Abstract
Objective:
The objective of this research is to determine the association of seven perfluoroalkyl and polyfluoroalkyl substances versus dental caries experience in US children, ages 3–11 years.
Methods:
A cross-sectional study design was used in the analysis of National Health and Nutrition Examination Survey 2013–2014 serological data of perfluoroalkyl and polyfluoroalkyl substances. The seven perfluoroalkyl and polyfluoroalkyl substances were: 2-(N-methyl-perfluorooctane sulfonamide) acetic acid; perfluorodecanoic acid; perfluorononanoic acid; perfluorohexane sulfonic acid; linear isomers of perfluorooctanoate; linear perfluorooctane sulfonate; and monomethyl branched isomers of perfluorooctane sulfonate. Two summative variables were created: monomethyl branch isomers of perfluorooctane sulfonic acid with linear isomer of perfluorooctane and branch isomers of perfluorooctanoate with linear isomer perfluorooctonate.
Results:
In unadjusted logistic regression, in which the comparison was between the less than 75th percentile reference group and the 75th and above percentile group, higher perfluorodecanoic acid was associated with dental caries experience [unadjusted odds ratio: 1.79 (95% CI: 1.19, 2.46; P = 0.0069); adjusted odds ratio: 1.54 (95% CI: 1.03, 2.30; P = 0.0385)].
Conclusions:
Of the seven examined perfluoroalkyl and polyfluoroalkyl substances, only perfluorodecanoic acid had an association with dental caries experience in an unadjusted model and adjusted logistic regression model.
Keywords: perfluorinated compounds, perfluoroalkyl, polyfluoroalkyl, C8, PFOS, PFOA
Introduction
Perfluoroalkyl and polyfluoroalkyl substances are ubiquitous, man-made chemicals with strong, stable carbon-fluoride bonds in their perfluoroalkyl moiety (CnF2n + 1−).1 They are used in water-resistant and soil-resistant items (carpeting, flooring, textiles, food contact paper, cardboard, or packaging used to prevent seepage, nonstick cookware, and so on) electronics, surfactants, lubricants, and paints. The substances are persistent in the environment.2,3 Human exposure to perfluoroalkyl or polyfluoroalkyl substances occurs by ingestion of contaminated food and/or water, occupational exposure, breathing contaminated air, dermal exposure, or hand-to-mouth exposure.4
Laboratory animal studies have provided much of the information about perfluoroalkyls and polyfluoroalkyl substances. From these studies, researchers identified adverse effects of perfluoroalkyl and polyfluoroalkyl substances as altered liver function (hepatomegaly, hepatic peroxisome proliferation), reduced testosterone production, changes in the thyroid stimulating hormone (TSH) and thyroxine levels, reduced birth weight, delayed growth and development, increases in pregnancy loss, neonatal mortality, altered inflammatory responses, and altered cytokine expression.4–6 For example, in 2002 and 2009, researchers Yang et al.7 and Guruge et al.8 found that there was suppression of an adaptive immune response and natural killer cell activity in mice exposed to perfluorooctanoate.
A variety of in vitro studies on perfluoroalkyl and polyfluoroalkyl substances have been conducted to gain an understanding of the toxic properties, effects, and modes of action of perfluoroalkyl and polyfluoroalkyl substances.9 Rainieri et al. (2007) found that 24 hours after being exposed to perfluorononanoic acid, macrophage cells (TLT cells) showed great cytotoxity and apoptosis induced by oxidative stress. Researchers examining healthy primary human CD4+ T cells showed that perfluorooctane sulfonate suppressed interleukin-2 production in doses within the high end of human exposure.10
In human studies, dietary intake of food contaminated with perfluoroalkyl and polyfluoroalkyl substances is the major exposure route.11 Perfluoroalkyl and polyfluoroalkyl substances can penetrate the placental barrier for fetal exposure.12 Prenatal exposure has been associated with adverse birth outcomes, allergic responses, and cancer.12,13 In the C8 Health Project conducted near Parkersburg, West Virginia, epidemiologists examined exposures in 69,030 adults. Researchers reported probable associations of perfluorooctanoate (C8) with hypercholesteremia, ulcerative colitis, thyroid dysfunction, testicular cancer, kidney cancer, preeclampsia, and elevated blood pressure during pregnancy.4
Mixed results in health outcomes occurred when researchers studied children. In Project Viva (Boston), researchers reported that early life exposure to perfluoroalkyl substances did not have an adverse effect on metabolic function, and actually had the effect of lowering insulin resistance.14 In another study, the Spanish INMA birth cohort study, no significant evidence of links of cardiometabolic risk and perfluoroalkyl substances were found in children.15 Conversely, prenatal exposure to perfluorooctonate, perfluorodecanoic acid, perfluorododecanoic acid, or perfluorohexane sulfonic acid was related to an increase in childhood atopic dermatitis (a disease associated with immune dysfunction) in females.16 Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances was associated with greater adiposity at age 8 years and a rapid increase in body mass index at ages 2–8 years in the prospective cohort Health Outcomes and Measures of the Environment study conducted in Cincinnati, Ohio.17
Research in humans is limited, inconsistent, and controversial. Perfluoroalkyl and polyfluoroalkyl substances have been found in blood, liver, urine, hair, cord blood, and nails and bioaccumulate in those tissues.18 The chemicals were reported to be toxic and carcinogenic for liver tissue, and may interfere with the immune system and the endocrine system.18 Their toxicity mechanism is currently unclear18 and the public health impact is currently unknown.19–21 In children, the research is even more challenging. In addition to determining exposure levels, researchers must also consider a) other factors known to influence health; b) potential confounders; and c) the effects of childhood periods of development in which exposure could have differing effects on health.22
Dental caries experience and perfluoroalkyl and polyfluoroalkyl substances
Dental caries is a complex disease involving many factors beyond the microbiome, dietary carbohydrates, plaque, brushing/flossing, and salivary output/buffering capacity/pH. Dental caries is related to several chronic conditions, and many of those conditions are associated with perfluoroalkyl and polyfluoroalkyl substances. Therefore, it is possible that dental caries itself is associated with perfluoroalkyl and polyfluoroalkyl substances.23 Several mechanisms of action have been postulated. Perfluoroalkyls and polyfluoroalkyl substances were associated with decreases in spinal bone mineral density in premenopausal women.24 As bone and dentin are similar in composition and structure, tooth mineralization could be affected by perfluoroalkyls and polyfluoroalkyl substances as bone mineral density is in premenopausal women.25 In addition, the perfluoroalkyls and polyfluoroalkyl substances affect TSH. Thyroid hormones influence tooth maturation. Low TSH is associated with enamel hypoplasia; therefore, perfluoroalkyl and polyfluoroalkyl substances could have the same effect through lowering TSH.
Other environmental factors (lead26 and tobacco smoke27) have been shown to be contributing factors in dental caries. The pathways of their effects may be like the effect of perfluoroalkyl and polyfluoroalkyl substances upon developing teeth. In addition, changes in a developing, immature immune system by these chemicals and the effect on saliva could result in physiologic changes on the vulnerability of teeth.27
Another plausible mechanism is that perfluoroalkyl and polyfluoroalkyl substances, which are suspected estrogen disruptors,28–32 disrupt estrogen in its role to upregulate carbonic anhydrase, an enzyme needed to properly build apatite enamel crystals.32 A change in crystal structure could increase the vulnerability of teeth for dental caries.
It is important to know if perfluoroalkyl and polyfluoroalkyl substances do affect tooth vulnerability to caries, particularly in early childhood. The purpose of this research is to determine the association of various perfluoroalky and polyfluoroalkyl substances with dental caries experience in children ages 3 years to 11 years.
Methods
Ethical statement
The West Virginia University Institutional Review Board has acknowledged this study as non-human subject research (Protocol number: 1806164862).
Study design
A cross-sectional research design was used for this study.
Data source
The data used for this study are from the National Health and Nutrition Examination Survey (NHANES) for the years 2013–2014. The data are publicly available from the NHANES website at https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2013. The NHANES is a survey of the National Health Center for Health Statistics at the Centers for Disease Control and Prevention on noninstitutionalized US population.
Study participants
Approximately one-third of the children, ages 3–11 years (n = 639), had serological specimens analyzed for perfluoroalkyl and polyfluoroalkyl substances in the NHANES 2013–2014. These children were included in the present study.
Description of procedures for variables of interest conducted in NHANES 2013–2014
NHANES scientists completed solid phase extraction of sera. The samples were coupled to high-performance liquid chromatography-turbo ion spray ionization-tandem mass spectrometry to determine 14 perfluoroalkyls and polyfluoroalkyl substances (Table 1).33 The scientists diluted samples with formic acid and injected 50 μL of each sample into a column switching system.33 The analytes were separated by chromatography and quantified with tandem mass spectrometry.33 If a perfluoroalkyl or polyfluoroalkyl substance value was below the limit of detection, NHANES scientists imputed a value equal to the lower limit of detection divided by the square root of 2.33 The tests are extremely adequate as they have a lower limit of detection of 0.1 ng/ml.
Table 1.
Perfluoroalkyl and Polyfluoroalkyls Substances Analyzed in US Children, Ages 3–11 Years, National Health and Nutrition Examination Survey, 2013–2014
| Abbreviation | Molecular formula | g/mol | |
|---|---|---|---|
| 1. Perfluorooctane sulfonamide | PFSA | C8H2F17NO2S | 499.14 |
| 2. 2-(N-methyl-perfluorooctane sulfonamide) acetic acid§ | MPAH | C11H6F17NO4S | 571.205 |
| 3. 2-(N-ethyl-perfluorooctane sulfonamide) acetic acid | EPAH | C12H8F17NO4S | 585.232 |
| 4. Perfluorodecanoic acid§ | PFDA | C10HF19O2 | 514.086 |
| 5. Perfluorobutane sulfonic acid | PFBS | C6HF13O3S | 400.11 |
| 6. Perfluoroheptanoic acid | PFHP | C7HF13O2 | 364.062 |
| 7. Perfluorononanoic acid§ | PFNA | C9HF17O2 | 464.078 |
| 8. Perfluoroundecanoic acid | PFUA | C11HF21O2 | 564.093 |
| 9. Perfluordodecanoic acid | PFDO | C12HF23O2 | 614.101 |
| 10. Perfluorohexane sulfonic acid§ | PFHxS | C6HF13O3S | 400.11 |
| 11. Linear isomers of perfluorooctanoate§ | NPFOA | CgF15NaO2* | 436.052 |
| 12. Branched isomers of perfluorooctanoate | BPFOA† | ||
| 13. Linear perfluorooctane sulfonate§ | NPFOS | C8HF17O3S | 500.126 |
| 14. Monomethyl branched isomers of perfluorooctane sulfonate§ | MPFOS‡ |
Sodium salt of the perfluoroocanate.
Branched isomers include perfluoro-3-methylheptanoic acid, perfluoro-4-methyheptanoic acid, perfluoro-5-methyheptanoic acid, perfluoro-6-methyheptanoic acid, perfluoro-4,4-dimethylhexanoic acid, perfluoro-5,5-dimethylhexanoic acid, perfluoro-3,5-dimethylhexanoic acid, and perfluoro-4,5-dimethylhexanoic acid.28
Monomethyl branched isomers of perfluorooctane sulfonate include perfluoro-3-methylheptane sulfonate, perfluoro-4-methylheptane sulfonate, perfluoro-5-methylheptane sulfonate, and perfluoro-6-methylheptane sulfonate.28
These perfluoroalkyl/polyfluoroalkyl substances were used in the study, and the other substances had sample sizes at or above the lower limit of detection that were too low for meaningful analyses.46
Bold items have P-values <.05.
Dental caries experience (any current dental caries and any dental restorations) in the NHANES was determined by calibrated US licensed dentists who had annual retraining, and were visited by the reference examiner up to three times per year to replicate 20–25 examinations.34 The dentists used a #5 reflecting mirror and a #23 dental explorer in identifying dental caries and dental restorations. The 1968 Radike criteria for coronal caries was used (a modified DMFS index).35
NHANES scientists ascertained fluoride water concentrations by testing water samples from participants’ households. The fluoride concentration was determined electrometrically with an ion-specific electrode having a limit of detection of 0.019 mg/l.34
Perfluoroalkyl and polyfluoroalkyl substances used in this study
Of the 14 perfluoroalkyl and polyfluoroalkyl substances analyzed by NHANES scientists, 7 were used in this study as the others had sample sizes at or below the lower limit of detection, which were too low for meaningful analyses36 (Table 1). In addition, two summative concentrations were used. The values of the monomethyl branch isomers of perfluorooctane sulfonic acid and linear isomer of perfluorooctane sulfonate were added to create the sum of perfluorooctane sulfonic acid isomers (PPFOS), and the values of the branch isomers of perfluorooctanoate and linear isomer perfluorooctonate were added to create the sum of perfluooctanoate isomers (PPFOA).
Dental variable used in this study
The variable, dental caries experience, was created from clinically observed current dental caries and/or dental restoration (yes, no), as described above.
Other variables used in this study
Other available variables known to be associated with dental caries were also included in this study. These were: age (≥3 to ≤5 years, >5 to ≤11 years); sex (female, male); race/ethnicity (Non-Hispanic white, Non-Hispanic black, Mexican American, other); tooth brushing frequency (once or less daily, twice or more daily); dental visit (within the previous year, more than 1 year or never); insurance status (yes, no); ratio of family income to poverty level guidelines (<2.00, 2 and above, missing; based upon the Department of Health and Human Services Poverty Guidelines),37 and fluoride in the child’s water (a continuous variable). Body mass index for children (underweight/normal weight, overweight/obese based upon CDC percentages) was also included (Figure 1).
Figure 1.
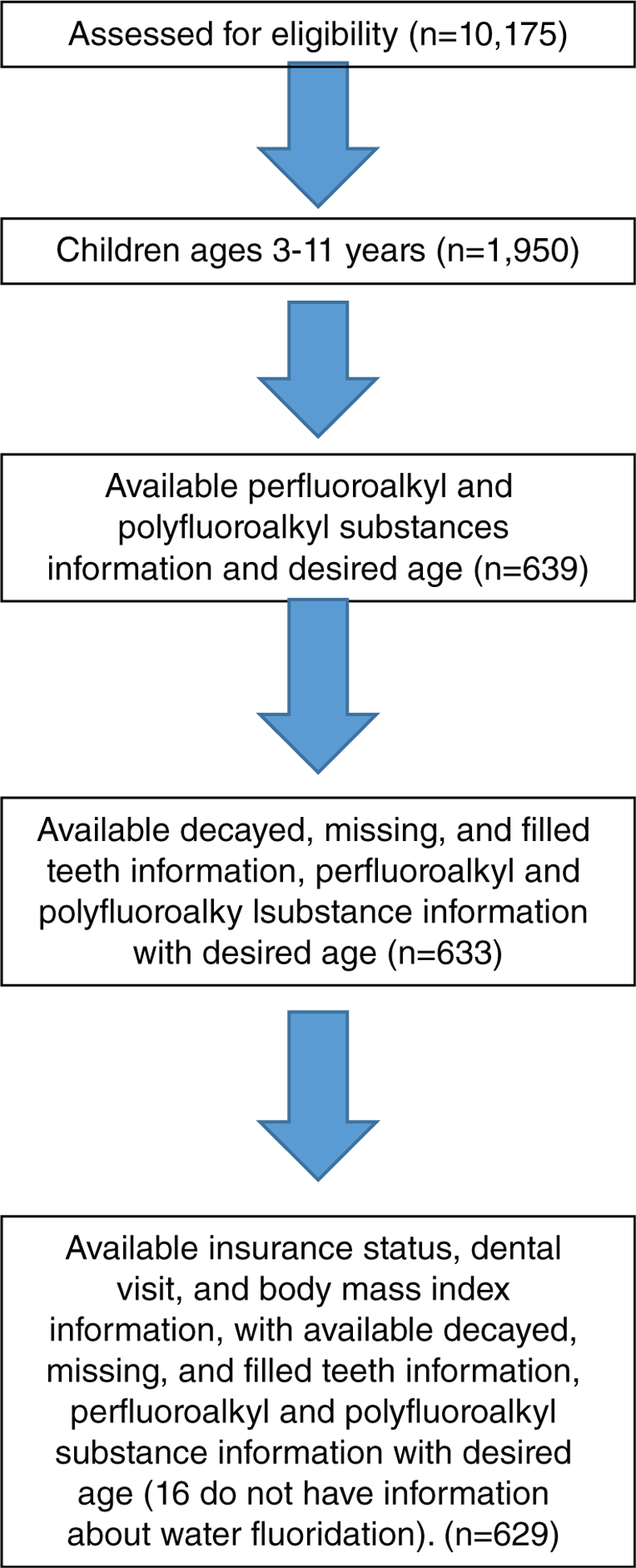
Eligible sample, US children ages 3–11 years, National Health and Nutrition Examination Survey, 2013–2014.
Statistical analysis
Statistical analyses were conducted with SAS version 9.4 (Cary, NC). Specific survey weights to analyze the perfluoroalkyl and polyfluoroalkyl substances (variable WTSS2YR in the SSPFAC_H NHANES dataset) and masked variance pseudoprimary sampling unit and masked variance pseudostratum were included to account for the NHANES survey design. An a priori level of significance was set at <0.05.
Data analyses included frequency determinations and weighted percentages of sample characteristics of the perfluoroalkyl and polyfluoroalkyl substances and bivariate (Rao-Scott Chi- square) evaluation of dental caries experience versus the variables. Geometric means of the perfluoroalkyl and polyfluoroalkyl substances were used as the data are skewed, whereas the arithmetic mean is sensitive to outliers (and would therefore not be a good summative statistic to use), the median is not, and, therefore, the geometric mean is more appropriate.38 It was calculated as:
Odds ratios and adjusted odds ratios were determined with logistic regression analyses. The adjusted model included epidemiological considerations as well as the results from the bivariate analyses in which variables failing to reach an a priori level of significance (<0.05) were excluded. The adjusted models included the specific perfluoroalkyl or polyfluoroalkyl substance, age, sex (which failed to reach significance in the bivariate analysis, but was included for epidemiological considerations), race/ethnicity, ratio of family-income-to-poverty-level guidelines, dental visit, tooth brushing frequency, and fluoride in the water. Percent of sugar in the diet, although an epidemiological factor, was not included due to the number of missing data points and the >90% to 10% split of the data for recommended sugar intake (see Table 2).
Table 2.
Sample Characteristics, National Health and Nutrition Examination Survey, 2013–2014
| N (weighted %) | |||
|---|---|---|---|
| Total Sample | 629 (100%) | ||
| Age in years | |||
| ≥3 to ≤5 | 178 (32.4%) | ||
| >5 to <11 | 451 (67.6%) | ||
| Sex | |||
| Female | 291 (49.0%) | ||
| Male | 338 (51.0%) | ||
| Race/ethnicity | |||
| Non-Hispanic white | 163 (51.5%) | ||
| Non-Hispanic black | 159 (13.9%) | ||
| Mexican American | 215 (24.7%) | ||
| Other | 92 (9.8%) | ||
| Ratio of family income to poverty guidelines | |||
| <2.00 | 391 (50.2%) | ||
| 2.00 and above | 206 (45.7%) | ||
| Missing | 32 (4.1%) | ||
| Body mass index for children* | |||
| Underweight/normal weight | 404 (68.0%) | ||
| Overweight/obese | 225 (32.0%) | ||
| Insurance | |||
| Yes | 597 (95.1%) | ||
| No | 32 (4.9%) | ||
| Dental caries experience | |||
| Yes | 321 (46.2%) | ||
| No | 308 (53.8%) | ||
| Tooth brushing frequency | |||
| ≤1 time per day | 209 (35.6%) | ||
| ≥2 times per day | 420 (64.4%) | ||
| Dental visit | |||
| Within the previous year | 540 (86.9%) | ||
| >1 year or no previous care | 89 (13.1%) | ||
| Fluoridated water as a continuous variable | |||
| Mean (95% CI) mg/l, SE | 0.54 (0.44, 0.64), 0.05 | ||
| (16 with missing data) | |||
| Percent of sugar in diet | |||
| <10% | 22 | 3.0% | |
| ≥10% | 495 | 80.5% | |
| Missing | 112 | 16.5% | |
| 2(N-methyl-perfluoro sulfon amide acetic) acid | Values 0.07†−6.75 ng/ml | ||
| Geometric mean not calculated‡ | |||
| Number less than limit of detection | 331 | 46.7 | <0.10 ng/ml |
| Detection to 75th percentile | 160 | 27.0 | ≥0.1 to <0.26 ng/ml |
| 75th—100th percentile | 138 | 26.2 | ≥0.26 |
| Perfluorodecanoic acid | Values 0.07†−2 ng/ml | ||
| Geometric mean not calculated‡ | |||
| Number less than limit of detection | 332 (52.2%) | <0.10 ng/ml | |
| Detection to 75th percentile | 118 (21.1%) | ≥0.10 to <0.17 ng/ml | |
| 75th—100th percentile | 179 (26.6%) | ≥0.17 ng/ml | |
| Perfluornononanoic acid | Values 0.07†−52.92 ng/ml | ||
| Geometric mean [95% Confidence Level] | 0.79 [0.68, 0.93] ng/ml | ||
| Up to 25th percentile | 160 (24.5%) | <0.50 ng/ml | |
| 25th-50th percentile | 149 (24.6%) | ≥0.50 to <0.70 ng/ml | |
| 50th-75th percentile | 153 (25.7%) | ≥0.70 to <1.07 ng/ml | |
| 75th—100th percentile | 167 (25.2%) | ≥1.07 ng/ml | |
| Perfluorohexane sulfonic acid | Values 0.07†−12.9 ng/ml | ||
| Geometric mean [95% Confidence level] | 0.84 [0.76, 0.94] ng/ml | ||
| Up to 25th percentile | 167 (23.8%) | <0.50 ng/ml | |
| 25th to 50th percentile | 182 (25.2%) | ≥0.50 to <0.81 ng/ml | |
| 50th to 75Th percentile | 130 (25.1%) | ≥0.81 to <1.24 ng/ml | |
| 75th to 100th percentile | 150 (25.8%) | ≥1.24 ng/ml | |
| Linear isomer of perfluorooctanoate | Values 0.07†−8.22 ng/ml | ||
| Geometric mean [95% Confidence level] | 1.81 [1.64, 2.01] ng/ml | ||
| Up to 25th percentile | 184 (24.4%) | <1.31 ng/ml | |
| 25th to 50th percentile | 171 (25.1%) | ≥1.31 to <1.82 ng/ml | |
| 50th to 75th percentile | 150 (25.2%) | ≥1.82 to <2.51 ng/ml | |
| 75th to 100th percentile | 124 (25.2%) | ≥2.51 ng/ml | |
| Linear isomer of perfluorooctane sulfonate | Values 0.5–26.54 ng/ml | ||
| Geometric mean [95% confidence level] | 2.51 [2.30, 2.74] ng/ml | ||
| Up to 25th percentile | 157 (24.2%) | <1.67 ng/ml | |
| 25th-50th percentile | 164 (25.3%) | ≥1.67 to <2.47 ng/ml | |
| 50th-75th percentile | 154 (25.3%) | ≥2.47 to <3.56 ng/ml | |
| 75th—100th percentile | 154 (25.1%) | ≥3.56 ng/ml | |
| Monomethyl branch isomers of perfluorooctanesulfonic acid | Values 0.18–10.68 ng/ml | ||
| Geometric mean [95% confidence level] | 1.23 [1.09, 1.40] ng/ml | ||
| Up to 25th percentile | 171 (25.0%) | <0.77 ng/ml | |
| 25th-50th percentile | 173 (24.6%) | ≥0.77 to 1.27 ng/ml | |
| 50th-75th percentile | 138 (25.1%) | ≥ 1.27 to 1.89 ng/ml | |
| 75th—100th percentile | 147 (25.2%) | ≥1.89 ng/ml | |
| (ΣPFOS) Monomethyl branch isomers of perfluorooctanesulfonic acid and linear isomer of perfluorooctane sulfonate | Values 0.18–26.54 ng/ml | ||
| Geometric mean [95% confidence level] | 3.88 [3.53, 4.27] ng/ml | ||
| Up to 25th percentile | 159 (24.0%) | <2.60 ng/ml | |
| 25th-50th percentile | 170 (25.8%) | ≥2.60 to 3.75 ng/ml | |
| 50th-75th percentile | 149 (25.2%) | ≥3.75 to 5.56 ng/ml | |
| 75th-100th percentile | 151 (25.0%) | ≥5.5 g ng/ml | |
| (ΣPFOA) Branch isomers-perfluorooctanoate and Linear isomer perfluorooctonate | |||
| Geometric mean [95% confidence level] | 1.92 [1.74, 2.11] ng/ml | ||
| Up to 25th percentile | 186 (24.9%) | < 1.39 ng/ml | |
| 25th-50th percentile | 173 (24.6%) | ≥ 1.39 to 1.93 ng/ml | |
| 50th-75th percentile | 136 (21.7%) | ≥1.93 to <2.67 ng/ml | |
| 75th-100th percentile | 135 (28.7%) | ≥2.67 ng/ml |
The body mass index for children was based upon NHANES determinations.
Values below the lower limit of detection (0.1 ng/ml) were imputed as 0.7 ng/ml () (Hornung, et al., 1990).
There were too many results below than the lower limit of detection for a valid calculation of the geometric mean.
sum of.
Results
There were 629 children, aged ≥3 to ≤11 years, whose data were used as the sample for this current study. The sample was 51.0% male; 51.5% Non-Hispanic white; 50.2% having a ratio of family-income-to-poverty level of less than 2.0;68.0% under or normal weight; 67.6% ages greater than 5–11 years; 95.1% with insurance, and 46.2% having had caries experience (Table 2). The highest geometric mean for a single perfluoroalkyl or polyfluoroalkyl substance was for perfluorooctane sulfonate with monoethyl branch isomers of perfluorooctane sulfonic acid. Its geometric mean and 95% confidence interval (95% CI) was 2.51 (95%CI: 2.30, 2.74) ng/ml. When the monomethyl branch isomers of perfluorooctane sulfonic acid were added, the summative perfluorooctane sulfonic acid geometric mean was 3.88 (95%CI: 3.53, 4.27) ng/ml (Table 2).
In bivariate, Chi-square analyses (Table 3), perfluorodecanoic acid was the only perfluoroalkyl or polyfluoroalkyl substance statistically significantly associated with dental caries experience (P = 0.0002). Older age (P = 0.0178), Mexican-American ethnicity (P = 0.0051), low-family-income-to-poverty ratio (P = 0.0039), and less tooth brushing frequency (P = 0.0005) having a dental visit within the year were also independently associated with dental caries experience in the bivariate analyses. Body mass index for children, insurance status, sugar in diet, and sex failed to reach significance with dental caries experience.
Table 3.
Dental Caries Experience Versus Selected Variables, National Health and Nutrition Examination Survey, 2013–2014
| Dental caries experience whole sample | Dental caries experience for children below limit of detection for perfluorodecanoic acid | |||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | P-value | Yes | No | P-value | |||
| Age in years | 0.0175 | 0.3847 | ||||||
| ≥3 to ≤5 | 66 (36.0%) | 112 (64.0%) | 39 (51.1%) | 47 (48.9%) | ||||
| >5 to ≤11 | 255 (51.1%) | 196 (48.9%) | 131 (58.5%) | 80 (41.5%) | ||||
| Sex | 0.2795 | 0.0664 | ||||||
| Female | 142 (43.5%) | 149 (56.5%) | 74 (50.4%) | 64 (49.6%) | ||||
| Male | 179 (48.8%) | 159 (51.2%) | 96 (62.1%) | 63 (37.9%) | ||||
| Race/ethnicity | 0.0054 | 0.1547 | ||||||
| Non-Hispanic white | 74 (40.6%) | 89 (59.4%) | 43 (52.8%) | 36 47.2%) | ||||
| Non-Hispanic black | 75 (45.0%) | 84 (55.0%) | 37 (46.0%) | 40 (54.0%) | ||||
| Mexican American | 127 (57.5%) | 88 (42.5%) | 99 (68.9%) | 31 (31.1%) | ||||
| Other | 45 (48.9%) | 47 (51.1%) | 24 (56.7%) | 20 (43.3%) | ||||
| Ratio of family income to poverty guidelines | 0.0216 | 0.2111 | ||||||
| <2.00 | 224 (52.8%) | 167 (47.2%) | 112 (60.4%) | 71 (40.6%) | ||||
| 2.00 and above | 81 (38.8%) | 125 (61.2%) | 48(51.9%) | 48 (48.1%) | ||||
| Missing | 16(47.1%) | 16(52.9%) | ||||||
| Body mass index for children* | 0.2065 | 0.0448 | ||||||
| Underweight/normal weight | 198 (43.6%) | 206 (56.4%) | 110(51.0%) | 95 (49.0%) | ||||
| Overweight/obese | 123 (51.8%) | 102 (48.2%) | 60 (68.4%) | 32 (31.6%) | ||||
| Insurance | 0.4831 | 0.6977 | ||||||
| Yes | 304 (45.9%) | 293 (54.1%) | † | † | ||||
| No | 17 (52.7%) | 15 (47.3%) | † | † | ||||
| Tooth brushing frequency | 0.0005 | 0.2014 | ||||||
| ≤1 time per day | 117 (52.9%) | 92 (47.1%) | 63 (61.0%) | 38 (39.0%) | ||||
| ≥2 times per day | 204 (42.5%) | 215 (57.5%) | 107 (52.7%) | 89(47.3%) | ||||
| Dental visit | 0.0184 | 0.0030 | ||||||
| Within the previous year | 287 | 48.6 | 253 | 51.4 | 153 (58.7%) | 107 (41.4%) | ||
| >1 year or no previous care | 34 | 30.2 | 55 | 69.8 | 17 (33.9%) | 20 (66.1%) | ||
| Sugar in diet | 0.1731 | 0.4379 | ||||||
| <10% | † | † | † | † | ||||
| ≥10% | † | † | † | † | ||||
| Missing | † | † | † | † | ||||
| 2-N-methyl-perfluorooctane | 0.8372 | |||||||
| sulfonamide acetic acid | ||||||||
| Less than limit of detection | 169 47.2 | 162 52.8 | ||||||
| Detection to 75th percentile | 76 43.4 | 84 56.6 | ||||||
| 75th-100th percentile | 76 47.3 | 62 52.7 | ||||||
| Perfluorodecanoic acid | 0.0002 | |||||||
| Less than limit of detection | 151 (37.3%) | 181 (62.7%) | ||||||
| Detection to 75th percentile | 69 (56.0%) | 49 (44.0%) | ||||||
| 75th-100th percentile | 101 (55.9%) | 78 (44.1%) | ||||||
| Perfluornononanoic acid | 0.7071 | |||||||
| Up to 25th percentile | 80 (44.6%) | 80 (55.4%) | ||||||
| 5th-50th percentile | 72 (46.7%) | 77 (53.3%) | ||||||
| 50th-75th percentile | 86 (50.4%) | 67 (49.6%) | ||||||
| 75th-100th percentile | 83 (43.0%) | 84 (57.0%) | ||||||
| Perfluorohexane sulfonic acid | 0.9801 | |||||||
| Up to 25th percentile | 92 (45.5%) | 75 (54.5%) | ||||||
| 25th-50th percentile | 86 (44.5%) | 96 (55.5%) | ||||||
| 50th-75th percentile | 68 (47.5%) | 62 (52.5%) | ||||||
| 75th-100th percentile | 75 (47.2%) | 75 (52.8%) | ||||||
| Linear isomer of perfluorooctanoate | 0.8181 | |||||||
| Up to 25th percentile | 98 (48.9%) | 86 (51.1%) | ||||||
| 25th-50th percentile | 87 (46.5%) | 84 (53.5%) | ||||||
| 50th-75th percentile | 70 (42.0%) | 80 (58.0%) | ||||||
| 75th-100th percentile | 66 (47.5%) | 58 (52.5%) | ||||||
| Linear isomer of perfluorooctane sulfonate | 0.5081 | |||||||
| Up to 25th percentile | 74 (42.1%) | 83 (57.9%) | ||||||
| 25th-50th percentile | 82 (44.9%) | 82 (55.1%) | ||||||
| 50th-75th percentile | 81 (47.0%) | 73 (53.0%) | ||||||
| 75th-100th percentile | 84 (50.6%) | 70 (49.4%) | ||||||
| Monomethyl branch isomers | 0.8495 | |||||||
| of perfluorooctanesulfonic acid | ||||||||
| Up to 25th percentile | 84 (44.9%) | 87 (55.1%) | ||||||
| 25th-50th percentile | 85 (43.5%) | 88 (56.5%) | ||||||
| 50th-75th percentile | 73 (48.5%) | 65 (51.5%) | ||||||
| 75th-100th percentile | 79 (48.0%) | 68 (52.0%) | ||||||
| Monomethyl branch isomers of perfluorooctanesulfonic acid and linear isomer of perfluorooctane sulfonate | 0.6812 | |||||||
| Up to 25th percentile | 79 (45.7%) | 80 (54.3%) | ||||||
| 25th-50th percentile | 84 (44.3%) | 86 (54.9%) | ||||||
| 50th-75th percentile | 72 (45.1%) | 77 (55.1%) | ||||||
| 75th-100th percentile | 86 (49.8%) | 65 (50.2%) | ||||||
| (ΣPFOA) Branch isomers-perfluorooctanoate and linear isomer perfluorooctonate | 0.76136 | |||||||
| Up to 25th percentile | 101 (50.5%) | 85 (49.5%) | ||||||
| 25th-50th percentile | 88 (45.8%) | 85 (54.2%) | ||||||
| 50th-75th percentile | 61 (40.4%) | 75 (59.6%) | ||||||
| 75th-100th percentile | 72 (47.4%) | 63 (52.6%) | ||||||
The body mass index for children was based upon NHANES determinations.
Cell size suppression as at least one cell is ≤10.
sum of; SE, standard error.
Values below the lower limit of detection (0.1 ng/ml) were imputed as 0.7 ng/ml () (Hornung, et al., 1990).
Results are from Rao-Scott Chi Square analyses utilizing masked pseudo-primary sampling unit, masked variance pseudo-stratum, and survey weights.
Dental caries experience is presented as the outcome variable and the other variables as predictors.
Bold items have P-values <.05.
In unadjusted logistic regression analyses in which the comparison was between the less than 75th percentile reference group and the 75th percentile and above group, higher perfluorodecanoic acid was associated with dental caries in an unadjusted model [odds ratio (UOR) 1.79 (95%CI: 1.19, 2.46; P = 0.0069)] and in an adjusted model [adjusted odds ratio (AOR) 1.54 (95%CI: 1.03, 2.30; P = 0.0385)] (Table 4). Other perfluoroalkyl and polyfluoroalkyl substances failed to reach significance in logistic regression analyses.
Table 4.
Logistic Regression on Dental Caries Experience With Various Perfluoroalkyls and Other Factors, National Health and Nutrition Examination Survey, 2013–2014
| Unadjusted OR (CI) | P-value | Adjusted OR (CI) | P-value | |
|---|---|---|---|---|
| 2-N-methyl-perfluorooctane sulfonamide acetic acid | ||||
| Less than 75th percentile | reference | reference | ||
| 75th to 100th percentile | 1.06 (0.54,2.10) | 0.8502 | 1.60 (0.81, 3.17) | 0.1635 |
| Perfluorodecanoic acid | ||||
| Less than to 75th percentile | reference | reference | ||
| 75th to 100th percentile | 1.71 (1.19, 2.46) | 0.0069 | 1.54 (1.03, 2.30) | 0.0385 |
| Perfluornononanoic acid | ||||
| Less than 75th percentile | reference | reference | ||
| 75th to 100th percentile | 1.04 (0.71, 1.54) | 0.8186 | 1.09 (0.68, 1.74) | 0.6943 |
| Perfluorohexane sulfonic acid | ||||
| Less than 75th percentile | reference | reference | ||
| 75th to 100th percentile | 1.05 (0.70, 1.58) | 0.8186 | 0.78 (0.45, 1.34) | 0.3429 |
| Linear isomer of perfluorooctanoate | ||||
| Less than 75th percentile | reference | reference | ||
| 75th to 100th percentile | 1.07 (0.64, 1.79) | 0.7715 | 1.38 (0.70, 2.70) | 0.3248 |
| Linear isomer of perfluorooctane sulfonate | ||||
| Less than 75th percentile | reference | reference | ||
| 75th to 100th percentile | 1.27 (0.80, 2.01) | 0.2898 | 1.49 (1.00, 2.24) | 0.0535 |
| Monomethyl branch isomers of perfluorooctanesulfonic acid | ||||
| Less than 75th percentile | reference | reference | ||
| 75th to 100th percentile | 1.09 (0.65, 1.81) | 0.7273 | 1.30 (0.62, 2.73) | 0.4643 |
| (ΣPFOS) Monomethyl branch isomers of perfluorooctanesulfonic acid and linear isomer of perfluorooctane sulfonate | ||||
| Less than 75th percentile | reference | reference | ||
| 75th to 100th percentile | 1.21 (0.86, 1.72) | 0.2511 | 1.41 (0.97, 2.05) | 0.0694 |
| (ΣPFOA) Branch isomers-perfluorooctanoate and Linear isomer perfluorooctonate | ||||
| Less than 75th percentile | reference | reference | ||
| 75th to 100th percentile | 1.07 (0.57, 2.03) | 0.85204 | 1.33 (0.70, 2.53) | 0.3516 |
| Age in years | ||||
| ≥3 to ≤5 | reference | reference | ||
| ≥5 to ≤11 | 1.85(1.07,3.21) | 0.0307 | 2.07 (1.17,3.67) | 0.0163 |
| Sex | ||||
| Female | reference | reference | ||
| Male | 1.24 (0.80, 1.92) | 0.3069 | 1.34 (0.84, 2.12) | 0.2007 |
| Race/ethnicity | ||||
| Non-Hispanic white | reference | reference | ||
| Non-Hispanic black | 1.20(0.69,2.11) | 0.4986 | 0.97 (0.56, 1.69) | 0.9046 |
| Mexican American | 1.99 (1.26, 3.13) | 0.0059 | 1.85 (1.13, 3.05) | 0.0185 |
| Other | 1.40 (0.69, 2.86) | 0.3316 | 1.16 (0.50, 2.68) | 0.7116 |
| Ratio of family income to poverty guidelines | ||||
| <2.00 | 1.76(1.11,2.80) | 0.0197 | 1.89 (1.27, 2.81) | 0.0039 |
| 2.00 and above | reference | reference | ||
| Tooth brushing frequency | ||||
| ≤1 time per day | 1.52(1.14, 2.02) | 0.0071 | 1.78 (1.23, 2.59) | 0.0049 |
| ≥2 times per day | reference | reference | ||
| Dental visit | ||||
| Within the previous year | 0.46 (0.22, 0.96) | 0.0409 | 0.43 (0.22, 0.86) | 0.0195 |
| >1 year or no previous care | reference | reference | ||
| Fluoride in water (beta) | 0.54 (0.32, 0.92) | 0.0271 | 0.47 (0.25, 0.87) | 0.0189 |
sum of; CI, confidence interval; OR, odds ratio.
Dental caries experience is presented as the outcome variable and the other variables as predictors.
Adjusted models are specific for the identified perfluoroalkyl and age, sex, race/ethnicity, ratio of family-income-to-poverty-level guidelines, tooth brushing frequency, dental visit, percentages of sugar in the diet, and fluoride in the water.
The adjusted data concerning the nonperfluoroalkyl factors (age, sex, race/ethnicity, ratio of family-income-to-poverty guidelines, tooth brushing frequency, fluoride, and dental visit) are the relationships from the eligible population associated with perfluorodecanoic acid.
Bold items have P-values <.05.
When logistic regression analyses were stratified by sex, the female analyses involved the data of 291 children and the male analyses involved the data of 338 children. For females, in the unadjusted logistic regression in which the comparison was between the less than 75th percentile reference group and the 75th percentile and above group for perfluorodecanoic acid, the UOR was 1.52 (95%CI: 0.75, 3.07, P = 0.2282), and it was 1.55 (95%CI: 0.85, 2.83; P = 0.1433) for the adjusted analysis.
For males, in the unadjusted logistic regression analysis in which the comparison was between the less than 75th percentile reference group and the 75th percentile and above group for perfluorodecanoic acid, the UOR was 1.95 (95%CI: 1.33, 2.86; P = 0.0020). The association remained positive for the 75th percentile and above group with an adjusted OR of 1.63 (95%CI: 0.96, 2.76; P = 0.0661); however, it was no longer statistically significant.
When logistic regression analyses were stratified by age, the data were from 178 children, ages 3 to and including 5 years in the younger analyses, and the data were from 461 children, ages 5 years to and including 11 years in the older analyses. For the younger group, in unadjusted logistic regression analysis in which the comparison was between the less than 75th percentile reference group and the 75th percentile and above group for perfluorodecanoic acid, the UOR was 1.84 (95%CI: 0.81, 4.17 P = 0.1332) and 1.64 (95%CI: 0.66, 4.05; P = 0.2627) for the adjusted analysis.
For the older children, in the unadjusted logistic regression analyses in which the comparison was between the less than 75th percentile reference group and the 75th percentile and above group for perfluorodecanoic acid, the UOR was 1.64 (95%CI: 1.21, 2.24; P = 0.0036). The association remained positive for the 75th percentile and above group with an adjusted OR of 1.52 (95%CI: 0.99, 2.33; P = 0.0541); however, it was no longer statistically significant.
Several other factors previously identified as being associated with dental caries were significant factors in the logistic regression analysis in this study. Age was significantly associated with dental caries [>5 to ≤11 years as compared with younger children, UOR = 1.85 (95%CI: 1.07, 3.21; P = 0. 0307); AOR =2.07 (95%CI: 1.17, 3.67; P = 0.0163)]. Mexican American status as compared with non-Hispanic white was associated with dental caries [UOR =1.99 (95%CI: 1.26, 3.13; P = 0.0059); AOR = 1.85 (95%CI: 1.13, 3.05; P = 0.0185)]. Having a federal poverty index less than 2.0 as compared with higher indices was significantly associated with dental caries [UOR = 1.76 (95%CI: 1.11, 2.80; P = 0.0197); AOR = 1.89 (95%CI: 1.27, 2.81; P = 0.0039)]. Brushing once or fewer times a day as compared with brushing more frequently was associated with dental caries [UOR = 1.52 (95%CI: 1.14, 2.02; P = 0.0071); AOR = 1.78 (95%CI: 1.23, 2.59; P = 0.0049)]. Having a dental visit within the previous year as compared with having a dental visit over a year ago was associated with a less likelihood of dental caries [UOR = 0.46 (95% CI: 0.22, 0.96; P = 0.0409)]. Higher fluoride in water content was also related to a less likelihood of dental caries [unadjusted beta = 0.54 (95%CI: 0.32, 0.92, P = 0.0271); adjusted = 0.47 (95% CI: 0.25, 0.87; P = 0.0189)].
Discussion
In this study of the association of dental caries experience and perfluoroalkyl and polyfluoroalkyl substances, perfluorodecanoic acid was significantly associated with dental caries experience in the unadjusted and adjusted logistic regression models. The observed values of each of the perfluoroalkyl and polyfluoroalkyl substances in this study were consistent with previous research specific to this age group36 and similar to those reported by the CDC.39 It should be noted that over half of the children in the study had levels of perfluorodecanoic acid below detection. This indicates a potential trend of decreased perfluorodecanoic acid exposure for children ages 3–11 years. As perfluorodecanoic acid is a large, stable perfluoroalkyl with associated adverse health effects, having a trend of fewer exposures is a public health benefit.36
Perfluoroalkyl and polyfluoroalkyl substances do not occur naturally; however, they are very persistence in the environment.40 Although they are no longer produced in the United States, there is much attention focused on their potential health impacts.40 There is an increased concern of the potential for health effects occurring at much lower levels than previously suspected. The most troubling perfluoroalkyl and polyfluoroalkyl substances are the large ones (with at least six carbon atoms) as they are the most stable and persistent.40
Epidemiological studies involving children and perfluoroalkyl and polyfluoroalkyl substances
As previously noted, there are few studies of perfluoroalkyl and polyfluoroalkyl substances involving the health of children. Food and water contamination are the two primary routes of human exposure to perlfuoroalkyl and polyfluoroalkyl substances, followed by dust inhalation and dust ingestion.41–43 There have been biomonitoring studies that have been performed in which the researchers indicated an association between the consumption of contaminated drinking water and high concentrations of perfluoroalkyl and polyfluoroalkyl substances in blood plasma and prenatal maternal cord blood.11,13
The attention to the presence of perfluoroalkyl and polyfluoroalkyl substances in water and air has led to several regulatory actions to limit such exposures in humans.2 Specific to this study and specific to perfluorodecanoic acid, researchers from the Agency for Toxic Substances and Disease Registry (an agency in the Department of Health and Human Services) indicated that there are insufficient data to determine the acute, intermediate, and chronic duration inhalation for the minimal risk level of exposure to perfluorodecanoic acid.40 Most of the research involving perfluorodecanoic acid has been limited to animal studies.40
Perfluorodecanoic acid may have several pathways to influence tooth growth and development. Though tooth growth and development have not been specifically studied with perfluorodecanoic acid, its effects on other cells may be similar to its effects on tooth growth and development. It activates peroxisome proliferator-activated receptor alpha and initiates oxidative stress in the metabolism of lipids, glucose, and amino acids.3 It promotes cell proliferation through a hypothesized change in gene expression in the vascular endothelial growth factor signaling pathway for gastric cells.44 Perfluorodecanoic acid was found to bind to and alter the structure of two hemoproteins— bovine hemoglobin and myoglobin indicating the potential of perfluorodecanoic acid to alter protein structures in general.45 In addition, as an estrogen disruptor,28–31 perfluorodecanoic acid may disrupt carbonic anhydrase, needed for sound enamel development.32
Although not a focus of this study, the typically accepted predictors of dental caries experience were significantly associated with dental caries for the participants. Dental caries was associated with brushing less than twice a day (P = 0.0049) as compared with brushing two or more times a day; no dental visits within the previous year (P = 0.0195) as compared with having a dental visit within the year; ages >5 to ≤11 (P = 0.0163) as compared with children ages 3–5; <2.0 ratio of family-income-to-poverty (P = 0.0039) as compared with higher income-to-poverty ratios; and non-Hispanic Black race/ethnicity (P = 0.0185) as compared with non-Hispanic White race/ethnicity.
Strengths and limitations
This study is limited by sample size. A larger sample size would have been desirable for an increase in power. However, only NHANES 2013–2014 had perfluoroalkyl and polyfluoralkyl substance data for children ages 3–11; the data were not available for additional years at this time. In addition, only a one-third subset of the children ages 3–11 years who were participants in 2013–2014 provided samples to be tested. Other factors that would have been helpful in this research were not available in the NHANES data source, or could not be included in the research due to the limitations imposed by the sample size/degrees of freedom/missing data. The data are cross sectional; therefore, the period of development in which the children were exposed and the degree to which an impact could have occurred were not available.
Nevertheless, the research was conducted with data from the National Health and Nutrition Examination Survey, which is a highly regarded national study in the United States with rigorous criteria and laboratory protocols. Data sample weights, masked variance pseudoprimary sampling unit, and the masked variance pseudostratum were used to adjust for the complex study design, and many known factors associated with dental caries experience were included in the analyses.
Conclusion
This is the first study, to the authors’ knowledge of the relationship of perfluoroalkyl and polyfluoroalkyl substances and dental caries experience in young children. Of the seven examined substances, only perfluorodecanoic acid was associated with dental caries experience in unadjusted and unadjusted logistic regression analyses. The results also support associations of greater likelihood of dental caries with older age, lower income, and Mexican American status (as compared with non-Hispanic white); and with the lesser likelihood of dental caries with brushing more than once a day, having a dental visit within a year (as compared with over a year), and water fluoridation.
Acknowledgment
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number U54GM104942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, vanLeeuwen SP. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011;7(4):513–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lilienthal H, Dieter HH, Hölzer J, Wilhelm M. Recent experimental results of effects of perfluoroalkyl substances in laboratory animals - relation to current regulations and guidance values. Int J Hyg Environ Health. 2017;220(4): 766–75. [DOI] [PubMed] [Google Scholar]
- 3.Luo M, Tan Z, Dai M, Song D, Lin J, Xie M, Yang J, Sun L, Wei D, Zhao J, Gonzalez FJ, Liu A. Dual action of peroxisome proliferator-activated receptor alpha in perfluorodecanoic acid-induced hepatotoxicity. Arch Toxicol. 2017;91(2):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ATSDR (Agency for Toxic Substances and Disease Registry). An Overview of Perfluoroalkyl and Polyfluoroalkyl Substances and Interim Guidance for Clinicians Responding to Patient Exposure Concerns. Interim Guidance National Center for Environmental Health, Agency for Toxic Substances and Disease Registry. Centers for Disease Control and Prevention; 2017. Available from: https://www.atsdr.cdc.gov/pfc/docs/pfas_clinician_fact_sheet_508.pdf [Google Scholar]
- 5.DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: recent developments. Toxicol Pathol. 2012;40:300–11. [DOI] [PubMed] [Google Scholar]
- 6.Frawley RP, Smith M, Cesta MF, Hayes-Bouknight S, Blystone C, Kissling GE, Harris S, Germolec D. Immunotoxic and hepatotoxic effects of perfluoro-ndecanoic acid (PFDA) on female Harlan Sprague-Dawley rats and B6C3F1/N mice when administered by oral gavage for 28 days. J Immunotoxicol. 2018;15(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Q, Abedi-Valugerdi M, Xie Y, Zhao X, Moller G, Nelson BD, DePierre JW. Potent suppression of the adaptive immune response in mice upon dietary exposure to the potent peroxisome proliferator, perfluorooctanoic acid. Int Immunopharmacol. 2002;2:389–97. [DOI] [PubMed] [Google Scholar]
- 8.Guruge KS, Hikono H, Shimada N, Murakami K, Hasegawa J, Yeung LW, Yamanaka N, Yamashita N. Effect of perfluorooctane sulfonate (PFOS) on influenza a virus-induced mortality in female B6C3F1 mice. J Toxicol Sci. 2009;34(6):687–91. [DOI] [PubMed] [Google Scholar]
- 9.Rainieri S, Conlledo N, Langerholc T, Madorran E, Sala M, Barranco A. Toxic effects of perfluorinated compounds at human cellular level and on a model vertebrate. Food Chem Toxicol. 2017;104:14–25. [DOI] [PubMed] [Google Scholar]
- 10.Midgett K, Peden-Adams MM, Gilkeson GS, Kamen DL. In vitro evaluation of the effects of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) on IL-2 production in human T-cells. J Appl Toxicol. 2015;35(5): 459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hölzer J, Midasch O, Rauchfuss K, Kraft M, Reupert R, Angerer J, Kleeschulte P, Marschall N, Wilhelm M. Biomonitoring of perfluorinated compounds in children and adults exposed to perfluorooctanoate-contaminated drinking water. Environ Health Perspect. 2008;116(5):651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liew Z, Ritz B, Bach CC, Asarnow RF, Bech BH, Nohr EA, Bossi R, Henriksen TB, Bonefeld-Jørgensen EC, Olsen J. Prenatal exposure to perfluoroalkyl substances and IQ scores at age 5; a study in the Danish National Birth Cohort. Environ Health Perspect. 2018;126(6):067004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu CY, Chen PC, Lien PC, Liao YP. Prenatal perfluorooctyl sulfonate exposure and Alu DNA hypomethylation in cord blood. Int J Environ Res Public Health. 2018;15(6):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleisch AF, Rifas-Shiman SL, Mora AM, Calafat AM, Ye X, Luttmann-Gibson H, Gillman MW, Oken E, Sagiv SK. Early-life exposure to perfluoroalkyl substances and childhood metabolic function. Environ Health Perspect. 2017;125(3):481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Iñiguez C, Martinez D, Romaguera D, Fernández Barrés S, Santa Marina L, Basterretxea M, Schettgen T. Prenatal exposure to perfluoroalkyl substances and cardiometabolic risk in children from the Spanish INMA birth cohort study. Environ Health Perspect. 2017; 125(9):097018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Q, Huang R, Hua L, Guo Y, Huang L, Zhao Y, Wang X, Zhang J. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and childhood atopic dermatitis: a prospective birth cohort study. Environ Health. 2018;17(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun JM, Chen A, Romano ME, Calafat AM,Webster GM, Yolton K, Lanphear BP. Prenatal perfluoroalkyl substance exposure and child adiposity at8 years of age: the HOME study. Obesity. 2016;24(1):231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Wu J, He W, Xu F. A review on perfluoroalkyl acids studies: environmetal behaviors, toxic effects, and ecological health risks. J Eco Health Sustain. 2019;5(1): 1558031. [Google Scholar]
- 19.Streeland K, Fletcher T, Savitz DA. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ Health Perspect. 2010;118:1100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emmett EA, Zhang H, Shofer FS, Freeman D, Rodway NV, Desai C, Shaw LM. Community exposure to perfluorooctanoate: relationships between serum levels and certain health parameters. J Occup Environ Med. 2006;48: 771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bach CC, Bech BH, Brix N, Nohr EA, Bonde JP, Henriksen TB. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit Rev Toxicol. 2015;45(1):53–67. [DOI] [PubMed] [Google Scholar]
- 22.Braun JM, Gray K. Challenges to studying the health effects of early life environmental chemical exposures on children’s health. PLoS Biol. 2017;15(12):e2002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey J. The relationship between polyfluorooctanoic acid and overall oral health (Masters of Science dissertation) Boston University; 2015. Available from: Open.bu.edu.https://hdl.handle.net/2144/16276. [Google Scholar]
- 24.Khalil N, Chen A, Lee M, Czerwinski SA, Ebert JR, DeWitt JC, Kannan K. Association of perfluoroalkyl substances, bone mineral density, and osteoporosis in the US population in NHANES 2009–2010. Environ Health Perspect. 2015;124(1):81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramesh NP, Arora M, Braun JM. Cross-sectional study of the association between serum perfluorinated alkyl acid concentrations and dental caries among US adolescents (NHANES 1999–2012). BMJ Open. 2019;9(2):bmjopen-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiener RC, Jurevic RJ. Association of blood lead levels in children 0–72 months living in mid-Appalachia: a semi-ecologic study. Rural Remote Health. 2016;16:3597 Available from: http://www.rrh.org.au [PMC free article] [PubMed] [Google Scholar]
- 27.Aligne CA, Moss ME, Auinger P, Weitzman M. Association of pediatric dental caries with passive smoking. JAMA. 2003;289(10):1258–64. [DOI] [PubMed] [Google Scholar]
- 28.Benninghoff AD, Field JA, Williams DE. Assessment of the estrogen activity of perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS) and other structurally diverse perfluorinated chemicals in rainbow trout, abstract 526. Toxicologist. 2007;96:110. [Google Scholar]
- 29.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99:366–94. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Tan YS, Haslam SZ, Yang C. Perfluorooctanoic acid effects on steroid hormone and growth factor levels mediate stimulation of peripubertal mammary gland development in C57BL/6 mice. Toxicol Sci. 2010;115: 214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen AA, Leffers H. Emerging endocrine disrupters: perfluoroalkylated substances. Int J Androl. 2008;31(2): 161–9. [DOI] [PubMed] [Google Scholar]
- 32.Kakei M, Sakae T, Yoshikawa M. Combined effects of estrogen deficiency and cadmium exposure on calcified hard tissues: animal model relating to itai-itai disease in postmenopausal women. Proc Jpn Acad Ser B. 2013;89(7): 340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CDC, 2016a. National Health and Nutrition Examination Survey. 2013–2014 Data Documentation, Codebook, and Frequencies. Perfluoroalkyl and Polyfluoroalkyl Substances in US children 3–11 Years of Age (SSPFAC_H) Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/SSPFAC_H.htm#Description_of_Laboratory_Methodology.
- 34.CDC, 2016b. National Health and Nutrition Examination Survey. 2013–2014 Data Documentation, Codebook, and Frequencies. Oral Health-Dentition (OHXDEN_H) Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/OHXDEN_H.htm
- 35.Radike AW. Criteria for diagnosis of dental caries Proceedings of the Conference on the Clinical Testing of Cariostatic Agents, American Dental Association, Chicago, Illinois, October 14–16, 1968. Chicago: ADA Council on Dental Research; 1972. p. 87–8. [Google Scholar]
- 36.Ye X, Kato K, Wong L, Jia T, Kalathil A, Latremouille J, Calafat A. Per- and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. Int J Hyg Environ Health. 2018;221:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Health and Nutrition Examination Survey. 2013–2014 Data Documentation, Codebook and Frequencies. Demographic Variables and Sample Weights (DEMO_H) Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/DEMO_H.htm#INDFMPIR
- 38.McChesney J You should summarize data with the geometric mean. 2016. Available from: https://medium.com/@JLMC/understanding-three-simple-statistics-for-data-visualizations-2619dbb3677a
- 39.CDC, 2019. Center for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals. Tables 2019 National Center for Environmental Health, Atlanta: Available from: https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf [Google Scholar]
- 40.Chou S, Ingerman L, Jones D, Barber L, Pohl HR. Carlson-Lynch H, Ruiz P, Citra M, Scinicariello F, Diamond GL, Buser M, Klotzbach J, Llados F, Plewak DJ. Toxicology Profile for Perfluoroalkyls: Draft for Public Comment 2018; U.S. Department of Health and Human Services. Agency for Toxic Substances and Disease Registry; Available from: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf [Google Scholar]
- 41.Hurley S, Goldberg D, Wang M, Park JS, Petreas M, Bernstein L, Anton-Culver H, Nelson DO, Reynolds P. Time trends in per-and polyfluoroalkyl substances (PFASs) in California women: declining serum levels, 2011–2015. Environ Sci Technol. 2017;52(1):277–87. [DOI] [PubMed] [Google Scholar]
- 42.Egeghy PP, Lorber M. An assessment of the exposure of Americans to perfluorooctane sulfonate: a comparison of estimated intake with values inferred from NHANES data. J Expo Sci Env Epid. 2011;21(2):150–68. [DOI] [PubMed] [Google Scholar]
- 43.Vestergren R, Berger U, Glynn A, Cousins IT. Dietary exposure to perfluoroalkyl acids for the Swedish population in 1999, 2005 and 2010. Environ Int. 2012;49:120–7. [DOI] [PubMed] [Google Scholar]
- 44.Dong T, Peng Y, Zhong N, Liu F, Zhang H, Xu M, Liu R, Han M, Tian X, Jia J, Chang LK. Perfluorodecanoic acid (PFDA) promotes gastric cell proliferation via sPLA2-IIA. Oncotarget. 2017;8(31):50911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin P, Liu R, Teng Y. Perfluorodecanoic acid binding to hemoproteins: new insights from spectroscopic studies. J Agric Food Chem. 2011;59(7):3246–52. [DOI] [PubMed] [Google Scholar]
- 46.Jain RB. Contribution of diet and other factors to the observed levels of selected perfluoroalkyl acids in serum among US children 3–11 years. Environ Res. 2018;161 (2018):268–75. [DOI] [PubMed] [Google Scholar]


