Abstract
Background and Objectives:
Many newly diagnosed breast cancer patients do not receive genetic counseling and testing at the time of diagnosis. We examined predictors of genetic testing (GT) in this population.
Methods:
Within a randomized controlled trial of proactive rapid genetic counseling and testing vs. usual care, patients completed a baseline survey within 6 weeks of breast cancer diagnosis but before definitive survey. We conducted a multinomial logistic regression to identify predictors of GT timing/uptake.
Results:
Having discussed GT with a surgeon was a dominant predictor (χ2 (2, N=320)=70.13, p < .0001). Among those who discussed GT with a surgeon, patients who had made a final surgery decision were less likely to receive GT prior to surgery compared to post-surgically (OR=.24, 95% CI =.12-.49) or no testing (OR=.28, 95% CI=.14-.56). Older patients (OR=.95, 95% CI=.91-.99) and participants enrolled in NY/NJ (OR=.22, 95% CI=.07 - .72) were less likely to be tested compared to receiving results prior to surgery. Those with higher perceived risk (OR=1.02, 95% CI=1.00–1.03) were more likely to receive results prior to surgery than to not be tested.
Conclusions:
This study highlights the role of patient-physician communication about GT as well as patient-level factors that predict presurgical GT.
Keywords: BRCA1, BRCA2, Genetic Testing, Genetic Counseling, Patient-Physician Communication
Introduction
Although most cases of breast cancer are sporadic, approximately 10% are hereditary [1, 2]. The majority of these hereditary breast cancers are due to mutations in the BRCA1 or BRCA2 (BRCA) genes [3–5]. Among women with breast cancer, the cumulative risk for developing a contralateral breast cancer 20 years after initial diagnosis is 40% for BRCA1 carriers and 26% for BRCA2 carriers [6]. Because of this high risk, many newly diagnosed breast cancer patients consider bilateral mastectomy to treat their existing cancer and reduce their risk for a future contralateral breast cancer. In addition to greatly reducing the risk of contralateral breast cancer, bilateral mastectomy also eliminates the need for adjuvant radiation treatment to the breast, and can yield improved breast reconstruction options [7–11]. Given these potential benefits, it is not surprising that the majority of newly diagnosed breast cancer patients who learn that they carry a BRCA mutation opt for a bilateral mastectomy [12–16].
Despite the documented impact of BRCA mutation status on bilateral mastectomy decisions, many newly diagnosed breast cancer patients do not receive genetic counseling and testing at the time of diagnosis. A recent study found that only about half of newly diagnosed breast cancer patients with a high pretest risk of a mutation received genetic testing [17]. Additionally, patients may not receive genetic testing until after their definitive breast cancer treatment.
Given the low percentage of newly diagnosed breast cancer patients receiving genetic testing and the clear impact of testing on decision-making, it is important to understand which patients are being tested. Few studies have prospectively examined the predictors of genetic testing uptake in newly diagnosed patients. Several years ago, our team found that physician testing recommendation and indecision about definitive treatment were associated with undergoing genetic testing [18]. A recent population-based retrospective survey found that lack of physician referral was the primary reason provided by patients for not obtaining genetic testing at the time of diagnosis, and that those who did not receive testing were older, and more likely to be of Asian ethnicity [17]. Another recent study confirmed that absence of a physician referral was a key barrier to testing in this population [19]. While several studies have identified physician referral as a key factor that is associated with genetic testing in breast cancer patients, there is an absence of data that prospectively incorporate patient-reported measures as predictors of genetic testing and evaluate factors associated with the receipt of a physician referral. To address this gap, we used data from a completed randomized controlled trial of proactive rapid genetic counseling [12, 20] to evaluate sociodemographic, clinical, and psychosocial factors that may predict the uptake and timing of genetic testing in newly diagnosed breast cancer patients.
Materials and Methods
Participants
Participants were newly diagnosed breast cancer patients enrolled in a parallel group, two-armed randomized trial comparing proactive rapid genetic counseling and testing (RGCT) to usual care (UC) [20]. From 2006–2012, we enrolled women from breast surgery clinics at Georgetown University Medical Center (Washington, DC), The Icahn School of Medicine at Mount Sinai (New York, NY), and Hackensack University Medical Center (Hackensack, NJ) as well as an affiliated private practice in Washington DC. Eligible women were aged 18–75, diagnosed with TNM stage 0 to IIIa breast cancer within the previous 6-weeks and had not undergone definitive breast cancer surgical treatment. In addition, they had to be at increased risk for carrying a BRCA mutation, defined as being diagnosed at <50 years of age or having a family history of one or more first- or second-degree relatives diagnosed with breast cancer at <50, ovarian cancer at any age or male breast cancer at any age. Women with a prior history of cancer (except non-melanoma skin cancer), bilateral, inflammatory, or metastatic breast cancer, or who had previously received BRCA counseling or testing were ineligible. Women who were pregnant, lacked the cognitive capacity to provide informed consent or could not communicate in English were also excluded.
Randomization
Participants were randomized to RGCT or UC in a 2:1 ratio using a computer-generated random number and stratified by study site.
Procedure
The institutional review boards at all study sites approved this study. Patients completed a family history form and provided consent for study contact. Research assistants (RA) reviewed clinic records to identify newly diagnosed patients. Potentially eligible patients were approached by an RA in clinic or by telephone shortly after the clinic visit. The RA introduced the study and obtained permission to contact the patient by telephone to complete the baseline interview. At the initial phone call, the RA confirmed eligibility, obtained verbal consent and completed the baseline survey. If the baseline survey was not completed within 6-weeks of diagnosis, the participant was considered a study decliner. Following the survey, participants were randomized to RGCT or UC in a 2:1 ratio. The interventions are explained in detail in a prior report [20]. Briefly, RGCT participants were proactively contacted within 72 hours of randomization to schedule an in-person or telephone genetic counseling session. UC participants were not proactively contacted but could contact the genetic counseling program for an appointment. We conducted follow-up surveys 1-, 6-, and 12-months post-randomization.
Measures
Sociodemographics.
At baseline, we assessed: age, education, employment, marital status, race/ethnicity.
Family/Personal Cancer History.
We used personal and family cancer history to calculate a priori objective mutation risk with the BRCAPRO model [21].
Clinical Variables.
From participants’ medical, genetic counseling and survey records we abstracted date of diagnosis, cancer stage, receptor status, and eligibility for breast conservation surgery.
Discussed Genetic Testing.
At baseline we used a single face-valid item to ascertain whether participants had discussed genetic testing with their surgeon.
Patient Surgical Decision Making.
At baseline, we used a single face-valid item to assess whether participants had made a final breast cancer surgical decision. We measured baseline decisional conflict regarding breast cancer surgery using the 10-item version of the Decisional Conflict Scale [22]. This version is highly reliable (Alpha = 0.86) and has been used in prior studies of breast cancer surgical and risk management decision making [23–25].
Knowledge.
At baseline, we measured knowledge of breast cancer and BRCA with a 10-item true/false scale created for this study (α= 0.68).
Distress.
We measured cancer-specific distress with the 15-item Impact of Events Scale [26]. We measured general distress with the 12-item Brief Symptom Inventory [27]. Reliability for both measures was high (α=.86 to .87).
Perceived Risk.
We assessed perceived risk for contralateral breast cancer by asking participants to rate their risk from 0 (definitely will not develop a new breast cancer) to 100 (definitely will develop a new breast cancer). We have used this item in prior research [28, 29].
Quality of Life.
We measured health-related quality of life with the 27-item Functional Assessment of Cancer Therapy-General (FACT-G) [30]. The FACT-G measures four quality of life domains: physical, emotional, functional, and social. We used the total score, where higher scores represent better quality of life (α=0.86).
Uptake/Timing of Genetic Testing.
We created a three-level categorical variable to distinguish participants who received test results prior to definitive surgery, received test results following definitive surgery and declined genetic testing.
Statistical Analyses
We conducted bivariate analyses (ANOVA and chi-square tests) to identify baseline predictors of the uptake/timing of genetic testing. These analyses revealed that having discussed genetic testing with a surgeon was a dominant predictor of genetic testing uptake/timing (χ2 (2, N = 320) = 70.13, p < .0001). Since discussing genetic testing with a surgeon at the time of diagnosis (i.e., prior to the baseline survey) was a functional prerequisite for subsequent genetic testing, we limited our multivariate analysis of test uptake/timing to those who reported having discussed genetic testing with their surgeon. For this analysis, we used multinomial logistic regression with backward elimination, including all variables with a p < 0.10 bivariate association with test uptake/timing. We also conducted analyses to identify variables associated with having discussed genetic testing with a physician. After identifying bivariate predictors with t-tests and chi-square tests, we used logistic regression with backward elimination to identify variables with an independent association with having discussed genetic testing with a surgeon.
All analyses were conducted using SAS, version 9.4 (Cary, NC).
Results
As displayed in Figure 1 and previously reported [20], of 717 potentially eligible women, 330 (46.0%) completed a baseline interview and were randomized to RGCT (N=222) vs. UC (N=108). This report focuses on pre-randomization predictors of the uptake and timing of genetic testing. We excluded 5 participants for whom the timing of genetic testing could not be verified, and 5 participants who did not respond to the outcome variable, bringing our sample size to 320.
Figure 1.
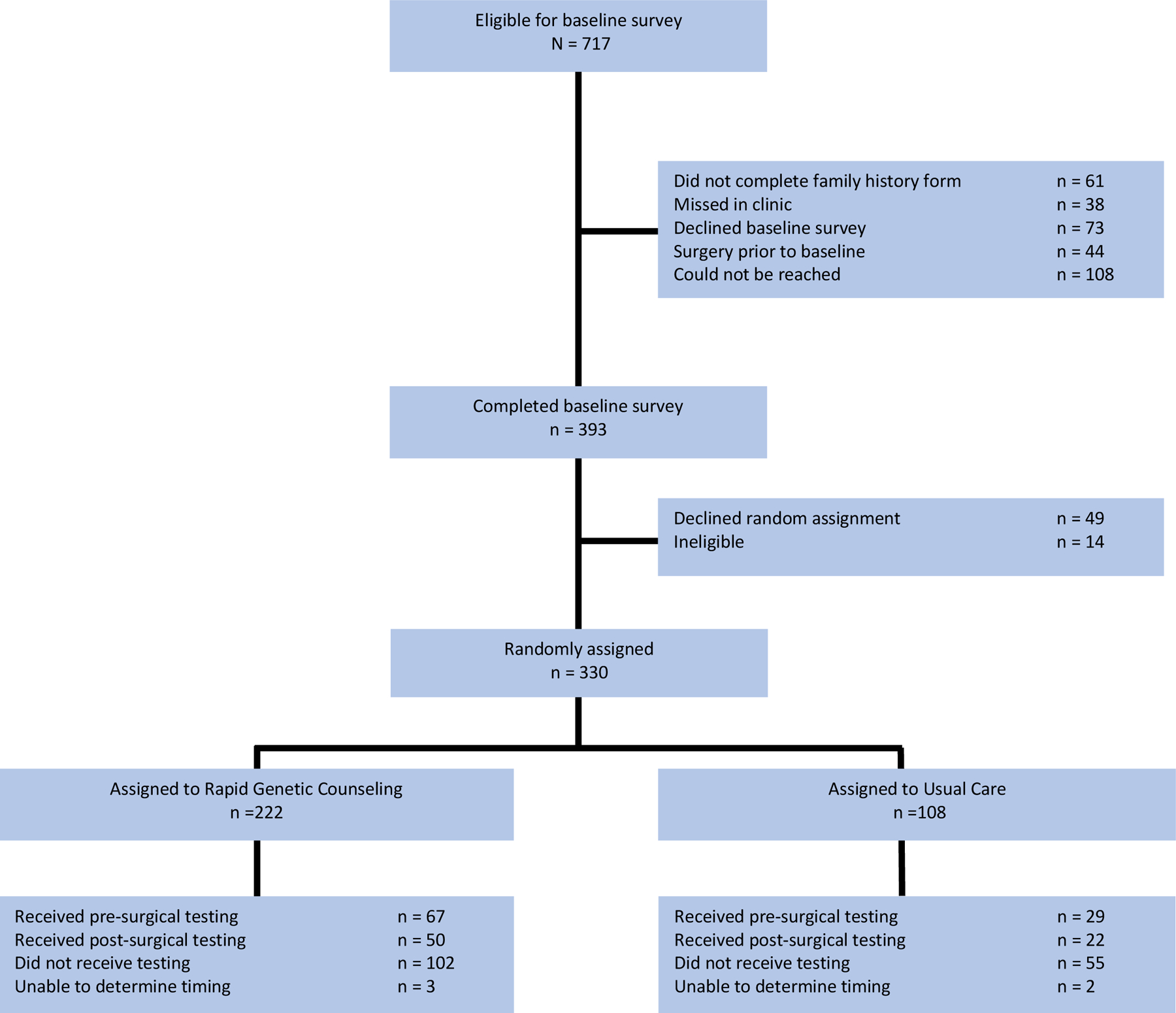
Study Flow Chart
Of the 320 participants, 29.7% received test results before their breast cancer surgery, 21.9% received test results after treatment, and 48.4% opted not to be tested. The strongest predictor of receipt of genetic testing was having discussed genetic testing with a surgeon prior to completion of the baseline survey. Of those who discussed genetic testing with their surgeon (n=222), 41.0% had pre-surgical testing, 25.7% had post-surgical testing, and 33.3% declined testing. Among the women who had not discussed genetic testing with their surgeon (n =98), 4.1% had pre-surgical testing, 13.3% received test results after treatment, and 82.7% were not tested (χ2 (2, N = 320) = 70.13, p < .0001). This association did not vary by randomization group.
Predictors of Genetic Testing
Among women who discussed genetic testing with their surgeon, the following variables had p < .10 associations with the uptake/timing of genetic testing: made a definitive surgical decision at the time of the baseline survey, being a candidate for breast conserving surgery, study site, knowledge, decision conflict, perceived breast cancer risk, and age (Table 1).
Table 1.
Bivariate Predictors of Genetic Testing Uptake and Timing
| PREDICTORS | Pre-Surgery N (%) | Post-Surgery N (%) | Untested N (%) | X2 | p |
|---|---|---|---|---|---|
| Randomization | |||||
| Treatment Decision | |||||
| Marital Status | |||||
| Employment | |||||
| Race/Ethnicity | |||||
| Study Site | |||||
| BCS Candidate | |||||
| ER/PR Status | |||||
| Cancer Stage | |||||
| Br Ca Detected | |||||
| Pre-Surgery M (SD) | Post-Surgery M (SD) | Untested M (SD) | F | P | |
| Objective Risk | 19.87 (26.07) | 12. 69 (23.66) | 14.79 (21.72) | 1.78 | .170 |
| Knowledge | 60.57 (17.41) | 55.58 (19.04) | 52.53 (21.07) | 3.50 | .032 |
| IES- Avoidance Total | 14.72 (8.59) | 14.93 (8.71) | 14.25 (10.06) | .10 | .907 |
| IES - Intrusion Total | 20.33 (9.79) | 20.45 (9.74) | 18.28 (9.65) | 1.15 | .320 |
| BSI - Total | 41.73 (12.23) | 40.29 (13.62) | 39.95 (15.11) | .40 | .674 |
| Decision Conflict | 23.28 (23.96) | 15.20 (24.31) | 15.71 (22.84) | 2.88 | .058 |
| Perceived Breast Cancer Risk | 47.51 (26.57) | 45.78 (26.11) | 35.11 (27.89) | 4.23 | .016 |
| FACT - G Total | 89.3 (8.7) | 89.3 (11.8) | 92.3 (9.7) | 2.33 | .100 |
| Age | 44.14 (8.79) | 43.98 (8.94) | 47.37 (8.56) | 3.48 | .032 |
RGC: rapid genetic counseling; UC: usual care; BCS: Breast conservation surgery; Br Ca: Breast cancer;
The results of our backward multinomial logistic regression are displayed in Table 2. With randomization group forced into the final model, independent predictors of genetic testing uptake/timing were: reached a final surgery decision, perceived breast cancer risk, age, and study site. Patients who had reached a final breast cancer surgery decision prior to study enrollment were less likely to receive genetic testing prior to surgery compared to post-surgically (OR = .24, 95% CI =.12-.49) or no testing (OR = .28, 95% CI = .14-.56). Older patients (OR = .95, 95% CI = .91-.99) and participants enrolled in NY/NJ (OR = .22, 95% CI = .07 - .72) were less likely to be tested compared to receiving results prior to surgery. Those with higher perceived risk (OR = 1.02, 95% CI = 1.00–1.03) were more likely to receive results prior to surgery than to not be tested.
Table 2.
Multivariate model of genetic testing uptake among those who discussed genetic testing with their surgeon
| Predictor | Odds Ratio | 95% Confidence Interval | X2 | p |
|---|---|---|---|---|
| Randomization Group | .06 | .969 | ||
| Made Treatment Decision | 20.07 | <.0001 | ||
| Perceived Risk* | 6.65 | .036 | ||
| Age* | 8.00 | .018 | ||
| Study Site | 7.35 | .025 | ||
The units on all continuous variables are a half standard deviation.
N = 222
Variables removed from the model: decision conflict (p = .78), breast conservation surgery candidate (p = .18), and knowledge (p = .21).
Predictors of Genetic Testing Discussion with Surgeon
Given the strength of the association between having discussed genetic testing with the surgeon and subsequent use of pre-surgical genetic testing, we explored factors that were associated with having discussed genetic testing. The following variables had bivariate associations (p < .10) with having discussed genetic testing with the surgeon: made a final breast cancer surgery decision, marital status, race/ethnicity, study site, breast conservation candidate, objective risk, knowledge, intrusive thoughts, psychological distress, perceived risk and age (Table 3).
Table 3.
Bivariate associations between categorical and continuous predictors and discussing genetic
| CATEGORICAL PREDICTRS | Discussed N (%) | Did Not Discuss N (%) | X2 | P |
|---|---|---|---|---|
| Randomization Group | ||||
| Made Treatment Decision | ||||
| Marital Status | ||||
| Employment | ||||
| Race/Ethnicity | ||||
| Study Site | ||||
| BCS Candidate | ||||
| ER/PR Status* | ||||
| Cancer Stage+ | ||||
| Breast Cancer Detected | ||||
| CONTINUOUS PREDICTORS | Discussed M (SD) | Did not Discuss M (SD) | t | P |
| Objective Risk (BRCAPRO Probability %) | 16.33 (24.16) | 7.41 (11.65) | 12.11 | .001 |
| Knowledge | 56.68 (19.31) | 44.43 (25.14) | 20.58 | <.0001 |
| IES - Avoidance Total | 14.57 (9.09) | 15.57 (10.60) | .86 | .391 |
| IES - Intrusion Total | 19.62 (9.84) | 16.50 (10.41) | 2.58 | .010 |
| Psychological Distress (BSI- Total) | 40.77 (13.56) | 35.51 (13.88) | 10.02 | .002 |
| Decision Conflict | 18.68 (23.89) | 15.46 (21.10) | 1.30 | .254 |
| Perceived Breast Cancer Risk | 42.99 (27.33) | 23.81 (23.24) | 32.22 | <.0001 |
| FACT-G Total | 90.30 (9.93) | 89.64 (12.08) | .52 | .607 |
| Age | 45.18 (8.85) | 48.11 (6.87) | 8.49 | .004 |
RGC: rapid genetic counseling; UC: usual care; BCS: Breast conservation surgery;
As shown in Table 4, the final logistic model predicting genetic testing discussion included: reached a final surgical decision (OR = .51, 95% CI = .28-.92), perceived breast cancer risk (OR = 1.20, 95% CI = 1.06–1.59), knowledge (OR = 1.22, 95% CI = 1.07–1.39), perceived stress (OR = 1.20, 95% CI = 1.05–1.39), objective risk (OR = 1.32, 95% CI = 1.14–1.54), breast conservation candidate (OR = .49, 95% CI = .25-.96) and study site (OR = .44, 95% CI = .21-.92).
Table 4.
Multivariable model of genetic testing discussion
| Predictor | Odds Ratio | 95% Confidence Interval | X2 | p |
|---|---|---|---|---|
| Made Treatment Decision | .51 | .28 – .92 | 4.98 | .026 |
| Perceived Breast Cancer Risk* | 1.30 | 1.06 – 1.59 | 13.26 | <.001 |
| Knowledge~ | 1.22 | 1.07 – 1.39 | 9.22 | .002 |
| BSI Total* | 1.20 | 1.05 – 1.39 | 6.66 | .010 |
| Objective Risk* | 1.32 | 1.14 – 1.54 | 6.15 | .013 |
| BCS Candidate | .49 | .25 – .96 | 4.38 | .036 |
| Study Site | .44 | .21 – .92 | 4.77 | .029 |
BCS: Breast conservation surgery
The units on these continuous variables are a half-standard deviation
The units on this variable is a 1-point difference on the measure.
Variables removed from the model: IES Intrusion (.91), race (p=.74), marital status (p=.29) and age (p=.07).
Discussion
Despite clear evidence that pre-surgical genetic testing results can inform breast cancer surgical decisions in women at increased risk for a BRCA gene mutation [13–15, 31, 32], rates of referral for genetic counseling and testing remain low [19, 33, 34]. We found that early discussion of genetic testing between the newly diagnosed patient and her surgeon was the strongest predictor of pre-surgical genetic testing. Overall, 41% of patients who reported an early discussion of genetic testing with their surgeon received genetic test results prior to surgery compared to only 4.1% who had not discussed genetic testing with their surgeon. This association did not vary by randomization group. Patients randomized to RGCT were proactively scheduled for genetic counseling by the research team, and these patients had the option to complete genetic counseling via telephone. While RGCT led to higher use of presurgical genetic counseling [20], it did not yield increased use of pre-surgical genetic testing. Even within the RGCT group, pre-surgical genetic testing was extremely rare in the absence of pre-randomization surgeon discussion. Thus, the absence of early genetic testing discussion by the surgeon remains a barrier to presurgical genetic testing despite proactively increasing utilization of genetic counseling.
Although patient-surgeon genetic testing discussion was the key predictor of genetic testing, there were other predictors among patients who had discussed testing with their surgeon. Compared to patients who had already made up their mind about surgical treatment, undecided patients were more likely to have presurgical genetic testing. This is consistent with the intended role of presurgical genetic testing in helping to guide treatment decisions. Since breast conservation is not contraindicated among mutation carriers [35, 36], patients who had already decided on lumpectomy may not have felt compelled to obtain their test results presurgically. Similarly, with increasing numbers of breast cancer patients opting for bilateral mastectomy [37–40], it is likely that some patients who had already decided to obtain a bilateral mastectomy saw no need for presurgical testing. Indeed, we have previously reported that 15.4% of untested patients and 28.9% of those who tested negative opted for bilateral mastectomy [12].
Perceived risk for a new breast cancer and younger age both predicted the receipt of pre-surgical testing vs. no testing. While these factors have been previously found to predict genetic testing in breast cancer patients and unaffected women [41, 42], in the current study, it is likely that they also reflect the discussion between the surgeon and the patient. It is likely that physician discussion of the option of genetic testing sensitized patients to their increased risk. Further, surgeons may have been more directive in younger patients who are at higher risk for contralateral breast cancer compared to older patients [19].
Given the central role of patient-surgeon discussion, we also examined patient-level factors that were associated with such discussions. Unlike the analyses focused on predictors of genetic testing, this analysis was not prospective so must be interpreted cautiously. Nonetheless, several suggestive findings emerged. It is reassuring that patient-surgeon genetic testing discussions were more likely when patients were younger and at higher objective risk for contralateral breast cancer. These patients are more likely to harbor a BRCA mutation and more likely to benefit from contralateral prophylactic mastectomy compared to older or lower risk patients. Further, these objective measures are not impacted by the discussion with the surgeon -- so we can be confident that they are true predictors.
Other patient factors that were related to whether or not a patient-surgeon discussion took place were: patient knowledge, perceived risk, perceived stress, and not having reached a decision about final surgery. We have previously reported that not having reached a final surgery decision predicted subsequent receipt of a contralateral prophylactic mastectomy [12]. The associations between higher perceived risk, perceived stress and knowledge could indicate that these patient-level variables drive the discussion with the surgeon. Patients who are concerned about their risk and more aware of genetic testing may raise the issue with their surgeon or may be more receptive to this discussion when raised by their surgeon. There is evidence that these patient characteristics can drive communication and shared decision making in other contexts [43]. On the other hand, it is also likely that the very discussion of genetic counseling and testing with their surgeon might have sensitized patients to their risk and yielded increases in perceived stress. Similarly, these patients may have sought additional information as a consequence of the discussion with their doctor – leading to higher knowledge. It is likely that these associations were bidirectional.
From a clinical standpoint, this study confirms the central role of patient-surgeon communication. This is notable because the strength of the patient-surgeon discussion was not diminished by providing proactive and highly flexible delivery of presurgical genetic counseling. This finding suggests that patient-facing interventions designed to facilitate the use of genetic testing may be insufficient in the absence of physician recommendation for testing. Thus, strengthening linkages between cancer physicians and genetics services may be the most effective approach to ensuring access for patients. Indeed, recent studies have found that embedded service models may be particularly promising in this regard [44–46]. Importantly, these data also indicate that surgeons are effectively referring patients based on their objective risk, the potential benefit of bilateral mastectomy and patient preferences (i.e., patients who are undecided about surgery). However, given the clear role of the surgeon in facilitating genetic testing, it would make sense for surgeons to raise testing as an option with all patients who meet referral criteria. Such routine referrals might be facilitated by recently updated guidelines by the American Society of Breast Surgeons recommending genetic referral for all new breast cancer patients [47].
There are several limitations to this study. These data were collected several years ago as part of a randomized controlled trial. However, recent studies indicate that genetic referral rates remain low [33, 34], suggesting that physician referral likely remains a key factor. However, the rise of multigene panel testing, recent discussion of universal testing, and the increased availability of telegenetic counseling and physician-performed testing [48–51], this issue may become less important moving forward. This study also included only a limited number of recruitment sites and surgeons. Thus, the data may not be fully representative of testing in the more comprehensive breast cancer surgical setting. Additionally, incomplete abstraction of medical records led to missing data for several cancer-related variables (hormone receptor status and cancer stage). Because of these missing data, we cannot definitely determine that these variables were not related to genetic testing uptake or to the patient-surgeon genetic testing discussion. Despite these limitations, this study highlights the central role of patient-physician communication about genetic testing as well as patient-level factors that predict the decision to undergo presurgical genetic testing.
Synopsis: Despite the documented impact of BRCA mutation status on bilateral mastectomy decisions, many newly diagnosed breast cancer patients do not receive genetic counseling and testing at the time of diagnosis. This report explores predictors of genetic testing uptake in newly diagnosed breast cancer patients. We found that discussing genetic testing with a surgeon was the dominant predictor of genetic testing timing/uptake, and among those who had this discussion, there were several additional patient-level predictors.
Acknowledgements:
The authors are grateful to all the women who participated in this study. The authors would like to acknowledge contributions of Dr. Colette Magnant, Dr. Elizabeth Feldman, Ms. Tamara Drazin and Ms. Aliza Zidell in providing access to their patients. We would also like to acknowledge the contributions of Dr. Kara-Grace Leventhal for conducting study telephone surveys.
Research Support: NCI Grants: R01 CA74861 and P30 CA051008.
Footnotes
Clinical Trial Registration: NCT00262899
Surgery After Genetic Evaluation Study Group Members: Rachel Nusbaum1, MS, CGC, Lina Jandorf2, MA, Sarah Kelleher1, PhD.
Data Availability Statement: The data that support the findings of this study are available upon reasonable request from the corresponding author [MDS]. The data are not publicly available due to the inclusion of information that could compromise research participant privacy/consent.
References
- [1].Norquist BM, Harrell MI, Brady MF, Walsh T, et al. , Inherited Mutations in Women With Ovarian Carcinoma, JAMA Oncol 2(4) (2016) 482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].American Cancer Society, Breast Cancer Facts and Figures 2019–2020, in: American Cancer Society (Ed.) American Cancer Society, American Cancer Society, Atlanta: GA, 2019. [Google Scholar]
- [3].Chong HK, Wang T, Lu HM, Seidler S, et al. , The validation and clinical implementation of BRCAplus: a comprehensive high-risk breast cancer diagnostic assay, PLoS One 9(5) (2014) e97408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Couch FJ, Shimelis H, Hu C, Hart SN, et al. , Associations Between Cancer Predisposition Testing Panel Genes and Breast Cancer, JAMA Oncol 3(9) (2017) 1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tung N, Lin NU, Kidd J, Allen BA, et al. , Frequency of Germline Mutations in 25 Cancer Susceptibility Genes in a Sequential Series of Patients With Breast Cancer, J Clin Oncol 34(13) (2016) 1460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, et al. , Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers, JAMA 317(23) (2017) 2402–2416. [DOI] [PubMed] [Google Scholar]
- [7].Domchek SM, Friebel TM, Singer CF, Evans DG, et al. , Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality, Jama 304(9) (2010) 967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Skytte AB, Cruger D, Gerster M, Laenkholm AV, et al. , Breast cancer after bilateral risk-reducing mastectomy, Clin Genet 79(5) (2011) 431–7. [DOI] [PubMed] [Google Scholar]
- [9].Evans DG, Baildam AD, Anderson E, Brain A, et al. , Risk reducing mastectomy: outcomes in 10 European centres, J Med Genet 46(4) (2009) 254–8. [DOI] [PubMed] [Google Scholar]
- [10].Metcalfe K, Gershman S, Ghadirian P, Lynch HT, et al. , Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis, Bmj 348 (2014) g226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Francken AB, Schouten PC, Bleiker EM, Linn SC, et al. , Breast cancer in women at high risk: the role of rapid genetic testing for BRCA1 and −2 mutations and the consequences for treatment strategies, Breast 22(5) (2013) 561–8. [DOI] [PubMed] [Google Scholar]
- [12].Tynan M, Peshkin BN, Isaacs C, Willey S, et al. , Predictors of contralateral prophylactic mastectomy in genetically high risk newly diagnosed breast cancer patients, Breast Cancer Res Treat 180(1) (2020) 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yadav S, Reeves A, Campian S, Sufka A, et al. , Preoperative genetic testing impacts surgical decision making in BRCA mutation carriers with breast cancer: a retrospective cohort analysis, Hered Cancer Clin Pract 15 (2017) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lokich E, Stuckey A, Raker C, Wilbur JS, et al. , Preoperative genetic testing affects surgical decision making in breast cancer patients, Gynecol Oncol 134(2) (2014) 326–30. [DOI] [PubMed] [Google Scholar]
- [15].Schwartz MD, Lerman C, Brogan B, Peshkin BN, et al. , Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients, J Clin Oncol 22(10) (2004) 1823–9. [DOI] [PubMed] [Google Scholar]
- [16].Evans DG, Lalloo F, Hopwood P, Maurice A, et al. , Surgical decisions made by 158 women with hereditary breast cancer aged <50 years, Eur J Surg Oncol 31(10) (2005) 1112–8. [DOI] [PubMed] [Google Scholar]
- [17].Kurian AW, Griffith KA, Hamilton AS, Ward KC, et al. , Genetic Testing and Counseling Among Patients With Newly Diagnosed Breast Cancer, JAMA 317(5) (2017) 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schwartz M, Lerman C, Brogan B, Peshkin B, et al. , Utilization of BRCA1/BRCA2 mutation testing in newly diagnosed breast cancer patients, CANCER EPIDEMIOLOGY BIOMARKERS &; PREVENTION 14(4) (2005) 1003–1007. [DOI] [PubMed] [Google Scholar]
- [19].Hafertepen L, Pastorino A, Morman N, Snow J, et al. , Barriers to genetic testing in newly diagnosed breast cancer patients: Do surgeons limit testing?, Am J Surg 214(1) (2017) 105–110. [DOI] [PubMed] [Google Scholar]
- [20].Schwartz MD, Peshkin BN, Isaacs C, Willey S, et al. , Randomized trial of proactive rapid genetic counseling versus usual care for newly diagnosed breast cancer patients, Breast Cancer Res Treat 170(3) (2018) 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Berry DA, Iversen ES Jr., Gudbjartsson DF, Hiller EH, et al. , BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes, J Clin Oncol 20(11) (2002) 2701–12. [DOI] [PubMed] [Google Scholar]
- [22].O’Connor AM, Validation of a decisional conflict scale, Med Decis Making 15(1) (1995) 25–30. [DOI] [PubMed] [Google Scholar]
- [23].Ladd MK, Peshkin BN, Senter L, Baldinger S, et al. , Predictors of risk-reducing surgery intentions following genetic counseling for hereditary breast and ovarian cancer, Transl Behav Med (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schwartz M, Valdimarsdottir H, DeMarco T, Peshkin B, et al. , Randomized Trial of a Decision Aid for BRCA1/BRCA2 Mutation Carriers: Impact on Measures of Decision Making and Satisfaction, HEALTH PSYCHOLOGY 28(1) (2009) 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schwartz MD, Valdimarsdottir HB, Peshkin BN, Mandelblatt J, et al. , Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer, J Clin Oncol 32(7) (2014) 618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Horowitz M, Wilner N, Alvarez W, Impact of Event Scale: a measure of subjective stress, Psychosom Med 41(3) (1979) 209–18. [DOI] [PubMed] [Google Scholar]
- [27].Derogatis LR, Melisaratos N, The Brief Symptom Inventory: an introductory report, Psychol Med 13(3) (1983) 595–605. [PubMed] [Google Scholar]
- [28].King L, O’Neill SC, Spellman E, Peshkin BN, et al. , Intentions for bilateral mastectomy among newly diagnosed breast cancer patients, J Surg Oncol 107(7) (2013) 772–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Graves KD, Vegella P, Poggi EA, Peshkin BN, et al. , Long-term psychosocial outcomes of BRCA1/BRCA2 testing: differences across affected status and risk-reducing surgery choice, Cancer Epidemiol Biomarkers Prev 21(3) (2012) 445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cella DF, Tulsky DS, Gray G, Sarafian B, et al. , The Functional Assessment of Cancer Therapy scale: development and validation of the general measure, J Clin Oncol 11(3) (1993) 570–9. [DOI] [PubMed] [Google Scholar]
- [31].Chiba A, Hoskin TL, Hallberg EJ, Cogswell JA, et al. , Impact that Timing of Genetic Mutation Diagnosis has on Surgical Decision Making and Outcome for BRCA1/BRCA2 Mutation Carriers with Breast Cancer, Ann Surg Oncol 23(10) (2016) 3232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weitzel JN, McCaffrey SM, Nedelcu R, MacDonald DJ, et al. , Effect of genetic cancer risk assessment on surgical decisions at breast cancer diagnosis, Arch Surg 138(12) (2003) 1323–8; discussion 1329. [DOI] [PubMed] [Google Scholar]
- [33].Childers CP, Childers KK, Maggard-Gibbons M, Macinko J, National Estimates of Genetic Testing in Women With a History of Breast or Ovarian Cancer, J Clin Oncol 35(34) (2017) 3800–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Powell CB, Littell R, Hoodfar E, Sinclair F, et al. , Does the diagnosis of breast or ovarian cancer trigger referral to genetic counseling?, Int J Gynecol Cancer 23(3) (2013) 431–6. [DOI] [PubMed] [Google Scholar]
- [35].Vallard A, Magne N, Guy JB, Espenel S, et al. , Is breast-conserving therapy adequate in BRCA 1/2 mutation carriers? The radiation oncologist’s point of view, Br J Radiol 92(1097) (2019) 20170657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].van den Broek AJ, Schmidt MK, van ‘t Veer LJ, Oldenburg HSA, et al. , Prognostic Impact of Breast-Conserving Therapy Versus Mastectomy of BRCA1/2 Mutation Carriers Compared With Noncarriers in a Consecutive Series of Young Breast Cancer Patients, Annals of surgery 270(2) (2019) 364–372. [DOI] [PubMed] [Google Scholar]
- [37].Jagsi R, Hawley ST, Griffith KA, Janz NK, et al. , Contralateral Prophylactic Mastectomy Decisions in a Population-Based Sample of Patients With Early-Stage Breast Cancer, JAMA surgery 152(3) (2017) 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kummerow KL, Du L, Penson DF, Shyr Y, et al. , Nationwide trends in mastectomy for early-stage breast cancer, JAMA surgery 150(1) (2015) 9–16. [DOI] [PubMed] [Google Scholar]
- [39].Wong SM, Freedman RA, Sagara Y, Aydogan F, et al. , Growing Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer, Annals of surgery 265(3) (2017) 581–589. [DOI] [PubMed] [Google Scholar]
- [40].Nash R, Goodman M, Lin CC, Freedman RA, et al. , State Variation in the Receipt of a Contralateral Prophylactic Mastectomy Among Women Who Received a Diagnosis of Invasive Unilateral Early-Stage Breast Cancer in the United States, 2004–2012, JAMA surgery 152(7) (2017) 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Allen CG, Roberts M, Guan Y, Exploring Predictors of Genetic Counseling and Testing for Hereditary Breast and Ovarian Cancer: Findings from the 2015 U.S. National Health Interview Survey, J Pers Med 9(2) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rosenberg SM, Ruddy KJ, Tamimi RM, Gelber S, et al. , BRCA1 and BRCA2 Mutation Testing in Young Women With Breast Cancer, JAMA Oncol 2(6) (2016) 730–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jung HP, Baerveldt C, Olesen F, Grol R, et al. , Patient characteristics as predictors of primary health care preferences: a systematic literature analysis, Health Expect 6(2) (2003) 160–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Senter L, O’Malley DM, Backes FJ, Copeland LJ, et al. , Genetic consultation embedded in a gynecologic oncology clinic improves compliance with guideline-based care, Gynecol Oncol 147(1) (2017) 110–114. [DOI] [PubMed] [Google Scholar]
- [45].Bednar EM, Oakley HD, Sun CC, Burke CC, et al. , A universal genetic testing initiative for patients with high-grade, non-mucinous epithelial ovarian cancer and the implications for cancer treatment, Gynecol Oncol 146(2) (2017) 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pederson HJ, Hussain N, Noss R, Yanda C, et al. , Impact of an embedded genetic counselor on breast cancer treatment, Breast Cancer Res Treat 169(1) (2018) 43–46. [DOI] [PubMed] [Google Scholar]
- [47].Manahan ER, Kuerer HM, Sebastian M, Hughes KS, et al. , Consensus Guidelines on Genetic` Testing for Hereditary Breast Cancer from the American Society of Breast Surgeons, Annals of Surgical Oncology 26(10) (2019) 3025–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].King MC, Levy-Lahad E, Lahad A, Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award, JAMA 312(11) (2014) 1091–2. [DOI] [PubMed] [Google Scholar]
- [49].Beitsch PD, Whitworth PW, Hughes K, Patel R, et al. , Underdiagnosis of Hereditary Breast Cancer: Are Genetic Testing Guidelines a Tool or an Obstacle?, J Clin Oncol (2018) JCO1801631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Interrante MK, Segal H, Peshkin BN, Valdimarsdottir HB, et al. , Randomized Noninferiority Trial of Telephone vs In-Person Genetic Counseling for Hereditary Breast and Ovarian Cancer: A 12-Month Follow-Up, JNCI Cancer Spectr 1(1) (2017) pkx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Armstrong J, Toscano M, Kotchko N, Friedman S, et al. , Utilization and Outcomes of BRCA Genetic Testing and Counseling in a National Commercially Insured Population: The ABOUT Study, JAMA Oncol 1(9) (2015) 1251–60. [DOI] [PubMed] [Google Scholar]


