Abstract
Background & Aims
Non-alcoholic steatohepatitis (NASH) leads to cirrhosis and is associated with a substantial socioeconomic burden, which, coupled with rising prevalence, is a growing public health challenge. However, there are few real-world data available describing the impact of NASH.
Methods
The Global Assessment of the Impact of NASH (GAIN) study is a prevalence-based burden of illness study across Europe (France, Germany, Italy, Spain, and the UK) and the USA. Physicians provided demographic, clinical, and economic patient information via an online survey. In total, 3,754 patients found to have NASH on liver biopsy were stratified by fibrosis score and by biomarkers as either early or advanced fibrosis. Per-patient costs were estimated using national unit price data and extrapolated to the population level to calculate the economic burden. Of the patients, 767 (20%) provided information on indirect costs and health-related quality of life using the EuroQOL 5-D (EQ-5D; n = 749) and Chronic Liver Disease Questionnaire – Non-Alcoholic Fatty Liver Disease (CLDQ-NAFLD) (n = 723).
Results
Mean EQ-5D and CLDQ-NAFLD index scores were 0.75 and 4.9, respectively. For 2018, the mean total annual per patient cost of NASH was €2,763, €4,917, and €5,509 for direct medical, direct non-medical, and indirect costs, respectively. National per-patient cost was highest in the USA and lowest in France. Costs increased with fibrosis and decompensation, driven by hospitalisation and comorbidities. Indirect costs were driven by work loss.
Conclusions
The GAIN study provides real-world data on the direct medical, direct non-medical, and indirect costs associated with NASH, including patient-reported outcomes in Europe and the USA, showing a substantial burden on health services and individuals.
Lay summary
There has been little research into the socioeconomic burden associated with non-alcoholic steatohepatitis (NASH). The GAIN study provides real-world data on the direct medical, direct non-medical, and indirect costs associated with NASH, including patient-reported outcomes in five European countries (UK, France, Germany, Spain, and Italy) and the USA. Mean total annual per patient cost of NASH was estimated at €2,763, €4,917, and €5,509 for the direct medical, direct non-medical, and indirect cost categories, respectively.
Keywords: Non-alcoholic steatohepatitis, Cost of illness, Cross-sectional studies, Europe
Abbreviations: CLDQ, Chronic Liver Disease Questionnaire; CLDQ-NAFLD, Chronic Liver Disease Questionnaire – Non-Alcoholic Fatty Liver Disease; CRF, case record form; EU5, five European; FIB-4, Fibrosis-4; GAIN, Global Assessment of the Impact of NASH; HRQoL, health-related quality of life; NAFL non-alcoholic fatty liver NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; OTC, over-the-counter; PPIE, Patient Public Involvement Engagement; T2DM, type 2 diabetes mellitus
Graphical abstract
Highlights
-
•
There has been little research into the socioeconomic burden associated with non-alcoholic steatohepatitis (NASH).
-
•
Direct medical, direct non-medical and indirect costs resulting from NASH were captured in a real-world setting.
-
•
Extrapolating the per-patient cost to a population level demonstrates the rising prevalence of NASH and related comorbidities.
Introduction
Non-alcoholic fatty liver disease (NAFLD) includes a spectrum of liver disease defined by an excessive accumulation of fat (triglycerides) in hepatocytes, ranging from hepatic steatosis (non-alcoholic fatty liver, NAFL) to non-alcoholic steatohepatitis (NASH), liver cirrhosis, and hepatocellular carcinoma. NAFLD does not result from alcohol consumption but rather is associated with obesity, insulin-resistance/type 2 diabetes mellitus (T2DM), hypertension, and dyslipidaemia.1,2 NAFLD is one of the most common causes of chronic liver disease worldwide,3 estimated to affect 25% of the adult population globally,4 including 20–30% of adults in the western world.5,6 Among the NAFLD population, NASH (the more progressive form of the disease) has a reported prevalence of 5–20%,7 but may occur more frequently in people who are obese or diabetic.8 Considering the global rise of obesity and the ageing population, NASH is a serious public health challenge that is predicted to become increasingly prevalent.4,9
NASH is distinguished from NAFLD histologically by the presence not only of steatosis, but also of inflammation and hepatocyte ballooning, with or without fibrosis.10 Therefore, liver biopsy remains the current standard for NASH diagnosis.11 However, this procedure is associated with complications and, thus, is limited by patient acceptability as well as by cost and sampling error.11 In that context, guidelines recommend the initial use of blood-based simple scores12 or biomarkers, such as Fibrosis-4 (FIB-4) or enhanced liver fibrosis scores and imaging techniques (e.g. transient elastography) to triage patients based on the likelihood of NASH being present with more advanced stages of fibrosis.13,14
NASH-associated mortality increases exponentially with fibrosis stage.8 However, there is substantial interpatient variation in disease progression and outcomes. Although understanding of the factors that affect interpatient variation in disease outcome remains incomplete, NAFLD is best considered a complex disease trait where environmental factors act upon a genetically and epigenetically determined background to modify natural history and outcome.15 In long-term follow-up studies, ∼40% of patients exhibited progressive disease, 40% stable disease, and 20% disease regression during follow-up.16,17 Initially, cirrhosis is not clinically apparent, and is termed ‘compensated cirrhosis’; however, emerging data suggested that health-related quality of life (HRQoL) is impaired during earlier stages of NASH.18 Over time, individuals experience hepatic decompensation, characterised by jaundice, ascites, variceal haemorrhage, and encephalopathy.19
The current standard of care for NASH management comprises lifestyle changes (i.e. diet and exercise) and medication for comorbidities, such as diabetes and hypertension.13 Individuals with early NAFLD are generally managed in primary care, with lifestyle modification. By contrast, patients with NASH and more advanced fibrosis require a multidisciplinary approach, and are currently optimally managed in a secondary-care setting.13 There is currently no pharmacological treatment licenced for NASH, although clinical guidelines recommend that specific antidiabetic and antioxidant drugs (e.g. pioglitazone and vitamin E) can be used in selected patients with fibrosing-steatohepatitis.13 Bariatric surgery induces weight loss and, thus, may also improve fibrosis.13 To date, the only curative treatment for cirrhosis is liver transplantation. In the USA, NASH cirrhosis-related liver transplantations increased from 1.2% in 2001 to 9.7% in 2009,20 and NASH is expected to become the leading need for liver transplantation in the near future.10,21,22
A recent study developed a steady-state prevalence model to estimate the economic burden of NAFLD. This suggested that more than 64 million people in the USA and around 52 million people in France, Germany, Italy and the UK have NAFLD.23 The annual direct medical costs associated with NAFLD were estimated to be US$103 billion/€89 billion (US$1,613/€1,430 per patient) in the USA and €35 billion (from €354 to €1,163 per patient) in Europe. Total costs were highest in patients aged between 45 and 65 years, and the burden was predicted to be significantly higher when societal costs were included, although these were not quantified.23
The overall aim of the Global Assessment of the Impact of NASH (GAIN) study was to determine the real-world humanistic and socioeconomic burden of NASH in adult patients in Europe and the USA. The primary objective was to explore the impact of NASH on patients' HRQoL using patient-reported outcome measures. The secondary objective was to estimate the total annualised cost of the disease, including all health-related items and non-health and indirect costs accrued by patients and their families. Patient stratification by country and fibrosis stage added granularity to the analyses.
Patients and methods
Study design
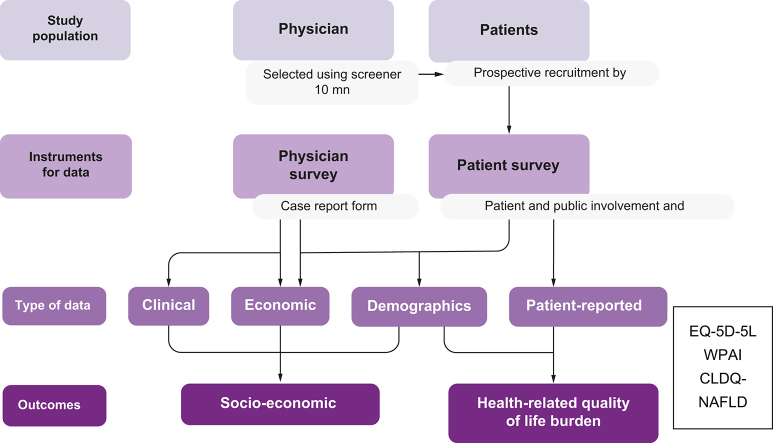
GAIN was a retrospective, cross-sectional study involving physicians and patients providing past and present clinical and economic data from five European (EU5) countries (France, Germany, Italy, Spain, and the UK) and the USA. Patients eligible for inclusion in the study were adults (18 years and over) found to have NASH, following exclusion of other potential liver diseases, confirmed at least 12 months before the date at which they were recruited. Although a definitive NASH diagnosis requires confirmation from liver biopsy, some patients either are unwilling to undergo a biopsy or have a contraindication to it. Restricting recruitment to only patients with a biopsy-confirmed NASH diagnosis would have biased the cohort towards patients under tertiary care and, thus, be unrepresentative of most real-world patients with NASH, who are managed in the primary and/or secondary-care setting. To address this, the expert Steering Committee (membership details are included in the supplemental information online) recommended that the diagnostic criteria for NASH be broadened to include: (i) histologically confirmed NASH with fibrosis; (ii) clinical biochemistry and/or serum biomarkers indicating advanced fibrosis (e.g. AST/ALT ratio, NAFLD fibrosis score, BARD score [derived from AST/ALT ratio, body mass index, and presence of diabetes], and FIB-4 score) in patients with metabolic syndrome risk factors; or (iii) imaging techniques (ultrasound or MRI elastography, or CT imaging) indicating advanced fibrosis and/or cirrhosis in patients with metabolic syndrome risk factors. These latter non-invasive diagnostic procedures are able to reliably discriminate between advanced fibrosis (F3–F4) and early-stage fibrosis (F0–F2),24 but do not reliably differentiate intermediate stages of fibrosis.
Specialists provided information on direct medical resource utilisation and clinical and demographic data based on recorded notes data using a web-based case record form (CRF). They recruited eligible patients consecutively as they attended a clinical appointment, regardless of the reason for their consultation. Additionally, several patients completed (on a voluntary basis) a survey called the Patient Public Involvement Engagement (PPIE) questionnaire following the consultation, providing information about indirect and direct non-medical resource use. patient-reported outcome measures were assessed using validated instruments. Generic HRQoL was evaluated using EuroQol EQ-5D-5L.25 Disease-specific HRQoL was evaluated with the Chronic Liver Disease Questionnaire (CLDQ)-NAFLD; this reports on six domains: abdominal symptoms, activity, emotions, fatigue, systemic symptoms, and worry.18
Through the above-mentioned CRFs, physicians collected relevant clinical and economic retrospective data at the time of patient consultation, which occurred between June and October 2018. Estimates of healthcare utilisation and costs were then calculated for the 12-month period. The online format of the CRFs ensured that numeric responses were kept within reasonable boundaries, and that infeasible responses (such as the ability to provide two mutually exclusive responses) were kept to a minimum. Nevertheless, a small amount of post-hoc data processing was conducted based on expert clinical guidance.
All patient participants provided written informed consent. The study protocol was approved by the Research Ethics Subcommittee of the Faculty of Health and Social Care within the University of Chester. The approval stipulated that the study was to be carried out in correspondence with regional and relevant guidelines, and the 1975 Declaration of Helsinki. For further details regarding the materials used, please refer to the CTAT table and supplementary information.
Cost estimation
Direct medical and non-medical costs were sourced from official country-specific data bases, as well as other non-country-specific sources, grey literature, and publicly available data (including commercial databases for non-medical costs and international websites, such as the World Health Organization and the Organisation for Economic Co-operation and Development databases). For instance, in the USA, the main sources of data were the Medicaid National Average Drug Acquisition Cost database for drug costs, the Centre for Medicare and Medicaid services for test procedures and surgeries, and the American Medical Association for consultations. For a list of all the used sources, refer to Table S1 in the supplemental information online. The choice of resources to be included in the GAIN study was defined by societal perspective. Cost categories included both NASH-related and non-NASH-related events. The total cost burden was categorised broadly as either direct or indirect expenditures. Direct medical costs included not only medication, hospitalisations, consultations, and surgical interventions, but also all tests and procedures used for diagnosis and follow-up of the disease. Direct non-medical costs included elements of professional and informal caregiving, alternative therapies, aids and home equipment/adaptations, and transportation and transfer payments (including state benefits or disability allowances). Over-the-counter (OTC) remedies were included in non-medical costs for practical reasons: not only were they only reported by the patient sample via the PPIE questionnaire (as opposed to all the above-mentioned medical items reported by physicians), but also most reported elements were dietary supplements, the medical therapy nature of which can be disputed. Finally, indirect costs were those that were less tangible in relation to the disease of interest but were nonetheless quantifiable. These included the loss of wages and productivity because of absenteeism or impairment while at work. We included loss of wages by patients, using the country-specific average salaries as a proxy for the opportunity costs.
Direct medical cost variables were obtained from the physician-reported CRF for all patients. Twenty percent of patients provided information about direct non-medical costs and indirect costs. All patients were categorised as having early (F0–F2) or advanced (F3–F4) fibrosis stage. Additionally, patients were classified by country to investigate possible differences in clinical and economic variables. Patients with a biopsy-confirmed diagnosis were included in the analysis, as well as patients with a NASH diagnosis using non-invasive diagnostic tools, such as ultrasound-based transient elastography. The use of these non-invasive tests that do not accurately differentiate intermediate stages of fibrosis imply a level of subjectivity in the physicians' evaluations and, therefore, the possibility of some misdiagnoses of NAFLD and NASH.
All local currency total costs were converted to Euros using the official conversion rate (1 US$ = €0.906756, 1 GB£ = €1.11799 as of September 12, 2019). Per-patient costs were calculated by multiplying the quantities of the resource used with the national unit price of each resource (updated to 2018 prices using inflation indicators via http://ec.europa.eu/eurostat/web/hicp/data/database). To extrapolate the sample costs to country population level, the per-patient costs were multiplied by national prevalence weights26:
where P = price, Q = resource use, and i = 1 − n (where n = number of cost items).
Results
CRFs were completed by 337 physicians, who recruited data from 3,754 patients. Among these, 1,619 patients (43%) had a histological diagnosis of NASH, staged by locally reported liver biopsy. Of the total patients, 767 (20%) completed a PPIE, which was matched to the corresponding CRF. Patient response rates ranged from 5% in the UK to 41% in Italy (Table S1 in the supplemental information online).
The main characteristics of the patients are summarised in Table 1. The demographics showed a slight preponderance of males, mean age in the low 50s (but higher in individuals with more advanced fibrosis) and a high level of obesity (BMI >30).
Table 1.
Study population and basic demographics.
| Demographic | NASH disease stage |
Overall | |
|---|---|---|---|
| Early (F0–F2) | Advanced (F3–F4) | ||
| No. of patients | 2,604 (69) | 1,150 (31) | 3,754 (100) |
| Male | 1,493 (57) | 657 (57) | 2,150 (57) |
| Female | 1,111 (43) | 493 (43) | 1,604 (43) |
| Age (n) | 2,604 | 1,150 | 3,754 |
| Mean (SD) | 52 (12.0) | 55 (11.4) | 53 (11.9) |
| Body mass index (n)a | 2,604 | 1,104 | 3,754 |
| Mean (SD) | 30.8 (9.0) | 30.7 (7.8) | 30.8 (8.7) |
| Country (n) | 2,604 | 1,150 | 3,754 |
| Germany | 364 (67) | 176 (33) | 540 (100) |
| Spain | 363 (70) | 159 (30) | 522 (100) |
| France | 346 (68) | 162 (32) | 508 (100) |
| UK | 322 (76) | 101 (24) | 423 (100) |
| Italy | 377 (70) | 163 (30) | 540 (100) |
| USA | 832 (68) | 389 (32) | 1,221 (100) |
Data are presented as n (%), unless otherwise indicated.
Calculated on a post hoc basis using information from patients' height and weight.
NAFL/NASH diagnosis and fibrosis scores
The pattern of use of NASH diagnostic tests was consistent across EU5 countries, although did differ from practice in the USA. Routine clinical tests, including liver biochemistry, were commonly performed. Overall, ultrasound imaging was the most frequently used diagnostic technique, with 2,550 (68%) patients undergoing an ultrasound. Serum biomarkers and non-invasive tests to risk-stratify NAFLD/NASH were less commonly applied, despite being advocated in the European Association for the Study of the Liver, European Association for the Study of Diabetes, and European Association for the Study of Obesity and American Association for the Study of Liver Diseases clinical guidelines. The most frequently used was AST/ALT ratio (23%), followed by NAFLD fibrosis score (9%), BARD score (3%), and the FIB-4 score (3%). Use of the cytokeratin-18 biomarker, an unvalidated diagnostic tool that detects apoptosis, was reported in 3% of patients. Transient elastography (Fibroscan™) was the most frequently used imaging test to stage disease in 1,228 (33%) patients.
Key differences in practice between the EU5 countries and USA were that imaging techniques were less frequently used in the USA: 618 (51%) patients received an ultrasound compared with an average of 76% in EU5 countries; and only a minority received transient elastography: 157 (13%) compared with a total of 1,071 (42%) in EU5 countries. CT and MRI are predominantly indicated during follow-up in more severely affected patients (e.g. for screening or diagnosing hepatocellular carcinoma) and had been used in 20% and 18% of the sample, respectively. The reported rate of liver biopsy was lowest in Spain (130 patients, 25%), and highest in the USA (697, 57%).
One or more comorbidities were reported in 64% of patients, with a higher prevalence in patients with more advanced disease. Consistent with the association of NAFLD/NASH with the metabolic syndrome, the most frequent comorbidities were obesity (35% of patients), dyslipidaemia (32%), T2DM (27%), and hypertension (27%). Comorbidity rates showed a relatively similar trend across countries. Obesity and dyslipidaemia were most frequent in French (48% and 42%, respectively) and British (41% and 38%, respectively) patients, whereas Italy had the lowest rates of both (24% and 27%, respectively). The rate of hypertension was similar in most countries but slightly higher in France (38%). Depression was the most common mental and behavioural disorder, reported by 8% of patients overall and ranging from 10% in Italy (10%) to 6% in the USA. Cardiovascular diseases (excluding hypertension) were most commonly reported in Germany (18%), and least commonly reported in the USA (4%).
HRQoL
The EQ-5D score decreased with fibrosis status (early vs. advanced) and varied by country (Table 2). In general, there was a clear pattern of decreasing HRQoL score with worsening fibrosis score in all countries, with the exception of France, where similar scores were seen in both early and advanced fibrosis.
Table 2.
EQ-5D index by country and fibrosis stagea.
| Country | NASH disease stage |
Overall | |
|---|---|---|---|
| Early (F0–F2) | Advanced (F3–F4) | ||
| France, n | 93 | 31 | 124 |
| Mean (SD) | 0.83 (0.15) | 0.82 (0.14) | 0.83 (0.15) |
| Germany, n | 94 | 37 | 131 |
| Mean (SD) | 0.81 (0.16) | 0.64 (0.26) | 0.76 (0.21) |
| Italy, n | 160 | 54 | 214 |
| Mean (SD) | 0.85 (0.12) | 0.5 (0.41) | 0.76 (0.28) |
| Spain, n | 119 | 47 | 166 |
| Mean (SD) | 0.7 (0.3) | 0.57 (0.36) | 0.66 (0.32) |
| United States, n | 58 | 36 | 94 |
| Mean (SD) | 0.80 (0.19) | 0.67 (0.23) | 0.75 (0.22) |
| All, n | 531 | 218 | 749 |
| Mean (SD) | 0.80 (0.2) | 0.62 (0.33) | 0.75 (0.26) |
EQ-5D, EuroQOL 5-D; NASH, non-alcoholic steatohepatitis.
UK data (n = 20) were not used for this question because of a low response rate.
The average CLDQ-NAFLD index was 4.9, with France having the highest score at 5.7 and the USA having the lowest score of 4.4 (Table 3). There was also a soft trend for CLDQ-NAFLD score to decrease with fibrosis stage although this was not observed in all territories.
Table 3.
CLDQ-NAFLD index by country and fibrosis stagea.
| Country | NASH disease stage |
Overall | |
|---|---|---|---|
| Early (F0–F2) | Advanced (F3–F4) | ||
| France, n | 91 | 28 | 119 |
| Mean (SD) | 5.7 (0.9) | 5.7 (0.7) | 5.7 (0.8) |
| Germany, n | 94 | 36 | 130 |
| Mean (SD) | 5.0 (1.1) | 4.2 (1) | 4.8 (1.1) |
| Italy, n | 152 | 53 | 205 |
| Mean (SD) | 5.1 (1) | 3.7 (1.5) | 4.7 (1.3) |
| Spain, n | 116 | 44 | 160 |
| Mean (SD) | 5.2 (1.2) | 4.6 (1.4) | 5.1 (1.3) |
| USA, n | 55 | 36 | 91 |
| Mean (SD) | 4.7 (1.05) | 4.0 (1.12) | 4.4 (1.12) |
| All, n | 513 | 210 | 723 |
| Mean (SD) | 5.2 (1.1) | 4.4 (1.4) | 4.9 (1.2) |
CLDQ-NAFLD, Chronic Liver Disease Questionnaire – Non-Alcoholic Fatty Liver Disease; NASH, non-alcoholic steatohepatitis.
UK data (n = 20) were not used for this question because of a low response rate.
Direct medical costs
Mean annual NASH-related direct medical costs per patient (see Table S2 in the supplemental information online for all subcategories) were €2,763 (Table 4); the direct costs for NASH and non-NASH elements (of which >70% were for medication) are provided in the Tables S3 and S4 in the supplemental information online, and the total combined direct costs were €4,754 (Table 4).
Table 4.
Mean annual total (NASH-related and non-related) direct costs by country (SD).
| Cost | NASH stage |
Country |
Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Early | Advanced | Germany | Spain | France | UK | Italy | USA | ||
| n | 2,604 | 1,150 | 540 | 522 | 508 | 423 | 540 | 1,221 | 3,754 |
| NASH-related (A) | €2,224 (19,956) | €3,983 (23,788) | €1,917 (7,715) | €2,162 (10,220) | €1,357 (5,427) | €1,851 (2,406) | €1,732 (7,799) | €4,881 (42,077) | €2,763 (21,216) |
| Non-NASH (B) | €1,659 (4,117) | €2,743 (6,107) | €1,712 (3,698) | €1,161 (3,200) | €1,082 (2,234) | €2,027 (2,897) | €1,006 (2,397) | €2,957 (7,170) | €1,991 (4,840) |
| Total (A+B) | €3,883 (20,634) | €6,727 (25,052) | €3,629 (8,795) | €3,323 (11,258) | €2,440 (6,241) | €3,879 (3,811) | €2,739 (8,296) | €7,258 (36,704) | €4,754 (22,117) |
NASH, non-alcoholic steatohepatitis.
Despite the lack of drugs specifically licenced for the treatment of NASH, the main component of NASH-related direct costs reported by physicians was pharmacological therapy. It is challenging to determine the exact motivation for prescribing specific agents in a multisystem disease, such as NASH, that is frequently associated with other metabolic diseases. However, separate to use for comorbidities, clinicians reported ‘off-label’ prescribing of a range of medication for presumed additional direct therapeutic benefit on NASH. These principally included medication ostensibly targeting obesity (orlistat [5.1% of patients]) or components of the metabolic syndrome (statins [18.4%], metformin [15.5%], losartan [4.2%], fenofibrate [4.3%], and omega-3 fatty acids [5.3%]), with or without evidence of additional liver-directed therapeutic benefits, and drugs directly targeting the liver (vitamin E [12.2%], ursodeoxycholic acid [5.7%], and pentoxifylline [1.1%]). Some agents (e.g. vitamin E) have clinical trial evidence to support their use and are included in clinical guidelines, whereas others lack strong evidence of direct hepatic effects but may have broader therapeutic benefits on metabolic disease. For example, metformin has been shown to reduce the development of T2DM[27], [28], [29]; thus, its use in patients without diabetes but with NASH may have been driven by this consideration and the evidence that there is a bi-directional relationship between NASH and T2DM.1 Surgical procedures, including weight-loss procedures and liver transplantation, were also reported (Table S3 in the supplemental information online).
Overall, costs for patients with advanced fibrosis were higher (€3,983) than for those with early-stage fibrosis (€2,224). Again, pharmacological costs were the highest cost item within the per-patient non-NASH-related costs, with up to 27 different drugs prescribed to treat comorbidities, and statins, metformin, and angiotensin-converting enzyme inhibitors being the most commonly prescribed drugs (in 21%, 18%, and 8% of all patients, respectively), likely reflecting the strong metabolic comorbidities. These costs were highest in the USA (€2,957) and lowest in France (€1,006).
Non-medical costs and indirect costs
Patients provided information about both direct non-medical and indirect (productivity) costs. The overall direct non-medical cost per patient was €4,917 (Table 5). Professional (34%) and informal (48%) caregivers accounted for most of these costs and these percentages increased with disease severity (fibrosis stage). At the country level, there were large differences in professional and informal caregiver costs among different countries. This variability may reflect not only cultural differences, but also the relatively low sample size in each country (see Table S1 in the supplemental information online, with a range of 5% of returned questionnaires from patients in the UK to 41% in Italy).
Table 5.
Mean annual non-medical and indirect costs (SD) reported by patients with NASH by country.
| Cost | NASH stage |
Country |
Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Early | Advanced | Germany | Spain | France | UK | Italy | USA | ||
| n | 546 | 221 | 132 | 169 | 130 | 20 | 219 | 97 | 767 |
| Non-medical | €1,704 (5,785) | €4,661 (9,839) | €2,695 (7,273) | €4,713 (10,828) | €213 (920) | €2,063 (3,707) | €1,977 (5,515) | €3,243 (7,685) | €2,556 (7,308) |
| Indirect | €5,939 (15,989) | €12,642 (21,813) | €11,624 (24,003) | €9,541 (18,223) | €2,530 (9,137) | €4,994 (8,166) | €5,451 (12,175) | €13,435 (29,493) | €7,871 (18,104) |
NASH, non-alcoholic steatohepatitis.
The remaining direct non-medical costs resulted from transport, disability allowances, alternative therapies, home alteration, and OTC remedies. Notably, these costs did not correlate with disease severity, and were evenly distributed among countries, with the exception of the UK and France, where costs of these items were proportionally higher. The UK has the highest cost reported for transportation and for OTC remedies; however, the sample size was the lowest of all countries and, thus, these findings should be interpreted with caution.
Total indirect costs were very high, reaching €5,509 (Table 5) and clearly correlating with disease severity. There was large variability between countries (Table 5), with the lowest costs in France (€2,530) and the highest costs in Germany (€11,624) and the USA (€13,435).
A major share of indirect costs resulted from early retirement or stopping working (74%), followed by time off work in the previous 12 months (26%). Patient indirect or productivity-related costs correlated positively with NASH severity, because patients with relatively more severe disease had to stop working (12% vs. 5% in patients with early-stage disease) or cited that their retirement was the result of NASH (13% vs. 5% in patients with early-stage disease); in addition, more patients with advanced NASH experienced work-related problems or sick leaves compared with patients with early-stage disease (74% vs. 53%, respectively).
Extrapolation of NASH medical costs to a national burden
The average NASH-related costs were €2,763, €4,917, and €5,509 per patient for direct medical, direct non-medical, and indirect cost categories, respectively. Adding the non-NASH-related costs borne by the patient increased the average total direct medical cost to €4,754 (Table 4).
The size and representativeness of the GAIN study CRF sample data was used to extrapolate the total medical cost of NASH using model-based prevalence estimations for the six countries from Estes et al.,4 where a further adjustment was performed to account for the different proportion or weights of fibrosis stages observed in clinical practice. The mean costs for patients with early or advanced-stage NASH in each country in the GAIN study were weighted by the respective proportions of patients with early or advanced-stage NASH following estimations from the aforementioned study (using information from their Table 1 and Fig. 24).
For example, in France, the adjusted prevalence of NASH was 2.3 million and the total per-patient NASH cost was €848 and €2,445 for patients with early or advanced-stage NASH, respectively, which, coupled with the prevalence estimations for a population with early or advanced-stage NASH would give a population cost of close to €2.6 billion annually. The adjusted NASH prevalence was much higher than in the USA than in France. Also, in the USA, 17 million people were estimated to have NASH and, therefore, the total expenditure on NASH was almost €80 billion annually. The national medical costs for patients with NASH are expected to be even higher if resource use related to treatment of concomitant conditions is added, including pharmacological treatment, consultations, and hospitalisations.
However, the population costs rely on an estimated prevalence that is assumed to be accurate and a close representation of the real prevalence of NASH in the countries included in this study. For example, in the paper by Younossi et al.,5 the prevalence of NASH ranged between 1.5% and 6.45% globally, which would provide similar cost projections for their mean point estimation (3.98%, in line with those from Table 6 and from the study by Estes et al.4). If we used the lower bounds of the prevalence figures from Younossi et al.5 (1.5%), the total costs projections would decrease proportionally, with total annual NASH costs ranging between €1 billion in France and €22.5 billion in the USA. These figures might be subject to change when new prevalence studies are published.
Table 6.
Total economic burden of NASH by country, using only medical NASH costs (SD).
| Characteristic | Country |
|||||
|---|---|---|---|---|---|---|
| Germany | Spain | France | UK | Italy | USA | |
| Country population (4) | 80,700,000 | 46,100,000 | 64,700,000 | 64,200,000 | 59,800,000 | 324,100,000 |
| NASH prevalence, % (4) | 4.10 | 3.90 | 3.60 | 4.10 | 4.40 | 5.30 |
| Total NASH cases | 3,308,700 | 1,797,900 | 2,329,200 | 2,632,200 | 2,631,200 | 17,177,300 |
| CRF sample, n | 540 | 522 | 508 | 423 | 540 | 1,221 |
| Direct NASH medical costs | €1,917 (7,715) | €2,162 (10,220) | €1,357 (5,427) | €1,850 (2,406) | €1,732 (7,799) | €4,880 (42,077) |
| NASH drug costs, Tx only | €655 (2,218) | €739 (3,047) | €502 (1,292) | €624 (1,733) | €431 (1,718) | €2,240 (6,116) |
| Costs, early-stage fibrosis | €909 (3,426) | €1,311 (3,001) | €848 (1,582) | €1,638 (2,072) | €1,189 (5,110) | €4,466 (34,876) |
| Early-stage NASH cases (4) | 2,680,047 | 1,402,362 | 1,968,174 | 2,079,438 | 2,052,336 | 13,702,124 |
| Costs, advanced-stage fibrosis | €4.002 (12,349) | €4,107 (17,840) | €2,445 (9,253) | €2,655 (3,139) | €2,989 (11,811) | €5,326 (37,112) |
| Advanced-stage NASH cases (4) | 628,653 | 395,538 | 361,026 | 552,762 | 578,864 | 3,475,176 |
| National medical costs of NASH | €4,952,604,679 | €3,462,650,582 | €2,551,899,368 | €4,873,746,196 | €4,170,141,834 | €79,701,054,554 |
| Conservative prevalence scenario of 1.50% NASH cases per country (5) | ||||||
| NASH prevalence (5) | 1.50% | 1.50% | 1.50% | 1.50% | 1.50% | 1.50% |
| Total NASH cases | 1,210,500 | 691,500 | 970,500 | 963,000 | 897,000 | 4,861,500 |
| National medical costs of NASH | €1,811,928,541 | €1,331,788,686 | €1,063,291,403 | €1,783,077,877 | €1,421,639,262 | €22,556,902,232 |
CRF, case record form; NASH, non-alcoholic steatohepatitis; Tx, treatment.
Discussion
The GAIN study aimed to provide comprehensive and accurate insight into the cost landscape for the real-world NASH population across the EU5 countries and the USA, and, to our knowledge, it is the first research of its kind to adopt a societal perspective.
Direct medical costs for non-NASH related events were almost as high as medical costs for NASH-related events for each of the countries. With the exception of France, direct non-medical and indirect costs for each country were high. This is consistent with the finding reported by Younossi et al.23 in their cost of illness study, where calculated societal costs were consistently higher than medical costs (despite the differences in methodology between the two studies). There is currently a remarkably paucity of data focussing on the costs of patients with NAFLD and NASH (whether including or excluding costs unrelated to NASH); nevertheless, Younossi et al.23 reported a NAFLD per-patient cost of €1,463 in the USA and in the range of €354–€1,163 in four EU countries (Germany, France, Italy, and the UK). These costs would lead to a total country cost of €93.4 billion in the USA and €35 billion for the four countries in Europe in the population with NAFLD. The costs predicted by GAIN for NASH only (excluding cost for concomitant conditions) were almost €80 billion in USA and €20 billion in the EU5 countries.
The only known literature review on the topic is that by Younossi and Henry,30 where 600 citations were reviewed but only six studies retrieved for detailed evaluation. However, only one of the six studies, performed by Ghamar Chehreh et al.31 in the Iranian population, used a similar methodology (i.e. cross-sectional study estimation of NAFLD cost per patient per year) with an annual average equivalent cost of US$5,043 per patient. Nevertheless, the comparability between these studies and the GAIN study must be interpreted with caution for several reasons, not only because NAFLD and NASH are different populations, but also because of the use of different methodologies for collecting resource use data and the use of different prevalence estimations in each country.
Caregivers, whether professional or informal, accounted for the greatest proportion of direct non-medical costs. Furthermore, indirect per patient costs were higher than medical direct costs in all countries.
The study also revealed differences in diagnostic practice between Europe and the USA. The use of ultrasound and other non-invasive techniques and serum biomarkers was more frequent in the EU5 countries, whereas, in the USA, proportionately more patients had their diagnosis confirmed by biopsy.
More advanced fibrosis was associated with higher levels of comorbidity and lower generic and disease-specific QoL scores. Costs also increased with increasing NASH severity.
Limitations
The patient PPIE sample was self-selected and, therefore, may not accurately represent the NASH population as a whole. Even though questions were framed in relation to NASH-specific burden (e.g. the productivity losses question was “Have you missed work because of NASH problems in the last 3 months?”), some physicians and patients might account for other conditions when responding to these questions and, thus, the possibility of double counting costs cannot be completely ruled out. Nevertheless, an effort was made to include non-NASH-specific elements separately in the CRF questionnaires (drugs, consultations, and hospitalisations exclusively for concomitant conditions). However, and despite having clearly separated questions for drugs prescribed for NASH and other comorbidities, it is difficult, as previously stated, to determine the exact motivation for prescribing specific agents in a multisystem disease, such as NAFLD/NASH, that is frequently associated with other metabolic diseases. Additionally, some of the subgroups analysed were small, particularly in the patient sample. No quota was enforced for PPIE questionnaires. As a result, there was a smaller number of patients reporting EQ-5D at both the earliest and most advanced fibrosis stage (F0, n = 35 and F4, n = 48). Therefore, the generalisation of these findings to individual countries requires caution; for example, no patients with F0 NASH in the UK and France provided HRQoL data from either EQ-5D or CLDQ-NAFLD. The possibility of recall bias for physicians is minimum, given that they looked at their patients records to provide CRF data. This might happen for patients, although the number of questions where recalling data was needed was kept to a minimum.
A possible limitation was the inclusion of misdiagnosed (i.e. incorrect distinction between NAFL and NASH) and misclassified NASH cases because of the use of non-invasive testing to diagnose disease. However, the aim of the GAIN study was to provide a broad picture of the current clinical practice and, therefore, including only patients with biopsy-confirmed disease would have focussed the data on a potentially unrepresentative cohort of patients in secondary and/or tertiary care, which would have missed the complexity and diversity of this condition. In fact, the GAIN study revealed that >50% of the overall population identified as NASH never underwent a biopsy to confirm their disease.
There is a scarcity of both QoL and costs (burden of illness) studies in the NASH field, as well as a wide variability of prevalence estimates within the general population. Nevertheless, given the current global rise in obesity and population ageing, NASH is considered to be a serious threat to public health and the associated burden is expected to increase over time. However, differences in population, and the methodology for collecting and computing cost and prevalence estimations, make it difficult to directly compare the results of GAIN and the study by Younossi et al.7
In conclusion, the GAIN study has successfully furthered our understanding of the cost of NASH in the EU5 countries and the USA, and how these costs differ among the participating countries. However, there is scope to delve deeper into the study data set to learn and understand more about the global economic burden of NASH. For example, further analysis would help stakeholders understand the drivers of cost and outcomes among this population, and better understand the causality of poorer outcomes and higher patient costs.
Financial support
This study was supported by Novo Nordisk and Gilead Sciences, Europe Ltd.
Authors' contributions
JO'H contributed to the study design and governance. AF was principal investigator, responsible for monitoring the progress of data collection and contributing to manuscript write-up. HD, LR-C, and BF were responsible for write-up and ongoing critical review of the article. GP and GM contributed to statistical analysis of the data and manuscript review. VH provided feedback for manuscript revision. BJ, MM, TR, IVT, VR, MR-G, EB, JMS, and QMA were responsible for ongoing study review and feedback regarding design, data collection, analysis, and critical review of the manuscript.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The draft paper was formatted for publication by Steve Chaplin, Medical Writer, HCD Economics. The data sets generated and/or analysed during the current study are held under license by the University of Chester and are not publicly available, but are available from the corresponding author on reasonable request.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100142.
Supplementary data
References
- 1.Anstee Q.M., Targher G., Day C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 2.Sanyal A.J. NASH: a global health problem. Hepatol Res. 2011;41:670–674. doi: 10.1111/j.1872-034X.2011.00824.x. [DOI] [PubMed] [Google Scholar]
- 3.Weiß J., Rau M., Geier A. Non-alcoholic fatty liver disease: epidemiology, clinical course, investigation, and treatment. Dtsch Arztebl Int. 2014;111:447–452. doi: 10.3238/arztebl.2014.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estes C., Anstee Q.M., Arias-Loste M.T., Bantel H., Bellentani S., Caballeria J. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 6.Bellentani S., Scaglioni F., Marino M., Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 7.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 8.Dulai P.S., Singh S., Patel J., Soni M., Prokop L.J., Younossi Z. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perumpail B.J., Khan M.A., Yoo E.R., Cholankeril G., Kim D., Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263–8276. doi: 10.3748/wjg.v23.i47.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyson J.K., Anstee Q.M., McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5:211–218. doi: 10.1136/flgastro-2013-100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thampanitchawong P., Piratvisuth T. Liver biopsy: complications and risk factors. World J Gastroenterol. 1999;5:301–304. doi: 10.3748/wjg.v5.i4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekstedt M., Franzén L.E., Mathiesen U.L., Thorelius L., Holmqvist M., Bodemar G. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence . NICE; London: 2016. Non-Alcoholic Fatty Liver Disease (NAFLD): Assessment and Management. NICE Clinical Guideline NG49. [PubMed] [Google Scholar]
- 14.Alkhouri N., McCullough A.J. Noninvasive diagnosis of NASH and liver fibrosis within the spectrum of NAFLD. Gastroenterol Hepatol. 2012;8:661–668. [PMC free article] [PubMed] [Google Scholar]
- 15.Anstee Q.M., Seth D., Day C.P. Genetic factors that affect risk of alcoholic and nonalcoholic fatty liver disease. Gastroenterology. 2016;150:1728–1744. doi: 10.1053/j.gastro.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 16.McPherson S., Hardy T., Henderson E., Burt A.D., Day C.P., Anstee Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 17.Singh S., Allen A.M., Wang Z., Prokop L.J., Murad M.H., Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber Y., Boyle M., Hallsworth K., Tiniakos D., Straub B.K., Labenz C. Health-related quality of life in nonalcoholic fatty liver disease associates with hepatic inflammation. Clin Gastroenterol Hepatol. 2019;17:2085–2092. doi: 10.1016/j.cgh.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Nusrat S., Khan M.S., Fazili J., Madhoun M.F. Cirrhosis and its complications: evidence based treatment. World J Gastroenterol. 2014;20:5442–5460. doi: 10.3748/wjg.v20.i18.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlton M.R., Burns J.M., Pedersen R.A., Watt K.D., Heimbach J.K., Dierkhising R.A. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 21.Sayiner M., Koenig A., Henry L., Younossi Z.M. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis. 2016;20:205–214. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Wong R.J., Aguilar M., Cheung R., Perumpail R.B., Harrison S.A., Younossi Z.M. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 23.Younossi Z.M., Blissett D., Blissett R., Henry L., Stepanova M., Younossi Y. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 24.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 25.EuroQol Group EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 26.Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clin Mol Hepatol. 2014;20:327–337. doi: 10.3350/cmh.2014.20.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musso G., Gambino R., Cassader M., Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 28.Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aroda V.R., Knowler W.C., Crandall J.P., Perreault L., Edelstein S.L., Jeffries S.L. Metformin for diabetes prevention: insights gained from the diabetes prevention program/diabetes prevention program outcomes study. Diabetologia. 2017;60:1601–1611. doi: 10.1007/s00125-017-4361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Younossi Z.M., Henry L. Economic and quality-of-life implications of non-alcoholic fatty liver disease. Pharmacoeconomics. 2015;33:1245–1253. doi: 10.1007/s40273-015-0316-5. [DOI] [PubMed] [Google Scholar]
- 31.Ghamar Chehreh M.E., Vahedi M., Pourhoseingholi M.A., Ashtari S., Khedmat H., Amin M. Estimation of diagnosis and treatment costs of non-alcoholic fatty liver disease: a two-year observation. Hepat Mon. 2013;13:e7382. doi: 10.5812/hepatmon.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.