Abstract
Changes in gonadotropin-releasing hormone (GnRH) release frequency from the brain help drive reproductive cycles. In polycystic ovary syndrome (PCOS), persistent high GnRH/luteinizing hormone (LH) frequency disrupts cycles and exacerbates hyperandrogenemia. Adult prenatally-androgenized (PNA) mice exhibit increased GnRH neuron firing rate, elevated ovarian androgens, and disrupted cycles, but before puberty, GnRH neuron activity is reduced in PNA mice compared with controls. We hypothesized that ovarian feedback mediates the age-dependent change in GnRH neuron firing rate in PNA vs control mice. Extracellular recordings of green fluorescent protein (GFP)-identified GnRH neurons were made 5 to 7 days after sham-surgery, ovariectomy (OVX), or, in adults, after OVX plus replacement of sub-male androgen levels with dihydrotestosterone implants (OVX + DHT). In 3-week-old mice, OVX did not affect GnRH neuron firing rate in either group. In adult controls, OVX increased GnRH neuron firing rate, which was further enhanced by DHT. In adult PNA mice, however, OVX decreased GnRH neuron firing rate, and DHT restored firing rate to sham-operated levels. In contrast to the differential effects of ovarian feedback on GnRH neuron firing rate, serum LH increased after OVX in both control and PNA mice and was not altered by DHT. Pituitary gene expression largely reflected changes expected with OVX, although in PNA but not control mice, DHT treatment increased Lhb expression. These results suggest prenatal androgen exposure programs marked changes in GnRH neuron regulation by homeostatic steroid feedback. PNA lowers GnRH neuron activity in low-steroid states (before puberty, OVX), and renders activity in adulthood dependent upon ongoing exposure to elevated ovarian androgens.
Keywords: polycystic ovary syndrome, androgen, puberty, neuroendocrine
Gonadotropin-releasing hormone (GnRH) neurons are the central drivers of the hypothalamo-pituitary gonadal axis governing reproduction. GnRH stimulates release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary; these hormones stimulate gonadal functions, including steroidogenesis. Gonadal steroids, in turn, exert feedback control of GnRH neuron firing rate and pulsatile hormone release. Pulsatile GnRH release, and, in females, modulation of its frequency, are required for proper LH and FSH release and thus fertility (1–4). Atypical GnRH/LH pulse frequency underlies several reproductive disorders, including polycystic ovary syndrome (PCOS) (5, 6). PCOS affects up to 20% of reproductive-age women, depending on the diagnostic criteria (7). In hyperandrogenemic forms of this disorder (about half of the total population with PCOS), persistent high-frequency LH, and presumably GnRH, pulses increase androgen levels, reducing the efficacy of progesterone negative feedback and disrupting reproductive cycles (6, 8, 9). Some of these changes emerge near or during puberty (10, 11).
It is not possible to study the central neurobiological mechanisms that contribute to altered GnRH/LH pulse frequency in PCOS patients, thus prenatally-androgenized (PNA) animal models, which mirror many aspects of PCOS, have been used (12–15). Similar to women with PCOS, PNA females exhibit increased LH pulse frequency, disrupted reproductive cyclicity, and elevated androgen levels in the blood circulation (16–20). In adult PNA mice, GnRH neuron firing rate is elevated, likely in part driven by increased gamma-aminobutyric acid (GABA)-related input (21–23), which is excitatory in these cells (24, 25). Interestingly, before puberty (as determined by vaginal opening), the GnRH firing rate is lower in PNA females than in controls (26), despite a concomitant increase in GABAergic transmission to these cells at this time (27).
Steroids can have both organizational programming effects during development to program neurophysiology and activational effects in later life. It is unclear which of these mechanisms contributes to the changes in GnRH neuron firing rate and GABA transmission in prepubertal and adult PNA mice. In the present study, we tested the influence of ovarian factors on GnRH neuron firing rate in control and PNA mice before puberty and in adulthood. Our hypotheses were (1) ovarian factors contribute to increased firing in adult PNA animals, (2) androgens are the bioactive ovarian factor contributing to this increase, and (3) ovarian factors do not affect GnRH neuron firing rate in prepubertal animals.
Methods
All chemicals were acquired from Sigma-Aldrich (St. Louis, Missouri) unless noted.
Animals
All mice were given ad libitum access to water and chow (Teklad 2916, breeders received higher protein 2919 chow) (Envigo, Madison, Wisconsin). Mice were housed on a 14L:10D light cycle with time of lights on at 0300 Eastern Standard Time (EST). PNA mice were generated as described (23, 26). In brief, male C57Bl/6J mice were crossed with female mice homozygous for the expression of green fluorescent protein (GFP) under control of the GnRH promoter (Tg(Gnrh1-EGFP)51Sumo MGI:6158457; GnRH-GFP mice, JAX 033639) (28). Pregnant GnRH-GFP female mice were injected subcutaneously with 225 µg dihydrotestosterone (DHT, 5α-Androstan-17β-ol-3-one) in sesame oil on days 16 to 18 of gestation (d1=copulatory plug). Control groups included mice injected with sesame oil vehicle and uninjected dams. No difference was observed between offspring of uninjected and vehicle-treated dams and these are combined and reported as controls (2-way analysis of variance [ANOVA], for 3-week: sham/ovariectomized [OVX] F [1,37] = 0.071, P = 0.7919; dam vehicle injection/no injection F [1,37] = 1.497, P = 0.2289; interaction F [1,37] = 0.01946, P = 0.8898; for adults sham/OVX F [2,25] = 0.9152, P = 0.4134; dam vehicle injection/no injection F [1,25] = 0.4786, P = 0.4954; interaction F [2,25] = 1.776, P = 0.1900). In all breeding cages, a CD1 dam was included for maternal and nutritional support to increase survival of PNA pups; combined litters were adjusted to < 15 pups by culling CD1 pups, which are phenotypically distinct, to normalize nutrition. The Institutional Animal Care and Use Committee of the University of Michigan approved all animal procedures (PRO00006816/PRO00008797).
Surgical procedures
To study the effects of ovarian factors, mice were ovariectomized (OVX) or sham-operated. For sham surgery, the ovary was externalized and reinserted in the peritoneal cavity. Ovariectomy or sham was performed at postnatal day 14 to 16 or in adults (14–30 weeks of age) under 1.5% to 2% isoflurane anesthesia with bupivacaine applied post operatively at the end of surgery as a local analgesic. To study the effects of androgen replacement, a Silastic (Dow Corning, Midland, Michigan) capsule containing 400 μg of DHT in sesame oil was implanted in the intrascapular region of some adult mice during ovariectomy (OVX + DHT). This implant is 18 mm long with an 8 mm column of DHT in oil flanked by 5 mm Silastic adhesive plugs; the outer diameter is 3.18 mm, the inner diameter is 2.0 mm (volume of oil column is 25 µl), producing sub-male levels of androgen exposure, as previously verified by the incomplete restoration of seminal vesicle mass in castrated males (29). A sub-male level of replacement was chosen, since our goal was to model androgen levels in PCOS. These women have elevated androgens in the blood circulation compared with typical women, but the circulating levels of androgen are considerably below typical male levels.
Experimental design
Brain slices were made from 3-week-old and adult female control and PNA mice 5 to 7 days after OVX. Three-week-old mice were not weaned before experiments to avoid abrupt changes in nutrition and separation stress. Adult sham-operated mice were in diestrus on the day of recording as determined by vaginal cytology. To assess the effects of treatments on firing rate, 1-hour extracellular recordings were performed at both ages. An additional group of unoperated 3-week-old control mice was tested to determine a potential effect of sham surgery at this age. To assess the effects of treatment on pituitary gene expression, pituitaries were collected from a separate group of adult mice and stored in RNAlater (Invitrogen/Thermo Fisher, Rockford, Illinois) until RNA was extracted and gene expression determined.
Slice preparation
Solutions were bubbled with 95% O2/5% CO2 throughout the duration of experiments and at least 15 minutes prior to tissue exposure to solutions. Slices were made between 8:00 and 14:00 EST. The brain was rapidly removed and placed in ice-cold sucrose saline solution containing (in mM): 250 sucrose, 3.5 KCl, 26 NaHCO3, 10 D-glucose, 1.25 NaH2PO4, 1.2 MgSO4, and 3.8 MgCl2. Coronal (300 µm) slices were cut with a Leica VT1200S (Leica Biosystems, Buffalo Grove, Illinois). Slices were incubated in a 1:1 mixture of sucrose saline and artificial cerebrospinal fluid (ACSF) containing (in mM): 135 NaCl, 3.5 KCl, 26 NaHCO3, 10 D-glucose, 1.25 Na2HPO4, 1.2 MgSO4, 2.5 CaCl2 (pH 7.4) for 30 min at room temperature (21ºC-23ºC) and then transferred to 100% ACSF for an additional 30 minutes at room temperature before recording. Recordings were performed 1 to 5 hours after brain slice preparation; no differences in firing patterns were evident based on time after brain slice preparation or time of day. No more than 3 cells per animal and 2 cells per slice were included for analysis, and at least 4 mice from at least 3 different litters were tested per group. Variation within an animal or among littermates was not less than among all animals within a group. Cell location was mapped to an atlas (30); no differences in recording parameters or outcomes were attributable to location.
Extracellular recordings
Extracellular recordings, which have minimal impact on the cell, were conducted to monitor spontaneous action potential firing (26, 31, 32). Slices were placed in a chamber continuously perfused with ACSF (2-3 mL/min) and heated to 30ºC to 32ºC with an inline-heating unit (Warner Instruments, Hamden, Connecticut). To identify GnRH neurons, an Olympus (Center Valley, Pennsylvania) BX51WI microscope was used to briefly illuminate GFP-positive cells in the preoptic area at 488 nm. A Flaming/Brown P-97 puller (Sutter Instruments, Novato, California) was used to pull borosilicate capillary glass (type 7052, 1.65 mm outer diameter; 1.12 mm inner diameter; World Precision Instruments, Inc. Sarasota, Florida) into recording micropipettes with a resistance of 2–4 MΩ. Micropipettes used for recordings were filled with HEPES-buffered solution containing (in mM): 150 NaCl, 10 HEPES, 10 D-glucose, 2.5 CaCl2, 1.3 MgCl2, and 3.5 KCl. All recordings were conducted on 1 channel of an EPC-10 dual patch clamp amplifier using Patchmaster software (HEKA Elektronik, Pfalz, Germany) running on a Macintosh computer. Low resistance seals (<30 MΩ) were formed between the pipette and neuron after first exposing the pipette to the slice tissue in the absence of positive pressure. Recordings were made in voltage-clamp mode with a 0-mV pipette holding potential; signals were acquired at 10 kHz and filtered at 5 kHz. Recording stability and loose seal resistance were checked every 10 minutes by current response to a 5-mV hyperpolarizing voltage step. Inactive cells were treated with high-potassium ACSF (20 mM K+) at the end of an experiment. Cells that exhibited action currents in response were verified to be viable and recordable, and all data were used. For cells not responding to K+, data analysis was truncated at the last observed action current.
Analysis
Action currents (events) were detected off-line using custom programs in Igor Pro 6.31 and 7.02 (Wavemetrics, Lake Oswego, Oregon). Data were binned at 60-second intervals and were transferred to Excel. Mean firing rate (Hz) was calculated by dividing the total number of events by the duration of recording. Short-term firing patterns (bursts), which can affect neurosecretion (33), were also compared. Events were considered to be part of a burst if they occurred within 0.36 seconds of the preceding event (26). Burst frequency, duration and number of spikes/burst were compared among groups.
Assays
All assays were performed by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. LH was measured in a terminal trunk blood sample taken from adult mice at the time the brain was collected for recordings. Serum was collected after clotting at 4°C and samples were kept at −20°C until LH assay, based on a method by Steyn et al. (34). The capture monoclonal antibody (anti-bovine LH beta subunit, 518B7; RRID AB_2665514) (35) was provided by Janet Roser, University of California. The detection polyclonal antibody (rabbit LH antiserum, AFP240580Rb; RRID AB_2665533) (36) was provided by the National Hormone and Peptide Program (NHPP). HRP-conjugated polyclonal antibody (goat anti-rabbit) was purchased from DakoCytomation (Glostrup, Denmark; D048701-2). Mouse LH reference prep (AFP5306A; NHPP) was used as the assay standard. The limit of quantitation (functional sensitivity) was 0.016 ng/mL, defined as the lowest concentration that demonstrates accuracy within 20% of expected values. Intraassay coefficient of variation (%CV) was 2.2% determined by serial dilutions of a defined sample pool. All LH samples were run in the same assay.
To ascertain if ovarian testosterone levels were similar between 3-week old control and PNA mice, ovaries were removed from a separate group of control and PNA mice that had been euthanized by decapitation under isoflurane anesthesia. Extraneous tissue was removed and ovaries snap-frozen on liquid nitrogen. Ovarian issue was homogenized in a pellet pestle (BioSmasher, Kimble-Chase, Rockwood, Tennessee) in 100 µl ice-cold PBS, sonicated for 1 to 2 minutes and then centrifuged to remove cellular debris (17 × g, 8 minutes). The supernatant was collected and stored at −20°C until assayed. A portion of the supernatant was reserved to measure ovarian protein concentration (in duplicate) by bicinchoninic acid assay (Pierce/ThermoFisher, Rockford, Illinois). Ovarian testosterone was quantified in singlicate following a 1:2 dilution using the IBL ELISA (IB79106, RRID: AB_2814981) (37); Minneapolis, Minnesota). The reportable range was 10 to 1600 ng/dL and the limit of quantitation was 10 ng/dL. Intraassay variability was 6%, all samples were measured in a single assay. Ovarian testosterone levels were normalized to µg protein.
PCR to assess pituitary gene expression
To assess pituitary gene expression, pituitaries were removed from adult mice at the time of brain and serum collection, and promptly placed into 400 µl RNAlater; pituitaries were stored at 4°C for 1 week and then moved to −20°C for long-term storage. Pituitary RNA was isolated using the RNeasy Plus Micro Kit with genomic DNA elimination (Qiagen, Valencia, California). As previously described, 500 ng of RNA and a standard curve of mouse pituitary RNA (2000, 500, 125, 31.25, 7.81, and 0 ng) was reverse transcribed (38, 39). Five nanograms of cDNA was amplified for Lhb, Fshb, Cga, Gnrhr, Esr1, Ar, and Pgr and 40 ng of cDNA for Egr1 and Fst utilizing Taqman based qPCR. All assays are PrimeTime qPCR Probe Assays from Integrated DNA Technologies (Coralville, Iowa). Transcript levels were normalized to housekeeping genes Actb and Fhl1 by the ΔΔCt method (40). One outlier was identified (ROUT test) for Cga (control sham), and 3 for Egr1 (control OVX, control OVX + DHT, PNA OVX); these were removed from analysis. The number of outliers for Egr1 may reflect its high sensitivity to recent GnRH exposure (41–44).
Statistics
Statistical analyses were performed using Prism 8 (GraphPad Software, La Jolla, California). Data are reported as individual values with mean ± standard error of the mean. Data distributions were tested using Shapiro-Wilk; nonnormal data (adult firing rate because of low values in PNA OVX group) were log-transformed. Statistical tests are detailed where results are presented. ANOVA parameters are presented in tables for firing rate and PCR data; post hoc P values < 0.1 are provided in the corresponding figures. The null hypothesis was rejected if P < 0.05.
Results
Confirmation of animal models
To verify effectiveness of PNA treatment, distance from the vaginal to the anal opening was measured (average of 3 measures per day over 3 days in adult mice). Anal-genital distance was increased in adult PNA vs control mice, confirming efficacy of PNA treatment (6.0 ± 0.1 mm control [n = 27]; 8.0 ± 0.2 mm PNA [n = 22]; 2-tailed, unpaired Student t test, F = 8.995, df = 47; P < 0.001). Uterine mass in adult OVX mice was reduced (P < 0.001) compared with sham-operated mice in both control and PNA mice; DHT treatment restored uterine mass to an intermediate value in both control and PNA mice (Table 1).
Table 1.
Uterine Mass and Statistical Parameters for Comparisons (2-way ANOVA/Sidak)
| Group | n | Mass (mg) | P vs sham | ANOVA | F, df | ANOVA P |
|---|---|---|---|---|---|---|
| Control sham | 14 | 62.7 ± 5.3 | n/a | control vs PNA | (1,73) 3.31 | 0.073 |
| Control OVX | 12 | 24.2 ± 1.1 | <0.0001 | OVX/DHT | (2,73) 43.93 | <0.0001 |
| Control OVX + DHT | 12 | 44.6 ± 2.8 | 0.0094 | interaction | (2,73) 0.011 | 0.989 |
| PNA sham | 15 | 69.1 ± 6.5 | n/a | |||
| PNA OVX | 14 | 31.0 ± 2.0 | <0.0001 | |||
| PNA OVX + DHT | 12 | 50.1 ± 2.4 | 0.0058 |
Abbreviations: ANOVA, analysis of variance; DHT, dihydrotestosterone; OVX, ovariectomy; PNA, prenatally androgenized.
Ovariectomy does not alter firing rate in 3-week-old PNA mice
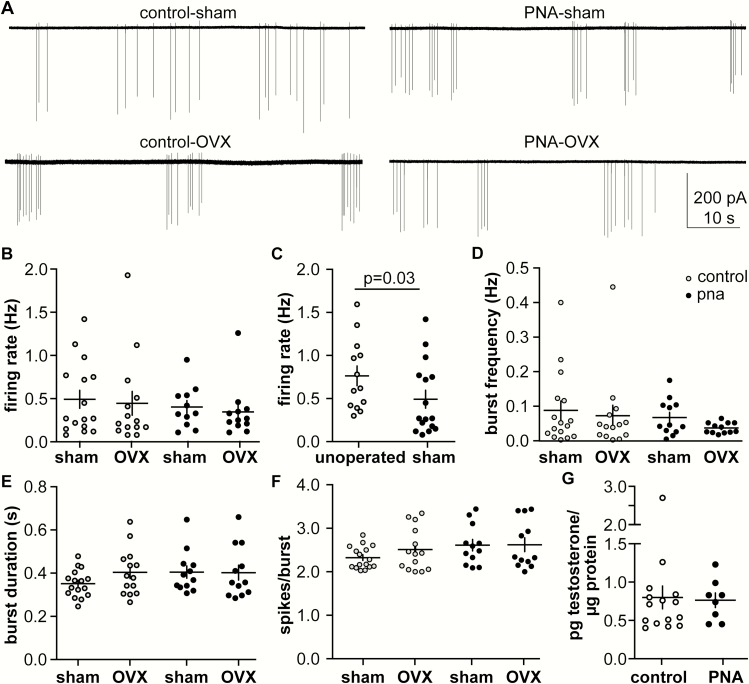
To test if gonadal factors influence firing rate in control and PNA mice before puberty, extracellular recordings were made of GnRH neurons from 3-week-old sham-operated and OVX mice. Representative recordings are shown in Fig. 1A. Ovariectomy did not affect firing rate in either control or PNA mice (Fig. 1B), (Table 2), (control sham, 16 cells from 8 mice, vs control OVX, 14 cells from 7 mice, and PNA sham, 12 cells from 6 mice, vs PNA OVX, 11 cells from 6 mice). Notably, firing rate of cells from sham-operated 3-week-old control mice was lower than reported (26) and was not different from 3-week-old PNA mice in the present study. We hypothesized that surgery at such a young age has an inhibitory influence on GnRH neuron firing rate that persists to at least 3 weeks of age. To test this, recordings were also made from nonoperated 3-week-old control mice. Firing rate was greater in mice not undergoing sham surgery (Fig. 1C), (n = 13 nonoperated, n = 16 sham-operated, 2-tailed Mann-Whitney U test, P = 0.0389, U = 57). These results indicate postoperative firing rate data in young mice must be interpreted with caution.
Figure 1.
Gonadal status does not alter firing rate of GnRH neurons in 3-week-old mice while sham surgery suppresses it. (A.) Representative traces from control (left) and PNA (right), with sham-operated on top and OVX on bottom. (B-G). Individual values and mean ± SEM for (B) firing rate, (C) firing rate in unoperated control mice compared with cells from the sham-operated controls in B, (D) burst frequency, (E) burst duration, (F) spikes/burst, (G) ovarian testosterone.
Table 2.
Two-Way ANOVA Parameters for Extracellular Recordings in 3-Week-Old Mice
| Comparison (Figure) | Control vs PNA | Sham vs OVX | Interaction |
|---|---|---|---|
| Firing rate, Hz (1B) | F (1,50) = 0.39, P = 0.5346 | F (1,50) = 0.50, P = 0.4818 | F (1,50) = 0.073, P = 0.7880 |
| Burst frequency, Hz (1D) | F (1,50) = 1.438, P = 0.2361 | F (1,50) = 0.9277, P = 0.3401 | F (1,50) = 0.0971, P = 0.7566 |
| Burst duration, s (1E) | F (1,50) = 0.9481, P = 0.3349 | F (1,50) = 0.8547, P = 0.3597 | F (1,50) = 1.045, P = 0.3166 |
| Spikes/burst (1F) | F (1,50) = 2.657, P = 0.1094 | F (1,50) = 0.6524, P = 0.4231 | F (1,50) = 0.5294, P = 0.4703 |
Abbreviations: ANOVA, analysis of variance; OVX, ovariectomy; PNA, prenatally androgenized.
We also examined short-term firing activity, also known as bursting activity, which is related to peptide neurosecretion (33, 45), an aspect postulated to be increased in both women with PCOS and PNA in models. Consistent with the lack of change in firing rate, burst parameters were also not different among groups in 3-week-old mice (Fig. 1D, 1E, and 1F), (Table 2).
Ovarian testosterone levels are not different between 3-week-old control and PNA mice
Although OVX did not alter GnRH neuron firing rate before puberty, the effect of surgery indicated the need to quantify a second parameter before an effect of ovarian factors on GnRH neuron firing rate could be rejected. Circulating testosterone levels in female mice, even in adulthood, are at or below the limit of detection of validated assays. We thus quantified ovarian testosterone content at 3 weeks of age in controls and PNA mice to estimate if a difference in circulating testosterone may exist. There was no difference in ovarian testosterone content (normalized to protein) between groups (Fig. 1G), (control n = 13, PNA n = 8; P = 0.16, 2-tailed, unpaired Student t-test, t = 1.477, df = 19).
Ovariectomy has different effects on firing rate of GnRH neurons from adult control vs PNA mice
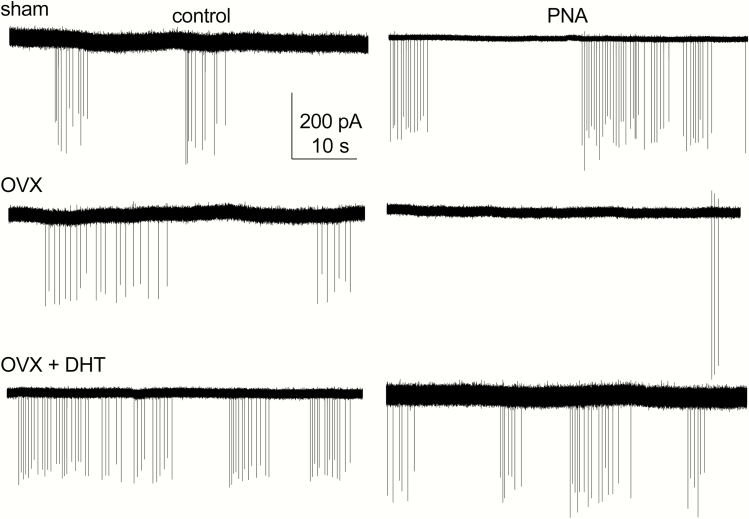
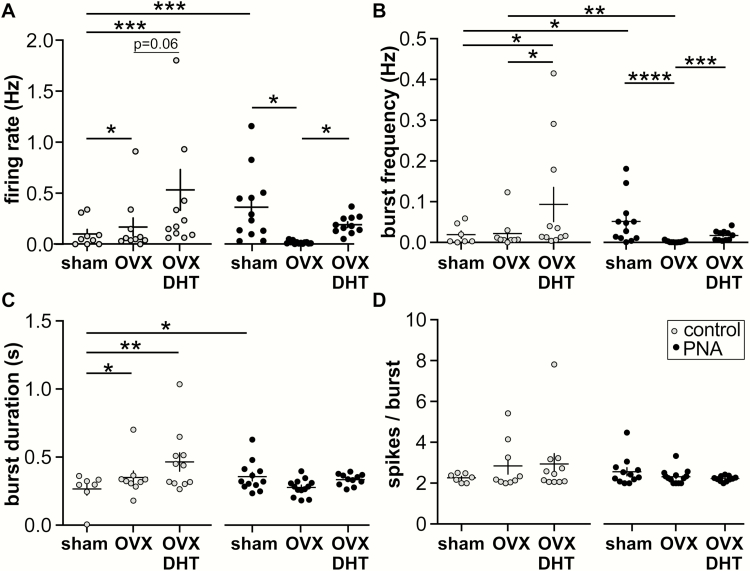
Ovarian feedback to the reproductive neuroendocrine circuitry matures during the pubertal process. To test whether ovarian factors contribute to the increased GnRH neuron firing rate in adult control vs PNA mice, extracellular recordings were made of action potential firing from sham-operated and OVX mice. Representative traces are shown in Fig. 2, summary data in Fig. 3A, and ANOVA parameters in Table 3. As reported (21, 26), cells from sham-operated PNA mice had a higher firing rate than cells from sham-operated controls (2-way ANOVA/Fishers, P < 0.0003). In control mice, OVX mildly increased firing rate, consistent with removal of steroid negative feedback (P = 0.0426, control sham 9 cells from 7 mice, control OVX 10 cells from 7 mice). In marked contrast, OVX reduced firing rate of GnRH neurons from PNA mice (P = 0.0107, PNA sham PNA 12 cells from 7 mice, PNA OVX 13 cells from 8 mice). These observations suggest that signals from the ovary drive increased GnRH neuron firing rate in PNA mice, and point to dysfunctional homeostatic feedback in these mice.
Figure 2.
Gonadal status and androgens affect long-term firing rate of GnRH neurons from both control (left) and PNA (right) adult mice. Representative traces from sham-operated (top), OVX (middle) and OVX + DHT (bottom).
Figure 3.
Effect of gonadal status and androgens on long-term and short-term (burst) firing properties of GnRH neurons from adult female mice. Individual values and mean ± SEM for (A) firing rate, (B) burst frequency, (C) burst duration, and (D) spikes/burst. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, Fisher’s LSD post hoc tests as indicated by horizontal bars. No other post hoc comparisons achieved P < 0.1. Two-way ANOVA F and P values can be found in Table 2.
Table 3.
Two-Way ANOVA Parameters for Extracellular Recordings in 3-Week-Old Mice. For Comparisons With Significant ANOVA, post hoc Test Results Are Provided in Fig. 3
| Comparison (Figure) | Control vs PNA | Sham vs OVX vs OVX + DHT | Interaction |
|---|---|---|---|
| Firing rate (Hz) (3A) | F (1,61) = 2.80, P = 0.1009 | F (2,61) = 5.60, P = 0.0058 | F (2,61) = 6.60, P = 0.0024 |
| Burst freq (Hz) (3B) | F (1,57) = 1.205, P = 0.2770 | F (2,57) = 9.196, P = 0.0003 | F (2,57) = 6.048, P = 0.0042 |
| Burst duration (s) (3C) | F (1,57) = 0.3117, P = 0.5788 | F (2,57) = 2.905, P = 0.0629 | F (2,57) = 4.049, P = 0.0227 |
| Spikes/burst (3D) | F (1,57) = 1.578, P = 0.2142 | F (2,57) = 0.1547, P = 0.8570 | F (2,57) = 1.969, P = 0.1490 |
Abbreviations: ANOVA, analysis of variance; DHT, dihydrotestosterone; OVX, ovariectomy; PNA, prenatally androgenized.
Hyperandrogenemia is common in women with PCOS and likely is a factor contributing to increased GnRH/LH pulse frequency (46). We thus hypothesized that replacement of androgen would increase GnRH neuron firing rate in control mice and would restore the elevated baseline firing rate in PNA mice. To test this, we used DHT implants that provide a level of androgen action that does not restore seminal vesicle mass in castrated males, thus is lower than in typical males (29); this mimics the mild ovarian androgen elevation observed in women with PCOS. Both of these hypotheses were supported. In control mice, OVX + DHT (12 cells from 7 mice) increased firing rate relative to sham-operated controls (P = 0.0002) and approached the level set for significance for an increase when compared with OVX controls (P = 0.0613). In cells from OVX + DHT PNA mice (11 cells from 6 mice), firing rates were elevated relative to OVX PNA (P = 0.0222), but were not different those in sham-operated PNA mice (P = 0.8247). Together these data suggest that androgens are the primary factor from the ovary contributing to increased firing in PNA mice, and further support that circulating androgens elevate GnRH neuron firing in female control mice (23, 47, 48).
Short-term firing patterns are altered by gonadal state in both control and PNA adult mice
Burst analysis in adults demonstrated an interaction between prenatal treatment and gonadal status/DHT treatment (2-way ANOVA/Fisher’s, ANOVA statistics Table 3). Both burst frequency (P = 0.0441) and duration (P = 0.0135) were greater in cells from sham-operated control vs PNA mice (Fig. 3B and 3C). In control mice, OVX alone had no effect on burst frequency, but DHT increased burst frequency relative to cells from sham-operated (P = 0.0201) or OVX-only (P = 0.0332) mice. Burst duration was increased relative to sham-operated controls by either OVX (P = 0.0270) or OVX + DHT (P = 0.0017) treatment. Consistent with the lower firing rate in cells from PNA OVX vs sham-operated PNA mice, burst frequency was also lower, but was restored by DHT treatment. No treatment affected burst duration in PNA mice (all P > 0.31) and no treatment studied altered the number of spikes per burst in either control or PNA mice (Fig. 3D, all P > 0.15).
Ovariectomy increases serum LH in both control and PNA mice
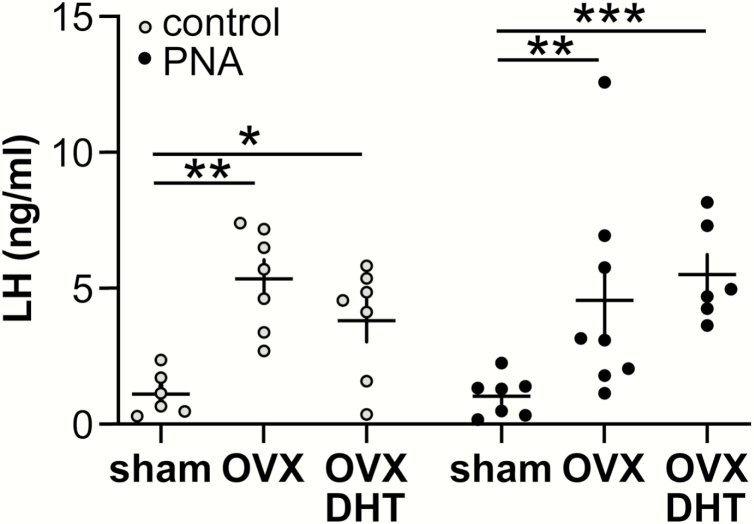
To test the overall output of the hypothalamo-pituitary unit, serum LH was assessed in trunk blood collected at the time of brain slice preparation (Fig. 4). Interestingly, serum LH responded differently to gonad and DHT manipulation than GnRH activity (2-way ANOVA/Fisher’s). In both control and PNA mice, OVX increased LH levels (P < 0.01) and DHT replacement did not affect post-OVX levels. These data suggest feedback in PNA mice produces different effects at the pituitary vs GnRH neurons.
Figure 4.
Serum LH values from adult mice used for extracellular recordings. Two-way ANOVA/Fisher’s: control vs PNA F (1,35) = 0.161, P = 0.6906; OVX/DHT F (2,35) = 12.87, P < 0.0001; interaction F (2,35) = 1.149, P = 0.3286. *P < 0.05, **P < 0.01, *** P < 0.01.
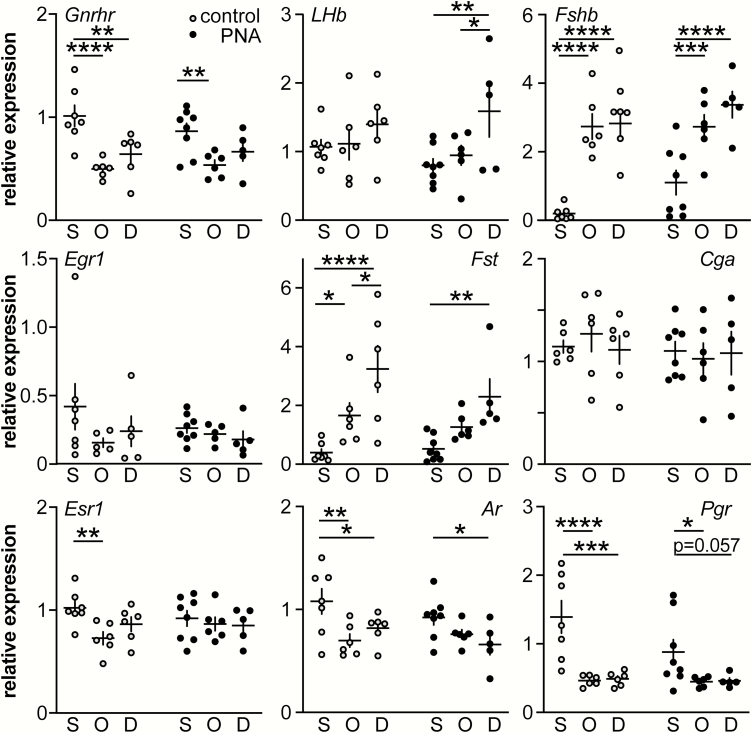
PNA treatment, gonadal status and DHT alter expression of pituitary genes
To begin to determine if altered pituitary gene expression could help explain the differential effects of ovariectomy at the hypothalamic and pituitary levels in control vs PNA mice, we measured expression levels of Gnrhr, Lhb, Fshb, Egr1, Fst, Cga, Esr1, Ar, and Pgr in pituitaries from adult control and PNA mice (Fig. 5), (Tables 4 and 5). For Gnrhr, Fshb, Fst, Esr1, Ar, and Pgr, OVX altered expression as reported (increasing Fshb, Fst; decreasing Gnrhr, Esr1, Ar, Pgr) (49–56). There were no changes in Lhb in control mice, but in PNA mice, DHT treatment increased expression of this transcript. The effect of prenatal androgen treatment approached the value accepted for significance (ANOVA P = 0.08) for increasing Fshb, and both control and PNA had similar increases in Fshb after OVX regardless of DHT. Neither Egr1 nor Cga expression were altered by any treatment.
Figure 5.
Pituitary gene expression in control and PNA adult mice. Normalized (to the average of Actb and Fhl1) relative expression of selected pituitary genes. S, sham; O, OVX; D, OVX + DHT. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, Fisher’s LSD post hoc tests. No other post hoc comparisons achieved P < 0.1. Two-way ANOVA F and P values can be found in Table 5.
Table 4.
Primers and IDT PrimeTime qPCRAssay ID Used to Quantify Expression of Pituitary Genes
| mRNA | Accession# | PrimeTime qPCRAssay ID | FWD | REV | Taqman Probe |
|---|---|---|---|---|---|
| Ar | NM_013476 | Mm.PT.47.17416675 | 5’CTGCCTTGTTATCTAGCCTCA3’ | 5’ATACTGAATGACCGCCATCTG3’ | 5’ACCACATGCACAAGCTGCCTCT3’ |
| Lhb | NM_008497 | Mm.PT.45.5612498 | 5’CCAGTCTGCATCACCTTCAC3’ | 5’GAGGCACAGGAGGCAAAG3’ | 5’AGTACTCGGACCATGCTAGGACAGT3’ |
| Fshb | NM_008045 | Mm.PT.45.17694677 | 5’TTCAGCTTTCCCCAGAAGAG3’ | 5’TCCAGCACCAGAATAAGATGC3’ | 5’AGCTACGTCCTGTGCAGTCAGC3’ |
| Cga | NM_009889 | Mm.PT.58.31855537 | 5’CCTCAGATCGACAATCACCTG3’ | 5’AGCATGACCAGAATGACAGC3’ | 5’CCCTCAAAAAGTCCAGAGCTTGCAGA3’ |
| Fst | NM_008046 | Mm.PT.45.6344184 | 5’GCATTCTGGATCTTGCAACTC3’ | 5’GGAAGAGATAGGAAAGCTGTAGTC3’ | 5’CGGAAGAAACGGAGGAAGAGGAGGA3’ |
| Gnrhr | NM_010323 | Mm.PT.45.16240237 | 5’TCAGCATTGTCTTTGCAGGA3’ | 5’TCACACATTGCGAGAAGACTG3’ | 5’TGATCTACCTAGCAGACGGCTCTGG3’ |
| Actb | NM_007393 | Mm.PT.39a.22214843.g | 5’GATTACTGCTCTGGCTCCTAG3’ | 5’GACTCATCGTACTCCTGCTTG3’ | 5’CTGGCCTCACTGTCCACCTTCC3’ |
| Egr1 | NM_007913 | Mm.PT.45.13313108 | 5’AGCGCCTTCAATCCTCAAG3’ | 5’GATAACTCGTCTCCACCATCG3’ | 5’ATGAGCACCTGACCACAGAGTCC3’ |
| Esr1 | NM_007956 | Mm.PT.47.16003033 | 5’CCTGTTTGCTCCTAACTTGCT3’ | 5’GAACCGACTTGACGTAGCC3’ | 5’TGCCTTCCACACATTTACCTTGATTCCT3’ |
| Fhl1 | NM_001077361 | Mm.PT.58.9685318 | 5’GTAGCGATTCTTATAATGCACCTC3’ | 5’GTCACTGCTGCCTGAAGT3’ | 5’TTGACAAGTTCTGCGCCAACACC3’ |
| Pgr | NM_008829 | Mm.PT.47.10254276 | 5’CGCCATACCTTAACTACCTGAG3’ | 5’CCATAGTGACAGCCAGATGC3’ | 5’AGATTCAGAAGCCAGCCAGAGCC3’ |
Table 5.
Two-Way Analysis of Variance (ANOVA) Parameters for Gene Expression Studies. For Comparisons With Significant ANOVA, post hoc Test Results Are Provided in Fig. 5
| Comparison (Gene) | Control vs PNA | Sham vs OVX vs OVX + DHT | Interaction |
|---|---|---|---|
| Actb | F (1,32) = 2.27 P = 0.141 | F (2,32) = 2.12 P = 0.136 | F (2,32) = 0.95 P = 0.398 |
| Fhl1 | F (1,32) = 2.27 P = 0.141 | F (2,32) = 2.12 P = 0.136 | F (2,32) = 0.95 P = 0.398 |
| Lhb | F (1,32) = 0.28 P = 0.601 | F (2,32) = 4.64 P = 0.017 | F (2,32) = 0.76 P = 0.475 |
| Fshb | F (1,32) = 3.19 P = 0.083 | F (2,32) = 34.44, P < 0.0001 | F (2,32) = 1.02 P = 0.373 |
| Cga | F (1,31) = 0.09 P = 0.358 | F (2,31) = 0.06 P = 0.938 | F (2,31) = 0.69 P = 0.372 |
| Gnrhr | F (1,32) = 0.02 P = 0.658 | F(2.32) = 15.54 P < 0.0001 | F (2,32) = 0.92 P = 0.408 |
| Egr1 | F (1,29) = 0.39 P = 0.537 | F (2,29) = 1.57 P = 0.225 | F (2,29) = 0.66 P = 0.525 |
| Fst | F (1,32) = 1.42 P = 0.243 | F (2,32) = 15.50 P < 0.0001 | F (2,32) = 0.44 P = 0.850 |
| Esr1 | F (1,32) = 0.02 P = 0.887 | F(2.32) = 3.39 P = 0.046 | F (2,32) = 1.56 P = 0.226 |
| Pgr | F (1,32) = 2.38 P = 0.133 | F (2,32) = 15.07 P < 0.0001 | F (2,32) = 2.01 P = 0.151 |
| Ar | F (1,32) = 1.50 P = 0.230 | F (2.32) = 7.33 P = 0.002 | F (2.32) = 1.12 P = 0.337 |
Discussion
Hyperandrogenemia is a common facet in many women with PCOS and can alter the feedback efficacy of other steroids (9). Here we demonstrate that prenatal androgen exposure, a model commonly used to mimic several characteristics of PCOS (16–20), has marked effects on the typical homeostatic operation of the hypothalamo-pituitary-ovarian axis. Ovarian androgens in adult PNA mice support the ongoing elevated firing rate of GnRH neurons and their removal with ovariectomy essentially silences these cells. Remarkably, ovarian regulation of GnRH neuron firing rate and serum LH are in opposite directions in adult PNA mice, with LH exhibiting the typical post-ovariectomy rise.
These studies support previous work that demonstrated elevated androgens can upregulate adult reproductive neuroendocrine output in PCOS patients (5) and in adult PNA models (23, 57). Mild androgen elevations occurring naturally in women with PCOS (58) and disrupt the efficacy of progesterone negative feedback to slow LH pulse frequency (9). Similar mild elevations of circulating androgens achieved in animal models have comparable activational effects (23, 59, 60). For example, impaired progesterone negative feedback has also been observed in PNA sheep (61) and rats (19), suggesting early exposure to androgens can disrupt later ovarian feedback. In young rhesus monkeys, mild androgen elevation increases LH pulse frequency (60); similarly, sub-male levels of androgen in combination with estradiol increased GnRH neuron firing rate in mice (48) and increased excitatory GABAergic drive to these cells (47). Postnatal treatment of rodents with the aromatase inhibitor letrozole also elevates androgens and LH levels and reduces progesterone receptor mRNA levels in the hypothalamus (62). Prenatal anti-Müllerian hormone (AMH) models were developed based on observations that AMH is increased in women with PCOS during the second trimester of pregnancy. Injection of AMH on days 16 to 18 of mouse pregnancy, the same days DHT was administered in the present PNA model, also results in disrupted cycles, increased LH pulse frequency, and elevated testosterone in adulthood (63). In both prenatal AMH and PNA mice, GABAergic input sites and spine density of GnRH neurons are increased, as is GABAergic transmission to GnRH neurons in the latter (22, 23, 63). Mild androgen elevation in the blood circulation is the common denominator among these diverse models and is consistently associated with increased reproductive neuroendocrine activity. In the present study, we chose DHT as our adult steroid manipulation with the goal of affecting primarily androgen receptors. We cannot exclude the possibility, however, that DHT is converted to diols that may signal specifically via the beta isoform of the estrogen receptor (64). Such action could also potentially account for the increase in uterine mass as the uterus expresses various estrogen receptor beta variants (65).
The present work extends the above findings by demonstrating that PNA treatment programs changes in the reproductive neuroendocrine system that markedly disrupt the typical operation of homoeostatic feedback at the central level. Specifically, in adult control mice ovariectomy resulted in the expected increase in GnRH neuron firing rate, due to removal of ovarian negative feedback. In marked contrast, in ovariectomized PNA mice, GnRH neuron firing rate plummeted. This suggests the postulate that PNA treatment programs the reproductive neuroendocrine system so that GnRH neuron function in the adults becomes dependent upon ongoing ovarian androgen exposure. This postulate was at least in part supported by the restoration of firing rate in ovariectomized PNA mice treated with DHT. Of interest, DHT also tended to increase firing rate in control mice. Together, present and past findings strongly indicate that mildly increased androgens in females upregulates reproductive neuroendocrine output and suggest similar changes may underlie phenotypes commonly observed in PCOS. These studies also compliment clinical studies in PCOS patients that have identified a role for the ovary. For example, ovarian surgeries such as wedge resection, ovarian drilling and electrocautery have been used to improve fertility outcomes in PCOS patients. In most studies, such surgeries were found to lower LH and testosterone levels (66), but not in all cases (67). Further, treatment with the androgen receptor blocker flutamide reduced clinical signs of androgen action and restored cycles in young women with PCOS (68). Similarly, flutamide restores cycles in PNA mice and ameliorates some effects of PNA treatment in sheep, suggesting circulating androgens are also active players in these animal models (23,57,69)
Examination of LH levels in these same mice on the day brain slices were prepared for recording revealed a differential regulation of the hypothalamus and pituitary gland. In control mice, the expected elevation of LH was observed following ovariectomy. In contrast to the inhibitory effects of removing the ovaries on GnRH neuron firing rate in PNA adults, LH levels rose after ovariectomy just as in controls. A similar discrepancy between activation in the hypothalamus and LH release at the pituitary gland in PNA mice was observed in a recent study. Optogenetic stimulation of arcuate GABA neurons that are upstream of GnRH neurons produced a smaller LH release in PNA than control females (70). Several possible explanations may underlie these observations in PNA mice (Fig. 6). First, negative feedback actions of other ovarian steroids may be more pronounced at the pituitary than the hypothalamus in PNA mice. This is consistent with the above-mentioned studies demonstrating that elevated androgens disrupt negative feedback at the hypothalamus. Second, pituitary response to GnRH may be increased in PNA mice as it is in other PNA models and in women with PCOS (18-20, 71). Third, the relationship between GnRH neuron firing activity and GnRH release could be altered by PNA, so that relatively little firing activity produces increased GnRH release. Fourth, PNA and/or ovariectomy may alter substances, such as gonadotropin-inhibitory hormone, pituitary adenylate-cyclase activating polypeptide (PACAP), or dopamine (either directly or via changes in prolactin), that can alter LH release independent of GnRH (72–76).
Figure 6.
Model depicting known and unknown differences in homeostatic feedback between control and PNA mice. Left, in 3-week-old mice, prenatal androgen exposure programs GnRH neurons, lowering activity; circulating steroids are low. Middle, in diestrous adults, ovarian androgens have a negative feedback effect on upstream components of the reproductive axis in controls (top), but in PNA mice (bottom) ovarian androgens feed forward to increase GnRH release. Ovarian feedback at the pituitary is negative. Right, removal of ovarian factors eliminates activational androgen drive to GnRH neurons and removes negative feedback from the pituitary, allowing LH to rise despite reduced GnRH neuron activity. Far right, addition of DHT is activating at the central level in both control and PNA mice but has no feedback action at the pituitary.
To begin to address changes at the pituitary, we examined expression of several pituitary genes. Although Pgr expression is reduced in the medial basal hypothalamus in both PNA and letrozole models of PCOS (19, 62, 77), PNA treatment had no effect on Pgr expression in the pituitary of sham-operated controls in the present study; this is similar to results in the letrozole model and suggests differential regulation of this gene at the pituitary and hypothalamus (62, 77). Ovariectomy had the expected effects on expression of genes examined (49, 50) and DHT treatment did not have effects other than to elevate Lhb exclusively in PNA mice. Of interest, both Lhb and Gnrhr expression were increased in pituitaries from letrozole-treated mice but were not different in the present study between sham-operated control and PNA mice; further in letrozole-treated mice, Fshb was reduced or unchanged (62, 77), whereas it approached an increase in the present study. The trend towards increased pituitary Fshb in sham-operated PNA mice is consistent with previous reports of the direct effects of androgens to augment or maintain Fshb in other rodent models (78–80). Both of these models recapitulate many aspects of PCOS thus these differences suggest latent mechanisms may exist that result in similar phenotypic endpoints in these mouse models and may contribute to phenotypic diversity in women with PCOS. Unfortunately, the present gene expression studies did not add to our understanding of why the response of the hypothalamic and pituitary components of the axis in adult PNA mice differ.
In contrast to adults, ovariectomy had no effect on firing rate of GnRH neurons from either control or PNA mice at 3 weeks of age. We urge caution in the interpretation of these results as sham surgery appears to have an inhibitory effect on GnRH neuron firing rate at this age. Androgen levels in the blood circulation of adult female mice are typically at or below the level of functional sensitivity in reliable immunoassays; these levels were expected to be even lower before puberty. Androgens, which are lipophilic, are not stored in vesicles for regulated secretion, thus ovarian levels should correlate with serum levels (81–83). There was no difference between control and PNA mice in ovarian testosterone content at 3 weeks of age, suggesting there are unlikely to be differential activational effects of ovarian androgens at this age. The low steroid levels and this lack of difference support the lack of an effect of ovariectomy on GnRH neuron activity before puberty. These results do not preclude other mechanisms from contributing to developmental changes in androgen action, such as altered androgen receptor expression or signaling (84) or altered central synthesis of androgens (85).
In addition to overall mean firing rate, short-term firing changes have implications for hormone release. For example, the duration and number of spikes in a burst is often correlated with a greater neuropeptide release (86). In the present study, changes in burst firing largely paralleled those in overall firing rate, adding further support to androgen alteration of neuroendocrine output. In cells from control mice, burst duration was shorter for sham-operated mice than for OVX or OVX + DHT. Sham-operated PNA mice also exhibited longer bursts than sham-operated controls. Together these results suggest that androgens in female mice increase burst length in GnRH neurons. This is in contrast to a recent study in orchidectomized male mice in which removal of gonadal factors resulted in increased burst length in arcuate kisspeptin neurons (87). Increased burst length could potentially generate a longer elevation in intracellular calcium and thus greater neuropeptide release (86). Androgen replacement reduced firing rate of arcuate kisspeptin neurons (87), but not GnRH neurons for which estradiol provided the primary negative feedback (29). Notably, the androgen replacement used in that study was male level as assessed by ability to restore seminal vesicle mass following orchidectomy. Male levels of androgens are not observed in PCOS patients, which led us to utilize a sub-male elevation of androgen in the present work. Mild increases in androgen action achieved as a consequence of PNA treatment or via low-level DHT replacement in OVX mice increased burst duration, which could potentially increase hormone release.
Disrupted neuroendocrine function and hyperandrogenemia emerge near the pubertal transition and may contribute to the foundations of increased reproductive neuroendocrine drive in adult PCOS. While no animal model perfectly replicates human disease, the present studies suggest that prenatal androgen exposure has programming (organizational) effects that markedly alter how the central aspects of the hypothalamo-pituitary gonadal axis respond to the activational effects of steroids in adulthood in terms of homeostatic feedback. This work adds to our understanding of feedback interactions within this axis and how they change with development and androgen exposure, and points to additional mechanisms that may be subject to regulation including excitation-secretion coupling in GnRH neurons, about which almost nothing is known.
Acknowledgments
We thank R. Anthony DeFazio and Elizabeth R. Wagenmaker for expert technical assistance.
Financial Support: Supported by National Institute of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development P50 HD28934 (Project III and the Ligand Assay and Analysis Core) and an NSF Graduate Research Program Fellowship and University of Michigan EDGE award to EAD.
Glossary
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AMH
anti-Müllerian hormone
- ANOVA
analysis of variance
- DHT
dihydrotestosterone
- FSH
follicle-stimulating hormone
- GABA
gamma-aminobutyric acid
- GFP
green fluorescent protein
- GnRH
gonadotropin-releasing hormone
- LH
luteinizing hormone
- OVX
ovariectomy
- PCOS
polycystic ovary syndrome
- PNA
prenatally androgenized
Additional Information
Disclosure Summary: The authors have nothing to disclose.
References
- 1. Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111(5):1737–1739. [DOI] [PubMed] [Google Scholar]
- 2. Moenter SM, Brand RM, Midgley AR, Karsch FJ. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology. 1992;130(1):503–510. [DOI] [PubMed] [Google Scholar]
- 3. Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202(4368):631–633. [DOI] [PubMed] [Google Scholar]
- 4. Wildt L, Häusler A, Marshall G, et al. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109(2):376–385. [DOI] [PubMed] [Google Scholar]
- 5. McCartney CR, Eagleson CA, Marshall JC. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med. 2002;20(4):317–326. [DOI] [PubMed] [Google Scholar]
- 6. Marshall JC, Eagleson CA, McCartney CR. Hypothalamic dysfunction. Mol Cell Endocrinol. 2001;183(1-2):29–32. [DOI] [PubMed] [Google Scholar]
- 7. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. [DOI] [PubMed] [Google Scholar]
- 8. Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998;83(2):582–590. [DOI] [PubMed] [Google Scholar]
- 9. Eagleson CA, Gingrich MB, Pastor CL, et al. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85(11):4047–4052. [DOI] [PubMed] [Google Scholar]
- 10. Burt Solorzano CM, McCartney CR, Blank SK, Knudsen KL, Marshall JC. Hyperandrogenaemia in adolescent girls: origins of abnormal gonadotropin-releasing hormone secretion. Bjog. 2010;117(2):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoo RY, Dewan A, Basu R, Newfield R, Gottschalk M, Chang RJ. Increased luteinizing hormone pulse frequency in obese oligomenorrheic girls with no evidence of hyperandrogenism. Fertil Steril. 2006;85(4):1049–1056. [DOI] [PubMed] [Google Scholar]
- 12. Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11(4):357–374. [DOI] [PubMed] [Google Scholar]
- 13. Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013;78(8):734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moore AM, Prescott M, Campbell RE. Estradiol negative and positive feedback in a prenatal androgen-induced mouse model of polycystic ovarian syndrome. Endocrinology. 2013;154(2):796–806. [DOI] [PubMed] [Google Scholar]
- 15. Roland AV, Moenter SM. Reproductive neuroendocrine dysfunction in polycystic ovary syndrome: insight from animal models. Front Neuroendocrinol. 2014;35(4):494–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW. Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab. 1998;9(2):62–67. [DOI] [PubMed] [Google Scholar]
- 17. Roland AV, Nunemaker CS, Keller SR, Moenter SM. Prenatal androgen exposure programs metabolic dysfunction in female mice. J Endocrinol. 2010;207(2):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373(1-2):8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72(6):1475–1483. [DOI] [PubMed] [Google Scholar]
- 20. Dumesic DA, Abbott DH, Eisner JR, Goy RW. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997;67(1):155–163. [DOI] [PubMed] [Google Scholar]
- 21. Roland AV, Moenter SM. Prenatal androgenization of female mice programs an increase in firing activity of gonadotropin-releasing hormone (GnRH) neurons that is reversed by metformin treatment in adulthood. Endocrinology. 2011;152(2):618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moore AM, Prescott M, Marshall CJ, Yip SH, Campbell RE. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci U S A. 2015;112(2):596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci U S A. 2004;101(18):7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type gamma-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16(12):2872–2891. [DOI] [PubMed] [Google Scholar]
- 25. Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol. 2011;23(7):557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dulka EA, Moenter SM. Prepubertal development of gonadotropin-releasing hormone neuron activity is altered by sex, age, and prenatal androgen exposure. Endocrinology. 2017;158(11):3943–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berg T, Silveira MA, Moenter SM. Prepubertal development of GABAergic transmission to Gonadotropin-Releasing Hormone (GnRH) neurons and postsynaptic response are altered by prenatal androgenization. J Neurosci. 2018;38(9):2283–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suter KJ, Song WJ, Sampson TL, et al. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141(1):412–419. [DOI] [PubMed] [Google Scholar]
- 29. Pielecka J, Moenter SM. Effect of steroid milieu on gonadotropin-releasing hormone-1 neuron firing pattern and luteinizing hormone levels in male mice. Biol Reprod. 2006;74(5):931–937. [DOI] [PubMed] [Google Scholar]
- 30. Sidman RL, Angevine JB, Pierce ET.. Atlas of the Mouse Brain and Spinal Cord. Cambridge, MA: Harvard University Press. [Google Scholar]
- 31. Nunemaker CS, DeFazio RA, Moenter SM. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proced Online. 2003;5:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alcami P, Franconville R, Llano I, Marty A. Measuring the firing rate of high-resistance neurons with cell-attached recording. J Neurosci. 2012;32(9):3118–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dutton A, Dyball RE. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979;290(2):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. RRID:AB_2665514, https://scicrunch.org/resolver/AB_2665514.
- 36. RRID:AB_2665533, https://scicrunch.org/resolver/AB_2665533.
- 37. RRID:AB_2814981, https://scicrunch.org/resolver/AB_2814981.
- 38. Glanowska KM, Burger LL, Moenter SM. Development of gonadotropin-releasing hormone secretion and pituitary response. J Neurosci. 2014;34(45):15060–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ruka KA, Burger LL, Moenter SM. Both estrogen and androgen modify the response to activation of Neurokinin-3 and κ-Opioid receptors in arcuate kisspeptin neurons from male mice. Endocrinology. 2016;157(2):752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29(1):23–39. [DOI] [PubMed] [Google Scholar]
- 41. Kowase T, Walsh HE, Darling DS, Shupnik MA. Estrogen enhances gonadotropin-releasing hormone-stimulated transcription of the luteinizing hormone subunit promoters via altered expression of stimulatory and suppressive transcription factors. Endocrinology. 2007;148(12):6083–6091. [DOI] [PubMed] [Google Scholar]
- 42. Burger LL, Haisenleder DJ, Aylor KW, Marshall JC. Regulation of Lhb and Egr1 gene expression by GNRH pulses in rat pituitaries is both c-Jun N-terminal kinase (JNK)- and extracellular signal-regulated kinase (ERK)-dependent. Biol Reprod. 2009;81(6):1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Halvorson LM, Kaiser UB, Chin WW. The protein kinase C system acts through the early growth response protein 1 to increase LHbeta gene expression in synergy with steroidogenic factor-1. Mol Endocrinol. 1999;13(1):106–116. [DOI] [PubMed] [Google Scholar]
- 44. Lawson MA, Tsutsumi R, Zhang H, et al. Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol. 2007;21(5):1175–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985;369:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Livadas S, Pappas C, Karachalios A, et al. Prevalence and impact of hyperandrogenemia in 1,218 women with polycystic ovary syndrome. Endocrine. 2014;47(2):631–638. [DOI] [PubMed] [Google Scholar]
- 47. Sullivan SD, Moenter SM. GABAergic integration of progesterone and androgen feedback to gonadotropin-releasing hormone neurons. Biol Reprod. 2005;72(1):33–41. [DOI] [PubMed] [Google Scholar]
- 48. Pielecka J, Quaynor SD, Moenter SM. Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology. 2006;147(3):1474–1479. [DOI] [PubMed] [Google Scholar]
- 49. Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol. 2004;33(3):559–584. [DOI] [PubMed] [Google Scholar]
- 50. Thompson IR, Kaiser UB. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Mol Cell Endocrinol. 2014;385(1-2):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dalkin AC, Knight CD, Shupnik MA, et al. Ovariectomy and inhibin immunoneutralization acutely increase follicle-stimulating hormone-beta messenger ribonucleic acid concentrations: evidence for a nontranscriptional mechanism. Endocrinology. 1993;132(3):1297–1304. [DOI] [PubMed] [Google Scholar]
- 52. Dalkin AC, Haisenleder DJ, Gilrain JT, Aylor K, Yasin M, Marshall JC. Regulation of pituitary follistatin and inhibin/activin subunit messenger ribonucleic acids (mRNAs) in male and female rats: evidence for inhibin regulation of follistatin mRNA in females. Endocrinology. 1998;139(6):2818–2823. [DOI] [PubMed] [Google Scholar]
- 53. Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17(6):1039–1053. [DOI] [PubMed] [Google Scholar]
- 54. Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Regulation of rat pituitary gonadotropin-releasing hormone receptor mRNA levels in vivo and in vitro. Endocrinology. 1993;133(2):931–934. [DOI] [PubMed] [Google Scholar]
- 55. Sánchez-Criado JE, Martín De Las Mulas J, Bellido C, Tena-Sempere M, Aguilar R, Blanco A. Biological role of pituitary estrogen receptors ERalpha and ERbeta on progesterone receptor expression and action and on gonadotropin and prolactin secretion in the rat. Neuroendocrinology. 2004;79(5):247–258. [DOI] [PubMed] [Google Scholar]
- 56. Shupnik MA, Gordon MS, Chin WW. Tissue-specific regulation of rat estrogen receptor mRNAs. Mol Endocrinol. 1989;3(4):660–665. [DOI] [PubMed] [Google Scholar]
- 57. Silva MS, Prescott M, Campbell RE. Ontogeny and reversal of brain circuit abnormalities in a preclinical model of PCOS. JCI Insight. 2018;3(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Azziz R. Androgen excess is the key element in polycystic ovary syndrome. Fertil Steril. 2003;80(2):252–254. [DOI] [PubMed] [Google Scholar]
- 59. Foecking EM, McDevitt MA, Acosta-Martínez M, Horton TH, Levine JE. Neuroendocrine consequences of androgen excess in female rodents. Horm Behav. 2008;53(5):673–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McGee WK, Bishop CV, Bahar A, et al. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Hum Reprod. 2012;27(2):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Robinson JE, Forsdike RA, Taylor JA. In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology. 1999;140(12):5797–5805. [DOI] [PubMed] [Google Scholar]
- 62. Kauffman AS, Thackray VG, Ryan GE, et al. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice. Biol Reprod. 2015;93(3):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tata B, Mimouni NEH, Barbotin AL, et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24(6):834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neurosci Lett. 2004;365(1):43–47. [DOI] [PubMed] [Google Scholar]
- 65. Poola I, Abraham J, Baldwin K. Identification of ten exon deleted ERbeta mRNAs in human ovary, breast, uterus and bone tissues: alternate splicing pattern of estrogen receptor beta mRNA is distinct from that of estrogen receptor alpha. FEBS Lett. 2002;516(1-3):133–138. [DOI] [PubMed] [Google Scholar]
- 66. Hendriks ML, Ket JC, Hompes PG, Homburg R, Lambalk CB. Why does ovarian surgery in PCOS help? Insight into the endocrine implications of ovarian surgery for ovulation induction in polycystic ovary syndrome. Hum Reprod Update. 2007;13(3):249–264. [DOI] [PubMed] [Google Scholar]
- 67. Judd HL, Rigg LA, Anderson DC, Yen SS. The effects of ovarian wedge resection on circulating gonadotropin and ovarian steroid levels in patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 1976;43(2):347–355. [DOI] [PubMed] [Google Scholar]
- 68. De Leo V, Lanzetta D, D’Antona D, la Marca A, Morgante G. Hormonal effects of flutamide in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83(1):99–102. [DOI] [PubMed] [Google Scholar]
- 69. Padmanabhan V, Veiga-Lopez A, Herkimer C, et al. Developmental programming: prenatal and postnatal androgen antagonist and insulin sensitizer interventions prevent advancement of puberty and improve lh surge dynamics in prenatal testosterone-treated sheep. Endocrinology. 2015;156(7):2678–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Silva MSB, Desroziers E, Hessler S, et al. Activation of arcuate nucleus GABA neurons promotes luteinizing hormone secretion and reproductive dysfunction: Implications for polycystic ovary syndrome. Ebiomedicine. 2019;44:582–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Graf MA, Bielfeld P, Distler W, Weiers C, Kühn-Velten WN. Pulsatile luteinizing hormone secretion pattern in hyperandrogenemic women. Fertil Steril. 1993;59(4):761–767. [DOI] [PubMed] [Google Scholar]
- 72. George JT, Hendrikse M, Veldhuis JD, Clarke IJ, Anderson RA, Millar RP. Effect of gonadotropin-inhibitory hormone on luteinizing hormone secretion in humans. Clin Endocrinol (Oxf). 2017;86(5):731–738. [DOI] [PubMed] [Google Scholar]
- 73. Tsutsui K, Ubuka T. How to contribute to the progress of neuroendocrinology: discovery of GnIH and progress of GnIH research. Front Endocrinol (Lausanne). 2018;9:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Halvorson LM. PACAP modulates GnRH signaling in gonadotropes. Mol Cell Endocrinol. 2014;385(1-2):45–55. [DOI] [PubMed] [Google Scholar]
- 75. Henderson HL, Townsend J, Tortonese DJ. Direct effects of prolactin and dopamine on the gonadotroph response to GnRH. J Endocrinol. 2008;197(2):343–350. [DOI] [PubMed] [Google Scholar]
- 76. Hodson DJ, Townsend J, Tortonese DJ. Characterization of the effects of prolactin in gonadotroph target cells. Biol Reprod. 2010;83(6):1046–1055. [DOI] [PubMed] [Google Scholar]
- 77. Ryan GE, Malik S, Mellon PL. Antiandrogen treatment ameliorates reproductive and metabolic phenotypes in the letrozole-induced mouse model of PCOS. Endocrinology. 2018;159(4):1734–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Haisenleder DJ, Burger LL, Aylor KW, et al. Testosterone stimulates follicle-stimulating hormone beta transcription via activation of extracellular signal-regulated kinase: evidence in rat pituitary cells. Biol Reprod. 2005;72(3):523–529. [DOI] [PubMed] [Google Scholar]
- 79. Wu S, Chen Y, Fajobi T, et al. Conditional knockout of the androgen receptor in gonadotropes reveals crucial roles for androgen in gonadotropin synthesis and surge in female mice. Mol Endocrinol. 2014;28(10):1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Thackray VG, Mellon PL. Synergistic induction of follicle-stimulating hormone beta-subunit gene expression by gonadal steroid hormone receptors and Smad proteins. Endocrinology. 2008;149(3):1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25(6):947–970. [DOI] [PubMed] [Google Scholar]
- 82. Auchus RJ. Chapter 8 - Human Steroid Biosynthesis. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction (Fourth Edition). San Diego: Academic Press; 2015:295–312. [Google Scholar]
- 83. McKenna NJ. Chapter 9 - Gonadal Steroid Action. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction (Fourth Edition). San Diego: Academic Press; 2015:313–333. [Google Scholar]
- 84. Brock O, De Mees C, Bakker J. Hypothalamic expression of oestrogen receptor α and androgen receptor is sex-, age- and region-dependent in mice. J Neuroendocrinol. 2015;27(4):264–276. [DOI] [PubMed] [Google Scholar]
- 85. Diotel N, Charlier TD, Lefebvre d’Hellencourt C, et al. Steroid transport, local synthesis, and signaling within the brain: roles in neurogenesis, neuroprotection, and sexual behaviors. Front Neurosci. 2018;12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76(1):98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vanacker C, Moya MR, DeFazio RA, Johnson ML, Moenter SM. Long-Term recordings of arcuate nucleus kisspeptin neurons reveal patterned activity that is modulated by gonadal steroids in male mice. Endocrinology. 2017;158(10):3553–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]