Abstract
Pseudomonas aeruginosa is a lethal pathogen that causes high mortality and morbidity in immunocompromised and critically ill patients. The type III secretion system (T3SS) of P. aeruginosa mediates many of the adverse effects of infection with this pathogen, including increased lung permeability in a Toll-like receptor 4/RhoA/PAI-1 (plasminogen activator inhibitor-1)-dependent manner. α-Tocopherol has antiinflammatory properties that may make it a useful adjunct in treatment of this moribund infection. We measured transendothelial and transepithelial resistance, RhoA and PAI-1 activation, stress fiber formation, P. aeruginosa T3SS exoenzyme (ExoY) intoxication into host cells, and survival in a murine model of pneumonia in the presence of P. aeruginosa and pretreatment with α-tocopherol. We found that α-tocopherol alleviated P. aeruginosa–mediated alveolar endothelial and epithelial paracellular permeability by inhibiting RhoA, in part, via PAI-1 activation, and increased survival in a mouse model of P. aeruginosa pneumonia. Furthermore, we found that α-tocopherol decreased the activation of RhoA and PAI-1 by blocking the injection of T3SS exoenzymes into alveolar epithelial cells. P. aeruginosa is becoming increasingly antibiotic resistant. We provide evidence that α-tocopherol could be a useful therapeutic agent for individuals who are susceptible to infection with P. aeruginosa, such as those who are immunocompromised or critically ill.
Keywords: vitamin E, pulmonary edema, type III secretion system, ExoY, antimicrobial resistance
Pseudomonas aeruginosa is a lethal pathogen that causes pneumonia in patients who are immunocompromised or critically ill (1, 2). The costs of treating nosocomial infections continues to climb, and the incidence of infection and antibiotic resistance is becoming more common (3, 4). One report specifically indicated that P. aeruginosa infection itself was an independent risk factor for mortality in nosocomial pneumonia (1). The increased mortality secondary to P. aeruginosa pneumonia is due, in part, to the development of acute lung injury characterized by flooding of the airspaces with protein-rich edema. Specific contributors to P. aeruginosa pathogenesis include, but are not limited to, flagella, pili, LPS, elastase, alkaline phosphatase, exotoxin A, and components of the type III secretion system (T3SS) (5–7). In addition to the detrimental effects from the bacterium itself, there is an increase in multidrug-resistant P. aeruginosa strains that are developing at an alarming rate (8–10). Even more concerning is the fact that there are very few antibiotics being discovered and tested by the pharmaceutical industry to combat these new multidrug-resistant strains (11). The T3SS plays a major role in distal lung injury caused by P. aeruginosa (12, 13), and four exoenzymes are currently known: ExoS, ExoT, ExoU, and ExoY. ExoY is a nucleotidyl cyclase that can generate cAMP, CGMP, and/or cUMP (14–16), and ExoU is a phospholipase that is correlated with high patient mortality (13, 17). ExoS and ExoT are N-terminal RhoGAPs that activate RhoA (18, 19) and cause ADP ribosylation of multiple cell proteins (20). Furthermore, we have previously published that ExoS and ExoT increase endothelial and epithelial paracellular permeability by a RhoA-dependent mechanism. The RhoA–mediated effects could be blocked both in vitro and in vivo by a specific RhoA inhibitor (21–23). Finally, clinical studies have shown elevated levels of PAI-1 in the BAL fluid of patients with P. aeruginosa pneumonia (24). Although knockout of PAI-1 in mice alleviates the in vivo effects of P. aeruginosa on lung permeability, PAI-1 deletion did not improve survival because of its inhibitory effects on neutrophil migration, endocytosis, and bacterial killing (23).
α-Tocopherol belongs to a family of lipid-soluble antioxidants including, but not limited to, α- and γ-tocopherols (25). In diseases such as asthma and allergic lung disease, α-tocopherol generally confers antiinflammatory effects, and γ-tocopherol mediates proinflammatory effects (26). For example, α-tocopherol can reduce airway hyperreactivity in mice (27) and block migration of leukocytes across endothelial cells (28). Furthermore, children and adults with asthma tend to have lower levels of α-tocopherol (29–31), and α-tocopherol is negatively associated with osteoarthritis (32).
Because P. aeruginosa pneumonia is often associated with multidrug resistance and there are few, if any, new antibiotic therapies in the discovery pipeline, new adjuncts that may attenuate the severity of P. aeruginosa pneumonia should be sought. Given the antiinflammatory properties of α-tocopherol and its salutary effects on lung inflammation in response to endotoxin (33) and sulfur and nitrogen mustards (34), we hypothesized that pretreatment of endothelial and epithelial cells with α-tocopherol would prevent increased paracellular permeability secondary to exposure to P. aeruginosa. We designed a series of experiments to test whether α-tocopherol may alleviate P. aeruginosa–mediated alveolar endothelial and epithelial paracellular permeability by inhibiting RhoA, in part, via PAI-1 activation (23), and increase survival in a mouse model of P. aeruginosa pneumonia. Furthermore, we tested the hypothesis that α-tocopherol decreases the activation of RhoA and PAI-1 by blocking the injection of T3SS exoenzymes into alveolar epithelial cells (35).
Methods
Reagents
All reagents were purchased from Fisher Scientific unless otherwise specified: Vital E-500 (α-tocopherol), Trolox, and γ-tocopherol (Stuart Products, Inc.); RhoA G-LISA (Cytoskeleton); plasminogen activator inhibitor (PAI)-1 ELISA (Innovative Research); mouse myeloperoxidase ELISA kit (Cell Sciences); 125I-HSA (Jeanatope ISO-TEX Diagnostics); Myc-Tag and GAPDH monoclonal antibodies (Cell Signaling); IRDye 680RD anti-rabbit and 800CW anti-mouse antibodies (LI-COR Biosciences).
Rat Endothelial Cell Isolation
Rat microvascular endothelial cells (RMVECs) were isolated as described (36).
Cell Culture
RMVECs were cultured in DMEM, and L2 cells (ATCC) were cultured in Ham’s F12-K medium with 10% FBS and 1% penicillin/streptomycin.
Preparation of P. aeruginosa
PAK and PAK-expressing GFP-fused or Myc-tagged ExoY were prepared as described (37).
Paracellular Resistance
Cellular barrier integrity was measured using Electric Cell–substrate Impedance Sensing as described (36).
Transepithelial Albumin Flux
L2 cells were assayed as described (23).
ELISA
RhoA and PAI-1 levels were measured according to manufacturer instructions.
Confocal Microscopy
Confocal microscopy was performed as described (37). Confluent cells were plated on coverslips, exposed to PAK, fixed, mounted, and viewed by fluorescence microscopy. RMVECs were additionally permeabilized and stained with rhodamine-phalloidin at 1:200.
Western Blotting
Western blotting was performed as described (38), with primary antibodies used at 1:1,000 and secondary antibodies used at 1:10,000.
Mice
C57BL/6 mice were purchased from Charles River Laboratories and housed in University of Alabama at Birmingham Animal Care Facilities. All use of animals was according to protocols approved by University of Alabama at Birmingham Animal Care and Use Committee.
Pneumonia
The mouse pneumonia model was performed as described (37).
In Vivo α-Tocopherol Levels
Mice were pretreated with α-tocopherol (3 units/kg). Six hours after PAK installation, mice were killed, desired organs were collected and homogenized, and α-tocopherol levels were measured by HPLC (39).
Lung Vascular Permeability
Lung endothelial permeability was measured as described (37).
Bacterial Lung Colony-Forming Units
Measurement of lung colony-forming units was performed as previously described (22).
BAL Protein Measurement and Cell Counts
Measurement of whole-lung protein was performed as previously described (40).
Lung Myeloperoxidase Measurements in Whole-Lung Tissue
Measurement of whole-lung myeloperoxidase (MPO) was performed as previously described (22).
Neutrophil Isolation
Peritoneal neutrophils were isolated as previously described (41).
Neutrophil Phagocytosis
Isolated neutrophils were pretreated with α-tocopherol and exposed to PAK, and phagocytosis was measured as previously described (41).
Statistical Analysis
All normal data are mean ± SEM. Nonparametric data are mean ± interquartile range. Normal distributions were verified using the Agostino-Pearson test. For normally distributed data, one-way ANOVA followed by Dunnett’s test was used to compare three or more groups and Student’s t test to compare two groups. Bonferroni correction was used to adjust multiple comparisons. Nonparametric data were compared with a Kruskal- Wallis test followed by a Tukey post hoc test. A Kaplan-Meier analysis followed by a log rank (Mantel-Cox) test was used to compare the survival between the two groups. A P value of <0.05 was considered statistically significant.
Results
α-Tocopherol Attenuates P. aeruginosa–mediated Increases in Lung Endothelial Paracellular Permeability
To test the hypothesis that α-tocopherol may be a useful adjunct in treatment of infection with P. aeruginosa, we first tested whether α-tocopherol (Vital E-500) could prevent PAK (wild-type strain of P. aeruginosa)-induced increases in lung endothelial permeability (Figure 1A). Although there was no change in permeability by α-tocopherol treatment alone, exposure to PAK increased paracellular permeability roughly fivefold. Pretreatment with 10 μM α-tocopherol significantly decreased PAK-mediated increases in endothelial paracellular permeability. To understand whether the effects of α-tocopherol were specific to this isoform, we performed the same experiment using γ-tocopherol and Trolox (water-soluble analog of α-tocopherol) (Figure 1A). Neither of these treatments had a significant effect on endothelial paracellular permeability. However, neither therapy was able to reduce PAK-mediated increases in permeability, indicating that the effects of α-tocopherol are both isoform- and fat soluble–specific.
Figure 1.
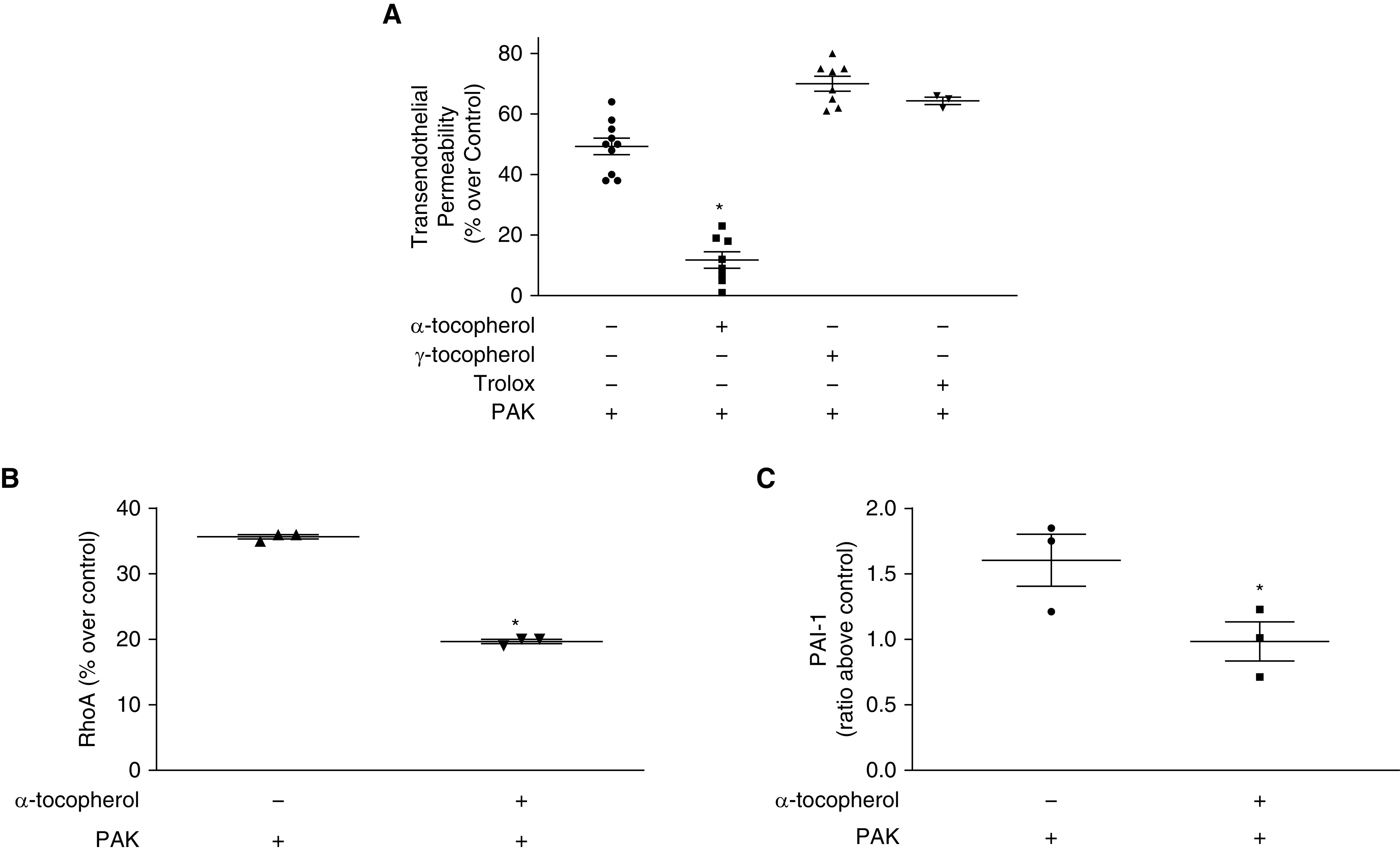
α-Tocopherol prevents endothelial paracellular permeability derangements and activation of RhoA and plasminogen activator inhibitor-1 (PAI-1) secondary to exposure to Pseudomonas aeruginosa. For all experiments, confluent rat microvascular endothelial cells were pretreated with 10 μM α-tocopherol, γ-tocopherol, or Trolox (or vehicle), exposed to PAK (wild-type strain of P. aeruginosa at a multiplicity of infection of 40) as appropriate, and parameters were measured as described below. (A) Transendothelial paracellular permeability was measured by Electric Cell-substrate Impedance Sensing for 24 hours. Vehicle alone baseline was 5% ± 2%, α-tocopherol alone was 6% ± 2%, γ-tocopherol alone was 3% ± 2%, and Trolox alone was 4% ± 2%. (B) RhoA was measured in the supernatant after 6 hours by ELISA. Baseline optical density 490 for vehicle was 0.13 ± 0.01 and for α-tocopherol was 0.135 ± 0.01. (C) PAI-1 was measured in the supernatant after 6 hours by ELISA. Baseline ng/ml for vehicle was 0.38 ± 0.1 and for α-tocopherol was 0.41 ± 0.2. Data are expressed as the ratio of PAK-treated levels divided by non–PAK-treated levels (α-tocopherol or vehicle alone, percentage over control or ratio above control). Data are expressed as mean ± SEM and are the result of three independent experiments. P ≤ 0.05. *Significant difference compared with cells treated with PAK alone.
α-Tocopherol Reduces P. aeruginosa–mediated Activation of RhoA and PAI-1 in Endothelial Cells and Prevents P. aeruginosa–mediated Stress Fiber Formation
Our previous work revealed that P. aeruginosa infection mediates its effects on lung permeability, in part, via the activation of RhoA and PAI-1, which can lead to actin stress fiber formation and increase in paracellular endothelial permeability (22, 23, 42). Therefore, we measured RhoA and PAI-1 levels in the supernatants of RMVECs pretreated with10 μM α-tocopherol and exposed to PAK for 6 hours (Figures 1B and 1C). Interestingly, pretreatment with α-tocopherol was able to significantly reduce activation of both of these mediators of endothelial paracellular permeability. Furthermore, α-tocopherol was able to prevent formation of PAK-induced stress fibers in endothelial cells (Figure 2). Finally, the protective effect of α-tocopherol on the lung endothelial permeability and RhoA and PAI-1 activation was not associated with an increase in bacterial killing (data not shown).
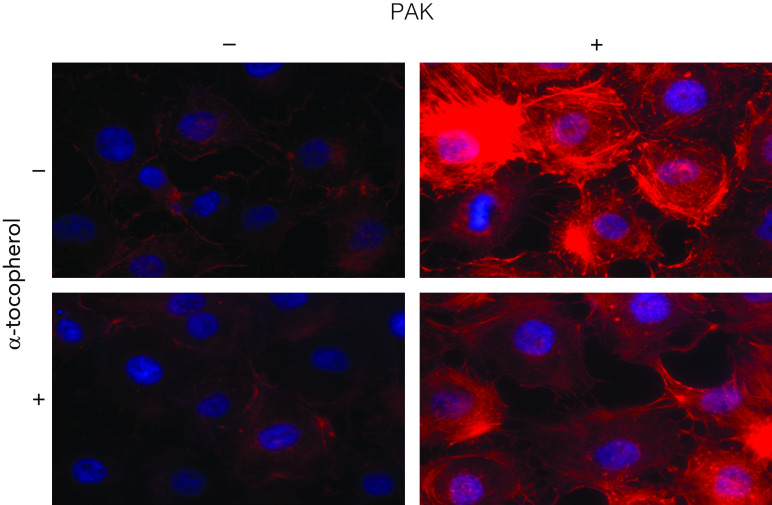
Figure 2.
α-Tocopherol blocks P. aeruginosa–mediated stress fiber formation. Rat microvascular endothelial cells were pretreated with 10 μM α-tocopherol and exposed to PAK. Six hours after PAK exposure, cells were fixed, permeabilized, stained with rhodamine-phalloidin, and mounted on slides with Heatshield/DAPI. Cells were imaged with confocal fluorescence microscopy. Experiments were performed in triplicate, and multiple areas of each slide were imaged for consistency. Representative images are shown.
α-Tocopherol Prevents Increases in Epithelial Paracellular Permeability and RhoA and PAI-1 Activation Induced by P. aeruginosa.
To determine whether α-tocopherol could prevent P. aeruginosa–induced derangements in alveolar epithelial cells as well, we exposed L2 cells to PAK after pretreatment with 10 μM α-tocopherol. P. aeruginosa–induced increase in transepithelial permeability (Figure 3A), RhoA activation (Figure 3B), and increased PAI-1 levels (Figure 3C) were ameliorated by pretreatment with α-tocopherol. These results indicate that α-tocopherol may be useful to protect both sides of the alveolar–capillary membrane.
Figure 3.
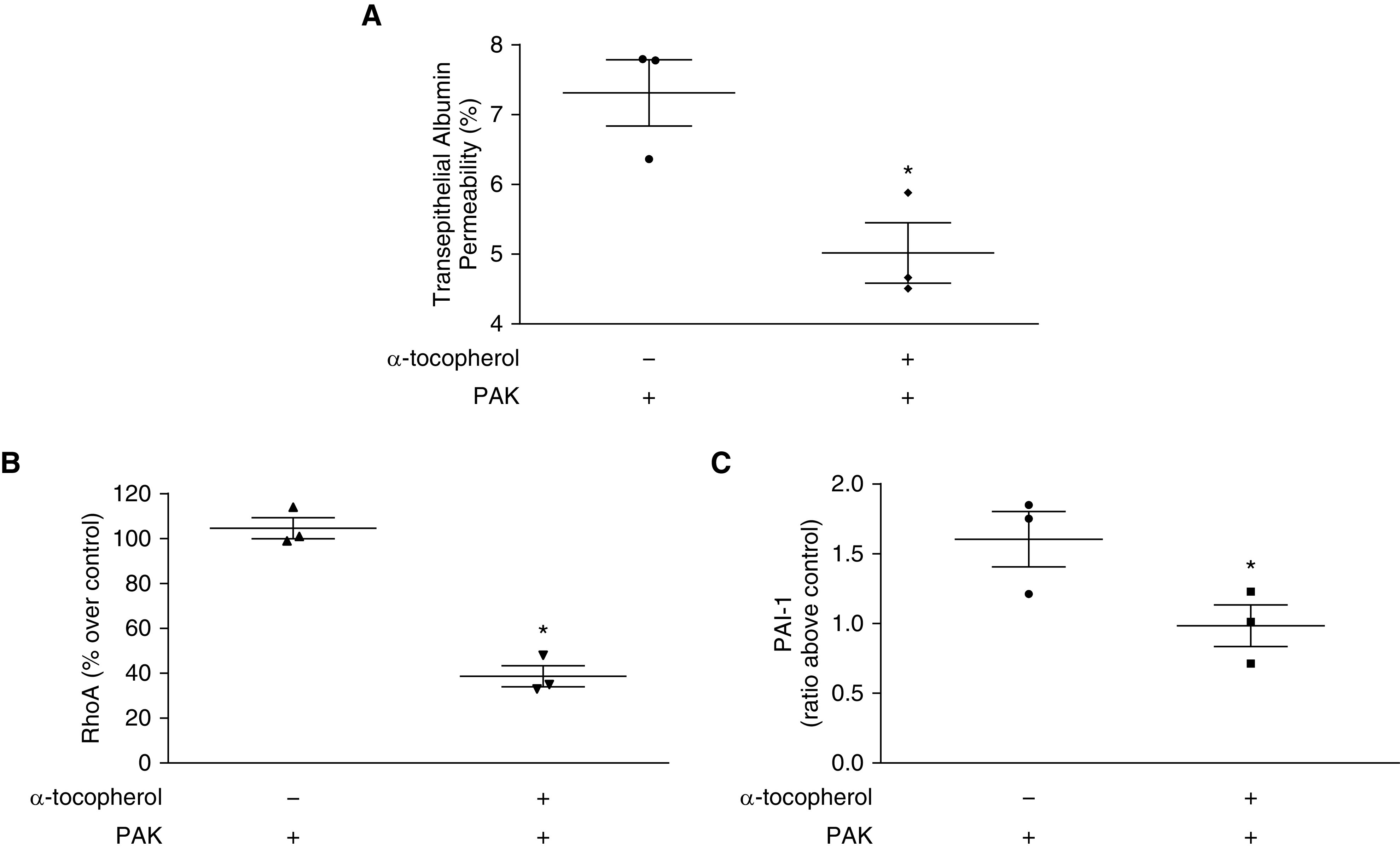
α-Tocopherol prevents epithelial paracellular permeability derangements and activation of RhoA and PAI-1 secondary to exposure to P. aeruginosa. (A) L2 (rat alveolar epithelial) cells were plated to Transwells and allowed to grow until they were polarized and could hold back media consistently. Transepithelial albumin permeability was performed as described, and cells were pretreated with 10 μM α-tocopherol and exposed to PAK. Permeability was measured as the ratio of radioactive albumin levels in the basolateral side after 60 minutes divided by the level after 3 minutes. Baseline level for vehicle alone was 3.7 ± 0.4 and for α-tocopherol was 4.2 ± 0.5. (B) Confluent L2 cells were pretreated with vitamin E and exposed to PAK. RhoA was measured in the supernatant after 6 hours by ELISA. Baseline OD490 for vehicle alone was 0.1 ± 0.01 and for α-tocopherol was 0.13 ± 0.01. (C) Confluent L2 cells were pretreated with α-tocopherol and exposed to PAK. PAI-1 was measured in the supernatant after 6 hours by ELISA. Baseline level for vehicle was 3.2 ± 0.4 and for α-tocopherol was 2.8 ± 0.3. Data are expressed as the ratio of PAK-treated levels divided by non–PAK-treated levels (α-tocopherol or vehicle alone, percentage over control or ratio above control). Data are expressed as mean ± SEM and are the result of three independent experiments. P ≤ 0.05. *Significant difference compared with cells treated with PAK alone.
α-Tocopherol Prevents Insertion of T3SS Exoenzymes into Alveolar Epithelial Cells
Previous studies have reported that T3SS exoenzymes are injected in parenchymal cells through insertion in lipid raft domains of the cell membrane (35). Furthermore, α-tocopherol has been shown to prevent accumulation of proatherogenic lipids in the lipid raft domains of the cell membrane (43). To further elucidate the mechanism by which α-tocopherol protects alveolar epithelial cells from P. aeruginosa–mediated injury, we examined whether α-tocopherol could prevent insertion of ExoY into L2 cells. ExoY is one of the exoenzymes of the T3SS of P. aeruginosa that plays an important role in mediating bacterial virulence (15, 44–46). Furthermore, wild-type strains of P. aeruginosa that express GFP-fused or Myc-tagged ExoY can be used to measure exoenzyme insertion into cells. PAK-Myc (PAK strain expressing Myc-tagged ExoY) and results of intracellular Myc (representing ExoY) was measured and quantified by Western blot (Figures 4A and 4B). α-Tocopherol prevented ExoY insertion by PAK-Myc. PAK-GFP (PAK strain expressing GFP-fused ExoY) alone was able to insert significant amounts of ExoY into L2 cells (Figure 4C). Alternatively, when L2 cells were pretreated with α-tocopherol there was near complete abrogation of ExoY insertion into cells by PAK-GFP (Figure 4D). The mean intensity of immunofluorescence was quantified in L2 cells exposed to the aforementioned treatments (Figure 4E). In both cases, α-tocopherol was able to significantly reduce the levels of ExoY inserted into alveolar epithelial cells by P. aeruginosa.
Figure 4.
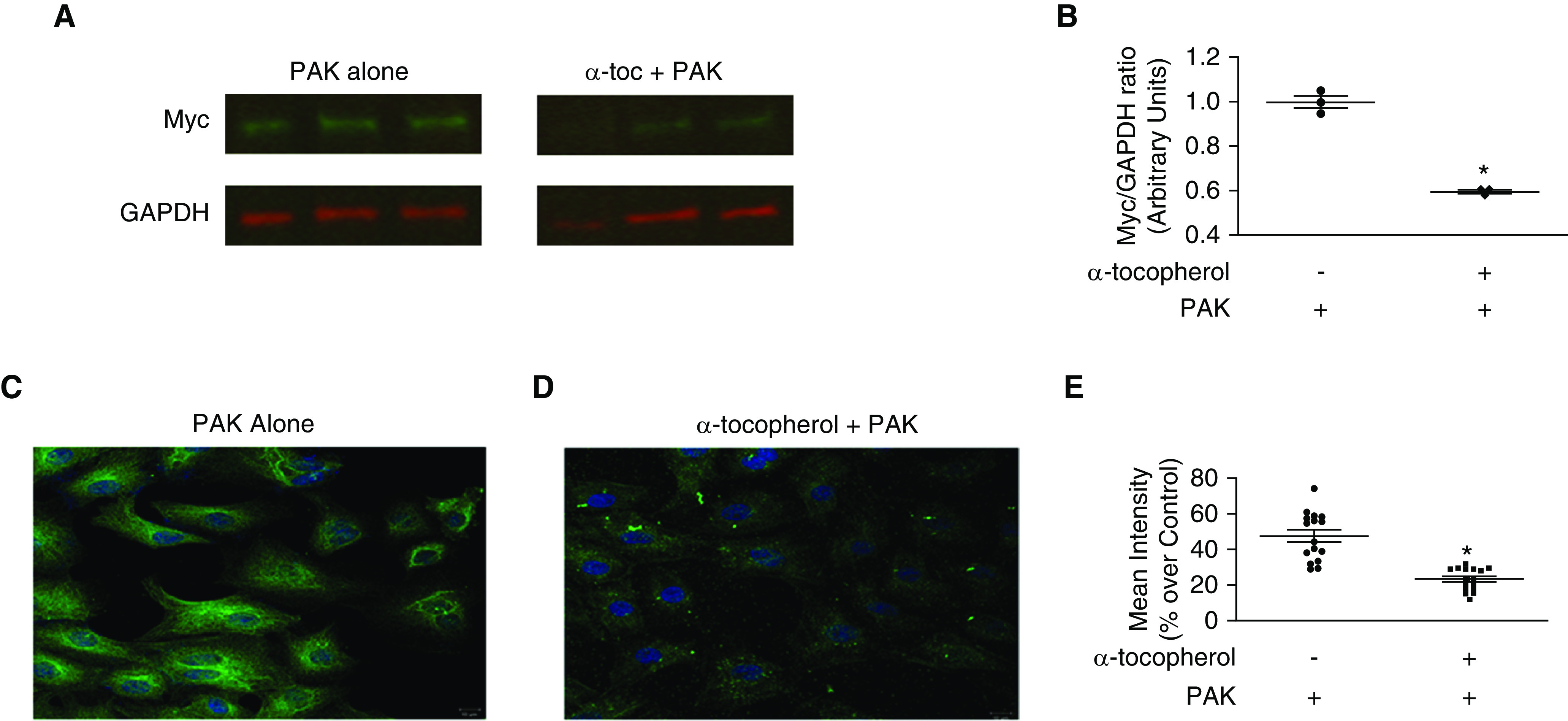
α-Tocopherol prevents insertion of myc- and GFP-tagged exoenzyme Y (ExoY) toxins into epithelial cells. Confluent L2 cells were pretreated with 10 μM α-tocopherol and exposed to PAK-GFP (wild-type strain of P. aeruginosa that expresses GFP-fused ExoY toxin) or PAK-Myc (wild-type strain of P. aeruginosa that expresses Myc-tagged ExoY toxin) at a multiplicity of infection of 40 for 6 hours. Cells were washed and treated with gentamicin (150 μg/ml) for 1 hour to eradicate extracellular PAK. (A and B) After appropriate treatment, cells were lysed and Western blotting using antibodies against Myc and GAPDH performed. (A) A representative blot is shown. (B) Quantification was performed using densitometry, and levels shown are Myc/GAPDH ratio. (C and E) After appropriate treatment with PAK-GFP, cells were fixed and mounted on slides with Vectashield/DAPI. Cells were imaged with confocal fluorescence microscopy. Experiments were performed in triplicate, and multiple areas of each slide were imaged for consistency. Representative images are shown. Quantification was performed by measuring mean intensity and is represented as its percentage over control (vehicle only). All quantified data are expressed as mean ± SEM and are the result of three independent experiments. P ≤ 0.05. *Significant difference compared with control.
α-Tocopherol Attenuates P. aeruginosa–mediated Lung Vascular Permeability, Reduces Bacterial Burden, and Prevents Mortality in a Murine Model of Pneumonia
We first sought to understand whether intraperitoneal administration of α-tocopherol to mice would achieve effective end-organ levels in a murine model of pneumonia. To this end, we treated mice with α-tocopherol with two separate injections 18 hours and 1 hour before PAK instillation. Mice were again injected with α-tocopherol 18 hours after PAK instillation. α-Tocopherol levels were measured in the plasma (see Figure E1A in the data supplement), lung (Figure E1B), kidney (Figure E1C), liver (Figure E1D), spleen (Figure E1E), and heart (Figure E1F) 6 hours after last α-tocopherol dose. In all organs measured, there was a 5- to 10-fold increase in α-tocopherol levels, indicating that α-tocopherol is found at therapeutic levels far exceeding control tissue concentrations in mice (47).
We next found that pretreatment with α-tocopherol was able to reduce lung permeability in a mouse model of P. aeruginosa pneumonia (Figure 5A). Interestingly, BAL protein was not decreased by α-tocopherol (Figure 5B). Lung bacterial burden, release of MPO in whole-lung tissue, and overall cells in the BAL were decreased by α-tocopherol (Figures 5C and 5E). Finally, isolated neutrophils did not have improved phagocytosis when pretreated with α-tocopherol (Figure 5F). To determine whether the decrease in lung permeability, reduced colony-forming units, MPO release, and cell influx could have clinically meaningful impact, we measured survival in our pneumonia model (Figure 6). Indeed, pretreatment with α-tocopherol significantly decreased mortality from P. aeruginosa in our murine model of bacterial pneumonia (Figure 6A); however, when one dose of α-tocopherol was given immediately after PAK instillation, there was no beneficial effect on survival (Figure 6B).
Figure 5.
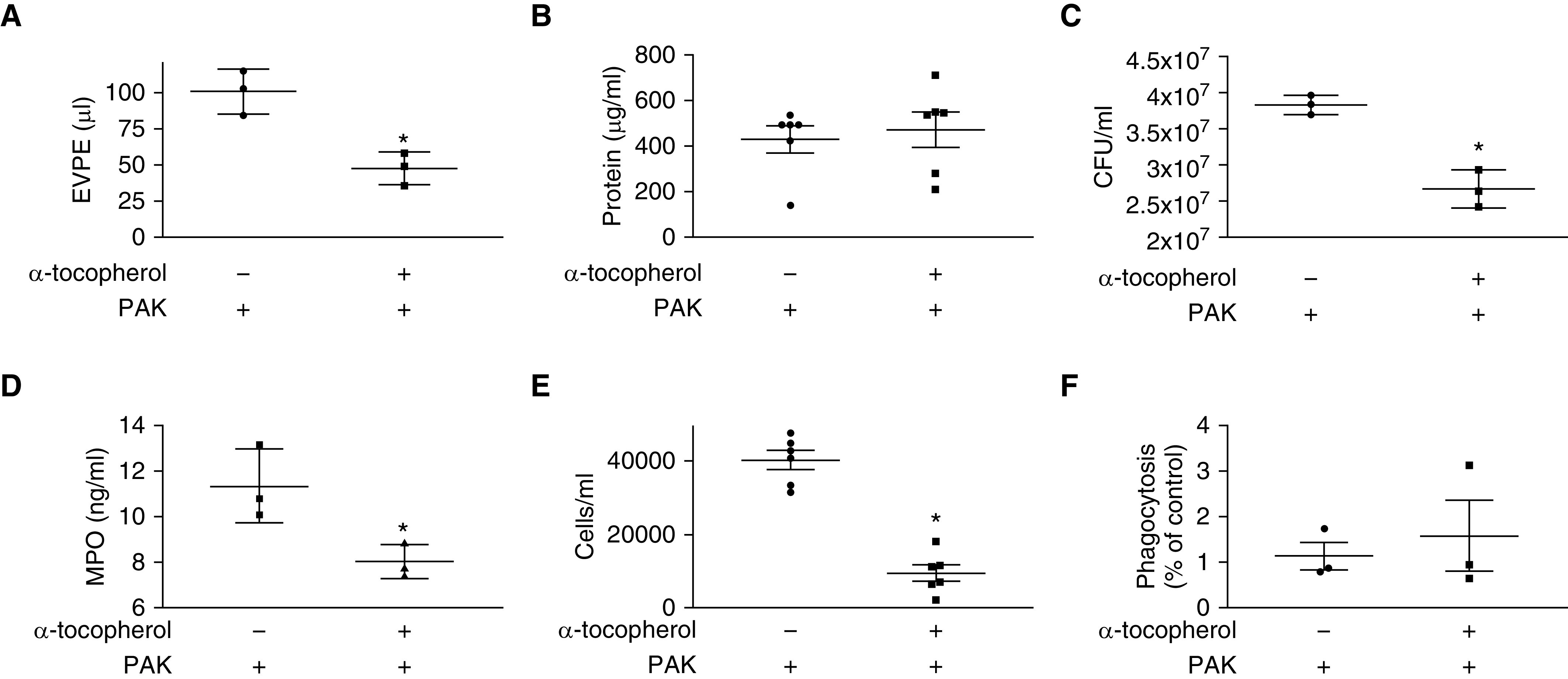
α-Tocopherol attenuates lung permeability and reduces lung bacterial burden in a murine model of pneumonia. For experiments A through E, C57BL/6 mice were intraperitoneally injected with α-tocopherol (3 units/kg) 18 and 1 hour before PAK (5 × 107 cfu) instillation and experiments were performed 6 hours after PAK installation. (A) Extravascular pulmonary edema (EVPE) was measured using 131l-albumin as described in Methods. Data are expressed as mean ± SEM and are the result of three independent experiments. (B) BAL protein was measured as described in Methods. Data are expressed as mean ± SEM and are the result of six independent experiments. (C) Colony-forming units (cfu) were measured as described in Methods. Data are expressed as mean ± SEM and are the result of three independent experiments. (D) Myeloperoxidase (MPO) in lung homogenates was measured as described in Methods. Measurements were made in duplicate, and data are expressed as mean ± SEM and are the result of three independent experiments. (E) Influx of cells in the BAL was measured as described in Methods. Data are expressed as mean ± SEM and are the result of six independent experiments. (F) Murine neutrophil was measured as described in Methods. Data are expressed as percentage of control, where vehicle or α-tocopherol groups were divided by cells infected with PAK to reduce variance between experiments. For all experiments, P ≤ 0.05. *Significant difference compared with control.
Figure 6.
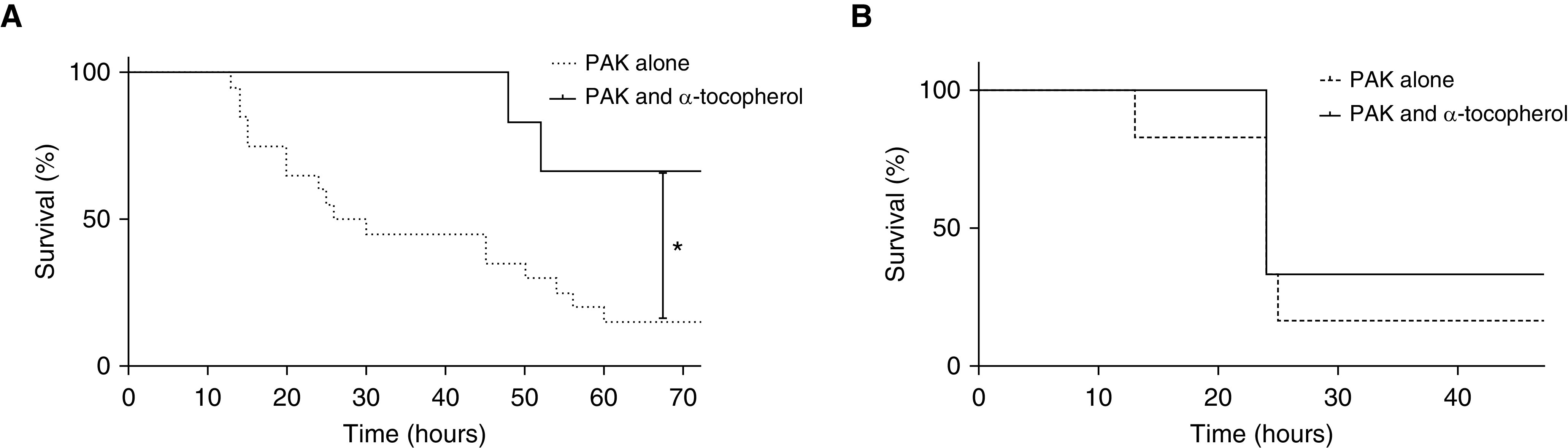
Pretreatment of mice with α-tocopherol, but not post-treatment, improves survival in a murine model of pneumonia. For experiments in A, C57BL/6 mice were intraperitoneally injected with α-tocopherol (3 units/kg) 18 and 1 hour before PAK (5 × 107 cfu) instillation and were given an additional dose of α-tocopherol 18 hours after PAK instillation. For experiments in B, C57BL/6 mice were intraperitoneally injected with α-tocopherol (3 units/kg) immediately after PAK instillation. (A) Survival was measured as described in Methods (n = 20 for PAK only, n = 8 for PAK + α-tocopherol). (B) Survival was measured as described in Methods (n = 6 for PAK only, n = 6 for PAK + α-tocopherol). P ≤ 0.05. *Significant difference compared with control.
Discussion
P. aeruginosa is an opportunistic pathogen that can cause lethal pneumonia in patients who are immunocompromised or critically ill (1, 2). Furthermore, multidrug resistance continues to be a clinical problem for those patients infected with P. aeruginosa, and there are few new antibiotics being discovered to combat the problem. α-Tocopherol is a lipid-soluble antioxidant compound that can attenuate inflammation in a variety of pathologic conditions, including, but not limited to, asthma, allergic lung disease, sterile inflammation, and burns, by reducing levels of proinflammatory mediators such as IL-8, IL-6, TNF-α, and transforming growth factor-β1 (25, 26, 48). The results of this study indicate that α-tocopherol significantly attenuates the severity of P. aeruginosa–induced pneumonia in mice by inhibiting the insertion of T3SS exoenzymes into the cytosol of lung parenchymal cells, thus decreasing lung vascular permeability, RhoA and PAI-1 activation, and stress actin fiber formation.
The first objective of our study was to test whether α-tocopherol could prevent P. aeruginosa–mediated increases in lung endothelial and alveolar epithelial paracellular permeability. We have previously demonstrated that P. aeruginosa increases in vitro and in vivo lung vascular permeability and the formation of actin stress fibers via a mechanism that is toll-like receptor 4/RhoA/PAI-1 dependent (22, 23) and results in the activation of αv-β5 and -6 integrins, respectively (21). Furthermore, a previous study has also shown that P. aeruginosa increases PAI-1 gene expression in alveolar epithelial cells, in part via a platelet-activating factor–dependent mechanism (49). In the present study, pretreatment with α-tocopherol attenuated the increase in paracellular permeability in both cell types. We then determined some of the mechanisms by which α-tocopherol reduced P. aeruginosa–mediated increases in paracellular permeability. Pretreatment with α-tocopherol prevented both RhoA and PAI-1 activation in lung endothelial and epithelial cells. This result is of importance, because increased PAI-1 concentrations in BAL fluids are associated with increased mortality in a cohort of patients with P. aeruginosa pneumonia (50). Furthermore, PAI-1 concentrations in BAL fluid can distinguish ventilator-associated pneumonia from colonization with P. aeruginosa in mechanically ventilated pediatric patients (24).
Our data also indicate that α-tocopherol, but not γ-tocopherol or Trolox, attenuated P. aeruginosa–induced increase in lung endothelial permeability, suggesting that this effect of α-tocopherol may not simply be due to its antiinflammatory properties. α-Tocopherol, but not γ-tocopherol, can accumulate in the lipid raft domains of the cell membrane (51, 52) and may prevent the incorporation of lipids in these domains (43). Furthermore, the T3SS is a complex molecular machinery used by P. aeruginosa to inject exoenzymes directly into eukaryotic cells (53–55). Three molecular partners (two hydrophobic and one hydrophilic) work together to make the T3SS needle that is capable of inserting exoenzymes. The injection of T3SS exoenzymes of P. aeruginosa into host cells is localized to the lipid raft domains of the cell membrane (35). Thus, the second objective of our study was to determine whether α-tocopherol could prevent T3SS exoenzyme (ExoY) insertion by P. aeruginosa into the lipid raft domains of the cell membrane. We demonstrated by both confocal microscopy and Western blot that insertion of ExoY could be prevented in alveolar epithelial cells by pretreatment with α-tocopherol. Taken together, these data suggest that α-tocopherol may actually be able to prevent insertion of the P. aeruginosa T3SS needle and prevent exoenzyme injection that leads to improper cellular activation and disintegration of lung barrier function.
The third objective was to determine whether α-tocopherol would decrease the severity of lung injury and improve survival in a murine model of P. aeruginosa pneumonia. The results showed that pretreatment with α-tocopherol improved survival after pretreatment, but α-tocopherol also improved lung permeability, decreased bacterial load, and reduced accumulation of neutrophils and other cells in the lung, as shown by the lower level of MPO in lung homogenates and decreased cells of mice with P. aeruginosa pneumonia. Pretreatment of neutrophils with α-tocopherol did not improve phagocytosis. However, α-tocopherol blocked the expression of intercellular adhesion molecule-1, which regulates the recruitment of neutrophils into the lung through PKC-α, and γ-tocopherol had the opposite effect (28, 56, 57). A follow-up study from the same research group suggested that α-tocopherol decreases neutrophil migration into the alveolar space, thereby reducing formation of radical oxygen species and preventing collateral damage to the intact barrier, but increases elastase activity of neutrophils and their ability to kill bacteria (58). Finally, BAL protein was not decreased by pretreatment with α-tocopherol. This would normally go against evidence that cells and MPO were decreased regarding lung injury; however, previous studies have demonstrated that this increased protein may be a marker of increased net alveolar fluid transport (59, 60). Together with reduced permeability measurements (extravascular pulmonary edema), this suggests that α-tocopherol may prevent epithelial injury and/or increase its rate of epithelial repair as a mechanism to reduce bacterial load and facilitate survival after bacterial pneumonia.
Does α-tocopherol protect against bacterial pneumonia in humans? One study reported that α-tocopherol reduced the incidence of pneumonia in elderly males (61). Interestingly, a secondary analysis of the ATBC (Alpha-Tocopherol, Beta-Carotene Cancer Prevention) Study of Finnish male smokers aged 50 to 69 years showed that vitamin E supplementation decreased pneumonia risk by 69% among participants who had the least exposure to smoking and exercised regularly, whereas vitamin E supplementation increased the risk of pneumonia in patients with the highest exposure to smoking and no exercise (62). Furthermore, it is important to point out that some studies suggest an increased rate of hemorrhagic stroke, elevated blood pressure, and increased all-cause mortality if >400 IU/d of vitamin E are used (63–68). The variable effectiveness of α-tocopherol in humans may be attributed to differences in patient population and doses of α-tocopherol used in these studies. In addition, there are polymorphisms in the genes implicated in vitamin E metabolism that include apolipoprotein E, lipoprotein lipase, CD36 scavenger receptor, scavenger receptor class B, and α-tocopherol transfer protein that may influence the end-organ level and effete of vitamin E supplementation (69). Importantly, these results do not mean that α-tocopherol cannot be a useful adjunct to antibacterial therapy, but dosing must be judicious and patients must be selected appropriately. Furthermore, although post-treatment did not protect mice from pneumonia in our study, it has been well described that certain populations, such as those who have experienced trauma with hemorrhage and resuscitation or severe neurologic injury, have an increased risk of secondary bacterial pneumonia. These populations may be significant targets for adjunct α-tocopherol therapy to prevent secondary bacterial pneumonia after these injuries.
Finally, our results have another important implication. We showed that α-tocopherol prevented the ExoY insertion into alveolar cells. These results suggest that α-tocopherol would also inhibit the intracellular injection of all T3SS exoenzymes of P. aeruginosa. Our recent data indicate that a complex of cytotoxic and prion-like proteins containing tau and β-amyloid are released from lung endothelial and alveolar epithelial cells in an ExoY- or ExoU-dependent manner (70). These cytotoxic proteins are present in vivo in the BAL fluid and plasma of patients with P. aeruginosa pneumonia. Experimental data showed that these cytotoxic proteins cause both lung and brain injury in mice. Therefore, it is possible that α-tocopherol might inhibit the release of these cytotoxic proteins by preventing the intracellular insertion of T3SS exoenzymes; this question is currently the focus of future investigations in our laboratory.
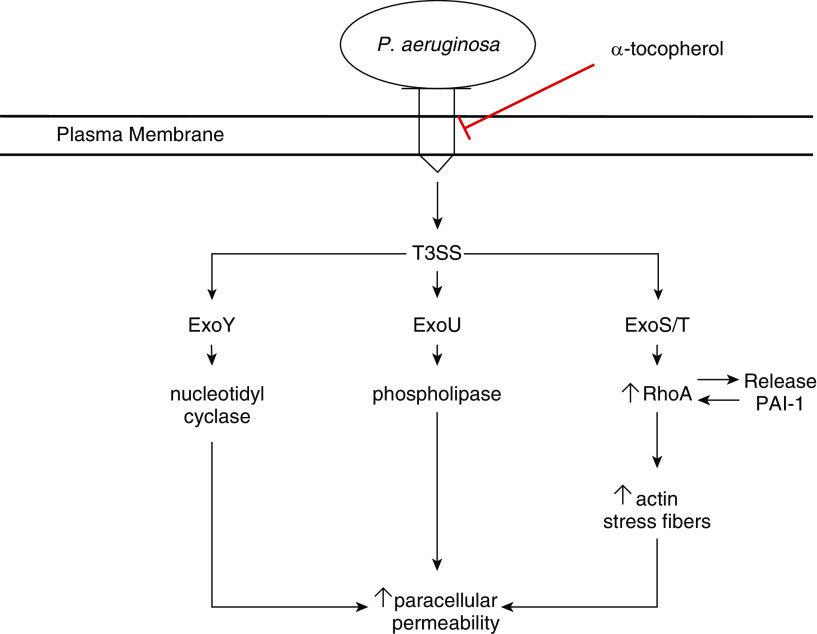
We have created a model for the findings of our present study (Figure 7). In this model, P. aeruginosa comes into contact with cells and can inject toxins (exoenzymes) of the T3SS into host cells. ExoS/T activates RhoA, leading to the release of PAI-1. In concert, these activators lead to increased formation of actin stress fibers. Injection of ExoU leads to increased phospholipase activity, and injection of ExoY leads to increases in cyclic nucleotides. All of the secondary consequences of exoenzyme injection can lead to increases in paracellular permeability and destruction of alveolar–capillary membrane integrity. Our model suggests that α-tocopherol prevents injection of T3SS exoenzymes by P. aeruginosa, thereby preventing the downstream effects of this bacterial toxin.
Figure 7.
Model of host cell intoxication by P. aeruginosa exoenzymes and α-tocopherol mechanism. Briefly, P. aeruginosa injects toxins (exoenzymes) into host cells. These exoenzymes activate pathways that lead to increased paracellular permeability. α-Tocopherol prevents increases in paracellular permeability by preventing injection of exoenzymes and activation of subsequent, deleterious pathways. T3SS = type III secretion system.
There are limitations to our study. First, we used labeled ExoY as a tool to demonstrate that α-tocopherol prevents injection of T3SS exoenzymes into the cytosol of alveolar epithelial cells. We are aware that, although PAK does express ExoY to intoxicate cells, there is no current evidence to suggest that host cell intoxication with ExoY and subsequent nucleotidyl cyclase formation activates the RhoA/PAI-1 pathway. This pathway has been classically activated by ExoS/T. However, previous studies have shown that the all T3SS exoenzymes are injected into the parenchymal cells by the same mechanism (reviewed in Reference 45). Second, in our in vivo studies, we demonstrate that there is decreased bacterial burden after infection with P. aeruginosa and pretreatment of mice with α-tocopherol. However, our data (not shown) also indicate that α-tocopherol has no direct antibacterial activity. Hence, another mechanism to decrease bacterial burden exists. As described above, α-tocopherol can activate certain neutrophil killing functions, although it decreases neutrophil influx into the alveolar airspace.
In summary, we report a novel mechanism by which α-tocopherol inhibits P. aeruginosa–mediated lung permeability, RhoA and PAI-1 activation, stress fiber formation, and insertion of the T3SS exoenzyme into alveolar epithelial cells. Given that P. aeruginosa typically infects critically ill and immunocompromised patients, administration of α-tocopherol could be a novel therapy for patients suffering from trauma, burns, cystic fibrosis, and other pathologies that leave a patient temporarily or permanently immunocompromised. Clinical trials should be considered in these patient populations to test whether α-tocopherol could attenuate the severity of lung infection with this lethal pathogen that is becoming resistant to extended-spectrum antibiotics, which can cause undesired complications of long-term antibiotic therapy.
Acknowledgments
Acknowledgment
The wild-type PAK strain of P. aeruginosa was a kind gift from Stephen Lory.
Footnotes
Supported by U.S. National Institutes of Health grant RO1 GM086416 (J.-F.P.) and a Mentored Research Training Grant from the Foundation for Anesthesia Education and Research (B.M.W.).
Author Contributions: Conceptualization: B.M.W., R.L.S., and J.-F.P. Methodology: B.M.W. and J.-F.P. Experimentation: B.M.W., N.A., C.E., A.B., J.H., and M.G.T. Data analysis: B.M.W., N.A., M.G.T., T.S., and J.-F.P. Resources: B.M.W., J.C., M.G.T., and J.-F.P. Writing (original draft preparation): B.M.W., N.A., and J.-F.P. Writing (review and editing): B.M.W., N.A., C.E., A.B., J.H., J.C., M.G.T., R.L.S., T.S., and J.-F.P.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0185OC on April 3, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rello J, Rué M, Jubert P, Muses G, Soñora R, Vallés J, et al. Survival in patients with nosocomial pneumonia: impact of the severity of illness and the etiologic agent. Crit Care Med. 1997;25:1862–1867. doi: 10.1097/00003246-199711000-00026. [DOI] [PubMed] [Google Scholar]
- 2.Rello J, Allegri C, Rodriguez A, Vidaur L, Sirgo G, Gomez F, et al. Risk factors for ventilator-associated pneumonia by Pseudomonas aeruginosa in presence of recent antibiotic exposure. Anesthesiology. 2006;105:709–714. doi: 10.1097/00000542-200610000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Barbier F, Andremont A, Wolff M, Bouadma L. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med. 2013;19:216–228. doi: 10.1097/MCP.0b013e32835f27be. [DOI] [PubMed] [Google Scholar]
- 4.Giske CG, Monnet DL, Cars O, Carmeli Y ReAct-Action on Antibiotic Resistance. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother. 2008;52:813–821. doi: 10.1128/AAC.01169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta SK, Berk RS, Masinick S, Hazlett LD. Pili and lipopolysaccharide of Pseudomonas aeruginosa bind to the glycolipid asialo GM1. Infect Immun. 1994;62:4572–4579. doi: 10.1128/iai.62.10.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee VT, Smith RS, Tümmler B, Lory S. Activities of Pseudomonas aeruginosa effectors secreted by the Type III secretion system in vitro and during infection. Infect Immun. 2005;73:1695–1705. doi: 10.1128/IAI.73.3.1695-1705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kipnis E, Sawa T, Wiener-Kronish J. Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Med Mal Infect. 2006;36:78–91. doi: 10.1016/j.medmal.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Kallen AJ, Srinivasan A. Current epidemiology of multidrug-resistant gram-negative bacilli in the United States. Infect Control Hosp Epidemiol. 2010;31:S51–S54. doi: 10.1086/655996. [DOI] [PubMed] [Google Scholar]
- 9.Pendleton JN, Gorman SP, Gilmore BF. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther. 2013;11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 10.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, et al. Infectious Diseases Society of America. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 11.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 12.Hauser AR, Cobb E, Bodi M, Mariscal D, Vallés J, Engel JN, et al. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med. 2002;30:521–528. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, et al. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis. 2001;183:1767–1774. doi: 10.1086/320737. [DOI] [PubMed] [Google Scholar]
- 14.Ochoa CD, Alexeyev M, Pastukh V, Balczon R, Stevens T. Pseudomonas aeruginosa exotoxin Y is a promiscuous cyclase that increases endothelial tau phosphorylation and permeability. J Biol Chem. 2012;287:25407–25418. doi: 10.1074/jbc.M111.301440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrow KA, Seifert R, Kaever V, Britain AL, Sayner SL, Ochoa CD, et al. Heterogeneity of pulmonary endothelial cyclic nucleotide response to Pseudomonas aeruginosa ExoY infection. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1199–L1207. doi: 10.1152/ajplung.00165.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kloth C, Schirmer B, Munder A, Stelzer T, Rothschuh J, Seifert R. The role of Pseudomonas aeruginosa ExoY in an acute mouse lung infection model. Toxins (Basel) 2018;10:E185. doi: 10.3390/toxins10050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juan C, Peña C, Oliver A. Host and pathogen biomarkers for severe Pseudomonas aeruginosa infections. J Infect Dis. 2017;215:S44–S51. doi: 10.1093/infdis/jiw299. [DOI] [PubMed] [Google Scholar]
- 18.Goehring UM, Schmidt G, Pederson KJ, Aktories K, Barbieri JT. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J Biol Chem. 1999;274:36369–36372. doi: 10.1074/jbc.274.51.36369. [DOI] [PubMed] [Google Scholar]
- 19.Kazmierczak BI, Engel JN. Pseudomonas aeruginosa ExoT acts in vivo as a GTPase-activating protein for RhoA, Rac1, and Cdc42. Infect Immun. 2002;70:2198–2205. doi: 10.1128/IAI.70.4.2198-2205.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riese MJ, Goehring UM, Ehrmantraut ME, Moss J, Barbieri JT, Aktories K, et al. Auto-ADP-ribosylation of Pseudomonas aeruginosa ExoS. J Biol Chem. 2002;277:12082–12088. doi: 10.1074/jbc.M109039200. [DOI] [PubMed] [Google Scholar]
- 21.Ganter MT, Roux J, Su G, Lynch SV, Deutschman CS, Weiss YG, et al. Role of small GTPases and alphavbeta5 integrin in Pseudomonas aeruginosa-induced increase in lung endothelial permeability. Am J Respir Cell Mol Biol. 2009;40:108–118. doi: 10.1165/rcmb.2007-0454OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carles M, Lafargue M, Goolaerts A, Roux J, Song Y, Howard M, et al. Critical role of the small GTPase RhoA in the development of pulmonary edema induced by Pseudomonas aeruginosa in mice. Anesthesiology. 2010;113:1134–1143. doi: 10.1097/ALN.0b013e3181f4171b. [DOI] [PubMed] [Google Scholar]
- 23.Goolaerts A, Lafargue M, Song Y, Miyazawa B, Arjomandi M, Carlès M, et al. PAI-1 is an essential component of the pulmonary host response during Pseudomonas aeruginosa pneumonia in mice. Thorax. 2011;66:788–796. doi: 10.1136/thx.2010.155788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivasan R, Song Y, Wiener-Kronish J, Flori HR. Plasminogen activation inhibitor concentrations in bronchoalveolar lavage fluid distinguishes ventilator-associated pneumonia from colonization in mechanically ventilated pediatric patients. Pediatr Crit Care Med. 2011;12:21–27. doi: 10.1097/PCC.0b013e3181e2a352. [DOI] [PubMed] [Google Scholar]
- 25.Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Vitamin E isoforms as modulators of lung inflammation. Nutrients. 2013;5:4347–4363. doi: 10.3390/nu5114347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook-Mills JM, Abdala-Valencia H, Hartert T. Two faces of vitamin E in the lung. Am J Respir Crit Care Med. 2013;188:279–284. doi: 10.1164/rccm.201303-0503ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCary CA, Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Supplemental and highly elevated tocopherol doses differentially regulate allergic inflammation: reversibility of α-tocopherol and γ-tocopherol’s effects. J Immunol. 2011;186:3674–3685. doi: 10.4049/jimmunol.1003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, et al. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol. 2009;182:4395–4405. doi: 10.4049/jimmunol.0803659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troisi RJ, Willett WC, Weiss ST, Trichopoulos D, Rosner B, Speizer FE. A prospective study of diet and adult-onset asthma. Am J Respir Crit Care Med. 1995;151:1401–1408. doi: 10.1164/ajrccm.151.5.7735592. [DOI] [PubMed] [Google Scholar]
- 30.Kalayci O, Besler T, Kilinç K, Sekerel BE, Saraçlar Y. Serum levels of antioxidant vitamins (alpha tocopherol, beta carotene, and ascorbic acid) in children with bronchial asthma. Turk J Pediatr. 2000;42:17–21. [PubMed] [Google Scholar]
- 31.Schünemann HJ, Grant BJ, Freudenheim JL, Muti P, Browne RW, Drake JA, et al. The relation of serum levels of antioxidant vitamins C and E, retinol and carotenoids with pulmonary function in the general population. Am J Respir Crit Care Med. 2001;163:1246–1255. doi: 10.1164/ajrccm.163.5.2007135. [DOI] [PubMed] [Google Scholar]
- 32.Jordan JM, De Roos AJ, Renner JB, Luta G, Cohen A, Craft N, et al. A case-control study of serum tocopherol levels and the alpha- to gamma-tocopherol ratio in radiographic knee osteoarthritis: the Johnston County Osteoarthritis Project. Am J Epidemiol. 2004;159:968–977. doi: 10.1093/aje/kwh133. [DOI] [PubMed] [Google Scholar]
- 33.Rocksén D, Ekstrand-Hammarström B, Johansson L, Bucht A. Vitamin E reduces transendothelial migration of neutrophils and prevents lung injury in endotoxin-induced airway inflammation. Am J Respir Cell Mol Biol. 2003;28:199–207. doi: 10.1165/rcmb.4899. [DOI] [PubMed] [Google Scholar]
- 34.Wigenstam E, Rocksén D, Ekstrand-Hammarström B, Bucht A. Treatment with dexamethasone or liposome-encapsuled vitamin E provides beneficial effects after chemical-induced lung injury. Inhal Toxicol. 2009;21:958–964. doi: 10.1080/08958370802596298. [DOI] [PubMed] [Google Scholar]
- 35.Verove J, Bernarde C, Bohn YS, Boulay F, Rabiet MJ, Attree I, et al. Injection of Pseudomonas aeruginosa Exo toxins into host cells can be modulated by host factors at the level of translocon assembly and/or activity. PLoS One. 2012;7:e30488. doi: 10.1371/journal.pone.0030488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jian MY, Alexeyev MF, Wolkowicz PE, Zmijewski JW, Creighton JR. Metformin-stimulated AMPK-α1 promotes microvascular repair in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2013;305:L844–L855. doi: 10.1152/ajplung.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carles M, Wagener BM, Lafargue M, Roux J, Iles K, Liu D, et al. Heat-shock response increases lung injury caused by Pseudomonas aeruginosa via an interleukin-10-dependent mechanism in mice. Anesthesiology. 2014;120:1450–1462. doi: 10.1097/ALN.0000000000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagener BM, Roux J, Carles M, Pittet JF. Synergistic inhibition of β2-adrenergic receptor-mediated alveolar epithelial fluid transport by interleukin-8 and transforming growth Factor-β. Anesthesiology. 2015;122:1084–1092. doi: 10.1097/ALN.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 39.Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res. 1996;37:893–901. [PubMed] [Google Scholar]
- 40.Zhou T, Yu Z, Jian MY, Ahmad I, Trempus C, Wagener BM, et al. Instillation of hyaluronan reverses acid instillation injury to the mammalian blood gas barrier. Am J Physiol Lung Cell Mol Physiol. 2018;314:L808–L821. doi: 10.1152/ajplung.00510.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lafargue M, Xu L, Carlès M, Serve E, Anjum N, Iles KE, et al. Stroke-induced activation of the α7 nicotinic receptor increases Pseudomonas aeruginosa lung injury. FASEB J. 2012;26:2919–2929. doi: 10.1096/fj.11-197384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Che P, Wagener BM, Zhao X, Brandon AP, Evans CA, Cai GQ, et al. Neuronal Wiskott-Aldrich syndrome protein regulates Pseudomonas aeruginosa-induced lung vascular permeability through the modulation of actin cytoskeletal dynamics. FASEB J. 2020;34:3305–3317. doi: 10.1096/fj.201902915R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Royer MC, Lemaire-Ewing S, Desrumaux C, Monier S, Pais de Barros JP, Athias A, et al. 7-ketocholesterol incorporation into sphingolipid/cholesterol-enriched (lipid raft) domains is impaired by vitamin E: a specific role for alpha-tocopherol with consequences on cell death. J Biol Chem. 2009;284:15826–15834. doi: 10.1074/jbc.M808641200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevens TC, Ochoa CD, Morrow KA, Robson MJ, Prasain N, Zhou C, et al. The Pseudomonas aeruginosa exoenzyme Y impairs endothelial cell proliferation and vascular repair following lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;306:L915–L924. doi: 10.1152/ajplung.00135.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrow KA, Frank DW, Balczon R, Stevens T. The Pseudomonas aeruginosa exoenzyme Y: a promiscuous nucleotidyl cyclase edema factor and virulence determinant. Handb Exp Pharmacol. 2017;238:67–85. doi: 10.1007/164_2016_5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traber MG, Siddens LK, Leonard SW, Schock B, Gohil K, Krueger SK, et al. Alpha-tocopherol modulates Cyp3a expression, increases gamma-CEHC production, and limits tissue gamma-tocopherol accumulation in mice fed high gamma-tocopherol diets. Free Radic Biol Med. 2005;38:773–785. doi: 10.1016/j.freeradbiomed.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 47.Singh U, Jialal I. Anti-inflammatory effects of alpha-tocopherol. Ann N Y Acad Sci. 2004;1031:195–203. doi: 10.1196/annals.1331.019. [DOI] [PubMed] [Google Scholar]
- 48.Machado GB, de Oliveira AV, Saliba AM, de Lima CD, Suassuna JH, Plotkowski MC. Pseudomonas aeruginosa toxin ExoU induces a PAF-dependent impairment of alveolar fibrin turnover secondary to enhanced activation of coagulation and increased expression of plasminogen activator inhibitor-1 in the course of mice pneumosepsis. Respir Res. 2011;12:104. doi: 10.1186/1465-9921-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song Y, Lynch SV, Flanagan J, Zhuo H, Tom W, Dotson RH, et al. Increased plasminogen activator inhibitor-1 concentrations in bronchoalveolar lavage fluids are associated with increased mortality in a cohort of patients with Pseudomonas aeruginosa. Anesthesiology. 2007;106:252–261. doi: 10.1097/00000542-200702000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Lemaire-Ewing S, Desrumaux C, Néel D, Lagrost L. Vitamin E transport, membrane incorporation and cell metabolism: is alpha-tocopherol in lipid rafts an oar in the lifeboat? Mol Nutr Food Res. 2010;54:631–640. doi: 10.1002/mnfr.200900445. [DOI] [PubMed] [Google Scholar]
- 51.Atkinson J, Harroun T, Wassall SR, Stillwell W, Katsaras J. The location and behavior of alpha-tocopherol in membranes. Mol Nutr Food Res. 2010;54:641–651. doi: 10.1002/mnfr.200900439. [DOI] [PubMed] [Google Scholar]
- 52.Matteï PJ, Faudry E, Job V, Izoré T, Attree I, Dessen A. Membrane targeting and pore formation by the type III secretion system translocon. FEBS J. 2011;278:414–426. doi: 10.1111/j.1742-4658.2010.07974.x. [DOI] [PubMed] [Google Scholar]
- 53.Burkinshaw BJ, Strynadka NC. Assembly and structure of the T3SS. Biochim Biophys Acta. 2014;1843:1649–1663. doi: 10.1016/j.bbamcr.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 54.Büttner D. Protein export according to schedule: architecture, assembly, and regulation of type III secretion systems from plant- and animal-pathogenic bacteria. Microbiol Mol Biol Rev. 2012;76:262–310. doi: 10.1128/MMBR.05017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Mechanisms for vascular cell adhesion molecule-1 activation of ERK1/2 during leukocyte transendothelial migration. PLoS One. 2011;6:e26706. doi: 10.1371/journal.pone.0026706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCary CA, Yoon Y, Panagabko C, Cho W, Atkinson J, Cook-Mills JM. Vitamin E isoforms directly bind PKCα and differentially regulate activation of PKCα. Biochem J. 2012;441:189–198. doi: 10.1042/BJ20111318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bou Ghanem EN, Lee JN, Joma BH, Meydani SN, Leong JM, Panda A. The alpha-tocopherol form of vitamin E boosts elastase activity of human PMNs and their ability to kill Streptococcus pneumoniae. Front Cell Infect Microbiol. 2017;7:161. doi: 10.3389/fcimb.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matthay MA. Resolution of pulmonary edema. Thirty years of progress. Am J Respir Crit Care Med. 2014;189:1301–1308. doi: 10.1164/rccm.201403-0535OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hendrickson CM, Abbott J, Zhuo H, Liu KD, Calfee CS, Matthay MA NHLBI ARDS Network. Higher mini-BAL total protein concentration in early ARDS predicts faster resolution of lung injury measured by more ventilator-free days. Am J Physiol Lung Cell Mol Physiol. 2017;312:L579–L585. doi: 10.1152/ajplung.00381.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hemilä H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin Interv Aging. 2016;11:1379–1385. doi: 10.2147/CIA.S114515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hemilä H. Vitamin E and the risk of pneumonia: using the I 2 statistic to quantify heterogeneity within a controlled trial. Br J Nutr. 2016;116:1530–1536. doi: 10.1017/S0007114516003408. [DOI] [PubMed] [Google Scholar]
- 62.Saremi A, Arora R. Vitamin E and cardiovascular disease. Am J Ther. 2010;17:e56–e65. doi: 10.1097/MJT.0b013e31819cdc9a. [DOI] [PubMed] [Google Scholar]
- 63.Leppälä JM, Virtamo J, Fogelholm R, Huttunen JK, Albanes D, Taylor PR, et al. Controlled trial of alpha-tocopherol and beta-carotene supplements on stroke incidence and mortality in male smokers. Arterioscler Thromb Vasc Biol. 2000;20:230–235. doi: 10.1161/01.atv.20.1.230. [DOI] [PubMed] [Google Scholar]
- 64.Miller ER, III, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 65.Paunio M, Heinonen OP, Virtamo J, Klag MJ, Manninen V, Albanes D, et al. HDL cholesterol and mortality in Finnish men with special reference to alcohol intake. Circulation. 1994;90:2909–2918. doi: 10.1161/01.cir.90.6.2909. [DOI] [PubMed] [Google Scholar]
- 66.Törnwall ME, Virtamo J, Korhonen PA, Virtanen MJ, Albanes D, Huttunen JK. Postintervention effect of alpha tocopherol and beta carotene on different strokes: a 6-year follow-up of the Alpha Tocopherol, Beta Carotene Cancer Prevention Study. Stroke. 2004;35:1908–1913. doi: 10.1161/01.STR.0000131750.60270.42. [DOI] [PubMed] [Google Scholar]
- 67.Azzi A, Brigelius-Flohé R, Kelly F, Lodge JK, Ozer N, Packer L, et al. On the opinion of the European Commission “Scientific Committee on Food” regarding the tolerable upper intake level of vitamin E (2003) Eur J Nutr. 2005;44:60–62. doi: 10.1007/s00394-005-0549-8. [DOI] [PubMed] [Google Scholar]
- 68.Mocchegiani E, Costarelli L, Giacconi R, Malavolta M, Basso A, Piacenza F, et al. Vitamin E-gene interactions in aging and inflammatory age-related diseases: implications for treatment. A systematic review. Ageing Res Rev. 2014;14:81–101. doi: 10.1016/j.arr.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 69.Morrow KA, Ochoa CD, Balczon R, Zhou C, Cauthen L, Alexeyev M, et al. Pseudomonas aeruginosa exoenzymes U and Y induce a transmissible endothelial proteinopathy. Am J Physiol Lung Cell Mol Physiol. 2016;310:L337–L353. doi: 10.1152/ajplung.00103.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sawa T, Shimizu M, Moriyama K, Wiener-Kronish JP. Association between Pseudomonas aeruginosa type III secretion, antibiotic resistance, and clinical outcome: a review. Crit Care. 2014;18:668. doi: 10.1186/s13054-014-0668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]