Abstract
Aberrant fatty acid (FA) metabolism is a hallmark of proliferating cells, including untransformed fibroblasts or cancer cells. Lipolysis of intracellular triglyceride (TG) stores by adipose triglyceride lipase (ATGL) provides an important source of FAs serving as energy substrates, signaling molecules, and precursors for membrane lipids. To investigate if ATGL-mediated lipolysis impacts cell proliferation, we modified ATGL activity in murine embryonic fibroblasts (MEFs) and in five different cancer cell lines to determine the consequences on cell growth and metabolism. Genetic or pharmacological inhibition of ATGL in MEFs causes impaired FA oxidation, decreased ROS production, and a substrate switch from FA to glucose leading to decreased AMPK-mTOR signaling and higher cell proliferation rates. ATGL expression in these cancer cells is low when compared to MEFs. Additional ATGL knockdown in cancer cells did not significantly affect cellular lipid metabolism or cell proliferation whereas the ectopic overexpression of ATGL increased lipolysis and reduced proliferation. In contrast to ATGL silencing, pharmacological inhibition of ATGL by Atglistatin© impeded the proliferation of diverse cancer cell lines, which points at an ATGL-independent effect. Our data indicate a crucial role of ATGL-mediated lipolysis in the regulation of cell proliferation. The observed low ATGL activity in cancer cells may represent an evolutionary selection process and mechanism to sustain high cell proliferation rates. As the increasing ATGL activity decelerates proliferation of five different cancer cell lines this may represent a novel therapeutic strategy to counteract uncontrolled cell growth.
Abbreviations: AMPK, AMP activated protein kinase; ABHD6, Alpha/beta-hydrolase domain containing 6; ATGL, Adipose triglyceride lipase; BM, Bone marrow; CD31, Cluster of differentiation 31; CGI-58, Comparative gene identification-58; DG, Diglyceride; DMSO, Dimethylsulfoxid; FA, Fatty acid; FAO, Fatty acid oxidation; G0S2, G0/G1 switch gene 2; H&E, Haematoxylin/eosin; HIG2, Hypoxia-inducible gene 2; HSL, Hormone-sensitive lipase; IHC, Immunohistochemistry; MEF, Mouse embryonic fibroblasts; MG, Monoglyceride; MGL, Monoacylglycerol lipase; mTOR, Mammalian target of rapamycin; NSCLC, Non-small-cell lung carcinoma; OCR, Oxygen consumption rate; PBS, Phosphate buffered saline; PCNA, Proliferating cell nuclear antigen; ROS, Reactive oxygen species; shRNA, small hairpin RNA; SVF, Stroma vascular fractions; TG, Triglyceride; WAT, White adipose tissue
Keywords: ATGL, Cancer, Lipolysis, Proliferation, Lipid
Highlights
-
•
ATGL deficiency increases cell proliferation and decreases phosphorylation of AMPK in murine embryonic fibroblasts.
-
•
ATGL silencing does not reduce lipolysis or increase proliferation of diverse cancer cells.
-
•
Increasing ATGL activity represses cell proliferation in a panel of cancer cells.
-
•
Atglistatin© exhibits an ATGL independent tumor suppressive effect.
1. Introduction
Although cancers are diverse in etiology and type, most cancer cells share the hallmark of metabolic reprogramming [1]. A well-defined metabolic transformation in cancer cells is the switch from an oxidative to a glycolytic phenotype. Moreover, cancer cells exhibit increased glutamine metabolism and fatty acid (FA) synthesis [2,3]. The role of intracellular lipolysis in cancer cell proliferation and metabolism has gained significant attention [4,5].
Intracellular lipolysis describes the biochemical pathway that is responsible for the hydrolysis of intracellularly stored triglycerides (TGs). The sequential process involves at least three enzymes and numerous regulatory proteins resulting in the formation of glycerol and FAs [6]. Although most active in adipocytes, lipolysis occurs also in all other cell types. Within these non-adipose cells, the released FAs are incorporated into membranes, serve as signaling lipids, or energy substrates. Adipose triglyceride lipase (ATGL) initializes the lipolytic breakdown of TGs by converting TGs to diglycerides (DGs) and FAs [7]. Comparative gene identification-58 (CGI-58; also called α/β-hydrolase domain-containing 5 or ABHD5) activates ATGL activity [8] while G0/G1 switch gene 2 (G0S2) and hypoxia-inducible gene 2 (HIG2) inhibit the enzyme [9,10]. The subsequent step in lipolysis is catalyzed by hormone-sensitive lipase (HSL) hydrolyzing DGs to monoglycerides (MGs) and FAs. Finally, MGs are hydrolyzed to FAs and glycerol by monoacylglycerol lipase (MGL) or alpha/beta-hydrolase domain containing 6 (ABHD6) [6,11].
Unlike studies focusing on MGL or ABHD6 unanimously assuming a positive correlation between MG hydrolase activity, tumor growth, and malignancy [[12], [13], [14], [15], [16], [17]], similar studies on the role of ATGL in cancer biology are less conclusive. Some studies suggested that ATGL acts as an oncogene in prostate cancer, colon cancer, hepatic cancer, and non-small-cell lung carcinoma (NSCLC) [[18], [19], [20], [21]]. The underlying molecular mechanism has only been demonstrated for NSCLC, where reduced ATGL expression altered TG catabolism and AMP-activated protein kinase (AMPK) signaling leading to apoptosis [20]. Other studies, obtained opposing conclusions showing that ATGL expression is downregulated in several cancer types and that mice lacking ATGL spontaneously develop pulmonary neoplasia [22,23]. In support of a tumor suppressor function of lipolysis, adipose-specific disruption of ATGL and HSL caused liposarcoma in mice [24]. The most recent study in colon and cervical cancer suggested that CRISPR/Cas9 mediated ATGL knockdown does not affect cancer cell proliferation or tumor growth [9].
Other studies investigated the role of ATGL-coregulators, particularly CGI-58 and G0S2, in malignancy with conflicting results. Loss of CGI-58 increased cancer incidence of both colon and prostate cancer [19,21,25]. The potential tumor suppressor role of CGI-58 was ascribed to ATGL-independent functions of CGI-58 [19,21,25,26]. Inconsistent results on the ATGL-G0S2 axis were reported in lung cancer. While the tumor suppressor role of G0S2 attenuating NSCLC cell proliferation depends on its inhibitory effect on ATGL [20], other studies concluded that the role of G0S2 in cancerogenesis is independent of ATGL [27,28]. Recently, another coregulator of ATGL, HIG2, has been identified to enhance cancer cell survival by inhibiting ATGL under hypoxic condition [9].
In view of these inconsistent findings, we revisited the role of ATGL in proliferation and metabolism of six cancer- and non-cancer cell lines by silencing or overexpressing ATGL and determine cell proliferation rates and parameters of lipid and glucose metabolism. We show that ATGL enzyme activity inversely correlates with the proliferation rate of mouse embryonic fibroblasts (MEFs) and various cancer cell lines suggesting a tumor suppressing role for the enzyme.
2. Materials and methods
2.1. Cell lines and drugs
Mouse embryonic fibroblasts (MEFs) were isolated from wild type (WT) mouse embryos or embryos globally lacking ATGL (AKO). Briefly, head, limbs, and organs of embryos (day 13) were removed using sterile forceps in 100-mm plates containing phosphate-buffered saline (PBS). The carcass was transferred to Dulbecco's modified eagle medium (DMEM, #41966, Thermo Fisher Scientific, Waltham, Massachusetts, USA), minced with a razor blade, and mechanically lysed 3–4 times using a 21-gauge needle. Cells were seeded in 0.1% gelatin coated 100-mm cell culture plates and cultivated in DMEM containing 10% FCS, and 100 IU/l penicillin, and 0.1 mg/l streptomycin. MEFs were grown until ~80% confluence and frozen in 10% DMSO at −80 °C for further experiments. Stromal vascular fraction (SVF) were isolated from inguinal white adipose tissue (WAT) of WT or AKO mice. Briefly, WATs of the sacrificed mice were excised using scalpel and minced into fine pieces. Minced samples were placed in DMEM medium. Collagenase type 2 (Worthington) was added into the tissue suspension at a final concentration of 2 mg/ml. Samples were incubated in an orbital shaker at 37 °C for 1 h. Once digestion was completed, samples were passed through a sterile 70 μm cell strainer and centrifuged at 1,000g for 5 min. The cell pellets were suspended in 5 ml of red blood cell lysis buffer for 3 min at room temperature and then washed three times using PBS. The cell pellets were collected as the SVF. Cells of SVF were seeded in 0.1% gelatin coated 100-mm cell culture plates and cultivated in DMEM containing 10% FCS, and 100 IU/l penicillin, and 0.1 mg/l streptomycin. Cells were grown until ~80% confluence for further experiments. Tumorigenic precursor B cell lines were established using murine stem cell virus derived vectors (pMSCV) that carried the human bcr-abl p185 oncogene. Essentially, mouse bone marrow (BM) was isolated from either WT or AKO animals at 4–8 weeks of age. BM was incubated with pMSCV-bcr-abl p185/IRES-GFP virus preparations in growth factor enriched media as described by Sexl et al. [29]. After 2–3 weeks, the outgrowth of immortalized cells was monitored. Proliferating cell populations were passaged every 2–3 days for 2–3 months, until stable proliferation was monitored. pMSCV-bcr-abl p185/IRES-GFP virus uptake was verified by GFP fluorescence. B16-F10 (ATCC# CRL 6475™), C26 (kindly provided by Graham Robertson, Anzac Institutes, Sydney), CT26 (ATCC# CRL 2638™), LLC (ATCC# CRL-1642™), HepG2 (ATCC# HB-8065™) cells, and WT/AKO transgenic precursor B cell lines were cultivated in RPMI 1640 medium (#A10491-01, Thermo Fisher Scientific). All media were supplemented with 10% fetal bovine serum (Thermo Fisher Scientific), 100 IU/l penicillin, and 0.1 mg/l streptomycin. At 70–80% confluency, cancer cells were used for experiments.
Cells were treated with Atglistatin© (40 μM, University of Technology, Graz, Austria), HSL inhibitor (Hi 760079, 25 μM, Novo Nordisk, Denmark), MGL inhibitor (JZL184, 20 μM, Cayman Chemical, Ann Arbor, USA), 5-fluoro-2′-deoxyuridine (10 μM, Sigma-Aldrich, St Louis, Missouri, USA) and uridine (10 μM, Sigma-Aldrich) dissolved in Dimethylsulfoxid (DMSO).
2.2. Animals and ethics
Mice were kept on a regular light/dark cycle (14/10) and fed a standard chow diet (Ssniff, Soest, Germany). 8–12 weeks old male C57Bl6J and CD2F/1 mice were injected with 0.5 × 106 cancer cells/100 μl 1xPBS subcutaneously into the right flank. For the control group, 100 μl 1xPBS were injected subcutaneously into the right flank of non-tumor bearing mice. For Atglistatin© treatment, mice were given 200 μmol/kg mouse weight Atglistatin© mixed into high fat diet powder (Ssniff) to obtain maximum bioavailability 14 days prior to cancer cells injection. Mice were sacrificed 14–16 days after cancer cell injection by cervical dislocation. Tumors were snap frozen in liquid nitrogen directly after excision or were fixed in 4% PBS buffered paraformaldehyde to perform IHC. All procedures in this study were in conformity with the public health service policy on the use of laboratory animals and were approved by local and national ethical committees (GZ 66.007/0002-II/3b/2014).
2.3. ATGL silencing and ATGL overexpression in cancer cells and MEFs
shControl (TR30021) and shATGL (TL302393B) expression constructs were purchased from OriGene (Rockville, Maryland, USA). The coding sequence of murine ATGL was amplified by PCR (primers 5′- GAT CCT CGA GGC CAC CAT GTT CCC GAG GGA GAC CAA-3′ and 5′- GAC TCC GCG GGC AAG GCG GGA GGC CAG GT-3′) [7]. The PCR product was digested with XhoI and SacII (NEB, Ipswich, Massachusetts, USA) and ligated to the vector pECFP-N1 (Clontech-Takara Bio, France). The resulting plasmid was digested with XhoI and NotI and the DNA fragment encoding for the ATGL-ECFP fusion protein was ligated to the lentiviral vector pLVX-IRES-Puro using T4 DNA ligase (NEB). A control lentiviral vector encoding for ECFP was generated by digesting pECFP-N1 with XhoI and NotI and ligating the DNA fragment encoding for ECFP to pLVX-IRES-Puro. The sequence was verified by DNA sequencing (Microsynth, Balgach, Switzerland) prior to applications. Lentivirus containing media was prepared with LVX Tet-Off advanced inducible expression system (Clontech-Takara Bio). Cancer cells used for ATGL knockdown and ATGL overexpression studies were seeded in 6-well plates. After the cells grew to confluence, aliquots of lentiviral stocks were thawed and mixed gently. Then, 4 μg/ml polybrene was added to the lentivirus containing medium. After 24 h incubation with lentivirus containing medium, the medium was replaced by fresh growth medium containing G418 (0.5 mg/ml) and puromycin (4 μg/ml) to select for transduced cells. Adenoviruses containing recombinant mouse ATGL or empty vectors were prepared using the AdEasy system according to the manufacturer protocol [30]. Twenty-four hours after viral infection, cells were washed with 1xPBS and collected for different analyses.
2.4. Cell proliferation assays
2500 cells/cm2 MEFs were seeded in 0.1% gelatin coated 6-well plates. 1000 cells/cm2 cancer cells were seeded in 6-well plates. Cells were counted every 24 h using a hemocytometer (Bio-Rad TC20). Live cells were distinguished from dead cells by trypan blue.
2.5. TG hydrolase assay and cellular lipid content
Cultured cells were harvested using a cell scraper and washed three times with 1xPBS. Cell pellets were disrupted in solution A (0.25 M sucrose containing 1 mM EDTA, 1 mM DTT, 20 μg/ml leupeptine, 2 μg/ml antipain, and 1 μg/ml pepstatin) by sonication. Cell debris and nuclei were removed by centrifugation at 1000 g and 4 °C for 10 min. Protein concentration was determined using Biorad protein assay (Bio-Rad, Hercules, California, USA) and BSA as standard. TG hydrolase activity of cell lysates was measured as described previously [31]. In brief, 100 μg protein of cell lysate were incubated with a substrate containing glycerol trioleate [9,10(N)-3H]-emulsified with phosphatidylglycerol/phosphatidylinositol (Perkin Elmer, Germany) in the presence or absence of 50 ng/ml purified murine CGI-58 produced in Escherichia coli, and 40 μM Atglistatin© for 1 h at 37 °C. Thereafter, FAs were extracted, and radioactivity determined by liquid scintillation counting (Packard BioScience Company, Meriden, Connecticut, USA).
Lipids of cell lysates were extracted using Folch extraction, CHCl3: MeOH (2:1). 20% volume of H2O was added to the samples and mixed. The extracts were centrifuged at 2000 g and 4 °C for 10 min. The lipid-containing organic phase was evaporated and reconstituted in H2O containing 2.5% Triton X-100. Acylglycerol content was measured using Infinity™ triglycerides reagent (Thermo Fisher) and glycerol (Sigma-Aldrich) as standard. FA content was measured using NEFA-HR2 reagent (Wako Diagnostics, Mountain View, California, USA).
2.6. Cellular FA uptake and FA oxidation (FAO)
MEFs were plated onto 0.1% gelatin-coated 6-well plates and cultured in DMEM. After reaching ~80% confluence, cellular FA uptake was determined by loading cells with 400 μM oleic acid (complexed to BSA, essentially FA-free, Sigma-Aldrich) and 0.4 μCi 14C-oleic acid (Hartmann analytic, Braunschweig, Germany) per well. After 1 min, cells were washed three times with 1xPBS. Thereafter, cells were lysed in 0.3 M NaOH/0.1% SDS, and an aliquot was used for liquid scintillation counting. Protein concentration of the lysate was determined using BCA reagent (Bio-Rad) and BSA as standard. FAO assay was performed according to the protocol of Hirschey et al. [32]. Briefly, MEFs were seeded in a 25-cm2 cell culture flask. After reaching ~80% confluence, MEFs were incubated with RPMI medium containing 0.8 mM oleic acid (complexed to FA-free BSA) for 6 h. Subsequently, MEFs were incubated with 0.8 mM oleic acid (complexed to FA-free BSA) and 0.05 μCi 14C-oleic acid (Hartmann Analytic) in RPMI medium per well for 2 h. For 14C-labeled CO2 trapping, a tube cap and a filter paper soaked with 20 μl 10 N NaOH was used. The covered filter paper was removed from the cap for scintillation counting. Cells were lysed in 0.25 ml 0.3 M NaOH/0.1% SDS for the determination of protein concentration using BCA reagent. Rates of FAO were calculated from the radioactivity that accumulated in the filter paper per hour and milligram cell protein.
2.7. Cellular glucose uptake
MEFs were plated onto 0.1% gelatin coated 6-well plates and cultured in DMEM. After reaching ~80% confluence, the medium was replaced by 1 ml of depletion medium (40 mM NaCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 4.7 mM KCl, 0.25 M CaCl2, 2% FA-free BSA) and incubated for 1 h at 37 °C. Subsequently, the medium was replaced by 1 ml of depletion medium with or without insulin (200 nM) and incubated for 15 min. Thereafter, the depletion medium was replaced by transport medium (40 mM NaCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 4.7 mM KCl, 0.25 M CaCl2, 5 mM 2-deoxyglucose) containing 0.5 μCi/well Deoxy-d-glucose, 2-[1,2-3H (N)] (Perkin Elmer, Germany). After incubation for 20 min, the glucose uptake was stopped by aspirating the medium. Cells were washed three times with 1xPBS and lysed with 0.3 M NaOH/0.1% SDS. An aliquot was used for liquid scintillation counting. Protein concentration of the lysate was determined using BCA reagent and BSA as standard.
2.8. Seahorse analysis and reactive oxygen species (ROS) measurement
Oxygen consumption rate (OCR) and ATP production were assessed using a seahorse XFe96 analyzer in combination with the Seahorse XF Cell Mito Stress Test kit according to a standard protocol [33]. In brief, MEFs were plated in XF96 polystyrene cell culture microplates (Seahorse Bioscience) at a density of 10,000 cells per well. After cells reach a high confluency, cells were washed and preincubated for 30 min in unbuffered XF assay medium (0.8 mM MgSO4, 1.8 mM CaCl2, 143 mM NaCl, 5.4 mM KCl, 0.91 mM NaH2PO4) supplemented with 2 mM glutamax (Thermo Fisher Scientific), 10 mM glucose, 1 mM sodium pyruvate (Thermo Fisher Scientific) at 37 °C in a non-CO2 environment. OCR was subsequently measured every 7 min using an XF96 extracellular flux analyzer (Seahorse Bioscience). Optimal concentrations of specific inhibitors/accelerators of the electron transport chain were determined in prior titration experiments and working concentrations used were 1.5 μM oligomycin, 1 μM carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP), and 2.5 μM antimycin A. Raw data were analyzed using Wave Desktop Software (Agilent, version 2.0). For ROS measurements, WT/AKO MEFs were washed with PBS and stained with 50 μM Dichloro-dihydro-fluorescein diacetate (DCF-DA) in PBS at 37 °C for 30 min. After the incubation, MEFs were washed three times with PBS. The DCF fluorescence of stained cells was measured using a fluorescence multi-detector. MEFs were lysed in 0.25 ml 0.3 M NaOH/0.1% SDS for the determination of protein concentration using BCA reagent and BSA as standard.
2.9. Immunohistochemistry (IHC) and haematoxylin/eosin (H&E) staining
Formalin-fixed, paraffin-embedded tissue samples were sectioned, and stained with H&E according to standard histopathological techniques [34]. For IHC of tumor tissue slides we used CD31 (cat#NCLKi67p, Novocastra, Newcastle, UK, 1:50 dilution). PCNA (cat#NCLKi67p, Novocastra, Newcastle, UK, 1:50 dilution), and active Caspase-3 (cat#NCLKi67p, Novocastra, Newcastle, UK, 1:50 dilution) antibodies. Antibody binding was visualized using AEC (cat#3464, Dako, Glostrup, Denmark).
2.10. Quantitative real-time PCR
Total RNA was extracted from murine tissues using TRIzol reagent according to the manufacturer's instruction (Life Technologies). Reverse transcription of RNA was performed using random primers (Life Technologies) and gene expression analyses were performed by qPCR using the CFX96 Real-Time PCR System (BioRad) and SYBR Green (Thermo Scientific) technology. Relative mRNA levels were quantified by ΔΔCt method. The following primers were used for PCR: Atgl (forward) 5′-GAGACCAAGTGGAACATC-3′ and (reverse) 5′-GTAGATGTGAGTGGCGTT-3′; Cgi-58 (forward) 5′-TGGTGTCCCACATCTACATCA-3′ and (reverse) 5′-CAGCGTCCATATTCTGTT TCC A-3′; G0s2 (forward) 5′-TAGTGAAGCTATACGTGCTGGGC-3′ and (reverse) 5′-GGCTGGCGGCTG TGAAAGGGT-3′.
2.11. Western blot analysis
Cultured cells were harvested using a cell scraper and washed three times with 1xPBS. Cell pellets were disrupted in solution A containing phosphatase inhibitors (Sigma-Aldrich) by sonication. Cell debris were removed by centrifugation at 1000 g and 4 °C for 10 min and the supernatant was collected. Protein concentration was determined using BioRad protein assay. Twenty μg protein were separated by SDS-PAGE and blotted onto PVDF membranes (Carl Roth). Proteins were detected by using the antibodies: GAPDH (Cat# 2118S), ATGL (Cat# 2138S), HSL (Cat#4107), mTOR (Cat# 2972), P-mTOR (Cat# 2974S), P-S6K (Cat# 9205), Bcl-XL (Cat# 2762), Bcl-2 (Cat# 2876S), AMPK and ACC Antibody Sampler Kit (Cat# 9957) from Cell Signaling (Massachusetts, USA), MGL (100035) from Cayman Chemical (Michigan, USA) and respective horseradish peroxidase conjugated secondary antibodies (A120-201P, Bethyl laboratories Inc., Texas, USA). Signal densities were analyzed using BioRad ChemiDoc MP system.
2.12. Flow cytometry to detect apoptosis
Apoptotic cells were detected by Caspase-3 staining and flow cytometry. MEFs were harvested, washed twice with PBS, and re-suspended in FACS buffer (1xPBS, 0.5% BSA, 0.1% sodium azide). Cells were blocked 30 min at 4 °C with blocking buffer (1xPBS containing 5% goat serum) and fixed in 100 μl fix buffer (4% formaldehyde) for 15 min at room temperature. After washing the cells with FACS buffer two times, cells were incubated with Caspase-3 primary antibody (Cat #9664, Cell signaling) diluted in IFA-Tx buffer (4% FCS, 150 nM NaCl, 10 nM HEPES, 0.1% sodium azide, 0.1% Triton X-100) for 60 min at 4 °C. After washing the cells with FACS buffer, cells were re-suspended and incubated with secondary antibody (DyLight 488, Cat# 35553, Thermo Fisher) diluted in IFA-Tx buffer for 30 min at 4 °C. Thereafter, cells were washed with 1xPBS and re-suspended in 200 μl FACS buffer for subsequent analysis using the BD LSRFortessa FACS system.
2.13. Statistics
The data are shown as means + standard deviation (SD). Differences between two groups were analyzed using an unpaired Student's t-test and p value ≤ 0.05 and ≤0.01 were considered significant. All analyses were performed using Prism 5 software.
3. Results
3.1. ATGL activity inversely correlates with MEF proliferation
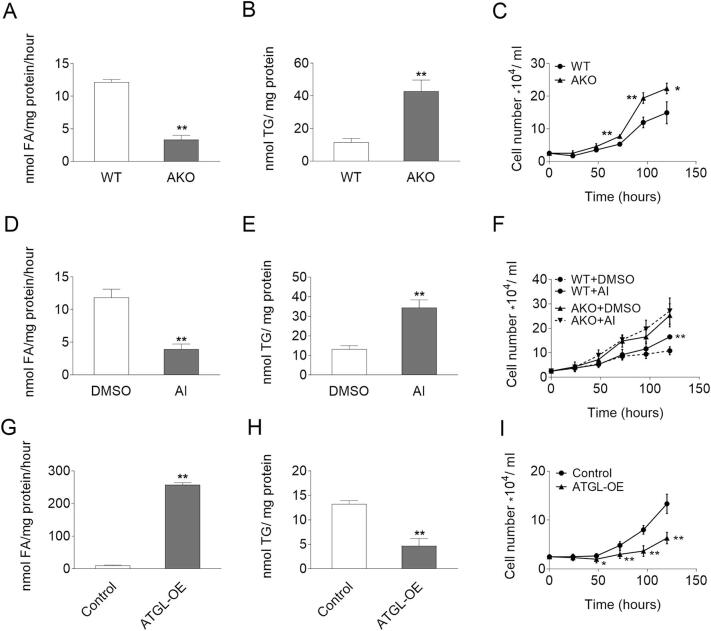
To investigate the potential impact of ATGL on the proliferation of non-cancerous cells, MEFs and fibroblasts of the stroma vascular fractions (SVFs) were isolated from wild-type (WT) and ATGL knockout (AKO) embryos or WAT of adult mice. Compared to WT, AKO cells exhibited reduced TG hydrolase activity (−72.6% and −48.6%), increased intracellular TG content (3.7-fold and 2.0-fold), and higher proliferation rates (Fig. 1A–C, Fig. S1A–C). To investigate whether ATGL interferes with cell proliferation via its enzymatic activity, we treated WT and AKO MEFs with Atglistatin©, a competitive ATGL inhibitor specific for murine ATGL [35,36]. Similar to genetic ATGL ablation, pharmacological inhibition reduced TG hydrolase activity by 66.8%, leading to an accumulation of TG (2.6-fold) (Fig. 1D–E). Atglistatin© treatment increased the proliferation of WT but not AKO MEFs (Fig. 1F). As genetic and pharmaceutical inhibition of ATGL consistently promoted cell proliferation of MEFs, we wondered whether overexpressing ATGL would reduce proliferation. MEFs were isolated from WT embryos and transduced with adenovirus coding for murine ATGL or control adenovirus (control MEFs). Differences in ATGL protein expression were determined by western blot analysis (Fig. S1D). Consistent with its function as a TG lipase, ATGL overexpression (ATGL-OE) increased TG hydrolase activity (24.3-fold) and reduced intracellular TG content (−64.8%) compared to control MEFs (Fig. 1G–H). ATGL overexpressing MEFs proliferated significantly slower compared to control MEFs (Fig. 1I). Taken together, these results indicate that ATGL activity negatively correlates with the proliferation rate of MEFs.
Fig. 1.
TG catabolism and proliferation of MEFs. (A, D, G) TG hydrolase activity of (A) WT/AKO MEFs, (D) WT MEFs, or (G) Control/ATGL-OE MEFs cell lysates in the absence or presence of 40 μM Atglistatin© (AI) or DMSO as vehicle control was determined using a radiolabeled TG substrate. (B, E, H) TG content of (B) WT/AKO MEF, (E) WT MEF or (H) Control/ATGL-OE MEFs in the absence or presence of 40 μM Atglistatin© or DMSO as vehicle control was determined after Folch extraction using a commercially available kit. (C, F, I) Proliferation of (C) WT/AKO MEFs, (F) WT/AKO MEFs in the absence or presence of 40 μM Atglistatin© or DMSO as vehicle control, or (I) Control/ATGL-OE MEFs was analyzed using a hemocytometer at the indicated timepoints. Data represent mean values of n = 3–6 ± sd. Significance was determined by student's t-test (*p ≤ 0.05, **p ≤ 0.01).
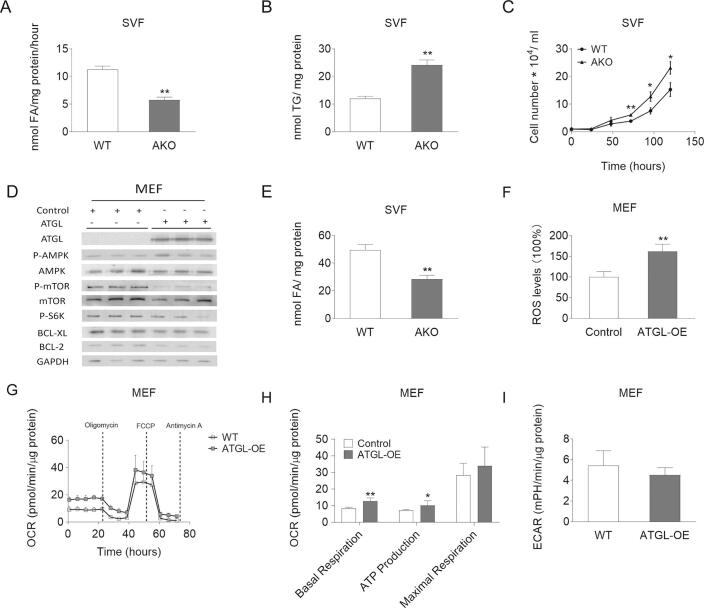
Fig. S1.
TG catabolism and proliferation of WT/AKO SVFs as well as AMPK phosphorylation and respiration of control/ATGL-OE MEFs. (A) TG hydrolase activity of WT/AKO SVFs was determined using a radiolabeled TG substrate. (B) TG content of WT/AKO SVFs was determined after Folch extraction using a commercially available kit. (C) Proliferation of WT/AKO SVFs was analyzed using a hemocytometer at the indicated timepoints. (D) Protein expression levels of ATGL, P-AMPK (Thr172), AMPK, P-mTOR (Ser2481), mTOR, P-S6K (Thr389), BCL-XL, and BCL-2 in control/ATGL-OE MEFs cell lysates were analyzed by western blot analysis. GAPDH was used as loading control. (E) FA content of WT/AKO SVFs was determined after Folch extraction using a commercially available kit. (F) For ROS measurements, control/ATGL-OE MEFs were washed with PBS and stained with 50 μM Dichloro-dihydro-fluorescein diacetate in PBS at 37 °C for 30 min. The fluorescence of stained cells was measured using a fluorescence multi-detector. (G-H) OCR and ATP production of control/ATGL-OE MEFs were determined by the seahorse XFe96 analyzer in combination with the Seahorse XF Cell Mito Stress Test kit. (I) Basal glycolytic rate was determined by the seahorse XFe96 analyzer. Data are presented as mean values of n = 3–6 ± standard deviation. Significance was determined by student's t-test (*p ≤ 0.05, **p ≤ 0.01).
3.2. Absence of ATGL leads to a metabolic adaptation resulting in reduced AMPK phosphorylation and decreased apoptosis in MEFs
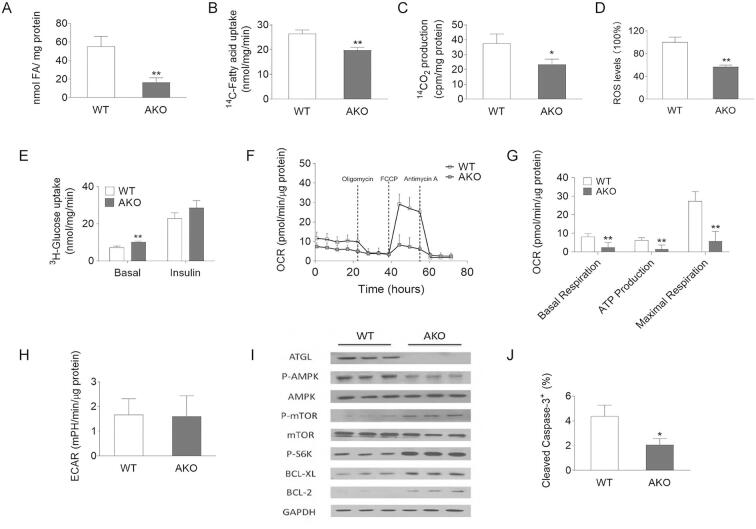
To assess whether the increased proliferation upon ATGL deletion is a consequence of metabolic adaptation, we first determined substrate utilization of WT and AKO MEFs. AKO MEFs exhibited decreased intracellular FA content (−69.8%) and reduced FA uptake rates (−25.3%), leading to reduced FA oxidation (FAO) (−37.9%) and reactive oxygen species (ROS) production (−43.5%) (Fig. 2A–D). In contrast, insulin independent glucose uptake was increased 1.4-fold (Fig. 2E). To examine whether reduced FAO affects cellular energy balance in AKO MEFs, we measured cellular respiration by seahorse analysis. Compared to WT MEFs, AKO MEFs exhibited reduced basal oxygen consumption (−70.2%) and ATP production (−75.5%), despite unchanged glycolytic rate, indicating limited mitochondrial capacity (Fig. 2F–H). Previous studies suggested that ATGL deficiency impairs phosphorylation of AMPK in MEFs [37]. A defect in AMPK signaling has been linked to enhanced cell proliferation of diverse cell types [21,37]. ATGL deficient MEFs exhibited reduced AMPK phosphorylation compared to WT MEFs (Fig. 2I). The master regulator of cell proliferation and cell growth, mTOR, is negatively regulated by P-AMPK [38,39]. Accordingly, phosphorylation of mTOR (Ser2481, P-mTOR) and its downstream target S6K (Thr389, P-S6K) were increased in AKO MEFs. The mTOR pathway suppresses apoptosis of cancer cells in response to energy stress [[40], [53]]. In accordance with reduced P-AMPK and activated mTOR, the anti-apoptotic proteins BCL-XL and BCL-2 were increased (Fig. 2I) and cleaved Caspase-3 was reduced in AKO MEFs by 52.7% compared to WT MEFs (Fig. 2J). On the contrary, in ATGL overexpressing MEFs, we observed increased ROS production (1.6-fold), increased oxygen consumption (1.5-fold) and ATP production (1.4-fold), in parallel with increased P-AMPK, decreased P-mTOR and P-S6K, and reduced expression of the anti-apoptotic BCL-XL and BCL-2 (Fig. S1D–I). Collectively, these data indicate that ATGL abundance determines intracellular FA concentrations, substrate utilization, and regulates apoptosis in primary cells, like MEFs.
Fig. 2.
Substrate utilization, respiration, AMPK phosphorylation, and apoptosis of WT/AKO MEFs. (A) FA content of WT/AKO MEFs was determined after Folch extraction using a commercially available kit. (B) For FA uptake, WT/AKO MEFs were incubated in the presence of 14C radiolabeled oleic acid. After 1 min radioactivity in the cell lysate was determined. (C) FAO of WT/AKO MEFs was determined using 14C labeled oleic acid and trapping of the generated radiolabeled CO2. (D) For ROS measurement, WT/AKO MEFs were washed with PBS and stained with 50 μM Dichloro-dihydro-fluorescein diacetate in PBS at 37 °C for 30 min. The fluorescence of stained cells was measured using a fluorescence multi-detector. (E) For glucose uptake, WT/AKO MEFs were incubated in the presence of 3H radiolabeled 2-deoxyglucose. After 15 min radioactivity in the cell lysate was determined. (F, G) OCR and ATP production were determined by the seahorse XFe96 analyzer in combination with the Seahorse XF Cell Mito Stress Test kit. (H) Basal glycolytic rate was determined by the seahorse XFe96 analyzer. (I) Protein expression levels of ATGL, P-AMPK (Thr172), AMPK, P-mTOR (Ser2481), mTOR, P-S6K (Thr389), BCL-XL, and BCL-2 in MEFs cell lysates were analyzed by western blot analysis. GAPDH was used as loading control. (J) Analysis of cleaved Caspase 3 by flow cytometry of WT/AKO MEFs. Data are presented as mean values of n = 3–5 ± standard deviation. Significance was determined by student's t-test (*p ≤ 0.05, **p ≤ 0.01).
3.3. ATGL silencing does not affect TG catabolism and proliferation of diverse cancer cells
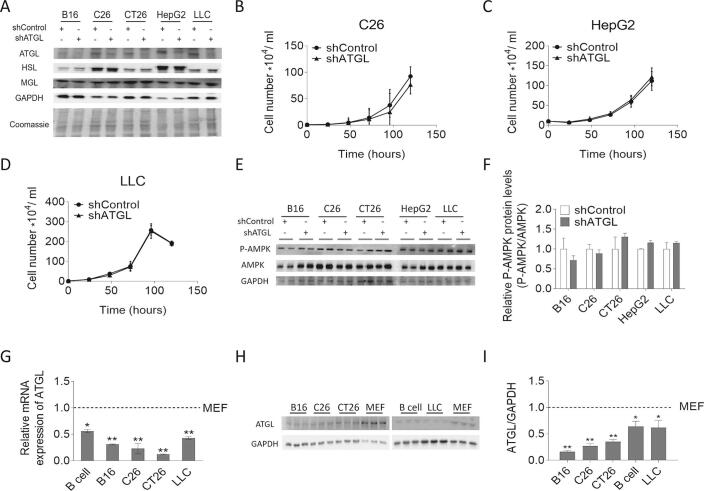
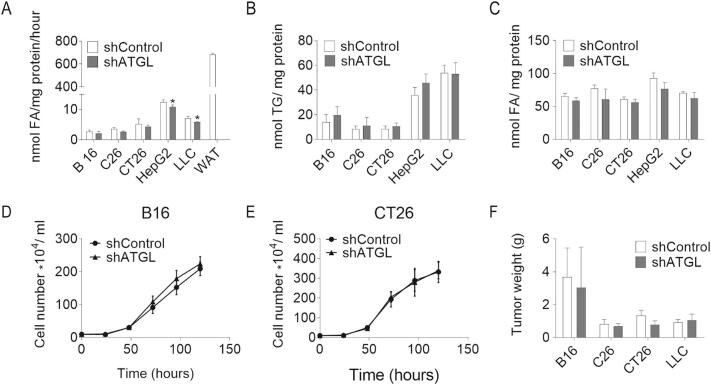
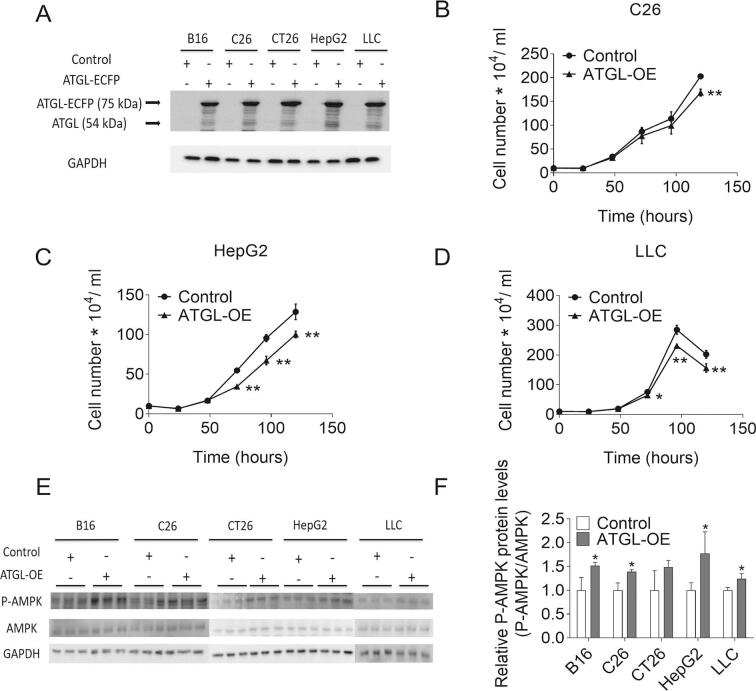
Prompted by the observation that reducing ATGL activity accelerates the proliferation of MEFs, we silenced ATGL expression in B16 (mouse melanoma), CT26 (mouse colon carcinoma), C26 (mouse colon carcinoma), LLC (mouse lung carcinoma), and HepG2 (human hepatic carcinoma) cancer cell lines by lentiviral small hairpin RNA (shATGL and shControl) (Fig. S2A). Protein expression levels of other lipases, like HSL and MGL, were not affected upon ATGL knock-down in these cancer cells. (Fig. S2A). ATGL silencing elicited negligible changes in TG hydrolase activity, intracellular TG content, and FA concentrations compared to control cells (Fig. 3A–C). ATGL silencing in B16, CT26, C26, HepG2, or LLC cells did not alter cell proliferation (Fig. 3D–E, Fig. S2B–D), or the P-AMPK/AMPK ratio as observed upon ATGL silencing in MEFs (Fig. S2E–F). The basal mRNA and protein levels of ATGL were low in these cancer cells (Fig. S2G–I). To examine whether ATGL silencing affects tumor growth in vivo, we subcutaneously injected different shATGL and shControl cancer cells into mice. In line with unaltered cell proliferation in vitro, we did not observe any significant difference in tumor weight between shControl and shATGL transduced B16, C26, CT26, and LLC allografts 14 days after cancer cell injection (Fig. 3F).
Fig. S2.
Proliferation and AMPK phosphorylation of shControl/shATGL cancer cells. (A) Protein expression levels of ATGL, HSL, and MGL in shControl/shATGL cancer cell lines were analyzed by western blot analysis. GAPDH was used as housekeeping protein. Equal protein loading was also verified by Coomassie blue staining of the membrane. (B-D) Proliferation of shControl/shATGL (B) C26, (C) HepG2, and (D) LLC cells was analyzed using a hemocytometer at the indicated timepoints. (E) P-AMPK (Thr172) and AMPK protein abundance in shControl/shATGL cancer cells was analyzed by western blot analysis. GAPDH was used as loading control. (F) Relative phosphorylation level of AMPK in shControl/shATGL cancer cells was determined by densitometric quantification of the immunoblots. (G) ATGL mRNA expression in murine cancer cell lines and MEFs was analyzed by qPCR. (H) ATGL protein abundance in murine cancer cell lines and MEFs was analyzed by western blot analysis. GAPDH was used as loading control. (I) Relative protein expression level of ATGL in mouse cancer cells and MEFs was determined by densitometric quantification of the immunoblots. Data are presented as mean values of n = 3–6 ± standard deviation. Significance was determined by student's t-test (*p ≤ 0.05, **p ≤ 0.01).
Fig. 3.
TG catabolism and proliferation of shControl/shATGL cancer cells. (A) TG hydrolase activity of shControl/shATGL cancer cells and white adipose tissue (WAT) was determined using cell/tissue lysates and a radiolabeled TG substrate. (B) TG content of shControl/shATGL cancer cells was determined after Folch extraction using a commercially available kit. (C) FA content of shControl/shATGL cancer cells was determined after Folch extraction using a commercially available kit. (D, E) Proliferation of shControl/shATGL (D) B16 and (E) CT26 cells was analyzed using a hemocytometer at the indicated timepoints. (F) Weights of shControl/shATGL allografts 14 days after cancer cell injection. Data are presented as mean values of n = 3–6 ± standard deviation. Significance was determined by student's t-test (*p ≤ 0.05, **p ≤ 0.01).
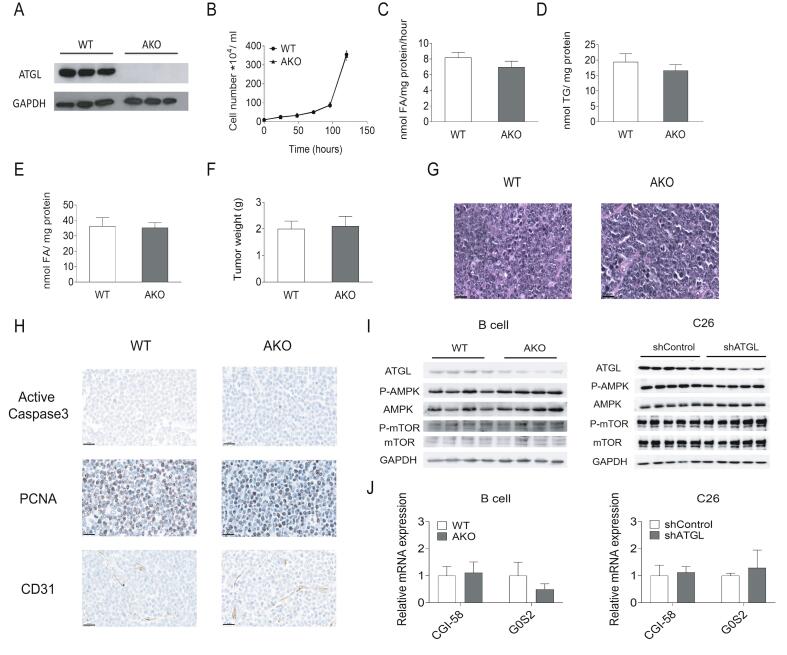
To exclude that residual ATGL activity in shATGL cells masks ATGL dependent effects, we established tumorigenic precursor B lymphoma cell lines from WT and AKO mice using murine stem cell virus derived vectors that carried the human bcr-abl p185 oncogene (Fig. S3A). Similar to shRNA mediated ATGL knockdown, the complete absence of ATGL did not affect cell proliferation, intracellular TG hydrolase activity, TG content, FA concentration, or tumor growth compared to WT B lymphoma cells (Fig. S3B–G). We also investigated tumors derived from C57Bl6J mice inoculated with WT or AKO B lymphoma cells, for vascularization (cluster of differentiation 31, CD31), proliferation (proliferating cell nuclear antigen, PCNA), and apoptosis (active Caspase-3) by IHC as well as AMPK phosphorylation by western blot. No consistent differences in vascularization, proliferation, or apoptosis between AKO and WT B lymphoma tumor tissues were detected (Fig. S3H–I). Additionally, we didn't observe any significant difference in the mRNA expression levels of CGI-58 and G0S2 in tumor tissues of mice bearing WT/AKO B lymphoma cells (Fig. S3J).
Fig. S3.
TG catabolism and proliferation of WT/AKO B lymphoma cells. (A) Protein expression level of ATGL in WT/AKO B lymphoma cells was analyzed by western blot analysis. (B) Proliferation of WT/AKO B lymphoma cells was analyzed using a hemocytometer at the indicated timepoints. (C) TG hydrolase activity of WT/AKO B lymphoma cells was determined using cell lysates and a radiolabeled TG substrate. (D) TG content of WT/AKO B lymphoma cells was determined after Folch extraction using a commercially available kit. (E) FA content of WT/AKO B lymphoma cells was determined after Folch extraction using a commercially available kit. (F) Weights of WT/AKO allografts 14 days after B lymphoma cell injection. (G) Representative images show H&E staining of WT/AKO allografts. Scale bar, 20 μm. (H) Representative images show IHC using antibodies against active Caspase-3, PCNA, and CD31 in WT/AKO allografts. Scale bar, 20 μm. (I) Protein expression levels of ATGL, P-AMPK (Thr172), AMPK, P-mTOR (Ser2481), and mTOR in WT/AKO B lymphoma tumor tissue lysates and shControl/shATGL C26 tumor tissue lysates were analyzed by western blot analysis. GAPDH was used as loading control. (J) CGI-58 and G0S2 mRNA expression levels in tumor tissues were analyzed by qPCR. Significance was determined by student's t-test (*p ≤ 0.05 versus MEF, **p ≤ 0.01 versus MEF). Data are presented as mean values of n = 3–6 ± standard deviation.
3.4. Atglistatin© suppresses proliferation of diverse cancer cells independent of ATGL activity
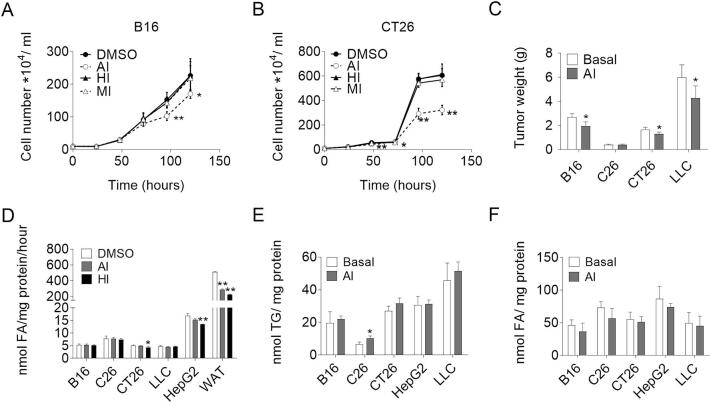
So far, we failed to detect any hint that the absence of ATGL interferes with cancer cell proliferation. As previous publications demonstrated that inhibition of ATGL activity by Atglistatin© affects tumor cell proliferation [20], we undertook a further set of experiments. To investigate the effect of lipase inhibition on cancer cell proliferation in more detail, we specifically inhibited ATGL (Atglistatin©, AI), HSL (Hi-76-0079, HI), and MGL (JZL184, MI) activities in five different cancer cell lines. Atglistatin© treatment significantly reduced proliferation of all cancer cells we examined (Fig. 4A–B, Fig. S4A–C), whereas inhibitors for HSL and MGL remained without any effect (Fig. 4A–B, Fig. S4A–C). To assess whether Atglistatin© also impairs tumor growth in vivo, we fed mice an Atglistatin© containing diet or a control diet before and after cancer cell injection. Atglistatin© treated mice had significantly reduced weights of B16 (−27.5%), LLC (−28%), and CT26 (−22%) tumors as compared to non-treated control mice (Fig. 4C). To understand the contradicting results of ATGL silencing and pharmacological ATGL inhibition on cancer cell proliferation, we first asked whether the anti-proliferative effect of Atglistatin© resulted from reduced TG hydrolase activity. Atglistatin© efficiently reduced murine WAT lipolysis (−52.1%) but failed to reduce TG hydrolase activities in lysates of all tested cancer cell lines (Fig. 4D). As a consequence, intracellular TG and FA concentrations were unaltered in Atglistatin©-treated compared to vehicle-treated cells except for a minor increase in the cellular TG content of C26 cells (Fig. 4E–F). In contrast, pharmaceutical inhibition of HSL significantly decreased TG hydrolase activity in lysates of WAT (−55.5%), CT26 cells (−15.1%), and HepG2 cells (−20.1%) (Fig. 4D).
Fig. 4.
The effect of Atglistatin on TG catabolism and proliferation of cancer cells. (A, B) Proliferation of (A) B16 and (B) CT26 cells in the presence and absence of 40 μM Atglistatin© (AI), 25 μM HSL inhibitor (HI), 20 μM MGL inhibitor (MI), or DMSO as control was determined with a hemocytometer at the indicated timepoints. (C) Tumor weights of mice injected with B16, C26, CT26, or LLC cancer cells and fed an HFD containing 200 μmol/kg Atglistatin© or a control HFD. (D) TG hydrolase activities of cancer cells treated with 40 μM Atglistatin©, 25 μM HI, or DMSO as vehicle control were determined using cell lysates and a radiolabeled TG substrate. (E) TG content of cancer cells treated with 40 μM Atglistatin© or DMSO as vehicle control was determined after Folch extraction using a commercially available kit. (F) FA content of cancer cells treated with 40 μM Atglistatin© or DMSO as vehicle control was determined after Folch extraction using a commercially available kit. Data are presented as mean values of n = 3–6 ± standard deviation. Significance was determined by student's t-test (*p ≤ 0.05, **p ≤ 0.01).
Fig. S4.
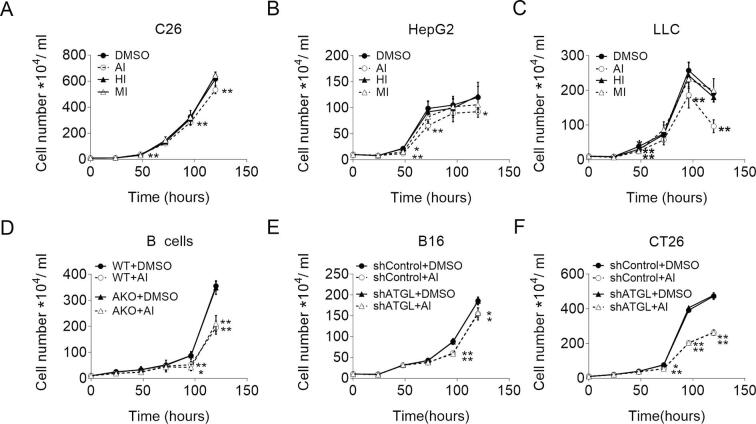
The effect of Atglistatin© on proliferation of cancer cells. (A-C) Proliferation of (A) C26, (B) HepG2, and (C) LLC cells in the presence and absence of 40 μM Atglistatin© (AI), 25 μM HSL inhibitor (HI), 20 μM MGL inhibitor (MI), or DMSO as control. (D-F) Proliferation of (D) WT/AKO B lymphoma cells, (E) shControl/shATGL B16, and (F) shControl/shATGL CT26 cells in the presence and absence of 40 μM Atglistatin©. Proliferation was determined by seeding equal amounts of cells and counting the cells using a hemocytometer at the indicated timepoints. Data are presented as mean values of n = 6 ± standard deviation. Significance was determined by student's t-test (*p ≤ 0.05, **p ≤ 0.01).
The absence of any effect of the ATGL inhibitor on TG catabolism, prompted us to ask whether the anti-proliferative effect of Atglistatin© was ATGL independent. Treatment of B lymphoma cells with Atglistatin© suppressed proliferation of WT and AKO B lymphoma cells (Fig. S4D). Similarly, the proliferation of shControl and shATGL transduced B16 and CT26 cancer cells was impaired upon Atglistatin© treatment to the same extent (Fig. S4E–F). Collectively, these results indicate that Atglistatin© reduces cell proliferation via a yet unknown mechanism independent of ATGL-mediated TG hydrolase activity.
3.5. Increasing ATGL mediated TG hydrolase activity represses proliferation of cancer cells
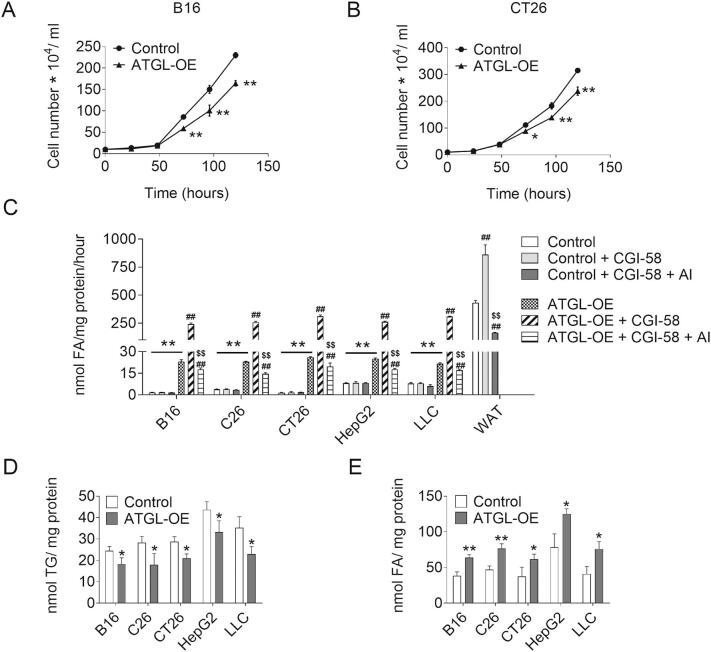
So far, our results suggest that cancer cells exhibit low ATGL activity to sustain high proliferation rates. This implies that increasing ATGL activity reduces proliferation in these cancer cells. To test this concept, we stably expressed ATGL in cancer cells using lentivirus (Fig. S5A). ATGL-OE consistently reduced proliferation of B16, C26, CT26, HepG2, and LLC cancer cells (Fig. 5A–B, Fig. S5B–D). ATGL-OE increased TG hydrolase activities in B16 (14.0-fold), C26 (6.0-fold), CT26 (18.0-fold), HepG2 (3.1-fold), and LLC cells (2.7-fold) (Fig. 5C). Addition of 50 ng/ml purified CGI-58 further increased TG hydrolase activities in ATGL-overexpressing B16 (10.5-fold), C26 (11.3-fold), CT26 (12.0-fold), HepG2 (10.4-fold), and LLC cells (14.3-fold), but not in cancer cells transduced with control vectors (Fig. 5C). Atglistatin© did not affect TG hydrolase activity of control cancer cells, but reduced CGI-58-stimulated TG hydrolase activity of ATGL-OE B16 (−92.7%), C26 (−94.3%), CT26 (−93.7%), HepG2 (−93.2%), and LLC cells (−94.4%). Increased TG hydrolase activities led to reduced intracellular TG content and increased FA concentrations in ATGL-OE B16 (−25.2% TG, 1.7-fold FA), C26 (−36.7% TG, 1.6-fold FA), CT26 (−26.8% TG, 1.6-fold FA), HepG2 (−23.8% TG, 1.6-fold FA), and LLC (−34.9%, 1.9-fold FA) cells (Fig. 5D–E). The increased TG catabolism was paralleled by increased levels of P-AMPK in ATGL-overexpressing cells compared to controls (Fig. S5E–F).
Fig. S5.
Proliferation and AMPK phosphorylation of Control/ATGL-OE cancer cells. (A) Protein expression level of ATGL in Control/ATGL-OE cancer cells was analyzed by western blot analysis. GAPDH was used as loading control. (B-D) Proliferation of Control/ATGL-OE (B) C26, (C) HepG2, and (D) LLC cells was determined by seeding equal amounts of cells and counting the cells using a hemocytometer at the indicated timepoints. (E) Protein expression levels of P-AMPK (Thr172) and AMPK in Control/ATGL-OE cancer cells were analyzed by western blot analysis. GAPDH was used as loading control. (F) Relative phosphorylation level of AMPK in shControl/shATGL cancer cells was determined by densitometric quantification of immunoblots. Data are presented as mean values of n = 3–6 ± standard deviation. Significance was determined by student's t-test (*p ≤ 0.05, **p ≤ 0.01).
Fig. 5.
TG catabolism and proliferation of Control/ATGL-OE cancer cells. (A, B) Proliferation of Control/ATGL-OE (A) B16 and (B) CT26 cells was analyzed with a hemocytometer at the indicated timepoints. (C) TG hydrolase activity of Control/ATGL-OE cancer cells and white adipose tissue (WAT) in the presence and absence of 50 ng/ml CGI-58 and 40 μM Atglistatin© (AI) was determined using cell/tissue lysates and a radiolabeled TG substrate. (D) TG content of Control/ATGL-OE cancer cells was determined after Folch extraction using a commercially available kit. (E) FA content of Control/ATGL-OE cancer cells was determined after Folch extraction using a commercially available kit. Data are presented as mean values of n = 3–6 ± standard deviation. Significance was determined by student's t-test (*p ≤ 0.05, **p ≤ 0.01 versus control group, #p ≤ 0.05, ##p ≤ 0.01 versus ATGL-OE group; $p ≤ 0.05, $$p ≤ 0.01 versus ATGL-OE + CGI-58 group).
In summary, these results indicate that, at least in the five different cancer cell lines we used, TG hydrolase activity is not activated by CGI-58 or inhibited by Atglistatin©. Introducing ATGL into these cancer cells increases TG catabolism and suppresses proliferation.
4. Discussion
Numerous studies have emphasized the fundamental role of ATGL in TG degradation in adipose and non-adipose tissues, providing new insights for cell physiology and the pathogenesis of metabolic diseases [41]. Our current knowledge on the role of ATGL on cell proliferation is controversial. Therefore, we explored how modifying ATGL expression affects cell proliferation and TG catabolism in various, highly proliferating cells including MEFs, stromal vascular fibroblasts, and cancer cell lines.
Our results demonstrate that ATGL activity negatively correlates with the proliferation rate of proliferating untransformed and transformed cancer cells. In MEFs, overexpression of ATGL decreased, while ATGL deficiency increased cellular TG levels and cell proliferation rates. These results are consistent with data suggesting that ATGL-mediated TG breakdown regulates cell cycle checkpoints and proliferation of primary hepatocytes [42]. Reduced ATGL expression and subsequently altered TG hydrolysis was shown to impair mitochondrial FAO, leading to a shift in substrate utilization in hepatocytes [[43], [44], [45]]. Using AKO MEFs, our study provides strong evidence for impaired FAO, decreased ROS production, and a substrate switch from FA to glucose oxidation as a compensatory mechanism to cope with the inability to hydrolyze TGs.
In many cell types AMPK activation inhibits cell proliferation via suppressing mTOR signaling and increasing apoptosis [38,39]. Several canonical and non-canonical regulations, including ATP/AMP ratio, glucose starvation, ligands binding, DNA damage, calcium, and ROS production, activate this critical cellular energy sensor [46]. MEFs cultured with Trolox, an antioxidant that reduces cellular ROS levels, were shown to display reduced basal AMPK activation [47]. The finding that reducing ATGL activity impairs FAO and ROS production in MEFs, prompted us to speculate that reduced ATGL expression would also have an impact on AMPK and mTOR activity. Indeed, ATGL overexpression led to increased AMPK phosphorylation and decreased mTOR activation while ATGL silencing caused the opposite effect. It is attractive to speculate that these ATGL-mediated changes regulate apoptosis and cell proliferation and provide a mechanistic explanation for our observations.
In line with increased proliferation rates in ATGL deficient cells, mice globally lacking the enzyme spontaneously develop pulmonary neoplasia and adenocarcinoma [22]. Deficiency of both ATGL and HSL in adipose tissue leads to liposarcoma, suggesting a potential tumor-suppressive function of ATGL [24]. This points towards a potential role of ATGL as regulator of cell growth in cancer cells. When we linked proliferation rates and parameters of lipid metabolism in a panel of cancer cell lines to ATGL activity, we found that the enforced expression of ATGL increased TG hydrolase activity and limited cell proliferation. In contrast, ATGL silencing failed to alter TG catabolism or cell proliferation of different cancer cell lines. Similarly, the complete absence of ATGL in BCR-ABL transformed B lymphoma cells derived from AKO mice did not alter TG catabolism or cell proliferation. This is most likely due to the lack of any detectable ATGL mediated TG hydrolase activity in all tested cancer cells.
In line, ATGL mRNA expression levels are reduced in a mouse model of induced hepatocellular carcinoma [23] and significantly decreased in 14 human malignancies compared with their matched non-cancerous tissues [22]. Low ATGL expression in malignant disease may result from reduced expression of FOXO1, a transcription factor known to directly regulate ATGL transcription [[48], [49], [50], [51]]. Alternatively, increased Snail1 expression, a frequently upregulated EMT marker and oncogene may contribute to the ATGL down regulation [52]. The inability of Atglistatin to reduce-, and of CGI-58 to increase TG hydrolase activity suggest that the small amount of enzyme present is inhibited, either by HIG2 [9], G0S2 [10], or a yet unknown inhibitor of ATGL in cancer cells.
Reducing or deleting ATGL expression had little effect on cell proliferation or TG catabolism of cancer cells. A previous study provided evidence that pharmacological inhibition of ATGL by Atglistatin© inhibits proliferation of some cancer cell lines in a concentration dependent manner [20]. We confirmed that Atglistatin© treatment reduced the proliferation of cancer cells in vitro and the growth of tumors in vivo. Notably, the ATGL inhibitor did not affect TG hydrolase activity but suppressed the proliferation of AKO B lymphoma cells as well as of shATGL B16 and CT26 cells to the same extent as the respective WT or shControl cells. These data are consistent with a report that showed that Atglistatin© also suppresses the proliferation of human hepatocellular cancer cell lines while not interfering with human ATGL activity [[20], [36]]. These findings link the anti-proliferative effect of Atglistatin© on murine and human cancer cells to a yet unknown target and define them as off-target effects of the inhibitor independent of ATGL.
Collectively, our study indicates that ATGL activity suppresses cell proliferation. Accordingly, cancerogenesis is accompanied by a substantial reduction in ATGL expression and activity to escape the tumor-suppressor function of the enzyme. Future work is needed to (i) delineate the signaling pathways accounting for ATGL downregulation in malignancy, (ii) identify the molecular targets linking ATGL downregulation to spontaneous neoplasms formation, and (iii) test whether re-expression of ATGL represents a pharmacological strategy to control cancer cell growth.
The following are the supplementary data related to this article.
CRediT authorship contribution statement
Hao Xie:Methodology, Investigation, Writing - original draft.Christoph Heier:Investigation, Methodology.Benedikt Kien:Methodology.Paul W. Vesely:Investigation, Methodology.Zhiyuan Tang:Investigation, Writing - review & editing.Veronika Sexl:Methodology, Writing - review & editing.Gabriele Schoiswohl:Investigation, Writing - review & editing.Isabelle Strießnig-Bina:Methodology.Gerald Hoefler:Funding acquisition, Investigation.Rudolf Zechner:Conceptualization, Funding acquisition, Project administration, Writing - review & editing.Martina Schweiger:Conceptualization, Project administration, Supervision, Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Astrid Steiner and Birgit Juritsch for animal care and genotyping. We also thank Silvia Schauer for histological analysis and Sabrina Huetter for western blot and qRT-PCR analyses. This work was supported by research grants from European Research Council LipoCheX [grant number 340896]; Leducq Foundation [grant number 12CVD04], Fondation Louis-Jeantet, SFB Lipid Hydrolysis [grant number F7302], DK Molecular Enzymology [grant number W901], Austrian Science Fund (FWF) stand-alone grant LipoLUNG [grant number P30968]; Austrian Science Fund (FWF) DK-MCD [grant number W1226].
Contributor Information
Rudolf Zechner, Email: rudolf.zechner@uni-graz.at.
Martina Schweiger, Email: tina.schweiger@uni-graz.at.
References
- 1.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menendez J. a, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 3.Wise D.R., Thompson C.B. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaidi N., Lupien L., Kuemmerle N.B., Kinlaw W.B., Swinnen J.V., Smans K. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog. Lipid Res. 2013;52:585–589. doi: 10.1016/j.plipres.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beloribi-Djefafli S., Vasseur S., Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5 doi: 10.1080/15384101.2016.1204850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zechner R., Zimmermann R., Eichmann T.O., Kohlwein S.D., Haemmerle G., Lass A., Madeo F. Fat signals–lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmermann R., Strauss J.G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 8.Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J.G., Gorkiewicz G., Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X., Saarinen A.M., Hitosugi T., Wang Z., Wang L., Ho T.H., Liu J. Inhibition of intracellular lipolysis promotes human cancer cell adaptation to hypoxia. Elife. 2017;6:1–24. doi: 10.7554/eLife.31132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X., Lu X., Lombès M., Rha G.B., Chi Y.I., Guerin T.M., Smart E.J., Liu J. The G0/G1 switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poursharifi P., Madiraju S.R.M., Prentki M. Monoacylglycerol signalling and ABHD6 in health and disease. Diabetes Obes. Metab. 2017;19:76–89. doi: 10.1111/dom.13008. [DOI] [PubMed] [Google Scholar]
- 12.Nomura D.K., Lombardi D.P., Chang J.W., Niessen S., Ward A.M., Long J.Z., Hoover H.H., Cravatt B.F. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem. Biol. 2011;18:846–856. doi: 10.1016/j.chembiol.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nomura D.K., Long J.Z., Niessen S., Hoover H.S., Ng S.-W., Cravatt B.F. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye L., Zhang B., Seviour E.G., Tao K., Liu X., Ling Y., Chen J., Wang G. Monoacylglycerol lipase (MAGL) knockdown inhibits tumor cells growth in colorectal cancer. Cancer Lett. 2011;307:6–17. doi: 10.1016/j.canlet.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J., Liu Z., Lian Z., Liao R., Chen Y., Qin Y., Wang J., Jiang Q., Wang X., Gong J. Monoacylglycerol lipase: a novel potential therapeutic target and prognostic indicator for hepatocellular carcinoma. Sci. Rep. 2016;6 doi: 10.1038/srep35784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grüner B.M., Schulze C.J., Yang D., Ogasawara D., Dix M.M., Rogers Z.N., Chuang C.-H., McFarland C.D., Chiou S.-H., Brown J.M., Cravatt B.F., Bogyo M., Winslow M.M. An in vivo multiplexed small-molecule screening platform. Nat. Methods. 2016;13:883–889. doi: 10.1038/nmeth.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Z., Xie H., Heier C., Huang J., Zheng Q., Eichmann T.O., Schoiswohl G., Chang P., Zechner R., Ni S., Hao H. Enhanced monoacylglycerol lipolysis by ABHD6 promotes NSCLC pathophysiology. EbioMedicine. 2020;53 doi: 10.2139/ssrn.3353706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X., Liang Y., Song R., Yang G., Han J., Lan Y., Pan S., Zhu M., Liu Y., Wang Y., Meng F., Cui Y., Wang J., Zhang B., Song X., Lu Z., Zheng T., Liu L. Long non-coding RNA NEAT1-modulated abnormal lipolysis via ATGL drives hepatocellular carcinoma proliferation. Mol. Cancer. 2018;17 doi: 10.1186/s12943-018-0838-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G., Zhou G., Aras S., He Z., Lucas S., Podgorski I., Skar W., Granneman J.G., Wang J. Loss of ABHD5 promotes the aggressiveness of prostate cancer cells. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-13398-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zagani R., El-Assaad W., Gamache I., Teodoro J.G. Inhibition of adipose triglyceride lipase (ATGL) by the putative tumor suppressor G0S2 or a small molecule inhibitor attenuates the growth of cancer cells. Oncotarget. 2015;6 doi: 10.18632/oncotarget.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ou J., Miao H., Ma Y., Guo F., Deng J., Wei X., Zhou J., Xie G., Shi H., Xue B., Liang H., Yu L. Loss of abhd5 promotes colorectal tumor development and progression by inducing aerobic glycolysis and epithelial-mesenchymal transition. Cell Rep. 2014;9:1798–1811. doi: 10.1016/j.celrep.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Zoughbi W., Pichler M., Gorkiewicz G., Guertl-Lackner B., Haybaeck J., Jahn S.W., Lackner C., Liegl-Atzwanger B., Popper H., Schauer S., Nusshold E., Kindt A.S.D., Trajanoski Z., Speicher M.R., Haemmerle G., Zimmermann R., Zechner R., Vesely P.W., Hoefler G., Al-Zoughbi W., Pichler M., Gorkiewicz G., Guertl-Lackner B., Haybaeck J., Jahn S.W., Lackner C., Liegl-Atzwanger B., Popper H., Schauer S., Nusshold E., Kindt A.S.D., Trajanoski Z., Speicher M.R., Haemmerle G., Zimmermann R., Zechner R., Vesely P.W., Hoefler G. Loss of adipose triglyceride lipase is associated with human cancer and induces mouse pulmonary neoplasia. Oncotarget. 2016;7 doi: 10.18632/oncotarget.9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Leo L., Vegliante R., Ciccarone F., Salvatori I., Scimeca M., Bonanno E., Sagnotta A., Grazi G.L., Aquilano K., Ciriolo M.R. Forcing ATGL expression in hepatocarcinoma cells imposes glycolytic rewiring through PPAR-α/p300-mediated acetylation of p53. Oncogene. 2018;38:1860–1875. doi: 10.1038/s41388-018-0545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J.W., Preuss C., Wang S.P., Yang H., Ji B., Carter G.W., Gladdy R., Andelfinger G., Mitchell G.A. Epistatic interaction between the lipase-encoding genes Pnpla2 and lipe causes liposarcoma in mice. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng Y., Miao H., Wu S., Yang W., Zhang Y., Xie G., Xie X., Li J., Shi C., Ye L., Sun W., Wang L., Liang H., Ou J. ABHD5 interacts with BECN1 to regulate autophagy and tumorigenesis of colon cancer independent of PNPLA2. Autophagy. 2016;12:2167–2182. doi: 10.1080/15548627.2016.1217380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ou J., Peng Y., Yang W., Zhang Y., Hao J., Li F., Chen Y., Zhao Y., Xie X., Wu S., Zha L., Luo X., Xie G., Wang L., Sun W., Zhou Q., Li J., Liang H. ABHD5 blunts the sensitivity of colorectal cancer to fluorouracil via promoting autophagic uracil yield. Nat. Commun. 2019;10:1–14. doi: 10.1038/s41467-019-08902-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yim C.Y., Sekula D.J., Hever-Jardine M.P., Liu X., Warzecha J.M., Tam J., Freemantle S.J., Dmitrovsky E., Spinella M.J. G0S2 suppresses oncogenic transformation by repressing a MYC-regulated transcriptional program. Cancer Res. 2016;76:1204–1213. doi: 10.1158/0008-5472.CAN-15-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yim C.Y., Bikorimana E., Khan E., Warzecha J.M., Shin L., Rodriguez J., Dmitrovsky E., Freemantle S.J., Spinella M.J. G0S2 represses PI3K/mTOR signaling and increases sensitivity to PI3K/mTOR pathway inhibitors in breast cancer. Cell Cycle. 2017;16:2146–2155. doi: 10.1080/15384101.2017.1371884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sexl V., Piekorz R., Moriggl R., Rohrer J., Brown M.P., Bunting K.D., Rothammer K., Roussel M.F., Ihle J.N. Stat5a/b contribute to interleukin 7-induced B-cell precursor expansion, but abl- and bcr/abl-induced transformation are independent of stat5. Blood. 2000;96:2277–2283. doi: 10.1182/blood.v98.10.2948. [DOI] [PubMed] [Google Scholar]
- 30.Luo J., Deng Z.L., Luo X., Tang N., Song W.X., Chen J., Sharff K.A., Luu H.H., Haydon R.C., Kinzler K.W., Vogelstein B., He T.C. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc. 2007;2 doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- 31.Schweiger M., Eichmann T.O., Taschler U., Zimmermann R. Methods in Enzymology. Vol. 538. Academic Press; 2014. Measurement of Lipolysis; pp. 171–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirschey M.D., Shimazu T., Goetzman E., Jing E., Schwer B., Lombard D.B., Grueter C.A., Harris C., Biddinger S., Ilkayeva O.R., Stevens R.D., Li Y., Saha A.K., Ruderman N.B., Bain J.R., Newgard C.B., Farese R.V., Alt F.W., Kahn C.R., Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Technologies A. 2019. Mito Stress Test Kit User Guide Seahorse. [Google Scholar]
- 34.Cook H.C. Tinctorial methods in histology. J. Clin. Pathol. 1997;50:716–720. doi: 10.1136/jcp.50.9.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayer N., Schweiger M., Romauch M., Grabner G.F., Eichmann T.O., Fuchs E., Ivkovic J., Heier C., Mrak I., Lass A., Höfler G., Fledelius C., Zechner R., Zimmermann R., Breinbauer R. Development of small-molecule inhibitors targeting adipose triglyceride lipase. Nat. Chem. Biol. 2013;9:785–787. doi: 10.1038/nchembio.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romauch M., Breinbauer R., Doler C., Eichmann T.O., Benedikt P., Knittelfelder O., Mayer N., Grabner G.F., De Cecco W., Diwoky C., Kotzbeck P., Schreiber R., Zimmermann R., Yamada S., Hütter S., Zechner R., Schweiger M. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat. Commun. 2017;8:1–15. doi: 10.1038/ncomms14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gauthier M.S., Miyoshi H., Souza S.C., Cacicedo J.M., Saha A.K., Greenberg A.S., Ruderman N.B. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J. Biol. Chem. 2008;283:16514–16524. doi: 10.1074/jbc.M708177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardie D.G. AMP-activated protein kinase: a cellular energy sensor with a key role in metabolic disorders and in cancer. Biochem. Soc. Trans. 2011;39:1–13. doi: 10.1042/bst0390001. [DOI] [PubMed] [Google Scholar]
- 39.Mihaylova M.M., Shaw R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim I.Y., He Y.Y. Targeting the AMP-activated protein kinase for cancer prevention and therapy. Front. Oncol. 2013;3:1–12. doi: 10.3389/fonc.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zechner R., Madeo F., Kratky D. Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat. Rev. Mol. Cell Biol. 2017;18:671–684. doi: 10.1038/nrm.2017.76. [DOI] [PubMed] [Google Scholar]
- 42.Wu H., Ploeger J.M., Kamarajugadda S., Mashek D.G., Mashek M.T., Manivel J.C., Shekels L.L., Lapiro J.L., Albrecht J.H. Evidence for a novel regulatory interaction involving cyclin D1, lipid droplets, lipolysis, and cell cycle progression in hepatocytes. Hepatol. Commun. 2019;3:406–422. doi: 10.1002/hep4.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopaschuk G.D., McNeil G.F., McVeigh J.J. Glucose oxidation is stimulated in reperfused ischemic hearts with the carnitine palmitoyltransferase 1 inhibitor. Etomoxir, Mol. Cell. Biochem. 1989 doi: 10.1007/BF00223440. [DOI] [PubMed] [Google Scholar]
- 44.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., Kratky D., Wagner E.F., Klingenspor M., Hoefler G., Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:737–747. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 45.Ong K.T., Mashek M.T., Bu S.Y., Mashek D.G. Hepatic ATGL knockdown uncouples glucose intolerance from liver TAG accumulation. FASEB J. 2013;27:313–321. doi: 10.1096/fj.12-213454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.González A., Hall M.N., Lin S.C., Hardie D.G. AMPK and TOR: the yin and yang of cellular nutrient sensing and growth control. Cell Metab. 2020;31:472–492. doi: 10.1016/j.cmet.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Rabinovitch R.C., Samborska B., Faubert B., Ma E.H., Gravel S.P., Andrzejewski S., Raissi T.C., Pause A., St.-Pierre J., Jones R.G. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 2017;21:1–9. doi: 10.1016/j.celrep.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 48.Chakrabarti P., Kandror K.V. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J. Biol. Chem. 2009;284:13296–13300. doi: 10.1074/jbc.C800241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lettieri Barbato D., Aquilano K., Baldelli S., Cannata S.M., Bernardini S., Rotilio G., Ciriolo M.R. Proline oxidase-adipose triglyceride lipase pathway restrains adipose cell death and tissue inflammation. Cell Death Differ. 2014;21:113–123. doi: 10.1038/cdd.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadav R.K., Chauhan A.S., Zhuang L., Gan B. FoxO transcription factors in cancer metabolism. Semin. Cancer Biol. 2018;50:65–76. doi: 10.1016/j.semcancer.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myatt S.S., Wang J., Monteiro L.J., Christian M., Ho K.K., Fusi L., Dina R.E., Brosens J.J., Ghaem-Maghami S., Lam E.W.F. Definition of microRNAs that repress expression of the tumor suppressor gene FOXO1 in endometrial cancer. Cancer Res. 2010;70:367–377. doi: 10.1158/0008-5472.CAN-09-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun C., Jiang L., Liu Y., Shen H., Weiss S.J., Zhou Y., Rui L. Adipose snail1 regulates lipolysis and lipid partitioning by suppressing adipose triacylglycerol lipase expression. Cell Rep. 2016;17:2015–2027. doi: 10.1016/j.celrep.2016.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villanueva-Paz M., Cotán D., Garrido-Maraver J., Oropesa-Ávila M., de la Mata M., Delgado-Pavón A., de Lavera I., Alcocer-Gómez E., Álvarez-Córdoba M., Sánchez-Alcázar J. AMP-Activated Protein Kinase. Springer, Cham; 2016. AMPK regulation of cell growth, apoptosis, autophagy, and bioenergetics. [DOI] [PubMed] [Google Scholar]