Abstract
Background
Many patients develop seroma after laparoscopic ventral hernia repair. It was hypothesized that leaving the hernial sac in situ may cause this complication.
Methods
In this patient‐ and outcome assessor‐blinded, parallel‐design single‐centre trial, patients undergoing laparoscopic intraperitoneal onlay mesh ventral hernia repair were randomized (1 : 1) to either conventional fascial closure or peritoneal bridging. The primary endpoint was the incidence of seroma 12 months after index surgery detected by CT, evaluated in an intention‐to‐treat analysis.
Results
Between September 2017 and May 2018, 62 patients were assessed for eligibility, of whom 25 were randomized to conventional closure and 25 to peritoneal bridging. At 3 months, one patient was lost to follow‐up in the conventional and peritoneal bridging groups respectively. No seroma was detected at 6 or 12 months in either group. The prevalence of clinical seroma was four of 25 (16 (95 per cent c.i. 2 to 30) per cent) versus none of 25 patients in the conventional fascial closure and peritoneal bridging groups respectively at 1 month after surgery (P = 0·110), and two of 24 (8 (0 to 19) per cent) versus none of 25 at 3 months (P = 0·235). There were no significant differences between the groups in other postoperative complications (one of 25 versus 0 of 25), rate of recurrent hernia within 1 year (none in either group) or postoperative pain.
Conclusion
Conventional fascial closure and peritoneal bridging did not differ with regard to seroma formation after laparoscopic ventral hernia repair. Trial registration: ClinicalTrials.gov (NCT03344575).
Fifty patients were enrolled and randomized to laparoscopic hernia repair with conventional fascial closure or a peritoneal bridging approach. Follow‐up by CT at 12 months (primary endpoint) revealed no seroma in either group, but seroma was detected clinically in the early postoperative period after 1 month (4 of 25 versus 0 of 25 patients in fascial closure and peritoneal bridging groups respectively) and 3 months (2 of 24 versus 0 of 25). The results suggest that peritoneal bridging is a safe and feasible alternative to conventional fascial closure, although larger studies are required to confirm this.
No difference in seroma formation, complications or rate of recurrent hernia.
Antecedentes
Tras la reparación laparoscópica de una eventración muchos pacientes desarrollan seromas. Se planteó la hipótesis de que dejar el saco herniario in situ puede ser causa de esta complicación.
Métodos
En este ensayo clínico unicéntrico, de grupos paralelos y ciego para el evaluador, se aleatorizaron (1:1) los pacientes en los que se realizó una reparación laparoscópica de una eventración mediante la colocación de una malla intraperitoneal (intraperitoneal onlay mesh, IPOM) con cierre convencional de la fascia o dejando el saco herniario. La variable principal fue la incidencia de seroma 12 meses después de la cirugía, detectada por tomografía computarizada. Se realizó el análisis por intención de tratamiento.
Resultados
Entre septiembre de 2017 y mayo de 2018, de 62 pacientes posibles, 25 se asignaron al grupo de cierre convencional y 25 al grupo en el que se dejaba el saco herniario. A los 6 y 12 meses de seguimiento, se perdieron un paciente de cada grupo. No se detectaron seromas en ninguno de los grupos a los 6 ó 12 meses. La prevalencia de seroma clínico a los 1 y 3 meses fue de 4/25 (16%, i.c. del 95% 2‐30%) versus 0/25 pacientes (P = 0,110) y 2/24 (8%, i.c. del 95% 0‐19%) versus 0/25 pacientes (P = 0,235) en el grupo de cierre fascial convencional versus el grupo en el que se dejó el saco peritoneal, respectivamente. No hubo diferencias significativas entre los grupos en otras complicaciones postoperatorias (1/25 versus 0/25), tasa de recidiva de la hernia al año (ninguna en ambos grupos), dolor postoperatorio o calidad de vida.
Conclusión
No hubo diferencias entre el cierre convencional de la fascia o dejando el saco herniario en la formación de un seroma tras la reparación laparoscópica de una eventración.
Introduction
Laparoscopic ventral hernia repair (LVHR), first described in 1993 by LeBlanc and Booth 1 , is a well established technique worldwide, but there is considerable controversy regarding the optimal approach. Two laparoscopic approaches are commonly used in LVHR: simple intraperitoneal onlay mesh (IPOM) and IPOM with defect closure before placement of mesh (IPOM‐plus).
Seroma formation is a common complication after LVHR, leading to an adverse aesthetic outcome, discomfort, pain and infections 2 , 3 . The published incidence of seroma after LVHR is highly variable. Previous studies have reported an incidence rate of seroma detected by clinical examination alone ranging from 0·5 per cent 4 to 35 per cent 5 . Various procedures have been suggested to reduce seroma formation between the mesh and the sac, but only conventional defect closure seems to reduce postoperative seroma significantly 3 . A recent study 6 reported seroma at the 1‐month visit, detected by clinical examination and (if inconclusive) abdominal CT, in 58 per cent of patients who underwent simple IPOM versus 25 per cent of those who had IPOM‐plus. It has been hypothesized that the lower incidence of seroma following defect closure is because of decreased dead space in the residual sac. Additionally, other complications such as recurrence and mesh bulging are less pronounced after conventional defect closure than open repair 7 , 8 or simple IPOM 3 , 9 , 10 , 11 . However, in comparisons of simple IPOM and IPOM‐plus, Clapp and colleagues 12 noted the incidence of seroma to be 27·8 versus 5·6 per cent respectively, whereas Zeichen et al.13 reported rates of 4·3 versus 11·4 per cent respectively. It is unclear whether these two studies used different criteria to define and measure postoperative seroma, which may explain the contradictory results. Although the incidence of seroma may be lower after IPOM‐plus than simple IPOM, it still remains a common complication and a major issue for surgeons 3 . IPOM‐plus may also cause more postoperative pain and discomfort as a result of the tension created by defect closure 3 , 12 .
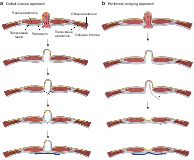
Instead of defect closure with suture, the part of the peritoneum constituting the hernial sac may be dissected and used to bridge the defect (Figure 1 ). This provides additional benefits of a near‐total decrease in the dead space by reducing the residual sac, thereby reducing serous fluid accumulation, while maintaining the tension‐free repair principle, resulting in less postoperative pain and discomfort. Furthermore, peritoneal bridging has the same advantage as defect closure in terms of a greater attachment area for mesh application, allowing improved mesh stabilization and thereby a low recurrence rate compared with simple IPOM 3 , 9 , 13 .
Figure 1.
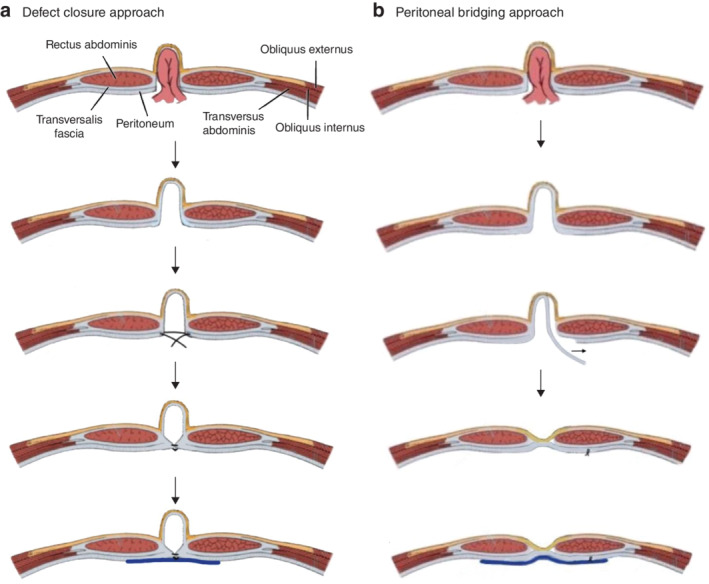
Schematic diagram of laparoscopic hernia repair methods a In the defect closure approach, the hernia is reduced, the fascial defect is closed with suture and the fascial closure reinforced with mesh. b In the peritoneal bridging approach, the hernia is reduced, the peritoneal layer in the hernia sac is dissected, and the protrusion of the hernia wall reduced and reinforced with mesh.
The main objective of the present trial was to assess whether the incidence of seroma was lower after peritoneal bridging compared with a conventional fascial closure approach in a small group of patients, in order to also assess the relative safety and feasibility of the trial during the assigned time interval. Should this trial provide evidence for the relative safety and feasibility of peritoneal bridging compared with the conventional approach, a subsequent trial with a large sample size will be undertaken to better assess whether there is a significant difference in seroma prevalence between the two approaches.
Methods
This was a single‐centre, outcome assessor‐ and patient‐blinded, two‐group parallel‐design RCT conducted at the Department of Surgery, Karlskoga hospital. Patient enrolment took place between September 2017 and May 2018. Patients aged between 18 and 80 years, undergoing elective laparoscopic hernia repair for a midline ventral hernia with a defect of 3–10 cm in diameter, were eligible for inclusion. Midline ventral hernia was defined as any hernia in the anterior abdominal wall between the xiphoid process and the superior pubic ramus. Exclusion criteria were: inability to provide informed consent; emergency surgery (for irreducible, strangulated or obstructed hernia); serious co‐morbidities; preoperative suspicion of extensive adhesions; pregnancy or intended pregnancy; BMI exceeding 40 kg/m2; and participation in any other study where concurrent involvement in the present study might place the patient at undue risk or confound the study data in the opinion of the chief investigator.
The study was approved by the Ethics Review Board of Uppsala University (2017/120), and all patients provided written informed consent more than 24 h before surgery. The trial was registered at ClinicalTrials.gov (NCT03344575). The CONSORT checklist 14 was followed for reporting of the study.
Trial randomization and blinding
The patients were randomized into two arms: IPOM with fascial closure (IPOM‐plus) and IPOM with peritoneal bridging (IPOM‐pb). Eligible patients were randomized by means of computerized simple sequence randomization. No blocking was done. The patients were enrolled by the surgeon on duty according to the protocol, and generation of the random allocation and assignment of participants according to sequence allocation was completed by the secretary of the department of surgery. The random allocation was concealed by using sequentially numbered, opaque, sealed envelopes. The envelope was later opened in sequence just before surgery. Patients and outcome assessors were blinded to the allocation for 12 months after surgery. The operating staff members were instructed not to disclose the procedure allocation to anyone.
Interventions
All procedures were performed by a single senior consultant with a wide experience of laparoscopic hernia surgery. Prophylactic antibiotics were given at the time of induction of anaesthesia. One 12‐mm and two 5‐mm ports were used. The fascial defect was measured during operation with a sterile tape measure at an intra‐abdominal pressure of 8 mmHg after adhesions had been dissected free. Adhesiolysis was carried out at an intra‐abdominal pressure of 12 mmHg. Fascial closure or peritoneal bridging was achieved at no more than 12 mmHg pressure (10–12 mmHg).
Each patient underwent midline ventral hernia repair using a laparoscopic IPOM technique. The hernial defect was repaired according to the allocation: IPOM‐plus or peritoneal IPOM‐pb. In the IPOM‐plus group, the hernia defect was closed with a 2/0 knotless polydioxanone suture before application of the mesh. In the IPOM‐pb group, the peritoneum was dissected, starting 2–3 cm from the edge of the defect along the path to the midpoint of the hernia sac. The free end of peritoneum was pulled to bridge the hernial defect and fixed with tackers.
The mesh was positioned with a peritoneal overlap. Its size was chosen to ensure at least 5 cm overlap on all sides of the defect. The edges of the fascial defect were fixed with the remaining tacks placed on the outer and inner rim along the closure line, to secure the mesh and to prevent folding (double‐crown technique).
In the event of intraoperative contamination, the laparoscopic procedure was abandoned and converted to open surgery. Patients whose operation was converted remained in the study and were analysed according to intention to treat.
Local anaesthetic (20 ml ropivacaine 7·5 mg/ml) infiltration was given routinely. The skin was closed with intradermal 4·0 Monocryl™ (Ethicon, Cornelia, Georgia, USA) suture. Wound dressings were applied at the end of the procedure according to the surgeon's usual practice, and remained unchanged for 24 h unless there was a clinical indication for changing the dressing. Patients were instructed to wear an abdominal elastic binder continuously for 14 days after index surgery, and only during the day for 14 days thereafter.
Patient assessments
Patients were assessed within 1 month of planned surgery, at operation, and at 1 week, 1 month ± 2 days, 3 months ± 3 days, 6 months ± 7 days and 12 months ± 14 days.
Outcomes
The primary endpoint was seroma detected by CT at the 12‐month visit after index surgery. The original primary outcome of seroma volume measured by CT at 12 months was changed after trial registration to better address the research question of whether there was a significant difference in incidence rate of seroma between IPOM‐plus and IPOM‐pb. This change to the protocol was made in November 2017 and updated in ClinicalTrials.gov on 26 January 2020.
Secondary outcomes included: duration of operation; duration of IPOM approach; duration of mesh fixation procedure; clinically detected seroma at 1‐, 3‐ and 6‐month visits; recurrence rate at 12‐month visit; postoperative pain measured by means of a visual analogue scale (VAS) at 1 week, 1 and 6 months, and 1 year after surgery; and adverse events.
Statistical analysis
The power calculation was based on the clinical presentation of seroma, which for the purpose of this study was defined as a palpable fluid swelling at the site of previous hernia during the postoperative period. Except for one study 15 reporting a 25 per cent incidence, there was a lack of previous reports with a large enough sample size based on the same clinical definition 16 . Therefore, based on the assumption that 25 per cent in the IPOM‐plus group would develop postoperative seroma and 5 per cent in the IPOM‐pb group, in a two‐sided test, a total of 48 procedures would have to be included to reach a 50 per cent chance of detecting a significant difference at the P < 0·050 level.
The incidence of seroma is presented as numbers (percentage; 95 per cent confidence interval), and other data as numbers or median (i.q.r.). Patients who were lost to follow‐up were not included in the analysis from the point of loss to follow‐up. Outcomes were analysed according to the intention‐to‐treat principle. The outcome data were not normally distributed (assessed by Kolmogorov–Smirnov test), so non‐parametric statistical tests were used. Levene's test was used to assess the assumption of homogeneity of variances for the Mann–Whitney U test that was used for continuous variables. Fisher's exact test was used for analysis of dichotomous variables. P < 0·050 was considered statistically significant (two‐sided α). Data were analysed using SPSS® software version 24 (IBM, Armonk, New York, USA).
Results
Between September 2017 and May 2018, 62 patients were assessed for eligibility, of whom 25 were randomized to IPOM‐plus and 25 to IPOM‐pb (Fig. 2 ). Two patients were lost to follow‐up: one patient in the IPOM‐plus group at 3 months, who died from cardiovascular co‐morbidities, and one patient in the IPOM‐pb group at 6 months, who had moved to another catchment area. These two patients were not included in the analysis of follow‐up times they had missed. The 12‐month intention‐to‐treat analysis included 48 patients, 24 in each group. There were no significant differences in patient characteristics or operating times between the groups (Tables 1 and 2 ).
Figure 2.
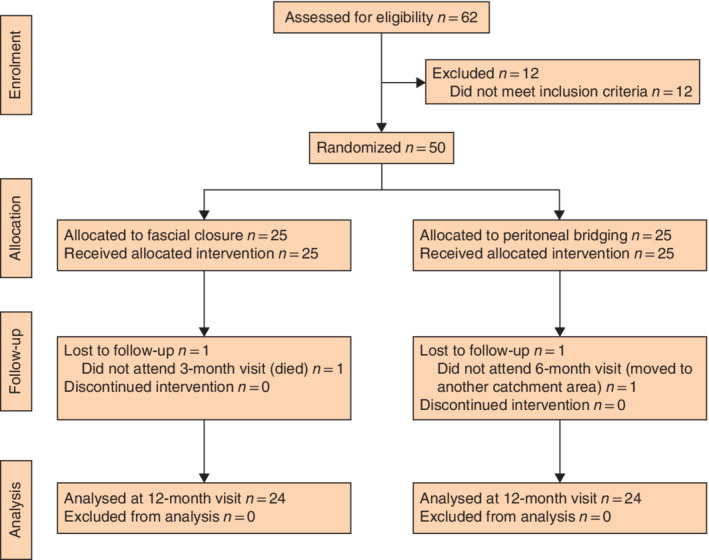
CONSORT diagram for the trial
Table 1.
Patient characteristics
| Defect closure (n = 25) | Peritoneal bridging (n = 25) | |
|---|---|---|
| Age (years) * | 58 (45–67) | 56 (46–62) |
| Sex ratio (M : F) | 13 : 12 | 16 : 9 |
| BMI (kg/m 2 ) * | 29 (28–32) | 30 (28–35) |
| ASA fitness grade | ||
| I | 8 | 7 |
| II | 13 | 15 |
| III | 4 | 3 |
| Largest defect size (EHS width classification) | ||
| W1 (< 4 cm) | 2 | 2 |
| W2 (≥ 4–6 cm) | 17 | 17 |
| W3 (≥ 6–10 cm) | 6 | 6 |
| Median (i.q.r.) (cm) | 5 (4–5·5) | 4·5 (4–5) |
| Type of hernia | ||
| Primary | 9 | 7 |
| Incisional | 16 | 18 |
Values are median (i.q.r.). EHS, European Hernia Society; W, width.
Table 2.
Overall outcomes
| Defect closure | Peritoneal bridging | P†† | |
|---|---|---|---|
| Seroma formation | |||
| Based on CT | |||
| 12 months | 0 of 24 | 0 of 24 | – |
| Based on clinical examination | |||
| 1 month* | 4 of 25 (16; 2, 30)‡ | 0 of 25 | 0·110‡‡ |
| 3 months* | 2 of 24 (8; 0, 19) | 0 of 25 | 0·235‡‡ |
| 6 months | 0 of 24 | 0 of 24 | – |
| Acute and chronic pain (VAS score)†§ | |||
| Acute pain | |||
| 1 week* | 6 (6–7) | 3 (3–4) | < 0·001 |
| 1 month* | 1 (0–2) | 0 (0–1) | 0·045 |
| Chronic pain | |||
| 6 months | 0 (0–0)¶ | 0 (0–0)# | 0·052 |
| 12 months | None reported | None reported | |
| Duration of procedure† | |||
| Overall (min) | 61 (56–70) | 63 (53–72) | 0·857 |
| IPOM approach (min)** | 20 (12–26) | 15 (11–20) | 0·116 |
| Mesh placement (min) | 11 (9–15) | 10 (9–14) | 0·424 |
*Values in parentheses are percentages with 95 per cent confidence intervals; †values are median (i.q.r.). ‡Seroma score according to Morales‐Conde classification: IIa (clinical seroma lasting less than 3 months), two patients; IIb (clinical seroma lasting more than 3 months), one patient; IVa (seroma that needs to be punctured to decrease symptoms), one patient in whom the seroma was drained after the 3‐month visit. §Measured on a visual analogue scale (VAS) ranging from 0 to 10. ¶VAS score 7 in one patient, 2 in three patients, 1 in two patients and 0 in the remaining patients. #VAS score 3 in one patient and 0 in the remaining patients. **Suturing the hernia defect in conventional defect closure approach, and hernial sac remodelling in peritoneal bridging approach. ††Mann–Whitney U test, except ‡‡Fisher's exact test.
The rate of clinical detection of seroma was four of 25 (16 (95 per cent c.i. 2 to 30) per cent) in the IPOM‐plus group versus 0 of 25 in the IPOM‐pb group at 1 month (P < 0·110), and two of 24 (8 (0 to 19) per cent) versus 0 of 25 respectively at 3 months (P = 0·235) (Table 2 ). No seroma was detected by CT at 12 months in either group. One of the two patients with seroma at 3 months in the IPOM‐plus group had an infected seroma that needed to be drained by puncture to decrease the symptoms (type IVa according to the Morales‐Conde classification). There were no significant differences between the groups in other postoperative complications (one of 25 versus 0 of 25).
Acute pain was significantly worse after IPOM‐plus than IPOM‐pb at 1 week and 1 month (Table 2 ). There was no significant difference between groups in chronic pain at 6 months, and no pain was reported at 12 months in either group. There was no recurrent hernia within the 1‐year follow‐up in either group.
Discussion
In this study, the near‐total reduction of the peritoneal sac by IPOM‐pb did not lower the incidence of seroma compared with IPOM‐plus.
Although most seromas cause few symptoms and resolve within couple of months 6 , 17 , they may persist in some patients, and cause discomfort and impaired cosmesis. Efforts to reduce the development of persisting seromas may therefore improve health‐related quality of life. In the present study, seroma detected by CT at 12 months was defined as the primary outcome, although defect closure also has a great impact on the risk of long‐term pain 17 .
The present patient‐ and outcome assessor‐blinded trial suggested that peritoneal bridging is feasible and safe. Apart from one patient in the IPOM‐plus group who had an infected seroma that needed to be drained to alleviate the symptoms, there were no other major complications in either group. However, the study did not have sufficient statistical power to assess recurrence rate or serious adverse events as outcome measures. Patients also reported less postoperative pain assessed by VAS score in the peritoneal bridging group. Pain after the index operation was greatest and most significantly different between the groups in the early postoperative period and declined over time. These results suggest that IPOM‐pb may be an alternative that merits further research.
There were some limitations of the trial. During the design of the study, the authors underestimated the feasibility of operating on over 50 patients within 1 year. A larger sample size would have been required to increase the power above the present 50 per cent chance of detecting a significant difference at the P < 0·050 level. To reduce the chance of type II error, future trials should have a greater sample size.
Furthermore, measurement of the primary endpoint could be improved in future trials. To reduce the chance of detection bias of the outcome assessors and increase the feasibility of the present study, patients were assigned randomly to a resident or consultant surgeon working in the department at the time of follow‐up. However, it was realized during the study that the ability to detect seroma clinically might have varied among the assessors, which could have contributed to increased random error as a result of human error. Future trials could reduce this error by measuring the volume of seroma at ultrasound examination performed by a single assessor blinded to the group allocation. Measuring the outcome by ultrasonography would also be better from an ethical and patient‐safety point of view for repeated follow‐up visits in the early postoperative period (within 6 months of operation) as the results suggest that most seromas resolve spontaneously beyond this period 5 , 16 .
Acknowledgements
No financial or material support was received from any commercial entity. The study was funded by Örebro University Hospital and Karolinska Institutet. This paper reports results of a preregistered study.
Disclosure: The authors declare no conflict of interest.
Funding information
No funding
References
- 1. LeBlanc KA, Booth WV. Laparoscopic repair of incisional abdominal hernias using expanded polytetrafluoroethylene: preliminary findings. Surg Laparosc Endosc 1993; 3: 39–41. [PubMed] [Google Scholar]
- 2. Edwards C, Angstadt J, Whipple O, Grau R. Laparoscopic ventral hernia repair: postoperative antibiotics decrease incidence of seroma‐related cellulitis. Am Surg 2005; 71: 931–935. [PubMed] [Google Scholar]
- 3. Suwa K, Okamoto T, Yanaga K. Closure versus non‐closure of fascial defects in laparoscopic ventral and incisional hernia repairs: a review of the literature. Surg Today 2016; 46: 764–773. [DOI] [PubMed] [Google Scholar]
- 4. Parker HH III, Nottingham JM, Bynoe RP, Yost MJ. Laparoscopic repair of large incisional hernias. Am Surg 2002; 68: 530–533. [PubMed] [Google Scholar]
- 5. Susmallian S, Gewurtz G, Ezri T, Charuzi I. Seroma after laparoscopic repair of hernia with PTFE patch: is it really a complication? Hernia 2001; 5: 139–141. [DOI] [PubMed] [Google Scholar]
- 6. Christoffersen MW, Westen M, Rosenberg J, Helgstrand F, Bisgaard T. Closure of the fascial defect during laparoscopic umbilical hernia repair: a randomized clinical trial. Br J Surg 2020; 107: 200–208. [DOI] [PubMed] [Google Scholar]
- 7. Misiakos EP, Machairas A, Patapis P, Liakakos T. Laparoscopic ventral hernia repair: pros and cons compared with open hernia repair. J Soc Laparoendosc Surg 2008; 12: 117–125. [PMC free article] [PubMed] [Google Scholar]
- 8. Sauerland S, Walgenbach M, Habermalz B, Seiler CM, Miserez M. Laparoscopic versus open surgical techniques for ventral or incisional hernia repair. Cochrane Database Syst Rev 2011; (3)CD007781. [DOI] [PubMed] [Google Scholar]
- 9. Banerjee A, Beck C, Narula VK, Linn J, Noria S, Zagol B et al Laparoscopic ventral hernia repair: does primary repair in addition to placement of mesh decrease recurrence? Surg Endosc 2012; 26: 1264–1268. [DOI] [PubMed] [Google Scholar]
- 10. Orenstein SB, Dumeer JL, Monteagudo J, Poi MJ, Novitsky YW. Outcomes of laparoscopic ventral hernia repair with routine defect closure using ‘shoelacing’ technique. Surg Endosc 2011; 25: 1452–1457. [DOI] [PubMed] [Google Scholar]
- 11. Palanivelu C, Jani KV, Senthilnathan P, Parthasarathi R, Madhankumar MV, Malladi VK. Laparoscopic sutured closure with mesh reinforcement of incisional hernias. Hernia 2007; 11: 223–228. [DOI] [PubMed] [Google Scholar]
- 12. Clapp ML, Hicks SC, Awad SS, Liang MK. Trans‐cutaneous closure of central defects (TCCD) in laparoscopic ventral hernia repairs (LVHR). World J Surg 2013; 37: 42–51. [DOI] [PubMed] [Google Scholar]
- 13. Zeichen MS, Lujan HJ, Mata WN, Maciel VH, Lee D, Jorge I et al Closure versus non‐closure of hernia defect during laparoscopic ventral hernia repair with mesh. Hernia 2013; 17: 589–596. [DOI] [PubMed] [Google Scholar]
- 14. Schulz KF, Altmana DG, Moher D; CONSORT Group . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. Open Med 2010; 4: e60–e68. [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma A, Mehrotra M, Khullar R, Soni V, Baijal M, Chowbey PK. Laparoscopic ventral/incisional hernia repair: a single centre experience of 1242 patients over a period of 13 years. Hernia 2011; 15: 131–139. [DOI] [PubMed] [Google Scholar]
- 16. Morales‐Conde S. A new classification for seroma after laparoscopic ventral hernia repair. Hernia 2012; 16: 261–267. [DOI] [PubMed] [Google Scholar]
- 17. Mercoli H, Tzedakis S, D'Urso A, Nedelcu M, Memeo R, Meyer N et al Postoperative complications as an independent risk factor for recurrence after laparoscopic ventral hernia repair: a prospective study of 417 patients with long‐term follow‐up. Surg Endosc 2017; 31: 1469–1477. [DOI] [PubMed] [Google Scholar]


