Summary
Objective
Recent research in knee osteoarthritis (OA) highlights the role of the meniscus in OA pathology. Our aim was to compare the proteomes of medial and lateral menisci from end-stage medial compartment knee OA patients, with reference menisci from knee-healthy deceased donors, using mass spectrometry.
Design
Tissue plugs of Ø3 mm were obtained from the posterior horns of the lateral and medial menisci from one knee of 10 knee-healthy deceased donors and 10 patients undergoing knee replacement. Proteins were extracted and prepared for mass spectrometric analysis. Statistical analysis was conducted on abundance data that was log2-transformed, using a linear mixed effects model and evaluated using pathway analysis.
Results
We identified a total of 835 proteins in all samples, of which 331 were included in the statistical analysis. The largest differences could be seen between the medial menisci from OA patients and references, with most proteins showing higher intensities in the medial menisci from OA patients. Several matrix proteins, e.g., matrix metalloproteinase 3 (MMP3) (4.3 times higher values [95%CI 1.8, 10.6]), TIMP1 (3.5 [1.4, 8.5]), asporin (4.1 [1.7, 10.0]) and versican (4.4 [1.8, 10.9]), all showed higher abundance in medial menisci from OA patients compared to medial reference menisci. OA medial menisci also showed increased activation of several pathways involved in inflammation.
Conclusion
An increase in protein abundance for proteins such as MMP and TIMP1 in the medial menisci from OA patients suggests simultaneous activation of both catabolic and anabolic processes that warrants further attention.
Keywords: Osteoarthritis, Meniscus, Mass spectrometry, Extracellular matrix
Introduction
Osteoarthritis (OA) is a chronic joint disease traditionally characterized by loss of articular cartilage and changes in the underlying bone. However, recent research in knee OA has highlighted an important role for the meniscus in OA etiology and pathogenesis1, 2, 3. The menisci are two wedge shaped semi-circular fibrocartilage structures interposed between the femoral and tibial condyles in the knee, and their main function is load transmission2,3. Studies have reported that damage to the meniscus, which can occur due to acute knee trauma or as a result of gradual degenerative changes, is strongly associated with increased risk for knee OA4, 5, 6. Slow degradation of meniscal tissue has been suggested to occur in early stages of OA, however little is known of the molecular processes7,8. There is also very limited understanding of the molecular composition of the meniscus. Thus, more knowledge is needed about the meniscus, both in health and OA.
Mass spectrometry (MS) coupled with liquid chromatography (LC) is one of the most powerful methods to analyze protein content in complex samples. With non-targeted MS, it is possible to identify thousands of proteins in a single analysis9,10. In a previous study we investigated the proteomic differences between three zones (peripheral, middle and inner) of normal human menisci, as a first step to gain new knowledge about the human meniscus11. In the present study, our aim was to gain further insight into the role of the meniscus in OA pathogenesis, by comparing tissue plugs from the posterior horn of normal human menisci and menisci taken from medial compartment knee OA patients, using a global MS approach.
Material and methods
Materials
N-Ethylmaleimide, 6-aminocaproic acid, benzamidine hydrochloride hydrate, dithiothreitol (DTT), iodoacetamide, ammonium bicarbonate (AMBIC), formic acid, and HPLC grade acetonitrile and Solvents A (0.1% formic acid) and B (0.1% formic acid in acetonitrile) for liquid chromatography-mass spectrometry (LC-MS) were from Sigma–Aldrich (St. Louis MO, USA). Guanidine hydrochloride (GdnHCl) and anhydrous sodium acetate (NaAc) were from Merck (Darmstadt, Germany). Trypsin Gold (MS grade) was purchased from Promega (Madison WI, USA). The Pierce Quantitative Colorimetric Peptide Assay and SOLAμ™ Solid Phase Extraction (SPE) HRP (horse radish peroxidase) 2mg/1 ml 96-well plates were from Thermo Fisher Scientific (Rockford IL, USA), and Nanosep® 30K Omega Centrifugal Devices were from Pall Life Sciences (Ann Arbor MI, USA).
Selection of meniscus samples
We sampled meniscal tissue from a previously described biobank of human knee tissues at Skåne University Hospital, Lund, Sweden12,13, following approval by the regional ethics committee in Lund (2015/39 and 2016/865).
Reference menisci: From the biobank, we selected both medial and lateral menisci from the right knees of 10 adult, deceased donors (five women, five men) (convenience sample) with no known diagnoses of rheumatoid arthritis or knee OA. All menisci were obtained within 48 h post-mortem, and the specimens were frozen at −80°C within 2 h of extraction. To be eligible as references, the donor menisci were required to be macroscopically intact (some minor calcifications were allowed) [Fig. 1(A)]. Further, we also inspected the femoral cartilage (the load bearing region) from the medial compartment of the same donors (also in the biobank) and required the cartilage to be macroscopically intact.
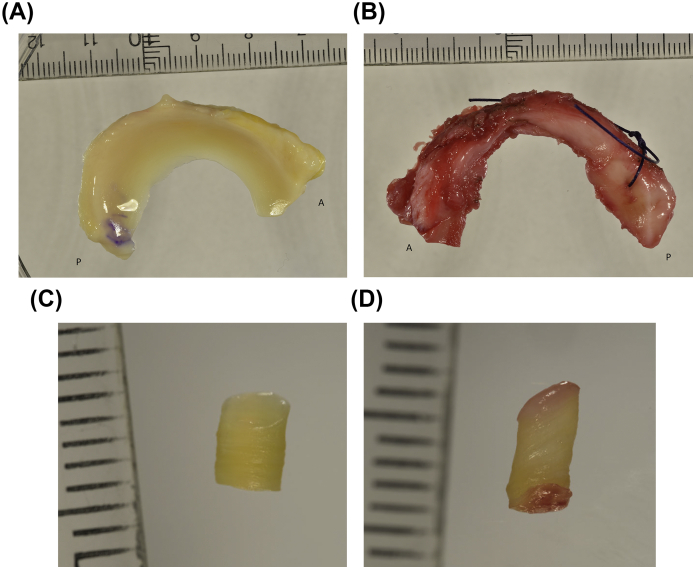
Fig. 1.
Representative images of A) reference meniscus (from a right knee), B) OA meniscus (from a left knee), C) plug from a reference meniscus and D) plug from an OA meniscus. In panels A and B, the annotations “A” and “P” refer to the anterior and posterior ends of the meniscus, respectively.
The donor menisci selected for this study will hereafter be called reference menisci, with samples from the medial and lateral menisci referred to as medialref and lateralref, respectively.
OA menisci: Medial and lateral menisci from 10 patients (five women, five men) (convenience sample) with medial compartment knee OA were selected from the biobank. The menisci were retrieved during total knee replacement (TKR) surgery and frozen at −80°C within 2 h after surgery at Trelleborg Hospital and later transported to Lund on dry ice for further storage at −80°C in the biobank. The surgeon classified the knee joint cartilage of all patients according to the Outerbridge classification system during surgery. In order to be classified as medial compartment knee OA, the Outerbridge grade was required to be IV (exposed bone) in the medial compartment and 0 (normal) or I (softening of cartilage) in the lateral compartment14. We further required the medial menisci from these patients to have at least two thirds of the substance of the posterior horn remaining (the inner one third was typically missing due to degeneration) [Fig. 1(B)]. The lateral OA menisci were usually intact at the macroscopic level.
Samples from the medial and lateral menisci from the medial compartment knee OA patients selected for this study will hereafter be referred to as medialOA and lateralOA, respectively.
Preparation of meniscal tissue for MS analysis
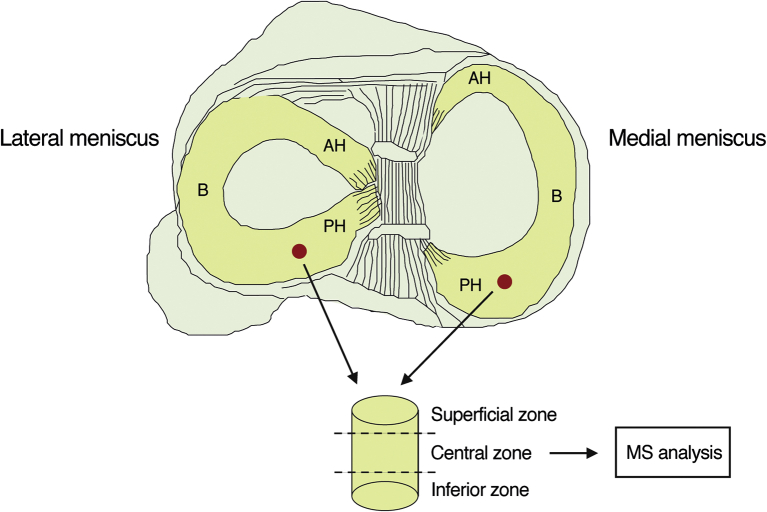
Menisci were thawed in phosphate-buffered saline (PBS). To prepare the tissue samples for MS analysis, a hole (3 mm in diameter) was punched vertically through the central part of the posterior horn (approximately 3–5 mm from the capsular insertion, i.e., in the thicker peripheral part of the meniscus) [Fig. 1, Fig. 2]. The superficial and inferior ends (1/4 each) of the plugs (n = 40) were cut off and stored at −80°C (Fig. 2). The central parts of the plugs were pulverized in liquid nitrogen using a pestle and mortal technique, after which the pulverized tissue was weighed. Proteins were extracted using 15 volumes (15 μL buffer/mg dry powder) of chaotropic buffer (4 M GdnHCl, 50 mM NaAc, 100 mM 6-aminocaproic acid, 5 mM benzamidine, 5 mM N-ethylmaleimide, pH 5.8), incubated for 24 h on an orbital shaker at +4°C, and centrifuged at 13,200×g at +4°C for 30 min. The resulting supernatant was used for further analysis. This process was repeated twice on the extraction pellet remaining after the centrifugation.
Fig. 2.
Top: Schematic figure showing the location of the collected meniscal plugs from lateral and medial menisci. AH: anterior horn, B: body, PH: posterior horn. Bottom: Of the different zones of the plugs, only the central zone was used for MS analysis.
Equal volumes from the three sequential extracts were pooled, resulting in 180 μL of extract per sample. For three of the samples it was only possible to take 20 μL from each extraction round due to sample losses during preparation. Pooled samples were reduced using 4 mM DTT, with shaking at +56°C for 30 min. Extracts were alkylated using 16 mM iodoacetamide for 1 h at room temperature in the dark. In order to remove residual salts, extracts were precipitated with 9 volumes of ethanol incubated for 4 h at 20°C, after which the precipitate was dried in a SpeedVac and suspended in 100 μL of 0.1 M AMBIC buffer, pH 8.5. All samples were digested using 2 μg Trypsin Gold, by incubating on a shaker at +37°C for approximately 16 h. Peptide concentrations of the digests were determined using the Pierce Quantitative Colorimetric Peptide Assay according to the manufacturer's instructions. Samples (30 μg) were diluted to 200 μL in 50 mM AMBIC buffer with 0.5 M sodium chloride (to minimize ionic interactions). In order to remove peptides with glycosaminoglycan (GAG) chains from the samples, they were centrifuged through Nanosep® 30K Omega Centrifugal Devices. Samples were subsequently desalted using Thermo Scientific™ SOLAμ™ SPE plates and eluted using 50% acetonitrile in 0.1% formic acid.
At the end of sample preparation, from the original 40 samples selected for analysis (i.e., 10 samples each of medialOA, lateralOA, medialref, and lateralref), seven had to be omitted due to sample preparation problems and lack of sufficient material. This resulted in n = 9 medialOA, n = 7 lateralOA, n = 8 medialref, and n = 9 lateralref samples for further MS analysis.
Mass spectrometry analysis
Digested samples were analyzed using a Q-Exactive™ HF-X quadrupole Orbitrap benchtop mass spectrometer (Thermo Fisher Scientific). Equal amounts of the protein digests were injected into an Easy nano-LC 1000 HPLC system (Thermo Fisher Scientific) equipped with an Acclaim PepMap® 100 nanoViper pre-column (Thermo Scientific, C18, 3 μm particles, 75 μm i.d. 2 cm long) and a PepMap® RSLC C18 analytical column (Thermo Scientific, C18, 2 μm particles, 75 μm i.d. 25 cm long) (RSLC: rapid separation liquid chromatography). On-line reversed-phase separation was performed using a flow rate of 300 nL/min. A binary gradient of 125 min was used, starting with a 5 min increase from 5% B to 7% B, then increasing to 20% B over 85 min, further increasing to 30% B over 20 min, and with a final 5 min increase to 90% B, after which it ended with 90% B isocratic for 10 min. The system was controlled by Xcalibur™ Software (Thermo Fisher Scientific), and blank runs were injected between every sample to avoid cross-contamination. A heated ion transfer setting of 280°C was used for desolvation, together with a spray voltage of +1850 V.
Data-independent acquisition (DIA)
We ran all samples in Data-independent acquisition (DIA) mode using a blocked randomization sequence. Each block contained one sample from each group, and the medial and lateral samples from each reference subject and OA patient were run in the same block. Two randomly selected samples from each group were selected to be run in duplicates to perform reliability assessment. All duplicates were run in a randomized order within the same block. The first replicate of each duplicate was used for statistical analysis.
For the MS settings, the full MS scan (350–1650 m/z) was set to a resolution of 120,000, with 3.0 × 106 automatic gain control (AGC), and 100 ms maximum ion injection time. This was followed by DIA collision-induced dissociation MS2 scans at a resolution of 45,000, with 3.0 × 106 AGC, and an automatic maximum ion injection time. A loop count of 26 was used with variable isolation windows (Table S1). To check for instrument performance, a pooled sample was injected every 10th injection.
Data-dependent acquisition (DDA)
Fourteen samples representing all sample groups (i.e., medialref, lateralref, medialOA and lateralOA) were randomly selected to be run in Data dependent acquisition (DDA) mode in order to make a combined spectral library using both DDA and DIA data for the quantitative data analysis. Eight of the DDA runs were run before the DIA runs, and six were run after. The run order was randomized. For the DDA analysis, the full MS scan (375–1400 m/z) was set to a resolution of 120,000, with 3.0 × 106 AGC, and 50 ms maximum ion injection time. This was followed by collision-induced dissociation MS2 scans with a resolution of 15,000, AGC of 1.0 × 105 and maximum injection time of 30 ms.
Data analysis
A total of 61 MS runs (14 DDA and 47 DIA) of the meniscal samples were converted to HTRMS format using HTRMS Converter (Biognosys AG, Switzerland) in order to decrease data analysis search time. A spectral library was created in Spectronaut X™ Pulsar (version 12.0.20491.15, Biognosys AG) using all DIA and DDA runs. Default settings (BGS factory settings) were used with additional modifications: cysteine carbamidomethylation as a fixed modification, and deamination, pyro-glutamic acid (N-term Glu to pyroglutamic acid), methionine oxidation, hydroxyproline and acetylation as variable modifications. The human protein database (20190416_UP152602_n20415) was used as the background proteome. A subsequent protein search was conducted in Spectronaut™ Pulsar using the recently created spectral library and the same human database as background proteome. Default settings were used for the search. Precursor quantitation was performed at MS2 level, and area under the curve was used as quantitation type. The top three peptides (proteotypic) for each protein were averaged to calculate protein abundance15.
Statistical analysis
In the statistical analysis, we included proteins with a maximum of one missing value per sample group (medialref, lateralref, medialOA and lateralOA), which provided a total of 331 proteins for analysis. All intensities were log2-transformed before the analysis. Thus, the presented estimates in figures and tables are differences between mean log2 intensities between groups, whose inverse 2x (where x is the estimate), provides the ratio of the geometric means (often referred to as fold-changes, which are the estimates presented in the text). We used a linear mixed-effects model, with the sample group, protein type, and their interactions as independent variables, and transformed intensities as the outcome. Subject identifiers were included as random effects to account for correlation between measurements from the same individual. We adjusted the model for the potential confounders age and body mass index (BMI). This adjustment has very limited impact on the within person comparisons (i.e., medialOA vs lateralOA and medialref vs lateralref), which are adjusted for person-invariant confounders by design. Using one multilevel model for simultaneous analysis of all included proteins results in more efficient estimation and better control of so-called type I error16. Presented estimates are with 95% confidence intervals (CI).
Functional enrichment analysis
A functional enrichment analysis was performed in Ingenuity® Pathway Analysis (IPA®, QIAGEN Bioinformatics, Fall release, 2019) to determine if any pathways were overrepresented in our data. In this analysis, we focused on the comparison between medialOA and medialref menisci, and only proteins with a log2 intensity difference of over 1.2 or under −1.2 (i.e., fold-changes over 2.3 or under 0.4) were included. IPA® also evaluates the proteins based on upstream regulators and calculates a z-score; positive for regulators with activating capacity and negative for those with inhibitory capacity. The z-score is a statistical measure of the match between the expected direction of the relationship and the observed protein expression, which is used to determine how strong the overrepresentation is. Default settings were used, with species set to human and all identified proteins in this study used as background proteome. Five proteins (hemoglobin subunit alpha (P69905), histone H4 (P62805), and immunoglobulin chains alpha-2 heavy (P0DOX2), gamma-1 heavy (P0DOX5) and kappa light (P0DOX7)) could not be mapped in IPA and therefore had to be excluded from the analysis.
Results
The 10 reference subjects had a median age of 51 years (range 18–77) and median BMI of 26.2 (range 16.4–42.4) (Table I). The nine OA patients (after exclusion of one patient due to sample loss) had a median age of 61 years (range 50–75) and median BMI of 28.7 (range 26.5–37.4) (Table I).
Table I.
Characteristics of the study sample
| Subject number | Group | Sex | Age (years)∗ | BMI (kg/m2) | Knee side | Medial (M)/Lateral (L)† |
|---|---|---|---|---|---|---|
| 1 | Reference | Female | 18 | 16.4 | Right | M |
| 2 | Reference | Female | 32 | 22.8 | Right | M/L |
| 3 | Reference | Female | 61 | 23.3 | Right | M |
| 4 | Reference | Female | 74 | 25.5 | Right | M/L |
| 5 | Reference | Female | 77 | 22.3 | Right | M/L |
| 6 | Reference | Male | 49 | 33.0 | Right | L |
| 7 | Reference | Male | 50 | 34.2 | Right | M/L |
| 8 | Reference | Male | 52 | 26.8 | Right | M/L |
| 9 | Reference | Male | 58 | 33.2 | Right | M/L |
| 10 | Reference | Male | 43 | 42.4 | Right | M/L |
| References: Mean (SD) |
– |
– |
51.4 (17.9) |
28.0 (7.6) |
– |
|
| 11 | OA | Female | 50 | 37.4 | Left | M/L |
| 12 | OA | Female | 53 | 22.5 | Left | M |
| 13 | OA | Female | 60 | 29.4 | Left | M/L |
| 14 | OA | Female | 61 | 30.5 | Right | M/L |
| 15 | OA | Female | 61 | 25.9 | Right | M |
| 16 | OA | Male | 61 | 30.4 | Right | M/L |
| 17 | OA | Male | 65 | 27.5 | Right | M/L |
| 18 | OA | Male | 72 | 28.7 | Left | M/L |
| 19 |
OA |
Male |
75 |
28.7 |
Right |
M/L |
| OA patients: Mean (SD) |
– |
– |
62 (8.0) |
29.0 (4.0) |
– |
|
| All subjects: Mean (SD) | – | – | 56.4 (14.7) | 28.5 (6.0) | – |
At time of death (references) or at the time of surgery (OA patients).
Menisci included in the final MS analysis.
In total, we identified a total of 835 proteins. However, in order to be included in the statistical analysis, proteins were allowed to have a maximum of one missing value per sample group, which resulted in 331 proteins remaining. Of these, 217 proteins had no missing values at all. There were on average more missing values in the reference menisci compared to the OA menisci (Supplementary Table S2).
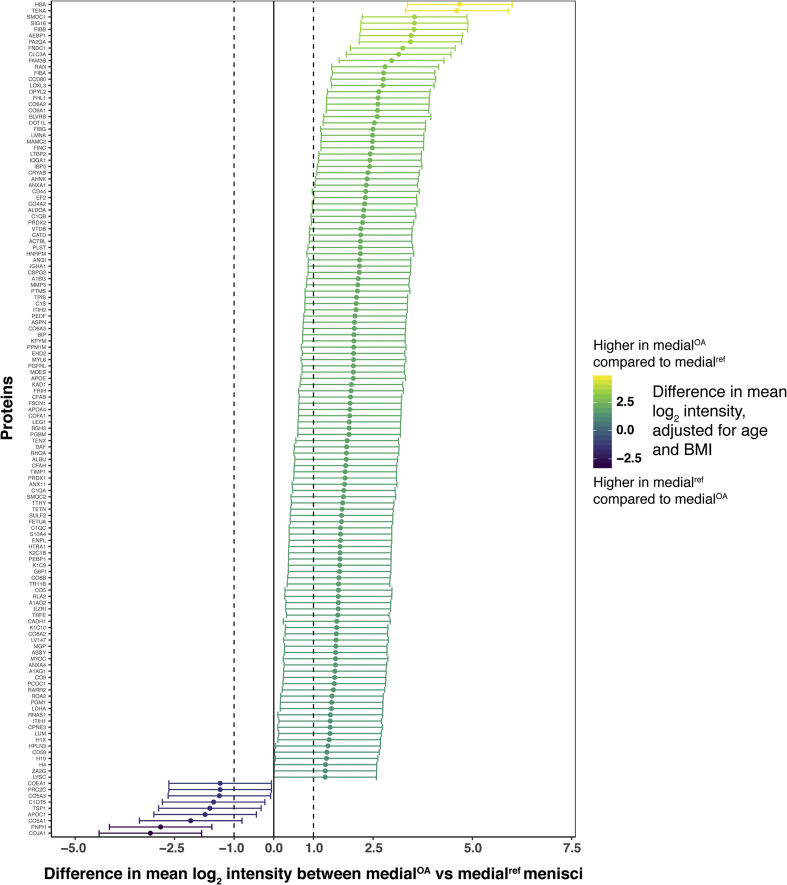
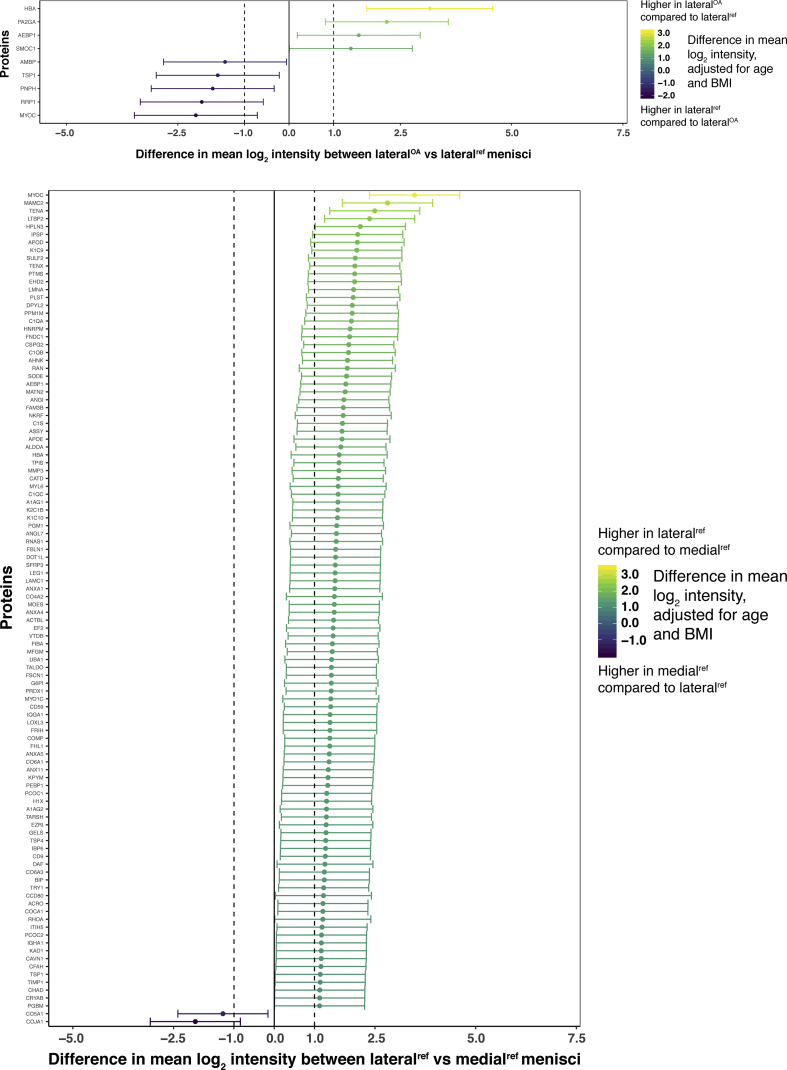
The biggest differences in protein intensities were found between the medialOA and medialref menisci, with a majority of proteins showing a higher mean log2 intensity in the medialOA group [Fig. 3(A)]. In contrast, very few differences were found between the lateralOA and lateralref menisci [Fig. 3(B)]. In within-person comparisons of lateral and medial menisci from the same knees, lateralref generally had higher log2 protein intensities than medialref among reference menisci [Fig. 3(C)], whereas most proteins had similar intensities in lateralOA and medialOA menisci [Fig. 3(D)]. All comparisons of proteins included in the statistical analysis is detailed in Table S3 and Figs. S1–4.
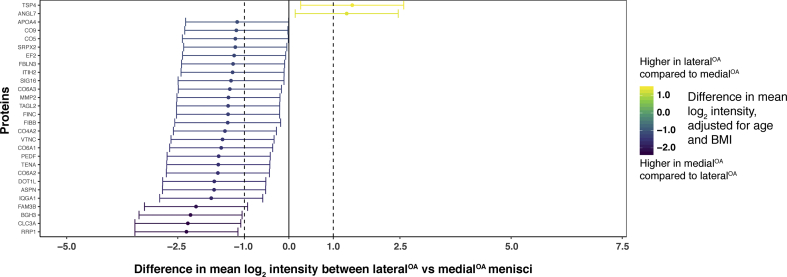
Fig. 3.
Visual representation of differentially expressed proteins (y-axis) in A) medialOA vs medialref menisci, B) lateralref vs medialref menisci, C) lateralOA vs medialOA menisci and D) lateralOA vs lateralref menisci. Log2-transformed mean intensity differences are displayed as point estimates with 95% confidence intervals as error bars (x-axis). The color of the point estimates and error bars represents the difference in log2 mean intensity between the groups, according to the scale bar on the right.
The protein with the largest fold-change between OA and reference menisci (both medial and lateral) was hemoglobin subunit alpha (HBA), with a higher mean intensity in the OA menisci (fold-change 25.63 with 95% CI [10.27, 64.0] for the medial menisci and 8.94 [3.36, 23.92] for the lateral) [Fig. 3(A) and (D)]. The protein with the largest fold-change between medialOA and medialref menisci, but with a higher mean intensity in the medialref menisci (0.12 [0.05, 0.28]) [Fig. 3(A)], as well as between lateralref and medialref menisci (0.26 [0.12, 0.56]) [Fig. 3(C)], was collagen alpha-1(XIX) chain (COJA1). Myocilin (MYOC) was the protein with the largest fold-change between lateralref menisci and lateralOA menisci (0.23 [0.09, 0.61]) (Fig. 3(B), as well as between medialref menisci and lateralref menisci (11.16 [5.13, 24.25]) [Fig. 3(C)]. Finally, the protein with the largest fold-change between lateralOA menisci and medialOA menisci was thrombospondin-4 (TSP4) (2.69 [1.21, 6.02]), whereas it was ribosomal RNA processing protein 1 homolog A (RRP1) in medialOA menisci compared to lateralOA menisci (0.20 [0.09, 0.45]) [Fig. 3(D)].
Several proteoglycans were identified in this study, including 10 major small leucine-rich proteoglycans (SLRPs). In comparing medialOA menisci to medialref menisci, all but one of the SLRPs had a higher intensity in the medialOA menisci. The two SLRPs with the largest fold-changes were asporin (4.08 [1.67, 9.99]) and lumican (2.68 [1.09, 6.54]) [Fig. 3(A)]. Among the SLRPs, decorin was the only protein with a lower mean intensity in the medialOA menisci compared to the medialref menisci, with a fold-change of 0.51 [0.21, 1.25]) (Table S3). All SLRPs had similar intensities in lateralOA and lateralref menisci (Table S3). In within-person comparisons between lateral and medial menisci, only asporin was differentially expressed between lateralOA and medialOA menisci (increased in medialOA with a fold-change of 0.31 [0.14, 0.70]), whereas it had a fold-change of 1.89 [0.87, 4.08] between the medialref and lateralref menisci [Fig. 3(C) and (D)], and chondroadherin was the only differentially expressed SLRP between lateralref and medialref menisci (increased in lateralref with a fold-change of 2.18 [1.0, 4.76]), with a similar fold-change between the lateralOA menisci and medialOA menisci (1.41 [0.63, 3.16]) [Fig. 3(C) and (D)].
The other proteoglycans identified in this study, aggrecan, versican, perlecan, and collagen alpha-1 (XVIII) chain, were all increased in medialOA compared to medialref menisci, where versican and perlecan had the largest fold-changes. Both were increased in medialOA compared to medialref menisci, with fold-changes of 4.44 [1.82, 10.85] and 3.73 [1.53, 9.13], respectively [Fig. 3(A)]. The same two proteoglycans were also elevated in lateralref vs medialref menisci from the same knees, with fold-changes of 3.61 [1.66, 7.84] and 2.17 [1.0, 4.72], respectively [Fig. 3(C)]. Both of these proteoglycans had similar intensities in lateralOA compared to lateralref menisci (versican: 0.82 [0.31, 2.13], perlecan: 1.07 [0.41, 2.79]) and lateralOA compared to medialOA menisci (versican: 0.66 [0.30, 1.48], perlecan: 0.63 [0.28, 1.40]) (Table S3).
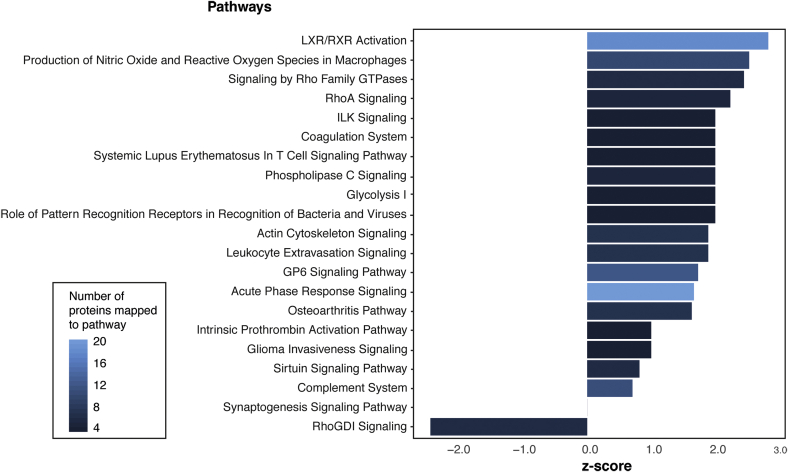
Furthermore, we identified several canonical pathways that were enriched in the comparison between medialOA and medialref menisci (Fig. 4 and Table S4). All pathways were increased in medialOA menisci except RhoGDI signaling (z-score −2.45) which was decreased. Liver X receptors (LXR)/retinoid X receptors (RXR) activation had the highest z-score among the pathways increased in medialOA menisci (z-score 2.83). In addition, many of the upregulated pathways were associated with inflammation.
Fig. 4.
Results from the pathway analysis on the proteins differing between the medialOA and medialref menisci, which was performed in IPA®. Bar plot displaying the most increased or decreased pathways mapped to the proteins. Z-score is a statistical measure of the match between the expected direction of the relationship and the observed protein expression and a higher z-score corresponds to a stronger overrepresentation. The color of the bars represents the number of proteins that could be mapped to that specific pathway.
Discussion
We compared the proteome of human meniscal posterior horns from knee-healthy references and end-stage medial compartment knee OA patients. Medial menisci from OA patients compared to references shows clear differences in protein expression, consistent with the disease pathology.
Several proteoglycans showed higher intensity in medialOA menisci compared to the medialref menisci. It might seem counter-intuitive to see an increase in proteoglycans in osteoarthritic tissue. However, in a previous study where we investigated the proteoglycan content in the same reference and OA menisci as in this study, by staining meniscus sections with Safranin-O-Fast Green staining, we observed increased staining with increasing degeneration of the menisci13. This finding is supported by others, and have been hypothesized to be an attempt at repair17,18.
Interestingly, HBA was the protein with the largest difference between OA and reference menisci (both medial and lateral), with a higher mean intensity in the OA menisci. In addition, several high abundant human plasma proteins also had higher mean intensities in the medialOA menisci. This might be due to the fact that there was intra-articular bleeding in the OA patients' knees during TKR surgery, with collected menisci contaminated with blood. However, before dissection, every meniscus was rinsed with PBS. The OA menisci should therefore, in theory, have been as free of blood on the surface as the reference menisci. Instead, since menisci are vascularized19, another possible explanation might be that there are internal hemorrhages within the menisci of OA patients, caused by tissue damage and degeneration.
The glycoprotein myocilin showed the highest mean intensity in lateralref menisci compared to lateralOA menisci. We previously identified myocilin in both articular cartilage and meniscus, found to be more abundant in the meniscus than articular cartilage20. In addition, a previous proteomic study reported higher expression of myocilin in synovial fluid from healthy controls compared to individuals with meniscal injuries or pathologies21, which is consistent with our findings. This indicates that myocilin might be involved in pathological processes of the meniscus, having a possible role in the pathogenesis of OA.
In the pathway analysis, several pathways could be mapped to the proteins that differed between the medialOA and medialref menisci. The pathway with the highest z-score was LXR/RXR activation, which was increased in the medialOA menisci. As members of the nuclear receptor superfamily, LXR and RXR can form heterodimers with each other, and function as transcription factors that regulate many different physiological processes22,23. Interestingly, studies have reported the involvement or dysregulation of LXR/RXR pathways in OA tissue24, 25, 26, 27. One of these studies reported that treatment of murine articular chondrocytes with RXR agonists decreased the expression of aggrecan and fibrillin-2 genes and increased the expression of MMP13 genes, changes associated with tissue degradation25. Interestingly, the same study also reported an opposite effect for treatment with LXR agonists, which decreased the expression of MMP13 and MMP2, which are genes associated with cartilage breakdown, indicating that LXR activation might act protectively on articular cartilage. We also observed a simultaneous increase of both the proteinase MMP3, which induces breakdown of cartilage, as well as its inhibitor TIMP1, in the medialOA menisci compared to the medialref menisci [Fig. 3(A)]. Taken together, these results indicate that there is an on-going degradation process in the OA menisci, but also a simultaneous attempt to stop the degradation.
Furthermore, one of the most striking results from the pathway analysis was the activation of several pathway associated with inflammation, such as production of nitric oxide and reactive oxygen species in macrophages, acute phase response signaling and complement system. Traditionally, OA has not been described as an inflammatory disease, however, it has been become widely accepted that inflammation plays a role in OA, with higher levels of inflammatory cytokines in synovial fluid and serum of OA patients28, 29, 30, 31. In addition, a study comparing the transcriptome of OA and non-OAOA menisci, reported an increase of genes involved in inflammation, in the OA menisci, supporting our findings.
Several of the differentially expressed proteins were collagens. However, this might not be a result of biological differences, but rather a result of the protein extraction method used in this study, which is based on guanidine hydrochloride. This method is not optimal for collagen extraction and depending on the extent of collagen cross-linking or subtypes in different samples, the results may vary32. It might therefore be premature to make any interpretation about collagens from our study, without further validation.
Finally, we would like to highlight some important limitations in our study. First, due to sample preparation issues, seven samples had to be omitted from the original 40 samples planned for analysis. However, since we consider this to be a pilot study, this was deemed acceptable and we acknowledge the need for future replication studies. Second, even though we use macroscopically intact menisci from donors without known OA, we recognize that there are variations among the donors in the extent of tissue degradation, which is why we prefer to refer to them as reference menisci, rather than “healthy” or “normal”. Further, unfortunately, the cause to the patients’ knee OA was not known, but is also in practice hard to ascertain as it is often a combination of hereditary and environmental factors. Furthermore, we observed a large amount of missing values in our dataset, and decided to use a very stringent approach of excluding proteins with more than one missing value per sample group, which decreased the number of proteins for analysis from 835 to 331. There are several possible reasons as to why there were so many missing values. One reason is that some proteins were of low abundance and could not be quantified at all, or only in some of the samples. However, several proteins exhibited interesting expression patterns, having many missing values in the reference samples and few in the OA samples (Table S2). This, together with the fact that the medial menisci appeared to have more missing values than the lateral menisci, indicates that there may be a biological explanation to the missing data. It would therefore be interesting to, in future studies, look more closely at the proteins with interesting patterns of missing data. Moreover, peptides with GAG chains were removed in order to avoid introducing GAG chains to the LC-MS system as they are detrimental for its performance. This could result in a potential loss of proteins via interactions with GAG chains (at peptide level), however this is circumvented by performing this step with high salt levels and thus these proteins should be detected as usual. Finally, there is unfortunately no material left for validation of our findings by other methods.
In conclusion, it appears that the largest differences in the meniscal proteome, as expected, could be seen between medialOA and medialref menisci. The majority of the proteins that differed, e.g., MMP3, TIMP1 and several proteoglycans, had higher intensities in the medialOA menisci, indicating that there is an overall activation of proteins in the menisci in the diseased OA compartment, both catabolic and anabolic.
Author contributions
Conception and design: EF, PÖ, AT and MEProvision of study materials and tissue preparation: ME, EF, VH, PÖ, JT, NA MS analysis: EF and PÖ Statistical analysis: EF, AT, NA and MR Interpretation of results: All co-authors Drafting of the article: EF Critical revision of the article for important intellectual content: MR, AT, PÖ, VH, JT, NA and ME Final approval of the article: All co-authors.
Conflict of interest
-
•
AT works as an associate editor (statistics) in Osteoarthritis and Cartilage.
-
•
ME reports grants from The European Research Council (ERC), The Foundation for Research in Rheumatology (FOREUM), The Swedish Research Council, The Alfred Österlund Foundation, The Crafoord Foundation, The Swedish Rheumatology Association, The IngaBritt and Arne Lundberg Research Foundation, The Greta and Johan Kock Foundation, and Governmental Funding of Clinical Research within the National Health Service (ALF)
-
•
Other authors (EF, PÖ, NA, JT, VH, MR) report no conflicts of interst.
Funding
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement #771121) (ME) and the Foundation for Research in Rheumatology (FOREUM) (ME). The study was also funded by grants from the Swedish Research Council (2014-2348 ME), the Alfred Österlund Foundation (ME, PÖ), the Crafoord Foundation (ME, PÖ), the Anna-Greta Crafoord Foundation (PÖ), the Swedish Rheumatology Association (ME, PÖ), the IngaBritt and Arne Lundberg Research Foundation (ME, PÖ, MS-instrument), the Krapperup Foundation (PÖ), the Olle Engkvist Foundation (PÖ), the Greta and Johan Kock Foundation (PÖ, ME), and Governmental Funding of Clinical Research within the National Health Service (ALF) (ME).
Role of funding source
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
We would like to thank the personnel at the Tissue Bank (Vävnadsbanken) at Skåne University Hospital in Lund and the Division of Forensic Medicine in Lund for the collection of reference menisci. We would also like to thank the personnel at the orthopaedic surgery department of Trelleborg Hospital for collection of menisci from OA patients.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.joca.2020.05.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Englund M., Haugen I.K., Guermazi A., Roemer F.W., Niu J., Neogi T. Evidence that meniscus damage may be a component of osteoarthritis: the Framingham study. Osteoarthritis Cartilage. 2016;24(2):270–273. doi: 10.1016/j.joca.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook J.L., Kuroki K., Stoker A.M., Monibi F.A., Roller B.L. Meniscal biology in health and disease. Connect Tissue Res. 2016 doi: 10.1080/03008207.2016.1243670. [DOI] [PubMed] [Google Scholar]
- 3.Fox A., Wanivenhaus F., Burge A.J., Warren R.F., Rodeo S.A. The human meniscus: a review of anatomy, function, injury, and advances in treatment. Clin Anat. 2015;28(2):269–287. doi: 10.1002/ca.22456. [DOI] [PubMed] [Google Scholar]
- 4.Melrose J., Fuller E.S., Little C.B. The biology of meniscal pathology in osteoarthritis and its contribution to joint disease: beyond simple mechanics. Connect Tissue Res. 2017 doi: 10.1080/03008207.2017.1284824. [DOI] [PubMed] [Google Scholar]
- 5.Kumm J., Roemer F.W., Guermazi A., Turkiewicz A., Englund M. Natural history of intrameniscal signal intensity on knee MR images: six years of data from the osteoarthritis initiative. Radiology. 2016;278(1):164–171. doi: 10.1148/radiol.2015142905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Englund M., Guermazi A., Roemer F.W., Aliabadi P., Yang M., Lewis C.E. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: the multicenter osteoarthritis study. Arthritis Rheum. 2009;60(3):831–839. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rai M., Brophy R.H., Sandell L.J. Osteoarthritis following meniscus and ligament injury. Curr Opin Rheumatol. 2019;31(1):70–79. doi: 10.1097/bor.0000000000000566. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Romero C., Rego-Perez I., Blanco F.J. What did we learn from ‘omics’ studies in osteoarthritis. Curr Opin Rheumatol. 2018;30(1):114–120. doi: 10.1097/bor.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 9.Schubert O.T., Röst H.L., Collins B.C., Rosenberger G., Aebersold R. Quantitative proteomics: challenges and opportunities in basic and applied research. Nat Protoc. 2017;12(7):1289. doi: 10.1038/nprot.2017.040. [DOI] [PubMed] [Google Scholar]
- 10.Old W.M., Meyer-Arendt K., Aveline-Wolf L., Pierce K.G., Mendoza A., Sevinsky J.R. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics. 2005;4(10):1487–1502. doi: 10.1074/mcp.m500084-mcp200. [DOI] [PubMed] [Google Scholar]
- 11.Folkesson E., Turkiewicz A., Hughes V., Ali N., Tjörnstrand J., Önnerfjord P. Mapping the protein distribution in zones of the healthy human medial meniscus body. Osteoarthritis Cartilage. 2019;27:S476–S477. doi: 10.1016/j.joca.2019.02.523. [DOI] [Google Scholar]
- 12.Olsson E., Folkesson E., Peterson P., Önnerfjord P., Tjörnstrand J., Hughes H.V. Ultra-high field magnetic resonance imaging parameter mapping in the posterior horn of ex vivo human menisci. Osteoarthritis Cartilage. 2018;27:476–483. doi: 10.1016/j.joca.2018.12.003. (Ann Rheum Dis 70 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kestilä I., Folkesson E., Finnilä M.A., Turkiewicz A., Önnerfjord P., Hughes V. Three-dimensional microstructure of human meniscus posterior horn in health and osteoarthritis. Osteoarthritis Cartilage. 2019 doi: 10.1016/j.joca.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Outerbridge R. The etiology of chondromalacia patellae. J Bone Jt Surg Br Vol. 1961;43-B(4):752–757. doi: 10.1302/0301-620x.43b4.752. [DOI] [PubMed] [Google Scholar]
- 15.Silva J.C., Gorenstein M.V., Li G.-Z., Vissers J.P., Geromanos S.J. Absolute quantification of proteins by LCMSE A virtue of parallel ms acquisition. Mol Cell Proteomics. 2006;5(1):144–156. doi: 10.1074/mcp.m500230-mcp200. [DOI] [PubMed] [Google Scholar]
- 16.Ji H., Liu S.X. Analyzing ’omics data using hierarchical models. Nat Biotechnol. 2010;28(4):337–340. doi: 10.1038/nbt.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams M.E., Billingham M.E., Muir H. The glycosaminoglycans in menisci in experimental and natural osteoarthritis. Arthritis Rheum. 1983;26(1):69–76. doi: 10.1002/art.1780260111. [DOI] [PubMed] [Google Scholar]
- 18.Herwig J., Egner E., Buddecke E. Chemical changes of human knee joint menisci in various stages of degeneration. Ann Rheum Dis. 1984;43(4):635. doi: 10.1136/ard.43.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnoczky S.P., Warren R.F. Microvasculature of the human meniscus. Am J Sports Med. 1982;10(2):90–95. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- 20.Folkesson E., Turkiewicz A., Englund M., Önnerfjord P. Differential protein expression in human knee articular cartilage and medial meniscus using two different proteomic methods: a pilot analysis. BMC Muscoskel Disord. 2018;19(1):416. doi: 10.1186/s12891-018-2346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roller B.L., Monibi F., Stoker A.M., Bal S.B., Cook J.L. Identification of novel synovial fluid biomarkers associated with meniscal pathology. J Knee Surg. 2014;29(1):47–62. doi: 10.1055/s-0034-1394165. [DOI] [PubMed] [Google Scholar]
- 22.Hiebl V., Ladurner A., Latkolik S., Dirsch V.M. Natural products as modulators of the nuclear receptors and metabolic sensors LXR, FXR and RXR. Biotechnol Adv. 2018;36(6):1657–1698. doi: 10.1016/j.biotechadv.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Willy P., Umesono K., Ong E., Evans R., Heyman R., Mangelsdorf D. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Gene Dev. 1995;9(9):1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 24.Wanner J., Subbaiah R., Skomorovska-Prokvolit Y., Shishani Y., Boilard E., Mohan S. Proteomic profiling and functional characterization of early and late shoulder osteoarthritis. Arthritis Res Ther. 2013;15(6):R180. doi: 10.1186/ar4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratneswaran A., Sun M., Dupuis H., Sawyez C., Borradaile N., Beier F. Nuclear receptors regulate lipid metabolism and oxidative stress markers in chondrocytes. J Mol Med. 2017;95(4):431–444. doi: 10.1007/s00109-016-1501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun M.M.-G., Beier F. Liver X Receptor activation delays chondrocyte hypertrophy during endochondral bone growth. Osteoarthritis Cartilage. 2014;22(7):996–1006. doi: 10.1016/j.joca.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Collins-Racie L.A., Yang Z., Arai M., Majumdarf M.K., Nagpal S., Mounts W.M. Global analysis of nuclear receptor expression and dysregulation in human osteoarthritic articular cartilage Reduced LXR signaling contributes to catabolic metabolism typical of osteoarthritis. Osteoarthritis Cartilage. 2009;17(7):832–842. doi: 10.1016/j.joca.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Sohn D., Sokolove J., Sharpe O., Erhart J.C., Chandra P.E., Lahey L.J. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14(1):R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sokolove J., Lepus C.M. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94. doi: 10.1177/1759720x12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mabey T., Honsawek S., Tanavalee A., Yuktanandana P., Wilairatana V., Poovorawan Y. Plasma and synovial fluid inflammatory cytokine profiles in primary knee osteoarthritis. Biomarkers. 2016;21(7):639–644. doi: 10.3109/1354750x.2016.1171907. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko S., Satoh T., Chiba J., Ju C., Inoue K., Kagawa J. Interleukin–6 and interleukin–8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6(2):71–79. doi: 10.1080/13684730050515796. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Li Y., Khabut A., Chubinskaya S., Grodzinsky A.J., Önnerfjord P. Quantitative proteomics analysis of cartilage response to mechanical injury and cytokine treatment. Matrix Biol. 2017;63 doi: 10.1016/j.matbio.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.