Abstract
Background/Aims:
To compare intraocular pressure (IOP) measurements using a prototype smartphone tonometer with other tonometers used in clinical practice.
Methods:
Patients from an academic glaucoma practice were recruited. The smartphone tonometer uses fixed force applanation and in conjunction with a machine-learning computer algorithm, is able to calculate the IOP. IOP was also measured using Goldmann applanation tonometry (GAT) in all subjects. A subset of patients were also measured using ICare®, pneumatonometry (upright and supine positions) and Tono-Pen® (upright and supine positions) and the results were compared.
Results:
92 eyes of 81 subjects were successfully measured. The mean difference (in mmHg) for IOP measurements of the smartphone tonometer versus other devices was +0.24 mmHg for GAT, −1.39 mmHg for ICare, −3.71 mmHg for pneumotonometry and −1.30 mmHg for Tono-Pen. The 95% limits of agreement (LoA) for the smartphone tonometer versus other devices was −4.35 to 4.83 mmHg for GAT, −6.48 to 3.70 mmHg for ICare, −7.66 to −0.15 mmHg for pneumotonometry and −5.72 to 3.12 mmHg for Tono-Pen. Overall, the smartphone tonometer results correlated best with GAT (R2=0.67, p < 0.001). Of the 92 videos, 90 (97.8%) were within ± 5 mmHg of GAT, and 58 (63.0%) were within ± 2 mmHg of GAT.
Conclusions:
Preliminary IOP measurements using a prototype smartphone-based tonometer was grossly equivalent to the reference standard
Keywords: Tonometer, tonometry, smartphone, intraocular pressure, machine learning
Synopsis/Precis
Intraocular pressure measurements using a prototype machine learning, smartphone-based tonometer compared similarly with Goldmann applanation tonometry and other clinical tonometers.
Introduction
In rural and remote areas, access to ophthalmological care can be challenging. Globally, a number of countries are estimated to have fewer than 1 ophthalmologist per million people and in spite of the increasing number of practitioners, the aging population is increasing at twice the rate of the profession.1 The standard ophthalmic exam requires large and expensive equipment, making it difficult to deploy in low resource areas.
As smartphones have become increasingly ubiquitous, a number of software programs and hardware attachments have been developed to allow a smartphone to perform various parts of the eye exam. Smartphone-based methods for measuring visual acuity have been validated.2,3 Attachments to obtain high quality anterior segment images have been developed and tested.4,5 A recent review and meta-analysis reported substantial agreement between smartphone funduscopic images and clinical gold standards6 in the grading of a number of conditions including diabetic retinopathy,7–9 cup-to-disc ratios10,11 and retinopathy of prematurity.12
Tonometry, or the measurement of intraocular pressure (IOP), is a critical component of the complete ophthalmologic exam. Elevated IOP is the greatest risk factor for the development of glaucoma and when left untreated can lead to irreversible vision loss; this may occur rapidly if IOP is very elevated. However, a simple, smartphone-based method for assessing intraocular pressure (IOP) has yet to be developed. One of the earliest methods for measuring IOP utilized fixed force applanation and was first introduced by Maklakov in 1885. His methods were later modified by Posner and Halberg in the 1960s and 70s.13,14 With fixed force applanation, a surface of known force is applied to the eye, which creates a circular impression. The diameter of this circular impression can be measured and using the Imbert-Fick relationship between pressure, force and area, an IOP can then be estimated.
In this pilot study, we built a smartphone-based fixed-force tonometer and compared its measurements against Goldmann Applanation Tonometry (GAT), considered the clinical gold standard, as well as other commonly used tonometers.
Methods
Smartphone-Based Tonometry
This study followed the tenets of the Declaration of Helsinski and was conducted in compliance with the Health Insurance Portability and Accountability Act. This study was approved by the Human Subjects Division of the University of Washington (UW) and written informed consent was obtained from all subjects. Patients presenting to the University of Washington glaucoma clinic were recruited. Patients with a history of eye surgery other than cataract surgery, history of corneal scarring or active infection of the eye were excluded. Demographic information including patient age, gender, and ocular history were collected.
The smartphone tonometer prototype is an attachment that is clamped onto an iPhone 6 (Apple, Cupertino, CA, USA) that aligns with both the smartphone camera and the smartphone flash (Figure 1A and 1B). Within the attachment is a clear a 5-gram weight that is rested on the surface of the eye to generate the applanation circle. The metal siding of the attachment in conjunction with a blue filter redirects the flash towards the tip of the 5-gram weight, casting a blue hue that highlights the applanation circle in green after fluorescein application to the eye.
Figure 1.
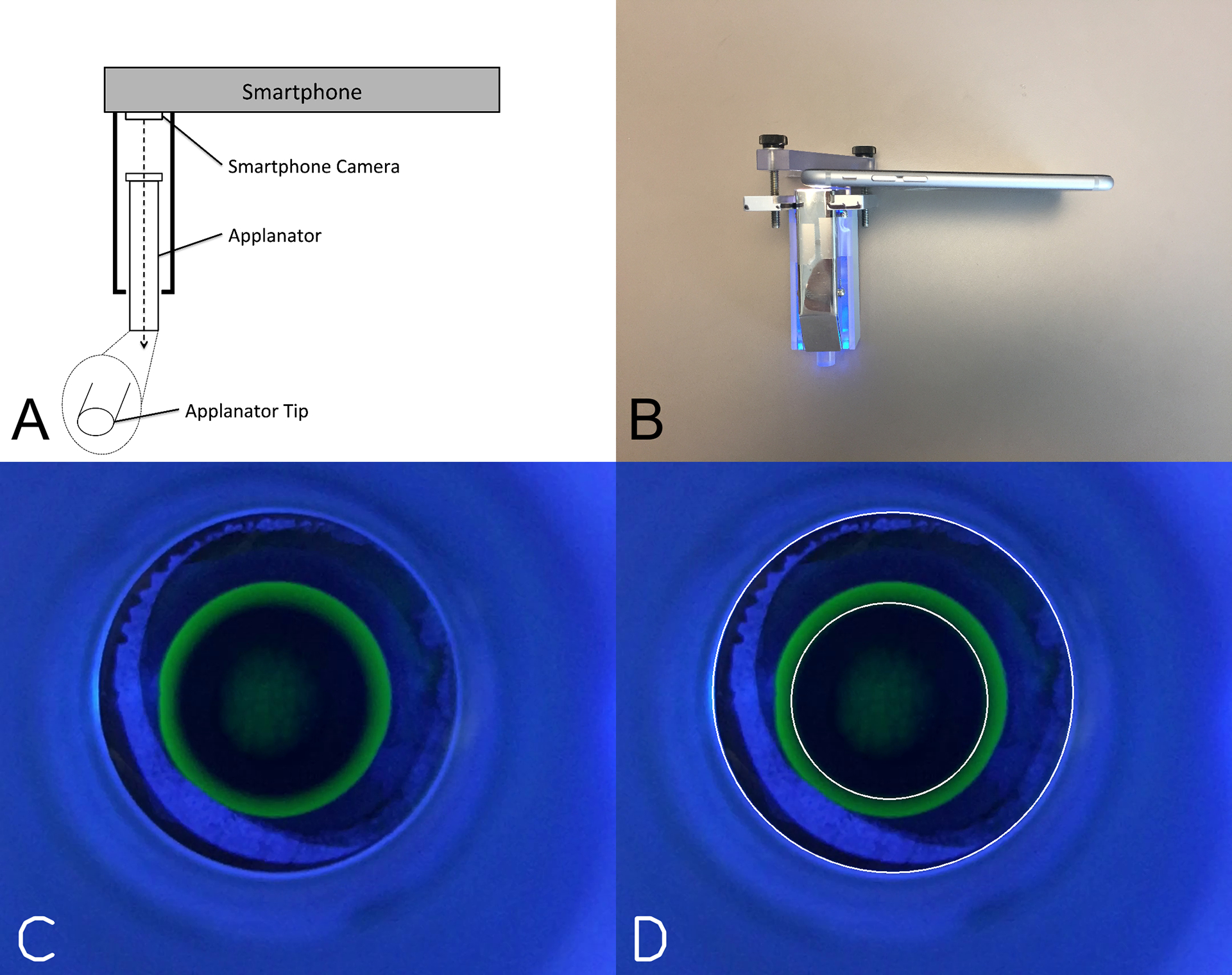
Prototype smartphone tonometer and machine learning image processing. (A) Prototype schematic showing the clear applanator aligned with the smartphone camera (B) The prototype smartphone tonometer used in this study. Metal siding in conjunction with a blue filter redirects the smartphone flash to illuminate the applanator tip with blue light. (C) Example frame from a video showing a typical appearance for the applanation tip and applanation mire. (D) Machine-Learning segmentation of the applanation tip circle and the applanation mire circle.
For each study subject, topical Fluress® was applied to both eyes and the IOP was measured using Goldmann Applanation Tonometry (GAT; Haag-Streit AG, Bern, Switzerland) by trained ophthalmic technicians, who each had experience performing > 100 GAT measurements. The patient was then laid supine for 5 minutes. The video mode with the flash set to “on” was initiated on the smartphone. The full weight of the clear 5-gram weight was then allowed to rest on the eye, creating a “fixed-force” and the size of the resulting applanation circle was recorded as a video. Three contacts with the applanator tip were recorded per video. The decision was made to obtain the GAT reading first because experience with Schiotz tonometry and tonography suggested that resting a weight on the eye would significantly decrease subsequent IOP due to the associated inducement of aqueous outflow. A single GAT measurement minimally displaces aqueous fluid and so by performing GAT first, the second IOP measurement is less affected.
In a subset of patients additional IOP measurements were obtained using other clinical tonometers including iCare Rebound ® tonometer (Tiolat Oy, Helsinki, Finland), Model 30™pneumatonometer (both upright and supine positions) (Reichert Technologies, Depew, NY, USA) and Tono-Pen AVIA® (both upright and supine positions) (Reichert Technologies, Depew, NY, USA). For iCare Rebound tonometry, 6 measurements were made per manufacturer recommendation and the final IOP was recorded only if there was minimal variation (highlighted in green on the device). The pneumatonometer IOP measurement was recorded once the IOP standard deviation was less than 1.0 mmHg for 3 seconds per manufacturer recommendation. For the Tono-Pen, only measurements with a standard error less than 5% were accepted. Each measurement typically took less than 10 seconds to obtain an acceptable measurement. Again, to minimize the IOP lowering effects of some tonometers more than others, the IOP measurements were performed in this order: GAT followed by upright iCare, upright TonoPen, upright pneumatonometer, supine pneumatonometer, supine TonoPen and smartphone tonometer. Tonometry measurements were taken approximately 30 seconds apart and supine measurements were taken after 5 minutes of laying supine.
Image Analysis
The videos taken by the smartphone tonometer were typically 30–60 seconds in length, which captured 25 frames/second and therefore yielded approximately 750–1500 frames per video. A typical frame taken while the tonometer tip is resting on the patient’s eye, is shown in Figure 1c. Two circles are seen: the larger circle is the circumference of the tonometer tip and the smaller greenish circle (applanation mire) results from contact of the tip with the fluorescein-coated ocular surface under blue illumination. In each frame, the 2 circles are identified and the diameter of the mire is calculated by taking the ratio of the mire to the known diameter of the tonometer tip. The diameter of the mire is then converted to an IOP estimate using the Imbert-Fick equation (Pressure = Force / Area). Because the supine nature of the smartphone tonometer measurements elevates the IOP relative to IOP in the upright position when measured with GAT, we adjusted for this by subtracting 4.1 mmHg from the smartphone tonometer measurements. This adjustment was based on studies that demonstrated a mean difference of 4.1 mmHg between upright and supine GAT IOP measurements.15
Machine Learning Methods
The task of the machine learning algorithm is to detect the tonometer circle and the mire for each frame in a smartphone measurement video, and thereby calculate the IOP. This task turned out to be challenging due to the lack of labelled data. To overcome the lack of labelled data, an unsupervised machine learning method based on kmeans, colour filtering and geometry was developed. The kmeans part was designed to detect the tonometer circle, while the green colour filter was developed to detect the fluorescein dyed mire.
First, for the tonometer circle, each frame of the smartphone videos were converted to grayscale. Then kmeans, with k=5, was applied to the grayscale frames. The number of clusters k was chosen to be 5, because visually there are four to five regions. The regions are the mire circle region, the mire to tonometer circle region, the tonometer circle boundary and the area outside the tonometer circle. Next, for each of the k intensity clusters, contours were detected using the OpenCV package and the minimal enclosing circle fit for each contour. These kmeans detected circles were then filtered for tonometer circle radius size. The range for tonometer circle radius size was chosen by manually checking tonometer circle size in two representative videos. Two videos were used because the video capture protocol changed once during the data collection process resulting in two camera focal lengths and thereby two groups of tonometer circle radii in the videos. Once the candidate circles were filtered for size, the tonometer circle was selected to be the smallest of the remaining candidate circles. Note the tonometer circle could not be simply fixed to a constant for the videos, as there is always patient motion as well as slight variations in setting up the smartphone video capture despite clear data collection protocols.
Second, each frame of smartphone videos in the original colour were filtered for the green colour using the HSV (hue, saturation, value) representation of the frame. The green colour range of the filter was determined from the mires in the two training videos. Then this green filter was applied to all videos. The filter transforms the original colour frame into a binary mask where the original colour image pixels that are within the green range are set to 1 and otherwise to 0. Effectively, this removes all but the green parts of the original image in the binary mask. Next, OpenCV was used to detect contours on the binary mask, and a minimal enclosing circle was fit to each contour. Again these circles were filtered for size by deriving the mire size range given the range of tonometer circle sizes from the two training videos for a wide range of possible IOPs (see Supplementary Figure 1). In addition, since the mire is composed of an inner and outer ring of green fluorescein, the candidate circles for the mire were filtered to be pairs of wholly overlapping circles. Finally, the mire was selected to be the smaller of the largest pair of these wholly overlapping circles.
Statistical analysis was performed using python and scipy code (python3.5.5 and scipy1.1.0). Linear correlation and Bland-Altman plots were used to compare measurements. Statistical significance was defined at the p<0.05 level.
Results
This study included 162 eyes from 81 subjects. The demographic information of these subjects is shown in Table 1. Mean (±SD) age was 65.2 ± 10.1 years and 70 eyes were female. Mean GAT IOP was 15.9 ± 4.7 mmHg and mean central corneal thickness was 554.5 ± 44.1 μm. The most common diagnoses were primary open angle glaucoma (31.5%), glaucoma suspect (30.8%), ocular hypertension (9.3%), primary angle closure or primary angle closure glaucoma (9.3%) and normal tension glaucoma (8.6%).
Table 1.
Baseline characteristics. Comparison of baseline characteristics between all measured eyes and eyes with videos that were adequately processed with the machine learning algorithm.
| Parameter | Total | Successfully Processed | P-value |
|---|---|---|---|
| Number of Eyes | 162 | 92 | - |
| Age, years ± SD | 65.2 ± 10.1 | 66.1 ± 9.1 | 0.54* |
| Age range, years | 38 – 88 | 42 – 88 | - |
| Sex (number female) | 70 (43.2%) | 47 (51.0%) | 0.24** |
| Mean IOP ± SD, mmHg | 15.9 ± 4.7 | 15.2 ± 4.0 | 0.22* |
| Mean Central Corneal Thickness, μm | 559.2 ± 40.5 | 554.5 ± 44.1 | 0.40* |
| Diagnosis, n (%) | 0.83*** | ||
| Glaucoma Suspect | 50 (30.9%) | 33 (35.9%) | |
| Primary Angle Closure (Glaucoma) | 15 (9.3%) | 6 (6.5%) | |
| Juvenile Open Angle Glaucoma | 2 (1.2%) | 0 (0%) |
T-Test
Fisher’s Exact Test
Chi-Square Test
Of the 162 smartphone videos obtained from the 162 eyes, the machine learning algorithm was successful in processing 92 videos (56.8%). The baseline characteristics of this subset of successfully processed eyes are also shown in Table 1. No significant differences in baseline characteristics were noted in this subset of eyes compared to all recruited subjects.
Figure 1C and 1D shows an example of a single frame and a single labeled frame. For each video, the IOP was calculated by taking the median of all the IOPs generated by the machine learning method from all the frames in the video. Figure 2A is a scatterplot of the median smartphone IOP measurements compared to the GAT IOP measurements and demonstrates a significant linear correlation (R2 = 0.67, p < 0.001). There was a +0.24 mmHg bias and 95% Limits of Agreement (LoA) was −4.35 mmHg to 4.83 mmHg when comparing smartphone IOP to GAT IOP (Figure 2B). Of the 92 videos, 90 (97.8%) were within ± 5 mmHg of GAT, and 58 (63.0%) were within ± 2 mmHg of GAT.
Figure 2.
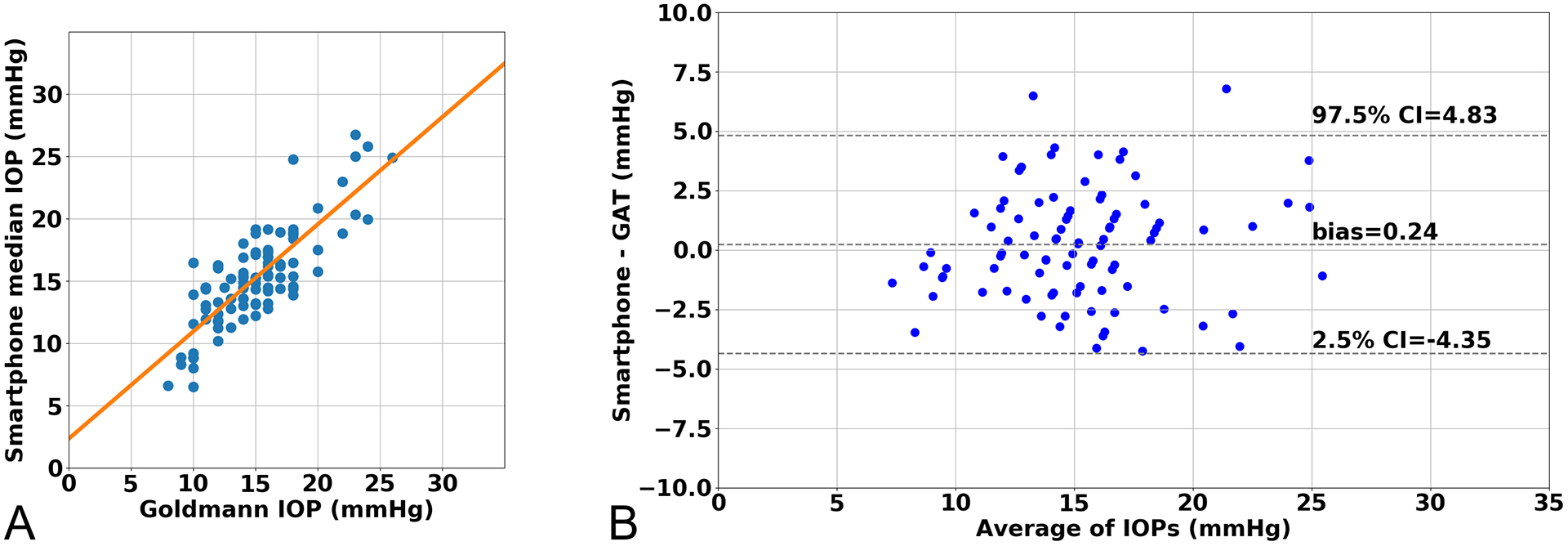
Comparison of smartphone tonometer with Goldmann applanation tonometer. (A) Linear correlation between smartphone tonometer intraocular pressure (IOP) and Goldmann applanation tonometer (GAT) (r=0.82, R2 = 0.67, p-value < 0.001) (B) Bland-Altman Plot comparing IOP measurements
In a subset of patients (n = 38), additional IOP measurements using a pneumatonometer, rebound tonometer and TonoPen were measured and compared with the smartphone tonometer (Table 2). Overall, the smartphone tonometer compared most similarly with GAT. Figure 3 includes all smartphone IOP measurements compared to other tonometers for each subject.
Table 2.
Comparison of the smartphone tonometer with other clinical tonometers in the upright position.
| Tonometer | Smartphone Tonometer Mean Difference (Bias) | Smartphone Tonometer 95% Limits of Agreement |
|---|---|---|
| Goldmann Applanation Tonometer | +0.24 | −4.35, 4.83 |
| Pneumatonometer | −3.91 | −7.66, −0.15 |
| TonoPen | −1.30 | −5.72, 3.12 |
| iCare | −1.39 | −6.48, 3.70 |
Figure 3.
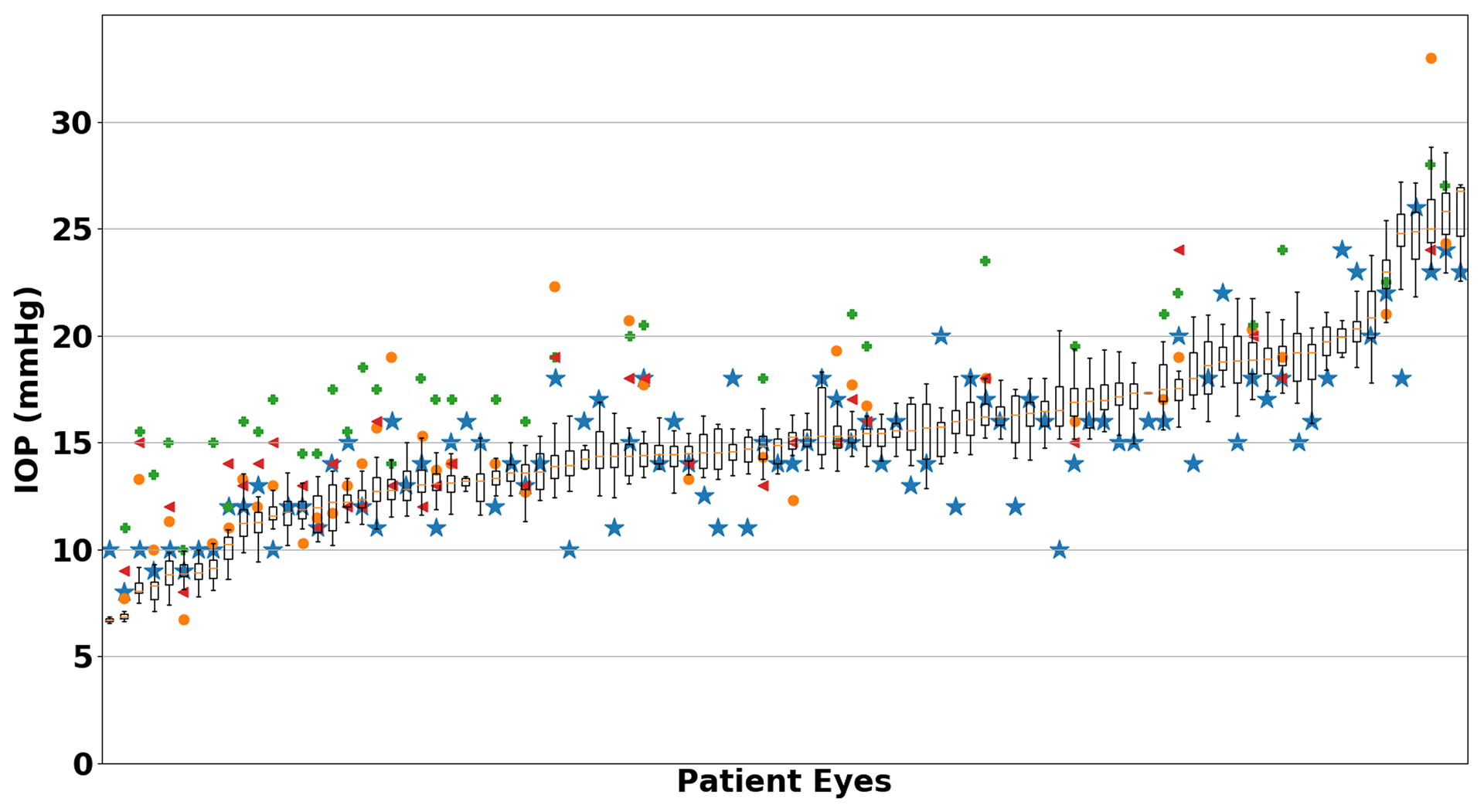
Smartphone tonometer intraocular pressure (IOP) measurements compared to other tonometers. The smartphone tonometer IOP is shown as a boxplot with the corresponding Goldmann (blue star), iCare (orange circle), Pneumatonometer (green cross) and Tonopen (red triangle) measurements.
While the median value of all IOP measurements within a video were taken to represent the overall IOP per video, viewing the scatterplots of the IOPs from all sequential frames of a video frequently revealed a sinusoidal pattern representing the ocular pulsations (Figure 4). A video simultaneously showing the segmented applanation mire in conjunction the calculated IOP as it is plotted can be found in Supplement 1.
Figure 4.
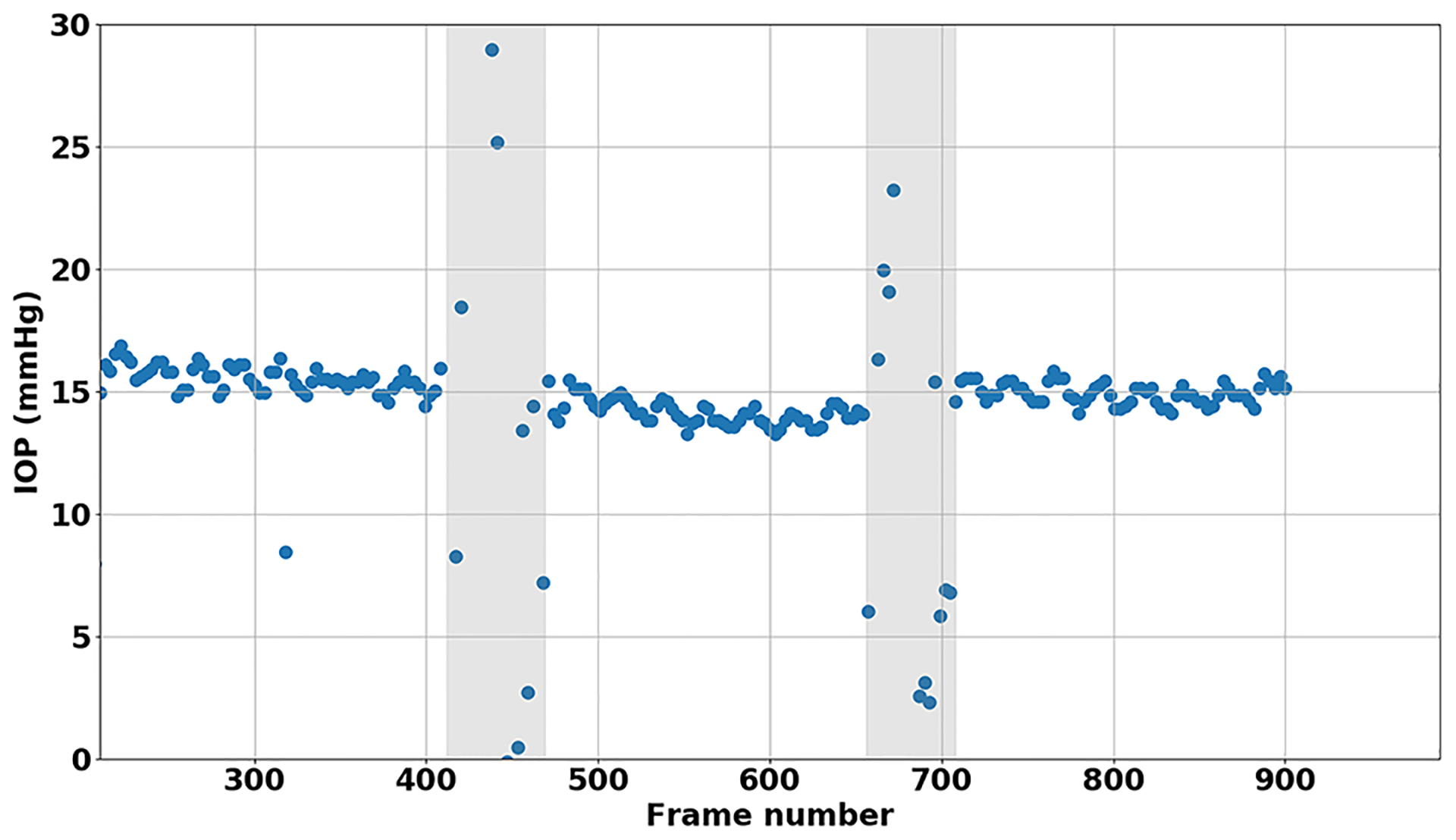
Example scatterplot of fully processed video demonstrating ocular pulsations. Shaded gray areas denote when the tonometer tip was lifted off the ocular surface.
Lastly, to better characterize the supine nature of the smartphone measurement, we compared the IOP measurements obtained from supine pneumatonometer and supine TonoPen with the supine smartphone measurements (Supplement Figure 1). The supine smartphone tonometer measurements had a mean bias of −4.83 and 95% LoA of −9.12 to −0.55 mmHg when compared to the supine pneumatonometer and a mean bias of +3.22 and 95% LoA of −3.29 to 9.73 mmHg when compared to the supine TonoPen. No significant proportional bias was observed in any of these comparisons.
Discussion
In this study, we compared the IOP measurements using a prototype smartphone based tonometer with GAT and a number of other common clinical tonometers. The prototype smartphone tonometer achieves comparable IOP measurements to GAT, which is considered the reference standard and compared favorably with a number of other clinical devices. We found that the mean bias of the smartphone tonometer compared to GAT was +0.24 mmHg with 95% LoA from −4.35 mmHg to 4.83 mmHg. The American National Standards ANSI Z80.10 sets forth the requirements for new tonometers, stipulating that no more than 5% of paired values can be greater than ± 5 mmHg when compared to the reference tonometer (Goldmann) across a range of IOP measurements. For our 92 eyes, only 2.2% of IOP measurements were outside of this tolerance range.
The mean bias and 95% LoA for the smartphone tonometer versus GAT agreed well with the reported mean bias and 95% LoA for other commonly used tonometers. In a large meta-analysis comparing various tonometers to GAT, Cook et al. reported a mean bias of +0.9 mmHg and 95% LoA of −4.3 to 6.1 mmHg for rebound tonometers and a mean bias of −0.2 mmHg and 95% LoA of −6.2 to 5.8 mmHg for Tono-Pens.(22578443) Compared to GAT, 52% of rebound tonometer measurements and 48% of Tono-Pen measurements were within 2.0 mmHg of the GAT measurement.16 Within our study, 63% of IOP measurements were within 2.0 mmHg. Studies evaluating pneumatonometer compared to GAT often report an even higher mean bias up to +5.5 mmHg and 95% LoA +10.0 to +1.5 mmHg.15,17
Fixed-force applanation tonometry was first described by Makalakov in 1885. With this method, the eye was anesthetized and the endplate of a known weight was coated with a mixture of argyrols, glycerin and water. The weight was then briefly rested on the cornea of the supine eye and lifted off. The resulting circular impression on the endplate of the weight was then measured and converted into an IOP. These methods were later modified by Posner and Halberg in the 1960s and 70s to transfer the inked impression to paper for measurement or to directly visualize and measure the applanation surface.13,14 Like the Halberg tonometer, our prototype smartphone tonometer directly visualizes and measures the applanation surface. In a study by Wind and Kaufman, the Halberg tonometer was found to correlate well with GAT (r = 0.961) with high reproducibility.13 However, the authors noted the considerable difficulty in using the Halberg tonometer and the ease at which large errors could be introduced. Automating the process of measuring the applanation surface using the smartphone minimizes these challenges. Anecdotally, the researchers in this study reported that the smartphone tonometer was as easy to use as other clinical tonometers and patients reported minimal discomfort.
Due to the supine position needed to obtain the smartphone tonometer measurement, an a priori correction factor was used to generate the smartphone IOP measurement. A number of studies using various tonometers have examined the effects of supine versus upright IOP measurements and have reported an increase in IOP ranging from 0.3 to 5.6 mmHg depending on the tonometer used.18,19 Since the smartphone tonometer measurements were all compared to GAT, the correction factor of 4.1 mmHg was selected as it best represented the effects of the supine positioning on GAT measurements.15,17 The subtraction of this factor from the smartphone tonometer measurement would therefore best approximate the upright IOP value as it compared to GAT. This corretion factor was determined a priori and applied universally to all measurements to minimize bias. However, to demonstrate the validity of the supine smartphone measurements, we also compared the uncorrected IOP values to the supine IOP measurements obtained using TonoPen and pneumatonometry (Supplementary Figure 1). The supine smartphone tonometer measurements were consistent with the other supine IOP measurements with a mean bias of −4.83 and 95% LoA of −9.12 to −0.55 mmHg when compared to the supine pneumatonometer and a mean bias of 3.22 and 95% LoA of −3.29 to 9.73 mmHg when compared to the supine TonoPen.
Interestingly when plotting the measured IOP from sequential frames within a video often generated a sinusoidal wave pattern as seen in Figure 4. The pulsation of the applanation mires can be seen in videos and are similar to the pulsations seen with GAT that are felt to represent the ocular pulsations. The ability to graphically depict these IOP pulsations suggests that the machine learning segmentation was fairly accurate. By measuring the amplitude of the ocular pulsations we could theoretically estimate the ocular pulse amplitude (OPA). Future research into these pulsations will be needed to better characterize them and compare them to other devices that measure OPA.
Furthermore, in many videos a gradual decline or downward slope of the IOP could be observed over time (Figure 4). Tonography is currently the only non-invasive method to measure outflow facility, where a 2- or 4- minute continuous IOP tracing is created while applying a constant weight to the supine eye. Because the smartphone tonometer utilizes a similar set up with a constant weight applied to the eye while a continuous recording is made, a longer tracing could provide information about outflow facility.
One of the main limitations of this study is that we were only able to successfully process 92 (56.8%) of the total acquired videos using the current machine learning approach. Our current method only successfully processed a portion of the videos because it was an unsupervised method that used only two videos to get some heuristic measures of frame colors and circle sizes. These heuristics were then applied to all videos. Given the variability in colors, focal lengths and circle sizes in all the videos, this unsupervised method was limited in its ability to successfully process all videos. However, Table 1 demonstrates that this subset did not different significantly from the total recruited subjects in terms of baseline characteristics and is therefore likely representative of the group. Furthermore, as a proof of concept, this approach demonstrates that the combination of fixed force applanation with a smartphone does achieve comparable IOP measurements to Goldmann applanation. However, improvements in the image processing are needed to improve the percent of videos that can be successfully processed. As part of future work, we are using the successfully segmented frames from our unsupervised machine learning method as labeled data for a deep learning algorithm to make the automatic detection of circles and thereby calculation of IOP more robust in different use conditions.
As this was a proof of concept study, the intra- and inter-observer reproducibility of the smartphone tonometer are currently unknown. In theory, due to the objective nature of the machine learning approach compared to the semi-subjective methods used in GAT, we anticipate that the reproducibility of the smartphone tonometer may be better than GAT. Future studies are needed to better address this.
In conclusion, our prototype smartphone based tonometer achieved comparable IOP measurements to the clinical gold standard GAT and compared favorably with other clinical tonometers. Future application of deep learning methods may improve successful image processing rates. A smartphone based tonometer complements other existing smartphone hardware attachments and apps to facilitate a portable ophthalmologic exam, which may improve access to ophthalmologic care in resource poor regions.
Supplementary Material
Figure S1. Bland-Altman Plots comparing intraocular pressure (IOP) measurements using (A) the supine pneumatonometer versus the supine smartphone tonometer, (B) the supine TonoPen versus the supine smartphone tonometer and (C) the supine pneumatonometer versus supine TonoPen. Proportional biases are shown (D-F) for each comparison, respectively. None were statistically significant.
Financial support:
Research to Prevent Blindness. University of Washington CoMotion Innovation Fund. AYL was supported by the NEI/NIH K23EY029246. The funding organizations had no role in the design or conduct of this research.
AYL has received grant support from Novartis and Carl Zeiss Meditec. AYL has received honoraria from Topcon and Verana Health. JCW has a patent pending US20170215728A1. None of the other authors have any competing interests related to this study.
Footnotes
This study was previously presented at the American Glaucoma Society Annual Meeting, San Francisco, CA, 2019
References:
- 1.Resnikoff S, Felch W, Gauthier T-M, Spivey B. The number of ophthalmologists in practice and training worldwide: a growing gap despite more than 200,000 practitioners. Br J Ophthalmol 2012;96:783–787. [DOI] [PubMed] [Google Scholar]
- 2.Pathipati AS, Wood EH, Lam CK, et al. Visual acuity measured with a smartphone app is more accurate than Snellen testing by emergency department providers. Graefes Arch Clin Exp Ophthalmol 2016;254:1175–1180. [DOI] [PubMed] [Google Scholar]
- 3.Bastawrous A, Rono HK, Livingstone IAT, et al. Development and Validation of a Smartphone-Based Visual Acuity Test (Peek Acuity) for Clinical Practice and Community-Based Fieldwork. JAMA Ophthalmol 2015;133:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig CA, Murthy SI, Pappuru RR, et al. A novel smartphone ophthalmic imaging adapter: User feasibility studies in Hyderabad, India. Indian J Ophthalmol 2016;64:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludwig CA, Newsom MR, Jais A, et al. Training time and quality of smartphone-based anterior segment screening in rural India. Clin Ophthalmol 2017;11:1301–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilela MA, Valença FM, Barreto PK, et al. Agreement between retinal images obtained via smartphones and images obtained with retinal cameras or fundoscopic exams - systematic review and meta-analysis. Clin Ophthalmol 2018;12:2581–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sengupta S, Sindal MD, Baskaran P, et al. Sensitivity and Specificity of Smartphone-Based Retinal Imaging for Diabetic Retinopathy: A Comparative Study. Ophthalmol Retina 2019;3:146–153. [DOI] [PubMed] [Google Scholar]
- 8.Rajalakshmi R, Arulmalar S, Usha M, et al. Validation of Smartphone Based Retinal Photography for Diabetic Retinopathy Screening. PLoS One 2015;10:e0138285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russo A, Morescalchi F, Costagliola C, et al. Comparison of smartphone ophthalmoscopy with slit-lamp biomicroscopy for grading diabetic retinopathy. Am J Ophthalmol 2015;159:360–4.e1. [DOI] [PubMed] [Google Scholar]
- 10.Russo A, Mapham W, Turano R, et al. Comparison of Smartphone Ophthalmoscopy With Slit-Lamp Biomicroscopy for Grading Vertical Cup-to-Disc Ratio. J Glaucoma 2016;25:e777–81. [DOI] [PubMed] [Google Scholar]
- 11.Bastawrous A, Giardini ME, Bolster NM, et al. Clinical Validation of a Smartphone-Based Adapter for Optic Disc Imaging in Kenya. JAMA Ophthalmol 2016;134:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goyal A, Gopalakrishnan M, Anantharaman G, et al. Smartphone guided wide-field imaging for retinopathy of prematurity in neonatal intensive care unit - a Smart ROP (SROP) initiative. Indian J Ophthalmol 2019;67:840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wind CA, Kaufman HE. Clinical evaluation of the Halberg hand applanation tonometer. Ann Ophthalmol 1972;4:634–641. [PubMed] [Google Scholar]
- 14.Posner A THE APPLANOMETER, A MODIFIED MAKLAKOV APPLANATION TONOMETER Eye Ear Nose Throat Mon 1965;44:77–80 PASSIM. [PubMed] [Google Scholar]
- 15.Barkana Y, Gutfreund S. Measurement of the difference in intraocular pressure between the sitting and lying body positions in healthy subjects: direct comparison of the Icare Pro with the Goldmann applanation tonometer, Pneumatonometer and Tonopen XL. Clin Experiment Ophthalmol 2014;42:608–614. [DOI] [PubMed] [Google Scholar]
- 16.Cook JA, Botello AP, Elders A, et al. Systematic review of the agreement of tonometers with Goldmann applanation tonometry. Ophthalmology 2012;119:1552–1557. [DOI] [PubMed] [Google Scholar]
- 17.Barkana Y Postural change in intraocular pressure: a comparison of measurement with a Goldmann tonometer, Tonopen XL, pneumatonometer, and HA-2. J Glaucoma 2014;23:e23–8. [DOI] [PubMed] [Google Scholar]
- 18.Tsukahara S, Sasaki T. Postural change of IOP in normal persons and in patients with primary wide open-angle glaucoma and low-tension glaucoma. Br J Ophthalmol 1984;68:389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson DR, Grant WM. The influence of position on intraocular pressure. Invest Ophthalmol 1973;12:204–212. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Bland-Altman Plots comparing intraocular pressure (IOP) measurements using (A) the supine pneumatonometer versus the supine smartphone tonometer, (B) the supine TonoPen versus the supine smartphone tonometer and (C) the supine pneumatonometer versus supine TonoPen. Proportional biases are shown (D-F) for each comparison, respectively. None were statistically significant.


