Abstract
The risk for metabolic disease, including metabolic syndrome, insulin resistance, and diabetes, increases with age. Altered plasma TG metabolism and changes in fatty acid partitioning are also major contributors to metabolic disease. Plasma TG metabolism itself is altered by age in humans and rodents. As discussed in this review, the age-induced changes in human TG metabolism include increased plasma TG levels, reduced postprandial plasma TG clearance rates, reduced postheparin LPL activity, decreased adipose tissue lipolysis, and elevated ectopic fat deposition, all of which could potentially contribute to age-associated metabolic diseases. Similar observations have been made in aged rats. We highlight the limitations of currently available data and propose that mechanistic studies are needed to understand the extent to which age-induced alterations in TG metabolism contribute to metabolic disease. Such mechanistic insights could aid in therapeutic strategies for preventing or managing metabolic disease in older individuals.
Keywords: lipolysis, lipoprotein metabolism, lipoprotein lipase, adipose tissue, chylomicrons, inflammation, metabolic syndrome, obesity
Age is a significant risk factor for metabolic syndrome and T2D. The percentage of individuals with metabolic syndrome significantly increases with age (1, 2). In the United States, over one-quarter of adults over 65 have diabetes, and simply being older than 45 years old is considered a risk factor for T2D (niddk.nih.gov; cdc.gov/diabetes/data/). Although age is clearly a risk factor for metabolic disease, the mechanisms that contribute to this age-related risk have not been fully elucidated. One major contributing factor to metabolic disease is aberrant TG metabolism and fat partitioning (3). Excessive delivery of fatty acids to tissues such as heart, skeletal muscle, and liver can lead to insulin resistance, cardiac lipotoxicity, and fatty liver disease (4–6). This ectopic lipid deposition often results from adipose dysfunction, wherein white adipose tissue no longer efficiently stores fat (7). Ectopic lipid deposition is associated with metabolic syndrome and T2D, and humans with metabolic syndrome or T2D have increased TG content in heart and liver (3).
Surprisingly, although age and aberrant TG metabolism are both associated with increased risk of metabolic disease, the two are rarely studied in combination. In this review, we examine what is currently known about how age effects plasma TG clearance, adipose tissue lipolysis, and the partitioning of fat. We review how the activity of LPL, the enzyme primarily responsible for plasma TG clearance, is altered by age. Aging in rodent models and how well these models reflect the human condition are also discussed. Finally, we consider the deficiencies in our current understanding of age-associated changes in TG metabolism and suggest how some of these deficiencies might be addressed.
AGE AND PLASMA TG LEVELS AND TG CLEARANCE
Plasma TGs levels are higher in older adults versus younger adults (8–10). At least some of this increase in plasma TG levels can be attributed to delayed postprandial TG clearance. In 1949, Becker, Meyer, and Necheles (11) gave a high-fat meal to 30 younger (average age 18) and 30 older (average age 76) fasted subjects and then measured serum chylomicrons over time. They found that in younger subjects, serum chylomicrons peaked at around 3 h and were cleared to fasting levels within 6 h. However, in older subjects, serum chylomicrons peaked later (∼9 h) and at a much higher level. Moreover, in older subjects, clearance of chylomicrons to fasted levels took nearly 24 h (11). In 1953, Herzstein, Wang, and Adlersberg (8) similarly found that clearance of total serum lipids was delayed in older (ages 51–71) compared with younger (ages 17–34) subjects after a fat-loaded meal. A more recent study by Vinagre et al. (12) suggested that the delayed TG clearance in older subjects is due, at least in part, to delayed clearance of remnant particles in the liver. In this study the authors used TG emulsions labeled with both 3H-TGs and 14C-cholesterol esters to distinguish fractional clearance through lipolysis (TGs only) from fractional clearance through remnant uptake (clearance of both TGs and cholesterol esters). They found that clearance through lipolysis was similar between healthy young (<30 years) and old (>60 years) subjects, but that remnant clearance was delayed in older subjects (12). However, it is important to note that these experiments were carried out in fasting (12 h) individuals and that clearance of TG emulsions may not reflect the behavior of endogenous lipoproteins. Interestingly, although the authors did not find a difference in lipolysis-driven clearance in older subjects, they did find that older individuals had lower postheparin LPL activity (12). The possible role of LPL in aging-induced changes in plasma TG metabolism is discussed below.
Rodent models are often used in aging studies due to their dramatically shorter lifespans. How well do rodent aging-models reflect the changes in TG metabolism observed in humans? Several studies have found that older male and female rats have elevated plasma TG levels compared with younger rats (13–16), though the degree of elevation appears to be strain dependent (14). Early studies also suggested that older rats, both male and female, have slower TG clearance rates than younger rats (13). Interestingly, older mice appear to have slightly lower plasma TGs than younger mice (17, 18). We are unaware of any study examining postprandial TG clearance in aged mice.
AGE AND LPL ACTIVITY
LPL is critical for the proper clearance of plasma TGs. LPL bound to the vascular lumen hydrolyzes the TGs of TG-rich lipoproteins, releasing fatty acids for uptake by tissues. The delayed clearance of plasma TGs in older humans suggests that LPL activity may be altered with age. Although a survey of tissue-specific LPL activity in younger and older humans is lacking, several studies have examined the levels of LPL activity released into the bloodstream by an injection of heparin (12, 19, 20). In each of these studies older subjects had significantly less postheparin LPL activity than younger subjects, suggesting that LPL activity does indeed decrease with age (12, 19, 20). There are caveats to these observations. First, two of the studies did not report the gender of subjects and so it is not clear whether both males and females were included (19, 20). The final study did include males and females but gender-separated data were not reported (12). Second, although heparin releases LPL into the circulation, there is scant evidence that heparin releases all vascular LPL or that LPL is released from all vascular beds with similar efficiency. There is also some evidence that heparin can release nonvascular LPL, LPL that would not normally participate in TG clearance (21). Therefore, although these studies suggest that LPL activity is altered in older humans, which tissues and pathways contribute to the change remain unclear.
More detailed studies of age-induced changes in tissue-specific LPL activity have been performed in rats. Unfortunately, observations in rats have not been consistent, in part due to the use of different rat strains and different feeding conditions (Table 1). Chen and Reaven (15) found no difference in fasted LPL activity between 40-day-old, 100-day-old, and 1-year-old Sprague-Dawley rats, but found that when the rats were fed, LPL activity was significantly lower in the 100-day-old rats compared with the 40-day-old rats. Interestingly, there was no further decrease in LPL activity in 1-year-old rats. A contemporaneous study looked at both Sprague-Dawley and Fischer-344 rats aged out to 2 years. In this study, fasting LPL activity in adipose tissue increased with age in the Sprague-Dawley rats, but decreased in the Fischer-344 rats (14). The same study found that LPL activity in heart did not change significantly with age in either rat strain, but that activity in the diaphragm muscle decreased with age in the Sprague-Dawley rats (14). Four years later, the same authors reported a more extensive study of LPL activity in aging male Fisher-344 rats, measuring fasting lipase activity at 6, 12, 15, 18, 21, and 24 months of age. In this study, they found a highly significant age-dependent decrease in LPL activity in white adipose tissue, diaphragm muscle, and cardiac muscle (16). Ursini et al. (22) found that in Wistar rats, fed LPL activity was significantly higher in adipose tissue at 15 months compared with 3 months. Bey et al. (23) looked at LPL activity specifically in skeletal muscle. In both Fischer-344 rats and the F1 progeny of a Fischer-344, Brown Norway cross, they found that LPL activity was reduced in the soleus muscle of older rats, but not in the tibialis anterior muscle. Although it is not clear why LPL activity observations vary so widely from study to study, undoubtedly differences in rat strain and age, sampling conditions, testing methods, and the highly dynamic regulation of LPL activity contributed to these discrepancies. In fact, there is evidence that feeding state regulation of LPL changes with age. Bergö, Olivecrona, and Olivecrona (24) found that in young rats (29 days), fasting results in a dramatic decrease in LPL activity in white adipose tissue, but a dramatic increase in activity in the soleus muscle. By eight months of age, these fasting-induced changes in activity had virtually disappeared (24). The mechanism behind this change has not been uncovered.
TABLE 1.
Effects of age on LPL activity in rats
| Tissue | Strain | Relevant Observation | Reference |
| Adipose tissue | Sprague-Dawley | Increasing fasted LPL activity with age (2, 12, 24 months) | (14) |
| No differences in fasted LPL activity at 40 days, 100 days, and 1 year of age | (15) | ||
| Increased fed LPL activity (compared with fasting) at 40 days of age, but no difference between fasting and fed activity at 100 days or 1 year | (15) | ||
| Fisher-344 | Decreasing fasted LPL activity with age (2, 12, 24 months) | (14) | |
| Decreasing fasted LPL activity with age (6, 12, 15, 18, 21, and 24 months) | (16) | ||
| Wistar | Increased LPL activity at 15 months compared with 3 months (feeding state not specified) | (22) | |
| Cardiac tissue | Sprague-Dawley | No change in LPL activity with age | (14) |
| Fisher-344 | No change in LPL activity with age | (14) | |
| Decreasing fasted LPL activity with age (6, 12, 15, 18, 21, and 24 months) | (16) | ||
| Diaphragm muscle | Sprague-Dawley | Decreased fasted LPL activity in 12- and 24-month-old rats compared with 2-month-old animals | (14) |
| Fisher-344 | No change in LPL activity with age | (14) | |
| Decreasing fasted LPL activity with age (6, 12, 15, 18, 21, and 24 months) | (16) | ||
| Skeletal muscle | Fisher-344 | Decreased LPL activity in 24-month-old rats compare with 2-month-old animals in soleus but not tibialis anterior muscles (feeding state not specified) | (23) |
| Fisher-344 × Brown Norway | Decreased LPL activity in 31-month-old rats compare with 18-month-old animals in soleus but not tibialis anterior muscles (feeding state not specified) | (23) |
Although mice have been used extensively to study the regulation of LPL activity, to our knowledge no systematic profiling of LPL activity in older mice has been performed.
AGE AND FAT PARTITIONING
Tissue-specific changes in LPL activity could lead to changes in lipid partitioning, altering the distribution of fat in the body. The location of TG storage has important metabolic consequences. Ectopic deposition of fat to tissues such as liver and skeletal muscle can lead to metabolic disease [reviewed in (3)]. The distribution of fat between adipose depots is also important. Both the Framingham Heart Study and the Jackson Heart Study found that excess visceral fat, independent of total or subcutaneous fat levels, is correlated with cardiovascular pathologies (25, 26). The determinants of body fat distribution have been reviewed recently in this journal (27). One important effector of fat distribution is age. Visceral adiposity increases with age (28–30). In females there is a striking shift from subcutaneous to visceral deposition of fat at menopause (31). However, it is important to note that even in premenopausal women, visceral adiposity increases with age (32). Ectopic lipid deposition also increases with age. Using magnetic resonance spectroscopy, Cree et al. (33) found that liver fat increased over 3-fold in elderly subjects (ages 65–74 years) compared with younger (ages 20–32 years) subjects. Intramyocellular lipids were also increased by ∼50% in the older subjects (33). The mechanisms behind these shifts remain unclear. Changes in tissue-specific LPL activity or remnant clearance in the liver could alter TG uptake into different tissue depots. Alternatively, tissue-specific LPL activity could become uncoupled from fatty acid uptake. In this scenario, adipose tissue depots fail to take up the fatty acids liberated by LPL and increasing levels of fatty acids spillover into the circulation where they are taken up by other tissues. These two possibilities are not mutually exclusive. Tracing studies have been used in the past to track TG processing, uptake, and spillover (34–36); however, to our knowledge, tracing studies comparing young and old individuals have not been performed.
TG METABOLISM AND AGE-INDUCED INFLAMMATION
Aging is characterized by persistent low grade inflammation in the absence of infection and is considered a risk factor for morbidity and mortality in elderly adults (37). Inflammation is associated with adipose tissue dysfunction and is an important link between obesity and dyslipidemia during aging [as reviewed in (38)]. How adipose dysfunction and inflammation alter plasma TG metabolism and how age-induced changes in plasma TG clearance contribute to adipose dysfunction and inflammation remain unclear. A major pro-inflammatory cytokine that plays a role in both aging and adipose tissue dysfunction is TNFα, which is increased in the plasma of elderly subjects (39). Increased TNFα levels can decrease human adipose tissue LPL expression and activity (40). In adipose tissue from obese female subjects, TNFα levels negatively correlated with LPL activity (41). In middle-aged men with metabolic syndrome or T2D, plasma levels of the LPL regulator, angiopoietin-like (ANGPTL)4, correlated with the pro-inflammatory marker C-reactive protein (42). In contrast, a study of male and female middle-aged subjects found an inverse correlation of ANGPTL4 with inflammation, but a positive correlation between the levels of ANGPTL3, another LPL regulator, and inflammation (43).
A recent study in mice linked inflammation, TG metabolism, and ANGPTL4, though the results raised as many questions as they answered. Mice lacking ANGPTL4 fed a diet rich in unsaturated fatty acids, cholesterol, and fructose drinking water had significantly more visceral fat and increased gene expression for pro-inflammatory genes in visceral fat compared with wild-type control mice fed the same diet (44). Despite increased inflammation and visceral fat, Angptl4−/− mice fed this high-fat diet had improved glucose tolerance and increased fasting plasma insulin levels compared with wild-type mice. Interestingly, when mice were treated with antibiotics, the increased visceral fat mass, visceral fat inflammation, and improvements in glucose homeostasis disappeared, identifying a role for gut bacteria in TG metabolism and glucose homeostasis (44). The role of ANGPTL4 in aged mice has not been investigated; however, recent work in mice has linked the gut microbiota with inflammation during aging. Conley et al. (45) compared 2-month-old and 26-month-old female mice and found an increase in the serum inflammatory marker, monocyte chemoattractant protein 1 (MCP-1), in the older mice. This increase was associated with age-induced changes in gut microbiota (45). A more recent study also identified a role for MCP-1 in ectopic lipid deposition in the liver during aging (46). An increase in liver weight, hepatic TGs, steatosis, and inflammatory gene expression was observed in 19-month-old female mice compared with 3-month-old controls. Liver TGs and inflammatory gene expression in the liver were attenuated in aged mice lacking the receptor for MCP-1 (46). Together, these data support the idea that there is an interaction between aging, inflammatory processes, and TG metabolism, but the mechanistic connections remain unclear.
AGING AND ADIPOSE TISSUE LIPOLYSIS
Although the relationships between inflammation and plasma TG metabolism remain ambiguous, the connection between age-induced inflammation and adipose tissue lipolysis have been explored in more depth. Adipose tissue lipolysis, wherein TGs stored in adipose are released as nonesterified fatty acids into the bloodstream, is an important counterpart to plasma TG clearance. Like plasma TG clearance, adipose tissue lipolysis is also altered in older individuals. Adipose tissue lipolysis occurs following activation of β-adrenergic G protein-coupled receptors by circulating hormones, namely, catecholamines such as norepinephrine released from sympathetic nerves. Early studies found that the responsiveness of adrenergic receptors in adipose tissue decreases with age (47). In a study looking at elderly (58–72 years) healthy nonobese subjects, catecholamine-stimulated lipolysis was reduced by 50% compared with young (21–35 years) subjects (48).
Lipolysis also decreases with age in mice and rats (49–52). Recent studies in mice have been particularly illuminating in connecting age-induced inflammation to decreased lipolysis. Camell et al. (49) found that the fasting-induced increases of sympathetic nerve-derived catecholamines and subsequent release of free fatty acids from adipose tissue was blunted in aged mice. Macrophages are major drivers of inflammation, and the authors found that during aging, adipose tissue macrophages inhibit norepinephrine-induced lipolysis in visceral adipose tissue (49). Transcriptional analysis of aged adipose tissue macrophages revealed that aging is associated with activation of the NOD-, LRR-, and pyrin domain-containing protein 3 (Nlpr3) receptor pathways (Nlpr3 inflammasome). Loss of Nlrp3 in mice resulted in protection from aging-associated impairment of fasting-induced lipolysis. Camell et al. (49) also found that the activation of the Nlrp3 inflammasome within the visceral adipose tissue macrophages in aged mice increased the enzyme, monoamine oxidase A, enhancing norepinephrine degradation and reducing lipolysis. In a later study, Camell et al. (53) also discovered a role for another key immune cell type during aging, B-cells. They found that with age there is an expansion in age-associated B-cells and fat-associated lymphoid clusters in visceral adipose, leading to impaired fasting-induced lipolysis (53). In aged (24 months) fasted mice, these changes were associated with lower expression of the adipocyte lipases, adipose TG lipase and hormone-sensitive lipase. When aged mice were treated with a monoclonal antibody against CD20 to deplete B-cells, fasting-induced lipolysis was still impaired, but there was an increase in the fasting levels of phosphorylated hormone-sensitive lipase and total adipose TG lipase. The authors also explored the role for the Nlpr3 inflammasome in B-cell-depleted mice. Nlpr3 inflammasome activation leads to the release of the pro-inflammatory cytokine, interleukin-1β (49). When 20-month-old female mice were depleted of B-cells and cotreated with an interleukin-1 receptor antagonist, there was an increase in fasting-induced lipolysis in the visceral adipose tissue of these mice (53). These data suggest that age-induced changes in inflammatory cell populations and numbers can reduce lipolysis, but that anti-inflammatory therapies can ameliorate these reductions.
CONCLUSIONS AND FUTURE DIRECTIONS
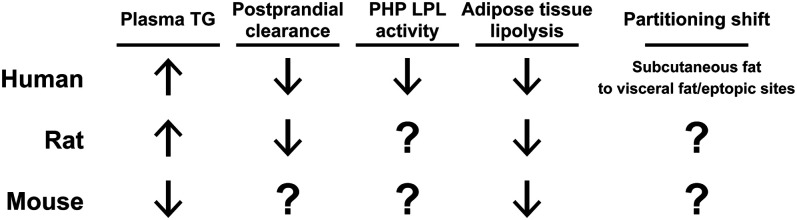
As reviewed above, age increases plasma TGs, decreases plasma TG clearance rates, decreases postheparin LPL activity, and decreases lipolysis in humans (Fig. 1). Moreover, age is associated with increased deposition of fat to visceral adipose tissue and ectopic sites. All of these changes point to an increased risk of metabolic disease. However, our knowledge of age-associated changes in TG metabolism are mostly observational. The mechanisms behind these changes, how these changes actually contribute to increased risk of metabolic disease with aging, and how reversing these changes might improve metabolic health remain relatively unknown.
Fig. 1.
A summary of age-associated changes in plasma TG metabolism. Arrows indicate the increase or decrease of plasma TG levels, plasma TG clearance, postheparin plasma (PHP) LPL activity, and lipolysis that occur with age in humans, rats, and mice. The shifts in TG partitioning that occur with age are also indicated.
Mechanistic studies exploring the biochemical, cellular, and physiological changes that lead to age-associated alterations in TG clearance and partitioning are not easy in humans. Experiments that trace the fate of TGs after a meal might be the most informative. As noted above, tracing studies have been used to track TG processing, uptake, and spillover in human subjects (34–36). Although these types of studies are not trivial to perform, they could be particularly useful in understanding the physiological changes in TG metabolism that occur with age. Coupling tracing studies with additional subject phenotypes, such as plasma TG levels, postheparin plasma LPL activity levels, and inflammatory state, could suggest cellular and biochemical mechanisms that alter TG clearance and partitioning with age.
Animal models are often very useful for dissecting mechanism. As discussed above and as shown in Fig. 1, rats have been used to study age-induced changes in TG metabolism. In most instances, the age-associated changes in plasma TG metabolism in rats are similar to those in humans (Fig. 1), but extensive mechanistic studies have not been performed. Mice, with their diversity of genetic tools, could be especially useful for interrogating mechanistic hypotheses; however, even basic descriptive studies of TG metabolism in aging mice are lacking (see Fig. 1). Such descriptive studies will be important for determining how well age-induced changes in mouse TG clearance and partitioning match those observed in humans. As with human subjects, tracing studies in rodents could reveal age-associated changes in TG and remnant clearance, tissue-specific fatty acid uptake, and spillover of fatty acids into the bloodstream. Rodents could be particularly useful in characterizing tissue-specific changes in LPL activity. Importantly, in rodent models, key proteins could be manipulated either genetically or pharmacologically to test mechanistic hypotheses.
Over the last two decades, many new regulators of TG metabolism have been characterized. For example, it is now known that generation of active LPL requires the LPL chaperone, lipase maturation factor 1, and that proper localization of LPL on the vascular lumen requires the endothelial cell transporter, glycosylphosphatidylinositol-anchored HDL binding protein 1 (54–56). ANGPTL3, ANGPTL4, and ANGPTL8 have emerged as important regulatory inhibitors of LPL activity (57). Syndecan-1 has been identified as a key mediator of remnant clearance in the liver (58). To date, there are no studies of how the expression or function of these proteins change with age. It is quite possible that some of these proteins contribute to age-induced alterations in TG metabolism. For example, as discussed above, Bergo, Olivecrona, and Olivecrona (24) found that in older rats, fasting no longer induced a reduction in LPL activity. Changes in the expression of ANGPTL4, the protein now known to be responsible for inhibiting adipose LPL activity in the fasted state (59), could potentially explain this observation.
In considering the effects of age on TG metabolism, additional factors require increased attention, including sex, diet, and physical activity. It is well established that males and females differ in their metabolism in many important ways including fuel utilization, energy expenditure in response to metabolic stress, fat partitioning, and risk for obesity and diabetes [reviewed in (60)]. Sex hormones are important determinants of body fat distribution (27). In addition, in their study of age and adipose tissue lipolysis, Camell et al. (53) found that the frequency and cellularity (cells per gram of tissue) of age-associated B-cells was more pronounced in female than male mice by 15 months of age, identifying sexual dimorphic differences in adipose tissue inflammatory changes during aging. Thus, there is ample reason to believe that age alters TG metabolism differently in males and females. Likewise, diet can dramatically alter TG metabolism as well as the extent of metabolic disease. Although accurately controlling for the diet consumed across decades by aging humans would be problematic, such controlled studies could be done in rodents. For example, using mice, Kim et al. (61) found that aged mice were more susceptible to high-fat diet-induced liver inflammation and fibrosis. It will be important to determine how different diets might exacerbate or blunt the effects of aging on TG metabolism, as well as the ability of dietary changes to improve or reverse adverse age-induced TG misregulation. Physical activity has many beneficial effects, including improving TG metabolism. Endurance training in both humans and rats decreases plasma TG levels in aged individuals (62–64). Studies in rats found that endurance training increases skeletal muscle LPL activity (65), providing a possible mechanism for improved plasma TG levels. These studies suggest that physical inactivity with age might be responsible for some of the changes in TG metabolism that occur with age and also suggest that exercise might reverse these changes. Additional studies of TG clearance and partitioning will be beneficial in determining whether exercise training can reduce age-related ectopic lipid deposition.
Ultimately, one of the most important questions is to what extent do the age-induced alterations in TG metabolism contribute to metabolic disease. Answering this question will require an increased understanding of how and why TG metabolism changes with age and of the mechanisms by which altered plasma TG levels, clearance, and partitioning increase the risk of metabolic disease. In turn, such an increase in understanding could aid in the design of strategies for combating metabolic disease in older patients.
Footnotes
This work was supported by National Institutes of Health Grant R01 HL130146 (B.S.J.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare that they have no conflicts of interest with the contents of this article.
REFERENCES
- 1.Ford E. S., Giles W. H., and Dietz W. H.. 2002. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA. 287: 356–359. [DOI] [PubMed] [Google Scholar]
- 2.Hildrum B., Mykletun A., Hole T., Midthjell K., and Dahl A. A.. 2007. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: the Norwegian HUNT 2 study. BMC Public Health. 7: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unger R. H., Clark G. O., Scherer P. E., and Orci L.. 2010. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta. 1801: 209–214. [DOI] [PubMed] [Google Scholar]
- 4.Kim J. K., Fillmore J. J., Chen Y., Yu C., Moore I. K., Pypaert M., Lutz E. P., Kako Y., Velez-Carrasco W., Goldberg I. J., et al. 2001. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc. Natl. Acad. Sci. USA. 98: 7522–7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wende A. R., Symons J. D., and Abel E. D.. 2012. Mechanisms of lipotoxicity in the cardiovascular system. Curr. Hypertens. Rep. 14: 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girousse A., Virtue S., Hart D., Vidal-Puig A., Murgatroyd P. R., Mouisel E., Sengenès C., and Savage D. B.. 2018. Surplus fat rapidly increases fat oxidation and insulin resistance in lipodystrophic mice. Mol. Metab. 13: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusminski C. M., Bickel P. E., and Scherer P. E.. 2016. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 15: 639–660. [DOI] [PubMed] [Google Scholar]
- 8.Herzstein J., Wang C-I., and Adlersberg D.. 1953. Fat-loading studies in relation to age. AMA Arch. Intern. Med. 92: 265–272. [DOI] [PubMed] [Google Scholar]
- 9.Cohn J. S., McNamara J. R., Cohn S. D., Ordovas J. M., and Schaefer E. J.. 1988. Postprandial plasma lipoprotein changes in human subjects of different ages. J. Lipid Res. 29: 469–479. [PubMed] [Google Scholar]
- 10.Cassader M., Gambino R., Ruiu G., Marena S., Bodoni P., and Pagano G.. 1996. Postprandial triglyceride-rich lipoprotein changes in elderly and young subjects. Aging (Milano). 8: 421–428. [DOI] [PubMed] [Google Scholar]
- 11.Becker G. H., Meyer J., and Necheles H.. 1949. Fat absorption and atherosclerosis. Science. 110: 529–530. [DOI] [PubMed] [Google Scholar]
- 12.Vinagre C. G., Freitas F. R., de Mesquita C. H., Vinagre J. C., Mariani A. C., Kalil-Filho R., and Maranhão R. C.. 2018. Removal of chylomicron remnants from the bloodstream is delayed in aged subjects. Aging Dis. 9: 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reaven G. M. 1978. Effect of age and sex on triglyceride metabolism in the rat. J. Gerontol. 33: 368–371. [DOI] [PubMed] [Google Scholar]
- 14.Carlile S. I., and Lacko A. G.. 1981. Strain differences in the age related changes of rat lipoprotein metabolism. Comp. Biochem. Phys. B. 70: 753–758. [Google Scholar]
- 15.Chen Y-D. I., and Reaven G. M.. 1981. Relationship between plasma triglyceride concentration and adipose tissue lipoprotein lipase activity in rats of different ages. J. Gerontol. 36: 3–6. [DOI] [PubMed] [Google Scholar]
- 16.Carlile S. I., and Lacko A. G.. 1985. Age-related changes in plasma lipid levels and tissue lipoprotein lipase activities of Fischer-344 rats. Arch. Gerontol. Geriatr. 4: 133–140. [DOI] [PubMed] [Google Scholar]
- 17.Houtkooper R. H., Argmann C., Houten S. M., Cantó C., Jeninga E. H., Andreux P. A., Thomas C., Doenlen R., Schoonjans K., and Auwerx J.. 2011. The metabolic footprint of aging in mice. Sci. Rep. 1: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calligaris S. D., Lecanda M., Solis F., Ezquer M., Gutiérrez J., Brandan E., Leiva A., Sobrevia L., and Conget P.. 2013. Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy. PLoS One. 8: e60931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niemi T., and Nikkila E. A.. 1957. Effect of age on the lipemia clearing activity of serum after administration of heparin to human subjects. J. Gerontol. 12: 44–47. [DOI] [PubMed] [Google Scholar]
- 20.Brodows R. G., and Campbell R. G.. 1972. Effect of age on post-heparin lipase. N. Engl. J. Med. 287: 969–970. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein M. M., Yin L., Beigneux A. P., Davies B. S. J., Gin P., Estrada K., Melford K., Bishop J. R., Esko J. D., Dallinga-Thie G. M., et al. 2008. Abnormal patterns of lipoprotein lipase release into the plasma in GPIHBP1-deficient mice. J. Biol. Chem. 283: 34511–34518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ursini F., Vugman M., Fernandes L. C., Curi C. M. O. N., and Curi R.. 1991. Metabolic changes of several adipose depots as caused by aging. Physiol. Behav. 50: 317–321. [DOI] [PubMed] [Google Scholar]
- 23.Bey L., Areiqat E., Sano A., and Hamilton M. T.. 2001. Reduced lipoprotein lipase activity in postural skeletal muscle during aging. J. Appl. Physiol. 91: 687–692. [DOI] [PubMed] [Google Scholar]
- 24.Bergö M., Olivecrona G., and Olivecrona T.. 1997. Regulation of adipose tissue lipoprotein lipase in young and old rats. Int. J. Obes. Relat. Metab. Disord. 21: 980–986. [DOI] [PubMed] [Google Scholar]
- 25.Liu J., Fox C. S., DeMarc H., Aurelian B., Jeffery C. J., and Taylor H. A.. 2011. Fatty liver, abdominal visceral fat, and cardiometabolic risk factors. Arterioscler. Thromb. Vasc. Biol. 31: 2715–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preis S. R., Massaro J. M., Robins S. J., Hoffmann U., Vasan R. S., Irlbeck T., Meigs J. B., Sutherland P., D’Agostino R. B., O’Donnell C. J., et al. 2010. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham Heart Study. Obesity (Silver Spring). 18: 2191–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank A. P., de Souza Santos R., Palmer B. F., and Clegg D. J.. 2019. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J. Lipid Res. 60: 1710–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn H. S., and Bullard K. M.. 2017. Indicators of abdominal size relative to height associated with sex, age, socioeconomic position and ancestry among US adults. PLoS One. 12: e0172245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hairston K. G., Scherzinger A., Foy C., Hanley A. J., McCorkle O., Haffner S., Norris J. M., Bryer-Ash M., and Wagenknecht L. E.. 2009. Five-year change in visceral adipose tissue quantity in a minority cohort: the Insulin Resistance Atherosclerosis Study (IRAS) family study. Diabetes Care. 32: 1553–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotani K., Tokunaga K., Fujioka S., Kobatake T., Keno Y., Yoshida S., Shimomura I., Tarui S., and Matsuzawa Y.. 1994. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int. J. Obes. Relat. Metab. Disord. 18: 207–202. [PubMed] [Google Scholar]
- 31.Lovejoy J. C., Champagne C. M., de Jonge L., Xie H., and Smith S. R.. 2008. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. (Lond.). 32: 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pascot A., Lemieux S., Lemieux I., Prud’homme D., Tremblay A., Bouchard C., Nadeau A., Couillard C., Tchernof A., Bergeron J., et al. 1999. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care. 22: 1471–1478. [DOI] [PubMed] [Google Scholar]
- 33.Cree M. G., Newcomer B. R., Katsanos C. S., Sheffield-Moore M., Chinkes D., Aarsland A., Urban R., and Wolfe R. R.. 2004. Intramuscular and liver triglycerides are increased in the elderly. J. Clin. Endocrinol. Metab. 89: 3864–3871. [DOI] [PubMed] [Google Scholar]
- 34.Binnert C., Pachiaudi C., Beylot M., Croset M., Cohen R., Riou J. P., and Laville M.. 1996. Metabolic fate of an oral long-chain triglyceride load in humans. Am. J. Physiol. 270: E445–E450. [DOI] [PubMed] [Google Scholar]
- 35.Evans K., Burdge G. C., Wootton S. A., Clark M. L., and Frayn K. N.. 2002. Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes. 51: 2684–2690. [DOI] [PubMed] [Google Scholar]
- 36.Piché M-E., Parry S. A., Karpe F., and Hodson L.. 2018. Chylomicron-derived fatty acid spillover in adipose tissue: a signature of metabolic health? J. Clin. Endocrinol. Metab. 103: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franceschi C., Garagnani P., Parini P., Giuliani C., and Santoro A.. 2018. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14: 576–590. [DOI] [PubMed] [Google Scholar]
- 38.Frasca D., Blomberg B. B., and Paganelli R.. 2017. Aging, obesity, and inflammatory age-related diseases. Front. Immunol. 8: 1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caso G., McNurlan M. A., Mileva I., Zemlyak A., Mynarcik D. C., and Gelato M. C.. 2013. Peripheral fat loss and decline in adipogenesis in older humans. Metabolism. 62: 337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fried S. K., and Zechner R.. 1989. Cachectin/tumor necrosis factor decreases human adipose tissue lipoprotein lipase mRNA levels, synthesis, and activity. J. Lipid Res. 30: 1917–1923. [PubMed] [Google Scholar]
- 41.Bulló M., García-Lorda P., Peinado-Onsurbe J., Hernández M., Castillo D. D., Argilés J. M., and Salas-Salvadó J.. 2002. TNFα expression of subcutaneous adipose tissue in obese and morbid obese females: relationship to adipocyte LPL activity and leptin synthesis. Int. J. Obes. Relat. Metab. Disord. 26: 652–658. [DOI] [PubMed] [Google Scholar]
- 42.Tjeerdema N., Georgiadi A., Jonker J. T., van Glabbeek M., Alizadeh Dehnavi R., Tamsma J. T., Smit J. W. A., Kersten S., and Rensen P. C. N.. 2014. Inflammation increases plasma angiopoietin-like protein 4 in patients with the metabolic syndrome and type 2 diabetes. BMJ Open Diabetes Res. Care. 2: e000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morinaga J., Zhao J., Endo M., Kadomatsu T., Miyata K., Sugizaki T., Okadome Y., Tian Z., Horiguchi H., Miyashita K., et al. 2018. Association of circulating ANGPTL 3, 4, and 8 levels with medical status in a population undergoing routine medical checkups: A cross-sectional study. PLoS One. 13: e0193731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janssen A. W. F., Katiraei S., Bartosinska B., Eberhard D., Willems van Dijk K., and Kersten S.. 2018. Loss of angiopoietin-like 4 (ANGPTL4) in mice with diet-induced obesity uncouples visceral obesity from glucose intolerance partly via the gut microbiota. Diabetologia. 61: 1447–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conley M. N., Wong C. P., Duyck K. M., Hord N., Ho E., and Sharpton T. J.. 2016. Aging and serum MCP-1 are associated with gut microbiome composition in a murine model. PeerJ. 4: e1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stahl E. C., Delgado E. R., Alencastro F., LoPresti S. T., Wilkinson P. D., Roy N., Haschak M. J., Skillen C. D., Monga S. P., Duncan A. W., et al. 2020. Inflammation and ectopic fat deposition in the aging murine liver is influenced by CCR2. Am. J. Pathol. 190: 372–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vestal R. E., Wood A. J., and Shand D. G.. 1979. Reduced beta-adrenoceptor sensitivity in the elderly. Clin. Pharmacol. Ther. 26: 181–186. [DOI] [PubMed] [Google Scholar]
- 48.Lönnqvist F., Nyberg B., Wahrenberg H., and Arner P.. 1990. Catecholamine-induced lipolysis in adipose tissue of the elderly. J. Clin. Invest. 85: 1614–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Camell C. D., Sander J., Spadaro O., Lee A., Nguyen K. Y., Wing A., Goldberg E. L., Youm Y-H., Brown C. W., Elsworth J., et al. 2017. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis in the aged. Nature. 550: 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu B. P., Bertrand H. A., and Masoro E. J.. 1980. Nutrition-aging influence of catecholamine-promoted lipolysis. Metabolism. 29: 438–444. [DOI] [PubMed] [Google Scholar]
- 51.Dax E. M., Partilla J. S., and Gregerman R. I.. 1981. Mechanism of the age-related decrease of epinephrine-stimulated lipolysis in isolated rat adipocytes: beta-adrenergic receptor binding, adenylate cyclase activity, and cyclic AMP accumulation. J. Lipid Res. 22: 934–943. [PubMed] [Google Scholar]
- 52.Carraro R., Li Z., and Gregerman R. I.. 1994. Catecholamine-sensitive lipolysis in the rat: different loci for effect of age on the lipolytic cascade in epididymal vs perirenal fat cells. J. Gerontol. 49: B140–B143. [DOI] [PubMed] [Google Scholar]
- 53.Camell C. D., Günther P., Lee A., Goldberg E. L., Spadaro O., Youm Y-H., Bartke A., Hubbard G. B., Ikeno Y., Ruddle N. H., et al. 2019. Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab. 30: 1024–1039.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beigneux A. P., Davies B. S. J., Gin P., Weinstein M. M., Farber E., Qiao X., Peale F., Bunting S., Walzem R. L., Wong J. S., et al. 2007. Glycosylphosphatidylinositol-anchored high density lipoprotein–binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 5: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies B. S. J., Beigneux A. P., Barnes R. H. II, Tu Y., Gin P., Weinstein M. M., Nobumori C., Nyrén R., Goldberg I., Olivecrona G., et al. 2010. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 12: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Péterfy M., Ben-Zeev O., Mao H. Z., Weissglas-Volkov D., Aouizerat B. E., Pullinger C. R., Frost P. H., Kane J. P., Malloy M. J., Reue K., et al. 2007. Mutations in LMF1 cause combined lipase deficiency and severe hypertriglyceridemia. Nat. Genet. 39: 1483–1487. [DOI] [PubMed] [Google Scholar]
- 57.Dijk W., and Kersten S.. 2016. Regulation of lipid metabolism by angiopoietin-like proteins. Curr. Opin. Lipidol. 27: 249–256. [DOI] [PubMed] [Google Scholar]
- 58.Stanford K. I., Bishop J. R., Foley E. M., Gonzales J. C., Niesman I. R., Witztum J. L., and Esko J. D.. 2009. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J. Clin. Invest. 119: 3236–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cushing E. M., Chi X., Sylvers K. L., Shetty S. K., Potthoff M. J., and Davies B. S. J.. 2017. Angiopoietin-like 4 directs uptake of dietary fat away from adipose during fasting. Mol. Metab. 6: 809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mauvais-Jarvis F. 2015. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex Differ. 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim I. H., Xu J., Liu X., Koyama Y., Ma H-Y., Diggle K., You Y-H., Schilling J. M., Jeste D., Sharma K., et al. 2016. Aging increases the susceptibility of hepatic inflammation, liver fibrosis and aging in response to high-fat diet in mice. Age (Dordr.). 38: 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seals D. R., Hagberg J. M., Hurley B. F., Ehsani A. A., and Holloszy J. O.. 1984. Effects of endurance training on glucose tolerance and plasma lipid levels in older men and women. JAMA. 252: 645–649. [PubMed] [Google Scholar]
- 63.Berman D. M., Rogus E. M., Busby-Whitehead M. J., Katzel L. I., and Goldberg A. P.. 1999. Predictors of adipose tissue lipoprotein lipase in middle-aged and older men: relationship to leptin and obesity, but not cardiovascular fitness. Metabolism. 48: 183–189. [DOI] [PubMed] [Google Scholar]
- 64.Barakat H. A., Dohm G. L., Shukla N., Marks R. H., Kern M., Carpenter J. W., and Mazzeo R. S.. 1989. Influence of age and exercise training on lipid metabolism in Fischer-344 rats. J. Appl. Physiol. 67: 1638–1642. [DOI] [PubMed] [Google Scholar]
- 65.Hamilton M. T., Etienne J., McClure W. C., Pavey B. S., and Holloway A. K.. 1998. Role of local contractile activity and muscle fiber type on LPL regulation during exercise. Am. J. Physiol. 275: E1016–E1022. [DOI] [PubMed] [Google Scholar]