To the Editor:
Patients with chronic obstructive pulmonary disease (COPD)/pulmonary emphysema often develop locomotor skeletal muscle dysfunction that involves muscle atrophy and lower force-generation capacity (1, 2). Muscle dysfunction is strongly associated with higher mortality and other poor outcomes in these patients (3–5); these associations persist after adjusting for pulmonary function and other covariables, suggesting that muscle dysfunction could partially contribute to the worse prognosis (1, 2). We have recently established a murine model of pulmonary emphysema–associated muscle dysfunction that recapitulates most of the features demonstrated by patients with this condition (6). A large-scale analysis of the mouse muscle proteome revealed that haptoglobin, hemopexin, and ceruloplasmin were the most significantly upregulated proteins (6). To our surprise, these same proteins have previously been described as serum diagnostic biomarkers of COPD (7). Thus, we hypothesized that their elevation in COPD reflects muscle integrity, potentially allowing interrogation of skeletal muscle dysfunction and its regulation under exercise via novel biomarkers.
Methods
Experiments were conducted using CC10-rtTA-IL-13 (IL13TG) doxycycline-inducible transgenic mice that develop chronic lung remodeling reminiscent of pulmonary emphysema upon induction (8). CC10-rtTA-IL-13 heterozygote animals were bred to C57BL/6 wild-type (WT) mice to obtain IL13TG and WT littermate control animals (6, 8). Both IL13TG (emphysema or COPD) and WT (nonemphysema, used as control littermates) mice were provided 0.5 g/L doxycycline in their drinking water together with sucrose 0.5 mg/ml, starting at 5 weeks of age for a total of 17 weeks (see Figure E1 in the data supplement). Further details of the methods can be found in the data supplement.
Results
Analysis of the extensor digitorum longus muscle and serum proteomes demonstrated an altered profile in the COPD animal: 61 of 2,337 proteins identified in muscle were dysregulated (42 upregulated and 19 downregulated) (Tables 1 and 2). In serum, 328 of 1,158 proteins were dysregulated (192 upregulated and 136 downregulated) (Tables 1 and 2). There were 22 proteins that were similarly up- or downregulated in both muscle and serum compartments (Tables 1 and 2). Among these, the candidate biomarkers haptoglobin, hemopexin, and ceruloplasmin were upregulated in IL13TG murine muscle and serum compared with WT mice. We validated the serum upregulation of these three proteins in IL13TG (COPD) versus WT mice using IB with specific antibodies, which demonstrated the same signature we identified by mass spectrometry analysis (Figure E2) and which is consistent with previously reported human data (7). To investigate the dynamic regulation in the muscle and serum proteomes, animals underwent an endurance/treadmill exercise protocol for 2 months. Analysis of skeletal muscle phenotype indicated a remarkable postexercise decrease in muscle dysfunction in COPD mice, as reflected by an increase in tibialis anterior and gastrocnemius muscle weight over tibia length (Figure 1A), extensor digitorum longus muscle mean cross-sectional area (Figure 1B), fiber distribution, fatigue test, hanging and grip-strength tests, isolated contractility, and oxygen consumption as measured by respirometry (Seahorse) technology (Figure E3A–E3F). Together with this muscle recovery, the three candidate biomarkers became not significantly different between COPD and WT mice in both muscle and serum (Figures 1C and 1D). Importantly, 13 proteins not previously described as COPD biomarkers were similarly normalized (Figure 1C and Table E1). Because exercise has been shown to mollify pulmonary injury in animal models of cigarette smoke–induced COPD (9, 10), we speculated that lung disruption could also be attenuated by endurance exercise and that this improvement could account for the biomarkers’ normalization. Histological scoring of pulmonary emphysema both at rest and after exercise showed no exercise-induced improvement of the magnitude of lung injury (Figure 1E). Moreover, immunoblot analyses of lung homogenates from pre- and postexercise mice demonstrated that the biomarkers’ pulmonary tissue upregulation was present in COPD murine lungs at baseline and was unaffected by chronic endurance exercise (Figure E4), ruling out the possibility that the biomarkers’ regulation in exercise is contributed by lung tissue turnover.
Table 1.
Number of Dysregulated Proteins, Including Down- and Upregulated Proteins, in Both Muscle and Serum from Wild-Type versus Chronic Obstructive Pulmonary Disease (IL13TG) Mice before Exercise
| Specimen | Proteins Identified | Dysregulated | Upregulated | Downregulated | Similarly Regulated |
|---|---|---|---|---|---|
| Serum | 1,158 | 328 | 192 | 136 | 22 |
| Muscle | 2,337 | 61 | 42 | 19 |
Definition of abbreviation: TG = transgenic.
Table 2.
Magnitude of Regulation of Described Human Chronic Obstructive Pulmonary Disease Biomarkers in Both Serum and Muscle before Exercise
| Baseline Samples | Serum Log2 Fold Change (IL13TG/WT) | Muscle Log2 Fold Change (IL13TG/WT) |
|---|---|---|
| Haptoglobin (Hp) | 2.7 | 6.45 |
| Hemopexin (Hpx) | 0.67 | 1.57 |
| Ceruloplasmin (Cp) | 0.86 | 0.9 |
Definition of abbreviation: WT = wild-type.
n = 4 (four experimental replicates). See statistical analysis in the Methods section.
Figure 1.
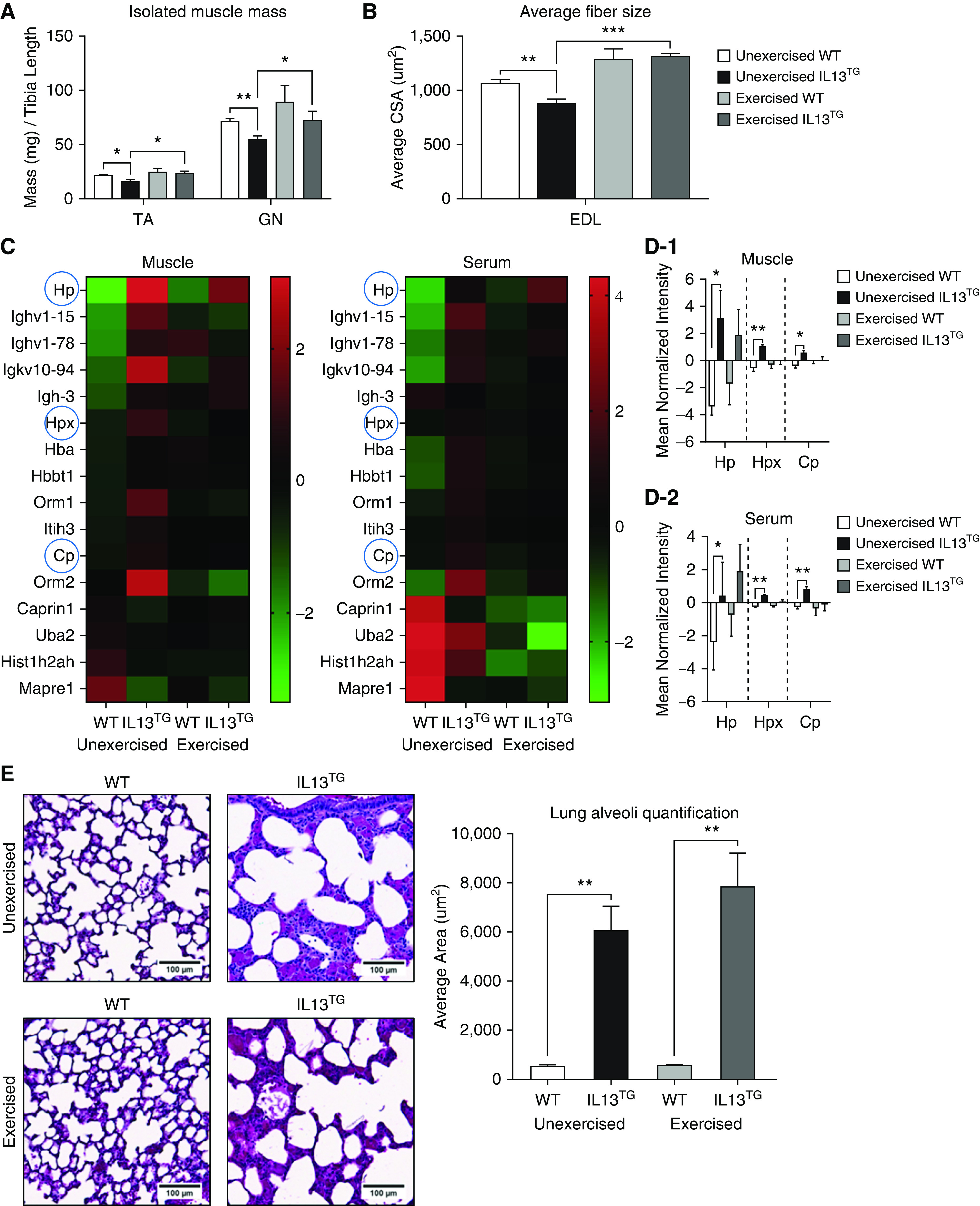
(A) Skeletal muscle mass of tibialis anterior (TA) (n = 4) and gastrocnemius (GN) (n = 4) from wild-type (WT) versus chronic obstructive pulmonary disease (COPD; IL13TG) mice before and after exercise for 2 months as described in the Methods. (B) Average IL13TG murine extensor digitorum longus (EDL) muscle, which is composed of type II (glycolytic) fibers, demonstrates a significant decrease in mean cross-sectional area compared with WT littermates that recovers after 2 months of endurance exercise (n = 4). (C) Heat map indicating similarly regulated proteins in both EDL muscle and serum under pre- and postexercise conditions. The three already described COPD biomarkers—haptoglobin (Hp), hemopexin (Hpx), and ceruloplasmin (Cp)—are highlighted with blue circles (n = 4). (D-1) The three described human COPD biomarkers are highly upregulated in EDL muscle and become not significantly differently regulated upon exercise. (D-2) These same biomarkers demonstrate a similar regulatory signature to the one shown by EDL muscle. (E) Hematoxylin and eosin staining of lungs freshly procured immediately after animals were killed demonstrates enlargement of distal airspace of IL13TG mice consistent with pulmonary emphysema, which is not attenuated by endurance exercise. n = 4. Error bars are SEM. Scale bars, 100 μm. *P < 0.05, **P < 0.01, and ***P < 0.001. CSA = cross-sectional area; Tg = transgenic.
Discussion
In this work, we used a recently established animal model of COPD-induced muscle dysfunction (6) to show that exercise leads to normalization of established COPD biomarkers haptoglobin, hemopexin, and ceruloplasmin expression levels in both muscle and serum, which correlates with muscle recovery and not with the magnitude of pulmonary emphysema. The biomarker expression levels were directly interrogated in lung tissue homogenates from WT and COPD mice after exercise and were found to be similar to preexercise levels, which is consistent with the lack of emphysema improvement after exercise. Moreover, other captured proteins potentially useful as biomarkers demonstrate similar regulation. These observations suggest that, although the aforementioned biomarkers are elevated in COPD, they are not specifically contributed by lung disruption in that setting, and these observations also suggest that the biomarkers could also represent surrogates of skeletal muscle dysfunction in these individuals. Our observations could have practical implications because these three proteins could be used as biomarkers of muscle status in COPD, allowing rapid and accurate patient stratification for muscle-strengthening rehabilitation programs via an accessible, unbiased, and easily measurable serum determination. The number and similarity of animals used for this study precluded modeling for independent effects of individual biomarkers on muscle recovery. Future human studies with multivariable modeling could determine whether these biomarkers have independent effects on muscle dysfunction. Persistence of these associations may lead to the development of an instrument that accurately assesses muscle dysfunction in COPD. The specific association between muscle dysfunction and upregulation of haptoglobin, hemopexin, and ceruloplasmin could have mechanistic implications. For example, haptoglobin-knockout mice demonstrate accelerated muscle wasting due to oxidative stress (11), which suggests that it participates in the pathophysiology of oxidative injury–driven muscle dysfunction (12). Similarly, genetic variations of these biomarkers could indicate more susceptibility to muscle dysfunction (13, 14). For instance, a haptoglobin polymorphism has been reported in patients with COPD (15).
In summary, we present data which suggest that haptoglobin, hemopexin, and ceruloplasmin are biomarkers of COPD that reflect skeletal muscle integrity in these patients. We also suggest that these and other similarly regulated proteins could be used to track skeletal muscle dysfunction in COPD and its response to exercise using a minimally invasive and unbiased method, which could have clinical and translational value in the treatment of patients with advanced COPD and chronic disability. Human studies will further elucidate the practical application of these biomarker determinations.
Acknowledgments
Acknowledgment
The authors thank Dr. Marc A. Judson for his important editorial comments.
Footnotes
Supported by the U.S. National Heart, Lung, and Blood Institute (NHLBI) of the U.S. National Institutes of Health (NIH) under award number K01-HL130704 (A.J.) and by the Collins Family Foundation Endowment (A.J.), NIH/NHLBI grant 5R01HL049426 (H.A.S.), P41 GM108538 (J.J.C.), NIH/NHLBI grant P01 HL114501 (J.A.E.), and R01 HL115813 (C.G.L.).
Author Contributions: J.B., C.E.V., and A.J. designed and performed experiments. J.B. and C.E.V. performed proteomic analyses. A.J.J. and L.A.D. performed IB experiments. J.B., J.J.C., C.G.L., J.A.E., H.A.S., and A.J. designed the experiments and wrote the manuscript.
This letter has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Jaitovich A, Barreiro E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease: what we know and can do for our patients. Am J Respir Crit Care Med. 2018;198:175–186. doi: 10.1164/rccm.201710-2140CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigaré R, et al. ATS/ERS Ad Hoc Committee on Limb Muscle Dysfunction in COPD. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:e15–e62. doi: 10.1164/rccm.201402-0373ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 4.Shrikrishna D, Patel M, Tanner RJ, Seymour JM, Connolly BA, Puthucheary ZA, et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J. 2012;40:1115–1122. doi: 10.1183/09031936.00170111. [DOI] [PubMed] [Google Scholar]
- 5.Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJ, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62:115–120. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balnis J, Korponay TC, Vincent CE, Singer DV, Adam AP, Lacomis D, et al. IL-13-driven pulmonary emphysema leads to skeletal muscle dysfunction attenuated by endurance exercise. J Appl Physiol (1985) 2020;128:134–148. doi: 10.1152/japplphysiol.00627.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verrills NM, Irwin JA, He XY, Wood LG, Powell H, Simpson JL, et al. Identification of novel diagnostic biomarkers for asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1633–1643. doi: 10.1164/rccm.201010-1623OC. [DOI] [PubMed] [Google Scholar]
- 8.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ, Jr, et al. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106:1081–1093. doi: 10.1172/JCI10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toledo AC, Magalhaes RM, Hizume DC, Vieira RP, Biselli PJ, Moriya HT, et al. Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur Respir J. 2012;39:254–264. doi: 10.1183/09031936.00003411. [DOI] [PubMed] [Google Scholar]
- 10.Toledo-Arruda AC, Vieira RP, Guarnier FA, Suehiro CL, Caleman-Neto A, Olivo CR, et al. Time-course effects of aerobic physical training in the prevention of cigarette smoke-induced COPD. J Appl Physiol (1985) 2017;123:674–683. doi: 10.1152/japplphysiol.00819.2016. [DOI] [PubMed] [Google Scholar]
- 11.Bertaggia E, Scabia G, Dalise S, Lo Verso F, Santini F, Vitti P, et al. Haptoglobin is required to prevent oxidative stress and muscle atrophy. PLoS One. 2014;9:e100745. doi: 10.1371/journal.pone.0100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puig-Vilanova E, Rodriguez DA, Lloreta J, Ausin P, Pascual-Guardia S, Broquetas J, et al. Oxidative stress, redox signaling pathways, and autophagy in cachectic muscles of male patients with advanced COPD and lung cancer. Free Radic Biol Med. 2015;79:91–108. doi: 10.1016/j.freeradbiomed.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Ragland MF, Benway CJ, Lutz SM, Bowler RP, Hecker J, Hokanson JE, et al. Genetic advances in chronic obstructive pulmonary disease: insights from COPDGene. Am J Respir Crit Care Med. 2019;200:677–690. doi: 10.1164/rccm.201808-1455SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald ML, Diaz AA, Ross JC, San Jose Estepar R, Zhou L, Regan EA, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease: a cross-sectional study. Ann Am Thorac Soc. 2014;11:326–334. doi: 10.1513/AnnalsATS.201307-229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun W, Kechris K, Jacobson S, Drummond MB, Hawkins GA, Yang J, et al. SPIROMICS Research Group; COPDGene Investigators. Common genetic polymorphisms influence blood biomarker measurements in COPD. PLoS Genet. 2016;12:e1006011. doi: 10.1371/journal.pgen.1006011. [DOI] [PMC free article] [PubMed] [Google Scholar]


