Abstract
Background and Aims:
As treatment for opioid use disorder (OUD) expands within general healthcare settings such as primary care, mechanisms to facilitate decision-making processes are increasingly necessary. Decision aids have the capacity to bolster existing resources in diverse treatment settings by increasing knowledge of treatment options and facilitating shared decision making. The aim of this study is to develop and test a patient decision aid for medication treatment for opioid use disorder (PtDA-MOUD) that assists individuals with OUD in making informed decisions about treatment at the time of initial clinical visit. Use of the PtDA-MOUD will be further tested in diverse treatment settings within the California Hub and Spoke System developed under the SAMHSA State Targeted Response to the Opioid Crisis grants.
Methods:
The PtDA-MOUD was iteratively developed with input from a scientific expert panel and both patient and provider focus groups, incorporating International Patient Decision Aid Standards. Thirty-six patients with OUD entering treatment pilot tested the PtDA and completed assessments, and results from clinical records were compared with matched controls who did not receive the PtDA. A clinical profile based on assessment data was created for use within the clinical visit.
Results:
The developed decision aid provides information on MOUD and captures patient characteristics relevant to medication treatment decisions. Feedback indicated that the PtDA-MOUD was feasible to implement and useful. Though the small sample size limited the ability to detect significant differences (p > .05), a greater number of individuals who reviewed the PtDA (37%) were inducted on MOUD than controls (11%) and received MOUD for more days (M = 14.0, SD = 24.7) than controls (M = 8.4; SD = 22.5). Moreover, the difference in means for days receiving MOUD had an approximately medium effect size (r = .25).
Conclusions:
Patient perceptions of the decision aid were favorable and it showed promise as a tool in the OUD treatment process. Pilot testing results suggested preliminary positive effects on MOUD initiation. Future phases of this study will further investigate the usefulness of this tool.
Keywords: decision aid, medication treatment, opioid use disorder, development, evaluation
1. INTRODUCTION
Escalating rates of opioid use disorder (OUD) and overdose deaths in the United States have become a public health crisis. Deaths associated with opioid use were six times higher in 2017 than in 1999, and fentanyl-related deaths rose by 45% since 2016 alone (CDC, 2018). Approved medications for the treatment of OUD include methadone, an opioid agonist, buprenorphine, a partial opioid agonist, and naltrexone, an opioid antagonist. Medications for opioid use disorder (MOUD) are recommended as first-line treatment options as they are most strongly associated with reductions in opioid relapse and overdose. Additional public health benefits of MOUD include improved productivity and reductions in infectious disease transmission, including HIV and hepatitis (Altice et al., 2011; MacArthur et al., 2012; Teesson et al., 2008; Woody et al., 2014).
Despite decades of robust evidence supporting the use of MOUD, there is widespread variability in acceptance and utilization of MOUD within the substance use disorder (SUD) treatment system (Knudsen, Roman, & Oser, 2010; Knudsen, Abraham, & Roman, 2011), and significant barriers exist for patients seeking treatment (Jones et al., 2015a; Jones et al., 2015b). Reasons include negative societal, institutional, and personal attitudes toward agonist treatment and limitations in access to medication and providers (Mayet et al., 2005; Mattick et al., 2009). Prior studies have identified areas that may influence patient decision making around MOUD, including treatment expectations and goals, prior experiences with MOUD, and concerns about addiction and pain control (Yarborough et al., 2016; Muthulingam et al., 2019). Furthermore, treatment goals may differ between patients and clinicians (Yarborough et al., 2016). Medication preference, positive experience with MOUD, and staff support/encouragement have been cited as factors that influence retention on MOUD (Teruya et al., 2014). Provider attitudes also affect recommendations around medication choice, duration, and dosing; these are influenced by perceived social norms, institutional support, and years of experience prescribing MOUD (Rieckmann et al., 2007; Reif, Thomas, & Wallack, 2007; Green et al., 2014). Thus, processes that improve patient knowledge, facilitate greater communication between patients and clinicians, address patient concerns, and incorporate individual patient goals have potential value in decision making around MOUD (Yarborough et al., 2016).
Patient decision aids (PtDAs) are established as interventional tools to support shared decision-making implementation (Stacey et al., 2017, Rehman, 2016). PtDAs are ‘interventions designed to help people make specific and deliberative choices among options by providing information on the options and outcomes relevant to a person’s health status and implicit methods to clarify values’ (Stacey et al., 2017), and are different from patient health education materials providing general health information about specific medical conditions. Decision aids are developed for specific populations or conditions in different formats (e.g. paper and pencil instruments, videos, web-based tools, interactive software), and can be used alone by patients or during interactions with clinicians. They include explanations about treatment options and describe benefits and harms based on scientific evidence. They also encourage patients to consider their own values and preferences regarding the benefits and risks of different treatment options, and how each option could influence their quality of life (Fagerlin et al., 2013; Charles & Gafni, 2014).
PtDAs have been developed for a variety of health conditions (e.g. diabetes, heart disease, depression). Systematic reviews suggest that PtDAs may reduce the proportion of people who are passive in their decision making or who remain undecided after deliberation (Stacey et al., 2017). In addition, PtDAs may improve patient-provider communication, provider knowledge about the patient, and satisfaction with visits (Stacey et al., 2017; Brener et al., 2009). Though research on PtDAs used within SUDs is limited, PtDAs are consistently associated with improved treatment adherence (Graff et al., 2009; Swift & Callahan, 2009). Furthermore, in some studies PtDAs have been associated with reduced substance use and psychiatric severity (Friedrichs et al., 2016; Joosten et al., 2009; Neumann et al., 2006).
Few PtDAs exist for MOUD designed for individuals with OUD with the exception of SAMHSA’s (2016) Decisions in Recovery: Treatment for Opioid Use Disorder, a free online decision support tool designed for individuals seeking recovery from OUD and for treatment providers. This tool provides materials that support decisions on starting treatment, choosing medication, and building recovery support. Yet to our knowledge no studies to date have assessed the effectiveness of SAMHSA’s or other decision support tools for MOUD or have developed a decision aid intended for facilitating shared decision making within a broad range of clinical settings including general healthcare systems.
The goal of the present study is to develop and test a decision aid that assists individuals with OUD in making informed decisions about medication treatment at the time of initial clinical visit within the California’s Hub & Spoke System (CA H&SS), a project developed under the SAMHSA State Targeted Response to the Opioid Crisis grants to expand MOUD within the state. Designed after Vermont’s H&SS, the CA H&SS is composed of opioid treatment program “Hubs” that are connected to multiple “Spokes”, which are offices or clinics with at least one buprenorphine prescriber. These treatment programs work together to provide the most appropriate treatment to individuals based on assessment of their clinical severity; patients who begin services at a Spoke and need a higher level of care may transition to a Hub, which is staffed by addiction treatment specialists and able to manage more clinically complex patients.
Given current efforts to expand MOUD delivery within primary care systems, we have designed a PtDA to provide information about MOUD and capture patient characteristics, needs, and preferences in order to facilitate shared decision making within diverse healthcare settings where providers may have limited time. A unique feature of our PtDA is the use of patient assessment data to generate a clinical profile of each patient that can be used to facilitate discussion with his or her treatment provider. The clinical profile includes patient preferences, health conditions, and perceived barriers to obtaining or remaining on MOUD. The purpose of this paper is to describe the development of this PtDA for MOUD (PtDA-MOUD), lessons learned from the development process, and anticipated implementation challenges.
2. METHODS
2.1. Study Design
According to the International Patient Decision Aid Standards (IPDAS), PtDAs should present information in a balanced manner, utilize a systematic development process (e.g., find out what users need to effectively discuss options, use peer review of the tool, conduct a field test), use current scientific evidence that is cited and referenced, and use plain language (IPDAS, 2005). Incorporating these standards, an iterative process guided by experts and key stakeholders (e.g., patients, OUD treatment service providers and other research/experts in the field) was used in the development and testing of the PtDA-MOUD. As illustrated in Figure 1, the development and evaluation of the PtDA was organized within three phases. Phase 1 consisted of initial development of the tool. Phase 2 included a small scale pilot test with patients in treatment for OUD. The primary objective of the pilot test was to determine feasibility and acceptability of the PtDA by examining both patient and provider response to the tool. This phase allowed for additional refinements to the tool. Finally, Phase 3 is planned as a large scale randomized trial to test short-term (e.g. MOUD uptake, adherence, urine tests) and long-term (e.g death records, recidivism, criminal justice involvement) outcomes among patients who utilized the PtDA-MOUD. These phases are described in more detail below.
Figure 1.
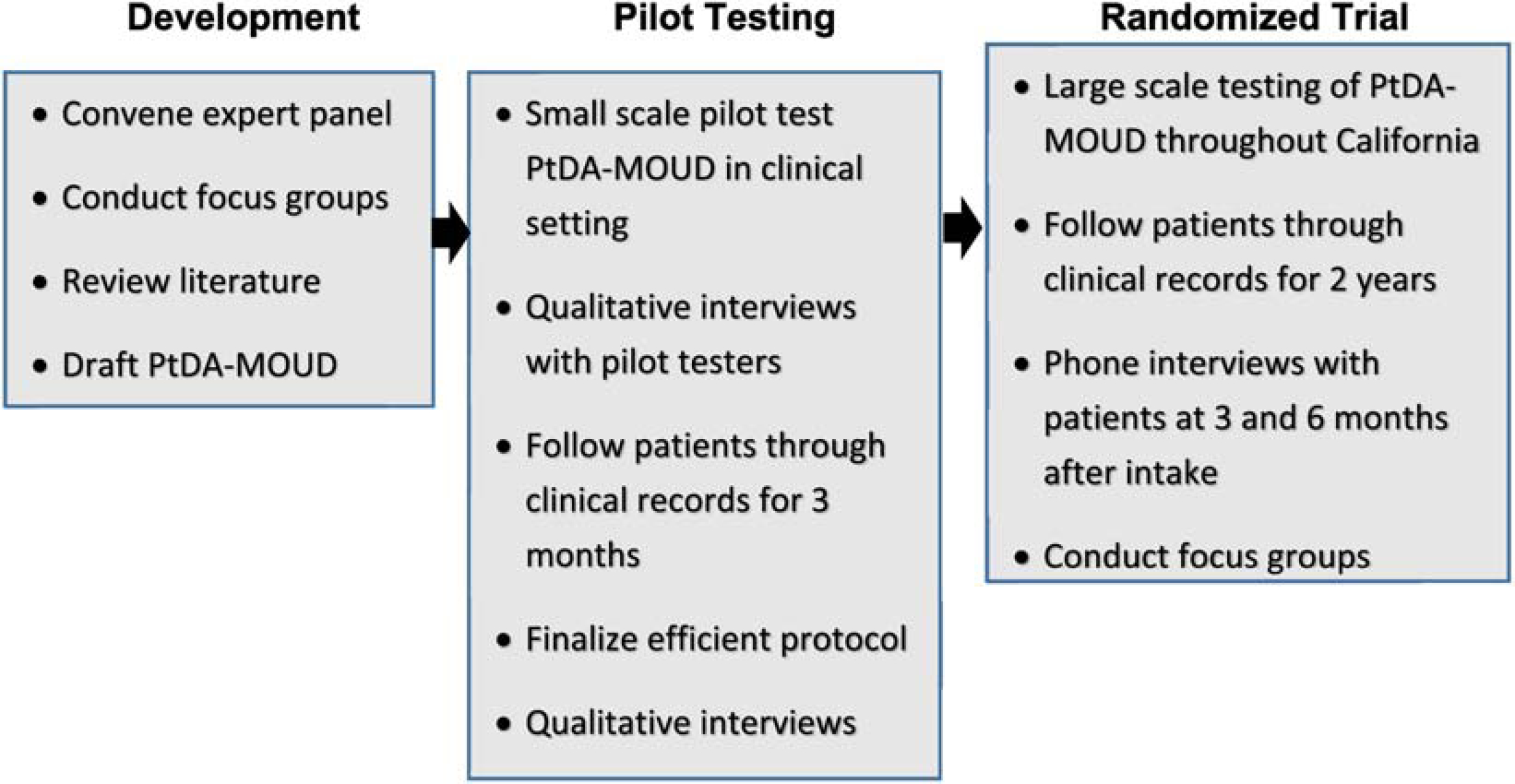
Design for developing and testing of PtDA-MOUD
2.2. Study Sites
Hub and Spoke System sites in California were asked to participant in all phases of the development and evaluation of PtDA-MOUD. California’s Hub & Spoke System is a clinical network designed to expand MOUD within the state. Two local H&SS sites participated in the development and pilot testing phase of the study.
2.3. Development Phase Procedures
Following the recommendations of IPDAS, a mixed methods approach was used to develop the PtDA-MOUD for use within the CA H&SS. Experts in MOUD and H&SS guided the development of the instrument. Focus groups were conducted separately with patients and clinicians to identify decisional needs for MOUD. Focus group topics included attitudes, perceptions, barriers, facilitators and factors that influence MOUD decision-making, patients’ health concerns, experience with shared decision-making, feedback, utility and acceptability of the PtDA as well as envisioned study implementation plans. Although topics were similar across focus groups, questions were tailored to elicit either patient or provider-specific feedback.
Literature reviews were conducted to help determine the best available evidence. An iterative draft-review-revise process was used until the PtDA-MOUD reached content and format ‘saturation’ (Curry et al., 2009). An initial draft PtDA was developed based on literature and current knowledge on MOUD. Six experts in MOUD and H&SS were convened to review the PtDA-MOUD. Experts reviewed the PtDA-MOUD content and study procedures, and provided feedback on factors that affected MOUD choice as well as perceived utility and acceptability of the PtDA-MOUD.
Focus group participants were recruited from two CA H&SS Hubs in Los Angeles in 2018. To allow for diverse feedback, inclusion criteria were broad. Interested individuals were eligible if they were 18 years or older and either a treatment provider for individuals with OUD or a patient in or seeking treatment for OUD. All focus group meetings were recorded and transcribed. In addition to these qualitative components, both patient and provider groups were asked to complete a MOUD knowledge assessment; feedback was used to revise quiz questions and answers so that they were understandable and reflective of PtDA-MOUD content.
These efforts culminated in a PtDA that includes descriptions of OUD and its consequences, a description of three medications approved for OUD (methadone, buprenorphine, and naltrexone) and their formulations, evidence for positive outcomes of MOUD, sections that incorporate patient values and address myths about MOUD, as well as guidance on communicating with providers and accessing additional resources. The guide presents these contents at an 8th grade reading level, avoiding text-heavy layouts, while using patient-centered questions for headers and infographics where possible. Although the guide is currently only available in paper format there is an intention to have an online version published.
2.4. Pilot Testing Phase Procedures
The PtDA-MOUD was pilot-tested at the time of clinical visits with OUD patients to assess its acceptability and feasibility. Qualitative interviews with selected patients provided further feedback on ways to improve the PtDA-MOUD. Furthermore, patients receiving PtDA-MOUD were tracked using their clinical and administration data over a 3-month period, and preliminary outcomes were compared to those of matched controls who did not use the PtDAT-MOUD.
Participants who completed pilot testing (N = 36) were recruited from intake reception rooms and inpatient units of two CA H&SS Hubs in Los Angeles throughout 2018. Interested individuals were deemed eligible if they were 18 years or older and self-identified as currently seeking or enrolled in MOUD. After confirming eligibility, research assistants consented participants and guided them through the study procedures. After completing study measures, participants reviewed the PtDA-MOUD and completed further assessments including a knowledge test. Afterwards, a clinical profile generated from assessment data was provided to clinicians so it could be reviewed with the patient. Participants were tracked through their treatment center’s clinical records for three months after their participation in the study. These records included treatment placement records, urine drug screen results, MOUD prescriptions/orders, physical and mental health diagnoses, and admission data for the episode in which the patient viewed the PtDA-MOUD and any subsequent admissions three months after interacting with the PtDA. Nineteen pilot-testing participants had an OUD diagnosis documented in their electronic health record (EHR) during the 3-month period. A conservative approach was taken where individuals without an OUD diagnosis (n = 17) over the 3-month span were omitted from the sample. Use of buprenorphine (solely Suboxone in our study) and methadone is only for opioid use disorder, while Vivitrol is only indicated for opioid and alcohol use disorders. Therefore, the sample without a recorded OUD would not have accurately reflected the PtDA’s effect on MOUD use in OUD populations. The MOUD treatment status is reported for these 19 patients.
A control group (N = 36) was constructed based on clinical data sets from the same facility and treatment time period. Controls were matched based on primary substance of treatment, gender, race, ethnicity, age, and treatment modality. Control participants did not review the PtDA-MOUD nor complete study assessments. As part of routine treatment procedures, these individuals would often have medication brochures available and meet with treatment providers to discuss MOUD and make treatment decisions. Three months of control participants’ clinical records, starting at admission, were obtained from the treatment facilities.
2.4.1. Measures for the Pilot Test
Treatment Outcomes Profile:
The Treatment Outcome Profile is a 20-item instrument designed for treatment outcomes monitoring (Marsden et al., 2008, Ryan et al., 2014, Castillo-Carniglia et al., 2015; Wang et al., 2017). The assessment captures days of substance use, days injected drugs or shared needles, employment status, education involvement, acute housing problems and risk of eviction in the past 4 weeks. It also captures ratings of physical health, psychological health, and quality of life.
Treatment Knowledge Questionnaire:
A treatment knowledge questionnaire was constructed to evaluate pre- and post-knowledge of the material presented in the PtDA. The measure was a 10-item multiple-choice questionnaire which assessed participants’ knowledge on topics presented within the PtDA material; consequences of opioid use, medications approved for OUD, and details about medication effects, dosing, and administration.
Treatment Needs Questionnaire:
Treatment Needs Questionnaire (TNQ) is a treatment placement tool developed for the Vermont H&SS. It is used during treatment intake to determine if a patient is better suited to receive services at the Hub or the Spoke (Brooklyn and Sigmon, 2017). A higher TNQ score indicates a higher level of treatment need and are seen in Hub settings. Individuals with lower TNQ sores are guided to a Spoke.
Barriers to Treatment Checklist:
The Barriers to Treatment Checklist was constructed using focus groups’ feedback on health conditions and barriers that played a role in treatment admittance and retention. Potential barriers included health conditions (e.g. pregnancy, chronic pain conditions, liver disease), other substance use, as well as mental health problems. Other concerns included treatment cost, taking time off from work, transportation, child care and stigma. Individuals noted whether or not each concern applied to them.
MOUD Experiences and Expectations:
A MOUD Treatment Experiences and Expectations questionnaire was developed to capture participants’ experience with MOUD. The measure was a 24-item questionnaire consisting of open ended and yes/no responses. Questions assessed prior experiences with MOUD, duration of prior treatment episodes, and reasons for or against consideration of MOUD initiation.
Participant Evaluation Form:
The Participant Evaluation Form (McGillion et al., 2016) is a 5-item questionnaire with a 4-point scale (ranging from “not at all true” = “0” to “very true” = “3”), which captures feasibility of delivery and overall acceptability after each decision. A modified version of this questionnaire was used to capture information about the PtDA and its relation to facilitation of treatment planning, decisions, and communication within the clinical visit.
2.4.2. Analyses of Pilot Test Results
To compare treatment outcomes between cases and controls in the pilot test, Wilcoxon-Mann-Whitney test was used for continuous variables and the Fisher’s exact test for categorical variables. An effect size was calculated as the standardized difference between two group means using pooled sample standard deviation (Sullivan and Feinn, 2012). Since data did not meet the requirements of parametric test, the point biserial correlation coefficient r was calculated to estimate effect size (Fritz, Morris, & Richler, 2012).
3. RESULTS
3.1. Development Phase Results
3.1.1. Expert Panel Feedback
The expert panel (n = 6) composed of experts in MOUD and H&SS were consulted throughout the development of the PtDA-MOUD. Panelist’s experiences included involvement in Vermont’s H&S model, CA H&SS implementation, shared decision making and PtDA development.
Content suggestions included changing graphs into infographics, ensuring that MOUD options were presented equally, focusing on patient-centered outcomes of MOUD, lowering reading level, including patient narratives, and conflicting suggestions about PtDA-MOUD length (i.e. more concise vs. exhaustive).
3.1.2. Focus Groups with Patients
Two focus groups were conducted with 19 patients who were in treatment for OUD. The patient focus group had an average age of 44.2 years (SD = 14.5 years), were predominantly still in treatment (95%), 5% students, 11% employed, 74% neither, and 10% did not respond. In this group 11% were in high school or were a high school dropout, 37% had a high school diploma, 21% had trade/technical training, 26% had a bachelor’s degree, and 5% did not report an education. Patients were 58% female, 21% Black, 42% White, 16% Hispanic, 16% Mixed-race, and 5% other race.
Patient focus groups were highly receptive to the PtDA-MOUD content. Feedback was mainly positive about readability, information, and length, with some criticism provided about the complexity of the graphs and how this might impede patient interpretation. Suggestions for additional content included cost, frequency of MOUD administration, and ancillary treatments. Patient feedback also guided the inclusion of treatment relevant factors to be captured in the PtDA-MOUD’s clinical profile. These additions included concerns about pregnancy, taking time off work, stigma, transportation, cost, and physical conditions. Patient group discussions also revealed important misconceptions about MOUD that would later be incorporated into the guide (e.g. that MOUD was simply substituting one addiction for another).
3.1.3. Focus Groups with Providers
Two focus groups were conducted with 16 providers with OUD treatment experience. The provider focus group sample was 13% nurse/physician assistant, 13% clinical supervisor/psychologist, 44% counselor/social worker, 19% manager/administrator, and 11% Other position. Participants had an average of 9.5 (SD = 9.4) years of experience treating patients with OUD. Providers were 44% female, 19% Black, 56% White, 6% Mixed-race, and 19% Hispanic race.
Providers gave similar feedback as patients on the PtDA-MOUD. While noting value in the PtDA-MOUD, concerns about reading level and length were voiced. Content suggestions included adding information about cost, MOUD forms, ancillary treatments, drug use’s influence on MOUD initiation, and the frequency of MOUD administration. Suggestions for the clinical profile included information on pregnancy, stigma, mental health issues, transportation, child care, housing, and when they last used. Providers also suggested including an MOUD misconception section that addresses dosing issues due to perceived stigma around higher doses reported by some individuals. Lastly, some providers expressed concern about the potential time burden imposed by assessments and review of the guide during typical clinic activities.
3.2. Pilot Test Results
The total PtDA pilot test group (N = 36) and matched controls (N = 36) are described in Table 1. Excluded participants, or those without a current OUD diagnosis indicated by EHR, were excluded from subsequent results but are characterized in Tables 1 and 2. The subsample of pilot test participants with a current diagnosis of OUD indicated in the EHR (n = 19) had an average age of 38.5 years, (SD = 12.0 years), and were 68% male, 5% Black, 63% White, 26% Hispanic, and 5% other race (see Table 1). The majority of participants were attending residential treatment (89%); 3% were students, 5% were employed, and 92% were not employed. In this group, 17% received less than a high school education, 61% had a high school diploma, 14% had a college degree, and 3% had a graduate school degree, while 5% did not report an education. The 19 matched control participants had an average age of 37 (SD = 11.2) and were 63% male, 5% Black, 63% White, 21% Hispanic, and 11% other race. Controls were predominantly attending residential treatment (89%).
Table 1.
Demographic characteristics of total pilot sample and controls.
| Total Sample | Subsample | |||
|---|---|---|---|---|
| Pilot (N=36) N (%) or M ± SD | Control (N=36) N (%) or M ± SD | Pilot (N=19) N (%) or M ± SD | Control (N=19) N (%) or M ± SD | |
| Age (year) | ||||
| 19–24 | 4 (11.1%) | 3 (8.3%) | 2 (10.5%) | 1 (5.3%) |
| 25–34 | 13(36.1%) | 16 (44.4%) | 7 (36.8%) | 9 (47.4%) |
| 35–44 | 9 (25.0%) | 10 (27.8%) | 4 (21.1%) | 6 (31.6%) |
| 45–54 | 6 (16.7%) | 3 (8.3%) | 3 (15.8%) | 0 (0.0%) |
| 55+ | 4 (11.1%) | 4 (11.1%) | 3 (15.8%) | 3 (15.8%) |
| Gender | ||||
| Male | 24 (66.7%) | 23 (63.9%) | 13 (68.4%) | 12 (63.2%) |
| Female | 12 (33.3%) | 13(36.1%) | 6 (31.6%) | 7 (36.8%) |
| Race/Ethnicity | ||||
| White | 16 (44.4%) | 19 (52.8%) | 12 (63.2%) | 12 (63.2%) |
| Black | 2 (5.6%) | 2 (5.6%) | 1 (5.3%) | 1 (5.3%) |
| Hispanic | 14 (38.9%) | 11 (30.6%) | 5 (26.3%) | 4 (21.1%) |
| Mixed | 1 (2.8%) | 0 (0%) | 0 (0.0%) | 0 (0%) |
| Other | 3 (8.3%) | 4 (11.1%) | 1 (5.3%) | 2 (10.5%) |
| Primary Language | ||||
| English | 34 (94.4%) | 18 (94.7%) | ||
| Spanish | 1 (2.8%) | 0 (0.0%) | ||
| Other | 1 (2.8%) | 1 (5.3%) | ||
| Number of Children | ||||
| 0 | 19 (52.8%) | 9 (47.4%) | ||
| 1 | 5 (13.9%) | 4(21.1%) | ||
| 2 | 7 (19.4%) | 5 (26.3%) | ||
| 3 | 2 (5.6%) | 0 (0.0%) | ||
| 4 | 3 (8.3%) | 1 (5.3%) | ||
| Mean (SD) | 1.011.3 | 0.9 ±1.1 | ||
| Health insurance | ||||
| Medicaid/Medi | 32 (88.9%) | 18 (94.7%) | ||
| Medicare | 1 (2.8%) | 1 (5.3%) | ||
| private | 3 (8.3%) | 0 (0.0%) | ||
| No insurance | 0 (0%) | 0 (0.0%) | ||
Table 2.
Depression severity, treatment needs, and overdose history of total pilot sample and subsamples.
|
Total (N=36) N (%) or M ± SD |
Subsample (n=19) N (%) or M ± SD |
Excluded (n=17) N (%) or M ± SD |
|
|---|---|---|---|
| Patient Health Questionnaire (PHQ) | |||
| Minimal depression | 6 (16.7%) | 4 (21.1%) | 2 (11.8%) |
| Mild depression | 10 (27.8%) | 6 (31.6%) | 4 (23.5%) |
| Moderate depression | 11 (30.6%) | 5 (26.3%) | 6 (35.3%) |
| Moderately severe depression | 6 (16.7%) | 2 (10.5%) | 4 (23.5%) |
| Severe depression | 3 (8.3%) | 2 (10.5%) | 1 (5.9%) |
| Mean score | 10.8 ±6.2 | 10.4 ±6.8 | 11.2 ±5.6 |
| Treatment Needs | |||
| Questionnaire (TNQ) | |||
| Excellent candidate for office based treatment | 2 (5.6%) | 1 (5.3%) | 1 (5.9%) |
| Good candidate for office based treatment with onsite counseling | 6 (16.7%) | 4 (21.1%) | 2 (11.8%) |
| Candidate for office based treatment in as tightly structured program | 20 (55.6%) | 11 (57.9%) | 9 (52.9%) |
| Opioid treatment program only | 8 (22.2%) | 3 (15.8%) | 5 (29.4%) |
| Mean Score | 12.313.8 | 11.6 ±3.2 | 13.1 ±4.4 |
| Overdose History | |||
| Had overdosed on opioids | 18 (50.0%) | 11 (57.9%) | 7 (41.2%) |
| Times overdosed on opioids | 1.7 ±2.2 | 2.0 ±2.4 | 1.4 ±2.0 |
Subsample = Participants with an OUD diagnosis of OUD in EHR over 3 months.
Excluded= Participants without an OUD diagnosis of OUD in EHR over 3 months.
Table 2 describes additional characteristics of the pilot group including depression severity, treatment needs, and overdose history. As rated by the PHQ-9 (Kroenke, Spitzer, & Williams, 2001), 21% had minimal depression, 32% mild depression, 26% moderate depression, 11% moderately severe depression, and 11% severe depression. According to recommendations provided by the TNQ (Brooklyn and Sigmon, 2017), 5% were excellent candidates for office-based opioid treatment (OBOT), 21% good candidates for OBOT with onsite counseling, 58% candidates for OBOT in a tightly structured program, and 16% recommended for opioid treatment programs only. Fifty-eight percent of the participants had a history of overdosing on opioids, with an average of two overdose experiences (SD = 2.4).
As indicated on a MOUD experiences and expectations questionnaire, 58% had previously taken methadone, 58% taken buprenorphine, and 11% taken extended-release naltrexone. While most patients preferred to initiate extended-release naltrexone (42%), 21% preferred methadone, 21% buprenorphine, and 15% had no preference for a specific MOUD. A majority of patients expected to take the medication for at least 3 months (39%), while 26% weren’t sure, and 37% would take it as long as their doctor recommended. The most common perceived barriers to MOUD treatment (see Table 3) included chronic pain (67%), substance use (63%), and mental health conditions (56%). Concerns about cost (37%) and potential judgment by others (37%) were also cited.
Table 3.
Perceived MOUD Barriers of total pilot sample and subsamples.
|
Total (N=36) N (%) |
Subsample (n=19) N (%) |
Excluded (n =17) N (%) |
|
|---|---|---|---|
| Health Concerns | |||
| Pregnant | 0 (0%) | 0 (0%) | 0 (0%) |
| Chronic pain condition | 22 (62.9%) | 12 (66.7%) | 10 (58.8%) |
| Chronic Pain intensity | |||
| Mild | 1 (4.6%) | 1 (8.3%) | 0 (0%) |
| Moderate | 9 (40.9%) | 7 (58.3%) | 2 (20.0%) |
| Severe | 12 (54.6%) | 4 (33.3%) | 8 (80%) |
| Liver disease (e.g., Hep.C) | 12 (33.3%) | 8 (42.1%) | 4 (23.5%) |
| Other substance use | 27 (75%) | 12 (63.2%) | 15 (88.2%) |
| Other health problems | 14 (38.9%) | 9 (47.4%) | 5 (29.4%) |
| Other medical concerns | 5 (14.3%) | 1 (5.6%) | 4 (23.5%) |
| Other mental health concerns | 22 (62.9%) | 10 (55.6%) | 12 (70.6%) |
| HIV positive | 2 (5.6%) | 0 (0%) | 2 (11.8%) |
| Other | 5 (15.2%) | 4 (23.5%) | 1 (6.3%) |
| Barriers to Treatment | |||
| Treatment cost | 14 (38.9%) | 7 (36.8%) | 7 (41.2%) |
| Take time off from work | 9 (25%) | 5 (26.3%) | 4 (23.5%) |
| Transportation | 13 (36.1%) | 5 (26.3%) | 8 (47.1%) |
| Child care | 2 (5.6%) | 1 (5.3%) | 1 (5.9%) |
| Others might judge me | 11 (30.6%) | 7 (36.8%) | 4 (23.5%) |
| Physical/Medical limitations | 7 (19.4%) | 4 (21.1%) | 3 (17.7%) |
| Other: | 9 (25.7%) | 6 (33.3%) | 3 (17.7%) |
Subsample = Participants with an OUD diagnosis in EHR over 3 months.
Excluded= Pilot testing participants without an OUD diagnosis in EHR over 3 months.
Sixteen out of 19 participants responded to questions about their perceptions of the PtDA (e.g., knowledge gained, ease of use, and value). After participants read the PtDA-MOUD, most participants “agreed” or “strongly agreed” that they knew which treatment options were available (88%), knew the benefits of each option (88%), knew the risks and side effects of each option (75%), were clear about the best choice for them (81%), had made an informed choice (94%), and were satisfied with their decision (88%). The majority of participants (n = 16) rated the PtDA “mostly true” or “all true” for easy to read (100%), informative (94%), and valuable in treatment planning with his or her provider (56%), with the remainder of responses rated as “somewhat true”. Lastly, comparisons of pre and post treatment-knowledge test scores revealed a significant difference in total score (p = .02), with post-tests scores indicating more knowledge of information provided in the PtDA-MOUD.
Pilot and control groups were highly comparable on treatment outcomes extracted from the three months of treatment records. Although more participants who used the PtDA were inducted on MOUD (37%) when compared to controls (11%), results were not significant (p = .12). Those in the PtDA group received MOUD (M = 14.0, SD = 24.7) for more days than controls (M = 8.4; SD = 22.5); the means between groups were not significantly different (p > .05), but had a medium effect size of r = .25 (see Figure 2 for the distribution of days in MOUD for the pilot test and control groups). Lastly, the pilot testing group had a higher number of individuals (n = 2) inducted on extended-release naltrexone than controls (n = 0) and more receiving methadone (n = 9), than controls (n = 2).
Figure 2.
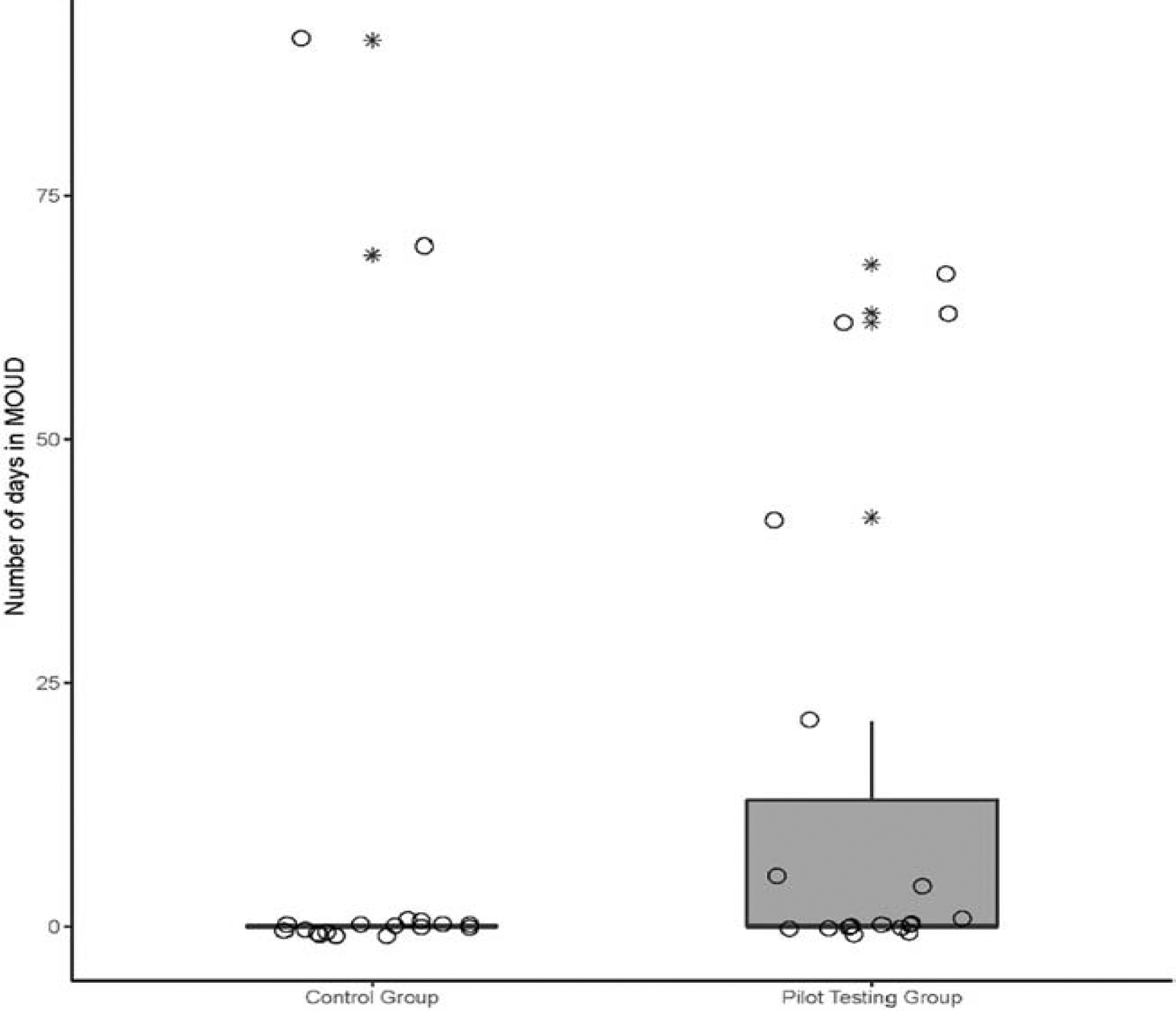
Distribution of the number of days in MOUD for pilot and control groups. Jittered circle (o) indicates participants. Star (*) indicates outliers.
4. DISCUSSION
Patient decision aids (PtDAs) are interventional tools designed to support shared decision making with clinicians (Stacey et al., 2017, Rehman, 2016). In light of the opioid crisis, utilization of the PtDA-MOUD developed in this study may provide opportunities to improve understanding and initiation of MOUD and reduce the consequences of opioid use. This manuscript describes the process of initial development and pilot testing of the PtDA-MOUD. Consistent with IPDAS recommendations for development of PtDAs using input from experts, user peer review, accurate and cited scientific evidence, pilot testing and plain language (IPDAS, 2005), key stakeholders (e.g., patients, service providers and other experts or researchers in the field) were consulted to inform the PtDA-MOUD content and development of the clinical profile. Feedback from patients and provider focus groups illuminated critical content to help inform patients’ decision making around MOUD and facilitate discussion with clinical providers. Stakeholder input also informed the PtDA-MOUD’s use of patient-friendly infographics. In addition, feedback highlighted content areas that may vary most across different treatment settings, such as cost and availability of MOUD. Stakeholders’ suggestions also helped shaped the clinical profile to include important treatment-relevant information, including health status, MOUD preference and experience, and perceived barriers to treatment.
While much of the feedback was consistent, incongruent suggestions regarding length and language were put forth and the PtDA-MOUD was adapted accordingly. While patients seemed to be comfortable with medical terminology, providers suggested patients might not be able to understand certain content, especially if seeking MOUD while in withdrawal. In an effort to make the PtDA-MOUD accessible to all individual in various situations, the guide revisions omitted medical jargon and its reading level was reduced. In addition, while patients and some experts believed the PtDA-MOUD’s length and comprehensiveness should not be limited, providers and other experts believed the guide should be kept short and concise. Given the understandable value of shorter decision aids, along with evidence demonstrating increased knowledge as a result of more detailed decision aids (Stacey et al., 2017), it was determined that a short and long version of the PtDA-MOUD would be maintained.
The small pilot sample limited the ability to detect significant differences between groups. Although the entire sample self-identified as seeking MOUD, a conservative approach was taken where those without an OUD diagnosis documented in the three months of available EHR data were excluded from analyses to ensure greater accuracy when examining effects of the PtDA on MOUD initiation. Though selection of participant samples using EHR- or claims-based OUD diagnoses over a specified time period is an approach used in prior studies (e.g. Gordon et al., 2015), the narrow 3-month window of available EHR data may limit the ability to accurately capture OUD. Although a missing OUD diagnosis could eliminate some individuals who may not have been well suited to test the PtDA, it could also reflect patients who did not disclose OUD symptoms, an incomplete EHR, or unrecorded OUD in remission. Neverthleless, to report the PtDA’s effects on participants with an established current diagnosis of OUD, the questionable sample without an OUD diagnosis was excluded.
Trends indicated that a greater number of individuals who received the PtDA-MOUD were inducted on MOUD and retained on MOUD (based on total days of use), and more received methadone and extended-release naltrexone than controls. The pilot testing phase also elucidated obstacles that would have to be overcome in order to implement study procedures with a much larger sample across multiple sites during the planned cluster-randomized trial. Study procedures will have to be efficient and minimally disruptive to clinic flow in order to encourage clinic participation and ultimately reach recruitment goals. Relatedly, the format of the PtDA-MOUD will have to be simplistic and ideally available in more than one format (e.g. paper, web-based) to allow flexibility and facilitate access. Data collection will have to be efficient and minimally burdensome, perhaps relying more heavily on electronic health records or having data collection take place outside of the treatment process.
Several limitations should be noted while interpreting the results of the study. Participants were recruited from two addiction specialty clinics, potentially limiting the applicability of findings to other populations such as primary care or other general healthcare settings. Although patients and providers were urged to use the clinical profile in decision making, there was no way to assess to what extent the generated clinical profile played a role in shared decision making due to a lack of follow up interviews with patients and clinicians. Participants were individuals who volunteered to participate in the pilot study rather than being randomly selected from the clinic population, possibly influencing preliminary results of the intervention. In an effort to compare PtDA-MOUD pilot test patients to a demographically-similar control group, participants were matched on demographic characteristics but not on potentially important factors such as such as previous treatment episodes or overdose history. Lastly, pilot test analyses were only conducted on participants who were identified as having OUD in the EHR, which may not accurately capture the total population with OUD due to limitations associated with EHR documentation. While this study illustrates promising effects of the PtDA in individuals with current OUD, we hope the PtDA will prove useful to a broader range of groups who have a prior history of OUD or those at high risk for OUD.
Altogether, this study demonstrated the feasibility of developing a patient decision aid for MOUD that integrates patient and clinician values and preferences with scientific evidence. Use of the PtDA-MOUD may increase patients’ understanding of possible medication risks, benefits, alternatives, and associated outcomes and facilitate shared decision making with clinicians around MOUD. The positive feedback regarding use of the tool is encouraging as practices move forward in improving and expanding the treatment of OUD.
The next phase of the study will help to further study the effectiveness of the PtDA-MOUD. After additional refinement, the planned stepped-wedge cluster-randomized trial will include more participants and take place in rural and urban areas of California. Participating H&SS treatment sites will be expanded to include federally qualified health centers, specialty care clinics, opioid treatment programs, rehabilitation centers along with other health centers. This phase will also address the limitations of the pilot test by recruiting a control group and randomizing the order of PtDA-MOUD implementation at the site level in a stepped-wedge cluster design. The trial will also work with participating treatment sites to verify participants seeking MOUD treatment and confirm eligibility before study procedures. Moreover, the anticipated procedures will mimic a real-world implementation of the PtDA-MOUD as staff involvement will be minimal and data collection will predominantly take place outside of the treatment setting after participants have interacted with the PtDA-MOUD.
Highlights.
Medication treatment for opioid use disorder (MOUD) is expanding in California.
Mechanisms to facilitate decision-making processes are increasingly important.
Patient decision aids (PtDAs) can bolster existing resources in treatment settings.
PtDA-MOUD was developed from stakeholder feedback and existing PtDA standards
Preliminary findings from a PtDA pilot test suggest feasibility and utility.
Acknowledgements:
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number R21/R33DA045844. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We are thankful for the assistance of staff a participating treatment programs in Los Angeles, California: Matrix Institute on Addictions and Tarzana Treatment Centers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifier: NCT03394261
References
- Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, … & Cajina A (2011). HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. Journal of Acquired Immune Deficiency Syndromes (1999), 56(Suppl 1), S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener L, Resnick I, Ellard J, Treloar C, & Bryant J (2009). Exploring the role of consumer participation in drug treatment. Drug and Alcohol Dependence, 105(1–2), 172–175. [DOI] [PubMed] [Google Scholar]
- Brooklyn JR, & Sigmon SC (2017). Vermont hub-and-spoke model of care for opioid use disorder: development, implementation, and impact. Journal of Addiction Medicine, 11(4), 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles C, & Gafni A (2014). The vexing problem of defining the meaning, role and measurement of values in treatment decision-making. Journal of Comparative Effectiveness Research, 3(2), 197–209. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Health Statistics (2018). Drug overdose deaths in the United States, 1999–2017. Retrieved from: https://www.cdc.gov/nchs/products/databriefs/db329.htm [PubMed]
- Fagerlin A, Pignone M, Abhyankar P, Col N, Feldman-Stewart D, Gavaruzzi T,K… Witteman HO (2013). Clarifying values: an updated review. BMC Medical Informatics and Decision Making, 13 Suppl 2: S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichs A, Spies M, Harter M, & Buchholz A (2016). Patient preferences and shared decision making in the treatment of substance use disorders: A systematic review of the literature. Public Library of Science One, 11(1), e0145817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz CO, Morris PE, & Richler JJ (2012). Effect size estimates: current use, calculations, and interpretation. Journal of experimental psychology: General, 141(1), 2. [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Lo-Ciganic WH, Cochran G, Gellad WF, Cathers T, Kelley D, & Donohue JM (2015). Patterns and quality of buprenorphine opioid agonist treatment in a large Medicaid program. Journal of Addiction Medicine, 9(6), 470–477. [DOI] [PubMed] [Google Scholar]
- Graff FS, Morgan TJ, Epstein EE, McCrady BS, Cook SM, Jensen NK, & Kelly S (2009). Engagement and retention in outpatient alcoholism treatment for women. American Journal on Addictions, 18(4), 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CA, McCarty D, Mertens J, Lynch FL, Hilde A, Firemark A, … Anderson BM (2014). A qualitative study of the adoption of buprenorphine for opioid addiction treatment. Journal of Substance Abuse Treatment, 46(3), 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Patient Decision Aids Standards (IPDAS) Collaboration. (2005). IPDAS 2005: criteria for judging the quality of patient decision aids. Retrieved from: http://ipdas.ohri.ca/ipdas_checklist.pdf
- Jones CM, Campopiano M, Baldwin G, & McCance-Katz E (2015a). National and state treatment need and capacity for opioid agonist medication-assisted treatment. American journal of public health, 105(8), e55–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Logan J, Gladden RM, & Bohm MK (2015b). Vital signs: demographic and substance use trends among heroin users—United States, 2002–2013. MMWR. Morbidity and mortality weekly report, 64(26), 719. [PMC free article] [PubMed] [Google Scholar]
- Joosten EA, de Jong CA, de Weert-van Oene GH, Sensky T, & van der Staak CP (2009). Shared decision-making reduces drug use and psychiatric severity in substance-dependent patients. Psychotherapy and Psychosomatics, 78(4), 245–253. [DOI] [PubMed] [Google Scholar]
- Knudsen HK, Roman PM, & Oser CB (2010). Facilitating factors and barriers to the use of medications in publicly funded addiction treatment organizations. Journal of Addiction Medicine, 4(2), 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Abraham AJ, & Roman PM (2011). Adoption and implementation of medications in addiction treatment programs. Journal of Addiction Medicine, 5(1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ‐9: validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, … & Hickman M (2012). Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ, 345, e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, & Davoli M (2009). Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database and Systematic Reviews (3), CD002209. [Google Scholar]
- Mayet S, Farrell M, Ferri M, Amato L, & Davoli M (2005). Psychosocial treatment for opiate abuse anddependence. Cochrane Database and Systematic Reviews (1), CD004330. [DOI] [PubMed] [Google Scholar]; Muthulingam D, Bia J, Madden LM, Farnum SO, Barry DT, & Altice FL (2019). Using nominal group technique to identify barriers, facilitators, and preferences among patients seeking treatment for opioid use disorder: A needs assessment for decision making support. Journal of Substance Abuse Treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann T, Neuner B, Weiss-Gerlach E, Tonnesen H, Gentilello LM, Wernecke KD, … Spies CD (2006). The effect of computerized tailored brief advice on at-risk drinking in subcritically injured trauma patients. Journal of Trauma - Injury, Infection and Critical Care, 61(4), 805–814. [DOI] [PubMed] [Google Scholar]
- Rehman B (2016). Shared decision-making and the use of patient decision aids. Medicines Optimization, Prescriber, 33–35. [Google Scholar]
- Reif S, Thomas CP, & Wallack SS (2007). Factors determining how early adopter physicians use buprenorphine in treatment. Journal of Addiction Medicine, 1(4), 205–212. [DOI] [PubMed] [Google Scholar]
- Rieckmann T, Daley M, Fuller BE, Thomas CP, & McCarty D (2007). Client and counselor attitudes toward the use of medications for treatment of opioid dependence. Journal of Substance Abuse Treatment, 32(2), 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2016). Decisions in recovery: Treatment for opioid use disorder. [Electronic Decision Support Tool] (HHS Pub No. SMA-16–4993), 2016.Retrieved from: www.samhsa.gov/brss-tacs/shared-decision-making
- Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, … & Trevena L (2017). Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews, (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, & Feinn R (2012). Using effect size—or why the P value is not enough. Journal of Graduate Medical Education, 4(3), 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift JK, & Callahan JL (2009). The impact of client treatment preferences on outcome: A meta‐analysis. Journal of Clinical Psychology, 65(4), 368–381. [DOI] [PubMed] [Google Scholar]
- Teesson M, Mills K, Ross J, Darke S, Williamson A, & Havard A (2008). The impact of treatment on 3 years’ outcome for heroin dependence: findings from the Australian Treatment Outcome Study (ATOS). Addiction, 103(1), 80–88. [DOI] [PubMed] [Google Scholar]
- Teruya C, Schwartz RP, Mitchell SG, Hasson AL, Thomas C, Buoncristiani SH, … Ling W (2014). Patient perspectives on buprenorphine/naloxone: A qualitative study of retention during the starting treatment with agonist replacement therapies (START) study. Journal of Psychoactive Drugs, 46(5), 412–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody G, Bruce D, Korthuis PT, Chhatre S, Hillhouse M, Jacobs P, … & Ling W (2014). HIV risk reduction with buprenorphine-naloxone or methadone: findings from a randomized trial. Journal of Acquired Immune Deficiency Syndromes (1999), 66(3), 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarborough BJ, Stumbo SP, McCarty D, Mertens J, Weisner C, & Green CA (2016). Methadone, buprenorphine and preferences for opioid agonist treatment: A qualitative analysis. Drug and Alcohol Dependence, 160, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]


