Abstract
The “whole genome” TMV-based expression system, Geneware®, was used in the cGMP production of the plant-made pharmaceutical Q-Griffithsin and demonstrates stable expression for up to a two-year period. Virion and plasmid banks which contained viral cDNA and a Q-Griffithsin sequence were able to produce >200 g of Q-Griffithsin. Data assessing the quality and stability of the product was measured through functional assessments of visual symptomology, amount of product produced, and densitometry.
Keywords: Q-Griffithsin, microbicide, TMV, plant-made pharmaceuticals, virion, stability
Background
Griffithsin (GRFT, UniProt P84801) is a lectin derived from the red algae Griffithsia. Its broad-spectrum antiviral properties are potent against enveloped viruses and GRFT is being developed as an HIV prophylactic [1]. A GRFT-based prophylactic could be used as a microbicide to significantly inhibit infection in populations with a high risk of exposure. The need for an HIV microbicide is great in countries with low resources and in populations at high risk of infection. Using plant-based transient protein expression as a production system, GRFT is viable for bulk production as an active pharmaceutical ingredient [2, 3]. We produced bulk quantities (>100g) of Q-GRFT (GenBank Accession # MT495604), an oxidation resistant variant of GRFT, to support an ongoing clinical trial. This letter provides analysis and discussion of the production, qualification, and stability of infectious recombinant Tobacco Mosaic Virus (TMV) expressing Q-GRFT, ‘master virion bank’ (MVB), and plasmid DNA containing the viral cDNA and the Q-GRFT DNA sequence, ‘master plasmid bank’ (MPB).
Plant-made pharmaceuticals provide advantages over other protein expression systems through speed and scalability. Additionally, some proteins express and fold more optimally in plants. This is the first report of plasmid and virion banks being constructed and monitored for stability with a “whole genome” TMV-based vector. The “whole genome” vector includes TMV coat protein, movement protein, RNA helicase, RNA polymerase, and Q-GRFT. The vector design is based upon the 30B vector described in [4] and commonly referred to as Geneware®. Geneware® was employed as opposed to “deconstructed” vectors because Q-GRFT downstream purification has been optimized with Geneware® expression and “deconstructed” vectors cultivated using agrobacteria would add endotoxin, possibly requiring reoptimization of downstream purification. We produced both MPB and MVB to support the cGMP production of Q-GRFT for a first-in-humans clinical trial and monitored the activity through functional assessment over a two-year period.
For bulk Q-GRFT production, transient expression in Nicotiana benthamiana is accomplished through inoculation with the MVB [5]. The MVB is composed of assembled virion particles carrying infectious RNA and should express Q-GRFT and be more stable than the naked RNA, making it more useful as an inoculum for bulk protein production. The MVB is first passage virion purified from plants infected with naked linear RNA transcribed from the MPB. Quality of plasmids and virion was assessed throughout a two-year −20° C storage period to determine if the stability of expression was consistent [2]. Assessment of MVB and MPB at room temperature was not performed, however informal observation shows at minimum short term stability.
Production of Master Plasmid Bank
An origin plasmid was created by inserting the synthetic gene for Q-GRFT (Atum formerly DNA 2.0, Newark, CA) into the Geneware® vector and subsequently verifying the sequence integrity with double strand sequencing of the entire plasmid. To prepare the MPB, the double-strand-sequenced origin plasmid was transformed into E. coli strain DH10β and replicated. After replication, plasmids were purified using an endotoxin-free prep to a final concentration of 242 ng/μL. The resulting plasmid prep was aliquoted, labeled MPB, and stored at −20° C.
Production of Master Virion Bank
Plants were infected with non-encapsidated rTMV transcribed from MPB using a T7 RNA transcription kit. Inoculated plants expressed fully assembled virion and the amplified virion was harvested 5-7 days after inoculation [2]. The virion produced in the plant tissue was extracted in an aqueous low pH buffer (21 mM Sodium Metabisulfite). Purified virion was precipitated using 4% PEG 6000 with respect to the final volume. Virion was isolated through a centrifugation step and resuspended in a sodium potassium phosphate buffer (10 mM NaKPO4) to remove remaining insoluble material [6]. The concentration of the isolated virion was estimated with UV optical density of 260 nm with an extinction coefficient of 3.06 [7]. The concentration of virion was adjusted to 25 – 100 μg/mL and then it was aliquoted and stored at −20° C [2]. Purified virion can be utilized for large scale manufacturing inoculations. Plants are inoculated with a high velocity pneumatic spray containing virion and diatomaceous earth, a mild and inert abrasive, targeting 50 ng of virus per plant.
Bank Qualification
To qualify the plasmid bank, the DNA sequence was confirmed using double-strand-sequencing and compared to the origin plasmid. A functional assessment was performed (Figure 1 A, B) using a T7 RNA transcription kit to transcribe the plasmid DNA into infectious RNA. The naked RNA produced was used to infect N. benthamiana by manually rubbing leaves with a mild abrasive and a phosphate buffered solution containing the naked rTMV RNA. Infected plants were assessed for TMV symptoms 14 days after initial inoculation [5]. The plasmid’s ability to produce Q-GRFT was assessed in planta (Figure 1B; 0-month time point).
Figure 1. Qualification and Stability of Virion and Plasmid Banks.
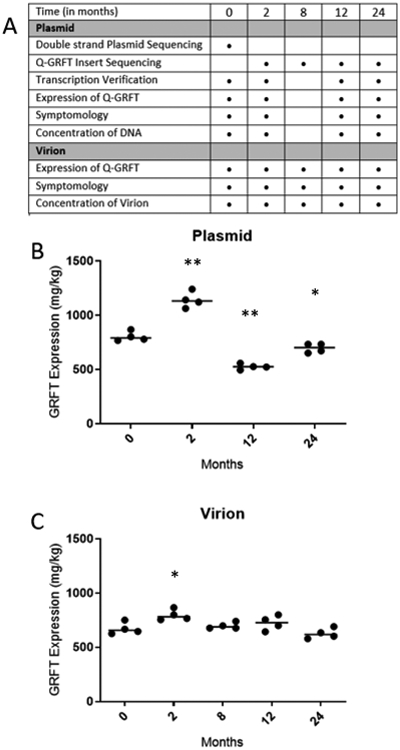
A. Figure A describes the qualification and stability testing process for both virion and plasmid banks. Dots indicate tests performed under various time points. The MPB was double strand sequenced in its entirety (>10kb) at time point zero. After the initial sequencing, only the Q-GRFT insert (366 bp) was sequence verified (at time points two, eight, twelve, and twenty-four months); and transcribed into RNA (at time points two, twelve, and twenty-four months). Expression levels of Q-GRFT from plasmid transcripts were measured at all time points using densitometry analyzed SDS-PAGE of clarified plant extracts. The virion qualification process differed in that there was no need to sequence or transcribe, and only a functional assessment of expression was performed to evaluate the quality of the virion.
B. Figure B displays the quantified results of the functional assessment of Q-GRFT expression performed on transcripts prepared from the MPB. Dots represent four samples taken from plants at each time point transcribing and expressing Q-GRFT from the same MPB. Q-GRFT expression levels (mg/kg) were quantified via SDS-PAGE using densitometry on clarified plant extracts. The average expression from plasmid at 0, 2, 12, and 24-month data points was; 804.1 ± 44.9 mg/kg, 1142.078 ± 73.7 mg/kg, 526.08 ± 25.4 mg/kg, 697.2 ± 41.96 mg/kg, respectively. Q-GRFT expression was analyzed by a repeated measures one-way ANOVA (p < 0.0001) with Bonferroni’s post-hoc tests comparing results to time 0. The MBP expressed Q-GRFT with significant variation from time 0 at all time points tested, but all were above the 200 mg/kg target. * p < 0.05, **p < 0.01.
C. Figure C displays the quantified results of the functional assessment of Q-GRFT expression performed on virions. Dots represent four samples taken from plants at each time point expressing Q-GRFT from the same MVB. Q-GRFT expression levels (mg/kg) were quantified via SDS-PAGE using densitometry on clarified plant extracts. The average expression from virion at 0, 2, 8, 12, and 24-month data points was; 672.1± 54.8, 796.4 ± 49.9, 697.98 ± 29.03, 723.9 ± 67.4, 626.4 ± 48.4, respectively. Q-GRFT expression was analyzed by a repeated measures one-way ANOVA (p = 0.0455) with Bonferroni’s post-hoc tests comparing results at each time point to time 0. Q-GRFT expression levels showed a significant difference at the two-month time point. However, all other time points appear to show consistent expression over the two-year observation period and are well above the 200 mg/kg qualification standard. * p < 0.05.
Plants were harvested 14 days after inoculation; 90% of plants must display visual viral symptoms to qualify. Densitometry on SDS-PAGE with Q-GRFT standards was used to calculate the expressed Q-GRFT from clarified green juice extract. Results from SDS-PAGE must show infected plants expressed Q-GRFT. However, the Q-GRFT expression level from plasmid was specified as information only with a target specification of >200 mg/kg of Q-GRFT because of the high degree of variation of the transcript infection. To be qualified the plasmid bank must: match the origin plasmid sequence, produce visual symptoms in plants, and produce Q-GRFT. After qualification, the plasmid was stored at −20° C [2].
The virion bank was qualified via a functional assessment of its induced expression level (Figure 1 A, C). Virion was spray inoculated on ten plants and harvested 14 days later. Q-GRFT was extracted and the Q-GRFT content assessed with SDS-PAGE and densitometry. A virion bank was qualified only if it produced >200 mg/kg of Q-GRFT [2].
Bank Stability
To monitor gene-sequence stability in plasmid banks, the Q-GRFT insert, rather than the entire plasmid, was sequenced at two, eight, twelve, and twenty-four months. The plasmid concentration was measured at two, twelve, and twenty-four months and transcribed using RNA transcription kit T7 for inoculation. Inoculated plants were harvested to assess plasmid function through Q-GRFT expression (Figure 1B). Additionally, the plasmid’s ability to produce visual symptomology in planta was also observed.
Virion was functionally assessed in its ability to produce Q-GRFT at two, eight, twelve, and twenty-four months (Figure 1C). The virion’s ability to produce product in planta was assessed through SDS-PAGE and densitometry to estimate Q-GRFT levels in biomass. Additionally, virion concentration and its ability to produce visual viral symptomology were evaluated at all time points (Figure 1A).
As seen in Figure 1B, plasmids produce Q-GRFT expression levels of 200 mg/kg or more but appear to be inconsistent between time periods. Expression data was analyzed by a repeated measures one-way ANOVA and showed significant differences in expression at all time points. MPB produced both the highest and lowest amount of product, which is likely related to transcription reaction efficiency, susceptibility of plants, or the stability of viral RNA. Virion, as seen in Figure 1C, produces more stable Q-GRFT expression over the two-year period. When expression data was analyzed by a repeated measures one-way ANOVA a significant increase in expression was observed at the two-month time point. The ANOVA has a p-value of 0.0455 and is on the cusp of significance. The data set may suffer from random variation and would likely benefit from increased sample numbers to more reliably assess the significance of variation in plant expression. In addition to providing stable expression, virion provides easier scalability than attempting to transcribe RNA for a large amount of plants. Virion reduces cost and time-associated with transcription of plasmids and provides more stable expression than RNA.
Conclusion
The Geneware® transient expression system in N. benthamiana using virion proves to stably express and function to guide future product bank development. The plasmid and virion banks were able to produce >200 g of Q-GRFT and inoculate more than >10,000 plants over a two-year period. Subsequent product was used to perform IND enabling animal toxicity and perform a clinical study. The virion expression system’s ability to cost-effectively manufacture stable and sufficient amounts of protein make it a viable system to produce other proteins [3, 8-10].
Highlights.
A TMV-based viral vector was used to express Q-Griffithsin in plants
Plasmid and virion banks were produced to support cGMP manufacturing of Q-Griffthsin
The quality of plasmid and virion banks was confirmed by assessing expression
Plasmid and virion banks maintain functionality for greater than 2 years
Acknowledgements
This work was funded by NIAID by US NIAID U19 Program Project Grant No. U19AI113182.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of Interest
KEP, JLF, and KTH are inventors on patents and patent applications that claim Q-GRFT composition and utility. In addition, KEP, JLF, and KTH are founders and equity holders in GROW Biomedicine LLC, which is commercializing Q-GRFT.
EH, JMC, JWS declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lusvarghi S, Bewley CA: Griffithsin: An Antiviral Lectin with Outstanding Therapeutic Potential. Viruses 2016, 8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuqua JL, Hamorsky K, Khalsa G, Matoba N, Palmer KE: Bulk production of the antiviral lectin griffithsin. Plant Biotechnol J 2015, 13(8):1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alam A, Jiang L, Kittleson GA, Steadman KD, Nandi S, Fuqua JL, Palmer KE, Tuse D, McDonald KA: Technoeconomic Modeling of Plant-Based Griffithsin Manufacturing. Front Bioeng Biotechnol 2018, 6:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shivprasad S, Pogue GP, Lewandowski DJ, Hidalgo J, Donson J, Grill LK, Dawson WO: Heterologous sequences greatly affect foreign gene expression in tobacco mosaic virus-based vectors. Virology 1999, 255(2):312–323. [DOI] [PubMed] [Google Scholar]
- 5.O'Keefe BR, Vojdani F, Buffa V, Shattock RJ, Montefiori DC, Bakke J, Mirsalis J, d'Andrea AL, Hume SD, Bratcher B et al. : Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc Natl Acad Sci U S A 2009, 106(15):6099–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asurmendi S, Berg RH, Smith TJ, Bendahmane M, Beachy RN: Aggregation of TMV CP plays a role in CP functions and in coat-protein-mediated resistance. Virology 2007, 366(1):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kathiria P, Sidler C, Golubov A, Kalischuk M, Kawchuk LM, Kovalchuk I: Tobacco mosaic virus infection results in an increase in recombination frequency and resistance to viral, bacterial, and fungal pathogens in the progeny of infected tobacco plants. Plant Physiol 2010, 153(4):1859–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pogue GP VF, Palmer KE, White E, Haydon H, Bratcher B : Production of Pharmaceutical Grade Recombinant Native Aprotinin and Non-oxidized Aprotinin Variants Under Greenhouse and Field Conditions . In: Commercial Plant-Produced Recombinant Protein Products. Edited by Howard J HE, vol. 68 Berlin, Heidelberg: Springer; 2014. [Google Scholar]
- 9.Pogue GP, Vojdani F, Palmer KE, Hiatt E, Hume S, Phelps J, Long L, Bohorova N, Kim D, Pauly M et al. : Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotechnol J 2010, 8(5):638–654. [DOI] [PubMed] [Google Scholar]
- 10.Moore L, Hamorsky K, Matoba N: Production of Recombinant Cholera Toxin B Subunit in Nicotiana benthamiana Using GENEWARE(R) Tobacco Mosaic Virus Vector. Methods Mol Biol 2016, 1385:129–137. [DOI] [PubMed] [Google Scholar]


