sexes aged under 50 years from the early 1990s (Fig. 3). Proximal cancer was more common in young men than distal colonic cancer (Fig. 3). In younger women, similar proportions of proximal and distal colonic cancer were observed, but with increasing age there were more proximal colonic cancers, consistent with a left‐to‐right shift (Fig. 3). In the most recent years there was an increase in proximal colonic cancers in all age groups regardless of sex, whereas distal and rectal tumours decreased or stabilized in patients aged 50 years or above (Figs 2, 3, 4).
Figure 3.
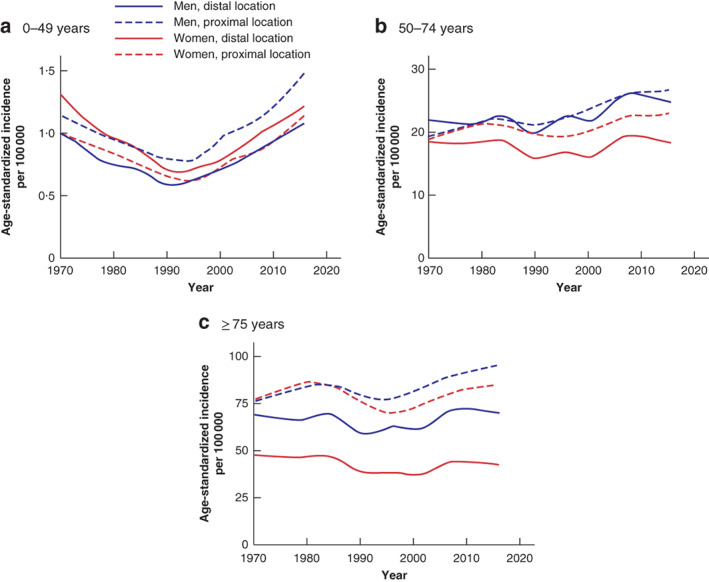
Age‐standardized incidence of colonic cancer in Sweden by age, sex and tumour location, 1970–2016 a 0–49 years; b 50–74 years; c 75 years and above.
Figure 4.
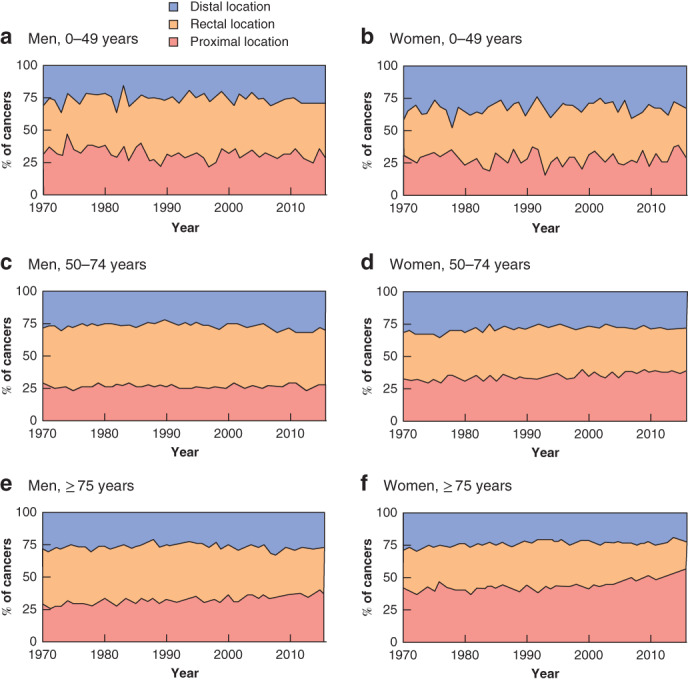
Proportion of colorectal cancer according to tumour location by age and sex a Men aged 0–49 years; b women aged 0–49 years; c men aged 50–74 years; d women aged 50–74 years; e men aged 75 years and above; f women aged 75 years and above.
Patients under the age of 50 years were more likely to present with more advanced disease (stage III–IV) than those in older age groups (66·2 per cent of patients aged less than 50 years versus 57·6 per cent in patients aged 50–74 years and 49·6 per cent in those aged 75 years or more). The stage of disease at presentation remained relatively stable between 2007 and 2016 (Figs 5 and 6).
Figure 5.
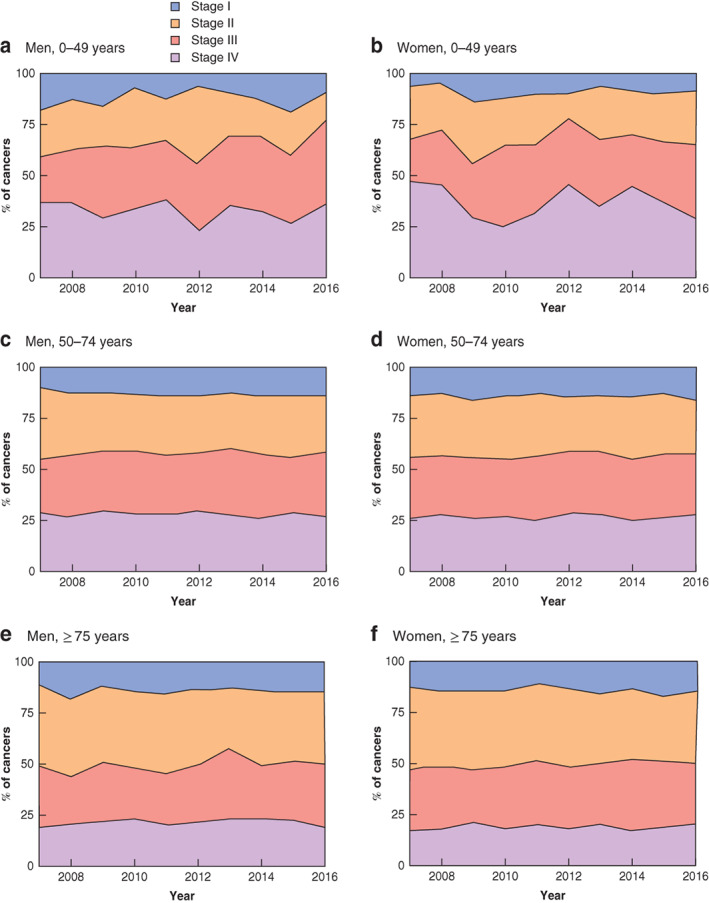
Proportion of colonic cancer according to UICC tumour stage at presentation by age and sex a Men aged 0–49 years; b women aged 0–49 years; c men aged 50–74 years; d women aged 50–74 years; e men aged 75 years and above; f women aged 75 years and above.
Figure 6.
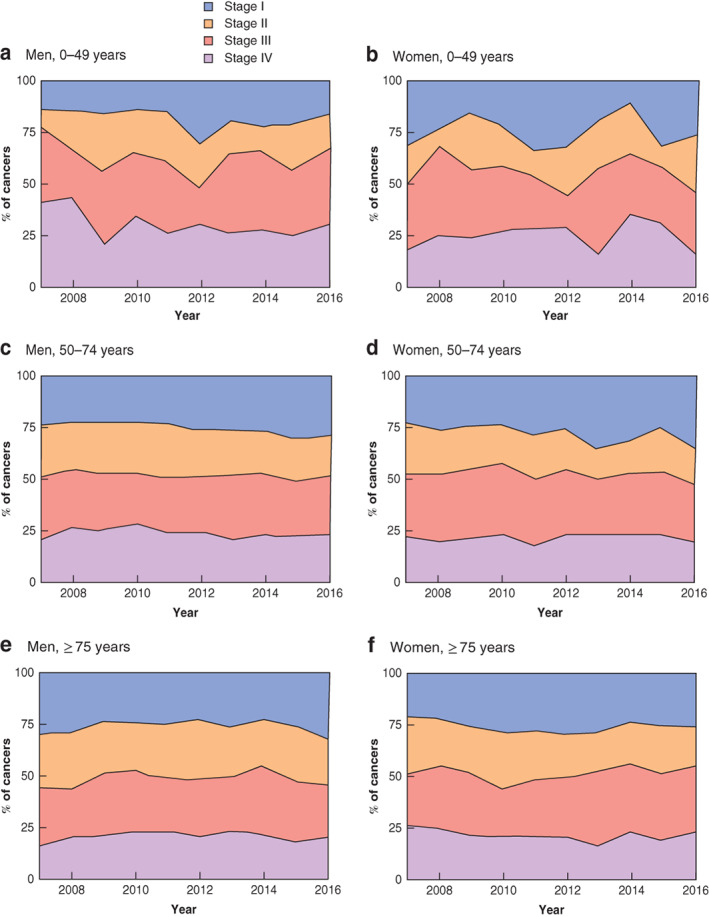
Proportion of rectal cancer according to UICC tumour stage at presentation by age and sex a Men aged 0–49 years; b women aged 0–49 years; c men aged 50–74 years; d women aged 50–74 years; e men aged 75 years and above; f women aged 75 years and above.
Rectal cancer
The overall incidence of rectal cancer in Sweden decreased steadily from 1985 to 2006, particularly in the oldest age group (Fig. 2). However, since 1990 there was an increase in rectal cancer among patients aged less than 50 years in both sexes, especially in women (2·01 (95 per cent c.i. −1·46 to 5·61) per cent versus 0·20 (−2·25 to 2·71) in men in 2006–2016) (Table 1). The greater increase in the AAPC rate was documented between 1995–2005 and 2006–2016 in women under 50 years of age: 3·14 (0·51 to 5·84) and 2·01 (−1·46 to 5·61) per cent respectively. This was higher than that in women aged 50–74 years (−1·80 per cent) and in those in the oldest age group (−2·22 per cent), although data analysis again disclosed limited/borderline values for the 95 per cent c.i. (Tables 1 and 2).
In men aged under 50 years the AAPC rate in 2006–2016 did not differ significantly between the 50–74 years and the oldest age group: 0·22 versus − 0·02 and 0·04 per cent respectively (Table 1).
The incidence of rectal cancer decreased in the oldest age group over time, largely attributed to the steady decline in incidence for women (−2·22 (95 per cent c.i. −3·22 to −1·21) per cent over the years 2006–2016) (Table 1). The incidence of rectal cancer was similar among men and women in the youngest age group, but was more common in men in patients aged over 50 years.
A greater proportion of patients under the age of 50 years presented with a more advanced tumour stage (stage III–IV): 61·2 per cent versus 54·3 and 51·3 per cent in the 50–74 and 75 years and over age groups respectively.
Discussion
Several studies have documented a general decrease in the incidence of colorectal cancer in developed countries4, 8. However, the literature has also documented an increased rate of colorectal cancer in patients under 50 years of age, mainly with a distal colonic and rectal localization4, 6, 18, 19. In the present study, the incidence of colorectal cancer – proximal, distal and rectal – was found to be increased in patients aged less than 50 years, especially in women. Similarly, the increasing trend in incidence of proximal colonic cancer (mainly in older women11, 13) has been reported by a number of authors, providing the basis for the left‐to‐right shift in colorectal cancer.
Although some investigators have proposed that this should be explained as the result of incomplete colonoscopies20, the overall increase in proximal tumours internationally and in the present study could also suggest an actual increase in proximal colonic cancer, possibly secondary to changes in environmental risk factors. The risk factors affecting the incidence of colorectal cancer are recognized to be multifactorial, inherited and acquired, including obesity, physical inactivity, diabetes, smoking, alcohol consumption and high red meat intake20, 21, 22. The increased incidence of obesity and red meat consumption could partly explain the increase in colorectal cancer in patients under the age of 50 years23. Smoking, however, decreased in Sweden24, and alcohol consumption has been relatively stable over time24.
The recent decrease in the overall incidence of colorectal cancer reported in previous studies3, 4, 8 has been related to the implementation of screening programmes. The present population‐based study shows an overall increase in incidence between 1970 and 2006, with a decrease observed in the last decade starting from 2006, consistent with the subsequent introduction of a regional population‐based screening programme in 2008.
The findings reported here of young patients presenting with more advanced disease (stage III–IV), regardless of tumour localization, are in line with those of a previously published study25.
Strengths of this study include the high quality of data from the Swedish Cancer Registry based on the entire Swedish population, and the enrichment of data from the Swedish Colorectal Cancer Registry regarding TNM staging. The separate reporting of synchronous and metachronous tumours could also be interpreted as a strength.
However, a potential limitation is in relation to stages of the disease, as these are based on clinical or pathological information. This is particularly important when considering rectal cancer, as the pTNM is more likely to be downstaged due to (chemo)radiotherapy.
These increasing rates of colorectal cancer in younger patients are of concern, and the factors relating to this increasing incidence require further investigation.
Statistical Editor's comments.
As a retired university teacher in medical statistics and epidemiology, I have always been intrigued by finding research publications with highly complex statistical methods combined with misuse of fundamental concepts. The two things often seem linked, and this study by Petersson and colleagues is not an exception.
First a brief background: the concepts of disease frequency, incidence rate, risk, and their calculations are not generally well understood1. Confused incidence calculations have already been described and criticized2.
The term cumulative incidence is a synonym for disease risk, the probability that an individual in a defined population will develop a disease. Cumulative incidence can be estimated directly in a cohort with fixed membership, for example as defined by a common event (such as having been randomized in a specific clinical trial) and followed up until disease endpoint or the study closing date. Cumulative incidence is estimated as a proportion, with the number of individuals developing the disease during follow‐up as the numerator and the number of cohort members at start of follow‐up as
the denominator.
In contrast, a dynamic population, for example the population of a country, varies during follow‐up because children are born and people die, others emigrate or immigrate. It is not practically possible to define a baseline, perform standardized follow‐up, and evaluate the disease endpoints as in a fixed‐membership cohort. However, another incidence measure, the incidence density, can be estimated. This measure is defined as a ratio with the same numerator as for the cumulative incidence but with a denominator consisting of the amount of person‐time at risk (the mean number of people at risk during follow‐up multiplied by the amount of time they have been at risk).
The number of person‐years at risk is for a specific calendar year, often calculated as the year's mid‐year population (multiplied by 1 year to get person‐years). The mid‐year population for a calendar year is defined as the arithmetic mean of that year's and the preceding years’ end‐of‐year populations.
In their article, Petersson and colleagues explain that they have estimated the cumulative incidence and that this is a reasonable approach: ‘Incidence was estimated as the number of cases divided by the population on 31 December the previous year (cumulative incidence)’ and ‘The cumulative incidence approach was considered reasonable because colorectal cancer is relatively rare and follow‐up is short (12 months).’
This description indicates a confusion between the two incidence measures. The population on 31 December the previous year is, because of the described population dynamics, not an adequate measure of the number of persons at risk during the next year, and the number of persons developing cancer during a year is not necessarily registered in the population register for 31 December the year before. In other words, the relation between the numerator and the denominator is not the one that is required for estimating cumulative incidence. Moreover, no adjustments to the number of registered cancer cases, or to the population sizes in order to achieve an adequate relation between numerator and denominator, are mentioned.
A more reasonable interpretation of the performed calculations is, therefore, that the authors, instead of the cumulative incidence, have calculated the incidence density, but with the amount of person‐time at risk based on previous end‐of‐year populations instead of mid‐year populations.
Given that the amount of person‐time at risk is then systematically miscalculated, that the studied population is ageing, and that the risk of the studied disease increases with age, the presented results are likely to be biased. The magnitude of this bias cannot easily be assessed without a statistical reanalysis. The bias may be minor, perhaps even negligible.
Nevertheless, the uncertainty of the results’ validity is an unnecessary limitation in this article, and this could easily have been avoided.
J. Ranstam
Statistical Editor, BJS
1 Vandenbroucke JP, Pearce N. Incidence rate in dynamic populations. Int J Epidemiol 2012; 41: 1472–1479.
2 Dobler CC. Cumulative incidence and incidence rate ratio for estimation of risk of tuberculosis in patients with cancer. Clin Infect Dis 2017; 65: 1423.
Authors’ reply to Statistical Editor's comments.
We are grateful to the Statistical Editor at BJS Open for scrutinizing our approach for estimating incidence (number of cases per 100 000). This will contribute to an increased awareness for the broader audience on proper estimation of incidence. The main objection against our approach to estimation using the previous end‐of‐year population is that the population is dynamic and will change during the 12‐month follow‐up. This should be accounted for using the average between the population at risk at the start and at the end of follow‐up (mid‐year population) as the at‐risk population (denominator).We have argued that, for large populations and rare diseases, both approaches will lead to similar results, but in smaller populations the differences may be larger.
To address the magnitude of discrepancy in the age‐standardized cumulative incidence between the two approaches, results of both methods are displayed in Fig. 1 below. All patients with colorectal cancer as well as subgroup analyses of men with colonic cancer are shown. The reader will note the close approximation of the curves for ‘mid‐year’ and ‘previous end‐of‐the‐year’ calculations. Fig. 1a corresponds to Fig. 1 in the main manuscript; Fig. 1b‐d correspond to the right‐side panels in Fig. 2 of the main manuscript.
To examine the discrepancies in more detail, percentage differences between the two approaches are displayed in Fig. 2 . For all patients (Fig. 2a ), the approach used in our article yields a slightly higher incidence. For example, in the year 2010, the estimates are 61⋅50 and 61⋅10 for our end‐of‐year approach and mid‐year approach respectively. The differences become slightly more pronounced in subgroup analysis. Amongst men aged 0–49 years (Fig. 2b ), the difference in incidence peaks in 1988, where the values are 1⋅11 and 1⋅09 respectively. Discrepancies of similar magnitude are seen amongst women and patients with rectal cancer.
In summary, we feel that the study results are highly unlikely to be significantly biased by the effects of person‐time at risk.We appreciate this opportunity for dialogue regarding the statistical methodology of our study.
Figure 1.
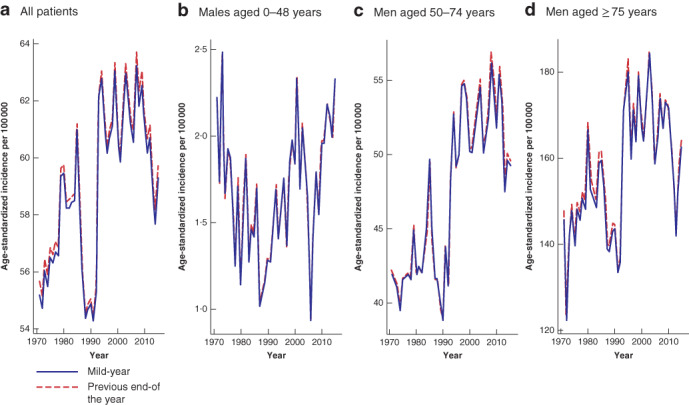
Comparison of mid‐year and previous end‐of‐the‐year calculations for age‐adjusted incidence of colorectal cancer, 1970–2016 a All patients. b Male patients aged 0–49 years, c men aged 50–74 years and d men aged 75 years or more with colonic cancer.
Figure 2.
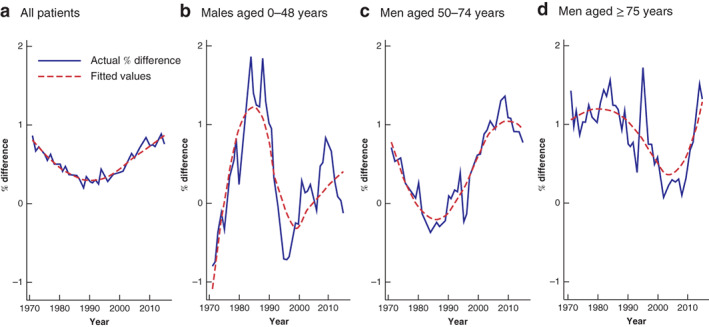
Percentage difference in colorectal cancer: previous end‐of‐the year versus mid‐year population a All patients. b Men aged 0–49 years, c men aged 50–74 years and d men aged 75 years or more with colonic cancer.
Acknowledgements
This study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG‐75070 and SLL Dnr 2017 – 1341), the Swedish Cancer Society (18 0523; 2016/509), Mag‐Tarmfonden and the Bengt Ihre Foundation.
Disclosure: The authors declare no conflict of interest.
Funding information
The ALF agreement, ALFGBG‐75070 and SLL Dnr 2017–1341
Swedish Cancer Society, 18 0523 (2016/509)
Mag‐Tarmfonden
Bengt Ihre Foundation
References
- 1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M et al Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019; 144: 1941–1953. [DOI] [PubMed] [Google Scholar]
- 2. Engholm G, Ferlay J, Christensen N, Hansen HL, Hertzum‐Larsen R, Johannesen TB et al NORDCAN: cancer incidence, mortality, prevalence and survival in the nordic countries, version 8.1 (28.06.2018). Association of the Nordic Cancer Registries. Danish Cancer Society. http://www.ancr.nu [accessed 12 December 2018]. [Google Scholar]
- 3. Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN et al Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116: 544–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A et al Colorectal cancer statistics, 2017. CA Cancer J Clin 2017; 67: 177–193. [DOI] [PubMed] [Google Scholar]
- 5. Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J et al The increasing incidence of young‐onset colorectal cancer: a call to action. Mayo Clin Proc 2014; 89: 216–224. [DOI] [PubMed] [Google Scholar]
- 6. Gandhi J, Davidson C, Hall C, Pearson J, Eglinton T, Wakeman C et al Population‐based study demonstrating an increase in colorectal cancer in young patients. Br J Surg 2017; 104: 1063–1068. [DOI] [PubMed] [Google Scholar]
- 7. Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez‐Bigas MA, Skibber JM et al Increasing disparities in the age‐related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg 2015; 150: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel P, De P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15–49‐year‐olds in Canada, 1969–2010. Cancer Epidemiol 2016; 42: 90–100. [DOI] [PubMed] [Google Scholar]
- 9. Abdelsattar ZM, Wong SL, Regenbogen SE, Jomaa DM, Hardiman KM, Hendren S. Colorectal cancer outcomes and treatment patterns in patients too young for average‐risk screening. Cancer 2016; 122: 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moghadamyeghaneh Z, Alizadeh RF, Phelan M, Carmichael JC, Mills S, Pigazzi A et al Trends in colorectal cancer admissions and stage at presentation: impact of screening. Surg Endosc 2016; 30: 3604–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cucino C, Buchner AM, Sonnenberg A. Continued rightward shift of colorectal cancer. Dis Colon Rectum 2002; 45: 1035–1040. [DOI] [PubMed] [Google Scholar]
- 12. Larsen IK, Bray F. Trends in colorectal cancer incidence in Norway 1962–2006: an interpretation of the temporal patterns by anatomic subsite. Int J Cancer 2010; 126: 721–732. [DOI] [PubMed] [Google Scholar]
- 13. Saltzstein SL, Behling CA. Age and time as factors in the left‐to‐right shift of the subsite of colorectal adenocarcinoma: a study of 213 383 cases from the California Cancer Registry. J Clin Gastroenterol 2007; 41: 173–177. [DOI] [PubMed] [Google Scholar]
- 14. Mattsson B, Wallgren A. Completeness of the Swedish Cancer Register. Non‐notified cancer cases recorded on death certificates in 1978. Acta Radiol Oncol 1984; 23: 305–313. [DOI] [PubMed] [Google Scholar]
- 15. Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T et al Colorectal cancer screening: recommendations for physicians and patients from the US multi‐society task force on colorectal cancer. Am J Gastroenterol 2017; 112: 1016–1030. [DOI] [PubMed] [Google Scholar]
- 16. European Colorectal Cancer Screening Guidelines Working Group , von Karsa L, Patnick J, Segnan N, Atkin W, Halloran S, Lansdorp‐Vogelaar I et al European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy 2013; 45: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heuer C. Modeling of time trends and interactions in vital rates using restricted regression splines. Biometrics 1997; 53: 161–177. [PubMed] [Google Scholar]
- 18. You YN, Dozois EJ, Boardman LA, Aakre J, Huebner M, Larson DW. Young‐onset rectal cancer: presentation, pattern of care and long‐term oncologic outcomes compared to a matched older‐onset cohort. Ann Surg Oncol 2011; 18: 2469–2476. [DOI] [PubMed] [Google Scholar]
- 19. Fairley TL, Cardinez CJ, Martin J, Alley L, Friedman C, Edwards B et al Colorectal cancer in US adults younger than 50 years of age, 1998–2001. Cancer 2006; 107: 1153–1161. [DOI] [PubMed] [Google Scholar]
- 20. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta‐analysis of prospective studies. Am J Clin Nutr 2007; 86: 556–565. [DOI] [PubMed] [Google Scholar]
- 21. Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M et al Association of obesity with risk of early‐onset colorectal cancer among women. JAMA Oncol 2019; 5: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta‐analysis. Br J Cancer 2009; 100: 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lööv H, Lannhard Öberg Å, Loxbo H, Lukkarinen J, Lindow K. Köttkonsumtionen i Siffror – Utveckling och Orsaker (Rapport 2013:2). Jordbruksverket: Jönköping, 2013. [Google Scholar]
- 24. Sundin J, Willner S. Social Change and Health in Sweden: 250 Years of Politics and Practice. Swedish National Institute of Public Health: Solna, 2007. [Google Scholar]
- 25. Orsini RG, Verhoeven RHA, Lemmens VEPP, van Steenbergen LN, de Hingh IHJT, Nieuwenhuijzen GAP et al Comparable survival for young rectal cancer patients, despite unfavourable morphology and more advanced‐stage disease. Eur J Cancer 2015; 51: 1675–1682. [DOI] [PubMed] [Google Scholar]


