Abstract
The dentate gyrus-CA3 circuit of the hippocampus is continuously modified by the integration of adult-born dentate granule cells (abDGCs). All abDGCs undergo a prolonged period of maturation, during which they exhibit heightened synaptic plasticity and refinement of electrophysiological properties and connectivity. Consistent with theoretical models and the known functions of the dentate gyrus-CA3 circuit, acute or chronic manipulations of abDGCs support a role for abDGCs in the regulation of memory interference. In this Review, we integrate insights from studies that examine the maturation of abDGCs and their integration into the circuit with network mechanisms that support memory discrimination, consolidation and clearance. We propose that adult hippocampal neurogenesis enables the generation of a library of experiences, each registered in mature abDGC physiology and connectivity. Mature abDGCs recruit inhibitory microcircuits to support pattern separation and memory indexing.
Experiences are encoded in memories that in turn guide future behavior. Newly formed episodic memories are constantly compared with, and discriminated from, previously encoded experiences, so that new memories are stored without overlap and previously encoded memories are updated to reflect new experiences. At a circuit level, encoding similar memories with shared features generate interference (such as trying to locate your car when you park it in a different spot every day). Studies in rodents, non-human primates and humans have suggested a role for the dentate gyrus (DG) in decreasing memory interference by promoting pattern separation1-10, a circuit mechanism by which similar inputs (or memories) are transformed or orthogonalized into more dissimilar outputs, thereby decreasing interference between similar memories. The DG transfers orthogonalized information of an experience to CA3, where a previously stored memory can be retrieved and updated or a novel experience can be consolidated as a new representation in hippocampal–cortical networks. The hippocampus does not store memories, but instead generates indexes in sparse ensembles or engrams that are linked to distributed cortical modules that encode the original experience in patterned neural activity10,11. Within this framework, activation of DG engram-bearing cells reinstates the original cortical activity patterns underlying the experience, thereby supporting memory recall. Thus, engram-bearing cells function as indexes, analogous to how library index cards are linked to books distributed on shelves11-15.
The DG–CA3 circuit of the hippocampus is continuously modified by the integration of abDGCs16. Many studies have demonstrated a link between acute or chronic manipulations of abDGCs and resolution of memory interference17,18. However, the underlying neural circuit mechanisms remain poorly understood. To address this knowledge gap, we present an updated synthesis of the literature by integrating findings regarding the physiological and behavioral functions of abDGCs. We propose that the unique developmental, physiological and circuit properties of abDGCs prime these cells to expand the hippocampus’ indexing capacity and promote pattern separation.
We first discuss how experience during a sensitive period of abDGC maturation modifies their physiological properties and connectivity, resulting in heterogeneous DGCs that have encoded specific features of prior experiences. We then examine evidence that abDGCs support hippocampus-dependent memory processes, specifically reduction of memory interference and promotion of consolidation and clearance. We next evaluate how abDGCs recruit synaptic competition and inhibitory microcircuits, thereby contributing to network mechanisms that modulate memory interference, such as pattern separation. In conclusion, we integrate insights from these sections to discuss how experience, inhibition and competition may dictate the contribution of abDGCs to hippocampal indexing and pattern separation.
A sensitive period for experience-dependent maturation of abDGCs
A protracted window of neuronal maturation.
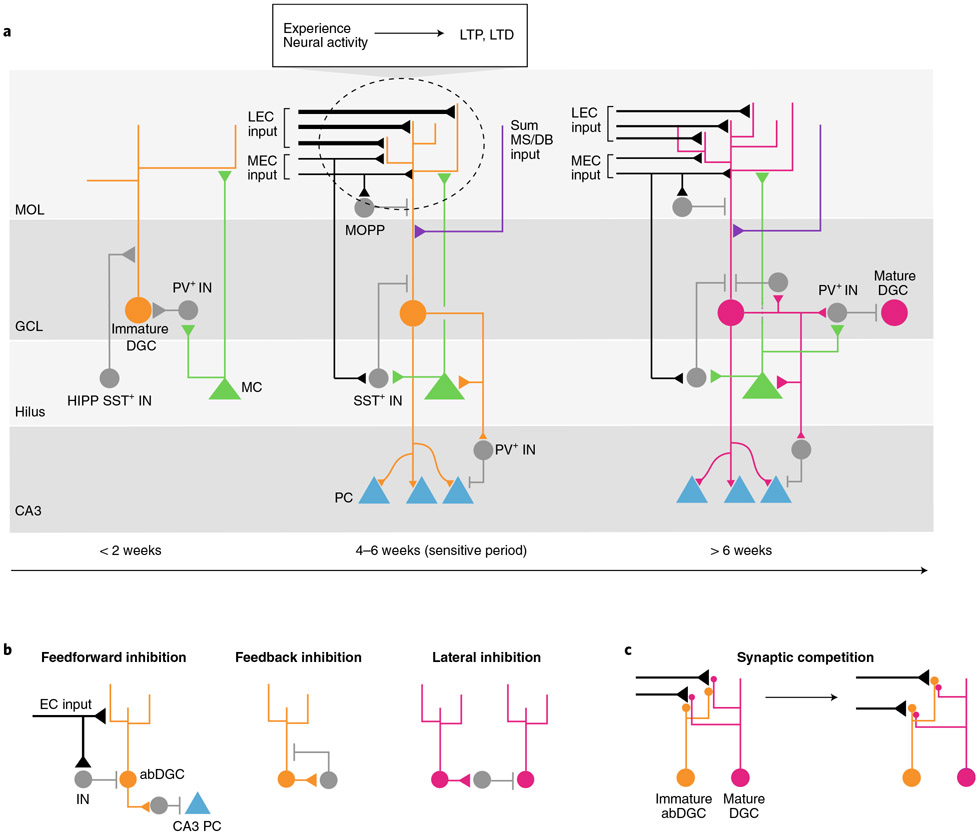
Adult-born DGCs transition through a series of maturation stages characterized by distinct physiological properties and synaptic connectivity (Fig. 1a). While this timeline is reminiscent of that seen during development, it is longer and more gradual19. Pioneering studies have demonstrated that dendritic GABAergic synaptogenesis promotes glutamatergic synapse formation onto immature DGCs (<2 weeks old) via depolarizing effects of GABA and coupling with Ca2+ signaling19-22. During this time, hilar mossy cells provide the first glutamatergic input onto newborn DGCs and, in coordination with mossy-cell-evoked disynaptic GABAergic depolarization, may synchronize activity-dependent excitatory synapse unsilencing23. Between 4 and 8 weeks, immature abDGCs exhibit inhibitory GABAergic signaling, phasic perisomatic inhibition and perforant path (PP)–DGC synapse formation19,20,24,25 (Fig. 1a,b). Maturation of glutamatergic inputs and perisomatic inhibition is accompanied by elaboration of dendritic spines and dendritic complexity over several months following birth of DGCs26-31.
Fig. 1 ∣. Development, experience and maturation of adult-born DGCs.
a, Left: during the first 2 weeks after their birth, immature abDGCs (orange circle) are low-spiking and are innervated by depolarizing GABA synapses (GABA+) from INs (gray circle), which subsequently promotes glutamatergic synapse formation from hilar mossy cells (MC). Mossy cells both directly innervate DGCs and provide disynaptic depolarization through synapses onto GABA+ interneurons. Middle: during the sensitive period 4–6 weeks after their birth, experience modifies inputs onto abDGCs. abDGCs receive inputs from medial EC (MEC) and, more strongly, from LEC. MEC and LEC synapses onto abDGCs are established via synaptic competition (see c), and their strength can be efficiently altered (LTP or long-term depression (LTD)) as a result of network activity and the animal’s experience (for example, enriched environment; see top inset). Additional excitatory input onto abDGCs comes from medial septum and diagonal band of Broca cholinergic neurons (MS/DB). Inhibitory microcircuits are also established during this period. This includes inhibitory GABAergic (GABA−) input from dendritically targeting SST+ INs in the hilus (HIPP), somatic-targeting PV+ INs in the granule cell layer (GCL; not shown and MOPP INs in the molecular layer (MOL). Interneurons receive input from MEC, LEC and mossy cells and thereby provide feedforward inhibition onto abDGCs. The abDGCs begin to establish glutamatergic (mossy fiber) synapses onto CA3 pyramidal cells (PC, blue) and progressively recruit PV+ INs to exert feedforward inhibition onto CA3. Right: after 6 weeks, additional inhibitory GABA+ synapses form, and the now-mature DGCs (pink circles) become highly input-specific. Mature DGCs provide lateral inhibition onto other DGCs via PV+ INs and may self-attenuate spiking via recruitment of feedback inhibition. b, Inhibitory microcircuit motifs. Feedforward inhibition: in EC–DG feedforward inhibition, interneurons are recruited by MEC and LEC inputs and mossy cell collaterals (not shown) to inhibit abDGCs. In DG–CA3 feedforward inhibition, abDGCs recruit PV+ INs to inhibit CA3 neurons. Feedback inhibition: DGCs undergo auto-inhibition via recruitment of interneurons by DGCs. Lateral inhibition: mDGCs recruit interneurons to inhibit neighboring DGCs. c, Synaptic competition: immature abDGCs (orange) compete with mDGCs (red) for PP inputs from LEC and MEC. Immature DGCs initially form multisynaptic boutons with pre-existing PP–DGC synapses (left) before forming monosynaptic connections with those PP terminals (right).
Integration of abDGCs into the hippocampal network is thought to subscribe to a synaptic-competition mechanism in which abDGCs compete with preexisting mature DGCs (mDGCs) for PP inputs (Fig. 1c). Deletion of the NMDA receptor in 2- to 3-week-old abDGCs impairs their survival, indicating that competition and abDGC integration is activity-dependent32. Immature abDGCs form multisynaptic boutons with pre-existing PP–mDGC synapses before they form monosynaptic connections with those PP terminals28. Indeed, selectively eliminating spines in mDGCs or enhancing spines in immature abDGCs resulted in increased neuronal integration of the immature abDGCs33-35. Maturing abDGCs establish mossy fiber synaptic connections with hilar inhibitory interneurons (INs), mossy cells and sequentially with pyramidal neurons in CA3c, Ca3b and CA3a over a period of at least 8 weeks27,29,36. However, as genetic silencing of mossy fiber synaptic release fails to induce axonal retraction, it seems that synaptic competition does not occur at DGC efferent excitatory synapses37.
Immature abDGCs exhibit increased intrinsic excitability and synaptic plasticity.
Electrophysiogical studies in rodents suggest that immature ~4- to 6-week-old abDGCs have greater excitability than mDGCs, owing to their higher input resistance19,38-40 and to their reduced GABAergic synaptic inhibition relative to excitation24,39,41. Additionally, conditions that induce activity-dependent synaptic plasticity in immature DGCs (PP–DGC and mossy fiber–CA3 synapses42) fail to do so in mDGCs. Specifically, in vivo and ex vivo studies have shown that PP–DGC synapses onto immature, 4- to 6-week-old abDGCs have a lower induction threshold and higher amplitude of associative long-term potentiation (LTP) than PP–mDGC synapses38,43. This form of LTP was shown to be dependent on NR2B-containing NMDA receptors in 4- to 6-week-old abDGCs43 and insensitive to GABAergic inhibition, consistent with field recordings41,44,45.
Physiological recruitment of immature abDGCs.
The window of heightened synaptic plasticity and intrinsic excitability during abDGC maturation has motivated enquiries into what physiological conditions might recruit these immature abDGCs. Ex vivo studies revealed that weak stimulation of medial PP preferentially activated immature abDGCs (~4 weeks old) over mDGCs and that immature DGCs showed greater spiking and broader tuning than mDGCs in response to a range of stimulus intensities24. Furthermore, this biased entorhinal cortical (EC) recruitment of immature abDGCs appeared to be governed by a higher ratio of feedforward excitation to inhibition during spike initiation in DGCs. This may reflect differences between immature abDGCs and mDGCs with regard to perisomatic inhibition mediated by parvalbumin (PV+) INs or CCK INs41,46 or increased inhibition of PV+ INs by neurogliaform and Ivy cells47. An alternative viewpoint posits that low excitatory cortical synaptic connectivity with immature abDGCs, coupled with smaller dendritic arbors and lower dendritic spine densities, constrains EC recruitment of ~4-week-old abDGCs and renders them finely tuned25,48,49. These contrasting views on tuning of immature abDGCs may arise from differences in experimental protocols.
In vivo studies afford a more physiological assessment of abDGC recruitment as they do not rely on artificial stimulation protocols and more accurately account for the contribution of long-range projecting cell types such as mossy cells50. Immediate early gene (IEG) analysis did not detect preferential activation of immature abDGCs relative to older DGCs during spatial learning51. In contrast, a similar study in rats did show increased activation of 4-month-old abDGCs relative to 7-month-old DGCs born during the first postnatal week52. In vivo two-photon calcium imaging revealed that immature ~3- to 6-week-old abDGCs were more active, less spatially tuned and fired with less spatial information than mDGCs53 and similarly to mossy cells54-56. However, a small population of the ~6-week-old abDGCs exhibited sufficient spatial tuning to permit differentiation of novel contexts. It is not clear why IEG analysis did not detect increased activity of ~6-week-old abDGCs51. The fine spatial tuning of mDGCs observed in calcium imaging studies55,56 is consistent with in vivo recordings in the DG of behaving rats documenting sparse activity and high input-specificity of mDGCs57. Although there is ex vivo evidence showing sparse EC–DGC connectivity and thus high input-selectivity in immature <6-week-old abDGCs, both in vivo calcium imaging data51 and alternative ex vivo evidence25,48,49 support the notion that immature abDGCs exhibit low input-specificity. Tetrode recordings of immature abDGCs in vivo are critically needed to resolve this debate.
Experience sculpts the connectome of abDGCs.
Rabies-virus based mapping studies of monosynaptic retrograde inputs have begun to identify the presynaptic partners of abDGCs during maturation. Mossy cells and different classes of INs in the DG—such as PV+ basket cells and axo-axonic cells, somatostatin (SST) hilar PP cells, hilar commissural associational pathway cells, neurogliaform cells and molecular PP cells35,58-60—are amongst the earliest presynaptic partners of abDGCs. Beginning at around 3 weeks, subcortical, entorhinal and intrahippocampal inputs are sequentially established58,59 (Fig. 1a). Lateral EC (LEC), rather than medial EC, seems to provide the dominant input to immature (4 weeks) abDGCs59,61, suggesting that immature and mature abDGCs may receive different information62.
Interestingly, the presynaptic connectome is modifiable by experience. Enriched experience and wheel running transiently increased inputs from CA3 and CA1 INs and permanently increased inputs from EC, the medial septum-diagonal band of Broca cholinergic neurons, medial mammillary nuclei and supramammilary nucleus63,64 in 2- to 6-week-old abDGCs. Enhancement of abDGC integration resulted in a scaled increase of mossy cell and hilar IN inputs, indicative of afferent structural plasticity35.
Little is known about the postsynaptic connectome of abDGCs. Mossy-fiber terminals of DGCs have filopodial protrusions that release glutamate onto different classes of hilar and stratum lucidum INs and convey feedback inhibition and lateral inhibition onto the DG and convey feedforward inhibition onto CA3–CA214,65-67. Interestingly, immature abDGCs exhibit the highest numbers of mossy-fiber terminal filopodia at 4 weeks of age68 (Guo and Sahay, unpublished observations). Because learning induces mossy-fiber terminal filopodial contacts with PV+ INs14,67, it is plausible that feedforward-, feedback- and lateral-inhibition connectivity may be more modifiable during a sensitive period (Fig. 1a,b; and see Box 1 for a glossary). Although abDGCs contact mossy cells and different CA3 subregions, the precise map of functional connectivity is yet to be defined.
Box 1 ∣ Glossary.
Sensitive period: the window of development or maturation during which circuit properties and functions exhibit heightened vulnerability to lasting experience-dependent modifications.
Pattern separation: a network mechanism by which cortical inputs are nonlinearly transformed into more divergent outputs, thereby minimizing interference between those inputs. Input–output transformations in EC–DG may be mediated by rate remapping (changes in firing rates of the same participant cells active in the same locations in two environments) or global remapping (independent populations of participant cells with differences in firing rate and firing fields) or by increased subsecond coupling of DGCs with inhibitory INs.
Pattern completion: a network mechanism by which a complete representation is retrieved based on a subset of its features.
Engram: a physical instantiation of an experiential event or memory in the brain, or an enduring change in physical state or structure of the brain in response to an experience or event.
Hippocampal indexing theory: Teyler and DiScenna posited11 that the hippocampus registers experiential events in engrams that are linked to distributed patterns of cortical and subcortical activity encoding those events. Activation of the engram bearing cells reinstates the cognate detailed cortical and subcortical memory traces through pattern completion to mediate memory retrieval.
Sparseness: a feature of a circuit that permits encoding of information in a small population of strongly activated cells.
Inhibitory microcircuits: networks of excitatory neurons and inhibitory INs whose organization dictates patterns of firing of excitatory principal neurons and oscillations.
Feedforward inhibition: activation of a principal cell preferential recruits IN discharge to dampen afferent excitation onto a principal cell. In a feedback inhibition or recurrent inhibitory motif, self-attenuation of principal cell firing occurs via recruitment of INs, which decreases principal cell output; a lateral inhibition motif enables a single principal cell to recruit INs to suppress firing of neighboring principal cells.
Memory clearance: transfer of memories out of the hippocampus to cortical sites for storage
Proactive interference: previously encoded memories interfere with recall of newer memories.
Retroactive interference: recent memories interfere with recalling previously encoded memories.
Together, these studies suggest that experience-dependent modifications of connectivity and, potentially, physiology during the sensitive period of abDGC development generates finely tuned heterogeneous mature abDGCs, each selective for distinct stimuli that were encoded during prior experiences69,70. Additionally, 6-week-old immature abDGCs appear to receive functional inputs and may also potentially contribute to encoding functions. We next discuss the contributions of abDGCs to memory processing as inferred from behavioral studies.
Adult-born DGCs and resolution of memory interference
Guided by theoretical models of DG functions in pattern separation1-3,71, behavioral studies have implicated the DG in reduction of memory interference4,5,72,73. Consistent with this and with computational modeling74, acute and chronic manipulations of abDGCs in mice and rats have demonstrated roles for abDGCs in hippocampal memory discrimination, consolidation and clearance, all of which, we argue, are critical to resolution of memory interference. Here we review convergent lines of evidence from many different laboratories supporting this idea.
Memory discrimination.
The delayed non-match-to-place radialarm-maze task and spatial-discrimination tasks are commonly used to study memory interference. For example, rodents more easily remember the reward-associated arm when there is a large space between the test and trial arms, i.e., less memory interference between the arms. Mice in which adult hippocampal neurogenesis was ablated by targeted X-irradiation (~< 12-week-old abDGCs)75 or genetic deletion of molecular factors (~7-week-old abDGCs)76,77 in neural stem cells failed to discriminate under high memory-interference conditions. Similarly, optogenetic silencing of 5- to 10-week-old abDGCs, but not 14- to 18-week-old abDGCs, in a spatial-discrimination task impaired performance only under high memory-interference conditions78.
Studies using X-irradiation79,80, chemical81, genetic76,82 and pharmacogenetic80 approaches to ablate or suppress adult hippocampal neurogenesis have provided evidence for a role for abDGCs in resolving proactive interference between new and previously learned items or locations in several tasks. Conversely, genetic expansion of the population of 5- to 8-week-old abDGCs reduced proactive interference in the reversal learning phase of the Morris water maze35.
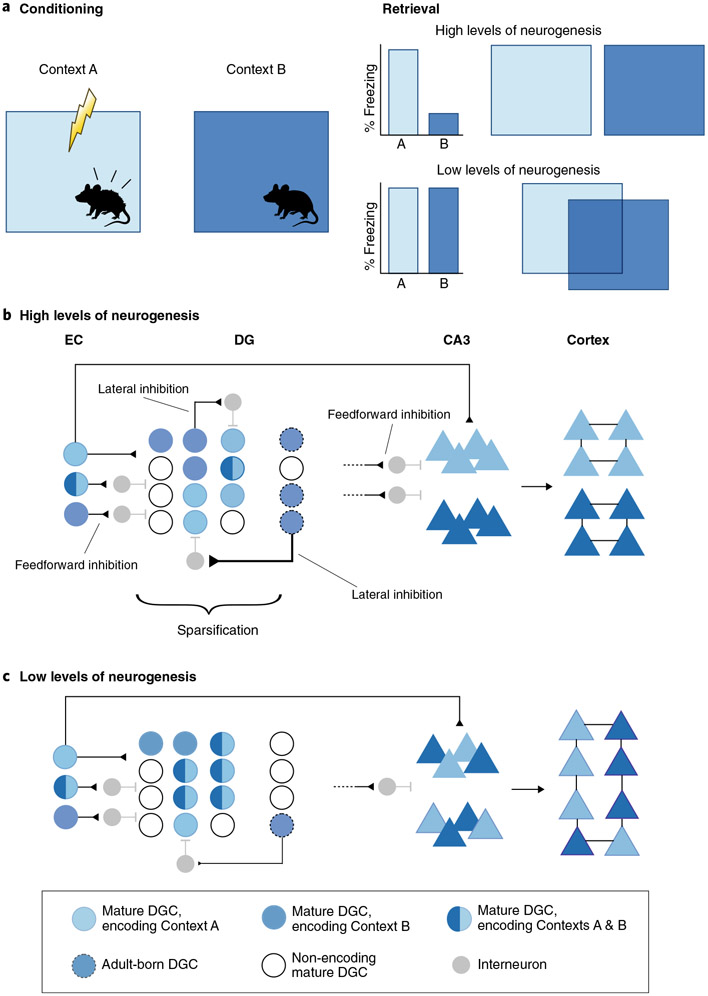
The contextual fear discrimination task permits assessment of whether abDGCs are necessary for encoding and distinguishing a context associated with a mild footshock from neutral but similar (high-interference) or neutral and distinct (low-interference) contexts (Fig. 2a). Chronic ablation of adult hippocampal neurogenesis (by genetic83, chemical84 or pharmacogenetic84 means), hippocampus-targeted X-irradiation45,85 or genetic disruption of synaptic plasticity in abDGCs86 impaired discrimination of a neutral, similar context from a context associated with a footshock in mice (Fig. 2a). Genetic enhancement of survival and integration of abDGCs improved discrimination between two similar contexts, but not between two distinct contexts35,45 (Fig. 2a). Remarkably, enhanced context discrimination was also observed following chronic genetic silencing of most mDGCs while sparing only the population of immature (~4-week-old) abDGCs85. Thus, the loss-of-function studies suggested a role for ~<10-week-old abDGCs in context discrimination, whereas the gain-of-function study narrowed this down to 4- to 8-week-old abDGCs. Adult-born DGCs may contribute to context discrimination through encoding of the training context and/or detection of mismatch between similar contexts. Some studies53,76,87, though not others42,88-90, have found that ablation or optogenetic silencing of abDGCs (but not a random population of mDGCs91) impaired encoding of the conditioning or a similar, neutral context, especially when a weak conditioning protocol was used. One interpretation is that abDGCs contribute to contextual encoding and subsequent mismatch detection under high cognitive demand and memory interference. Furthermore, abDGC-associated impairments in extinction of contextual fear76,88 may reflect deficits in reducing interference between the original trace and the new memory trace generated by extinction learning92.
Fig. 2 ∣. Adult-born DGCs reduce memory interference and promote consolidation through inhibitory microcircuits.
a, Schematic of contextual fear conditioning paradigm using two similar but nonidentical contexts (here indicated by two different shades of blue). With high levels of adult neurogenesis, mice efficiently discriminate between the two similar contexts, as indicated by increased freezing in the shock-paired chamber (indicated in light blue) but not in the non-paired but similar chamber (indicated in dark blue). With low levels of neurogenesis, discrimination is impaired, as indicated by similar levels of freezing in the two chambers. b, Adult-born DGCs (outlined with dotted border) facilitate separation of DG engrams encoding similar contexts. Top: EC inputs are decorrelated in DG via feedforward inhibition and lateral inhibition; abDGCs, competing with mature DGCs for perforant path inputs, exert high levels of lateral inhibition onto other DGCs through local inhibitory microcircuits; this facilitates sparse activity in the DG and thereby enables the establishment of non-overlapping engrams for the shock-associated context (light blue) and the similar but neutral context (dark blue); white cells do not encode either context. abDGCs recruit feedforward inhibition to transfer engrams of both contexts in non-overlapping populations of CA3 (triangles). Following decorrelation of engrams of both contexts in DG–CA3, each representation is consolidated in independent cortical ensembles. Through these mechanisms, high levels of adult neurogenesis can reduce memory interference. Bottom: with low levels of neurogenesis, similar contexts are represented by overlapping engrams (light and dark blue shades) in the DG and CA3 owing to inefficient synaptic competition, decreased feedforward inhibition, decreased lateral inhibition and reduced sparsification of DG activity. This increased interference in turn results in overlap and linkage of representations of both contexts in the cortex.
Memory consolidation.
Loss-of-function and gain-of-function studies have linked abDGCs with maintenance of long-term and remote memories. Pre-training ablation of abDGCs impaired long-term contextual fear memory and spatial memory76,93. Post-training optogenetic silencing of 4- or 6-week-old abDGCs, or genetic ablation of 7-week-old abDGCs, that were present during training produced deficits in contextual fear, visual discrimination and spatial memory89. Interestingly, ablation of abDGCs not present during training, but present after contextual-fear conditioning, also decreased remote memory retrieval76. These data suggest that abDGCs may affect ongoing consolidation processes even if they were not available during memory encoding. Genetic enhancement of survival of ~5- to 8-week-old abDGCs enhanced remote contextual fear, object and spatial memory35,94. Adult-born DGCs, like mDGCs, may regulate memory interference in CA3 to promote consolidation in hippocampal-cortical networks and remote-memory precision14 (Fig. 2b,c). This function of abDGCs may in turn promote discrimination between future experiences by promoting consolidation of prior experiences.
Memory clearance.
Following hippocampal encoding, memories are transferred to cortical sites for consolidation. Several lines of evidence suggest a role for adult hippocampal neurogenesis in modulating this process. Whole-brain irradiation to ablate neurogenesis extended the window of hippocampus-dependence for expression of remote memories95. Pharmacogenetic reduction of <6-week-old abDGCs following learning prevented forgetting of the platform location in the Morris water maze and contextual fear memory deficits96,97. Conversely, genetically increasing the population of <4-week-old immature abDGCs following training, but not 4 weeks later, impaired the expression of contextual fear memories, suggesting a window of memory vulnerability to neurogenesis-induced clearance or forgetting98. Consistently, partial reduction of adult hippocampal neurogenesis following wheel running was found to abrogate running-induced forgetting98 and impair subsequent reversal learning, indicative of increased pro-active interference between old and new memories97. Interestingly, wheel running, which is potently pro-neurogenic but also induces numerous changes in the hippocampus, is only modestly effective in inducing forgetting when mice are trained using a strong fear-conditioning protocol96. These studies suggest that memory strength and time post-learning dictate the extent of memory vulnerability to neurogenesis-induced clearance. Through regulation of memory clearance, adult hippocampal neurogenesis may maintain bandwidth in the DG to encode new memories without increasing interference with those previously encoded.
Together, these studies demonstrate that abDGCs contribute to DG functions in decreasing memory interference. To understand the precise role of abDGCs in mediating this function, we need to integrate synaptic connectivity and physiology of abDGCs with circuit and network mechanisms that support memory discrimination, consolidation and clearance, as discussed below.
Adult-born DGCs in pattern separation
One network mechanism recognized as a substrate for resolving memory interference is pattern separation, the process by which small changes in EC inputs are nonlinearly transformed into divergent DG outputs and relayed to CA31-8,99. Attractor dynamics in CA3’s recurrent collateral circuitry determine whether it performs pattern separation to store a representation as new or performs pattern completion to retrieve or update a previously stored memory trace, based on the balance of the direct EC inputs and the transformed DG outputs100. Some evidence suggests that DGCs and mossy cells have a role in decorrelating similar inputs from EC by rate remapping (changes in firing rates) and global remapping (changes in firing rate and location) of place cells6,7,54-56,100,101. Additionally, subsecond coupling between place cells and INs underlies spatial-memory discrimination8. Guided by physiological properties of the DG and EC–DG–CA3 connectivity, computational models and theoretical studies3,102-104 have suggested a role for sparseness, expansion recoding and high-dimensionality coding105 in facilitating decorrelation of cortical inputs. The general idea is that overlapping inputs maybe segregated in non-overlapping cells because of the relatively larger size and sparser activity of the output layer (DG) relative to input layer (EC). Additionally, expanding the circuit’s capacity to encode neural representations in activity patterns (high-dimensionality coding) is also thought to minimize overlap or promote decorrelation of overlapping patterns105. Consistent with these proposals, in vivo recordings57,106, calcium imaging studies54-56 and IEG analysis107 have suggested that at any given time only 2–5% of the DGCs are active, providing further evidence for sparseness. Computational and experimental studies support a role for GABAergic inhibition in this sparsification of DG activity104,108 through a ‘winner-take-all’ model, where the most strongly activated ‘winner’ DGCs suppress surrounding ‘competitor’ DGCs to facilitate encoding of unique elements of similar inputs in non-overlapping populations and consequently decrease the overlap between those inputs109,110.
Evidence linking abDGCs with sparseness and decorrelation.
A few studies have provided direct evidence linking adult hippocampal neurogenesis with sparseness and population-based coding mechanisms that support pattern separation in memory-discrimination tasks. One study found that genetic expansion of a population of 5- to 8-week-old abDGCs decreased overlap between DGC ensembles activated by two similar contexts in a contextual fear-discrimination task in mice35. Interestingly, both control and experimental groups showed greater ensemble overlap in the posterior DG than in anterior DG, suggesting that spatial discrimination is differentially processed by the dorsal and the ventral DG111. Moreover, mice with greater numbers of 5- to 8-week-old abDGCs also exhibited increased sparseness of activity in DG that was dependent on exposure to a similar context, but not the same context, following training. This suggests the existence of an abDGC-dependent mismatch-detection mechanism that suppresses activity of DGCs to enhance sparseness35. These findings align with those of a study showing that genetic ablation of <8-week-old abDGCs reduced mismatch-dependent regulation of sparseness80. Furthermore, partial genetic ablation of <4-week-old abDGCs increased overlap of CA3 ensembles activated by two similar contexts, and this was accompanied by elevated numbers of active CA3 neurons following exposure to the similar context84. Additionally, pharmacological suppression of adult hippocampal neurogenesis increased firing rates of DG neurons and impaired response selectivity of DG neurons to temporally separated contexts112—although this study could not distinguish between DGCs and mossy cells.
Together, these findings suggest that abDGCs promote population-based coding and sparseness during discrimination under conditions of high memory interference. Because the studies above did not examine the activity of neuronal ensembles in the EC, it is not clear whether abDGC-dependent modulation of population-based coding in DG and CA3 reflects the input–output transformation that is emblematic of pattern separation. It is also unknown whether abDGCs contribute to circuit mechanisms supporting pattern separation in tasks beyond contextual fear discrimination. Thus, abDGCs contribute to sparseness and decorrelation of inputs and, therefore, may partake in in pattern separation, but very little is known about the underlying circuit mechanisms.
Adult-born DGCs and inhibitory microcircuits
Interneurons and mossy cells participate in both guiding abDGC maturation and governing functions of abDGCs. Hilar mossy cells and distinct IN subtypes embedded within EC–DG–CA3 circuit architecture may function as putative arbiters of information transformation and transfer functions that underlie sparseness and decorrelation46,66,113,114. Excitatory inputs from PP and hilar mossy cells onto the DG are balanced by recruitment of local networks of diverse INs that coordinate feedforward, feedback and lateral inhibition onto DGCs. Distinct INs may differentially contribute to these inhibitory mechanisms depending on their dendritic and axonal distribution within the DG. Additionally, INs differentially integrate excitatory inputs depending on their morphology and physiology. Mossy cells mediate DGC-dependent lateral excitation onto DGCs, but also exert potent ipsilateral and contralateral disynaptic inhibition onto hundreds of DGCs via PV+ INs50. Genetic ablation of mossy cells induces hyperexcitability of the DG and impairs context discrimination115, and optogenetic silencing of mossy cells increases DGC firing56. Although mossy-cell-dependent DGC inhibition is thought to prevail over DGC excitation, this balance may be modulated50. Here we focus on evidence linking abDGCs with different IN-dependent circuit mechanisms that support pattern separation.
Several indirect lines of evidence hint at a role for abDGC-dependent reorganization of local inhibitory circuits. First, a study in anaesthetized mice showed that X-irradiation-induced ablation of <10-week-old abDGCs decreased responses to PP stimulation and increased gamma burst activity116—a form of network oscillation that is thought to be dependent on PV+ basket cells117. Second, genetic ablation of adult hippocampal neurogenesis blocked picrotoxin-independent LTP at EC–DGC synapses, which was restored over time by a compensatory decrease in GABAergic tone118. Third, genetic enhancement of adult hippocampal neurogenesis decreased DGC excitability in acute slices, whereas hippocampal X-irradiation-induced ablation of abDGCs increased DGC excitability119. Fourth, hippocampal irradiation-induced ablation of <12- to 16-week-old abDGCs increased DG network excitability120. Fifth, acute optogenetic silencing of 7-week-old, but not 16-week-old, abDGCS in resting mice elevated both blood-oxygen level-dependent (BOLD) signals in the DG–CA3 area and the power of high-frequency hippocampal oscillations, suggestive of increased disinhibition78. Next, we discuss studies that directly investigate the contribution of inhibitory microcircuits within EC–DG–CA3 circuit architecture toward abDGC functions.
Feedforward inhibition in the EC–DG circuit.
Studies performing simultaneous recordings of DGCs and fast-spiking INs in mice have shown that medial PP stimulation preferentially induces spiking in fast-spiking INs over DGCs, which have a significantly higher spike threshold121. DG afferent inputs provide feedforward inhibition by recruiting perisomatic-projecting PV+ INs or the molecular layer perforant path-associated (MOPP) cells, which in turn inhibit DGCs, facilitating efficient summation of incoming EC signals by restricting the window in which spikes are evoked by excitation before onset of inhibition (Fig. 1a,b). Neurogliaform cells also mediate feedforward inhibition onto DGCs and, additionally, inhibit PV+ basket cells47. Fast-spiking somatic-targeting INs and non-fast-spiking dendritic-targeting INs maybe differentially recruited by sparse and strong presynaptic activity122.
How do abDGCs contribute to this feedforward inhibition in the EC–DG circuit (Fig.1b)? During early stages of abDGC maturation, GABA is excitatory; however, strong GABAergic activity can shut down spiking in young DGCs by shunting inhibition123. Thus, some of the earliest presynaptic INs of abDGCs, such as neurogliaform cells, may regulate spiking of abDGCs through shunting inhibition. Electrical stimulation of the PP drives MOPP-mediated disynaptic inhibition onto abDGCs. Whole-cell recording studies have detected inhibitory postsynaptic currents in ~3-week-old abDGCs following activation of local uncaging of glutamate in MOPP cells60. Theoretical modeling and experimental data demonstrate that immature abDGCs receive weaker MOPP-mediated feedforward inhibition compared to mDGCs48,124. Consistently, 4-week-old abDGCs, unlike mDGCs, are recruited by a range of medial PP stimulation intensities, and this broad tuning is thought to reflect a higher ratio of feedforward excitation to inhibition during spike initiation24. Thus, mature (>6-week-old) abDGCs are likely to recruit EC–DG feedforward inhibition to modulate sparseness and decorrelation in the DG.
Lateral inhibition in the DG.
DGCs may reciprocally or unidirectionally recruit local INs to attenuate their own excitation (feedback inhibition) or that of neighboring DGCs (lateral inhibition)46,66,113. DG INs are robustly activated during exploration of novel environments when the DG is likely to be engaged125. Simultaneous recordings in slices have demonstrated that lateral inhibition predominates over feedback inhibition and is mediated largely by PV+ INs and potentially, SST+ INs109,126 (Fig. 1a,b). This lateral inhibition may support a winner-take-all logic to promote decorrelation of similar inputs. Several studies have found that >6-week-old abDGCs can recruit lateral inhibition70,127. In addition to local lateral inhibition, DGCs receive axo-axonic and dendritically targeted inhibitory inputs from distally located INs in CA3 and CA1114.
How do abDGCs contribute to lateral inhibition (Fig. 1b)? Optogenetic stimulation of 7-week-old, but not 4-week-old abDGCs, paired with medial PP electrical stimulation reduced spiking of mDGCs, and this effect was dependent on GABAergic inhibition likely mediated by PV+ basket cells70. Furthermore, <4-week-old abDGCs, unlike mDGCs, were insensitive to lateral inhibition70. Another optogenetics study showed that <7-week-old abDGCs can exert lateral inhibition onto DGCs127. Similarly, optogenetic stimulation of 6- or 8-week-old, but not 4-week-old abDGCs, activated DG and hilar INs in vivo68. In addition to recruitment of INs, <6-week-old abDGCs may also inhibit mDGCs via monosynaptic excitatory synapses62. However, it is not clear how this abDGC–DGC synaptic mechanism meaningfully contributes to sparseness in the face of strong tonic inhibition exerted by local INs. Together, these studies suggest that >6-week-old abDGCs recruit lateral inhibition ex vivo; whether abDGCs recruit this inhibitory circuit mechanism to promote decorrelation of similar inputs remains to be determined.
Feedforward inhibition in the DG–CA3 circuit.
Mossy-fiber synapses onto thorny excrescence-like spines of CA3 neurons convey monosynaptic strong excitatory drive that demonstrates robust facilitation in response to repetitive stimulation128. Mossy fibers form a larger number of synaptic contacts with hilar and stratum lucidum INs such as PV+ and SST+ INs via filopodial extensions65,66. The pattern of DGC disynaptic inhibitory inputs onto CA3 neurons appears to be randomly organized and independent of DGC-dependent monosynaptic excitation, such that feedforward inhibition governs the excitability of a large number of CA3 pyramidal neurons129 (Fig. 1a,b). PV+ INs relay feedforward inhibition onto CA3, and this has been suggested to promote memory consolidation in hippocampal–cortical networks through modulation of sharp-wave ripples130. Feedforward inhibition connectivity is inversely correlated with remote-memory precision67, and acutely increasing feedforward inhibition onto CA3 decreased remote-memory interference in CA3–anterior cingulate cortex–basolateral amygdala networks14. Although differences in recurrent excitation–inhibition between proximal and distal CA3 may bias them toward pattern separation and pattern completion, respectively131,132, it is not known whether feedforward inhibition differences along the CA3 transverse axis also contribute to a pattern separation–completion continuum. Thus, the balance between feedforward excitation and inhibition in the DG–CA3 circuit may modulate memory retrieval dynamics and interference and thereby determine whether an experience is consolidated as a new memory or updates a previously encoded representation.
How do abDGCs contribute to this feedforward inhibition in DG–CA3 (Fig. 1b)? Findings from optogenetic stimulation of abDGCs in combination with whole-cell recordings in CA3 in slices suggested that 4-week-old abDGCs, like 8-week-old abDGCs, can recruit feedforward inhibition onto CA370. In contrast, in vivo optogenetic stimulation of 4-week-old, but not 6- or 8-week-old abDGCs, resulted in activation of CA3 INs68. Furthermore, abDGCs exhibit highest number of mossy-fiber terminal filopodia, the anatomical substrate for feedforward inhibition, at 4 weeks68 (Guo and Sahay, unpublished observations). Pharmacogenetic and X-irradiation-induced abDGC ablation increased the overlap between two similar context-associated ensembles and decreased reactivation of long-term memory traces in CA3, respectively84,133. Consistent with the dynamic regulation of DGC–PV+ IN connectivity by learning, engram-bearing DGCs exhibit greater connectivity with PV+ INs than non-engram-bearing DGCs14. Thus, abDGCs may contribute to memory consolidation and reduce memory interference by recruitment of PV+ INs and feedforward inhibition in DG–CA3.
Role of synaptic competition in sparseness and memory clearance
The influential Marr–Albus theory of pattern separation invoked a role for ‘expansion recoding’ in pattern separation, whereby the ‘fanning out’ of cortical inputs onto the DG promotes decorrelation of inputs onto non-overlapping DGCs1. While absolute numbers of DGCs exceed EC cells by only a factor of five (approximately), computational models suggest that sparse synaptic connectivity between EC and DGCs facilitates pattern separation104. Network integration of abDGCs is thought to involve synaptic competition with mDGCs for PP inputs (Fig. 1c) and reduction of excitatory inputs onto mature DGCs28,33,35. In response to novel stimuli, immature abDGCs may successfully compete for PP inputs with pre-existing PP–mDGC synapses. This competition would redistribute PP–DGC synaptic weights, and integration of abDGCs may increase sparseness of EC–mDGC connectivity.
Such synaptic competition is also likely to mediate clearance of memories encoded in PP–DGC synapses that are out-competed. The precise mechanisms and synaptic signals by which synapse competition is mediated are poorly understood134. LTP decay at EC–DG synapses over a few weeks may signify completion of consolidation which then, via a cortical signal, promotes weakening of PP–DGC synapses that originally encoded the memory. This, in turn, would clear the memory trace by rendering the synapse vulnerable to synaptic competition with abDGCs. We speculate that EC–DGC engram synapses linked to inefficiently consolidated representations in cortical networks are most vulnerable to clearance.
In sum, abDGCs may recruit feedforward and lateral inhibition in EC–DG–CA3 and synaptic competition to generate a sparse code that supports encoding of similar contexts in non-overlapping populations of DGCs and that promotes cortical consolidation of experiences (Fig. 2b,c).
A model for abDGC contribution to hippocampal indexing and pattern separation
The maturation of abDGCs occurs along a continuum, during which afferent and efferent connectivity is established together with recruitment of distinct inhibitory microcircuits. Integration of abDGCs remodels the EC–DG–CA3 network to promote sparseness and memory clearance and decrease interference. This remodeling is reminiscent of how maturing INs modify excitatory synaptic competition during the critical period of visual system135. Based on our understanding of physiology and connectivity of abDGCs, we propose that immature and mature abDGCs make distinct contributions to DG functions in decreasing memory interference, namely by encoding novelty or mismatch by and indexing memories, respectively (Fig. 3).
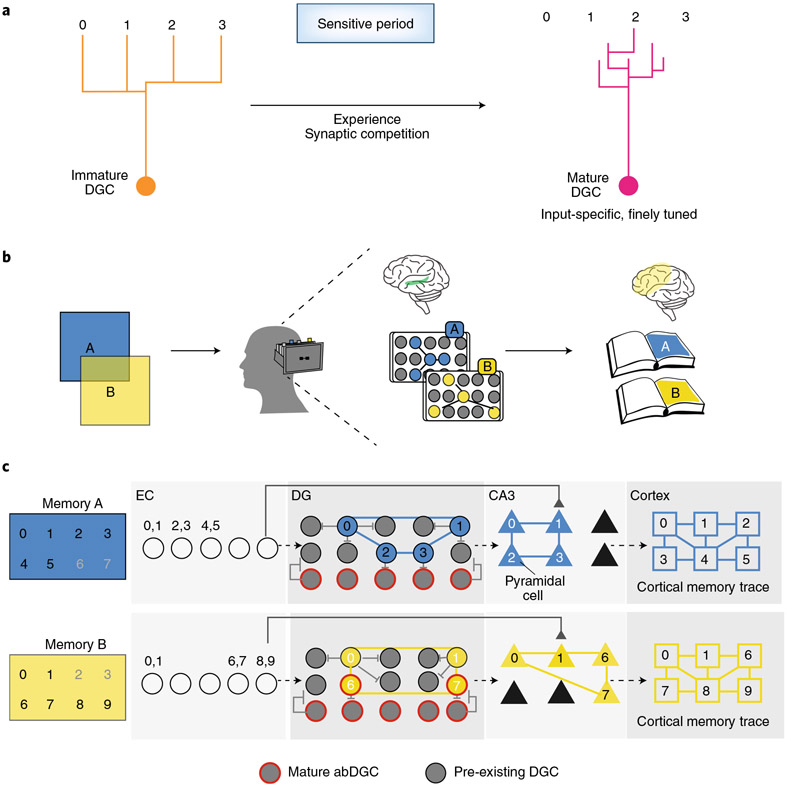
Fig. 3 ∣. Proposed role of adult-born DGCs in indexing and pattern separation.
a, Refinement of abDGC input specificity during the sensitive period. Immature abDGCs (orange) mature into abDGCs (red) with high input-selectivity and respond to specific features experienced during the sensitive period (here only stimulus 2 of stimuli 0–3). b, Schematic of indexing. Overlapping experiences (A and B) are encoded in distinct engrams (or ‘indexes’) in the hippocampus (green). These indexes are linked to detailed representations of A and B that are distributed in downstream cortical and subcortical regions (yellow), much like the way a library index card corresponds to a book on the shelves of the library. c, Experiences A (blue) and B (yellow) represented by unique permutations of inputs (examples: 0–9) in association sensory cortices are relayed to the EC. A and B are transformed into distinct non-overlapping engrams or indexes in the DG through decorrelation of EC inputs in the DG, where inhibitory microcircuits sparsify DG activity through a winner-take-all circuit motif. Different combinations of mature abDGCs (outlined in black) are flexibly allocated into each engram (or index), with shared features recruiting the same DGCs (here: features 0 and 1). Feature-responsive DGCs (yellow and blue) inhibit neighboring DGCs (gray) to further refine the engram through the winner-take-all mechanism. Immature, <6-week-old abDGCs (outlined in yellow) encode novel features of B (here features 6 and 7) and contribute to the index of B while also exerting lateral inhibition. DGC recruitment of feedforward inhibition onto CA3 facilitates the transfer of the ensemble onto non-overlapping pyramidal cells in CA3 (triangles). The balance between DG and EC inputs in CA3 dictates whether pattern completion or separation occurs. The DG–CA3 engram represents an index of the memory, but does not encode all of its features; these are instead stored in the corresponding linked cortical traces (yellow and black squares). Activation of the index in DG–CA3 reinstates the cortical memory trace through pattern completion to mediate retrieval.
Adult neurogenesis expands the capacity for hippocampal indexing.
As described earlier, experience sculpts the connectivity and, potentially, physiology of immature 4- to 6-week-old abDGCs during the sensitive period of maturation. As 6-week-old abDGCs mature, they acquire high input selectivity; this presumably involves Hebbian mechanisms of synaptic refinement and maturation, as well as activity-dependent transcriptional programs (Fig. 3a). Information encoded by abDGCs is consolidated in hippocampal–cortical networks14,70 by recruitment of feedforward inhibition onto CA3. The predominance of LEC inputs onto abDGCs at this stage permits encoding of local and egocentric information. Importantly, we speculate that cohorts of mature abDGCs differ from each other and other DGCs based on the experience-related inputs that they encountered during their maturation. Thus, the DG can be thought of as a ‘library of experiences’ that are registered in the connectivity and input selectivity of mDGCs. What function might this serve? Building on previous models69,70,136,137, we propose that adult hippocampal neurogenesis ensures generation of mature abDGCs that are representative of previously encoded experiences. Each mature abDGC encodes a specific feature of past experiences and different combinations of mature abDGCs are allocated into engrams of new memories. These engrams serve as hippocampal indexes which permit faithful memory retrieval.
Our proposal is based on hippocampal memory indexing theory11-15, which posits that the hippocampus does not encode experiential details but instead registers experiences in engram-bearing cells that are linked to distributed cortical and subcortical loci that have encoded details of the experience in temporal and spatial patterns of neural activity11(Fig. 3b). Activation of engram-bearing cells reinstates cortical and subcortical patterns of activity, thereby mediating recall of the experience. Recent studies have lent support to the idea that engram-bearing cells function as indexes. First, optogenetic stimulation of engram-bearing DGCs induces recall of precise contextual information13. Second, silencing of engram-bearing cells in CA1 impairs reinstatement of previously encoded cortical and subcortical representations during retrieval138. Third, in vivo recordings from engram-bearing cells suggest that these cells are distinct from place cells, in that they encode experiential information rather than just spatial locations12. Fourth, genetic inhibition of outputs of engram-bearing DGCs impaired maturation of the cortical ensemble15. Fifth, engram-bearing DGCs recruit feedforward inhibition, resulting in decreased memory interference in CA3 and increased memory consolidation in hippocampus–anterior cingulate cortex–basolateral amygdala networks14.
According to our model, mature abDGCs contribute to indexing functions of the hippocampus by responding to distinct experience-related inputs. We consider abDGCs >6 weeks of age as ‘mature’ because this time point seems to coincide with the decline of the sensitive period and because in vivo studies suggest that >6-week-old abDGCs are more similar to mature DGCs in terms of input selectivity and recruitment of lateral inhibition. Mature abDGCs are likely to respond to inputs that they have previously experienced and exercise a winner-take-all process via lateral inhibition (Fig. 3c). This enables activated DGCs to retrieve the memory trace in recurrent CA3 networks and reinstate the original patterns of activity underlying the experience in distributed cortical and subcortical modules by pattern completion. The more diverse the history of prior experiences recorded in mature abDGCs, the greater the representation of stimulus features in the input-space of DGCs and, consequently, the greater the potential number of flexible combinations of mDGCs allocated to new engrams or indexes. Thus, allocation of mature abDGCs into engrams is dependent on features previously encoded; in a laboratory setting, this may reflect housing and testing conditions.
New memories always share some features with those previously encoded. We propose that shared features of similar memories reactivate mature abDGCs and DGCs that have previously encoded those stimulus features, whereas novel features, local cues and egocentric information are encoded in immature <6-week-old abDGCs (Fig. 3c). This amalgam of activated immature and mature DGCs suppresses other DGCs via lateral inhibition126, resulting in the generation of an index of the similar memory in DG–CA3 that is linked to the detailed representation in the cortex.
Expanding the population of 5- to 8-week-old abDGCs promotes DGC reactivation following context re-exposure and mismatch-dependent sparseness, resulting in decreased overlap between ensembles encoding similar contexts35. Engram-bearing DGCs, through recruitment of feedforward inhibition onto CA3, ensure orthogonalization of the similar memory trace in CA3 and consolidation in hippocampal–cortical networks14. Finally, it is likely that cognitive demands may modulate DGC-dependent recruitment of INs during encoding. With low levels of adult hippocampal neurogenesis, coverage of the input space is low in mature abDGCs; as a consequence, there are fewer combinations of indexes available for encoding new memories in DG. Therefore, there will be greater interference between indices and linked memory traces in hippocampal–cortical networks and increased pattern completion of similar inputs.
A role for maturing abDGCs and lateral inhibition in novelty and mismatch detection.
Conservatively, it seems that the recruitment of inhibition by immature abDGCs to promote their differentiation and ultimately, to govern spiking minimizes the cost of network remodeling. This is evident in how synaptic competition both mediates network integration of abDGCs and also promotes memory clearance and sparseness of activity as discussed previously. Assuming that 6-week-old abDGCs are broadly tuned in vivo53, we suggest that 6-week-old abDGCs may function like mossy cells to exert lateral inhibition and facilitate recruitment of mDGCs into non-overlapping indexes. Innervation by LEC inputs may preferentially mediate abDGC responses to local and egocentric cues139 to facilitate detection of mismatch. Adult-born DGCs may also recruit CA3c to exert disynaptic inhibition onto DGCs140. Alternatively, if 6-week-old abDGCs already exhibit high input-selectivity in vivo as has been suggested25,48,49, then these cells may encode novel information while exerting lateral inhibition. Thus, immature abDGCs may transiently contribute to DG functions in pattern separation even during their maturation.
Outlook
Adult hippocampal neurogenesis appears to be a conserved form of structural plasticity in non-human primates and humans141-146 (but also see ref. 147). However, it is not clear why numbers of abDGCs as ascertained by immunohistochemistry in human postmortem tissue appear to differ substantially from numbers of abDGCs gleaned from birthdating studies141,142. It is plausible that that putative abDGCs in primates and humans may mature over much longer periods than that seen in rodents144,148 or that mature DGCs may express markers of structural plasticity associated with immature DGCs. Since birthdating experiments in humans are unethical, resolving this debate necessitates deployment of other approaches, such as quantification of neural stem cells and singlecell RNA sequencing to examine signatures of neurogenesis in the adult human hippocampus . In addition, retroviral targeting of neurogenesis in adult non-human primates will enable assessment of abDGCs connectivity, physiology and function.
Substantial work is needed to deconstruct the complexity of IN microcircuits and dissect the effects of experience on the integration and functions of abDGCs as they relate to hippocampal memory processes. To what extent is the inhibitory microcircuit architecture conserved in non-human primates and humans? Given the differential contributions of hippocampal circuits along the septotemporal axis to memory processing and emotion149, future studies must integrate differences in abDGC connectivity and physiology along the septotemporal axis in rodents and non-human primates. Such efforts will illuminate how abDGC-dependent regulation of memory interference gates recruitment of different hippocampal targets in cortex and subcortical circuits to influence a range of behaviors.
Memory impairments associated with age-related cognitive decline, mild cognitive impairment and psychiatric disorders are characterized by increased memory interference and disrupted memory consolidation17,18,99,136,150. Thus, strategies that stimulate adult hippocampal neurogenesis may improve information processing in DG–CA3, cognition and emotional regulation. Additional outstanding research questions are listed in Box 2.
Box 2 ∣. Outstanding questions.
How does experience modify physiological properties and output connectivity of abDGCs during the sensitive period?
Does gene expression in mature abDGCs reflect heterogeneity and input selectivity based on prior experience?
What patterns of activity are displayed by immature and mature abDGCs as assessed using in vivo electrophysiological recordings?
How do abDGCs recruit inhibitory microcircuits to support pattern separation in vivo?
How does abDGC connectivity with different inhibitory neurons vary along the septotemporal axis?
How does abDGC-dependent regulation of memory interference gate recruitment of subcortical and cortical circuits?
How do abDGCs integrate inputs from LEC and medial EC to support memory processing in vivo?
Does increasing or ablating neurogenesis affect the capacity of the DG to generate engrams of different memories?
Acknowledgements
We thank members of Sahay lab for discussions and L.M.S. Sahay for help with manuscript editing. A.S. acknowledges support from NIH-R01MH104175, NIH–R01AG048908, NIH-1R01MH111729, the James and Audrey Foster MGH Research Scholar Award, the Ellison Medical Foundation New Scholar in Aging, the Whitehall Foundation, an Inscopix Decode award, a NARSAD Independent Investigator Award, Ellison Family Philanthropic support, the Blue Guitar Fund, a Harvard Neurodiscovery Center–MADRC Center Pilot Grant award, Alzheimer’s Association Research Grant, a Harvard Stem Cell Institute Development grant, and an HSCI seed grant. The authors apologize to scientists whose works could not be cited due to limits on the number of references.
Footnotes
Competing interests
The authors declare no competing financial or non-financial interests.
Reprints and permissions information is available at www.nature.com/reprints.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marr D Philos. Trans. R. Soc. Lond. B Biol Sci 262, 23–81 (1971). [DOI] [PubMed] [Google Scholar]
- 2.Treves A & Rolls ET Hippocampus 2, 189–199 (1992). [DOI] [PubMed] [Google Scholar]
- 3.O’Reilly RC & McClelland JL Hippocampus 4, 661–682 (1994). [DOI] [PubMed] [Google Scholar]
- 4.Berron D et al. J. Neurosci 36, 7569–7579 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker A, Kirwan CB, Miller M & Stark CE Science 319, 1640–1642 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using an incidental encoding task, the authors showed that the DG–CA3 circuit in humans is preferentially recruited under conditions of high mnemonic interference. [Google Scholar]
- 6.Leutgeb JK, Leutgeb S, Moser MB & Moser EI Science 315, 961–966 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Neunuebel JP & Knierim JJ Neuron 81, 416–427 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; Recordings from EC, DG and CA3 demonstrated input–output transformation functions in DG and retrieval dynamics in CA3 consistent with their proposed functions in pattern separation and completion. [Google Scholar]
- 8.van Dijk MT & Fenton AA Neuron 98, 832–845.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakon JJ & Suzuki WA Proc. Natl. Acad. Sci. USA 116, 9634–9643 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClelland JL, McNaughton BL & O’Reilly RC Psychol. Rev 102, 419–457 (1995). [DOI] [PubMed] [Google Scholar]
- 11.Teyler TJ & DiScenna P Behav. Neurosci 100, 147–154 (1986). [DOI] [PubMed] [Google Scholar]
- 12.Tanaka KZ et al. Science 361, 392–397 (2018). [DOI] [PubMed] [Google Scholar]; Activity of c-Fos-expressing engram-bearing cells in CA1 is distinct from that of place cells and reliably predicts contextual identity. [Google Scholar]
- 13.Liu X et al. Nature 484, 381–385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo N et al. Nat. Med 24, 438–449 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Engram-bearing DGCs recruit PV+INs to convey feedforward inhibition onto CA3, stabilize the engram and modulate memory interference and consolidation in hippocampal–cortical–basolateral amygdala networks. [Google Scholar]
- 15.Kitamura T et al. Science 356, 73–78 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman J & Das GD J. Comp. Neurol 124, 319–335 (1965). [DOI] [PubMed] [Google Scholar]
- 17.Besnard A & Sahay A Neuropsychopharmacology 41, 24–44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAvoy KM & Sahay A Neurotherapeutics 14, 630–645 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Espósito MS et al. J. Neurosci 25, 10074–10086 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge S et al. Nature 439, 589–593 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overstreet-Wadiche LS, Bensen AL & Westbrook GL J. Neurosci 26, 2326–2334 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagasia R et al. J. Neurosci 29, 7966–7977 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chancey JH et al. J. Neurosci 33, 6614–6622 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marín-Burgin A, Mongiat LA, Pardi MB & Schinder AF Science 335, 1238–1242 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; Ex vivo study showing that immature abDGCs, unlike mature DGCs, respond to a wide range of inputs due to delayed recruitment of feedforward inhibition in the EC–DG circuit. [Google Scholar]
- 25.Dieni CV et al. Nat. Commun 7, 11313 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; Ex vivo study showing that sparse functional EC connectivity and excitatory drive onto immature abDGCs limits their recruitment in response to a broad range of cortical inputs. [Google Scholar]
- 26.Zhao C, Teng EM, Summers RG Jr., Ming GL & Gage FH J. Neurosci 26, 3–11 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toni N et al. Nat. Neurosci 11, 901–907 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toni N et al. Nat. Neurosci 10, 727–734 (2007). [DOI] [PubMed] [Google Scholar]; Electron microscopy analysis of EC–abDGC synapse formation. [Google Scholar]
- 29.Sun GJ et al. J. Neurosci 33, 11400–11411 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonçalves JT et al. Nat. Neurosci 19, 788–791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemaire V et al. J. Neurosci 32, 3101–3108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tashiro A, Sandler VM, Toni N, Zhao C & Gage FH Nature 442, 929–933 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Adlaf EW et al. eLife 6, e19886 (2017).28135190 [Google Scholar]; Genetically enhancing or ablating abDGCs decreases or enhances excitatory synaptic inputs onto mature DGCs by altering synaptic competition dynamics. [Google Scholar]
- 34.Krzisch M et al. Cereb. Cortex 27, 4048–4059 (2016). [DOI] [PubMed] [Google Scholar]
- 35.McAvoy KM et al. Neuron 91, 1356–1373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; Genetic elimination of dendritic spines in mature DGCs enhances functional integration of abDGCs and promotes context discrimination and population-based coding. [Google Scholar]
- 36.Faulkner RL et al. Proc. Natl. Acad. Sci. USA 105, 14157–14162 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez CM et al. Front. Neural Circuits 6, 85 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt-Hieber C, Jonas P & Bischofberger J Nature 429, 184–187 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Mongiat LA, Espósito MS, Lombardi G & Schinder AF PLoS One 4, e5320 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Overstreet Wadiche L, Bromberg DA, Bensen AL & Westbrook GL J. Neurophysiol 94, 4528–4532 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Aimone JB, Xu X, Callaway EM & Gage FH Proc. Natl. Acad. Sci. USA 109, 4290–4295 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu Y et al. Nat. Neurosci 15, 1700–1706 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ge S, Yang CH, Hsu KS, Ming GL & Song H Neuron 54, 559–566 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]; Immature abDGCs exhibit heightened synaptic plasticity at EC–DG synapses during a sensitive period in their maturation. [Google Scholar]
- 44.Snyder JS, Kee N & Wojtowicz JM J. Neurophysiol 85, 2423–2431 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Sahay A et al. Nature 472, 466–470 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; First study to demonstrate that genetically enhancing adult hippocampal neurogenesis is sufficient to improve memory processing specifically, decreasing contextual memory interference. [Google Scholar]
- 46.Bartos M, Alle H & Vida I Neuropharmacology 60, 730–739 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Overstreet-Wadiche L & McBain CJ Nat. Rev. Neurosci 16, 458–468 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dieni CV, Nietz AK, Panichi R, Wadiche JI & Overstreet-Wadiche LJ Neurosci. 33, 19131–19142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L et al. eLife 6, e23612 (2017).28826488 [Google Scholar]; Ex vivo study showing that immature abDGCs, like mDGCs, exhibit sparse patterns of activity. [Google Scholar]
- 50.Scharfman HE & Myers CE Front. Neural Circuits 6, 106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stone SS et al. Hippocampus 21, 1348–1362 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Tronel S, Lemaire V, Charrier V, Montaron MF & Abrous DN Brain Struct. Funct 220, 645–661 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Danielson NB et al. Neuron 90, 101–112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; Immature abDGCs are more active and more broadly tuned than mature DGCs in vivo, and their activity permits decoding of contextual information. [Google Scholar]
- 54.Danielson NB et al. Neuron 93, 552–559.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.GoodSmith D et al. Neuron 93, 677–690.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Senzai Y & Buzsáki G Neuron 93, 691–704.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neunuebel JP & Knierim JJ J. Neurosci 32, 3848–3858 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deshpande A et al. Proc. Natl. Acad. Sci. USA 110, E1152–E1161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vivar C et al. Nat. Commun 3, 1107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y et al. Proc. Natl. Acad. Sci. USA 110, 9106–9111 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woods NI et al. J. Neurosci 38, 5843–5853 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luna VM et al. Science 364, 578–583 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Adult-born DGCs establish monosynaptic excitatory contacts with mature DGCs. [Google Scholar]
- 63.Bergami M et al. Neuron 85, 710–717 (2015). [DOI] [PubMed] [Google Scholar]; Experience sculpts presynaptic connectome of abDGCs during a sensitive period. [Google Scholar]
- 64.Vivar C, Peterson BD & van Praag H Neuroimage 131, 29–41 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Acsády L, Kamondi A, Sik A, Freund T & Buzsáki GJ Neurosci. 18, 3386–3403 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pelkey KA et al. Physiol. Rev 97, 1619–1747 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruediger S et al. Nature 473, 514–518 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Restivo L, Niibori Y, Mercaldo V, Josselyn SA & Frankland P W. J. Neurosci 35, 10600–10612 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aimone JB, Deng W & Gage FH Neuron 70, 589–596 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Temprana SG et al. Neuron 85, 116–130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McNaughton B & Morris R Trends Neurosci. 10, 408–415 (1987). [Google Scholar]
- 72.Gilbert PE, Kesner RP & Lee I Hippocampus 11, 626–636 (2001). [DOI] [PubMed] [Google Scholar]
- 73.McHugh TJ et al. Science 317, 94–99 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Wiskott L, Rasch MJ & Kempermann G Hippocampus 16, 329–343 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Clelland CD et al. Science 325, 210–213 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; The first study to implicate abDGCs in resolution of memory interference in a behavioral task. [Google Scholar]
- 76.Pan YW, Chan GC, Kuo CT, Storm DR & Xia ZJ Neurosci. 32, 6444–6455 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J et al. J. Neurosci 34, 5184–5199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhuo JM et al. eLife 5, e22429 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wojtowicz JM, Askew ML & Winocur G Eur. J. Neurosci 27, 1494–1502 (2008). [DOI] [PubMed] [Google Scholar]
- 80.Burghardt NS, Park EH, Hen R & Fenton AA Hippocampus 22, 1795–1808 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garthe A, Behr J & Kempermann G PLoS One 4, e5464 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swan AA et al. Hippocampus 24, 1581–1591 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tronel S et al. Hippocampus 22, 292–298 (2012). [DOI] [PubMed] [Google Scholar]
- 84.Niibori Y et al. Nat. Commun 3, 1253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakashiba T et al. Cell 149, 188–201 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kheirbek MA, Tannenholz L & Hen RJ Neurosci. 32, 8696–8702 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huckleberry KA et al. Neuropsychopharmacology 43, 2487–2496 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deng W, Saxe MD, Gallina IS & Gage FH J. Neurosci 29, 13532–13542 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA & Frankland PW J. Neurosci 31, 15113–15127 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Snyder JS et al. J. Neurosci 29, 14484–14495 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park S et al. Neuropsychopharmacology 41, 2987–2993 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lacagnina AF et al. Nat. Neurosci 22, 753–761 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Snyder JS, Hong NS, McDonald RJ & Wojtowicz JM Neuroscience 130, 843–852 (2005). [DOI] [PubMed] [Google Scholar]
- 94.Wang W et al. J. Neurosci 34, 2130–2147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kitamura T et al. Cell 139, 814–827 (2009). [DOI] [PubMed] [Google Scholar]
- 96.Akers KG et al. Science 344, 598–602 (2014). [DOI] [PubMed] [Google Scholar]
- 97.Epp JR, Silva Mera R, Köhler S, Josselyn SA & Frankland PW Nat. Commun 7, 10838 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; Post-training ablation of abDGCs decreases forgetting of previously learned spatial information. [Google Scholar]
- 98.Gao A et al. J. Neurosci 38, 3190–3198 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leal SL & Yassa MA Nat. Neurosci 21, 163–173 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Knierim JJ & Neunuebel JP Neurobiol. Learn. Mem 129, 38–49 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deng W, Mayford M & Gage FH eLife 2, e00312 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McClelland JL & Goddard NH Hippocampus 6, 654–665 (1996). [DOI] [PubMed] [Google Scholar]
- 103.Barak O, Rigotti M & Fusi SJ Neurosci. 33, 3844–3856 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chavlis S, Petrantonakis PC & Poirazi P Hippocampus 27, 89–110 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cayco-Gajic NA & Silver RA Neuron 101, 584–602 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jung MW & McNaughton BL Hippocampus 3, 165–182 (1993). [DOI] [PubMed] [Google Scholar]
- 107.Chawla MK et al. Hippocampus 15, 579–586 (2005). [DOI] [PubMed] [Google Scholar]
- 108.Engin E et al. J. Neurosci 35, 13698–13712 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Espinoza C, Guzman SJ, Zhang X & Jonas P Nat. Commun 9, 4605 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Simultaneous octuple recordings in DG ex vivo demonstrate that lateral inhibition predominates over feedback inhibition and is primarily mediated by PV+ INs. [Google Scholar]
- 110.de Almeida L, Idiart M & Lisman JE J. Neurosci 29, 7504–7512 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jung MW, Wiener SI & McNaughton BL J. Neurosci 14, 7347–7356 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rangel LM et al. Nat. Commun 5, 3181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Freund TF & Buzsáki G Hippocampus 6, 347–470 (1996). [DOI] [PubMed] [Google Scholar]
- 114.Szabo GG et al. Cell Reports 20, 1262–1268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jinde S et al. Neuron 76, 1189–1200 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lacefield CO, Itskov V, Reardon T, Hen R & Gordon JA Hippocampus 22, 106–116 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bartos M, Vida I & Jonas P Nat. Rev. Neurosci 8, 45–56 (2007). [DOI] [PubMed] [Google Scholar]
- 118.Singer BH et al. Proc. Natl. Acad. Sci. USA 108, 5437–5442 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ikrar T et al. Front. Neural Circuits 7, 204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park EH, Burghardt NS, Dvorak D, Hen R & Fenton AA J. Neurosci 35, 11656–11666 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ewell LA & Jones MV J. Neurosci 30, 12597–12607 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu YC, Cheng JK & Lien CC J. Neurosci 34, 1344–1357 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Heigele S, Sultan S, Toni N & Bischofberger J Nat. Neurosci 19, 263–270 (2016). [DOI] [PubMed] [Google Scholar]
- 124.Ferrante M, Migliore M & Ascoli GA Proc. Natl. Acad. Sci. USA 106, 18004–18009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nitz D & McNaughton BJ Neurophysiol. 91, 863–872 (2004). [DOI] [PubMed] [Google Scholar]
- 126.Stefanelli T, Bertollini C, Lüscher C, Muller D & Mendez P Neuron 89, 1074–1085 (2016). [DOI] [PubMed] [Google Scholar]
- 127.Drew LJ et al. Hippocampus 26, 763–778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chamberland S, Evstratova A & Tóth KJ Neurosci. 37, 4913–4927 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Neubrandt M, Oláh VJ, Brunner J & Szabadics J Hippocampus 27, 1034–1039 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gan J, Weng SM, Pernia-Andrade AJ, Csicsvari J & Jonas P Neuron 93, 308–314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun Q et al. Neuron 95, 656–672.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee H, Wang C, Deshmukh SS & Knierim JJ Neuron 87, 1093–1105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Denny CA et al. Neuron 83, 189–201 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bergami M & Berninger B Dev. Neurobiol 72, 1016–1031 (2012). [DOI] [PubMed] [Google Scholar]
- 135.Sun Y et al. Neuron 92, 160–173 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sahay A, Wilson DA & Hen R Neuron 70, 582–588 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McAvoy K, Besnard A & Sahay A Front. Syst. Neurosci 9, 120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tanaka KZ et al. Neuron 84, 347–354 (2014). [DOI] [PubMed] [Google Scholar]
- 139.Wang C et al. Science 362, 945–949 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Myers CE & Scharfman HE Hippocampus 19, 321–337 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Spalding KL et al. Cell 153, 1219–1227 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eriksson PS et al. Nat. Med 4, 1313–1317 (1998). [DOI] [PubMed] [Google Scholar]
- 143.Boldrini M et al. Cell Stem Cell 22, 589–599.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moreno-Jiménez EP et al. Nat. Med 25, 554–560 (2019). [DOI] [PubMed] [Google Scholar]; Analysis of postmortem human tissue documenting DGCs across different stages of maturation in adulthood, aging and Alzheimer’s disease. [Google Scholar]
- 145.Knoth R et al. PLoS One 5, e8809 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gould E et al. Proc. Natl. Acad. Sci. USA 96, 5263–5267 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sorrells SF, et al. Nature 555, 377–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kohler SJ, Williams NI, Stanton GB, Cameron JL & Greenough WT Proc. Natl. Acad. Sci. USA 108, 10326–10331 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Strange BA, Witter MP, Lein ES & Moser EI Nat. Rev. Neurosci 15, 655–669 (2014). [DOI] [PubMed] [Google Scholar]
- 150.Anacker C & Hen R Nat. Rev. Neurosci 18, 335–346 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]