Abstract
Background
Urinary catheters are placed after rectal surgery to prevent urinary retention, but prolonged use may increase the risk of urinary tract infection (UTI). This review evaluated the non‐inferiority of early urinary catheter removal compared with late removal for acute urinary retention risk after rectal surgery.
Methods
MEDLINE, Embase and the Cochrane Central Register of Controlled Trials were searched from January 1980 to February 2019. RCTs comparing early versus late catheter removal after rectal surgery were eligible. Primary outcomes were acute urinary retention and UTI; the secondary outcome was length of hospital stay. Early catheter removal was defined as removal up to 2 days after surgery, with late removal after postoperative day 2. The non‐inferiority margin from an included trial was used for analysis of change in urinary retention (ΔNI = 15 per cent). Pooled estimates of risk differences (RDs) were derived from random‐effects models. Risk of bias was assessed using a modified Cochrane risk‐of‐bias tool.
Results
Four trials were included, consisting of 409 patients. There was insufficient evidence to conclude non‐inferiority of early versus late catheter removal for acute urinary retention (RD 9 (90 per cent c.i. −1 to 19) per cent; P NI = 0·31). Early catheter removal was superior for UTI (RD −11 (95 per cent c.i. −17 to −4) per cent; P = 0·001). Results for length of stay were mixed. There were insufficient data to conduct subgroup analyses.
Conclusion
The existing literature is inconclusive for non‐inferiority of early versus late urinary catheter removal for acute urinary retention. Early catheter removal is superior in terms of reducing the risk of UTI.
Urinary catheters are placed after rectal surgery to prevent urinary retention, but prolonged catheterization may increase the risk of urinary tract infection. A systematic review and non‐inferiority meta‐analysis was performed of RCTs comparing early (up to postoperative day 2) and late (after postoperative day 2) urinary catheter removal. Based on four trials, there was insufficient evidence to conclude non‐inferiority of early catheter removal for urinary retention (risk difference (RD) 9 (90 per cent c.i. −1 to 19) per cent; P NI = 0·31; ΔNI = 15 per cent). However, early removal was superior compared with late removal for urinary tract infection (RD −11 (95 per cent c.i. −17 to −4) per cent).
![]()
Early removal reduces risk of infection
Antecedentes
Las sondas urinarias se colocan después de la cirugía rectal para prevenir la retención urinaria, pero su uso prolongado puede aumentar el riesgo de infección del tracto urinario. Esta revisión evaluó si la retirada precoz de la sonda urinaria no fue inferior a la retirada tardía del catéter en cuanto al riesgo de retención urinaria aguda tras cirugía rectal.
Métodos
Se realizaron búsquedas en las bases de datos MEDLINE, Embase y en el Registro Central Cochrane de Ensayos Controlados desde enero de 1980 hasta febrero de 2019. Se consideraron elegibles los ensayos controlados aleatorizados que comparaban la retirada precoz y tardía de la sonda tras cirugía rectal. Las variables principales fueron la retención urinaria aguda y la infección del tracto urinario. La variable secundaria fue la duración de la estancia hospitalaria. Se consideró retirada precoz cuando ésta ocurrió hasta el segundo día postoperatorio mientras que más allá de ese tiempo se consideró retirada tardía. El margen de no inferioridad de uno de los ensayos incluidos se utilizó para el análisis de la retención urinaria (ΔNI = 15%). Las estimaciones agrupadas de las diferencias de riesgo se derivaron de los modelos de efectos aleatorios. El riesgo de sesgo se evaluó utilizando una herramienta de riesgo de sesgo Cochrane modificada.
Resultados
Se incluyeron cuatro ensayos que incluyeron un total de 409 pacientes. No se encontraron evidencias suficientes para concluir la no inferioridad de la retirada precoz del catéter versus la retirada tardía para la retención urinaria aguda (diferencia de riesgo, risk difference, RD 9%, i.c. del 90% ‐1% a 19%, valor de la P para no inferioridad, P value for non‐inferiority, PNI = 0,31). La retirada precoz del catéter fue superior con relación a la infección del tracto urinario (RD ‐11%, IC del 95%: ‐17% a ‐4%, P = 0,001). Los resultados de la duración de la estancia hospitalaria fueron mixtos. No hubo datos suficientes para realizar análisis de subgrupos.
Conclusión
La literatura existente no es concluyente para determinar la no inferioridad de la retirada precoz de la sonda urinaria versus la retirada tardía con relación a la retención urinaria aguda. La retirada precoz de la sonda es superior y reduce el riesgo de infección del tracto urinario.
Introduction
Acute urinary retention (AUR) is common after abdominopelvic surgery, affecting up to 24 per cent of men and 15 per cent of women1. Older patients are at increased risk of AUR from age‐related neuronal degeneration2, 3, and men are at increased risk as a result of benign prostatic hyperplasia4. The mechanism by which pelvic surgery results in AUR is unclear, but two theories have been proposed3. Pelvic dissection can stimulate a reflex involving the pudendal nerve, sacral spinal cord and sympathetic pelvic nerves, resulting in inhibition of the detrusor muscle3, 5. Postoperative sympathetic drive can cause bladder outlet obstruction by activating α‐adrenergic receptors at the bladder neck3, 6.
Transurethral urinary catheters are placed during rectal surgery to prevent AUR and allow accurate measurement of perioperative urine output7. Urinary catheters are, however, not without risk. Transurethral catheterization is associated with a risk of urinary tract infection (UTI) that increases with duration of catheterization8. Historically, catheters have been left in place for 3–5 days after rectal surgery, but this practice restricts mobility, results in potentially avoidable UTIs requiring antimicrobial therapy, and may increase length of hospital stay (LOS)7, 8, 9. Several randomized10, 11 and non‐randomized12, 13 studies have attempted to determine the optimal duration of catheterization after rectal surgery. The results of these studies have varied, and the risk–benefit trade‐off of early catheter removal remains uncertain.
A recent systematic review14 compared catheter removal on day 1 with removal on days 3 and 5 for AUR using a superiority approach. Despite pooling of RCTs and observational studies, this review did not demonstrate a statistically significant difference in AUR rates between day 1 and day 3 removal. The review14 had important methodological limitations, with implications for the conclusions drawn. Superiority approaches to the analysis of a single trial or pooled estimates in the context of a meta‐analysis are appropriate where an intervention plausibly improves an outcome compared with the standard of care15. In the setting of catheter removal, it is not physiologically plausible that earlier catheter removal improves AUR. Furthermore, an evaluation of the effectiveness of early catheter removal must acknowledge that the intervention involves trading off AUR for UTI. Superiority approaches do not inherently consider efficacy trade‐offs.
In contrast with the superiority approach, the non‐inferiority approach acknowledges that the novel treatment (early catheter removal) is not expected to improve the primary efficacy outcome for which catheters are used (AUR)16. Furthermore, it assumes that the novel treatment is associated with potential secondary benefits (reducing UTI, increasing mobility, decreasing LOS) that make it worth accepting a prespecified reduction in the primary efficacy outcome compared with the existing treatment (late catheter removal). This diminution in efficacy for the primary outcome is termed the non‐inferiority margin16. Given the clinical context, a non‐inferiority approach is therefore more appropriate to determine the optimal timing for urinary catheter removal after rectal surgery.
The aim of this review was to synthesize the existing evidence evaluating the effect of early transurethral urinary catheter removal after rectal surgery in adults on AUR, UTI and LOS using a non‐inferiority meta‐analytical approach.
Methods
This systematic review and meta‐analysis was registered on PROSPERO, the international Prospective Register of Systematic Reviews (CRD42019121059), and is reported according to the PRISMA guidelines17.
Searches
The search strategy was developed with a senior information specialist. Observational studies were initially eligible, but were subsequently excluded as sufficient RCTs were identified. MEDLINE, Embase and the Cochrane Central Register of Controlled Trials were searched from January 1980 to February 2019, including studies in English, French and Portuguese. Owing to limited access to professional translation services, language restrictions were applied, although considered unlikely to introduce bias18. Citation lists of included articles and the first ten pages of Google Scholar results were also searched. The search strategy was reviewed by a second information specialist in accordance with the Peer Review of Electronic Search Strategies (PRESS) guidelines19. The final MEDLINE search strategy is shown in Appendix S1 (supporting information).
Two investigators independently screened study titles and abstracts before completing independent full‐text reviews of remaining studies. Discrepancies were resolved by discussion and mediated by a third investigator as required. Screening was performed using DistillerSR™ software20.
Eligibility criteria
RCTs of adults aged 18 years or over undergoing rectal surgery for benign or malignant indications were eligible. Studies must have included an experimental group consisting of transurethral urinary catheter removal on postoperative day (POD) 1 or 2, with or without premedication with an α‐blocker, and a control group of removal on POD 3 or later. Studies with fewer than 20 patients, conference abstracts, studies not reporting the present outcomes of interest, studies that included only patients in the ICU, and studies for which the full text was not available despite interlibrary loan requests were excluded.
Outcomes
The primary outcomes were the incidence of postoperative AUR and UTI during the index hospital admission. The secondary outcome was LOS.
Definitions
Rectal surgery was defined as any colorectal operation including rectal dissection below the peritoneal reflection. For all studies, patients who had urinary catheters removed on POD 1 or 2 were combined to form the early removal group, and those who had catheters removed on POD 3 or later were combined to form the late removal group. AUR was defined as the inability to void adequately after catheter removal, requiring recatheterization with either intermittent or indwelling catheters. UTI was defined in the primary analysis as the presence of a positive urine culture with or without UTI symptoms (any of dysuria, frequency, urgency, suprapubic pain, haematuria or testicular pain). A sensitivity analysis included trials that failed to differentiate between symptomatic and asymptomatic bacteriuria. LOS was restricted to the index hospital admission.
Data extraction
Two investigators independently extracted study‐level data using piloted data extraction forms. Discrepancies were resolved by discussion and mediated by a third investigator as required. Study authors were contacted for clarification as necessary. Data extracted included study and patient‐level characteristics. Data for both intention‐to‐treat and per‐protocol analyses were extracted when possible, in keeping with the non‐inferiority approach16.
Assessment of bias
Study quality was assessed using the Cochrane risk‐of‐bias tool21, supplemented by additional assessments of bias specific to non‐inferiority studies for the primary outcome of AUR16. Two investigators independently completed the risk‐of‐bias assessment for each outcome. Discrepancies were resolved by discussion and mediated by a third investigator as required. A funnel plot was not constructed as fewer than ten studies were included.
Statistical analysis
Descriptive synthesis was used to summarize study characteristics, patient characteristics and study results. The included trials did not present per‐protocol data, and the meta‐analyses were therefore performed with the available modified intention‐to‐treat data. The pooled risk difference (RD) was calculated for the primary outcome of AUR. To test for non‐inferiority of early catheter removal for AUR, a one‐sided Z test with α = 0·05 was used. The pooled risk difference is presented with its 90 per cent confidence interval. One of the included trials6 was a non‐inferiority trial and used a consensus between three surgeons to determine a non‐inferiority margin increase (ΔNI) of 15 per cent in absolute risk of AUR associated with early catheter removal (personal communication). This non‐inferiority margin was used for the meta‐analysis.
A standard meta‐analytical superiority approach was used for the remaining outcomes. Pooled RDs were calculated and 95 per cent c.i. estimated from weights in a Mantel–Haenszel random‐effects model. LOS was extracted with all reported measures of variance and central tendency. Analyses stratified by sex and epidural use were planned a priori, but were unable to be performed owing to a paucity of data.
Statistical heterogeneity was quantified using I 2 scores and Forest plots. I 2 scores of 25 per cent or less were considered as low heterogeneity, 25–50 per cent as moderate, and above 50 per cent as high22. P < 0·050 was considered statistically significant. Statistical analyses were performed using the metafor package in R Studio® v1.0.136 (RStudio, Boston, Massachusetts, USA), RevMan® 5 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and SAS® version 9.4 (SAS Institute, Cary, North Carolina, USA).
Results
The search strategy identified 3904 unique articles. After screening and full‐text review, four RCTs6, 10, 11, 23 involving 409 patients were included in the final analysis (Fig. 1). Study characteristics are presented in Table 1.
Figure 1.

PRISMA diagram for the systematic review
Table 1.
Characteristics of included RCTs comparing early versus late urinary catheter removal
| No. of randomized patients | |||||||
|---|---|---|---|---|---|---|---|
| Reference | Setting | Study definition of rectal surgery | Trial design | Intervention groups | Outcomes and definitions | Early removal | Late removal |
| Patel et al.6 (2018) | Single centre | Dissection of infraperitoneal portion of rectum | Non‐inferiority | POD 1 catheter removal with α‐blocker versus POD 3 removal | AUR: inability to urinate 8 h after catheter removal or difficulty voiding with PVR > 300 ml*, managed by catheterization | 71 | 71 |
| UTI: positive urine culture with symptoms | |||||||
| LOS: days | |||||||
| Coyle et al.23 (2015) | Single centre | Anterior resection/proctectomy, low anterior resection, APR | Superiority | Catheter removal at 48 h after surgery versus removal within 12 h of withdrawal of epidural analgesia | AUR: inability to pass urine requiring reinsertion of urethral catheter with residual > 400 ml after catheter reinsertion | 10 | 13 |
| UTI: positive urine culture | |||||||
| Zmora et al.11 (2010) | Multicentre | Any abdominal surgery involving mobilization of rectum below level of sacral promontory | Superiority | POD 1 versus POD 3 versus POD 5 catheter removal | AUR: inability to void despite urge and attempting for at least 30 min or failure to void 8 h after catheter removal with > 250 ml urine residual on catheter reinsertion | 41 | 77 |
| UTI: positive urine culture with symptoms | |||||||
| Benoist et al.10 (1999) | Single centre | Total or subtotal proctectomy with dissection of infraperitoneal rectum | Superiority | POD 1 versus POD 5 catheter removal | AUR: inability to void after catheter removal with full bladder or 12 h after catheter removal, even after single IOC | 64 | 62 |
| UTI: positive routine urine culture | |||||||
| LOS: days | |||||||
Acute urinary retention (AUR) definition changed from registered protocol definition of inability to void 6 h after removal or postvoid residual (PVR) greater than 200 ml. POD, postoperative day; UTI, urinary tract infection; LOS, length of stay; APR, abdominoperineal resection; IOC, in‐and‐out catheterization.
Patient characteristics are described in Table 2. In three RCTs10, 11, 23, the majority of patients had surgery for cancer, whereas in the remaining trial6 only 30 (21·1 per cent) of the 142 patients underwent cancer surgery. With the exception of one RCT23, in which men comprised 20 (91 per cent) of the 22 patients in the early removal arm and ten of the 22 (45 per cent) of those in the delayed removal arm, all RCTs were well balanced with respect to patient sex. Epidural analgesia was used for all patients in one RCT23, whereas the others either excluded patients who required epidural analgesia or made no reference to epidural analgesia.
Table 2.
Characteristics of patients in included RCTs comparing early and late urinary catheter removal
| Reference | No. of randomized patients | Male sex (%) | Age (years)* | Epidural use (%) | Cancer (%) | IBD (%) |
|---|---|---|---|---|---|---|
| Patel et al. 6 | ||||||
| Early removal | 71 | 56 | 43 (31·5–56·5) | 0 | 15 | 77 |
| Late removal | 71 | 52 | 44 (29·0–60·0) | 0 | 27 | 68 |
| Coyle et al. 23 | ||||||
| Early removal | 22 | 91¶ | 63·5¶ | 100¶ | 73¶ | 14¶ |
| Late removal | 22 | 46¶ | 62¶ | 100¶ | 77¶ | 14¶ |
| Zmora et al. 11 | ||||||
| Early removal | 41 | 56 | 57 (18–85)† | Unclear | 73 | 2 |
| Late removal | 77 | 58 | 54 (22–81)† | 74 | 8 | |
| Benoist et al. 10 | ||||||
| Early removal | 64 | 52 | 55(18)‡ | 0 | 69 | 13 |
| Late removal | 62 | 47 | 56(17)‡ | 0 | 63 | 26 |
Values are median (i.q.r.) unless indicated otherwise;
values are mean (range);
values are mean (s.d.).
Characteristic as randomized, including patients undergoing both colon and rectal surgery; some patients were excluded after randomization and the authors did not differentiate baseline characteristics by type of surgery. IBD, inflammatory bowel disease.
Of four RCTs, three10, 11, 23 used a superiority approach and one6 used a non‐inferiority approach to AUR. Patients in the non‐inferiority trial were younger and had rectal surgery for inflammatory bowel disease more often than those in the other trials6, 10, 11, 23. One trial23 included patients undergoing colonic and rectal surgery, but reported AUR by group, so was included in the analysis.
Risk of bias
The risk‐of‐bias assessment for AUR showed that all trials6, 10, 11, 23 were at low risk of bias for sequence generation, and none of the trials blinded participants and personnel to treatment allocation. With regard to specific risk of bias for non‐inferiority trials, all four trials had a low risk of bias for participant selection bias, where inconsistent application of inclusion/exclusion criteria could lead to anticipated non‐response to the intervention. Two trials6, 23 were found to be at high risk of performance bias. Patel and colleagues6 changed the definition of AUR between the trial protocol24 and final manuscript, and Coyle and co‐workers23 had less precision around catheter removal timing in the late compared with the early removal arm (12 h after epidural removal versus 48 h after surgery). Owing to the subjective nature of AUR assessment among unblinded study personnel, all trials6, 10, 11, 23 were considered at high risk of measurement bias. Two11, 23 were at high risk of attrition bias for failure to use and report intention‐to‐treat and per‐protocol analyses. Risk‐of‐bias results for UTI and LOS are shown in Tables S1 and S2 (supporting information).
Study interventions
Patel et al.6 randomized participants to catheter removal on POD 1 with an α‐blocker (prazosin 1 mg orally) given 6 h before catheter removal on POD 3. Zmora and colleagues11 randomized participants to catheter removal on POD 1, 3 or 5. The POD 3 and POD 5 arms were combined to calculate the pooled estimates for late catheter removal. Benoist and co‐workers10 randomized participants to catheter removal on POD 1 or 5. Coyle et al.23 randomized participants to catheter removal on POD 2 or delayed removal 12 h after epidural removal. The latter group had catheter removal a median of 85 h after surgery.
Acute urinary retention
All included RCTs reported AUR. Among the four studies6, 10, 11, 23, 35 (18·8 per cent) of 186 patients in the early catheter removal group experienced AUR, compared with 21 (9·4 per cent) of 223 patients in the late removal group. The pooled RD between groups was inconclusive for non‐inferiority of early versus late catheter removal for AUR (RD 9 (90 per cent c.i. −1 to 19) per cent; ΔNI = 15 per cent; P NI = 0·31) (Fig. 2). Statistical heterogeneity was high (I 2 = 63 per cent). Only one trial6 included an α‐blocker in the experimental group. As this could be considered a substantively different intervention from that in the other trials, a sensitivity analysis was performed excluding this trial. This analysis was also inconclusive (RD 14 (5 to 23) per cent; ΔNI = 15 per cent; P NI = 0·46) (Fig. 3), with low statistical heterogeneity (I 2 = 22 per cent). Relative measures of effect were similarly inconclusive.
Figure 2.

Forest plot showing non‐inferiority meta‐analysis of acute urinary retention on the risk difference scale in patients who had rectal surgery with early or late urinary catheter removal A Mantel–Haenszel random‐effects model was used for meta‐analysis. Risk differences are shown with 90 per cent confidence intervals. The dashed line indicates the risk difference non‐inferiority margin set at 15 per cent (ΔNI).
Figure 3.
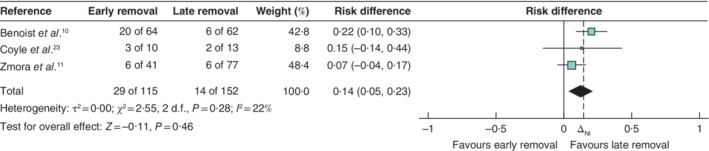
Forest plot showing non‐inferiority meta‐analysis of acute urinary retention as a sensitivity analysis excluding Patel et al.6 on the risk difference scale in patients who had rectal surgery with early or late urinary catheter removal A Mantel–Haenszel random‐effects model was used for meta‐analysis. Risk differences are shown with 90 per cent confidence intervals. The dashed line indicates the risk difference non‐inferiority margin set at 15 per cent (ΔNI).
Urinary tract infection
All four trials reported UTI as an outcome and were included in the primary analysis. Among these trials6, 10, 11, 23, 18 (9·7 per cent) of the 186 patients in the early removal group were diagnosed with a UTI, compared with 47 (21·1 per cent) of the 223 in the late group. The pooled analysis showed early catheter removal to be superior to late removal for risk of UTI (RD −11 (95 per cent c.i. −17 to −4) per cent; P = 0·001 (Fig. 4). The statistical heterogeneity was low (I 2 = 12 per cent). Relative measures of effect demonstrated similar findings.
Figure 4.
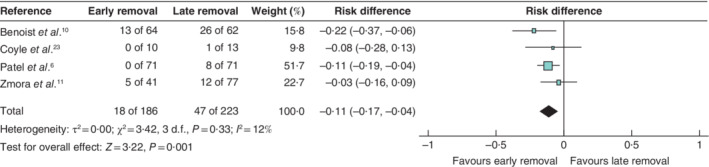
Forest plot showing meta‐analysis of urinary tract infection on the risk difference scale in patients who had rectal surgery with early or late urinary catheter removal A Mantel–Haenszel random‐effects model was used for meta‐analysis. Risk differences are shown with 95 per cent confidence intervals.
Coyle and colleagues23 observed a single UTI in the late removal group. Although the authors reported AUR stratified by colonic or rectal surgery, they failed to do so for UTI. Thus, a sensitivity analysis excluding this trial was performed, the results of which did not differ from the primary analysis ( Fig. S1 , supporting information).
Two trials6, 11 defined UTI as the presence of typical symptoms and a positive urine culture, and did not include asymptomatic bacteriuria in the outcome definition. The result of a second sensitivity analysis restricted to these two trials did not differ from the primary analysis ( Fig. S2 , supporting information).
Length of hospital stay
Two RCTs6, 10 reported LOS as an outcome. Because the trials were conducted nearly 20 years apart, the findings were considered too heterogeneous for quantitative meta‐analysis. One study10 reported mean(s.d.) LOS of 13(5) days in the early removal group and 15(11) days in the late removal group (mean difference −2·00 (95 per cent c.i. −5·00 to 1·00) days; P = 0·19). The other trial6 reported a median LOS of 4 (i.q.r. 3–6) days in the early and 5 (4–7) days in the late removal group (P = 0·03).
Discussion
This systematic review and non‐inferiority meta‐analysis of four RCTs6, 10, 11, 23 comparing early versus late catheter removal after rectal surgery could not conclude non‐inferiority of early removal for the risk of AUR. Early catheter removal resulted in a significantly reduced risk of UTI compared with late removal. In one of two trials6, 10 reporting LOS, this was shortened by early catheter removal.
A recent systematic review and meta‐analysis14 concluded that catheter removal on POD 1 may be as safe as removal on POD 3. The present review differs from that analysis in a number of ways. This review used a non‐inferiority rather than a superiority approach to account for the clinical trade‐off inherent to early catheter removal, did not pool RCTs and observational studies, performed a risk‐of‐bias assessment specific for non‐inferiority trials, presented risk‐of‐bias assessments for each outcome25, and performed multiple sensitivity analyses around the definition of UTI.
The findings of this non‐inferiority meta‐analysis differ subtly, but importantly, from those of the earlier study14. On the basis of the superiority analysis, it was concluded that early catheter removal on POD 1 may be as safe as removal on POD 3 based on the absence of a significant difference in AUR rates between day 1 and day 3 removal (relative risk 1·36, 95 per cent c.i. 0·83 to 2·21)14. That conclusion could be interpreted as supporting recommendations of catheter removal within 2 days of mid/lower rectal resection26. Null results from superiority analysis must be interpreted cautiously, as readers cannot conclude, based on the absence of a significant difference in AUR rates between catheter removal on POD 1 and POD 3, that the two treatments are similar for AUR, only that they had insufficient power to detect a difference.
In contrast, a non‐inferiority approach allows conclusions to be drawn about whether or not early catheter removal results in an acceptable increase in AUR. The results of the present review indicate that there is insufficient evidence to conclude early removal on days 1–2 is non‐inferior to removal on days 3–5. Instead of supporting recommendations for catheters to be removed within 2 days of rectal surgery, the present results suggest that the recommendations of existing perioperative care pathways7, 26 for patients having rectal surgery should reflect greater uncertainty around the safety of early catheter removal.
In contrast to the findings of this non‐inferiority meta‐analysis, the sole non‐inferiority trial6 concluded that early catheter removal was non‐inferior to late catheter removal for AUR. In that study, patients in the early removal arm were pretreated with α‐blockers, which may have resulted in a lower risk of AUR after early catheter removal. Additionally, the median age of patients was lower than in the other trials, and most operations were performed for inflammatory bowel disease rather than cancer. These factors may reduce the risk of AUR in both groups, leading to underestimation of the difference between groups and a higher likelihood of a non‐inferiority conclusion4, 27. Because of the paucity of data found in the present systematic review, subgroup analyses stratified by age and surgical indication could not be conducted, and thus the influence of surgical indication and age on AUR rates could not be assessed quantitatively.
That early catheter removal after rectal surgery is superior to late removal for avoiding UTI is an important finding. Although previous studies7, 9, 28 have found a positive association between the risk of UTI and duration of catheterization in other surgical contexts, rectal surgery is associated with a particularly high risk of AUR26, which may itself contribute to UTI through recatheterization. An observational study12 of 205 patients undergoing rectal surgery did not find an association between UTI and earlier catheter removal. In contrast, the present analysis indicated that, in the setting of an RCT, catheter removal on or before POD 2 decreased the risk of UTI. This review has demonstrated the robustness of this finding through sensitivity analyses that accounted for heterogeneity in trial definitions of UTI and uncertainty about whether the single UTI observed in one trial23 occurred in a patient who had rectal surgery.
Strengths of this review include the systematic nature of the search and quantitative synthesis of the existing evidence. Heterogeneity in interventions and outcome definitions was accounted for through several sensitivity analyses. The non‐inferiority approach acknowledges the trade‐off between AUR and beneficial outcomes of reduced UTI risk, increased patient comfort and earlier ambulation. In addition, the analysis used the non‐inferiority margin previously defined by Patel et al.6, which was derived by consensus, and a risk‐of‐bias framework specific to non‐inferiority trials was used.
This meta‐analysis does have limitations. All trials were deemed to be at high risk of bias owing to issues with blinding as well as poorly described secondary exposures, such as epidural analgesia, that may have altered the effect of early removal on AUR. Acknowledging the subjective nature of voiding ‘urge’ and some UTI symptoms, all trials in the primary analysis did include objective criteria to assess AUR and UTI, such as postvoid residual volume and urine culture. Although the presence of measurement bias cannot be excluded completely, objective criteria likely minimized these biases. Limited data meant that interactions between early removal and known risk factors for AUR could not be examined, nor could a meta‐regression be undertaken to explore quantitatively the influence of patient sex and epidural use on outcomes. Future RCTs should report their results in a manner that allows stratification of results by sex, surgical indication and epidural use. Ideally, such RCTs would also be powered to identify differences between these subgroups. Additional RCT data are also needed to determine the optimal α‐blocker regimen in patients who have early catheter removal.
Supporting information
Appendix S1: Supporting information
Acknowledgements
M.C. and C.S. contributed equally and share first authorship.
Disclosure: The authors declare no conflict of interest.
Funding information
No funding
References
- 1. Eveno C, Lamblin A, Mariette C, Pocard M. Sexual and urinary dysfunction after proctectomy for rectal cancer. J Visc Surg 2010; 147: e21–e30. [DOI] [PubMed] [Google Scholar]
- 2. Baldini G, Bagry H, Aprikian A, Carli F. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology 2009; 110: 1139–1157. [DOI] [PubMed] [Google Scholar]
- 3. Toyonaga T, Matsushima M, Sogawa N, Jiang SF, Matsumura N, Shimojima Y et al Postoperative urinary retention after surgery for benign anorectal disease: potential risk factors and strategy for prevention. Int J Colorectal Dis 2006; 21: 676–682. [DOI] [PubMed] [Google Scholar]
- 4. Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 1984; 132: 474–479. [DOI] [PubMed] [Google Scholar]
- 5. Church JM, Raudkivi PJ, Hill GL. The surgical anatomy of the rectum – a review with particular relevance to the hazards of rectal mobilisation. Int J Colorectal Dis 1987; 2: 158–166. [DOI] [PubMed] [Google Scholar]
- 6. Patel DN, Felder SI, Luu M, Daskivich TJ, Zaghiyan KN, Fleshner P. Early urinary catheter removal following pelvic colorectal surgery: a prospective, randomized, noninferiority trial. Dis Colon Rectum 2018; 61: 1180–1186. [DOI] [PubMed] [Google Scholar]
- 7. Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N et al Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations: 2018. World J Surg 2019; 43: 659–695. [DOI] [PubMed] [Google Scholar]
- 8. Nagle D, Curran T, Anez‐Bustillos L, Poylin V. Reducing urinary tract infections in colon and rectal surgery. Dis Colon Rectum 2014; 57: 91–97. [DOI] [PubMed] [Google Scholar]
- 9. Okrainec A, Aarts M‐A, Conn LG, McCluskey S, McKenzie M, Pearsall EA et al; members of the iERAS Group . Compliance with urinary catheter removal guidelines leads to improved outcome in enhanced recovery after surgery patients. J Gastrointest Surg 2017; 21: 1309–1317. [DOI] [PubMed] [Google Scholar]
- 10. Benoist S, Panis Y, Denet C, Mauvais F, Mariani P, Valleur P. Optimal duration of urinary drainage after rectal resection: a randomized controlled trial. Surgery 1999; 125: 135–141. [PubMed] [Google Scholar]
- 11. Zmora O, Madbouly K, Tulchinsky H, Hussein A, Khaikin M. Urinary bladder catheter drainage following pelvic surgery – is it necessary for that long? Dis Colon Rectum 2010; 53: 321–326. [DOI] [PubMed] [Google Scholar]
- 12. Kwaan MR, Lee JT, Rothenberger DA, Melton GB, Madoff RD. Early removal of urinary catheters after rectal surgery is associated with increased urinary retention. Dis Colon Rectum 2015; 58: 401–405. [DOI] [PubMed] [Google Scholar]
- 13. Lee SY, Kang S‐B, Kim D‐W, Oh H‐K, Ihn MH. Risk factors and preventive measures for acute urinary retention after rectal cancer surgery. World J Surg 2015; 39: 275–282. [DOI] [PubMed] [Google Scholar]
- 14. Lee Y, McKechnie T, Springer JE, Doumouras AG, Hong D, Eskicioglu C. Optimal timing of urinary catheter removal following pelvic colorectal surgery: a systematic review and meta‐analysis. Int J Colorectal Dis 2019; 34: 2011–2021. [DOI] [PubMed] [Google Scholar]
- 15. Mauri L, D'Agostino RB. Challenges in the design and interpretation of noninferiority trials. N Engl J Med 2017; 377: 1357–1367. [DOI] [PubMed] [Google Scholar]
- 16. Treadwell J, Uhl S, Tipton K, Singh S, Santaguida L, Sun X et al Assessing Equivalence and Noninferiority. US Agency for Healthcare Research and Quality: Rockville, 2012. [PubMed] [Google Scholar]
- 17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62: e1–e34. [DOI] [PubMed] [Google Scholar]
- 18. Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M et al The effect of English‐language restriction on systematic review‐based meta‐analyses: a systematic review of empirical studies. Int J Technol Assess Health Care 2012; 28: 138–144. [DOI] [PubMed] [Google Scholar]
- 19. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016; 75: 40–46. [DOI] [PubMed] [Google Scholar]
- 20.Evidence Partners. DistillerSR https://www.evidencepartners.com/ [accessed 13 March 2019].
- 21. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et al; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions; version 5.1.0, 2011. http://handbook‐5‐1.cochrane.org/ [accessed 28 January 2019].
- 23. Coyle D, Joyce KM, Garvin JT, Regan M, McAnena OJ, Neary PM et al Early post‐operative removal of urethral catheter in patients undergoing colorectal surgery with epidural analgesia – a prospective pilot clinical study. Int J Surg 2015; 16: 94–98. [DOI] [PubMed] [Google Scholar]
- 24. ClinicalTrials.gov. Optimal Duration of Indwelling Urinary Catheter Following Pelvic Surgery https://clinicaltrials.gov/ct2/show/NCT01923129 [accessed 31 May 2019].
- 25. Dekkers OM, Vandenbroucke JP, Cevallos M, Renehan AG, Altman DG, Egger M. COSMOS‐E: guidance on conducting systematic reviews and meta‐analyses of observational studies of etiology. PLoS Med 2019; 16: e1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carmichael JC, Keller DS, Baldini G, Bordeianou L, Weiss E, Lee L et al Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum 2017; 60: 761–784. [DOI] [PubMed] [Google Scholar]
- 27. Lange MM, Maas CP, Marijnen CAM, Wiggers T, Rutten HJ, Kranenbarg EK et al; Cooperative Clinical Investigators of the Dutch Total Mesorectal Excision Trial. Urinary dysfunction after rectal cancer treatment is mainly caused by surgery. Br J Surg 2008; 95: 1020–1028. [DOI] [PubMed] [Google Scholar]
- 28. Nicolle LE. Catheter‐related urinary tract infection. Drugs Aging 2005; 22: 627–639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


