Abstract
Lung cancer remains the leading cause of cancer-related death due to its advanced stage at diagnosis. Early detection of lung cancer can be improved by better defining who should be screened radiographically, and determining which imaging-detected pulmonary nodules are malignant. Gene expression biomarkers measured in normal-appearing airway epithelium provide an opportunity to use lung cancer associated molecular changes in this tissue for early detection of lung cancer. Molecular changes in the airway may result from an etiologic field of injury and/or field cancerization. The etiologic field of injury reflects the aberrant physiological response to carcinogen exposure that creates a susceptible microenvironment for cancer initiation. In contrast, field cancerization reflects effects of “first-hit” mutations in a clone of cells from which the tumor ultimately arises or the effects of the tumor on the surrounding tissue. These fields might have value both for assessing lung cancer risk and diagnosis. Cancer-associated gene expression changes in the bronchial airway have recently been used to develop and validate a 23-gene classifier that improves the diagnostic yield of bronchoscopy for lung cancer among intermediate-risk patients. Recent studies have demonstrated that these lung cancer-related gene expression changes extend to nasal epithelial cells that can be sampled non-invasively. While the bronchial gene expression biomarker is being adopted clinically, further work is necessary to explore the potential clinical utility of gene-expression profiling in the nasal epithelium for lung cancer diagnosis, risk assessment, and precision medicine for lung cancer treatment and chemoprevention.
Introduction
Lung cancer is the leading cause of cancer-related death both in the United States and the world with an estimated 222,500 new lung cancer cases and 155,870 deaths in the US in 2017 (1). Lung cancer is most often lethal, with a 5-year survival rate which was only 18% between 2005 and 2011 (1). Clearly, the improvements in survival realized in other malignancies have yet to be achieved in lung cancer. This is due, in part, to our frequent failure to detect lung cancer at its earliest and potentially curable stage; less than 20% of lung cancer patients are diagnosed at stage 1 (2).
While 90% of lung cancer cases in the US occur in individuals with a history of tobacco smoke exposure, only 15-20% of lifetime smokers will develop lung cancer and we are currently limited in our ability to identify those at highest risk (3–5). Over the past 5 years, the early detection landscape for lung cancer has been transformed by the results of the National Lung Screening Trial (NLST) which compared low-radiation dose helical computed tomography (CT) with chest radiography (6). They reported a 20.3% reduction in lung cancer mortality in the CT arm as well as a statistically significant reduction of 6.9% in all-cause mortality. Currently, annual CT screening has been recommended for individuals at highest risk for developing lung cancer (National Comprehensive Cancer Network Criteria: aged 55-74 years old with ≥30 pack-years smoking history) (7). However, over 70% of lung cancer cases in the US occur in individuals who fall outside of the NLST criteria (8) suggesting that better methods are needed to identify those who ultimately will benefit from CT screening.
Beyond identifying those who should be screened for lung cancer, there is also the challenge of identifying which abnormalities detected by CT represent lung cancer. It is estimated that 1.5 million indeterminate pulmonary nodules are found in the U.S. annually either incidentally or on screening chest CT, representing a significant diagnostic dilemma given that the overwhelming majority of these nodules will ultimately be determined to be benign (9). Recent studies have found that many of these subjects undergo unnecessary invasive biopsies that incur significant risks and cost (10,11). There is thus an urgent need to develop approaches that can better discriminate benign versus malignant pulmonary nodules in this setting to aid decision making with regard to appropriate follow-up.
Addressing these unmet needs would improve the early detection of lung cancer and thereby have the potential to decrease lung cancer mortality. There have been significant recent efforts to develop molecular biomarkers for lung cancer detection primarily in the setting of screen-detected or incidental lung nodules using a variety of analytes (genomic, transcriptomic, epigenomic, and proteomic) measured in a number of different biospecimens (12–30). This review focuses on a promising approach using gene-expression profiling of the relatively accessible airway epithelium as a window for sampling carcinogen-exposed tissue that could reflect the molecular signatures of cancer-associated processes. The use of genome-wide gene expression approaches as part of the biomarker discovery process allows for a broad search for genes with cancer-associated gene expression for further biomarker development, and it can generate hypotheses as to the biological processes responsible for many of the observed gene expression differences. The utility of airway epithelium is based on two distinct, yet related, concepts: the “Etiological Field of Injury” and “Field Cancerization” (figure 1). We will focus on reviewing potential mechanisms underlying these two “field effects”, the clinical application of airway gene expression for the early detection of lung cancer and highlight some of the critical challenges and opportunities that lie ahead for this field.
Figure 1:
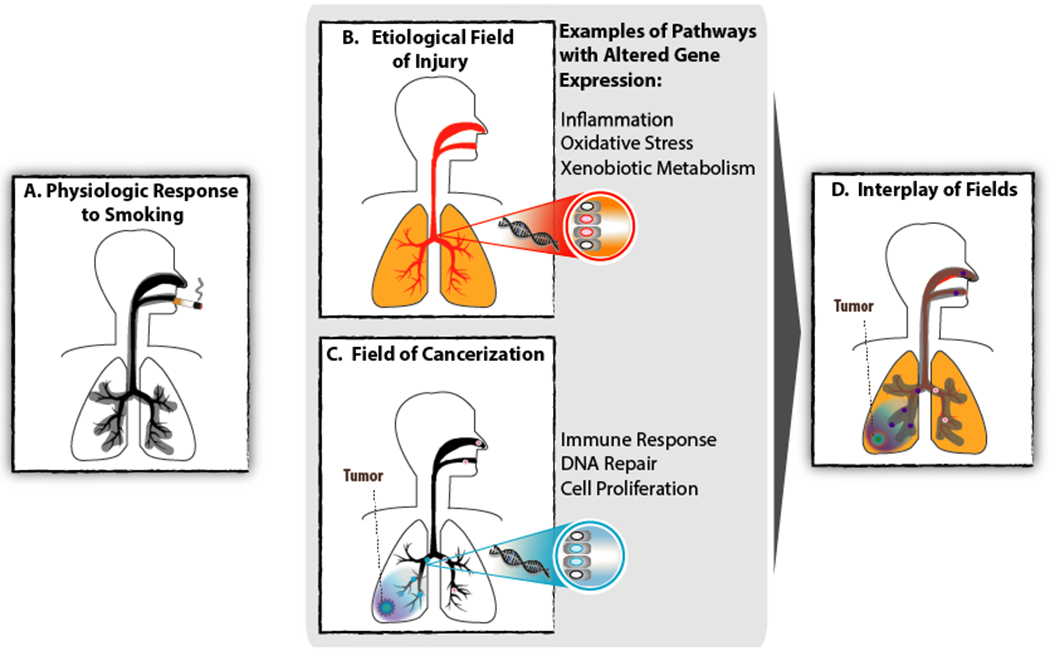
Molecular alterations found throughout the respiratory tract can be attributed to field effects. A) The physiologic effect of smoking occurs predictably, commonly, immediately, and largely reversibly. This field of injury consists of molecular alterations present throughout the respiratory tract upon exposure to tobacco smoke. B) The etiological field of injury consists of processes at the molecular level that contribute to lung carcinogenesis. Molecular alterations related to inflammation, oxidative stress, and xenobiotic metabolism are among these processes which potentiate the establishment and expansion of cells harboring the genetic or epigenetic alterations that are directly required for tumorigenesis. C) Field cancerization refers to tumor-associated abnormalities (e.g. gene expression pathways related to immune response, DNA repair, and cell proliferation) in tumor-adjacent normal appearing cells. These abnormalities may reflect clonal expansion of cells harboring cancer-initiating alterations that are insufficient, on their own, to lead to transformation; or may be a consequence of the tumor on surrounding tissue. D) These fields are not mutually exclusive, and histologically normal-appearing tissue exhibiting cancer-associated alterations due to the etiologic field of injury and / or field cancerization can be leveraged for developing gene-expression based biomarkers for the early detection of lung cancer.
Field of Injury
Tobacco smoke induces molecular alterations in epithelial cells throughout the respiratory tract that are exposed to its carcinogens. These molecular alterations represent a smoking-related field of injury that reflects a complex physiologic response to toxins and mechanical injury (31) characterized by increased inflammation, oxidative stress, and xenobiotic metabolism, (32). Several groups have characterized the gene-expression correlates of this physiologic response. Hackett et al. demonstrated upregulation of antioxidant-related genes in active smoker epithelium compared with nonsmokers (33) that generally fell into one of four categories: catalase/superoxide dismutase, glutathione metabolism (GPX2, GSR), redox balance (ADH7, AKR1C3), and the pentose phosphate cycle (TALDO1, PGD). Furthermore, they identified subgroups of smokers that differed in their expression levels of these antioxidant-related genes. Similarly, Spira et al. used genome-wide gene-expression profiling to broadly characterize alterations in healthy current smokers and identified smoking-associated differences in genes involved in xenobiotic metabolism (CYP1B1 and DBDD), oxidative stress (GPX2 and ALDH3A1), and immune response (34). Subsequent analysis demonstrated that while the expression of the vast majority of these genes returns to normal after smoking cessation, a number of genes (e.g. CEACAM5, SULF1, UPK1B) have persistently altered expression for years after smoking cessation (35). Chari et al. similarly found that the expression levels of many genes altered by tobacco smoking return to baseline levels after smoking cessation, including genes related to xenobiotic metabolism (e.g. ALDH3A1, ALDH7, AKR1C2, CYP1B1, NQO1) while also finding that others (e.g. GSK3B and COX2) remain persistently altered (36). Overall, these studies suggest that the physiologic response to tobacco smoke occurs commonly, immediately, and largely reversibly in individuals who smoke. As such, these smoking-associated changes are not directly useful for assessing the presence of lung cancer, and are distinct from those changes due to an etiologic field of injury associated with lung carcinogenesis.
However, the identification of persistent effects of smoking on airway epithelium is intriguing as it may relate to the persistent risk of many smoking-associated diseases (including lung cancer) among former smokers. Furthermore, variability in the response to tobacco smoke exposure and variability in the injury it induces (e.g. immune modulation, oxidative damage, microbiome) might contribute to differences in risk for lung cancer (37). These processes reflect what has been referred to as an etiological field of injury. The etiological field of injury reflects the processes triggered by environmental exposures that potentiate the molecular alterations (e.g. genetic and epigenetic changes) that directly lead to cancer development (38). Markers of the etiological field of injury may therefore be used to assess lung cancer risk or detect lung cancer. As one example, Blomquist et al measured bronchial airway epithelium expression of 14 genes related to antioxidant and DNA repair pathways among smokers with suspect lung cancer including, CEBPG, E2F1, ERCC4, ERCC5, GPX1, and GPX3 (39). Combining the levels of these genes into a single score resulted in a biomarker that could reliably differentiate individuals with and without lung cancer (80% accuracy, odds ratio of 12.8). This biomarker is currently being validated as a screening tool in the Lung Cancer Risk Test trial, a prospective cohort study evaluating the clinical validity and utility of a 15-gene classifier from bronchial epithelial cells of individuals with and without screen-detected lung cancer (40). Leveraging the cancer-associated transcriptomic field of injury for the assessment of lung cancer risk and cancer detection could improve our ability to detect lung cancer earlier in the disease process.
Field Cancerization
Field cancerization, a concept first introduced by Slaughter et al. in the context of oral cancers, suggests that both the neoplasm and adjacent grossly normal-appearing tissue share some histological abnormalities such as epithelial hyperplasia and hyperkeratinization (41). This concept has evolved to broadly reflect tumor-related changes in adjacent normal tissue, including molecular alterations. These tumor-related changes can either be due to “first-hit” genetic alterations in a clone of cells from which the tumor ultimately develops, or effects of the tumor on the local microenvironment. As an example of the former, Nelson et al. found K-ras mutations in both Non-Small Cell Lung Cancer (NSCLC) tumor tissue and adjacent normal tissue (42). Similarly, Franklin et al. analyzed DNA from micro-dissected airway epithelial cells of sites along the entire tracheobronchial tree of an autopsy specimen in a patient with widespread dysplastic lesions and identified widespread p53 mutations despite the absence of an overt carcinoma. This suggests “field carcinogenesis” (or at least the expansion of clones of cells harboring cancer-associated mutations) can occur prior to tumor development in which a single abnormal progenitor clone may expand to populate broad areas of the bronchial mucosa (43). This hypothesis is supported by several observations. In 1997, Wistuba et al. identified genetic changes (i.e. Loss of Heterozygosity, allelic loss, and microsatellite alterations) in normal and abnormal bronchial epithelium from current smokers compared to former and never smokers (44). Similarly, Mao et al. investigated microsatellite DNA from airway biopsies of chronic smokers and identified genetic alterations in putative tumor-suppressor genes (45). Furthermore, Tang et al. found Epidermal Growth Factor Receptor (EGFR) mutations in histologically normal respiratory epithelium that were concordant with the EGFR mutations found within the actual adenocarcinoma tissue (46). Also, phosphatidylinositol 3-kinase (PI3K) pathway activation has been demonstrated in the cytologically normal bronchial airway epithelium of smokers with lung cancer (including patients with adenocarcinoma or squamous cell carcinoma) and smokers with airway dysplasia, a process thought to reflect an early event in tumorigenesis (47).
Several other studies provide evidence of field cancerization. Kadara et al. conducted serial expression profiling on the airway epithelium from patients with lung cancer following resection and found that gene expression patterns in airway epithelium adjacent to previously resected tumors persisted for at least three years after definitive surgery. These differences could reflect field cancerization arising from somatic mutations in the adjacent field, or be due to the persistence of the etiological field even after tumor resection, or some combination of the two (48). However, this study did not include pre-surgical baseline specimens and it is difficult to determine whether the genes were altered due to the malignancy or were altered in response to surgery (49). A more definitive demonstration of the impact of field cancerization on gene expression comes from another study by Kadara et al. that performed gene expression profiling on resected lung tumor tissue, matched uninvolved normal lung tissue, and adjacent airways at varying distances from the tumor (50). They found a number of genes with tumor-associated changes in gene expression (e.g. TGFBR2, VIPR1, NETO2, LAPTM4B) that are similarly changed in the matched airway samples. For some of these genes, the extent of altered airway expression was greater in more tumor-proximal airways (presumably reflecting field cancerization), while for other genes, airway expression was consistently altered regardless of tumor proximity (presumably reflecting the etiologic field of injury). These observations illustrate a relationship between molecular changes present in airway epithelium and the tumor and provides a basis for using less invasive tissue sampling for lung cancer detection remote to the actual tumor. Key studies related to the etiological field of injury and field cancerization are summarized in table 1.
Table 1.
Summary of Key Findings from Select Papers in the Field
| Paper | Study Population | Tissue | Analytes | Significant Genes/Pathways | Major Findings |
|---|---|---|---|---|---|
| Slaughter et al. 1953 (41) | Oral cancer cases | Resected oral tumor | Histology | N/A | Non-malignant epithelium surrounding tumors is abnormal |
| Blomquist et al. 2009 (39) | Ever smokers undergoing bronchoscopy for suspect lung cancer | Bronchial Airway epithelial cells via bronchoscopy | mRNA | Antioxidant and DNA repair pathways (CAT, CEBPG, E2F1, ERCC4, ERCC5, GPX1, GPX3, GSTM3, GSTP1, GSTT1, GSTZ1, MGST1, SOD1, and XRCC1) | Variability in the expression of antioxidant, DNA repair and transcription factor genes in normal airway epithelium identifies the presence of lung cancer |
| Spira et al. 2007 (54) | Ever smokers undergoing bronchoscopy for suspect lung cancer | Bronchial Airway epithelial cells via bronchoscopy | mRNA | Inflammation (PLA2G4A, DEFB1, C6, FCGR3A, IL8, SERPINA1) Cell Cycle (PPBP, DUSP6, TOB1) Antioxidant (BACH2, DUOX1) | An 80-gene classifier derived from cytologically normal large airways of ever smokers can distinguish lung cancer vs. benign disease |
| Franklin et al. 1997 (43) | Lung premaligancy | Bronchial epithelial cells at multiple sites during autopsy | DNA | p53 | A p53 tumor suppressor gene mutation was identified at several sites throughout the airway epithelium that exhibited only squamous metaplasia and mild to moderate atypia by histology. |
| Tang et al. 2005 (46) | Lung cancer cases | Resected lung tumor | DNA | EGFR mutations | EGFR mutations are found in normal epithelium of EGFR-positive lung cancer patients. |
| Kadara et al. 2014 (50) | Lung cancer cases | Resected lung tumor | mRNA | TGFBR2, VIPR1, NETO2, LAPTM4B | Histologically normal-appearing adjacent airway exhibits a gradient of tumor-associated gene expression that decreases with increasing distance from the tumor as well as a set of genes that are consistently altered regardless of tumor proximity |
| Silvestri et al. 2015 (57) | Ever smokers undergoing bronchoscopy for suspect lung cancer | Bronchial Airway epithelial cells via bronchoscopy | mRNA | Immune Response (BST1, CD177) Tumor related (TSPAN2, NOVA1, CDR1, MCAM) Xenobiotic Metabolism (EPHX3) Cell Cycle (SDC2, NKX3-1, CGREF1) Others (RPS4Y1, SLC7A11, CLND10, TKT, RUNX1T1, AKR1C2, CD177.2, ATP12A, GABBR1, CLND22, LYPD2, MIA, RNF150) | A 23-gene-expression classifier measured in cytologically normal mainstem bronchus improved the diagnostic performance of bronchoscopy for the detection of lung cancer. |
Bronchial Airway Gene-Expression Biomarker for Lung Cancer Detection
The etiologic field of injury and field cancerization are potentially relevant for the diagnosis of lung cancer as they suggest the possibility of detecting lung cancer via abnormalities in more potentially accessible non-tumor tissue. This could have considerable clinical utility in settings where the probability of lung cancer is too low to justify the risk of directly assessing the suspicious lesion. In the current diagnostic algorithm for indeterminate pulmonary nodules, the American College of Chest Physicians (ACCP) recommends that clinicians estimate the pretest probability of malignancy either qualitatively using their clinical judgment and/or quantitatively using a validated model (3,4,51,52). In those individuals with a lower pretest probability, surveillance with serial CT scans may be appropriate. In those individuals with a high pretest probability, further work-up is indicated and typically involves direct pathological assessment of lesion tissue obtained via bronchoscopy, needle biopsy or surgical resection (figure 2a). Individuals with an intermediate pretest probability often pose a diagnostic challenge, though current guidelines recommend functional imaging (e.g. Positron Emission Tomography) to help direct further management such as invasive biopsy versus surveillance imaging. Bronchoscopy to collect cells from the lesion is often used in the setting of intermediate risk indeterminate pulmonary nodules as it is only minimally invasive. However it is challenging to acquire lesion tissue from peripheral nodules by bronchoscopy, and its sensitivity for lung cancer is estimated to be 50-60% (53). When bronchoscopy is non-diagnostic, molecular biomarkers that leverage cancer-associated abnormalities in histologically normal epithelium provide an opportunity to detect malignant nodules with increased sensitivity. Furthermore, molecular changes in airway epithelium could also play a role in lung cancer risk assessment as some might occur at early stages of lung tumorigenesis before an incipient tumor is readily detectable by imaging (figure 2b).
Figure 2:
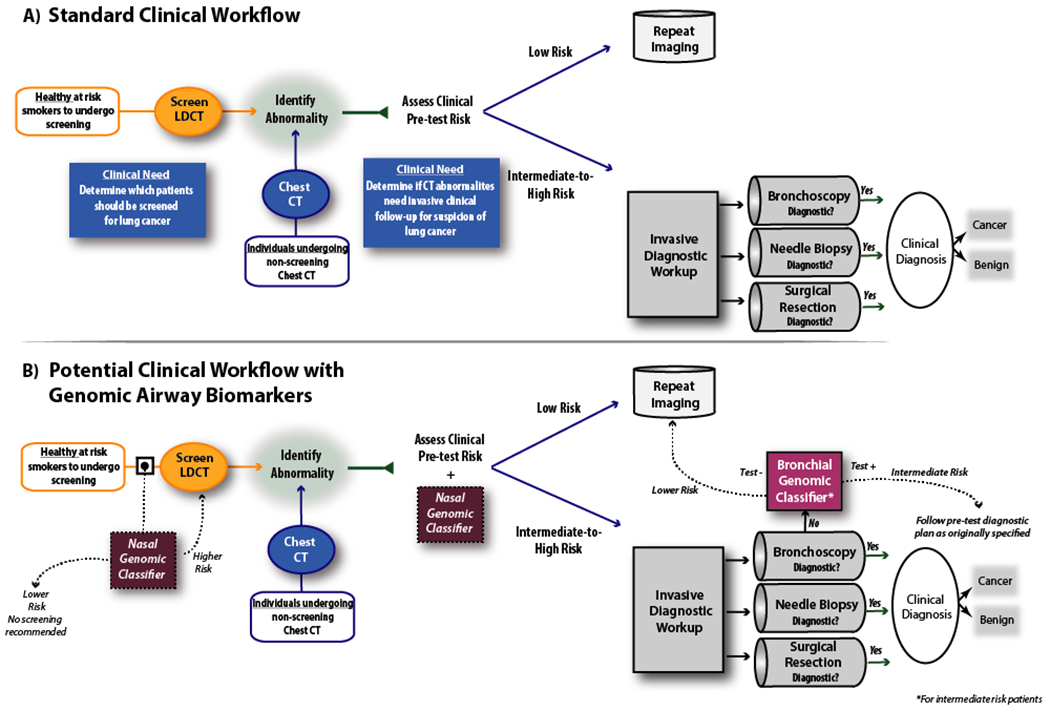
Potential clinical applications of airway molecular biomarkers for the early detection of lung cancer. A) In current practice, pulmonary nodules are discovered either due to lung cancer screening based on clinical risk factors, as findings incidental to other imaging studies, or during the work up of symptomatic patients. In the current diagnostic algorithm for indeterminate pulmonary nodules established by the American College of Chest Physicians (ACCP), based on the pretest probability of malignancy, patients determined to be low risk will typically undergo continued surveillance while higher risk patients will undergo invasive testing. However, there is a clinical unmet need to better stratify those subjects at intermediate pre-test risk into CT surveillance vs. invasive biopsy as well as an unmet need to identify which subjects should undergo CT screening. B) Molecular biomarkers have the potential to impact the current diagnostic workflow of patients with indeterminate pulmonary nodules. Current biomarkers measured in histologically normal-appearing bronchial airway tissue can serve to identify patients with non-diagnostic bronchoscopies who are at low risk for lung cancer and can be followed with continued imaging surveillance. Future biomarkers measured in histologically normal-appearing nasal airway tissue could help identify at-risk individuals for CT-based lung cancer screening or direct diagnostic workup in individuals with suspect lung nodules who are not otherwise undergoing bronchoscopy. CT = Computed Tomography. LDCT = Low Dose Computed Tomography.
Based on both the etiologic field of injury and field cancerization, there has been considerable effort to determine if there are gene expression alterations in normal appearing bronchial airway epithelium that might have clinical utility for lung cancer detection. Enthusiasm for this approach stems from an early study by Spira et al. which profiled gene-expression in bronchial airway epithelium from the mainstem bronchus of current and former smokers undergoing bronchoscopy for suspect lung cancer using microarrays (54). These patients were then followed prospectively until a definitive diagnosis of cancer or non-cancer was established. Spira et al. identified a number of gene expression differences between patients with and without cancer in normal appearing airway cells from the mainstem bronchus, establishing the existence of a cancer-associated gene expression signature in the proximal airway (54). In support of the biological relevance of these gene expression changes, Kadara et al. showed that these genes are enriched for genes whose expression is altered both in the tumor and airways regardless of the distance from the tumor (48). Beyond demonstrating cancer-associated gene expression alterations in the mainstem bronchus, Spira et al. also demonstrated that they could function as a biomarker that achieved high sensitivity and negative predictive value in two independent test sets (54). As these airway epithelial biospecimens were collected during a clinical procedure for suspect lung cancer that has modest sensitivity, the high sensitivity of the bronchial airway genomic biomarker suggested the potential clinical utility of combining the biomarker with routine bronchoscopy to serve as a rule-out test for lung cancer. Supporting this potential application, Spira et al. found that the performance of the gene-expression biomarker is independent of whether bronchoscopy resulted in a diagnosis; and Beane et al. showed that the biomarker is independent of a number of clinical risk factors for lung cancer (55).
Key to the further refinement and validation of this approach were two large multicenter clinical cohorts of current and former smokers undergoing bronchoscopy for suspect lung cancer: AEGIS1 and AEGIS2. These studies were similar in study design to the original study by Spira et al. and included collection of pre-diagnostic bronchial epithelial cells from the mainstem bronchus and prospective follow-up of patients for up to one year after bronchoscopy until a final diagnosis was obtained. Samples were profiled using a substantially different microarray platform, so a portion of the samples from AEGIS1 were used to derive a new 23-gene biomarker. Consistency with the prior study was demonstrated by showing that the new biomarker could distinguish patients with and without lung cancer in the previous dataset from Spira et al. The strategy used by Whitney et al. to develop the 23-gene biomarker is noteworthy in that in addition to including 17 genes with cancer-associated expression, they also included genes that serve as surrogates of clinical risk factors: including sex, current smoking status, and cumulative tobacco exposure (56). These gene-expression correlates were incorporated to capture the physiological effects of these clinical factors that might more accurately reflect their effect on disease risk and minimize the effect of inaccurate patient self-report. For the important risk factor of patient age, they were unable to identify a robust gene expression surrogate, so the clinically reported value is used directly in the calculation of the biomarker score. For determining predicted cancer status, they chose a score threshold that resulted in a sensitivity of 93% and specificity of 57% in the training set. In addition to demonstrating that this biomarker had similar performance in the original dataset from Spira et al., it was subsequently validated by Silvestri et al. in two additional independent datasets: the remainder of the samples from AEGIS1 that were not used for biomarker development (n = 298) and all of the samples from AEGIS2 (n = 341) (57). This study demonstrated that the combination of bronchoscopy with the gene-expression classifier performed well (97% sensitivity) compared to bronchoscopy alone (75% sensitivity). The performance of the gene-expression classifier is striking in those individuals with an inconclusive bronchoscopy and intermediate pre-test physician assessed risk of lung cancer (88% sensitivity and 91% negative predictive value).
These data suggest that combining the gene-expression classifier with bronchoscopy has several potential benefits. By detecting most cancers (i.e., high sensitivity and high negative predictive value), a negative test result in patients with an intermediate pre-test risk of lung cancer lowers the post-test probability of lung cancer sufficiently that repeat imaging could be pursued as a follow up strategy rather than invasive diagnostic procedures (figure 2b); which may lower cost and the risk of iatrogenic complications including respiratory failure, bronchospasm, bleeding, or in the case of CT-Fine Needle Aspiration, over 20% rate of pneumothorax (58,59). However, given the modest specificity and positive predictive value of the biomarker, this test cannot reliably “rule in” a diagnosis of cancer. Therefore, Silvestri et al. caution that a positive result should not be used to alter the decision between an invasive or conservative diagnostic approach. Moreover, in patients with a high pre-test assessed risk of lung cancer, a negative biomarker result may not lower the post-test risk of lung cancer sufficiently to safely alter the pre-test diagnostic plan. The authors noted several other limitations that narrow the intended use population including exclusion of lifetime non-smokers and individuals with a concurrent cancer or a prior history of lung cancer. It should also be noted that the biomarker does not incorporate a number of lung cancer risk factors that have been used in risk calculators such as co-morbid Chronic Obstructive Pulmonary Disease (COPD) or emphysema, nodule fluorodeoxyglucose-positron emission tomography (FDG-PET) avidity, size, location, spiculation, or texture; but these factors contribute to the physician’s pre-test risk assessment.
The genomic classifier developed and validated in the AEGIS trials has since been commercialized as the Percepta® Bronchial Genomic Classifier, a Clinical Laboratory Improvement Amendments (CLIA)-based 23-gene genomic classifier. It is possible that this biomarker captures the effects of the etiological field of injury, field cancerization, or some combination of the two. However, because these gene-expression changes are readily detected in the airway epithelium, this biomarker may be used as a clinical decision-making tool in the evaluation of a subset of patients undergoing bronchoscopy due to suspect lung cancer. With a high sensitivity and high negative predictive value, clinicians are encouraged to follow a more conservative strategy of serial imaging in those bronchoscopy-negative patients with an intermediate pre-test probability of disease whose Percepta® test is negative; and the clinical utility of the biomarker comes from reducing the burden of procedures in patients without cancer. A reanalysis of the AEGIS data by Vachani et al. suggests that 50% of the invasive biopsies performed in AEGIS participants who were then determined not to have lung cancer could have been avoided by using Percepta negativity to reassign intermediate-risk bronchoscopy-negative patients to repeat imaging (60). Encouragingly, data from a decision impact study by Ferguson et al. based on a survey of pre- and post-test diagnostic management plans suggests the willingness of physicians to alter their management plans in intermediate risk patients based on a negative Percepta result: when the surveyed clinicians were blinded to the Percepta result, the rate of recommended invasive procedures was 57%, but only 18% when they were unblinded (61). Finally, a recent study has provided an estimate of the cost effectiveness of this diagnostic test (62).
Recent work has extended these lung cancer associated transcriptomic alterations to microRNA (miRNA), a class of small non-coding RNA that can regulate gene expression and protein translation. Previous work has demonstrated the effects of smoking on miRNA expression and the potential for these smoking-related differences in miRNA expression to regulate cigarette smoking-related gene expression (63–65). More recent work has identified four miRNA (miR-146a-5p, miR-324-5p, miR-223-3p, and miR-223-5p) with decreased expression in the bronchial airway of patients with lung cancer. The predicted mRNA targets exhibit of each of these miRNA are enriched among the genes most increased in the bronchial airway of lung cancer patients, suggesting that these miRNA may be regulators of genes with cancer-associated expression patterns (66). Furthermore, of the four isoforms identified, incorporating miR-146a-5p into the 23-gene biomarker led to significantly improved performance suggesting a potential benefit to a multi-omic biomarker strategy.
Gene-Expression Biomarkers for Lung Cancer in Extra-Thoracic Airway Epithelium
While the bronchial genomic classifier may serve an important role in determining the clinical management of patients undergoing bronchoscopy for suspect lung cancer, there are patients with suspect lesions who do not undergo bronchoscopy (e.g. patients with small peripheral nodules), and the risks and costs associated with bronchoscopy preclude using it to establish cancer risk in the setting of broad lung cancer screening. One hypothesis that might provide an alternative approach is that the etiologic field of injury and / or field cancerization that give rise to the molecular signature of lung cancer detectable via bronchial airway epithelial gene expression might extend into the extra-thoracic airway epithelium in the nose and mouth that can be collected less invasively.
Early work demonstrated that the majority of genes whose expression is altered in current smokers are altered similarly in bronchial and nasal epithelium (67), suggesting that if the lung cancer etiological field effect results from the long-term consequences of the response to tobacco smoke it might evolve similarly in bronchial and nasal epithelium. More recent work has directly addressed whether there is a gene expression signature of lung cancer in nasal epithelium. Perez-Rogers et al. profiled gene expression in nasal epithelium samples collected from patients in the AEGIS trials and identified a number of genes whose expression differed between patients with and without lung cancer (68). Moreover, there was a high degree of concordance between nasal and bronchial cancer-associated gene expression as determined using Gene Set Enrichment Analysis: genes with either increased or decreased expression in nasal epithelium of patients with lung cancer were significantly enriched among the genes most significantly increased or decreased in bronchial epithelium of patients with cancer. These findings support the hypothesis that there is a common gene expression signature of lung cancer that extends from the bronchial epithelium into the nose. Samples from AEGIS1 were used to define a 30-gene biomarker that differed significantly between patients with and without lung cancer. When combined with known clinical risk factors and applied to patients from AEGIS2, the resulting clinico-genomic model performed better in identifying patients with and without lung cancer than a model containing only clinical risk factors: achieving a Receiver Operating Characteristic (ROC) curve Area Under the Curve (AUC) of 0.81, a sensitivity of 91% and a specificity of 52%.
While the study by Perez-Rogers et al. demonstrates the ability to detect lung cancer on the basis of nasal gene expression, considerable work remains to be done before a nasal biomarker could be used clinically. Importantly, the reported nasal classifier was derived in the context of patients undergoing bronchoscopy for suspicion of lung cancer. It is unclear how the classifier would perform in a setting where the prevalence of lung cancer is lower: which is the clinical context in which a nasal biomarker would have most utility relative to a bronchoscopy-based test. Furthermore, in the context of low disease prevalence, there may be more clinical utility for a test with high specificity that could trigger appropriate invasive follow up (figure 2b). Additional work, especially in cohorts that more closely mirror the potential intended use population(s) (e.g. at risk individuals prior to screening and individuals with screen-detected indeterminate pulmonary nodules), will be necessary to 1) identify biomarkers and / or combinations of biomarkers and clinical factors that work well in these settings and 2) determine intended uses based on the biomarker’s performance in these settings.
Another site for minimally invasive biomarkers within the airway transcriptome is the mouth. Although this site is challenged by the degree of RNA degradation present in buccal epithelial cells obtained by scraping the cheek, recent studies suggest that there may be relevant gene-expression signatures that can be measured at that site. While smoking related gene-expression alterations in the buccal epithelium are less pronounced that those in the nose and bronchus, there is a common set of smoking-related gene-expression changes across all three sites (69). Further, Wang et al. recently profiled buccal gene expression in a cohort of women from rural China who have an elevated risk of lung cancer due to indoor household cooking with smoky coal (70,71). They identified buccal gene-expression alterations associated with exposure to smoky coal with similar molecular changes as those seen with tobacco smoke exposure. The similarity between the field of injury induced by smoky coal and tobacco smoke in buccal epithelium suggest that there may be potential to develop clinically-relevant biomarkers at this site.
Conclusion and Future Directions for the Field
Identifying lung cancer associated differences in normal-appearing tissue throughout the lung and airway has created an opportunity to detect lung cancer via sampling of non-tumor tissue. Understanding the processes which give rise to these lung-cancer-associated molecular signatures is challenging but two leading hypotheses are that they are related to 1) an etiologic field of injury in which processes that promote carcinogenesis (including the response to carcinogen exposure) occur throughout the lung and that the progression of these processes can therefore be measured in non-tumor tissue like airway epithelium; and / or 2) that the effects of field cancerization can extend beyond the immediate tumor microenvironment and can be detected in non-tumor tissue like airway epithelium. The airway gene-expression biomarkers described in this review may reflect a molecular signature resulting from either an etiologic field of injury, field cancerization, or both (figure 1).
Further work may lead to a better understanding of the processes that give rise to these airway gene expression signatures and / or potentially expand their clinical applications (figure 2b). For instance, little is known about how the field is affected by surgical resection which might have practical implications for the utility of these biomarkers in patients with a prior history of lung cancer. It is also unclear whether there is an etiologic field of injury or field cancerization in never smokers who develop lung cancer which could be leveraged to develop lung cancer biomarkers for this patient population. Recent studies have identified gene-expression alterations in the normal-appearing airway of smokers who have premalignant squamous cell lesions and that changes in the magnitude of these gene expression alterations is associated with lesion progression (72), but little is known about the time course during which these airway molecular changes occur relative to when these lesions appear. This might have implications for the role of an etiologic field of injury as a cause of these molecular changes as well as practical implications for the utility of these biomarkers in the setting of assessing lung cancer risk.
Further downstream is determining how the lung cancer signature detectable in the airways differs between patients with different types of lung cancer. This could ultimately provide information about field cancerization and / or how different etiologic fields of injury predispose to different types of lung cancer. It would also enable using airway gene expression for precision medicine approaches to lung cancer treatment (or chemoprevention) by serving as a biomarker for selecting therapy. While there is certainly much to be learned about the processes that contribute to lung cancer associated changes in airway gene expression, and many of the potential clinical applications that can be envisioned are yet to be explored, the initial successful translation of this approach into a tool with clinical utility for the diagnostic evaluation of patients with suspect lung cancer is a promising sign that there will be other tools to improve lung cancer outcomes that leverage the ability to detect cancer-associated molecular changes in airway epithelium.
Acknowledgement:
The authors thank Sarah Mazzilli for her contributions to the figures of this paper.
Grant Support: This work was supported by grants to MEL and AES from the NCI Early Detection Research Network (U01CA214182), the National Cancer Institute (R01CA210360) and the Department of Defense (DOD W81XWH-11-2-0161).
Footnotes
Conflicts of Interest: A.E.S. and M.E.L. are consultants to Veracyte, Inc. Boston University owns patents related to the subject matter of this manuscript. The other authors have no conflicts of interest to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al. Cancer Statistics Review, 1975–2014 - SEER Statistics [Internet]. Bethesda, MD: National Cancer Institute; 2016. November Available from: https://seer.cancer.gov/csr/1975_2014/ [Google Scholar]
- 3.Gould MK, Ananth L, Barnett PG. A Clinical Model To Estimate the Pretest Probability of Lung Cancer in Patients With Solitary Pulmonary Nodules. Chest 2007;131:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tammemagi CM, Pinsky PF, Caporaso NE, Kvale PA, Hocking WG, Church TR, et al. Lung cancer risk prediction: Prostate, Lung, Colorectal And Ovarian Cancer Screening Trial models and validation. J Natl Cancer Inst. 2011;103:1058–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spitz MR, Hong WK, Amos CI, Wu X, Schabath MB, Dong Q, et al. A Risk Model for Prediction of Lung Cancer. J Natl Cancer Inst. 2007;99:715–26. [DOI] [PubMed] [Google Scholar]
- 6.Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-Small Cell Lung Cancer Version 7.2017. [Internet]. [cited 2017 Jul 7]. Available from: https://www.tri-kobe.org/nccn/guideline/lung/english/lung_screening.pdf
- 8.Pinsky PF, Berg CD. Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancers would be covered? J Med Screen. 2012;19:154–6. [DOI] [PubMed] [Google Scholar]
- 9.Gould MK, Tang T, Liu I- LA, Lee J, Zheng C, Danforth KN, et al. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med. 2015;192:1208–14. [DOI] [PubMed] [Google Scholar]
- 10.Barnett PG, Ananth L, Gould MK, Veterans Affairs Positron Emission Tomography Imaging in the Management of Patients with Solitary Pulmonary Nodules (VA SNAP) Cooperative Study Group. Cost and outcomes of patients with solitary pulmonary nodules managed with PET scans. Chest. 2010;137:53–9. [DOI] [PubMed] [Google Scholar]
- 11.Lokhandwala T, Bittoni MA, Dann RA, D’Souza AO, Johnson M, Nagy RJ, et al. Costs of Diagnostic Assessment for Lung Cancer: A Medicare Claims Analysis. Clin Lung Cancer. 2017;18:e27–34. [DOI] [PubMed] [Google Scholar]
- 12.Kikuchi T, Hassanein M, Amann JM, Liu Q, Slebos RJC, Rahman SMJ, et al. In-depth proteomic analysis of nonsmall cell lung cancer to discover molecular targets and candidate biomarkers. Mol Cell Proteomics MCP. 2012;11:916–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ocak S, Chaurand P, Massion PP. Mass Spectrometry–based Proteomic Profiling of Lung Cancer. Proc Am Thorac Soc. 2009;6:159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pecot CV, Li M, Zhang XJ, Rajanbabu R, Calitri C, Bungum A, et al. Added value of a serum proteomic signature in the diagnostic evaluation of lung nodules. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2012;21:786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassanein M, Rahman JSM, Chaurand P, Massion PP. Advances in Proteomic Strategies toward the Early Detection of Lung Cancer. Proc Am Thorac Soc. 2011;8:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belinsky SA. Gene-promoter hypermethylation as a biomarker in lung cancer. Nat Rev Cancer. 2004;4:707–17. [DOI] [PubMed] [Google Scholar]
- 17.Lin H, Tong Z- H, Xu Q- Q, Wu X- Z, Wang X- J, Jin X- G, et al. Interplay of Th1 and Th17 cells in murine models of malignant pleural effusion. Am J Respir Crit Care Med. 2014;189:697–706. [DOI] [PubMed] [Google Scholar]
- 18.Su Y, Fang H, Jiang F. Integrating DNA methylation and microRNA biomarkers in sputum for lung cancer detection. Clin Epigenetics. 2016;8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su Y, Guarnera MA, Fang H, Jiang F. Small non-coding RNA biomarkers in sputum for lung cancer diagnosis. Mol Cancer [Internet]. 2016. [cited 2017 Apr 20];15 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4866414/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rauch TA, Wang Z, Wu X, Kernstine KH, Riggs AD, Pfeifer GP. DNA methylation biomarkers for lung cancer. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2012;33:287–96. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Xu Y, Liao X, Liao R, Zhang L, Niu K, et al. Plasma miRNAs in predicting radiosensitivity in non-small cell lung cancer. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2016;37:11927–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao L, Hruban RH, Boyle JO, Tockman M, Sidransky D. Detection of oncogene mutations in sputum precedes diagnosis of lung cancer. Cancer Res. 1994;54:1634–7. [PubMed] [Google Scholar]
- 23.Belinsky SA, Grimes MJ, Casas E, Stidley CA, Franklin WA, Bocklage TJ, et al. Predicting gene promoter methylation in non-small-cell lung cancer by evaluating sputum and serum. Br J Cancer. 2007;96:1278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Xiao H, Zhou H, Santiago S, Lee JM, Garon EB, et al. Development of transcriptomic biomarker signature in human saliva to detect lung cancer. Cell Mol Life Sci CMLS. 2012;69:3341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamandis EP, Goodglick L, Planque C, Thornquist MD. Pentraxin-3 is a novel biomarker of lung carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2011;17:2395–9. [DOI] [PubMed] [Google Scholar]
- 26.Ajona D, Pajares MJ, Corrales L, Perez-Gracia JL, Agorreta J, Lozano MD, et al. Investigation of complement activation product c4d as a diagnostic and prognostic biomarker for lung cancer. J Natl Cancer Inst. 2013;105:1385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boeri M, Verri C, Conte D, Roz L, Modena P, Facchinetti F, et al. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci U S A. 2011;108:3713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotunno M, Hu N, Su H, Wang C, Goldstein AM, Bergen AW, et al. A gene expression signature from peripheral whole blood for stage I lung adenocarcinoma. Cancer Prev Res Phila Pa. 2011;4:1599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montani F, Marzi MJ, Dezi F, Dama E, Carletti RM, Bonizzi G, et al. miR-Test: a blood test for lung cancer early detection. J Natl Cancer Inst. 2015;107:djv063. [DOI] [PubMed] [Google Scholar]
- 30.Ilie M, Hofman V, Long-Mira E, Selva E, Vignaud J- M, Padovani B, et al. “Sentinel” Circulating Tumor Cells Allow Early Diagnosis of Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease. PLOS ONE. 2014;9:e111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steiling K, Ryan J, Brody JS, Spira A. The field of tissue injury in the lung and airway. Cancer Prev Res Phila Pa. 2008;1:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, Taneja V, Vassallo R. Cigarette Smoking and Inflammation. J Dent Res. 2012;91:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hackett NR, Heguy A, Harvey B- G, O’Connor TP, Luettich K, Flieder DB, et al. Variability of Antioxidant-Related Gene Expression in the Airway Epithelium of Cigarette Smokers. Am J Respir Cell Mol Biol. 2003;29:331–43. [DOI] [PubMed] [Google Scholar]
- 34.Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, et al. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci U S A. 2004;101:10143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beane J, Sebastiani P, Liu G, Brody JS, Lenburg ME, Spira A. Reversible and permanent effects of tobacco smoke exposure on airway epithelial gene expression. Genome Biol. 2007;8:R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chari R, Lonergan KM, Ng RT, MacAulay C, Lam WL, Lam S. Effect of active smoking on the human bronchial epithelium transcriptome. BMC Genomics. 2007;8:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Chorley BN, Pittman GS, Kleeberger SR, Brothers J, Liu G, et al. Genetic variation and antioxidant response gene expression in the bronchial airway epithelium of smokers at risk for lung cancer. PloS One. 2010;5:e11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lochhead P, Chan AT, Nishihara R, Fuchs CS, Beck AH, Giovannucci E, et al. Etiologic field effect: reappraisal of the field effect concept in cancer predisposition and progression. Mod Pathol Off J U S Can Acad Pathol Inc. 2015;28:14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blomquist T, Crawford EL, Mullins D, Yoon Y, Hernandez D- A, Khuder S, et al. Pattern of antioxidant and DNA repair gene expression in normal airway epithelium associated with lung cancer diagnosis. Cancer Res. 2009;69:8629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crawford EL, Levin A, Safi F, Lu M, Baugh A, Zhang X, et al. Lung cancer risk test trial: study design, participant baseline characteristics, bronchoscopy safety, and establishment of a biospecimen repository. BMC Pulm Med. 2016;16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. Clinical implications of multicentric origin. Cancer. 1953;6:963–8. [DOI] [PubMed] [Google Scholar]
- 42.Nelson HH, Christiani DC, Mark EJ, Wiencke JK, Wain JC, Kelsey KT. Implications and Prognostic Value of K-ras Mutation for Early-Stage Lung Cancer in Women. JNCI J Natl Cancer Inst. 1999;91:2032–8. [DOI] [PubMed] [Google Scholar]
- 43.Franklin WA, Gazdar AF, Haney J, Wistuba II, Rosa FGL, Kennedy T, et al. Widely dispersed p53 mutation in respiratory epithelium. A novel mechanism for field carcinogenesis. J Clin Invest. 1997;100:2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wistuba II, Lam S, Behrens C, Virmani AK, Fong KM, LeRiche J, et al. Molecular Damage in the Bronchial Epithelium of Current and Former Smokers. J Natl Cancer Inst. 1997;89:1366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao L, Lee JS, Kurie JM, Fan YH, Lippman SM, Lee JJ, et al. Clonal genetic alterations in the lungs of current and former smokers. J Natl Cancer Inst. 1997;89:857–62. [DOI] [PubMed] [Google Scholar]
- 46.Tang X, Shigematsu H, Bekele BN, Roth JA, Minna JD, Hong WK, et al. EGFR Tyrosine Kinase Domain Mutations Are Detected in Histologically Normal Respiratory Epithelium in Lung Cancer Patients. Cancer Res. 2005;65:7568–72. [DOI] [PubMed] [Google Scholar]
- 47.Gustafson AM, Soldi R, Anderlind C, Scholand MB, Qian J, Zhang X, et al. Airway PI3K pathway activation is an early and reversible event in lung cancer development. Sci Transl Med. 2010;2:26ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadara H, Shen L, Fujimoto J, Saintigny P, Chow C- W, Lang W, et al. Characterizing the molecular spatial and temporal field of injury in early-stage smoker non-small cell lung cancer patients after definitive surgery by expression profiling. Cancer Prev Res Phila Pa. 2013;6:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomperts BN, Walser TC, Spira A, Dubinett SM. Enriching the Molecular Definition of the Airway “Field of Cancerization:” Establishing New Paradigms for the Patient at Risk for Lung Cancer. Cancer Prev Res (Phila Pa). 2013;6:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kadara H, Fujimoto J, Yoo S- Y, Maki Y, Gower AC, Kabbout M, et al. Transcriptomic architecture of the adjacent airway field cancerization in non-small cell lung cancer. J Natl Cancer Inst. 2014;106:dju004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Detterbeck FC, Lewis SZ, Diekemper R, Addrizzo-Harris D, Alberts WM. Executive Summary:Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. CHEST. 2013;143:7S–37S. [DOI] [PubMed] [Google Scholar]
- 52.Haaf K ten, Jeon J, Tammemägi MC, Han SS, Kong CY, Plevritis SK, et al. Risk prediction models for selection of lung cancer screening candidates: A retrospective validation study. PLOS Med. 2017;14:e1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ost DE, Ernst A, Lei X, Kovitz KL, Benzaquen S, Diaz-Mendoza J, et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med. 2016;193:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spira A, Beane JE, Shah V, Steiling K, Liu G, Schembri F, et al. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med. 2007;13:361–6. [DOI] [PubMed] [Google Scholar]
- 55.Beane J, Sebastiani P, Whitfield TH, Steiling K, Dumas Y- M, Lenburg ME, et al. A prediction model for lung cancer diagnosis that integrates genomic and clinical features. Cancer Prev Res Phila Pa. 2008;1:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitney DH, Elashoff MR, Porta-Smith K, Gower AC, Vachani A, Ferguson JS, et al. Derivation of a bronchial genomic classifier for lung cancer in a prospective study of patients undergoing diagnostic bronchoscopy. BMC Med Genomics. 2015;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silvestri GA, Vachani A, Whitney D, Elashoff M, Porta Smith K, Ferguson JS, et al. A Bronchial Genomic Classifier for the Diagnostic Evaluation of Lung Cancer. N Engl J Med. 2015;0:null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stahl DL, Richard KM, Papadimos TJ. Complications of bronchoscopy: A concise synopsis. Int J Crit Illn Inj Sci. 2015;5:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boskovic T, Stanic J, Pena-Karan S, Zarogoulidis P, Drevelegas K, Katsikogiannis N, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis. 2014;6:S99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vachani A, Whitney DH, Parsons EC, Lenburg M, Ferguson JS, Silvestri GA, et al. Clinical Utility of a Bronchial Genomic Classifier in Patients With Suspected Lung Cancer. Chest. 2016;150:210–8. [DOI] [PubMed] [Google Scholar]
- 61.Ferguson JS, Van Wert R, Choi Y, Rosenbluth MJ, Smith KP, Huang J, et al. Impact of a bronchial genomic classifier on clinical decision making in patients undergoing diagnostic evaluation for lung cancer. BMC Pulm Med. 2016;16:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feller-Kopman D, Liu S, Geisler BP, DeCamp MM, Pietzsch JB. Cost-Effectiveness of a Bronchial Genomic Classifier for the Diagnostic Evaluation of Lung Cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2017;12:1223–32. [DOI] [PubMed] [Google Scholar]
- 63.Etheridge A, Lee I, Hood L, Galas D, Wang K. Extracellular microRNA: a new source of biomarkers. Mutat Res. 2011;717:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perdomo C, Campbell JD, Gerrein J, Tellez CS, Garrison CB, Walser TC, et al. MicroRNA 4423 is a primate-specific regulator of airway epithelial cell differentiation and lung carcinogenesis. Proc Natl Acad Sci U S A. 2013;110:18946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schembri F, Sridhar S, Perdomo C, Gustafson AM, Zhang X, Ergun A, et al. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:2319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pavel AB, Campbell JD, Liu G, Elashoff DA, Dubinett SM, Smith K, et al. Alterations in bronchial airway microRNA expression for lung cancer detection. Cancer Prev Res (Phila Pa). 2017;canprevres.0098.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X, Sebastiani P, Liu G, Schembri F, Zhang X, Dumas YM, et al. Similarities and differences between smoking-related gene expression in nasal and bronchial epithelium. Physiol Genomics. 2010;41:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.AEGIS Study Team. Shared Gene Expression Alterations in Nasal and Bronchial Epithelium for Lung Cancer Detection. J Natl Cancer Inst. 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sridhar S, Schembri F, Zeskind J, Shah V, Gustafson AM, Steiling K, et al. Smoking-induced gene expression changes in the bronchial airway are reflected in nasal and buccal epithelium. BMC Genomics. 2008;9:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang TW, Vermeulen RCH, Hu W, Liu G, Xiao X, Alekseyev Y, et al. Gene-expression profiling of buccal epithelium among non-smoking women exposed to household air pollution from smoky coal. Carcinogenesis. 2015;36:1494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mumford JL, He XZ, Chapman RS, Cao SR, Harris DB, Li XM, et al. Lung cancer and indoor air pollution in Xuan Wei, China. Science. 1987;235:217–20. [DOI] [PubMed] [Google Scholar]
- 72.Beane J, Mazzilli SA, Tassinari AM, Liu G, Zhang X, Liu H, et al. Detecting the Presence and Progression of Premalignant Lung Lesions via Airway Gene Expression. Clin Cancer Res. 2017;clincanres.2540.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


