Abstract
The incidence of cyanobacterial harmful algal blooms (CHABs) and their potentially toxic secondary metabolites is increasing in Italy and worldwide and several studies demonstrated that the climate change may be playing a role.
The method described in this work allows to detect simultaneously 21 cyanotoxins of different classes (including 12 Microcystins, 5 Microginins, 2 Cyanopeptolins, and 2 Anabaenopeptins) in water samples for human consumption by an Ultra Performance Liquid Chromatography coupled with a Q-TOF mass spectrometer.
Water samples were freezed, filtered, spiked with Nodularin used as internal standard (I.S.) and then extracted with a SPE Carboghaph 4 cartridge.
The extracted sample were analysed injecting 10 µL into the UPLC-HRMS/MS system: the chromatographic separation was obtained using acetonitrile and water as mobile phases, both containing 10 mM formic acid, and a UPLC C18 column, acquiring the experiments in positive ionization and Sensitivity Mode.
The described method allows to separate and detect all the selected analytes in short time of analysis (16 minutes) with a good resolution for all the analytes.
-
•
simultaneous determination of 21 cyanotoxins belonging to different classes in water sample
-
•
low injection volume
-
•
short time of analysis
Keywords: Drinking water, Mass spectrometry, Microcystins
Graphical abstract
Specifications Table
| Subject Area: | Chemistry |
| More specific subject area: | Analytical chemistry |
| Method name: | High resolution mass spectrometry |
| Name and reference of original method: | |
| Resource availability: | Acetonitrile, MilliQ, formic acid, cyanotoxins standard, Nodularin standard, Carbograph 4 cartridges, Dichloromethane, methanol, TFA, HCl, vacuum pump |
Method details
This work describes an advanced analytical method for simultaneous determination of 21 cyanotoxins of four different class, including 12 Microcystins (MC), 2 Cyanopeptolins(CYP),2 Anabaenopeptins (ANAB) and 5 Microginins (MICRO).
For the analysis of total Cyanotoxins content, 250 mL of water sample were freezed, filtered, spiked with Nodularin (used as internal standard) at 1 µg/L and then extracted using Carbograph 4 SPE cartridge.
For the sample extraction a previous method [1] was updated adding Cyanopeptolins, Anabeanenopeptins and Microginins as analytes and the extraction efficiency was optimised for all the selected cyanotoxins.
All cyanotoxins standards were purchased by Alexis® Biochemicals (La Jolla, 96 CA, USA) while the reagents used were RS grade and the water was obtained from a Milli-Q system (Millipore Bedford, MA, USA).
The instruments used in this work were:
-
•
Acquity UPLC system (Waters Corp., Milford, MA, USA)
-
•
Acquity UPLC BEH C18 column (2,1 mm ID × 100 mm, 1,7 µm, Waters Corp., Milford, MA, USA)
-
•
XEVO G2S Q-TOF (Waters Corp., Milford, MA, USA)
The presence and the quantization of the selected analytes were obtained used:
-
•
a standard solution containing 0.1 μg/L of all the analytes and 1 μg/L of Nodularin used as internal standard (IS), prepared in 70:30 H2O: ACN 10 mM formic acid without any extraction was used
-
•
four water samples spiked at four different concentration levels (0.1, 0.5, 1.0 and 2.0 μg/L) to prepare the calibration curve for each analytes
All the condition applied to obtain the best chromatographic separation for all the analytes was reported in Table 1.
Table 1.
Phase A: water 10 mM formic acid; Phase B: acetonitrile 10 mM formic acid; %As: % phase A start time; %Af: % phase A end time.
| Start time → end time (min) | Column temp (°C) | Flux (µl/min) | %As→ %Af |
|---|---|---|---|
| 0 → 4 | 40 | 450 | 90 →70 |
| 4 → 8 | 40 | 450 | 70 → 30 |
| 8 → 10 | 40 | 450 | 30 → 0 |
| 10 → 12 | 40 | 450 | 0 → 0 |
| 12 → 13 | 40 | 450 | 0 → 90 |
| 13 → 16 | 40 | 450 | 90 → 90 |
Mass Spectrometry analyses were operated in:
-
•
sensitivity mode and positive ionization
-
•
scan time of 0.5 s
-
•
source temperature of 130°C and desolvation temperature of 500°C
-
•
desolvation gas flow of 1000.0 L/Hr
The lock mass signal was used to correct automatically the analytes mass detected during the experiment.
The mass experiment was carried out acquiring
-
•
the molecular ion with a full scan 50-1200
-
•
the specific fragment for each toxins applying the experimental condition reported in Table 2.
Table 2.
Experimental conditions for the selected cyanotoxins; * bicharged ion.
| Toxin | Molecular ion | CE (eV) | Fragment | Retention time (min) | LOD (μg/L) |
|---|---|---|---|---|---|
| MC-RR | 519.7902* | 50 | 135.0806 | 5.44 | 0.002 |
| [D-Asp3]-MC-RR | 512.7824* | 30 | 135.0806 | 5.25 | 0.002 |
| MC-YR | 1045.5353 | 60 | 135.0806 | 6.96 | 0.002 |
| [D-Asp3]-MC-LR | 981.5404 | 80 | 135.0806 | 7.22 | 0.020 |
| MC-LR | 995.556 | 80 | 135.0806 | 7.15 | 0.002 |
| MC-LA | 910.492 | 70 | 135.0806 | 8.26 | 0.017 |
| MC-LY | 1002.5183 | 60 | 135.0806 | 8.33 | 0.004 |
| MC-LW | 1025.5342 | 60 | 135.0806 | 8.63 | 0.022 |
| MC-LF | 986.5233 | 60 | 135.0806 | 8.73 | 0.031 |
| MC-WR | 1068.5513 | 70 | 135.0806 | 7.46 | 0.022 |
| MC-Htyr | 1059.551 | 60 | 135.0806 | 7.03 | 0.004 |
| MC-HilR | 1009.5717 | 100 | 135.0806 | 7.38 | 0.002 |
| CYP-1041 | 1041.4807 | 60-90 | 150.0918 | 7.18 | 0.012 |
| CYP-1007 | 1007.5197 | 50-90 | 150.0918 | 6.61 | 0.012 |
| Microginin-690 | 691.3371 | 50-80 | 128.1436 | 4.56 | 0.008 |
| Microginin-690 me | 705.3528 | 60-90 | 128.1436 | 5.34 | 0.034 |
| Microginin 527 | 528.2738 | 30-50 | 128.1436 | 4.00 | 0.023 |
| Microginin 527 me | 542.2894 | 50-90 | 128.1436 | 4.62 | 0.009 |
| Microginin-704 | 705.3528 | 60-90 | 128.1436 | 5.34 | 0.047 |
| Anab B | 837.4618 | 90 | 201.0987 | 4.29 | 0.004 |
| Anab A | 844.424 | 60 | 84.0860 | 5.98 | 0.003 |
| Nodularin (I.S.) | 825.4505 | 60 | 135.0806 | 6.26 |
It is necessary the presence of molecular and fragment ion, at the same retention time (reported in Table 2), to be sure to detect the analites.
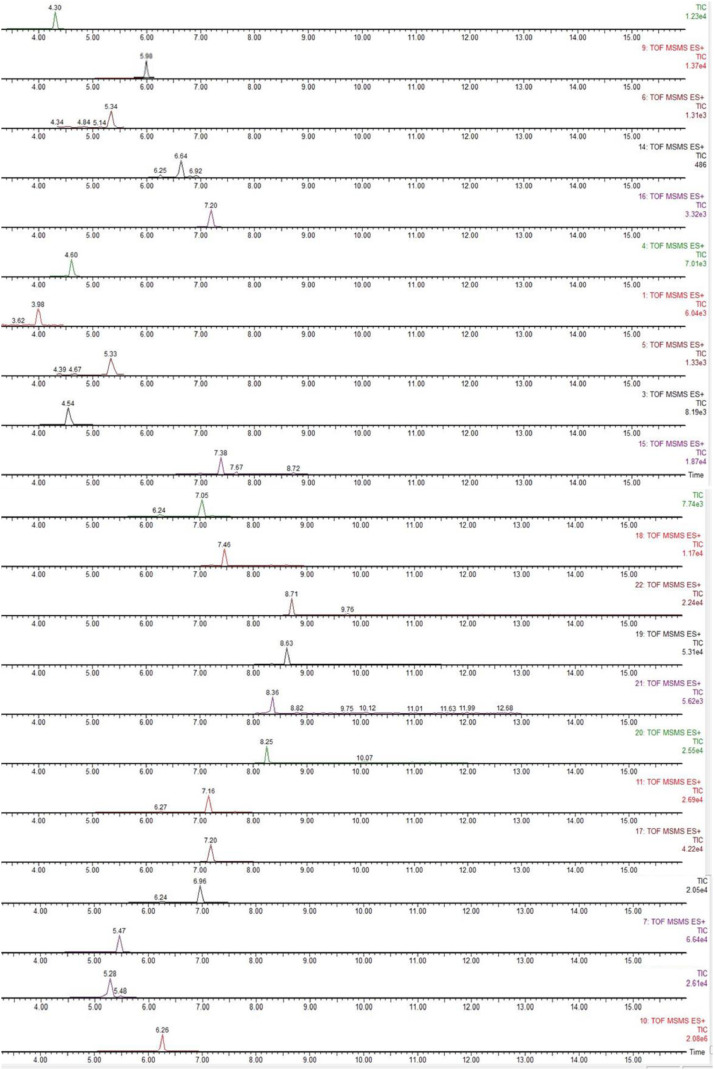
The chromatograms obtained analyzing a solution containing all the selected toxins 0.1 µg/L and Nodularin 1 µg/L is reported in Fig 1 (MS mode) and 2 (MS/MS mode).
Fig. 1.
Chromatogram obtained from the analysis of a standard solution containing all analytes at 0.1 µg/L in MS mode.
Fig. 2.
Chromatogram obtained from the analysis of a standard solution containing all analytes at 0.1 µg/L in MS/MS mode.
The method has proven to be robust, precise and accurate with recovery percentages above 85% and with relative standard deviations ≤16%, fitting for the intended purposes at the concentrations of interest.
No matrix effect ware observed for all the selected analytes.
The LODs were in the range 0.002 to 0.047 µg/L, as reported in Table 2, and all the obtained values at least 20-fold lower than the guideline value proposed by WHO for drinking water (1.0 µg/L for microcystin-LR). [2]
The limit of quantitation (LOQ) could be calculated by multiplying for 3 the LODs values.
The described method allows to detect and quantify the presence of 21 cyanotoxins of different classes in water samples for human consumption simultaneously, in short time of analysis (16 minutes) with a good resolution for all the analytes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
Research project presented in this paper was funded by Caprarola municipality (ISS Protocol 15/02/2011 0007349).
References
- 1.Nigro Di Gregorio F., Bogialli S., Ferretti E., Lucentini L. First evidence of MC-HtyR associated to a Plankthothrix rubescens blooming in an Italian lake based on a LC-MS method for routinely analysis of twelve microcystins in freshwaters. Microchem. J. 2017;130:329–335. doi: 10.1016/j.microc.2016.10.012. [DOI] [Google Scholar]
- 2.World Health Organization, Guidelines for drinking-water quality: fourth edition incorporating the first addendum., 2017. http://apps.who.int/iris/bitstream/handle/10665/254637/9789241549950-eng.pdf;jsessionid=B7A8C40C69D1C876A6809D9572C6B29C?sequence=1. [PubMed]