Highlights
-
•
Production of functional hydrolysate from wheat bran.
-
•
Xylanase hydrolyze wheat bran insoluble dietary fibre.
-
•
The hydrolysate is rich in reducing sugars, xylooligosaccharides and phenolic acids.
-
•
The hydrolysate showed excellent DPPH scavenging activity (92.7 %) and antioxidant activity.
Abbreviations: XOS, xylooligosaccharides; DPPH, 1,1-diphenyl-2-picrylhydrazyl; WB, wheat bran; WBIDF, wheat bran insoluble dietary fibre; X2, xylobiose; X3, xylotriose; X4, xylotetraose; X5, xylopentose; X6, xylohexose; DNS, dinitrosalicylic acid; ANOVA, analysis of variance; DAD, diode array detector; AAE, ascorbic acid equivalents; DP, degree of polymerization
Keywords: Wheat bran, Hydrolysis optimization, Reducing sugars, Xylooligosaccharides, Phenolic acids, Antioxidant capacity
Abstract
The aim was to enhance production of functional hydrolysate from wheat bran (WB). WB was hydrolyzed with 3000 U/mL ɑ-amylase and 1200 U/mL alkaline protease to prepare WB insoluble dietary fibre (WBIDF). Functional hydrolysate production from the extract containing crude xylan of WBIDF by xylanase was optimized by Taguchi method. The optimal condition for xylan degradation and functional substances production was 78.50 U/mL xylanase, pH 10.0, 50 °C, and reaction time 6 h. The maximum yield of reducing sugars was 614.0 μg/mL, xylobiose increased from 12.9 μg/mL to 213.3 μg/mL, xylotriose increased from 34.9 μg/mL to 174.0 μg/mL, ferulic acid 13.1 μg/mL made up 57.5 % of the total identifiable phenolic pool in the hydrolysate. The total antioxidant activity of hydrolysate was 141.8 mg ascorbic acid equivalents g−1 crude xylan, and the highest 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity reached 92.7 %. The hydrolysate exhibited great potential in agricultural and food industry application.
1. Introduction
Wheat bran (WB) is the main by-products from the processing of wheat to produce flour. Approximately 150 million tons of WB is produced annually and only a small part is utilized [1,2]. Generally, WB comprises 13.2–18.4 % protein, 13.8–24.9 % starch, about 56.0 % carbohydrate, and is regard as important source of minerals and salts in animal feed [3,4]. The analysis of data revealed that dietary fiber and phenolic compounds containing in WB are key factors in determining whole grain health benefits. However, non-starch polysaccharides in WB, the major components of plant cell walls, increase digesta viscosity and reduce nutrient digestibility, therefore, limit the utilization of WB [5].
The carbohydrate in WB comprises starch, hemicellulose and cellulose. Arabinoxylan is the major component of hemicellulose, accounting for 10.9 %–26.0 % of total WB based on dry matter [3]. It is composed of linear β-d-1,4-linked xylopyranose backbones, and the xylose units in the backbone can be substituted, monosubstituted at C-(O)-3- or di-substituted at C-(O)-2 and/or C-(O)-3 with L-arabinofuranose [6], other substituents such as 4-O-methylglucuronic acid,
D-galactose and glucuronic acid have also been reported [7]. In addition, phenolic acids randomly esterify with α-L-arabinofuranose residues in C-5, and arabinoxylan always crosses neighboring components of lignin, cellulose, and proteins, which make more complexity to the xylan [7,8]. Xylan can be degraded into xylooligosaccharides (XOS) consisting of 2–10 xylose units [9], and XOS possess favorable physicochemical features, such as resistance to heat and low caloric values [10]. XOS increase Bifidobacteria population and reduce the number of aberrant crypt foci in the colon of 1,2-dimethylhydrazine-treated rats [11], show an high 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging activity and antimicrobial activity against Klebsiella pneumonia and Pseudomonas aeruginosa [12]. XOS also shows benefit on prevention of atherosclerosis, inhibition of melanoma cell proliferation, treatment of atopic dermatitis, enhancement of collagen production, antihyperlipidemic, anti-inflammatory and immunomodulating activity [13]. XOS are being considered as FOSHU (Foods for Specified Health Use) in Japan, and being provided commercially [14].
WB is rich of bioactive components such as phenolic compounds. The main phenolic compound, phenolic acid containing one aromatic ring is hydroxycinnamic acid including ferulic acid, sinapic acid, and p-coumaric acid. Apart from hydroxycinnamic acid, hydroxybenzoic acid is another kind of phenolic acid in WB, whose derivatives include p-hydroxybenzoic acid, vannilic acid, syringic acid, and gallic acid [15]. Most of the phenolic acids bound to cell wall polysaccharides or to lignin, and can be released by hydrolyzation under alkaline or adidic conditions [3]. Phenolic acids in WB play an important role in antioxidation, which may be attributed to the electron donation and hydrogen atom transfer to free radicals [16], and/or associated with modifying some cellular signaling processes [17], further alleviate oxidative stress, reduce cellular damage or death [18], and decrease the risk of chronic diseases of body [19]. Phenolic acids also possess antimicrobial, anti-inflammatory, anti-thrombosis and anti-carcinogenic activities [20].
Some pretreatments have been developed to alter the structure of plant cell wall for reducing the resistance of lignocellulose to increase the release of XOS, such as alkali, acid and sodium hypochlorite treatment [21,22]. Autohydrolysis, using water as the sole fractionation reagent, is an environment friendly way to help to degrade xylan-rich biomass into XOS [23,24]. Ultrasonic and microwave assisted reaction have also been employed [25,26]. Xylanase degrades xylan into various sizes by catalyzing the endohydrolysis of the β-1,4-glycosidic bonds in the main chain of xylan randomly, which is an efficient method for production of XOS [27].
In this study, WB was hydrolyzed with ɑ-amylase and alkaline protease to produce WB insoluble dietary fibre (WBIDF), and then WBIDF was processed through ultrasonic assisted treatment and autohydrolysis. Taguchi method was employed to optimize the release of reducing sugars from extract of WBIDF by xylanase expressed in Pichia pastoris (P. pastoris). Functional products XOS and phenolic acids in the hydrolysate were characterized by HPLC and LC-ESI-MS. Furthermore, the antioxidant activity of hydrolysate was investigated.
2. Materials and methods
2.1. Materials and reagents
WB was obtained from a local factory (Beijing, China). Alkaline protease (CAS No. 9014-01-1) and ɑ-amylase (CAS No. 9000-85-5) were purchased from Worthington (Colorado, USA). Alkaline protease was 200 units/mg, and ɑ-amylase was 500 units/mg. Beechwood xylan, L-ascorbic acid, 1-phenyl-3-methyl-5-pyrazolone (PMP) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (Saint Louis, USA). Ammonium molybdate was purchased from Coolaber (Beijing, China). Arabinose, xylose, xylobiose (X2), xylotriose (X3), xylotetraose (X4), xylopentose (X5) and xylohexose (X6) used as standards were purchased from Qingdao BZ Oligo Bio-tech Co., Ltd (Qingdao, China). HPLC-grade phenolic acids were procured from Sigma-Aldrich. Glucose was purchased from Amresco (Texas, USA). All other reagents used in this work were of analytical grade and commercially available.
2.2. Preparation of WBIDF and crude xylan
WB was milled and passed through a 0.2 mm screen sieve, and subsequently mixed with sterile distilled water at a ratio of 1:15 (w/v). Then 0.2, 0.4, 0.6, 0.8 or 1.0 % (w/v) ɑ-amylase (500 units/mg) was added to the suspension to hydrolyze starch at 60 °C for 30 min. Reducing sugars content released was measured by the dinitrosalicylic acid (DNS) method at 540 nm with glucose as a standard reference. After boiling for 10 min and cooling down, the suspension was adjusted to pH 10.0 with 1.0 mol/L NaOH, and then 0.2, 0.4, 0.6, 0.8 or 1.0 % (w/v) alkaline protease (200 units/mg) was added, followed by incubation at 50 °C for 2 h. The suspension was boiled for 10 min and cooled down again, and then followed by centrifugation. The sediment was washed twice with distilled water, and three times with 95 % (v/v) ethanol, and then dried at 60 °C to a constant weight to get WBIDF. Kjeldahl method was employed to determine protein residues. The WBIDF was mixed with distilled water at a ratio of 1:20 (w/v), and ultrasonicated in an ultrasonic reaction system (JY92-II, Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China) at 330 W for 20 min. Subsequently, the mixture was autoclaved at 121 °C for 1 h, filtered, and the filtered solution was lyophilized. The resulted crude xylan was stored at −20 °C for all subsequent use.
2.3. Compositional analysis of WB and WBIDF
According to the methods of Association of Official Analytical Chemists (AOAC, 2000), WB and WBIDF were analyzed for crude protein (procedure 954.01), neutral detergent fibre, and acid detergent fiber (procedure 4.6.03). Neutral detergent fiber and acid detergent fiber were determined using fiber analyzer equipment (fiber Analyzer, Ankom Technology, Macedon, NY).
2.4. Xylanase production
The xylanase gene (xynA) from Thermobiafida fusca YX was cloned and expressed in P. pastoris X-33 [27]. Cultivation of the engineered P. pastoris X-33 was performed in basal salt medium (pH 6.0) in a 50-L jacket fermentor (Baoxing Co., Shanghai, China), at 30 °C for 5 days. The supernatant was obtained by centrifugation (12 000 rpm, 5 min) and used as crude enzyme solution. The activity of xylanase in the supernatant was assayed with beechwood as substrate [28], and the produced reducing sugar was determined with DNS (3,5-dinitrosalicylic acid) method [29]. The reaction mixture contained 20 μL of appropriately diluted crude enzyme solution and 180 μL of 1% (w/v) beechwood xylan in 0.05 mol/L KH2PO4-NaOH buffer (pH 8.0). After incubation at 80 °C for 20 min, the reaction was terminated by adding 200 μL of DNS for boiling 10 min. The absorbance of 200 μL of sample was measured at 540 nm. One unit of xylanase activity was defined as the amount of xylanase releasing 1 μmole of reducing sugar per minute under the assay condition (with xylose as a standard reference). All analytical measurements were performed in triplicate.
2.5. Xylan hydrolyzation
The one-factor-at-a-time method and Taguchi method were used to obtain the best combination of enzyme reaction variables for xylan hydrolyzation. Five variables, including substrate concentration, xylanase, temperature, pH and reaction time were selected for optimization (Table 1), and 16 different combinations were run as shown in Table 2. The result of each combination was calculated by the MINITAB® statistical software package (Design Expert, version 8.0). The statistically significant factors were determined by analysis of variance (ANOVA) method by the statistical program JMP 10.0 software package (SAS Institute Inc., Cary, NC). A verification test was performed to check the optimal combination of the factors whose levels having the highest main effect value. The enzymatic hydrolysate was boiled at 100 °C for 10 min to inactivate xylanase after reaction, and then the reducing sugars released by xylanase were determined by DNS method using xylose as a standard reference.
Table 1.
Variables and their levels in Taguchi experimental design for optimization of reducing sugars production by xylanase.
| Factor | Level |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Xylanase (U/mL) | 44.86 | 56.07 | 67.28 | 78.50 |
| Substrate (mg/mL) | 0.336 | 0.420 | 0.504 | 0.588 |
| pH | 7 | 8 | 9 | 10 |
| Temperature (°C) | 45 | 50 | 55 | 60 |
| Reaction time (h) | 4 | 5 | 6 | 7 |
Table 2.
Matrix layout of the L16 Taguchi orthogonal array design for optimization of reducing sugars production by xylanase.
| Run | Level of Variables |
Reducing Sugarsa (μg/mL) |
||||
|---|---|---|---|---|---|---|
| Xylanase | Substrate | pH | Temperature | Reaction time | ||
| 1 | 1 | 1 | 1 | 1 | 1 | 85.9 ± 0.44 |
| 2 | 1 | 2 | 2 | 2 | 2 | 94.4 ± 0.90 |
| 3 | 1 | 3 | 3 | 3 | 3 | 118.0 ± 1.81 |
| 4 | 1 | 4 | 4 | 4 | 4 | 124.2 ± 1.75 |
| 5 | 2 | 1 | 2 | 3 | 4 | 95.8 ± 1.02 |
| 6 | 2 | 2 | 1 | 4 | 3 | 104.2 ± 1.40 |
| 7 | 2 | 3 | 4 | 1 | 2 | 120.5 ± 0.77 |
| 8 | 2 | 4 | 3 | 2 | 1 | 130.6 ± 1.37 |
| 9 | 3 | 1 | 3 | 4 | 2 | 111.5 ± 0.22 |
| 10 | 3 | 2 | 4 | 3 | 1 | 119.2 ± 0.96 |
| 11 | 3 | 3 | 1 | 2 | 4 | 110.5 ± 3.69 |
| 12 | 3 | 4 | 2 | 1 | 3 | 123.8 ± 1.89 |
| 13 | 4 | 1 | 4 | 2 | 3 | 120.0 ± 3.40 |
| 14 | 4 | 2 | 3 | 1 | 4 | 119.3 ± 4.08 |
| 15 | 4 | 3 | 2 | 4 | 1 | 114.6 ± 1.03 |
| 16 | 4 | 4 | 1 | 3 | 2 | 114.7 ± 4.91 |
Values are mean ± SD (n = 3).
2.6. Analysis of XOS in the hydrolysate
Analysis of XOS was performed according to Pu et al. [30]. Briefly, after mixing 100 μL of appropriate concentration of sample with 100 μL of 0.3 mol/L NaOH, the mixture was added 120 μL of 0.5 mol/L methanolic solution of PMP, and then incubated at 70 °C for 1 h. After cooling down to room temperature, the whole mixture was neutralized by adding 100 μL of 0.3 mol/L HCl and extracted with chloroform for three times. The aqueous phase was obtained by discarding the chloroform layer and filtered through 0.2-μm filter. All aqueous phase samples were analyzed using a Dionex UltiMate 3000 HPLC system equipped with a Kromasil C18 column (150 × 4.6 mm, 5 μm) and a Diode array detector (DAD). A mixture of acetonitrile (A) and 10 mmol/L ammonium acetate buffer (B, pH 5.5) in a ratio of 20:80 (v/v, %) was used as the eluent, with a constant flow rate of 0.8 mL/min. Column temperature was maintained at 30 °C. The injection volume was 20 μL. The absorbance of the effluent was monitored at 245 nm. × 2, X3, X4, X5, and X6 were used as standards. The samples were also spiked with known concentrations of X2, X3, X4, X5, and X6 to confirm peaks in the HPLC analysis.
2.7. Analysis of phenolic acids in the hydrolysate
Phenolic acids in the hydrolysate were analyzed by LC-ESI-MS with a Waters Quattro Premier XE triple quadrupole-mass spectrometer connected to an LC-2695e chromatograph (Milford, MA, USA). Phenolic acids were determined using the method described by Zhang et al. with slightly modifications [31]. Source temperature was 150 °C and desolvation temperature was 400 °C. Phenolic acids taken in methanol were separated on a BEH C18 (50 × 2.1 mm, 1.7 μm) column. A mixture of acetonitrile (A) and 0.05 % (v/v) ammonia solution (B) in a ratio of 60:40 (v/v, %) was used as mobile phase at a flow rate of 0.3 mL/min. Quantification of phenolic acids in the hydrolysate was carried out by measuring the area under respective peaks.
2.8. Scavenging activity of hydrolysate on DPPH
The hydrogen atoms or electrons donation ability of the corresponding compounds was measured through the bleaching of 0.004 % (w/v) purple colored methanol solution of DPPH [32]. One milliliter of various concentrations of hydrolysate was mixed with 4 mL methanol solution of DPPH. The mixture was standed for 30 min in dark and the absorbance was measured at 517 nm with a spectrophotometer (TU-1810, Beijing, China). The percentage of Inhibition activity was calculated using the following equation (Eq. (1)):
| I (%) = [A0-(Ai-Aj)]/A0 × 100 % | (1) |
Where A0 is the absorbance of the control (1 mL distilled water +4 mL methanol solution of DPPH), Ai is the absorbance of the test sample (1 mL hydrolysate +4 mL methanol solution of DPPH), Aj is the absorbance of the test sample without DPPH solution (1 mL hydrolysate +4 mL absolute methanol). The scavenging activity of the samples was expressed as 50 % inhibitory concentration (IC50) which represented the concentration of the sample having a 50 % of DPPH radical-scavenging effect.
2.9. Evaluation of total antioxidant activity of hydrolysate
The total antioxidant activity was determined by phosphomolybdenum method [32]. The mixture of 0.3 mL of appropriate concentration of the hydrolysate and 3 mL reagent solution (0.6 mol/L sulfuric acid, 28.0 mmol/L sodium phosphate and 4.0 mmol/L ammonium molybdate) was incubated at 95 °C for 90 min, and then the absorbance was measured at 695 nm with a spectrophotometer. The antioxidant activity was expressed as ascorbic acid equivalents (mg AAE g−1 crude xylan).
3. Results and discussion
3.1. Composition of WB and WBIDF
WB was processed to get WBIDF. The optimal added quantity of both ɑ-amylase and alkaline protease was confirmed to be 0.6 % (w/v) by measuring the released reducing sugars in supernatant and protein residues in solid residue. WBIDF accounted for 41.5 % of WB. The composition of WB and WBIDF was measured and shown in Table 3. The water content of WB and WBIDF was 2.17 % and 2.23 %, respectively. Neutral detergent fibre mainly contains hemicellulose, cellulose, lignin and insoluble ash, and it accounted for 82.0 % of WBIDF. Acid detergent fibre includes cellulose, lignin and acid-insoluble ash, which accounted for 25.8 % of WBIDF. WB includes parts of the endosperm and the aleurone layer [2]. Most of the starches and proteins were degraded by addition of ɑ-amylase and alkaline protease in the pretreatment processes.Maltose from starch and peptides from protein could be used as carbon source and nitrogen source for industrial fermentation and/or incorporation as feed additives [33].The chemical composition analysis had important implications for following processing of WBIDF.
Table 3.
Composition of wheat bran and wheat bran (WB) insoluble dietary fibre (WBIDF).
| WB (100 % original) |
WBIDF (41.50 % original) |
|
|---|---|---|
| Crude protein (g/100 g dry weight) | 19.60 | 5.41 |
| NDF (g/100 g dry weight) | 34.60 | 83.82 |
| ADF (g/100 g dry weight) | 9.51 | 26.34 |
| Water (%) | 2.17 | 2.23 |
NDF: neutral detergent fibre, ADF: acid detergent fibre. Values were calculated with a relative standard deviation <5 %.
3.2. Xylan hydrolyzation with the recombinant xylanase
The quantity of reducing sugars in the hydrolysate was employed to estimate the hydrolysis of xylan. Effects of xylanase, substrate, temperature, pH and reaction time on reducing sugars production were studied by Taguchi method. As shown in Table 2, the maximum average yield of reducing sugars was 130.6 μg/mL in the eighth run among 16 runs, whose conditions were as follows: 10 % xylanase, 0.588 mg/mL substrate, pH 9.0, 50 °C, and reaction time 4 h. Table 4A depicted the main effect of each factor. These factors (xylanase, substrate and pH) showed significant effects on reducing sugars production, however, temperature and reaction time were the two least important factors. When treating the factors temperature and reaction time as error, the results also showed xylanase, substrate and pH were the main factors for reducing sugars production, and R-square was 0.9588 (Table 4B). The levels of each factor were shown in Table 2, and the optimal hydrolysis conditions for reducing sugars production suggested by calculations were as follows: 78.50 U/mL xylanase, 0.588 mg/mL substrate, pH 10.0, 50 °C, and reaction time 6 h. The predicted yield of reducing sugars was 138.5 μg/mL, and the actual reducing sugars reached 136.6 μg/mL. The difference between them was only 1.37 %, which was acceptable. Comparing with 130.6 μg/mL, a further increase of 4.59 % was achieved (Table 5).
Table 4.
Main effects of factors and ANOVA of main effects of factors.
| A | ||||
|---|---|---|---|---|
| Term | SS | DF | F | P |
| Xylanase | 290.72 | 1 | 13.34 | 0.0044 |
| Substrate | 890.66 | 1 | 40.8777 | < 0.0001 |
| pH | 821.92 | 1 | 37.7228 | 0.0001 |
| Temperature | 0.62 | 1 | 0.0284 | 0.8695 |
| Reaction time | 6.92 | 1 | 0.3174 | 0.5856 |
| Error | 217.88 | 10 | ||
| Total | 2228.71 | 15 | ||
| R-square | 0.9022 | |||
| B | |||||
|---|---|---|---|---|---|
| Term | SS | DF | MS | F | P |
| Xylanase | 330.26 | 3 | 110.09 | 7.19 | 0.0206 |
| Substrate | 892.84 | 3 | 297.61 | 19.44 | 0.0017 |
| pH | 913.75 | 3 | 304.58 | 19.89 | 0.0016 |
| Error | 91.86 | 6 | |||
| Total | 2228.71 | 15 | |||
| R-square | 0.9588 | ||||
SS: Sum of squares, DF: Degrees of freedom, MS: Mean of Squares.
Table 5.
Validation of Taguchi experimental data values for reducing sugars production.
| Factor | Taguchi optimum | Experiment optimum |
|---|---|---|
| Xylanase (U/mL) | 78.50 | 56.07 |
| Substrate (mg/mL) | 0.588 | 0.588 |
| pH | 10 | 9 |
| Temperature (°C) | 50 | 50 |
| Reaction time (h) | 6 | 4 |
| Predicted reducing sugars (μg/mL) | 138.5 | |
| Experimental reducing sugars (μg/mL) | 136.6 | 130.6 |
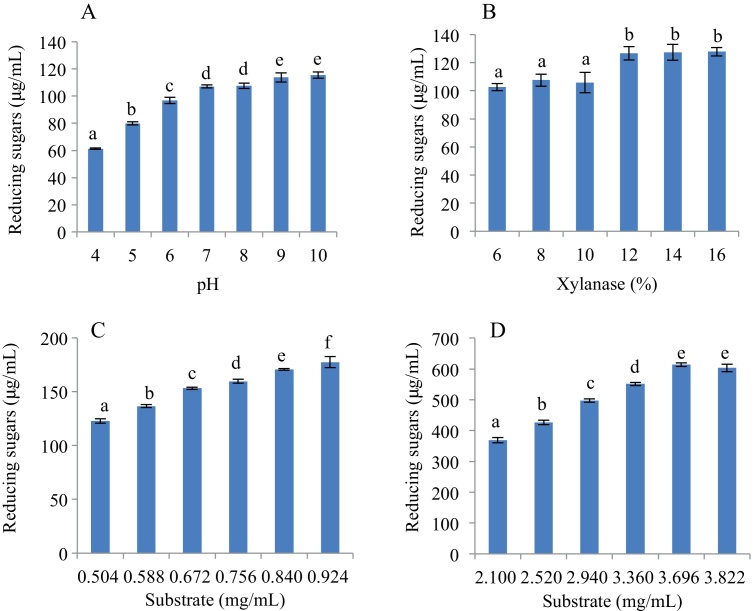
The effects of the main factors including xylanase, substrate and pH on reducing sugars production were also investigated by one factor at a time. As shown in Fig. 1A, the optimal pH for reducing sugars production was pH 10.0. When 56.07 U/mL, 67.28 U/mL or 78.50 U/mL xylanase was added, there was no difference in reducing sugars production, and the reducing sugars were 126.7 μg/mL, 127.4 μg/mL and 127.8 μg/mL, respectively (Fig. 1B). The yield of reducing sugars increased with the increasing of substrate concentration, and the maximum yield of reducing sugars was 614.0 μg/mL with substrate was 3.696 mg/mL (Fig. 1C, D). Therefore, the optimal experiment conditions for reducing sugars production were proved to be 78.50 U/mL xylanase, 3.696 mg/mL substrate, pH 10.0, 50 °C, and reaction time 6 h.
Fig. 1.
Effect of main factors, which was studied by one-factor-at-a-time, on reducing sugars production. (A) pH; (B) xylanase; (C) lower concentration of substrate; (D) higher concentration of substrate. Bars represent means for 3 replications per treatment ± SEM. Bars with different letters differ significantly (P < 0.05).
3.3. Characterization of XOS in the hydrolysate
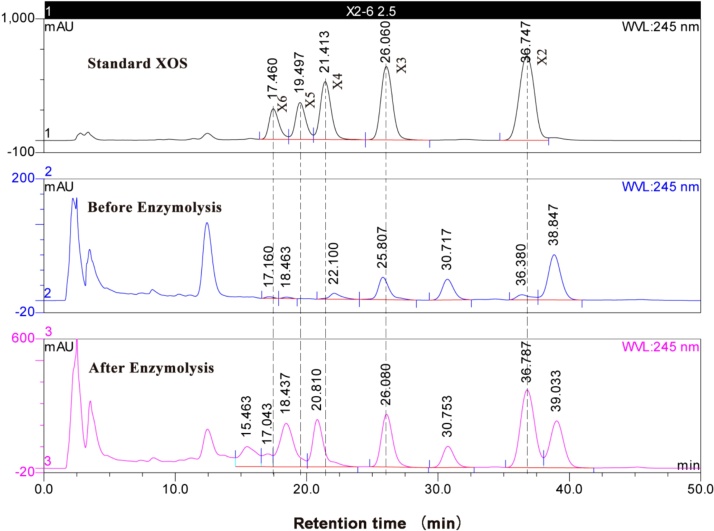
XOS with low degree of polymerization (DP 2–6) were considered as potential prebiotic oligosaccharides [34]. The result of HPLC analysis showed that X2 and X3 were the main oligosaccharides in the hydrolysate. X2 increased from 12.9 μg/mL to 213.3 μg/mL and X3 increased from 34.9 μg/mL to 174.0 μg/mL after enzymolysis (Table 6). X2 and X3 were the major components in xylan hydrolysate, which is consistent with XOS generated by xylanase previously reported [10,[35], [36], [37]]. The potential prebiotic (xylose/XOS) was 0.614 mg/mL in 6 h hydrolyzation. The xylanase from various organisms was reported to generate higher concentration of XOS from WB in 24 h, however, we did not use purified enzyme in only 6 h hydrolyzation [[38], [39], [40]]. There were some unknown peaks shown in Fig. 2, which may be formed by the presence of substituted XOS, because xylan is made of xylose units lined by β-1,4-xylosidic bonds and the xylosyl residues are variably substituted by acetyl, arabinosyl and glucuronyl groups [34]. XOS, especially X2, stimulate the proliferation of probiotics such as Bifidobacteria and Lactobacilli, and they are believed to play an important role in maintenance of a healthy intestinal microflora and alleviating disease symptoms such as diabetes, arteriosclerosis and colon cancer, beneficially affecting host health [[41], [42], [43]]. The xylose, arabinose and glucose in the hydrolysate were also analyzed by HPLC, and they were 23.8 μg/mL, 15.6 μg/mL, and 17.3 μg/mL, respectively. Glucose is frequently-used carbon source for microorganism growth. Xylose and arabinose are pentose, can be fermented by C5-fermenting microorganisms [44]. Xylose also is a precursor for the synthesis of chemical compounds xylitol [45] and succinic acid [46]. Therefore, after purification, XOS in the hydrolysate could be widely used as prebiotics in the food industry as ingredients, even the hydrolysate could be use feed additives.
Table 6.
Concentration of XOS in the hydrolyzate.
| XOS | Concentration (μg/mL) | Ratio (%) |
|---|---|---|
| Before Enzymolysis | ||
| X2 | 12.9 ± 0.10 | 6.2 ± 0.13 |
| X3 | 34.9 ± 0.23 | 20.1 ± 0.12 |
| After Enzymolysis | ||
| X2 | 213.3 ± 2.53 | 26.2 ± 0.03 |
| X3 | 174.0 ± 3.72 | 14.3 ± 0.16 |
Fig. 2.
HPLC chromatograms of XOS in the hydrolysate.
X2: xylobiose, X3: xylotriose, X4: xylotetraose, X5: xylopentose, X6: xylohexose.
3.4. Characterization of phenolic acids in the hydrolysate
The main phenolic acids in WB are ferulic acid, sinapic acid, p-coumaric acid, p-hydroxybenzoic acid, vanillic acid, syringic acid, and gallic acid [15]. The characterization of phenolic acids standards was shown in Table 7. Ferulic acid was the dominant phenolic acid in the hydrolysate, whose concentration was 13.1 μg/mL, making up 57.5 % of the total identifiable phenolic pool. This result was in agreement with Verma et al. [18]. The vanillic acid, syringic acid, p-hydroxybenzoic acid, p-coumaric acid, and sinapic acid were 3.8 μg/mL, 2.3 μg/mL, 1.6 μg/mL, 1.5 μg/mL and 0.5 μg/mL, and relative ratios were 16.67 %, 10.09 %, 7.02 %, 6.58 % and 2.19 %, respectively. Feruloyl esterases (FAEs) are key enzymes for ester-bond hydrolyzation between polysaccharides and ferulic acid [47]. In this study, no FAE was added to the reaction mixture, but ferulic acid and other phenolic acids were detected in the hydrolysate. It is speculated that free phenolic acids were produced during treating process. As we know, three natural bifunctional xylanase/feruloyl esterase enzyme have been reported [47,48], we are inspecting the esterase activity in XynA we used, although no esterase activity was reported in XynA from Thermobiafida fusca YX.
Table 7.
Characterization of phenolic acids standards.
| Phenolic acids | Regression equations | Determination |
|---|---|---|
| (Y, mAu × min; X, μg/mL) | coefficient R2 | |
| Ferulic acid | Y = 19223.9X + 4801.44 | 0.9951 |
| Sinapic acid | Y = 22447.3X | 0.9904 |
| p-Coumaric acid | Y = 27208.2X | 0.9921 |
| p-Hydroxybenzoic acid | Y = 21270.8X | 0.9969 |
| Vanillic acid | Y = 15837.4X + 1213.7 | 0.9995 |
| Syringic acid | Y = 17683X | 0.9919 |
3.5. Antioxidant capacity of hydrolysate
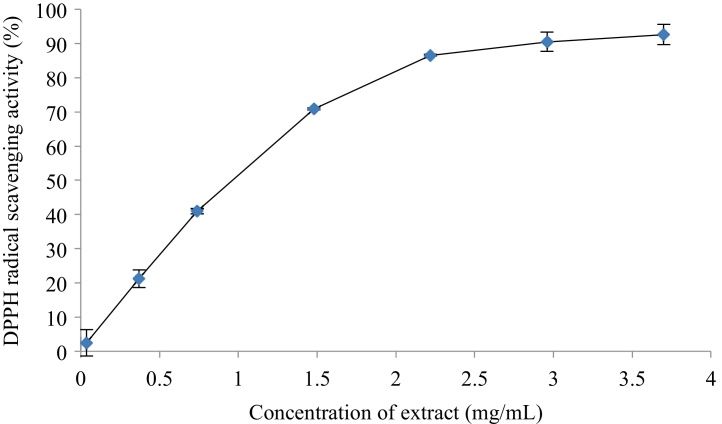
The stable organic radical DPPH was employed to measure the free radical scavenging activity of hydrolysate, and the activity was indicated by the reduction of purple picrylhydrazyl to the corresponding pale yellow picrylhydrazine [32]. The color change reflects the capacity of the hydrolysate transferring electrons or hydrogen atoms [49]. As shown in Fig. 3, the hydrolysate showed DPPH radical scavenging activity in a concentration-dependent manner. The hydrolysate showed the highest DPPH radical scavenging activity (92.7 %) when the substrate (crude xylan) concentration was 3.696 mg/mL, and the IC50 values was 0.967 mg/mL crude xylan. The total antioxidant activity of hydrolysate was also determined by phosphomolybdenum method with ascorbic acid as a standard reference, and it was 141.8 mg g−1 crude xylan. Hydroxycinnamic acids such as ferulic acid and p-coumaric acid have been reported to be in good correlation between antioxidant activity and their concentrations [50]. Unsaturated chain in hydroxycinnamic acids makes them have good hydrogen donating ability, which stabilizes radical and terminates free radical chain reactions, so hydroxycinnamic acids show high antioxidant activity [51]. Hydroxybenzoic acids were proved to activate endogenous antioxidant mechanisms, and decrease oxidative stress of body [52]. Antioxidant activity of XOS have also been reported in previous work [10,22,53]. Xylanase hydrolyzes xylan chains into XOS with varying DP and substitution depending on the xylanase used and xylan source. The substitution and length of XOS were proved to affect its antioxidant activity [[54], [55], [56], [57]]. The time of xylanase catalyzed hydrolyzation affects the length of XOS, and the antioxidant activity of the XOS decreased as the prolongation of the enzyme incubation in xylan hydrolyzation [57]. In the present study, the hydrolyzation was stopped at 6 h, although the XOS concentration was not so high as previously reported, the antioxidant capacity of the hydrolysate was high.
Fig. 3.
DPPH radical scavenging activity of the hydrolysate.
4. Conclusion
WBIDF was prepared by hydrolysis of WB with ɑ-amylase and alkaline protease. The extract containing crude xylan was got from WBIDF with ultrasonic assisted treatment and autohydrolysis. Taguchi method was employed to optimize reducing sugars production from extract by recombinant xylanase, and the maximum yield was 614.0 μg/mL. XOS and phenolic acids in the hydrolysate were characterized by HPLC and LC-ESI-MS. The hydrolysate rich in reducing sugars, XOS and phenolic acids showed favorable biological function, can be potentially applied in dietary supplement, food and agricultural industry.
CRediT authorship contribution statement
Dayong Si: Conceptualization, Writing - review & editing. Tingting Shang: Conceptualization, Methodology, Writing - original draft. Xuhui Liu: Formal analysis, Resources. Zhaojun Zheng: Formal analysis, Resources. Qingyong Hu: Visualization, Writing - original draft. Cong Hu: Visualization, Writing - original draft. Rijun Zhang: Funding acquisition, Project administration, Supervision.
Declaration of Competing Interest
None.
Acknowledgments
This work was supported by the National Key R&D Program of China (No. 2018YFD0500600) and by the ‘Five-twelfth’ National Science and Technology Support Program of China (2011BAD26B0403).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00511.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Rakotoarivonina H., Revol P.V., Aubry N., Remond C. The use of thermostable bacterial hemicellulases improves the conversion of lignocellulosic biomass to valuable molecules. Appl. Microbiol. Biotechnol. 2016;100(17):7577–7590. doi: 10.1007/s00253-016-7562-0. [DOI] [PubMed] [Google Scholar]
- 2.Pruckler M., Siebenhandl-Ehn S., Apprich S., Holtinger S., Haas C., Schmid E. Wheat bran-based biorefinery 1: composition of wheat bran and strategies of functionalization. LWT-Food Sci. Technol. 2014;56(2):211–221. doi: 10.35376/10324/33475. [DOI] [Google Scholar]
- 3.Apprich S., Tirpanalan O., Hell J., Reisinger M., Bohmdorfer S., Siebenhandl-Ehn S. Wheat bran-based biorefinery 2: valorization of products. LWT-Food Sci. Technol. 2014;56(2):222–231. doi: 10.35376/10324/33475. [DOI] [Google Scholar]
- 4.Dornez E., Gebruers K., Wiame S., Delcour J.A., Courtin C.M. Insight into the distribution of arabinoxylans, endoxylanases, and endoxylanase inhibitors in industrial wheat roller mill streams. J. Agric. Food Chem. 2006;54(22):8521–8529. doi: 10.1021/jf061728n. [DOI] [PubMed] [Google Scholar]
- 5.Stefanello C., Vieira S.L., Santiago G.O., Kindlein L., Sorbara J.O.B., Cowieson A.J. Starch digestibility, energy utilization, and growth performance of broilers fed corn-soybean basal diets supplemented with enzymes. Poultry Sci. 2015;94(10):2472–2479. doi: 10.3382/ps/pev244. [DOI] [PubMed] [Google Scholar]
- 6.Gullon B., Gullon P., Tavaria F., Pintado M., Gomes A.M., Alonso J.L. Structural features and assessment of prebiotic activity of refined arabinoxylooligosaccharides from wheat bran. J. Funct. Foods. 2014;6:438–449. doi: 10.1016/j.jff.2013.11.010. [DOI] [Google Scholar]
- 7.Broekaert W.F., Courtin C.M., Verbeke K., Van de Wiele T., Verstraete W., Delcour J.A. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit. Rev. Food Sci. 2011;51(2):178–194. doi: 10.1080/10408390903044768. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Orozco R., Li L., Harflett C., Shewry P.R., Ward J.L. Effects of Environment and genotype on phenolic acids in wheat in the health grain diversity screen. J. Agric. Food Chem. 2010;58(17):9341–9352. doi: 10.1021/jf102017s. [DOI] [PubMed] [Google Scholar]
- 9.Nakakuki T. Development of functional oligosaccharides in Japan. Trends Glycosci. Glycotechnol. 2003;15(82):57–64. doi: 10.1201/9781315371061-3. [DOI] [Google Scholar]
- 10.Bian J., Peng F., Peng X.P., Peng X.P., Xu F., Sun R.C. Structural features and antioxidant activity of xylooligosaccharides enzymatically produced from sugarcane bagasse. Bioresour. Technol. Rep. 2013;127:236–241. doi: 10.1016/j.biortech.2012.09.112. [DOI] [PubMed] [Google Scholar]
- 11.Hsu C.K., Liao J.W., Chung Y.C., Hsieh C.P., Chan Y.C. Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J. Nutr. 2004;134(6):1523–1528. doi: 10.1093/jn/134.6.1523. [DOI] [PubMed] [Google Scholar]
- 12.Kallel F., Driss D., Chaabouni S.E., Ghorbel R. Biological activities of xylooligosaccharides generated from garlic straw xylan by purified xylanase from Bacillus mojavensis UEB-FK. Appl. Biochem. Biotechnol. 2015;175(2):950–964. doi: 10.1007/s12010-014-1308-1. [DOI] [PubMed] [Google Scholar]
- 13.Moure A., Gullon P., Dominguez H., Parajo J.C. Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals. Process Biochem. 2006;41(9):1913–1923. doi: 10.1016/j.procbio.2006.05.011. [DOI] [Google Scholar]
- 14.Vazquez M.J., Alonso J.L., Dominguez H., Parajo J.C. Xylooligosaccharides: manufacture and applications. Trends Food Sci. Technol. 2000;11(11):387–393. doi: 10.1016/s0924-2244(01)00031-0. [DOI] [Google Scholar]
- 15.Laddomada B., Caretto S., Mita G. Wheat bran phenolic acids: bioavailability and stability in whole wheat-based foods. Molecules. 2015;20(9):15666–15685. doi: 10.3390/molecules200915666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sevgi K., Tepe B., Sarikurkcu C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 2015;77:12–21. doi: 10.1016/j.fct.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Ghasemzadeh A., Ghasemzadeh N. Flavonoids and phenolic acids: role and biochemical activity in plants and human. J. Med. Plants Res. 2011;5(31):6697–6703. doi: 10.5897/jmpr11.363. [DOI] [Google Scholar]
- 18.Verma B., Hucl P., Chibbar R.N. Phenolic acid composition and antioxidant capacity of acid and alkali hydrolysed wheat bran fractions. Food Chem. 2009;116(4):947–954. doi: 10.1016/j.foodchem.2009.03.060. [DOI] [Google Scholar]
- 19.Gianotti A., Danesi F., Verardo V., Serrazanetti D.I., Valli V., Russo A. Role of cereal type and processing in whole grain in vivo protection from oxidative stress. Front. Biosci. Landmark. 2011;16:1609–1618. doi: 10.2741/3808. [DOI] [PubMed] [Google Scholar]
- 20.Ou S.Y., Kwok K.C. Ferulic acid: pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004;84(11):1261–1269. doi: 10.1002/jsfa.1873. [DOI] [Google Scholar]
- 21.Escarnot E., Aguedo M., Paquot M. Characterization of hemicellulosic fractions from spelt hull extracted by different methods. Carbohydr. Polym. 2011;85(2):419–428. doi: 10.1016/j.carbpol.2011.03.005. [DOI] [Google Scholar]
- 22.Gowdhaman D., Ponnusami V. Production and optimization of xylooligosaccharides from corncob by Bacillus aerophilus KGJ2 xylanase and its antioxidant potential. Int. J. Biol. Macromol. 2015;79:595–600. doi: 10.1016/j.ijbiomac.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 23.Immerzeel P., Falck P., Galbe M., Adlercreutz P., Karlsson E.N., Stalbrand H. Extraction of water-soluble xylan from wheat bran and utilization of enzymatically produced xylooligosaccharides by Lactobacillus, Bifidobacterium and Weissella spp. LWT-Food Sci. Technol. 2014;56(2):321–327. doi: 10.1016/j.lwt.2013.12.013. [DOI] [Google Scholar]
- 24.Montane D., Nabarlatz D., Martorell A., Torne-Fernandez V., Fierro V. Removal of lignin and associated impurities from xylo-oligosaccharides by activated carbon adsorption. Ind. Eng. Chem. Res. 2006;45(7):2294–2302. doi: 10.1021/ie051051d. [DOI] [Google Scholar]
- 25.Kawee-ai A., Srisuwun A., Tantiwa N., Nontaman W., Boonchuay P., Kuntiya A. Eco-friendly processing in enzymatic xylooligosaccharides production from corncob: influence of pretreatment with sonocatalytic-synergistic Fenton reaction and its antioxidant potentials. Ultrason. Sonochem. 2016;31:184–192. doi: 10.1016/j.ultsonch.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Aguedo M., Ruiz H.A., Richel A. Non-alkaline solubilization of arabinoxylans from destarched wheat bran using hydrothermal microwave processing and comparison with the hydrolysis by an endoxylanase. Chem. Eng. Process. 2015;96:72–82. doi: 10.1016/j.cep.2015.07.020. [DOI] [Google Scholar]
- 27.Zhao L.M., Geng J., Guo Y.Q., Liao X.D., Liu X.H., Wu R.J. Expression of the Thermobifida fusca xylanase Xyn11A in Pichia pastoris and its characterization. BMC Biotechnol. 2015;15 doi: 10.1186/s12896-015-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey M.J., Biely P., Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992;23(3):257–270. doi: 10.1016/0168-1656(92)90074-j. [DOI] [Google Scholar]
- 29.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 30.Pu J.H., Zhao X., Wang Q.C., Wang Y.D., Zhou H. Development and validation of a HPLC method for determination of degree of polymerization of xylo-oligosaccharides. Food Chem. 2016;213:654–659. doi: 10.1016/j.foodchem.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L.Q., Li Y., Liang Y., Liang K.H., Zhang F., Xu T. Determination of phenolic acid profiles by HPLC-MS in vegetables commonly consumed in China. Food Chem. 2019;276:538–546. doi: 10.1016/j.foodchem.2018.10.074. [DOI] [PubMed] [Google Scholar]
- 32.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E1. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 33.Wood I.P., Cook N.M., Wilson D.R., Ryden P., Robertson J.A., Waldron K.W. Ethanol from a biorefinery waste stream: saccharification of amylase, protease and xylanase treated wheat bran. Food Chem. 2016;198:125–131. doi: 10.1016/j.foodchem.2015.09.108. [DOI] [PubMed] [Google Scholar]
- 34.Reddy S.S., Krishnan C. Production of high-pure xylooligosaccharides from sugarcane bagasse using crude beta-xylosidase-free xylanase of Bacillus subtilis KCX006 and their bifidogenic function. LWT-Food Sci. Technol. 2016;65:237–245. doi: 10.1016/j.lwt.2015.08.013. [DOI] [Google Scholar]
- 35.Ding C.H., Li M.X., Hu Y.Q. High-activity production of xylanase by Pichia stipitis: purification, characterization, kinetic evaluation and xylooligosaccharides production. Int. J. Biol. Macromol. 2018;117:72–77. doi: 10.1016/j.ijbiomac.2018.05.128. [DOI] [PubMed] [Google Scholar]
- 36.Li Q., Sun B.G., Jia H.Y., Hou J., Yang R., Xiong K. Engineering a xylanase from Streptomyce rochei L10904 by mutation to improve its catalytic characteristics. Int. J. Biol. Macromol. 2017;101:366–372. doi: 10.1016/j.ijbiomac.2017.03.135. [DOI] [PubMed] [Google Scholar]
- 37.Rahmani N., Kahar P., Lisdiyanti P., Lee J., Yopi, Prasetya B. GH-10 and GH-11 Endo-1,4-beta-xylanase enzymes from Kitasatospora sp produce xylose and xylooligosaccharides from sugarcane bagasse with no xylose inhibition. Bioresour. Technol. 2019;272:315–325. doi: 10.1016/j.biortech.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Dai X.J., Liu M.Q., Jin H.X., Jing M.Y. Optimisation of solid-state fermentation of Aspergillus niger JL-15 for xylanase production and xylooligosaccharides preparation. Czech J. Food Sci. 2011;29(5):557–567. doi: 10.17221/103/2010-cjfs. [DOI] [Google Scholar]
- 39.Liu M.Q., Huo W.K., Xu X., Weng X.Y. Recombinant Bacillus amyloliquefaciens xylanase A expressed in Pichia pastoris and generation of xylooligosaccharides from xylans and wheat bran. Int. J. Biol. Macromol. 2017;105:656–663. doi: 10.1016/j.ijbiomac.2017.07.073. [DOI] [PubMed] [Google Scholar]
- 40.Reque P.M., Pinilla C.M.B., Gauterio G.V., Kalil S.J., Brandelli A. Xylooligosaccharides production from wheat middlings bioprocessed with Bacillus subtilis. Food Res. Int. 2019;126 doi: 10.1016/j.foodres.2019.108673. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Z.Q., Deng W., Zhu Y.P., Li L.T., Sheng Y.J., Hayashi K. The recombinant xylanase B of Thermotoga maritima is highly xylan specific and produces exclusively xylobiose from xylans, a unique character for industrial applications. J. Mol. Catal. B-Enzym. 2004;27(4-6):207–213. doi: 10.1016/j.molcatb.2003.11.012. [DOI] [Google Scholar]
- 42.Reddy S.S., Krishnan C. Production of xylooligosaccharides in SSF by Bacillus subtilis KCX006 producing beta-xylosidase-free endo-xylanase and multiple xylan debranching enzymes. Prep. Biochem. Biotechnol. 2016;46(1):49–55. doi: 10.1080/10826068.2014.970694. [DOI] [PubMed] [Google Scholar]
- 43.Manisseri C., Gudipati M. Prebiotic activity of purified xylobiose obtained from Ragi (Eleusine coracana, Indaf-15) bran. Indian J. Microbiol. 2012;52(2):251–257. doi: 10.1007/s12088-011-0176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sedlak M., Ho N.W.Y. Production of ethanol from cellulosic biomass hydrolysates using genetically engineered Saccharomyces yeast capable of cofermenting glucose and xylose. Appl. Biochem. Biotechnol. 2004;113:403–416. doi: 10.1385/abab:114:1-3:403. [DOI] [PubMed] [Google Scholar]
- 45.Rao L.V., Goli J.K., Gentela J., Koti S. Bioconversion of lignocellulosic biomass to xylitol: an overview. Bioresour. Technol. Rep. 2016;213:299–310. doi: 10.1016/j.biortech.2016.04.092. [DOI] [PubMed] [Google Scholar]
- 46.Liu R.M., Liang L.Y., Li F., Wu M.K., Chen K.Q., Ma J.F. Efficient succinic acid production from lignocellulosic biomass by simultaneous utilization of glucose and xylose in engineered Escherichia coli. Bioresource Technol. 2013;149:84–91. doi: 10.1016/j.biortech.2013.09.052. [DOI] [PubMed] [Google Scholar]
- 47.Blum D.L., Kataeva I.A., Li X.L., Ljungdahl L.G. Feruloyl esterase activity of the Clostridium thermocellum cellulosome can be attributed to previously unknown domains of XynY and XynZ. J. Bacteriol. 2000;182(5):1346–1351. doi: 10.1128/jb.182.5.1346-1351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dodd D., Kocherginskaya S.A., Spies M.A., Beery K.E., Abbas C.A., Mackie R.I. Biochemical analysis of a beta-D-xylosidase and a bifunctional xylanase-ferulic acid esterase from a xylanolytic gene cluster in Prevotella ruminicola 23. J. Bacteriol. 2009;191(10):3328–3338. doi: 10.1128/jb.01628-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez-Jimenez J., Arranz S., Tabernero M., Diaz-Rubio M.E., Serrano J., Goni I. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: extraction, measurement and expression of results. Food Res. Int. 2008;41(3):274–285. doi: 10.1016/j.foodres.2007.12.004. [DOI] [Google Scholar]
- 50.Reddy S.S., Krishnan C. Characterization of enzyme released antioxidant phenolic acids and xylooligosaccharides from different graminaceae or poaceae members. Food Biotechnol. 2013;27(4):357–370. doi: 10.1080/08905436.2013.840787. [DOI] [Google Scholar]
- 51.Kylli P., Nousiainen P., Biely P., Sipila J., Tenkanen M., Heinonen M. Antioxidant potential of hydroxycinnamic acid glycoside esters. J. Agric. Food Chem. 2008;56(12):4797–4805. doi: 10.1021/jf800317v. [DOI] [PubMed] [Google Scholar]
- 52.Juurlink B.H.J., Azouz H.J., Aldalati A.M.Z., AlTinawi B.M.H., Ganguly P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr. J. 2014;13 doi: 10.1186/1475-2891-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mandelli F., Brenelli L.B., Almeida R.F., Goldbeck R., Wolf L.D., Hoffmam Z.B. Simultaneous production of xylooligosaccharides and antioxidant compounds from sugarcane bagasse via enzymatic hydrolysis. Ind. Crop Prod. 2014;52:770–775. doi: 10.1016/j.indcrop.2013.12.005. [DOI] [Google Scholar]
- 54.Rao R.S.P., Muralikrishna G. Water soluble feruloyl arabinoxylans from rice and ragi: changes upon malting and their consequence on antioxidant activity. Phytochemistry. 2006;67(1):91–99. doi: 10.1016/j.phytochem.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 55.Pristov J.B., Mitrovic A., Spasojevic I. A comparative study of antioxidative activities of cell-wall polysaccharides. Carbohydr. Res. 2011;346(14):2255–2259. doi: 10.1016/j.carres.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 56.Malunga L.N., Beta T. Antioxidant capacity of water-extractable arabinoxylan from commercial barley, wheat, and wheat fractions. Cereal Chem. 2015;92(1):29–36. doi: 10.1094/cchem-11-13-0247-r. [DOI] [Google Scholar]
- 57.Valls C., Pastor F.I.J., Vidal T., Roncero M.B., Diaz P., Martinez J. Antioxidant activity of xylooligosaccharides produced from glucuronoxylan by Xyn10A and Xyn30D xylanases and eucalyptus autohydrolysates. Carbohydr. Polym. 2018;194:43–50. doi: 10.1016/j.carbpol.2018.04.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.