Abstract
Purpose
Myeloid sarcoma (MS) of the orbit is an uncommon condition in occurring in children, generally coupled to myeloproliferative neoplasms.
Observations
We describe two rare cases of orbital MS in young boys with aggressive local symptoms but without evidence of acute myeloid leukemia (AML), both patients underwent orbitotomy for gross-tumor resection and biopsy. At follow up, there was no evidence of recurrence nor evolution of the myeloproliferative neoplasms clinically and by radiological and laboratory work-up. We also provide a detailed description of the magnetic resonance imaging presentation, with an extensive pathological analysis correlation.
Conclusions and importance
A comprehensive revision of the literature on isolated orbital MS was carried out with particular emphasis on clues for differential diagnosis and treatment options, stressing the need to consider MS even in the absence of sign and symptoms of an underlying myeloproliferative disorders.
Keywords: Myeloid sarcoma, Acute myeloid leukemia, Orbital pathology, Pediatric tumor, Magnetic resonance imaging
1. Introduction
Myeloid sarcoma (MS) is an extra-medullary solid tumor caused by an abnormal proliferation of primitive immature precursors of the granulocytic series of white blood cells.1 First described in 1811, MS is also called “chloroma” because of its green color secondary to the presence of intracellular myeloperoxidase.2,3 Subsequently, because of its macroscopic appearance variability, the tumor was renamed granulocytic sarcoma in 1966.4
MS is a rare disease, often related to other underlying unrecognized myeloproliferative conditions. Indeed, MS occurs in 2.5–9.1% of patients with acute myeloid leukemia (AML).5 Less frequently it occurs as a harbinger of AML in non-leukemic patients, or in association with myelodysplastic disorders or chronic myeloid leukemia (CML) with impending blast crisis.1,2 In pediatric population, orbit is one of the most common sites of occurrence.1
The correct diagnostic assessment of orbital MS is challenging due to its uncommon presentation and to the high number of possible mimickers, by both clinical and radiological examination. Nevertheless, a prompt diagnosis is important especially in patients with a non-leukemic presentation, because AML-type chemotherapy and or allogeneic hematopoitic cell transplantation improves overall free survival.6
Herein we report two rare cases of orbital MS in a 2 young male patients without evidence of AML, detailing their magnetic resonance imaging (MRI) features and highlighting possible pitfalls and useful clues in neuroradiological differential diagnosis. A comprehensive literature review of all included reports were available from PubMed, PMC and MEDLINE database of references and abstracts.
Only pediatric-onset MS were included in the literature revision. Orbit involvement was considered positive when solid tissue was documented at CT/MRI examination within the orbital pyramid, independently of possible site of origin; association with AML or other myeloproliferative diseases (MDs) was considered positive both when myeloproliferative neoplasm preceded, co-occurred with or followed the diagnosis of MS. Publications in other languages than English and previous literature reviews have not been considered in this analysis.
2. Case 1
A caucasian 14-year-old boy came to the Orbital Pathology Department with one-month history of left eye upper and lower eyelid swelling, refractory to corticosteroid therapy. His previous ocular, personal and family history was negative. At physical examination eyelid edema, conjunctive hyperemia and inferior displacement of the left eye were detected, with exotropic deviation of about 2mm at Hirschberg test. Upright clinical exophthalmometry revealed a severe protrusion (33mm) of the left eyeball. At infrared oculography voluntary eye movements were unilaterally restricted in all directions, excepted for adduction that was preserved. Visual acuity on Early Treatment Diabetic Retinopathy Study chart rows and fundus examination were normal.
Ultrasonography revealed a well-circumscribed mass characterized by heterogeneous mild echogenicity, with moderate intra-lesional vascularity at doppler US, located in the upper and lateral quadrant of the orbit. MRI examination confirmed the presence of a supero-lateral extra-conal orbital mass (maximum diameters 38 × 20 × 37mm, approximate volume 10 cm3) between the superior and lateral rectus muscles occupying in the lacrimal fossa (Fig. 1a and b). The lesion was isointense on T2w and slightly hyperintense on T2w images compared to muscle tissue, with restricted water diffusion on diffusion weighted imaging (DWI) indicating hypercellularity (Fig. 1c and d). Lacrimal gland was not clearly dissociable from the mass, and focal bony erosion and invasion of the lateral wall of the orbit were also noted (Fig. 2a, white arrow). After gadolinium injection the lesion presented with in homogeneous and vivid contrast enhancement with a small inner necrotic core (Fig. 2b–d, black arrow). Dynamic contrast-enhanced (DCE) MRI perfusion demonstrated fast contrast media wash-in and wash-out, highly suggestive for malignancy. The mass caused displacement without infiltration of adjacent muscles, as well as minimal dislocation of the optic nerve. A locally infiltrating solid tumor, possibly sarcomatous, was then hypothesized. The patient underwent left lateral orbitotomy for biopsy, and possibly lesion maximal resection. A whitish fish-flesh tumor with some internal hemorrhage compressing the adjacent structures was found, and a gross total tumor resection was carried out.
Fig. 1.
Axial (a) and coronal (b–d) T2w images showing expansive extra-conal orbital lesion arising along the external border of superior and lateral rectus muscles and superior oblique muscle. The lesion presents mild hyperintensity with inhomogeneous core, and slightly restricted water diffusion on DWI (c) indicating hypercellularity. Extra-ocular muscles and optic nerve are displaced (white dotted line); marked left eye proptosis is also present. The lesion does not spear lacrimal gland that seems to be infiltrated (not shown); bony erosion and invasion of the lateral wall of the orbit are clearly visible (white arrow).
Fig. 2.
Axial pre (a) and post-contrast (b) T1w showing lesion vivid enhancement and inner un-enhancing necrotic area (black arrow); the great wing of the sphenoid seems to be infiltrated (white arrow). Multi-planar reconstruction on coronal plane shows lesion extent from the lacrimal fossa of the frontal bone (c) (black arrowhead) to the orbital apex (d) (white arrowhead).
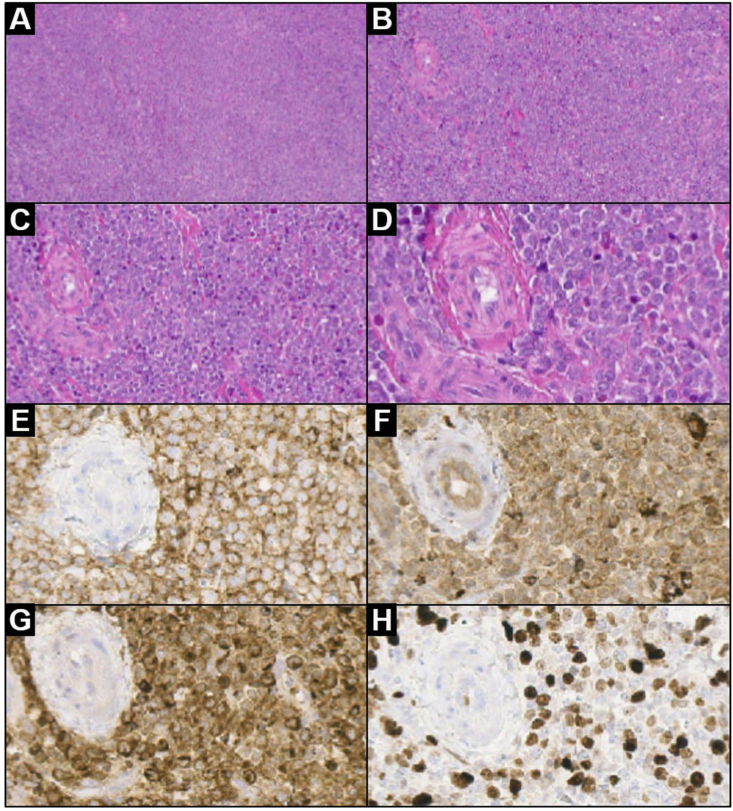
Pathological examination revealed a cohesive proliferation of small to medium-sized cells with large oval and often indented nuclei, prominent nucleoli and scant cytoplasm (myeloblast-like), mixed with a discrete number of eosinophils (Fig. 3a–d). Neoplastic cells expressed leucocyte common antigen (LCA/CD45RO), CD68 and myeloperoxidase (MPO); there was also a focal reactivity for CD34. Ki-67 staining was 50% (Fig. 3e–h). Final diagnosis was consistent with MS. Due to the common association with AML, blood sampling was collected to document the presence of altered white blood cell count; final results did not showed peripheral blood abnormalities. Bone marrow aspirate was negative for tumor infiltration.
Fig. 3.
Low and high magnification lesion histology after surgery: (a) low magnification showing a dense, diffuse infiltrate (H&E 4×); (b) the tumor consists of a cohesive proliferation of small/medium-sized cells (H&E 10×); (c) a discrete number of eosinophils are admixed with tumor cells (H&E 20×); (d) neoplastic cells show large oval nuclei, prominent nucleoli and poor cytoplasm (H&E 40×). Lesion immunophenotype: (e–g) neoplastic cells diffusely expressed CD45RO/LCA (anti-CD45RO/LCA, 40×), CD68 (anti-CD68, 40×) and myeloperoxidase (anti-MPO, 40×); (h) Ki-67 proliferative index is positive in about 50% of neoplastic cells (anti-Ki-67, 40×).
Legend: H&E = hematoxylin and eosin stain.
After surgery followed by 1 month of induction chemotherapy according to European Leukemia Network recommendations for AML treatment, proptosis resolved and MRI demonstrated no evidence of residual and/or recurrent local disease. The patient is under clinical, laboratory and radiological follow-up with no sign of recurrence after one year from surgery.
3. Case 2
4 year old boy presented to Emergency room at King Khaled Eye Specialist Hospital with a growing mass in right upper lid over the last one month. He is medically free and his previous ocular, personal and family history was negative. On examination visual acuity in the right eye was 20/50 and left eye was 20/30. Extraocular muscle movement was full in both eyes. Pupil round, regular and reactive with no relative afferent pupillary defect on both sides. Examination grossly of the right eye showed well circumscribed upper and medial mass about 3 × 2 cm in size with smooth surface. It was hard in consistency but non-tender or mobile. The globe was displaced inferiorly with exotropia. The overlying skin was normal with no discoloration. Slit lamp examination showed normal anterior segment and fundus examination was unremarkable in both eyes.
MRI examination confirmed the presence of an extraocular solid mass involving the superior aspect of the right orbit with intermediate signal intensity on T1-weighted and T2-weighted image which was seen infiltrating and extending through the pre-septal into the post-septal part. It was a well-defined mass and measured approximately 3.5 × 2.4 cm in diameter with subsequent minimal displacement of the related part of the right globe. The mass lesion had marked restricted pattern on diffusion weighted image(ADC value equal to 0.475 × 10(−3) mm(2)/sec) (Fig. 4a–d). The mass was seen separated from the medial rectus muscle and abutting the superior rectus muscle and displacing the superior oblique with evidence of mild degree of prominent vascularities. There was no evidence of bony infiltration and no evidence of intraspinous nor intracranial extension. Rhabdomyosarcoma was clinically suspected. The patient underwent incisional biopsy which showed a yellow-grey mass.
Fig. 4.
Axial CT scan orbit (a) and sagittal T1 fat suppressed post contrast (b,c) and axial DWI (D) images showing solid lobulated extra-conal orbital lesion arising superiorly along the orbital roof and nasal infiltrating the recti muscles and superior oblique muscle. The lesion presents marked restricted water diffusion on DWI (c) indicating high degree of cellularity. The lesion abutting the lacrimal gland with no definite line of separation.
Pathological examination revealed a highly vascular soft tissue of poorly differentiated malignant cells infiltrating the adipose tissue with few eosinophils suggestive of malignant orbital tumor in the right eye, The tumor cells expressed the following immunohistochemical markers: CD45, 34, 43, 117, Lysozyme, CD68 (KP1) and Myeloperoxidase indicating myeloid lineage but did not show expression of CD20 and CD3 (Fig. 5a–d).
Fig. 5.
Low and high magnification lesion histology after surgery: (a) high magnification showing a highly vascular soft tissue of poorly differentiated malignant cells infiltrating the adipose tissue with eosinophils (H&E 400×); (b) low magnification showing a dense, diffuse infiltrate (H&E 100×); (c) CD34 expression in neoplastic cells (CD34, ×100) (d) neoplastic cells diffusely expressed CD45RO/LCA (anti-CD45RO/LCA, 100×).
Legend: H&E = hematoxylin and eosin stain.
Final diagnosis was consistent with MS and because of common association with AML, blood sampling was collected to document the presence of altered white blood cell count; final results was unremarkable except for below normal neutrophils. The patient referred to King Faisal Specialist Hospital and Research center and started systemic chemotherapy with a complete remission at 6 months follow up. At present, the patient is under clinical, laboratory and radiological follow-up with no sign of recurrence at four years from surgery.
4. Discussion
MS is a rare condition occurring in 2.5–9.1% patients with AML; it is characterized by the presence of one or more tumor masses in extra-medullary sites such as bone, subcutaneous tissues, orbit, lymph nodes, gastro-intestinal tract and central nervous system7 In pediatric population skin and orbit constituted the most common sites of invasion.8 Scattered isolated cases of pediatric orbital MS have been described, frequently associated to a high misdiagnosis rate. Isolated orbital MS frequently exhibits clinical features mimicking inflammatory process or lymphoproliferative disease.9, 10, 11, 12, 13, 14 The most frequent manifestation is the unilateral exophthalmos; other possible signs include ptosis, painful lacrimal gland swelling, conjunctival mass, retinal hemorrhages, diffuse iris or uveal alterations.8 We are presenting two cases with isolated orbital MS that were associated with dystopia.
In patients whose anamnesis is positive for hematologic malignancies, the diagnosis of MS is relatively easy to evoke, while diagnosis of primary MS with no AML can be challenging15 and imaging assessment become then crucial. In these cases, differential diagnosis with orbital infections and inflammations can be performed with the use of diffusion weighted and contrast-enhanced imaging techniques.16, 17, 18, 19, 20 Other more challenging mimickers to be considered include vascular lesion, lymphoma (especially African Burkitt), metastatic neuroblastoma and rhabdomyosarcoma. Dynamic contrast-enhanced Computed Tomography (CT) or MRI can be very helpful in demonstrating the vascular nature of these lesions because of their progressive enhancement, starting from a small and generally central portion and then filling up the entire mass; eventually this enhancement pattern is typically associated to benign findings.21 Orbital lymphoid tumors are rare in children, with the only exception of Burkitt lymphoma; in this setting, lymphoid tissue commonly shows lower apparent diffusion coefficient values compared to other neoplastic lesions.21 Neuroblastoma metastases are not rare in pediatric population, but they are generally associated with important focal bony destruction and invasion of adjacent structures.16 Rhabdomyosarcoma imaging features might be not clearly distinguishable from MS. Few clues may help the radiologist in correct assessment, such as a marked involvement of muscles that is not frequently observed in MS.22
It has been reported that orbital MS on CT appears as a well-defined mass, isodense or hyperdense to brain tissue, with homogeneous enhancement after contrast media injection. However, in some cases it can exhibit heterogeneous enhancing with non-enhancing areas corresponding to the inner necrotic areas, which is considered by some as a sign of rapid growth.23 On MRI images, the orbital lesion appears isointense or slightly hypointense to the brain both on T1w and T2w images. With gadolinium contrast enhancement, it can present with a more or less homogeneous enhancement depending on the presence of necrotic areas within the mass. Associated bone marrow involvement and low signal intensity on T2w imaging may be helpful in differentiating these tumors from other lesions.20, 24 MRI has been proved to provide useful and more comprehensive information for lesion characterization compared to CT scan, avoiding any exposure to ionizing radiation.19, 20, 25 In the first case the MRI showed mild hyperintensity on T2w images, with inhomogeneous core and slightly restricted water diffusion due to hypercellularity; vivid enhancement and inner un-enhancing necrotic area after contrast administration along with adjacent bone infiltration helped in differential diagnosis of malignancy (Fig. 1, Fig. 2). While in the second case the solid mass lesion showed intermediate signal intensity on T1-weighted and T2-weighted image which was seen infiltrating and extending through the pre-septal into the post-septal part. Diffusion weighted image (DWI) demonstrating high signal at the site of the mass indicating restricted diffusion, likely reflecting increased cellularity (Fig. 4). Differential considerations include lymphoma, rhabdomyosarcoma or other malignant mass with high dense cellularity.26
However, despite the guidance given by an accurate neuroradiological examination, biopsy remains the only method for a final diagnostic assessment particularly in patients who develop granulocytic sarcoma in absence of a diagnosis of leukemia. In this study, immunohistochemical staining for myeloid markers, such as CD45RO/LCA (anti-CD45RO/LCA) CD68 (anti-CD68, and myeloperoxidase (anti-MPO) allowed the diagnosis. Cell surface markers including CD4, CD30, CD34, TdT, and glycophorin A are also useful for diagnosis of MS.27 Among the various markers, MPO, lysozyme, and CD68 are the most sensitive and essential markers for myeloid differentiation in addition to molecular genetic.22,28
Alkatan and Chaudhry,29 documented that FISH using the DNA probe for t(8; 21) was positive in only 2% of the cells, which was significant for the diagnosis and expected prognosis since the finding of such translocation is expected to be associated with higher chance for the development of systemic leukemia.
When comparing our observation to current literature reports, out of the 243 cases reported from 1978 to present time (Table 1), around 10% patients (n = 25) presented with an isolated lesion with no evidence of AML. Indeed, the majority (n = 218) were preceded, accompanied or followed by the evidence of generalized hematopoietic malignancies. Interestingly, two isolated cases had history of orbital trauma preceding the onset,21 proposed to be a possible trigger event for the onset of this type of malignancy; however, further evidences need to be collected to verify this hypothesis. A more detailed description of literature review in term of location of orbital mass, key finding, laterality, risk factor, association with acute myelocytic leukemia and myelodysplastic disease, type of treatment and prognosis on pediatric myeloid sarcoma is provided in (Table 2).
Table 1.
Summary of main findings described in current scientific literature on pediatric myeloid sarcoma (MS) with orbital involvement.
| n | % | ||
|---|---|---|---|
| Total Orbital MS Reports | 243 | 100 | |
| No MDs | 25 | 10.3 | |
| MDs | 218 | 89.7 | |
| AML | 215 | 88.4 | |
| CML | 1 | 0.41 | |
| Other | 2 | 0.82 | |
| Risk factors | Absent | 216 | 88.8 |
| Trauma | 2 | 0.82 | |
| Surgery | 0 | 0 | |
| Other | 0 | 0 | |
Legend: MS = Myeloid Sarcoma; MD = Myeloproliferative Disease; AML = Acute Myeloid Leukemia; CML = Chronic Myeloid Leukemia.
Table 2.
Articles included in the literature review on Pediatric myeloid sarcoma (MS) with orbital involvement until 2019.
| Author(s) | Year of Publication | Type of Study | N | Location | Key Findings | Unilateral/Bilateral | Risk Factor | AML/Other MDs | Type of Treatment | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|
| Lim et al. | 2018 (Turkey) | Case report | 1 | Extra-conal | Multifocal involvement | Unilateral | NTR | AML | CT | Median remission 30 m |
| Cheng et al. | 2018 (China) | Case report | 1 | Intra-conal | NTR | Unilateral | Trauma | Absent | CT + RT | Disease-free at 24 m follow up |
| Wang et al. | 2018 (USA) | Case report | 1 | Sphenoid wing | Multifocal skeletal involvement | Unilateral | NTR | Absent | CT | NA |
| Gupta et al. | 2017 (India) | Case report | 1 | Retro-conal | NTR | Unilateral | NTR | AML | CT | Relapse at 12 m follow up |
| Siraj et al. | 2017 (India) | Case series | 1 | Extra-conal | NTR | Unilateral | NTR | AML | CT + RT | Disease-free at 18 m follow up |
| Qian et al. | 2016 (USA) | Case report | 1 | Extra-conal | NTR | Unilateral | NTR | Absent | CT | NA |
| Mohanlal et al. | 2016 (South Africa) | Case report | 1 | Extra-conal, peri-orbital | Glycophorin A positive | Unilateral | NTR | PEL | CT | NA |
| Huanh et al. | 2015 (Taiwan) | Case report | 1 | Extra-ocular muscles | NTR | Bilateral | NTR | AML | CT | Disease-free at 12 m follow up |
| Karmegaraj et al. | 2014 (India) | Case report | 1 | Peri-orbital | Flu-like onset | Bilateral | NTR | AML | CT | Disease-free at follow up |
| Aggarwal et al. | 2014 (India) | Original article | 23 | Either intra- or extra-conal | NA | Unilateral | NA | 23 AML | CT | Median remission 36 m |
| Thakur et al. | 2013 (India) | Case report | 2 | NA; NA | NTR | Unilateral; Unilateral | NTR | 2 AML | NA; NA | NA; NA |
| Dinand et al. | 2013 (India) | Case report | 1 | Extra-conal, intra-conal | NTR | Unilateral | NTR | Absent | CT | Disease-free at 6 m follow up |
| Chaudhry et al. | 2012 (Saudi Arabia) | Case report | 1 | Extra-conal | NTR | Unilateral | NTR | AML | CT | Disease-free at 10 m follow up |
| Johnston et al. | 2012 (USA) | Original article | 23 | NA | 15/23 CNS involvement | NA | NA | 19 AML; 4 Absent | CT + RT | Remission at 12 m follow up |
| Isik et al. | 2011 (Turkey) | Case report | 2 | Retroconal; Intra-conal, | NTR | Unilateral | NTR | 2 Absent | CT; CT | Both disease-free at follow up |
| Baldwin et al. | 2010 (USA) | Case report | 1 | Extra-conal | NTR | Unilateral | NTR | AML | CT + RT | Disease-free at 20 m follow up |
| Alkatan et al. | 2008 (Saudi Arabia) | Case report | 1 | Extra-conal | NTR | Unilateral | NTR | Absent | NA | NA |
| Hmidi et al. | 2007 (Tunisia) | Case report | 1 | Extra-conal | NTR | Unilateral | NTR | AML | CT | Disease-free at 24 m follow up |
| Janic et al. | 2007 (Serbia) | Case report | 1 | NA | Kidney involvement | Bilateral | NTR | Absent | CT | NA |
| Choo et al. | 2006 (USA) | Case report | 1 | Extra-conal | NTR | Unilateral | NTR | AML | NA | NA |
| Bhat et al. | 2005 (India) | Case report | 1 | Extra-conal | NTR | Unilateral | Trauma | Absent | S + CT + RT | Relapse 3 m after treatment |
| Porto et al. | 2004 (Germany) | Case series | 3 | Extra-conal; Extra- and intra-conal; Extra-conal | Previous neuroblastoma; NTR; NTR | Unilateral; Unilateral; Unilateral | NTR; NTR; NTR | 3 AML | CT; CT; CT | Deceased few months from diagnosis; Remission; NA |
| Söker et al. | 2003 (turkey) | Case report | 1 | Extra-conal | NTR | Bilateral | NTR | AML | NA | NA |
| Shields et al. | 2003 (USA) | Case report | 1 | Extra-conal | NTR | Bilateral | NTR | AML | CT | Disease-free at 3 m follow up |
| Steinwexler et al. | 2002 (USA) | Case report | 1 | Extra-conal | NTR | Unilateral | NTR | AML | CT | Disease-free at 1 m follow up |
| Bönig et al. | 2002 (Germany) | Case report | 1 | Extra- and retro-conal | NTR | Unilateral | NTR | AML | CT + RT | Disease-free at 20 m follow up |
| Fisgin et al. | 2002 (Turkey) | Case report | 1 | Extra- and intra-conal | PVB19 infection | Bilateral | NTR | AML | CT | Remission at follow up |
| Hung et al. | 2002 (Taiwan) | Case report | 1 | Extra- and intra-conal | NTR | Bilateral | NTR | AML | CT | Disease-free at 19 m follow up |
| Bisschop et al. | 2001 (NL) | Original article | 35 | NA | NA | NA | NA | 35 AML | CT | NA |
| Uyesugi et al. | 2000 (USA) | Case report | 1 | Extra- and intra-conal | NTR | Bilateral | NTR | AML | CT + RT | Deceased 1 m from diagnosis |
| Lakhkar et al. | 2000 (India) | Case report | 1 | Extra-conal | NTR | Bilateral | NTR | NA | CT | Remission at follow up |
| Felice et al. | 1999 (Argentina) | Original article | 5 | Either intra- or extra-conal | NA | NA | NA | 5 AML | 1 S + CT; 1 RT + CT; 3 CT |
7 Remission at follow up; 1 Deceased within 1y from diagnosis |
| Puri et al. | 1999 (UK) | Case report | 1 | Extra-ocular muscles | NTR | Unilateral | NTR | AML | CT | NA |
| Schwyzer et al. | 1999 (South Africa) | Original article | 9 | All extra-conal and peri-orbital | NA | Either unilateral or bilateral | NA | 9 AML | CT | 8 Remission at follow up; 1 No Remission |
| Luckit et al. | 1998 (UK) | Case report | 1 | Retro-conal | NTR | Unilateral | NTR | AML | CT | Disease-free at 42 m follow up |
| Tanigawa et al. | 1998 (Japan) | Case report | 1 | Extra-conal | NTR | Unilateral | NTR | AML | CT | Remission at follow up |
| Stockl et al. | 1997 (Canada) | Original article | 7 | NA | NTR | Unilateral | NTR | 7 AML | CT | Remission at follow up |
| Girardot et al. | 1996 (Morocco) | Case report | 1 | Extra-conal | NTR | Unilateral | NTR | AML | CT + RT | Disease-free at 36 m follow up |
| Hiçsönmez et al. | 1996 (Turkey) | Case report | 1 | NA | NTR | Unilateral | NTR | AML | CT | Disease-free at 36 m follow up |
| Bulas et al. | 1995 (USA) | Case report | 1 | Extra-conal | NTR | Bilateral | NTR | AML | CT | No remission al follow up |
| Pui et al. | 1994 (USA) | Original article | 31 | NA | NA | NA | NA | 30 AML; 1 CML |
18 CT; 9 CT + RT; 3 S + CT; 1 S + RT + CT |
16 Deceased 2.5–143.9 m from diagnosis; 15 Disease-free |
| Cavdar et al. | 1993 (Turkey) | Case report | 1 | Intra-conal | NTR | Bilateral | NTR | AML | CT | Deceased 7 m from diagnosis |
| Cavdar et al. | 1993 (Turkey) | Original article | 10 | Either intra- or extra-conal | NA | Either unilateral or bilateral | NA | 10 AML | CT | NA |
| Kalmanti et al. | 1991 (Greece) | Case report | 2 | Retroconal; Extra-conal | NTR | Unilateral; Unilateral | NTR | 2 AML | CT; CT | Disease-free at 8y follow up; Deceased 2y from diagnosis |
| Banna et al. | 1991 (Saudi Arabia) | Case series | 4 | Either intra- or extra-conal | NTR | 2 Unilateral; 2 Bilateral | NTR | 4 AML | CT + RT | NA |
| Cavdar et al. | 1989 (Turkey) | Original article | 33 | Either intra- or extra-conal | NA | 17 Bilateral; 16 Unilateral | NA | 21 AML; 12 Absent | CT | All deceased within 20 m from diagnosis |
| Davis et al. | 1985 (USA) | Case report | 1 | Extra-ocular muscles | CNS involvement | Unilateral | NTR | AML | CT | Remission at follow up |
| Rajantie et al. | 1984 (Finland) | Case report | 1 | Extra-conal | NTR | Unilateral | NTR | AML | S + CT | Deceased 11 m from diagnosis |
| Cavdar et al. | 1978 (Turkey) | Original article | 20 | NA | NA | 11 Unilateral; 9 Bilateral | NA | 20 AML | CT | Median remission 9 m |
Legend: N = Number of patients; AML = Acute Myelocytic Leukemia; MDs = Myelodysplastic Disease; NTR = Nothing To Report; CT = Chemotherapy; RT = Radiotherapy; S = Surgery NA = Not Available; PEL = Pure Erythroid Leukemia; CNS = Central Nervous System.
Since orbital MS has been generally thought to be an antecedent disease entity able to evolve into AML, treatment strategies have been mainly focused on inducing a remission to prevent evolution to AML: isolated MS left untreated, commonly evolves into AML within 1 year.23 Regarding therapeutic options, due to tumor rarity, no universal consensus on the best treatment planning has yet been reached and no unified protocol has been identified. Out of 6 patients with isolated orbital MS who initially received high-dose methylprednisolone treatment, followed by the Acute Myeloid Leukemia-Berlin Frankfurt Munster 2004 treatment protocol, 2 (33.3%) later developed AML; on the other hand, out of 12 patients with isolated MS who received only external beam radiotherapy, 11 (91.7%) developed AML during the follow-up period.30
Lee et al. reported that 22.2% of patients with isolated MS who had undergone only local treatment (such as surgery and/or local radiotherapy) did not progress to AML, particularly with a complete remission durations of 1.8 months whose treatment was surgery alone and 83.9 months for those who received radiotherapy; at the same time these Authors also reported that, in contrast, 44.4% of patients who received systemic chemotherapy treatment had evolved to AML within a median time of 13.4 months.5 Tsimberidou et al. reported a review of 20 cases of non-leukemic MS in which the combined treatment with chemotherapy and radiotherapy resulted in better survival than chemotherapy alone.31 Therefore local treatment, such as surgery or radiotherapy, might play an important role in controlling primary disease and relieving symptoms, without significant toxicity and additional risk of evolution into AML.32,33 Nevertheless, although the number of isolated orbital lesions is very limited and the treatment results reported in literature are still confusing, it seems that combining systemic and local treatment for patients with isolated orbital MS might be a more promising therapeutic strategy to achieve complete remission compared with any other treatment alone. In this light, after gross surgical resection the first patient received systemic chemotherapy with complete remission at one-year follow-up and the second patient received a systemic chemotherapy with complete remission at 6 months follow-up and disease free at four years follow up.
5. Conclusion
MS generally occurs in patients with AML, but it can also occasionally precede myeloproliferative disorders within a matter of months in patients with no evidence of hematological disease at the time of bone marrow aspiration and biopsy at the initial diagnosis (isolated, primary or non-leukemic MS). Particularly in these patients, a prompt diagnosis of MS is essential to the most effective clinical management, because conventional AML-type chemotherapy and or allogeneic hematopoitic cell transplantation improves overall free survival. In this light it is important to recognize the few radiological features that can guide the radiologist in differentiating orbital MS from other pediatric orbital masses. Eventually the use of multi-planar and multi-parametric MRI, with particular reference to diffusion weighted and contrast-enhanced sequences, may be crucial to the purpose.
Patient consent
The patient(s)/patient's legal guardian consented to publication of the case in writing/orally.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Declaration of competing interest
The following authors have no financial disclosures (MP, MA, CR, HA, AM, SE, RM, MM, AE, LR, RC, DS).
Acknowledgements
The author thanks King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia, for financial support.
References
- 1.Guermazi A., Feger C., Rousselot P. Granulocytic sarcoma (chloroma): imaging findings in adults and children. AJR. 2002;178(February):319–325. doi: 10.2214/ajr.178.2.1780319. [DOI] [PubMed] [Google Scholar]
- 2.Lee B., Fatterpekar G.M., Kim W., Som P.M. Granulocytic sarcoma of the temporal bone. AJNR. 2002;23(October):1497–1499. [PMC free article] [PubMed] [Google Scholar]
- 3.Maka E., Lukáts O., Tóth J., Fekete S. Orbital tumour as initial manifestation of acute myeloid Leukemia : granulocytic sarcoma: case report. Pathol Oncol Res. 2008;14:209–211. doi: 10.1007/s12253-008-9028-x. [DOI] [PubMed] [Google Scholar]
- 4.Thakur B., Varma K., Misra V., Chauhan S. Granulocytic sarcoma presenting as an orbital mass: report of two cases. J Clin Diagn Res. 2013;7(8):1704–1705. doi: 10.7860/JCDR/2013/5582.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J.Y., Chung H., Cho H. Clinical characteristics and treatment outcomes of isolated myeloid sarcoma without bone marrow involvement: a single-institution experience. Blood Res. 2017;52(3):184–192. doi: 10.5045/br.2017.52.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitan M., He W., Zhang M. Transplantation for children with acute myeloid leukemia: a comparison of outcomes with reduced intensity and myeloablative regimens. Blood. 2014;123(10):1615–1620. doi: 10.1182/blood-2013-10-535716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pileri S.A., Ascani S., Cox M.C. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21:340–350. doi: 10.1038/sj.leu.2404491. [DOI] [PubMed] [Google Scholar]
- 8.Samborska M., Derwich K., Skalska-Sadowska J., Kurzawa P., Wachowiak J. Myeloid sarcoma in children – diagnostic and therapeutic difficulties. Contemp Oncol. 2016;20(6):444–448. doi: 10.5114/wo.2016.65602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavazza S., Bocciolini C., Laudadio P., Tassinari G., Dall'Olio D. Two anomalous localizations of mucocele: clinical presentation and retrospective review. Acta Otorhinolaryngol Ital. 2007;27:208–211. [PMC free article] [PubMed] [Google Scholar]
- 10.Kiratli H., Sekeroglu M.A., Tezel G.G. Orbital heterotopic glial tissue presenting as exotropia. Orbit. 2008;27:165–168. doi: 10.1080/01676830701523889. [DOI] [PubMed] [Google Scholar]
- 11.Strianese D., Elefante A., Matarazzo F., Panico A., Ferrara M., Tranfa F. Orbital lymphoma mimicking lacrimal gland pleomorphic adenoma. Case Rep Ophtalmol. 2013;4:109–113. doi: 10.1159/000354963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chirapapaisan N., Chuenkongkaew W., Pornpanich K., Vangveeravong S. Orbital pseudotumor: clinical features and outcomes. Asian Pac J Allergy Immunol. 2007;25(4):215–218. [PubMed] [Google Scholar]
- 13.Strianese D., Piscopo R., Elefante A. Unilateral proptosis in thyroid eye disease with subsequent contralateral involvement: retrospective follow-up study. BMC Ophthalmol. 2013;13(21) doi: 10.1186/1471-2415-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ugga L., Stilo S., Napolitano P. Orbitofrontal cholesterol granuloma: case report and review of the literature. Quant Imaging Med Surg. 2017;7(3):373–377. doi: 10.21037/qims.2017.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yilmaz A.F., Saydam G., Sahin F., Baran Y. Granulocytic sarcoma: a systematic review. Am J Blood Res. 2013;3(4):265–270. [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed S., Goel S., Khandwala M., Agrawal A., Chang B., Simmons I. Neuroblastoma with orbital metastasis: ophthalmic presentation and role of ophthalmologists. Eye. 2006;20:466–470. doi: 10.1038/sj.eye.6701912. [DOI] [PubMed] [Google Scholar]
- 17.Jakobiec F.A. Granulocytic sarcoma. AJNR. 1991;12(March/April):263–264. [PMC free article] [PubMed] [Google Scholar]
- 18.Noh B.W., Park S., Chun J., Kim J.H., Kim H.J., Lim M.K. Granulocytic sarcoma in the head and neck: CT and MR imaging findings. Clin Exp Otorhinolaryngol. 2009;2(2):66–71. doi: 10.3342/ceo.2009.2.2.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tortora F., Prudente M., Cirillo M. Diagnostic accuracy of short-time inversion recovery sequence in Graves' ophthalmopathy before and after prednisone treatment. Neuroradiology. 2014;56(5):353–361. doi: 10.1007/s00234-014-1332-4. [DOI] [PubMed] [Google Scholar]
- 20.Purohit B.S., Vargas M.I., Ailianou A. Orbital tumours and tumour-like lesions: exploring the armamentarium of multiparametric imaging. Insights Imaging. 2015 doi: 10.1007/s13244-015-0443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung E.M., Smirniotopoulos J.G., Specht C.S., Schroeder J.W., Cube R. From the archives of the AFIP pediatric orbit tumors and tumorlike lesions: nonosseous lesions of the extraocular orbit. Radiographics. 2007;27(6):1777–1800. doi: 10.1148/rg.276075138. [DOI] [PubMed] [Google Scholar]
- 22.Alexiev B.A., Wang W., Ning Y. Myeloid sarcomas: a histologic, immunohistochemical, and cytogenetic study. Diagn Pathol. 2007;42(2):1–8. doi: 10.1186/1746-1596-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamauchi K., Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma. Report of two cases and a review of 72 cases in the literature. Cancer. 2002;94(6):1739–1746. doi: 10.1002/cncr.10399. [DOI] [PubMed] [Google Scholar]
- 24.Hakyemez B., Yildirim N., Taskapilioglu O. Intracranial myeloid sarcoma: conventional and advanced MRI findings. Br J Radiol. 2007;80(June):109–112. doi: 10.1259/bjr/16630393. [DOI] [PubMed] [Google Scholar]
- 25.Comerci M., Elefante A., Strianese D. Semiautomatic regional segmentation to measure orbital fat volumes in thyroid-associated ophthalmopathy. A validation study. Neuroradiol J. 2013;26(4):373–379. doi: 10.1177/197140091302600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razek A.A., Elkhamary S., Mousa A. Differentiation between benign and malignant orbital tumors at 3-T diffusion MR-imaging. Neuroradiology. 2011;53:517–522. doi: 10.1007/s00234-011-0838-2. [DOI] [PubMed] [Google Scholar]
- 27.Campidelli C., Agostinelli C., Stitson R., Pileri S.A. Myeloid sarcoma. Extramedullary manifestation of myeloid disorders. Am J Clin Pathol. 2009;132:426–437. doi: 10.1309/AJCP1ZA7HYZKAZHS. [DOI] [PubMed] [Google Scholar]
- 28.Janečková V., Semerád L., Ježíšková I. Molecular genetic testing for acute myeloid leukemia. Klin Onkol. 2016;29(6):411–418. doi: 10.14735/amko2016411. [DOI] [PubMed] [Google Scholar]
- 29.Alkatan H., Chaudhry I. Myeloid sarcoma of the orbit. Ann S Med. 2008;28(6):461–465. doi: 10.4103/0256-4947.51679. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal E., Mulay K., Honavar S.G. Orbital extra-medullary granulocytic sarcoma: clinicopathologic correlation with immunohistochemical features. Surv Ophthalmol. 2014;59(2):232–235. doi: 10.1016/j.survophthal.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Tsimberidou A., Kantarjian H.M., Estey E. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia. 2003;17:1100–1103. doi: 10.1038/sj.leu.2402958. [DOI] [PubMed] [Google Scholar]
- 32.Antic D., Elezovic I., Milic N. Is there a “‘gold’” standard treatment for patients with isolated myeloid sarcoma? Biomed Pharmacother. 2013;67(1):72–77. doi: 10.1016/j.biopha.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Bakst R., Wolden S., Yahalom J. Radiation therapy for chloroma (granulocytic sarcoma) Int J Radiat Oncol Biol Phys. 2012;82(5):1816–1822. doi: 10.1016/j.ijrobp.2011.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]