Abstract
Invasive strains of Streptococcus pyogenes with significantly reduced susceptibility to β-lactam antibiotics have been recently described. These reports have caused considerable concern in the international infectious disease, medical microbiology, and public health communities because S. pyogenes has remained universally susceptible to β-lactam antibiotics for 70 years. Virtually all analyzed strains had single amino acid replacements in penicillin-binding protein 2X (PBP2X), a major target of β-lactam antibiotics in pathogenic bacteria. We used isogenic strains to test the hypothesis that a single amino acid replacement in PBP2X conferred a fitness advantage in a mouse model of necrotizing myositis. We determined that when mice were administered intermittent subtherapeutic dosing of benzylpenicillin, the strain with a Pro601Leu amino acid replacement in PBP2X that confers reduced β-lactam susceptibility in vitro was more fit, as assessed by the magnitude of colony-forming units recovered from disease tissue. These data provide important pathogenesis information that bears on this emerging global infectious disease problem.
Streptococcus pyogenes, alias group A Streptococcus (GAS), causes >700 million human infections each year worldwide.1,2 This organism is a strict human pathogen that is responsible for a remarkable array of diverse health problems, ranging from relatively mild pharyngitis to life-threatening infections, such as septicemia and severe soft tissue infections.3, 4, 5, 6, 7 GAS is also responsible for acute rheumatic fever, a disease that remains the most common cause of preventable pediatric heart disease globally.6 No licensed vaccine against GAS is available despite 100 years of effort, and no promising vaccine is on the immediate horizon.8, 9, 10, 11
Firstline treatment of GAS infections typically involves a β-lactam antibiotic, such as penicillin or ampicillin, either alone or in conjunction with other classes of antibiotics (eg, macrolides or fluoroquinolones).3,7,12, 13, 14 Pediatric GAS pharyngitis and attendant oral prescriptions account for enormous antibiotic use in the United States and many other countries.12, 13, 14 For reasons that remain poorly understood and contrast with many bacterial pathogens, GAS has remained universally susceptible to β-lactam antibiotics.15 However, GAS strains with significantly decreased susceptibility to β-lactams in vitro were described in two recent publications.16,17 The levels of decreased susceptibility did not exceed the threshold of frank in vitro resistance, but nevertheless, identification of these strains has resulted in widespread concern in the international infectious disease, microbiology, and public health communities.
Decreased susceptibility to β-lactams in GAS and other pathogenic streptococci is primarily associated with nonsynonymous (amino acid–altering) mutations in the pbp2x gene encoding penicillin-binding protein 2X (PBP2X), a major target of these antibiotics in many pathogens.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 In the case of GAS, virtually all strains with altered susceptibility have only one amino acid replacement in PBP2X.16 Molecular genetic techniques were used to construct an isogenic mutant strain and showed that one of these changes (Pro601Leu in PBP2X) was the molecular cause of decreased in vitro susceptibility to β-lactams.16 Many questions remain unanswered about GAS strains with PBP2X amino acid replacements linked to decreased β-lactam susceptibility. One important area that has not been addressed is the effect, if any, of these amino acid changes on strain virulence or fitness. To begin to explore this association, isogenic strains were used to test the hypothesis that a single amino acid replacement in PBP2X alters fitness and/or virulence in mouse necrotizing myositis, a widely used model of deep tissue infection.38, 39, 40, 41, 42 We found that in infected mice given intermittent subtherapeutic dosing of benzylpenicillin, a naturally occurring strain with the Pro601Leu amino acid replacement in PBP2X was more fit than a strain with the wild-type (WT) PBP2X variant, as assessed by the number of colony-forming units (CFUs). The pilot studies described herein will form the basis for a more in-depth analysis of the relationship between β-lactam susceptibility and virulence in vivo, an area of GAS research that is greatly lagging compared with other pathogenic bacteria.
Materials and Methods
Construction of Isogenic Strain 27213-PBP2X-WT
MGAS27213 is a serotype M89 GAS strain recovered in 2012 in the United States from a patient with invasive infection.43 This strain has a single amino acid replacement (Pro601Leu) that results in significantly decreased susceptibility to penicillin G, ampicillin, and other β-lactam antibiotics.16 We refer to this strain as 27213-PBP2X-mutant (MT). The isogenic derivative strain was constructed to restore a proline residue at position 601 in the PBP2X protein, which represents the WT variant of PBP2X that is fully susceptible to β-lactam antibiotics. Molecular construction and phenotypic characterization of isogenic strain 27213-PBP2X-WT has been described recently.16 Whole genome sequencing of 27213-PBP2X-WT confirmed that the constructed strain lacked spurious mutations.
Mouse Model of Necrotizing Myositis with Subtherapeutic Antibiotic Dosing
The mouse model of necrotizing myositis used in these experiments has been previously described.40, 41, 42 The model was modified to include intermittent subtherapeutic benzylpenicillin treatment. Mice were treated on the day of infection with 7.3 IU/g benzylpenicillin (MilliporeSigma, Burlington, MA), and day 10 after infection with 11 IU/g, both of which were subtherapeutic doses of benzylpenicillin injected intramuscularly. The antibiotic was given intramuscularly as a 100-μL injection in an identical manner as the GAS inoculation. The first dose was given at/near the GAS injection site in the right rear lower limb, as outlined in Figure 1; the second dose was given in the contralateral limb.
Figure 1.
Group A Streptococcus inoculation and benzylpenicillin dosing schedule. Mice were inoculated with strain 27213–penicillin-binding protein 2X (PBP2X)–wild type (WT) or 27213-PBP2X-mutant (MT) followed by a subtherapeutic dose of benzylpenicillin via i.m. injection at the site of bacterial inoculation. A second dose was administered on day 10 in the contralateral limb. The mice were sacrificed on day 17, and infected tissue was harvested for colony-forming unit (CFU) counts. n = 40.
Near Mortality, CFU Determination, and Histopathology
For mouse survival experiments, infected mice were monitored for near mortality at least once daily for up to 17 days. CFU determination from infected muscle was assessed by culturing tissue homogenates, as previously described.41,44 Briefly, diseased muscle recovered from the infected limb was weighed and homogenized (Omni International, Kennesaw, GA) in 1 mL phosphate-buffered saline, serially diluted, plated on sheep blood agar, cultured overnight, and counted. For histologic evaluation, tissue taken from the inoculation site was fixed, embedded, and processed using standard automated instruments. Statistical differences between strain groups were determined with the U-test. Mouse experiments were approved by the Institutional Animal Care and Use Committee of Houston Methodist Research Institute (protocol AUP-0318-0016).
Growth of GAS Strains in THY Broth
Growth of GAS strains in Todd Hewitt broth supplemented with 0.5% yeast extract (THY) with or without a subinhibitory concentration of benzylpenicillin (6 ng/mL) was performed, as described previously.16 Liquid GAS cultures were incubated for 12 hours at 37°C in 5% CO2. Growth was determined by measuring the OD at 600 nm.
Results
To test the hypothesis that a single amino acid replacement in PBP2X alters virulence in vivo, a mouse model of necrotizing myositis that has been used extensively was employed.38, 39, 40, 41, 42 The naturally occurring strain MGAS27213 that has reduced susceptibility to β-lactams in vitro was compared with its isogenic derivative that differs in a single amino acid in PBP2X. The isogenic strain has a proline at amino acid position 601 of PBP2X, converting it to the WT PBP2X variant and a fully susceptible strain.16 We refer to these two strains as 27213-PBP2X-WT and 27213-PBP2X-MT.
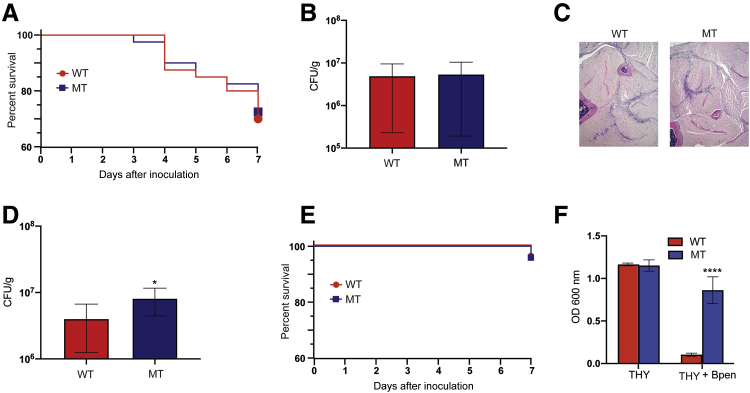
Ten CD-1 outbred mice were inoculated with 1.5 × 108 CFUs of either 27213-PBP2X-MT or the isogenic derivative 27213-PBP2X-WT in the right hind limb, and near mortality was assessed at day 7 after infection. The Kaplan-Meier survival curves for mice infected with these two strains were essentially superimposable (Figure 2A). Mice were sacrificed on day 7 after inoculation, and homogenates of the infected limbs were plated on sheep red blood cell agar and incubated overnight, and colonies were counted. The same numbers of CFUs were recovered from diseased tissue from the wild-type and 27213-PBP2X-MT strains (Figure 2B). Sections of diseased tissue were fixed, stained, and analyzed for histopathology; both strains caused nearly identical lesions (Figure 2C). Taken together, the results show that in the absence of β-lactam treatment, the two isogenic strains had identical fitness and virulence, as assessed by the ability to replicate and persist (CFUs), induce tissue necrosis (histopathology), and cause near mortality.
Figure 2.
Comparison of 27213–penicillin-binding protein 2X (PBP2X)–wild type (WT) and 27213-PBP2X-mutant (MT) strains of Group A Streptococcus in a mouse model of necrotizing myositis in the absence and presence of benzylpenicillin (Bpen). A: Kaplan-Meier survival curves for mice inoculated with strain 27213-PBP2X-WT (WT) and the reduced susceptibility to β-lactam strain 27213-PBP2X-MT (MT). No significant differences are observed in near mortality. B: Colony-forming units (CFUs) recovered from the hind limbs of infected animals are similar. C: Histologic examination of hind limb sections from mice show no differences in bacterial dissemination or tissue destruction (hematoxylin and eosin stain). A–C: All analyses were performed with 10 mice in the absence of benzylpenicillin and at day 7 after infection. D: CFUs recovered from mice infected with strains 27213-PBP2X-WT and 27213-PBP2X-MT at day 17 after infection. Mice were injected following the schedule depicted in Figure 1. E: Kaplan-Meier survival curves for mice inoculated with WT and MT strains in the presence of benzylpenicillin. No significant difference in near mortality is observed. F: Growth of group A Streptococcus strains in Todd Hewitt broth supplemented with 0.5% yeast extract (THY) with or without a subinhibitory concentration of Bpen. n = 40 (D). ∗P < 0.05, ∗∗∗∗P < 0.0001 versus WT (U-test). Original magnification, ×4 (C).
We next tested the hypothesis that strain 27213-PBP2X-MT, which shows reduced in vitro sensitivity to β-lactams, would have a fitness advantage in the presence of subtherapeutic treatment with benzylpenicillin (penicillin G). This hypothesis is based on the clinical observation that patients with prolonged intermittent treatment with β-lactams can be infected by bacterial strains with significantly decreased susceptibility to this class of antibiotics.17,20,27,45,46 A pilot experiment was performed to test this hypothesis. Mice (n = 10 animals per strain) were inoculated in the right hind limb with bacteria, and a subtherapeutic dose of benzylpenicillin (7.3 IU/g) was administered by i.m. injection. The mice were followed up for 10 days, and then a second subtherapeutic dose of benzylpenicillin (11 IU/g) was administered (Figure 1). These doses were chosen on the basis of the recommended benzylpenicillin therapeutic dose of 22 IU/g for mice47; the timing of the benzylpenicillin doses was somewhat arbitrary. Mice were sacrificed on day 17 after inoculation, and homogenates of the infected limbs were plated for CFU determination. Compared with mice infected with isogenic strain 27213-PBP2X-WT, more CFUs/g of mouse tissue were recovered from limbs infected with the 27213-PBP2X-MT strain. That is, mice infected with the strain with significantly reduced susceptibility to β-lactam antibiotics in vitro yielded more CFUs (data not shown). Thus, in the context of the intermittent subtherapeutic antibiotic dosing, the PBP2X mutant strain trended toward increased fitness, but the data did not reach statistical significance.
Using the previous infection and dosing strategy (Figure 1) and applying a power calculation, the study was repeated with 40 mice per GAS strain. Consistent with the data described in the previous paragraph, mice infected with strain 27213-PBP2X-MT yielded significantly higher CFUs than mice infected with the 27213-PBP2X-WT susceptible strain (Figure 2D). When benzylpenicillin was present, mortality (Figure 2E) and extent of tissue damage (data not shown) in mice infected with the 27213-PBP2X-WT and 27213-PBP2X-MT strains did not significantly differ. Consistent with the in vivo data, strain 27213-PBP2X-MT grew to a significantly higher OD than strain 27213-PBP2X-WT in THY broth supplemented with benzylpenicillin (Figure 2F). These data show that in the presence of subtherapeutic levels of benzylpenicillin, the PBP2X mutant strain is significantly more fit than the wild-type, susceptible strain.
Discussion
The goal of this study was to test the hypothesis that a single amino acid replacement in PBP2X significantly alters fitness in a mouse model of necrotizing myositis. The study data show that in the absence of intermittent subtherapeutic benzylpenicillin, there was no difference in fitness or virulence between the isogenic PBP2X wild-type and mutant strains. In contrast, the introduction of intermittent dosing with subtherapeutic levels of benzylpenicillin showed that strain 27213-PBP2X-MT, with significantly decreased in vitro susceptibility to this β-lactam, was more fit in this mouse model of necrotizing myositis. These data provide important new fitness information about GAS strains that differ by a single amino acid replacement in PBP2X that confers altered β-lactam susceptibility. Many naturally occurring strains with single PBP2X polymorphisms have recently been identified, which has generated significant concern globally.16,17
The relationship between resistance to antimicrobial agents and bacterial fitness and virulence is complicated and has not been assessed in pathogenic streptococci using isogenic strains. Work in other organisms, such as Mycobacterium tuberculosis, Staphylococcus aureus, and Streptococcus pneumoniae, has not yielded a simple definitive theme: in some bacteria, investigators have reported no change in fitness in animal infection models, whereas other investigators have reported altered fitness.48, 49, 50, 51, 52, 53, 54, 55, 56 Although our study is the first analysis of altered antimicrobial susceptibility and fitness in GAS using isogenic strains, clearly more work is warranted given the spectrum of PBP2X amino acid polymorphisms and different diseases caused by GAS.
A large number of single amino acid replacements in PBP2X that are associated with decreased in vitro susceptibility to β-lactams were recently identified.16 Thus far, our studies have been confined to single amino acid changes in PBP2X identified by analysis of GAS strains cultured from patients with serious invasive infections.16 Currently, there are no data about the frequency of occurrence of such changes in strains causing pharyngitis or recovered from asymptomatic carriers. In this regard, pbp2x mutations in strains from these two clinical sources were recently identified, information that will likely add to the complexity of altered susceptibility to β-lactam antibiotics in GAS.
In addition, the extent of variation in pbp1a that may contribute to β-lactam resistance in GAS is unknown. Amino acid substitutions in PBP1A have been shown to contribute to β-lactam resistance in group B Streptococcus (Streptococcus agalactiae) and other pathogenic streptococci.23,25,27,36,57, 58, 59 The extent of amino acid substitutions in PBP1A that contribute to β-lactam resistance in GAS clinical isolates will need to be assessed to determine the full complement of changes contributing to reduced susceptibility in GAS to this important class of antibiotics.
The experiments described herein are important to assess potential changes in fitness and virulence associated with reduced susceptibility to β-lactams, an emerging problem that has raised concern in the microbiology, infectious disease, and public health communities. Future analyses will require construction, confirmation, and phenotypic characterization (eg, in vitro growth and β-lactam minimum inhibitory concentration) of additional isogenic strains, followed by in vivo pathogenesis and fitness studies in various mouse models of infection. Finally, it will be important to assess the relative fitness of PBP2X and PBP1A isogenic strains in the upper respiratory tract of nonhuman primates, the gold standard infection model for GAS pharyngitis.
Acknowledgments
We thank Stephen B. Beres for input on study design; Frank R. DeLeo for critical reading of the manuscript and suggesting improvements; Concepcion C. Cantu and Matthew Ojeda Saavedra for technical support; and Kathryn Stockbauer for editorial assistance.
Footnotes
Supported in part by NIH grants AI146771-01 and AI139369-01 and the Fondren Foundation, Houston Methodist Hospital and Research Institute (J.M.M.).
Disclosures: None declared.
Author Contributions
R.J.O. planned and conducted the mouse infection experiments and analyzed the resulting data. L.Z. generated and characterized the isogenic mutant strain. J.M.M. designed the studies, analyzed experiments, and oversaw the project. All authors wrote the manuscript and contributed to the intellectual atmosphere that resulted in this study. J.M.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Carapetis J.R., Steer A.C., Mulholland E.K., Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 2.Ralph A.P., Carapetis J.R. Group a streptococcal diseases and their global burden. Curr Top Microbiol Immunol. 2013;368:1–27. doi: 10.1007/82_2012_280. [DOI] [PubMed] [Google Scholar]
- 3.Bisno A.L. Acute pharyngitis. N Engl J Med. 2001;344:205–211. doi: 10.1056/NEJM200101183440308. [DOI] [PubMed] [Google Scholar]
- 4.Stevens D.L., Bryant A.E. Necrotizing soft-tissue infections. N Engl J Med. 2017;377:2253–2265. doi: 10.1056/NEJMra1600673. [DOI] [PubMed] [Google Scholar]
- 5.Walker M.J., Barnett T.C., McArthur J.D., Cole J.N., Gillen C.M., Henningham A., Sriprakash K.S., Sanderson-Smith M.L., Nizet V. Disease manifestations and pathogenic mechanisms of group A Streptococcus. Clin Microbiol Rev. 2014;27:264–301. doi: 10.1128/CMR.00101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins D.A., Johnson C.O., Colquhoun S.M., Karthikeyan G., Beaton A., Bukhman G., Forouzanfar M.H., Longenecker C.T., Mayosi B.M., Mensah G.A., Nascimento B.R., Ribeiro A.L.P., Sable C.A., Steer A.C., Naghavi M., Mokdad A.H., Murray C.J.L., Vos T., Carapetis J.R., Roth G.A. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017;377:713–722. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 7.Wessels M.R. Clinical practice: streptococcal pharyngitis. N Engl J Med. 2011;364:648–655. doi: 10.1056/NEJMcp1009126. [DOI] [PubMed] [Google Scholar]
- 8.Osowicki J., Vekemans J., Kaslow D.C., Friede M.H., Kim J.H., Steer A.C. WHO/IVI global stakeholder consultation on group A Streptococcus vaccine development: report from a meeting held on 12-13 December 2016. Vaccine. 2018;36:3397–3405. doi: 10.1016/j.vaccine.2018.02.068. [DOI] [PubMed] [Google Scholar]
- 9.Schodel F., Moreland N.J., Wittes J.T., Mulholland K., Frazer I., Steer A.C., Fraser J.D., Carapetis J. Clinical development strategy for a candidate group A streptococcal vaccine. Vaccine. 2017;35:2007–2014. doi: 10.1016/j.vaccine.2017.02.060. [DOI] [PubMed] [Google Scholar]
- 10.Steer A.C., Carapetis J.R., Dale J.B., Fraser J.D., Good M.F., Guilherme L., Moreland N.J., Mulholland E.K., Schodel F., Smeesters P.R. Status of research and development of vaccines for Streptococcus pyogenes. Vaccine. 2016;34:2953–2958. doi: 10.1016/j.vaccine.2016.03.073. [DOI] [PubMed] [Google Scholar]
- 11.Vekemans J., Gouvea-Reis F., Kim J.H., Excler J.L., Smeesters P.R., O'Brien K.L., Van Beneden C.A., Steer A.C., Carapetis J.R., Kaslow D.C. The path to group A Streptococcus vaccines: World Health Organization research and development technology roadmap and preferred product characteristics. Clin Infect Dis. 2019;69:877–883. doi: 10.1093/cid/ciy1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dooling K.L., Shapiro D.J., Van Beneden C., Hersh A.L., Hicks L.A. Overprescribing and inappropriate antibiotic selection for children with pharyngitis in the United States, 1997-2010. JAMA Pediatr. 2014;168:1073–1074. doi: 10.1001/jamapediatrics.2014.1582. [DOI] [PubMed] [Google Scholar]
- 13.King L.M., Bartoces M., Fleming-Dutra K.E., Roberts R.M., Hicks L.A. Changes in US outpatient antibiotic prescriptions from 2011-2016. Clin Infect Dis. 2020;70:370–377. doi: 10.1093/cid/ciz225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pichichero M.E. Group A beta-hemolytic streptococcal infections. Pediatr Rev. 1998;19:291–302. doi: 10.1542/pir.19-9-291. [DOI] [PubMed] [Google Scholar]
- 15.Horn D.L., Zabriskie J.B., Austrian R., Cleary P.P., Ferretti J.J., Fischetti V.A., Gotschlich E., Kaplan E.L., McCarty M., Opal S.M., Roberts R.B., Tomasz A., Wachtfogel Y. Why have group A streptococci remained susceptible to penicillin? report on a symposium. Clin Infect Dis. 1998;26:1341–1345. doi: 10.1086/516375. [DOI] [PubMed] [Google Scholar]
- 16.Musser J.M., Beres S.B., Zhu L., Olsen R.J., Vuopio J., Hyyrylainen H.L., Grondahl-Yli-Hannuksela K., Kristinsson K.G., Darenberg J., Henriques-Normark B., Hoffmann S., Caugant D.A., Smith A.J., Lindsay D.S.J., Boragine D., Palzkill T. Reduced in vitro susceptibility of Streptococcus pyogenes to beta-lactam antibiotics associated with mutations in the pbp2x gene is geographically widespread. J Clin Microbiol. 2020;58:e01993-19. doi: 10.1128/JCM.01993-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vannice K., Ricaldi J., Nanduri S., Fang F.C., Lynch J., Bryson-Cahn C., Wright T., Duchin J., Kay M., Chochua S., Van Beneden C., Beall B. Streptococcus pyogenes pbp2x mutation confers reduced susceptibility to beta-lactam antibiotics. Clin Infect Dis. 2019;70:1265. doi: 10.1093/cid/ciz1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banno H., Kimura K., Seki T., Jin W., Wachino J.I., Yamada K., Nagano N., Arakawa Y. High isolation rate and multidrug resistance tendency of penicillin-susceptible group B Streptococcus with reduced ceftibuten susceptibility in Japan. Eur J Clin Microbiol Infect Dis. 2018;37:1511–1519. doi: 10.1007/s10096-018-3278-7. [DOI] [PubMed] [Google Scholar]
- 19.Dahesh S., Hensler M.E., Van Sorge N.M., Gertz R.E., Jr., Schrag S., Nizet V., Beall B.W. Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to beta-lactam antibiotics. Antimicrob Agents Chemother. 2008;52:2915–2918. doi: 10.1128/AAC.00461-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuursted K., Stegger M., Hoffmann S., Lambertsen L., Andersen P.S., Deleuran M., Thomsen M.K. Description and characterization of a penicillin-resistant Streptococcus dysgalactiae subsp. equisimilis clone isolated from blood in three epidemiologically linked patients. J Antimicrob Chemother. 2016;71:3376–3380. doi: 10.1093/jac/dkw320. [DOI] [PubMed] [Google Scholar]
- 21.Gordon E., Mouz N., Duee E., Dideberg O. The crystal structure of the penicillin-binding protein 2x from Streptococcus pneumoniae and its acyl-enzyme form: implication in drug resistance. J Mol Biol. 2000;299:477–485. doi: 10.1006/jmbi.2000.3740. [DOI] [PubMed] [Google Scholar]
- 22.Haenni M., Galofaro L., Ythier M., Giddey M., Majcherczyk P., Moreillon P., Madec J.Y. Penicillin-binding protein gene alterations in Streptococcus uberis isolates presenting decreased susceptibility to penicillin. Antimicrob Agents Chemother. 2010;54:1140–1145. doi: 10.1128/AAC.00915-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura K., Nagano N., Arakawa Y. Classification of group B streptococci with reduced beta-lactam susceptibility (GBS-RBS) based on the amino acid substitutions in PBPs. J Antimicrob Chemother. 2015;70:1601–1603. doi: 10.1093/jac/dkv022. [DOI] [PubMed] [Google Scholar]
- 24.Kimura K., Suzuki S., Wachino J., Kurokawa H., Yamane K., Shibata N., Nagano N., Kato H., Shibayama K., Arakawa Y. First molecular characterization of group B Streptococci with reduced penicillin susceptibility. Antimicrob Agents Chemother. 2008;52:2890–2897. doi: 10.1128/AAC.00185-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura K., Wachino J., Kurokawa H., Matsui M., Suzuki S., Yamane K., Nagano N., Shibayama K., Arakawa Y. High cephalosporin resistance due to amino acid substitutions in PBP1A and PBP2X in a clinical isolate of group B Streptococcus. J Antimicrob Chemother. 2013;68:1533–1536. doi: 10.1093/jac/dkt060. [DOI] [PubMed] [Google Scholar]
- 26.Koide S., Hayashi W., Taniguchi Y., Tanaka H., Kimura K., Nagano Y., Arakawa Y., Nagano N. Potential effect of selective pressure with different beta-lactam molecules on the emergence of reduced susceptibility to beta-lactams in group B Streptococci. Microbiol Immunol. 2019;63:65–76. doi: 10.1111/1348-0421.12667. [DOI] [PubMed] [Google Scholar]
- 27.Longtin J., Vermeiren C., Shahinas D., Tamber G.S., McGeer A., Low D.E., Katz K., Pillai D.R. Novel mutations in a patient isolate of Streptococcus agalactiae with reduced penicillin susceptibility emerging after long-term oral suppressive therapy. Antimicrob Agents Chemother. 2011;55:2983–2985. doi: 10.1128/AAC.01243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metcalf B.J., Chochua S., Gertz R.E., Jr., Hawkins P.A., Ricaldi J., Li Z., Walker H., Tran T., Rivers J., Mathis S., Jackson D., Glennen A., Lynfield R., McGee L., Beall B. Active bacterial core surveillance t: short-read whole genome sequencing for determination of antimicrobial resistance mechanisms and capsular serotypes of current invasive Streptococcus agalactiae recovered in the USA. Clin Microbiol Infect. 2017;23:574.e7–574.e14. doi: 10.1016/j.cmi.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Mouz N., Di Guilmi A.M., Gordon E., Hakenbeck R., Dideberg O., Vernet T. Mutations in the active site of penicillin-binding protein PBP2x from Streptococcus pneumoniae: role in the specificity for beta-lactam antibiotics. J Biol Chem. 1999;274:19175–19180. doi: 10.1074/jbc.274.27.19175. [DOI] [PubMed] [Google Scholar]
- 30.Mouz N., Gordon E., Di Guilmi A.M., Petit I., Petillot Y., Dupont Y., Hakenbeck R., Vernet T., Dideberg O. Identification of a structural determinant for resistance to beta-lactam antibiotics in gram-positive bacteria. Proc Natl Acad Sci U S A. 1998;95:13403–13406. doi: 10.1073/pnas.95.23.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagano N., Nagano Y., Kimura K., Tamai K., Yanagisawa H., Arakawa Y. Genetic heterogeneity in pbp genes among clinically isolated group B Streptococci with reduced penicillin susceptibility. Antimicrob Agents Chemother. 2008;52:4258–4267. doi: 10.1128/AAC.00596-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagano N., Nagano Y., Toyama M., Kimura K., Tamura T., Shibayama K., Arakawa Y. Nosocomial spread of multidrug-resistant group B streptococci with reduced penicillin susceptibility belonging to clonal complex 1. J Antimicrob Chemother. 2012;67:849–856. doi: 10.1093/jac/dkr546. [DOI] [PubMed] [Google Scholar]
- 33.Nagano N., Nagano Y., Toyama M., Kimura K., Shibayama K., Arakawa Y. Penicillin-susceptible group B streptococcal clinical isolates with reduced cephalosporin susceptibility. J Clin Microbiol. 2014;52:3406–3410. doi: 10.1128/JCM.01291-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pares S., Mouz N., Petillot Y., Hakenbeck R., Dideberg O. X-ray structure of Streptococcus pneumoniae PBP2x, a primary penicillin target enzyme. Nat Struct Biol. 1996;3:284–289. doi: 10.1038/nsb0396-284. [DOI] [PubMed] [Google Scholar]
- 35.Pernot L., Chesnel L., Le Gouellec A., Croize J., Vernet T., Dideberg O., Dessen A. A PBP2x from a clinical isolate of Streptococcus pneumoniae exhibits an alternative mechanism for reduction of susceptibility to beta-lactam antibiotics. J Biol Chem. 2004;279:16463–16470. doi: 10.1074/jbc.M313492200. [DOI] [PubMed] [Google Scholar]
- 36.Piccinelli G., Carlentini G., Gargiulo F., Caruso A., De Francesco M.A. Analysis of point mutations in the pbp2x, pbp2b, and pbp1a genes of Streptococcus agalactiae and their relation with a reduced susceptibility to cephalosporins. Microb Drug Resist. 2017;23:1019–1024. doi: 10.1089/mdr.2017.0013. [DOI] [PubMed] [Google Scholar]
- 37.Zapun A., Contreras-Martel C., Vernet T. Penicillin-binding proteins and beta-lactam resistance. FEMS Microbiol Rev. 2008;32:361–385. doi: 10.1111/j.1574-6976.2007.00095.x. [DOI] [PubMed] [Google Scholar]
- 38.Hirose Y., Yamaguchi M., Okuzaki D., Motooka D., Hamamoto H., Hanada T., Sumitomo T., Nakata M., Kawabata S. Streptococcus pyogenes transcriptome changes in the inflammatory environment of necrotizing fasciitis. Appl Environ Microbiol. 2019;85:e01428-19. doi: 10.1128/AEM.01428-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu L., Charbonneau A.R.L., Waller A.S., Olsen R.J., Beres S.B., Musser J.M. Novel genes required for the fitness of Streptococcus pyogenes in human saliva. mSphere. 2017;2:e00460-17. doi: 10.1128/mSphereDirect.00460-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu L., Olsen R.J., Beres S.B., Eraso J.M., Saavedra M.O., Kubiak S.L., Cantu C.C., Jenkins L., Charbonneau A.R.L., Waller A.S., Musser J.M. Gene fitness landscape of group A streptococcus during necrotizing myositis. J Clin Invest. 2019;129:887–901. doi: 10.1172/JCI124994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu L., Olsen R.J., Lee J.D., Porter A.R., DeLeo F.R., Musser J.M. Contribution of secreted NADase and streptolysin O to the pathogenesis of epidemic serotype M1 Streptococcus pyogenes infections. Am J Pathol. 2017;187:605–613. doi: 10.1016/j.ajpath.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu L., Olsen R.J., Nasser W., Beres S.B., Vuopio J., Kristinsson K.G., Gottfredsson M., Porter A.R., DeLeo F.R., Musser J.M. A molecular trigger for intercontinental epidemics of group A Streptococcus. J Clin Invest. 2015;125:3545–3559. doi: 10.1172/JCI82478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beres S.B., Kachroo P., Nasser W., Olsen R.J., Zhu L., Flores A.R., de la Riva I., Paez-Mayorga J., Jimenez F.E., Cantu C., Vuopio J., Jalava J., Kristinsson K.G., Gottfredsson M., Corander J., Fittipaldi N., Di Luca M.C., Petrelli D., Vitali L.A., Raiford A., Jenkins L., Musser J.M. Transcriptome remodeling contributes to epidemic disease caused by the human pathogen Streptococcus pyogenes. mBio. 2016;7:e00403-16. doi: 10.1128/mBio.00403-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen R.J., Sitkiewicz I., Ayeras A.A., Gonulal V.E., Cantu C., Beres S.B., Green N.M., Lei B., Humbird T., Greaver J., Chang E., Ragasa W.P., Montgomery C.A., Cartwright J., Jr., McGeer A., Low D.E., Whitney A.R., Cagle P.T., Blasdel T.L., DeLeo F.R., Musser J.M. Decreased necrotizing fasciitis capacity caused by a single nucleotide mutation that alters a multiple gene virulence axis. Proc Natl Acad Sci U S A. 2010;107:888–893. doi: 10.1073/pnas.0911811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaudreau C., Lecours R., Ismail J., Gagnon S., Jette L., Roger M. Prosthetic hip joint infection with a Streptococcus agalactiae isolate not susceptible to penicillin G and ceftriaxone. J Antimicrob Chemother. 2010;65:594–595. doi: 10.1093/jac/dkp458. [DOI] [PubMed] [Google Scholar]
- 46.Nagano N., Kimura K., Nagano Y., Yakumaru H., Arakawa Y. Molecular characterization of group B streptococci with reduced penicillin susceptibility recurrently isolated from a sacral decubitus ulcer. J Antimicrob Chemother. 2009;64:1326–1328. doi: 10.1093/jac/dkp374. [DOI] [PubMed] [Google Scholar]
- 47.Quesenberry K.E., Carpenter J.W. ed 3. Elsevier/Saunders; St. Louis, MO: 2012. Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery. [Google Scholar]
- 48.Azoulay-Dupuis E., Rieux V., Muffat-Joly M., Bedos J.P., Vallee E., Rivier C., Isturiz R., Carbon C., Moine P. Relationship between capsular type, penicillin susceptibility, and virulence of human Streptococcus pneumoniae isolates in mice. Antimicrob Agents Chemother. 2000;44:1575–1577. doi: 10.1128/aac.44.6.1575-1577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bjorkman J., Hughes D., Andersson D.I. Virulence of antibiotic-resistant Salmonella typhimurium. Proc Natl Acad Sci U S A. 1998;95:3949–3953. doi: 10.1073/pnas.95.7.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dewan K.K., Skarlupka A.L., Rivera I., Cuff L.E., Gestal M.C., Taylor-Mulneix D.L., Wagner S., Ryman V.E., Rodriguez C., Hamidou Soumana I., Levin B.R., Harvill E.T. Development of macrolide resistance in Bordetella bronchiseptica is associated with the loss of virulence. J Antimicrob Chemother. 2018;73:2797–2805. doi: 10.1093/jac/dky264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farhat M.R., Shapiro B.J., Kieser K.J., Sultana R., Jacobson K.R., Victor T.C., Warren R.M., Streicher E.M., Calver A., Sloutsky A., Kaur D., Posey J.E., Plikaytis B., Oggioni M.R., Gardy J.L., Johnston J.C., Rodrigues M., Tang P.K., Kato-Maeda M., Borowsky M.L., Muddukrishna B., Kreiswirth B.N., Kurepina N., Galagan J., Gagneux S., Birren B., Rubin E.J., Lander E.S., Sabeti P.C., Murray M. Genomic analysis identifies targets of convergent positive selection in drug-resistant Mycobacterium tuberculosis. Nat Genet. 2013;45:1183–1189. doi: 10.1038/ng.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee J.H., Ammerman N.C., Nolan S., Geiman D.E., Lun S., Guo H., Bishai W.R. Isoniazid resistance without a loss of fitness in Mycobacterium tuberculosis. Nat Commun. 2012;3:753. doi: 10.1038/ncomms1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Margolis E., Levin B.R. Within-host evolution for the invasiveness of commensal bacteria: an experimental study of bacteremias resulting from Haemophilus influenzae nasal carriage. J Infect Dis. 2007;196:1068–1075. doi: 10.1086/520934. [DOI] [PubMed] [Google Scholar]
- 54.Rieux V., Carbon C., Azoulay-Dupuis E. Complex relationship between acquisition of beta-lactam resistance and loss of virulence in Streptococcus pneumoniae. J Infect Dis. 2001;184:66–72. doi: 10.1086/320992. [DOI] [PubMed] [Google Scholar]
- 55.Rifat D., Campodonico V.L., Tao J., Miller J.A., Alp A., Yao Y., Karakousis P.C. In vitro and in vivo fitness costs associated with Mycobacterium tuberculosis RpoB mutation H526D. Future Microbiol. 2017;12:753–765. doi: 10.2217/fmb-2017-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith K.L., Saini D., Bardarov S., Larsen M., Frothingham R., Gandhi N.R., Jacobs W.R., Jr., Sturm A.W., Lee S. Reduced virulence of an extensively drug-resistant outbreak strain of Mycobacterium tuberculosis in a murine model. PLoS One. 2014;9:e94953. doi: 10.1371/journal.pone.0094953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmadi A., Yaghoubi S., Irajian G. Molecular analysis of PBP1A in Streptococcus pneumoniae isolated from clinical and normal flora samples in Tehran, Iran: a multicenter study. Microb Drug Resist. 2019;25:39–46. doi: 10.1089/mdr.2017.0326. [DOI] [PubMed] [Google Scholar]
- 58.Diawara I., Nayme K., Katfy K., Barguigua A., Kettani-Halabi M., Belabbes H., Timinouni M., Zerouali K., Elmdaghri N. Analysis of amino acid motif of penicillin-binding proteins 1a, 2b, and 2x in invasive Streptococcus pneumoniae nonsusceptible to penicillin isolated from pediatric patients in Casablanca, Morocco. BMC Res Notes. 2018;11:632. doi: 10.1186/s13104-018-3719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu E.Y., Chang J.C., Lin J.C., Chang F.Y., Fung C.P. Important mutations contributing to high-level penicillin resistance in Taiwan(19F)-14, Taiwan(23F)-15, and Spain(23F)-1 of Streptococcus pneumoniae isolated from Taiwan. Microb Drug Resist. 2016;22:646–654. doi: 10.1089/mdr.2015.0261. [DOI] [PubMed] [Google Scholar]