Abstract
The incidence and prevalence of nontuberculous mycobacteria (NTM) lung disease is rising worldwide and accounts for most clinical cases of NTM disease. NTM infections occur in both immunocompetent and immunocompromised hosts. Macrophages are the primary host cells that initiate an immune response to NTM. Defining the molecular events that govern the control of infection within macrophages is fundamental to understanding the pathogenesis of NTM disease. Here, we review key macrophage host signaling pathways that contribute to the host immune response to pulmonary NTM infections. In this review, we focus primarily on NTM that are known to cause lung disease, including Mycobacterium avium intracellulare, M. abscessus, and M. kansasii.
Keywords: macrophage, Mycobacterium avium, Mycobacterium abscessus, autophagy, inflammasome
The term “nontuberculous mycobacteria (NTM)” refers to mycobacteria other than Mycobacterium tuberculosis (MTB) and M. leprae. NTM are gram-positive, acid-fast, aerobic bacilli that are ubiquitous in the environment and can be normal inhabitants of natural and drinking water systems, pools, hot tubs, bird droppings, dust, milk, laboratory equipment, and soil (1, 2). NTM is acquired from the environment and is not transmitted from person to person, with few exceptions such as in immunocompromised patients or patients with cystic fibrosis (CF) (3). The incidence and prevalence of NTM lung disease (NTM-LD) are rising worldwide, particularly in Western countries, and account for most clinical cases of NTM infection (see Table 1 for representative data [4–8]). In particular, the number of cases of NTM-LD has doubled in both the United States and Canada over a 10-year period (4, 6). Generally, areas that are considered high-risk for NTM-LD tend to be large, densely populated, urban areas (9, 10). Although NTM infection can be common and can affect almost any organ system, pulmonary infection is more prevalent than disseminated disease, which is usually seen only in immunocompromised patients (11, 12).
Table 1.
Annual Prevalence of Nontuberculous Mycobacterial Lung Disease in Select Western Countries
| Study | Country | Database | Annual Prevalence at Study Start | Annual Prevalence at Study End |
|---|---|---|---|---|
| Adjemian et al. (4) | United States | Medicare Part B beneficiaries | 1997: 20 per 100,000 | 2007: 47 per 100,000 |
| Winthrop et al. (5) | United States | National managed care claims database (Optum) | 2008: 6.78 per 100,000 | 2015: 11.7 per 100,000 |
| Age ≥ 65: 30.27 per 100,000 | Age ≥ 65: 47.48 per 100,000 | |||
| Ringshausen et al. (7) | Germany | Health Risk Institute health services database of statutory health insurance | 2009: 2.3 per 100,000 | 2014: 3.3 per 100,000 |
| Brode et al. (6) | Canada (Ontario) | Public Health Ontario Laboratory culture results | 1998: 4.65 per 100,000 | 2010: 9.07 per 100,000 |
| Santin et al. (8) | Spain (Catalonia) | Bellvitge University Hospital mycobacteria laboratory | 1994: 2.2 per 100,000 | 2014: 2 per 100,000 |
There are several subspecies of NTM that are broadly divided into rapid and slow growers. The rapid growers include Mycobacterium abscessus (MAB), M. chelonae, M. fortuitum, and M. smegmatis, whereas slow growers include M. avium complex (MAC), M. kansasii, M. xenopi, and M. marinum, among others. The most virulent strains of NTM include MAC (comprised of MAI [M. avium and M. intracellulare] and M. chimaera), MAB, and M. kansasii. Not surprisingly, the more virulent strains are also the most common strains that contribute to opportunistic NTM infections in individuals with acquired immunodeficiency syndrome (13).
Clinically, pulmonary NTM infections present as one of three phenotypes: nodular bronchiectasis, cavitary type, and hypersensitivity pneumonitis (14). Risk factors for developing NTM pulmonary disease include genetic abnormalities that increase susceptibility, immune dysfunction (whether primary immunodeficiency or iatrogenic), and disease due to ciliary abnormalities (whether structural or functional) (15). In particular, ciliary defects in patients with CF or primary ciliary dyskinesia have been studied extensively. Abnormal ciliary beat frequency can be affected by nitric oxide (NO) production and bacterial or viral TLR (Toll-like receptor) agonists, indicating that NTM infection may further worsen these patients’ ability to clear bacteria (16). Immunocompetent individuals with comorbid pulmonary diseases, such as chronic obstructive pulmonary disease and non-CF bronchiectasis, can also be susceptible to pulmonary NTM infections (17). NTM-LD in immunocompetent, older white women is of particular interest because little is known about the reasons for their increased susceptibility, other than risk factors such as a tall, lean body habitus; thoracic skeletal abnormalities; and use of immunomodulatory medications (18).
Disseminated NTM infections are usually seen in immunocompromised patients, such as patients with human immunodeficiency virus, CF, or malignancies; transplant recipients; and patients treated with biologics, such as TNF-α (tumor necrosis factor-α) blockers (19, 20). Macrophages that have phagocytosed mycobacteria release IL-12 and IL-18 in response to bacterial virulence factors, which stimulate the production of IFN-γ from T cells and natural killer cells. IFN-γ then activates TNF-α production, as well as production of other cytokines and chemokines (21). A variety of genetic defects in the IFN-γ/IL-12 pathway have been described in patients with NTM infections (22). In particular, IL12B, IL12RB1, and ISG15 result in decreased IFN-γ production (23), whereas IFNGR1, IFNGR2, STAT1, and IRF8 IFN regulatory factors lead to an inadequate response to the cytokine (24). In addition, GATA2 deficiency and anti–IFN-γ autoantibodies have been associated with disseminated NTM infections (22). Recently, the use of biologics, particularly anti-TNF therapies, has been associated with increased mycobacterial susceptibility, and in one study of patients who received anti–TNF-α therapy, nearly half of the patients exhibited extrapulmonary or disseminated disease (25).
The epidemiology and clinical presentation of NTM-LD, as well as risk factors and treatment options for this disease, have been previously reviewed (12, 20). Less is known about the host factors, and particularly the molecular mechanisms, that contribute to the pathogenesis of NTM. NTM are intracellular pathogens, and macrophages are of central importance in the innate immune response. Macrophages express pattern-recognition receptors (PRRs), generate toxic nitrogen and oxygen radicals, and induce autophagy and apoptosis to eliminate pathogens, among other mechanisms of innate immunity. Here, we review the macrophage molecular mechanisms that contribute to the host response to pulmonary NTM infection.
Role of Macrophages in the NTM Immune Response
Innate immunity involves a coordinated effort by multiple cell types, including respiratory tract epithelium and resident and recruited phagocytic cells, and plays a key role in the activation of the host response to mycobacterial infection (26). Alveolar macrophages provide the first line of defense against organisms that reach the lower airways. The macrophage host response to mycobacteria, particularly MTB, was reviewed extensively by Awuh and Flo (27); however, the host response to NTM is less well characterized (28).
The process begins with the engagement of PRRs, such as Toll-like receptors (TLRs) and C-type lectins, at the plasma membrane by bacterial products, known as pathogen-associated molecular patterns (PAMPs). PAMPs bind to these cell-surface receptors and intracellular receptors such as NLRs (nucleotide-binding oligomerization domain [NOD]-like receptors). PAMP binding initiates inflammatory signaling, followed by phagocytosis. Phagocytosis of mycobacteria leads to the development of phagosomes that mature and eventually merge with lysosomes to create phagolysosomes that are responsible for degradation of microbes (29). Activated macrophages synthesize protein and lipid mediators, and generate toxic nitrogen and oxygen radicals through activation of NOX (nicotinamide adenine dinucleotide phosphate oxidase) and iNOS (inducible NO synthase) signaling, which contributes to clearance of ingested pathogens. However, mycobacteria are capable of evading host antimicrobial processes and persisting within the macrophages through mechanisms involving arrest of phagosomal maturation and lysosomal delivery, or by perturbing host cellular trafficking (27).
Maturation of phagosomes into phagolysosomes involves acidification accomplished by proton pumps, oxidative stress, and activation of degradative enzymes, such as hydrolases and cathepsins, after lysosomal fusion. Evolved mycobacteria can disrupt acidification to make the phagosomal environment more favorable to the microbe. They have also developed mechanisms to maintain a tight apposition between mycobacterial tsurface lipids and the phagosome membrane to prevent lysosomal fusion (30). Mycobacterial type VII secretion systems (also known as ESX [ESAT6 secretion] systems) allow for the secretion of mycobacterial virulence factors or even phagosomal escape into the cytosol of macrophages, where the Mycobacterium can take control of host cell signaling and replicate within the cell, resulting in host cell death (27). These mechanisms are reviewed in further detail below.
Cytosolic NLRs play an important role in forming the inflammasome, which in turn activates caspases that cleave pro versions of the inflammatory cytokines IL-1β and IL-18 into active forms. The detection of microbial PAMPs by NOD proteins results in activation of NF-κB and MAP (mitogen-activated protein) kinases to induce proinflammatory cytokine production, and also leads to activation of autophagy. Mycobacteria have developed mechanisms to block inflammasome activation by either decreasing triggers of inflammasome activation or inhibiting inflammasome assembly, allowing the survival of intracellular pathogens (27). Understanding these host–pathogen interactions is critical for developing host-directed therapies that can enhance the clearance of bacteria.
Lastly, macrophages play a role in granuloma formation that is best described in MTB and M. marinum models of MTB. Specifically, granulomas are collections of infected and uninfected phagocytic cells and T-lymphocytes that begin with epithelioid transformation of infected macrophages (31, 32). The earlier prevailing view of granulomas as host-protective mechanisms has been challenged in recent years, and it is now believed that, at least in the initial stages, granulomas can be a means by which MTB replicate and disseminate (31). The epithelization of granulomas involving E-cadherin expression, among other epithelial cell junction proteins, has been reviewed as well (32). Less is known about granuloma formation in NTM and whether its pathogenesis is like that of MTB. Regev and colleagues showed that HO-1 (heme oxygenase-1) plays an important role in regulating granuloma formation and preventing disseminated MAI infection in mice (33). The same group later showed that HO-1 levels were attenuated in older mice and in peripheral blood monocytes from elderly patients, leading to enhanced cell death by cell necrosis rather than programmed apoptosis and subsequent proliferation of MAI infection (34). Mechanistically, a deficient HO-1 response resulted in upregulation of SOCS3 and inhibition of Bcl2, leading to programmed cell death of macrophages. These studies suggest that HO-1 is a cytoprotective enzyme that regulates several important immune processes, including host responses to mycobacterial infection and granuloma formation. Bernut and colleagues showed that granuloma formation in zebrafish embryos by either R or S variants of MAB was mediated by TNF-α expression and IL-8–dependent neutrophil mobilization (35). Furthermore, defects in the TNF receptor resulted in fewer and morphologically altered granulomas. Separately, mmpL8 (mycobacterial membrane protein L8) was found to play a role in the virulence of MAB, and the presence of functional mmpL8 was associated with granuloma formation as well (36). Because granuloma formation is a part of the macrophage inflammatory response upon infection with NTM, Matsumura and colleagues explored the role of the antioxidant tannin (derived from persimmons) and found that it had a bacteriostatic effect on MAC and reduced granuloma formation (37). Taken together, these studies appear to indicate that granuloma formation is associated with a proinflammatory state that enhances the pathogenesis of NTM by allowing it to further propagate. However, more definitive studies are needed to determine how granuloma formation can mechanistically be altered to fight NTM infection.
Role of PRRs in the NTM Response
The activation of PRRs triggers macrophage antimicrobial responses, including cytokines, chemokines, and reactive oxygen species (ROS), that can lead to destruction of microbial membranes, DNA, and important microbial residues, such as thiol and tyrosine residues. Different mycobacterial ligands are recognized by distinct PRRs present in the various cellular compartments (38, 39). TLR1, TLR2, and TLR6 are expressed on the plasma membrane and recognize mycobacterial lipoproteins, proteins, and glycolipids, whereas TLR3, TLR7, and TLR8, which are present intracellularly, traffic from the endoplasmic reticulum to endolysosomal compartments where they detect mycobacterial nucleic acids (40). TLR9 recognizes mycobacterial DNA. When engaged, TLR/ligand complexes recruit adaptors such as TIRAP (Toll/IL-1 receptor [TIR] domain-containing adaptor protein) to signal via molecules such as MyD88 (myeloid differentiation primary response gene 88) and TRIF (TIR-domain-containing adapter-inducing IFN-β), leading to activation of transcription factors such as NF-κB and IFN regulatory factors, with subsequent production of inflammatory mediators, type I IFNs, and antimicrobial programs (41). By altering the balance of pro- and antiinflammatory cytokines, intracellular bacteria attempt to improve their chances of survival.
Fremond and colleagues found that MyD88−/− mice were unable to control MTB infection (42), and similarly, Feng and colleagues showed that mice deficient in MyD88 were more susceptible to infections with MAI (43). Interestingly, the profound defect in IFN signaling seen in MyD88−/− mice was not evident in TLR2−/− or TLR4−/− mice, suggesting that MyD88 is necessary for host inflammatory and IFN-γ responses to mycobacteria. Alternatively, the marked susceptibility to MAI infection of MyD88−/− relative to TLR2−/− or TLR4−/− mice may reflect the requirement for multiple TLRs in the host response to mycobacterial infection. Shin and colleagues investigated the role of TLR2- and TLR4-induced cytokine signaling in MAB infection. Using TLR2-deficient mice, they showed that MAB activated ERK1/2 (extracellular signal-regulated kinase 1/2) and p38 MAP kinases, and secretion of TNF-α, IL-6, and IL-12p40 in murine macrophages via TLR2 (44). In addition, the physical binding and colocalization of dectin-1 with TLR2 were shown to be required for efficient phagocytosis of MAB and regulation of the innate immune response. Roux and colleagues examined the roles of TLR2 and TLR4 in cytokine production in the smooth (S) and rough (R; more virulent due to loss of surface glycopeptidolipids) variants of MAB (45). The R forms induced higher levels of TNF-α in wild-type and TLR4 knockout macrophages in an NF-κB–dependent manner, but not in TLR2 knockout macrophages, suggesting that the R morphotype modifies the innate immune response via TLR2 signaling (45).
Role of ROS in the NTM Response
PRR activation also induces the production of ROS via NOXs, particularly NOX2, as protection against infection (46). However, excessive oxidative signaling can damage host cells, and mechanisms such as Keap1 (Kelch-like ECH-associated protein 1) serve to limit the oxidative damage. Awuh and colleagues showed that Keap1 downregulated the inflammatory response necessary for controlling MAI infection, thereby allowing intracellular growth of MAI within macrophages (47). Thus, although antioxidant defenses like Keap1 are important for avoiding overwhelming inflammation, they provide another opportunity for microbes to develop resistance mechanisms.
ROS production also plays a crucial role in inducing apoptosis of host cells by NTM infection. Lee and colleagues showed that the MAI protein MAV2052 induced apoptosis via TLR4 binding and subsequent production of ROS and loss of mitochondrial transmembrane potential (48). This eventually led to activation of MAP kinases and the ASK1 (apoptosis signal-regulating kinase 1)/JNK (c-Jun N-terminal kinase) pathway, resulting in caspase-dependent apoptosis. Similarly, Whang and colleagues showed that ROS production contributed to loss of mitochondrial transmembrane potential in MAB, with subsequent induction of apoptosis of infected macrophages (49). Interestingly, the presence of glycopeptidolipids (GPLs) on the MAB cell surface (i.e., the smooth S phenotype) inhibited ROS-mediated apoptosis.
Similarly, the generation of reactive nitrogen intermediates (RNIs) via IFN-γ–mediated stimulation of iNOS (and in human macrophages, endothelial NOS) plays an immunoregulatory role in activation and inhibition of kinases, caspases, transcription factors, and cytokines (50). Interestingly, in one study, RNI generation in MTB was shown to have enhanced antimicrobial activity, whereas a lack of iNOS in MAC led to more resistant disease (28). However, in another study, even in MTB, maximal levels of RNI production were insufficient to restrict intracellular growth, and increased nitrate levels were favorable for mycobacterial survival (51). Thus, the mechanisms by which RNIs affect NTM survival remain elusive and are a crucial area of investigation.
Role of Autophagy in the Host Response to NTM
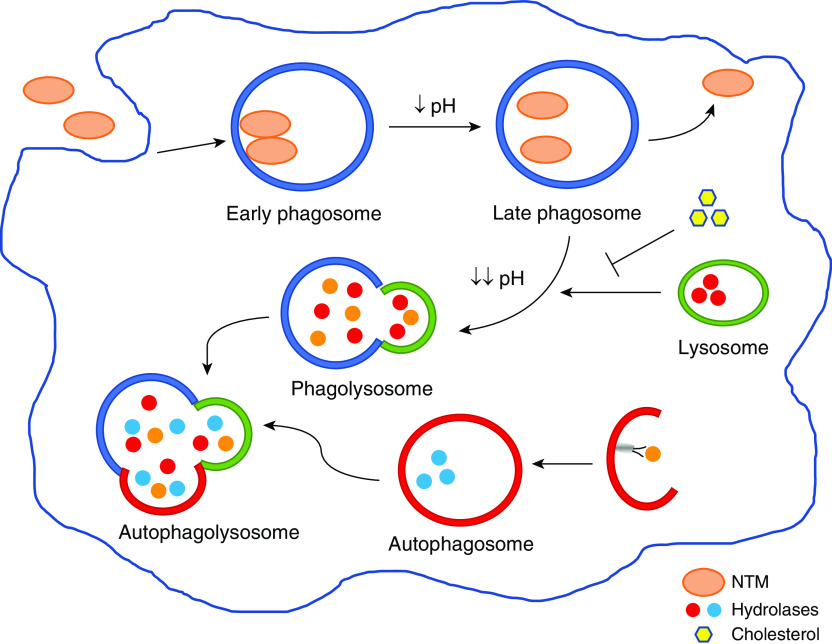
Another important mechanism by which host cells eliminate intracellular pathogens, especially those that escape the phagolysosome, is autophagy (52). Phagosomal maturation and its interaction with autophagy is depicted in Figure 1. This interaction is a prime target for the development of resistance mechanisms by mycobacteria. Induction of autophagy can occur by recognition of microbial factors that are then ubiquinated and recognized by SLRs (sequestosome 1-like receptors), or it can occur by production of ROS and IFN-γ–mediated proteolysis and autophagosome formation. If mycobacteria are able to survive autophagic killing, the host cell’s last line of defense is induction of apoptotic cell death. Although this can lead to caspase activation and initiation of lytic and inflammatory responses that stimulate neutrophils, cell death can also be a mechanism by which mycobacteria infect neighboring macrophages and propagate pathogenicity (27). Investigators have begun to explore many of these mechanisms with regard to the interaction between macrophages and MAI and MAB.
Figure 1.
Phagosomal maturation and autophagy. Nontuberculous mycobacteria (NTM) are taken up by the macrophage by phagocytosis. The bacteria aggregate into social phagosomes, with more than one bacillus within each phagosome. Tight apposition between the mycobacterial cell wall and the phagosomal membrane prevents phagosomal maturation and is a virulence mechanism by which NTM can survive intracellularly. As phagosomes mature, the pH becomes increasingly more acidic in an attempt to curb, and even kill, bacterial growth. The mature phagosomes fuse with lysosomes, and hydrolases degrade mycobacterial components, initiating autophagy and resulting in phagolysosome fusion with autophagosomes. The presence of cholesterol enables NTM to survive by keeping the bacteria closely apposed to the phagosomal membrane, thus preventing phagosomal fusion with lysosomes. Occasionally, NTM can also escape from late phagosomes or phagolysosomes and survive in a separate compartment within the cell.
As stated above, MAB can transform from a smooth (S) morphotype to a rough (R) morphotype that enhances virulence. As far back as 1999, Byrd and Lyons showed the existence of an S type that spontaneously dissociated from the R type (53). They noted that the R type had growth characteristics similar to those of virulent MTB and led to “phagocyte aggregates” that were not present with the S type (53). Since then, both types have been studied further, and it is now known that the R type gets its phenotype from a lack of GPLs, whereas the S type contains GPLs (54). The S type can form biofilms and is less infectious and more commonly found in the environment than the R morphotype, which is more virulent and leads to highly persistent and difficult-to-treat infections (54).
Differences in phagosomal uptake and acidification, and therefore bacterial survival, also exist between the R and S morphotypes. Roux and colleagues showed that the R variants, in contrast to the S variants, which were usually taken up as a single bacterium by “loner” phagosomes, often aggregated into connected, or social, phagosomes with multiple bacilli within each phagosome (55). Phagosomes containing R-type MAB were significantly more acidic than those containing S-type bacteria, suggesting that maturation of phagosomes into phagolysosomes does not occur with the S type. Lastly, the two phenotypes of MAB also triggered apoptosis and autophagy differently, in that the S type was less apoptotic and was less likely to induce autophagy based on the small number of S-containing phagosomes colocalized with LC3. The authors concluded that the presence or lack of GPLs impacts the ability of mycobacteria to withstand the usual mechanisms of bacterial clearance.
The importance of phagosomal acidification was also examined in MAI by de Chastellier and Thilo, who found that cholesterol plays an important role in the maintenance of phagosome membrane integrity and prevention of phagolysosome fusion (56). Cholesterol depletion led to loosening of the contact between the mycobacterial surface and phagosome membrane, which then allowed fusion with lysosomes. The loner phagolysosomes fused with one another to create social phagolysosomes with multiple mycobacteria. The pH of the phagolysosomes was more acidic than that of immature phagosomes, leading to phagolysosome-initiated autophagy. Interestingly, mycobacteria remained intact throughout the cholesterol-depleted state, and after the addition of cholesterol again became viable mycobacteria within longer phagosomes with closely apposed membranes. This suggests that despite exposure to hydrolytic, acidic conditions, the mycobacteria were not killed and could rescue themselves from autophagolysosomes.
These findings were corroborated by another group that investigated differences between UC22, a rough variant of MAB, and ATCC 19977, a smooth variant, and their effects on autophagy flux (57). UC22-infected macrophages had enhanced autophagy, as indicated by an increase in LC3 proteins, and were also found to have larger and more numerous puncta. Inhibition of autophagic degradation and autophagy flux are both important for intracellular survival of MAB, and blocking 3-methyladenine (an early-phase inhibitor of autophagy) decreased colocalization of LC3, whereas bafilomycin A1 (which inhibits autophagosome–lysosome fusion) increased accumulation of LC3.
Interruptions in phagolysosome fusion and acidification, as well as survival within vacuoles, are crucial mechanisms by which NTM survive within infected macrophages. Early and colleagues determined that apoptosis of infected macrophages led to autophagy by other macrophages and intracellular proliferation of MAI (58). Loss of integrity of the vacuolar membrane surrounding MAC allowed the mycobacteria to escape the apoptotic body, and MAC-infected macrophages showed an LC3 staining pattern consistent with increased autophagy. Furthermore, MAC survival increased if apoptosis was inhibited, and when apoptotic macrophages were added to fresh macrophages, the number of intracellular bacteria eventually increased after an initial killing phase.
Gidon and colleagues investigated how MAI elicit inflammatory signaling and the mechanisms involved in phagosomal maturation arrest and mycobacterial survival within macrophages (59). MAI phagosomes are not arrested at an early endosomal stage, and mature normally into phagolysosomes from which a fraction of the bacteria escape and reestablish themselves in a new compartment. In addition, to avoid degradation in phagolysosomes, MAI evade inflammatory signaling (59).
Although most NTM studies are done in macrophages, Ribeiro and colleagues compared the intracellular growth of MAB and phagosomal characteristics in macrophages and A549 type II alveolar epithelial cells (60). Although uptake of both a clinical MAB ssp massiliense isolate and a reference MAB ssp abscessus ATC 19977 strain was similar in macrophages and epithelial cell lines, the epithelial cell line had decreased survival rates when infected by the reference ATC 19977 strain as compared with the clinical isolate. The authors concluded that the clinical isolate behaved differently depending on the host cell type. In the epithelial cell line, it behaved like an R type with a closely apposed phagosome membrane, whereas in the macrophages, it behaved more like an S type with a loosely apposed membrane.
Similar to what has been observed with dormant MTB, MAI promote the conversion of macrophages to foamy macrophages by forming intracytoplasmic lipid inclusions (ILIs). Caire-Brändli and colleagues showed that macrophages became foamy owing to the presence of triacylglycerol, for which very-low-density lipoprotein (VLDL) is a good source (61). Endocytosed VLDL lipid bodies fused with immature phagosomes containing mycobacteria, and mycobacterial breakdown of triacylglycerol led to the formation of ILIs. Mycobacteria did not replicate while they were in ILIs; however, after withdrawal of VLDL, foamy macrophages lost ILIs and lipid bodies, and mycobacterial replication resumed. This provides insight into the formation of foamy macrophages as a mechanism by which MAI can survive.
More recently, Danelishvili and colleagues found that bacterial survival and lipid export in macrophages occurred in association with voltage-dependent anion channels (VDACs) of the MAI phagosome (62). Macrophages treated with cyclosporine A, a known VDAC blocker, or 4,4′-diisothiocyano-2,2′-disulfonic acid stilbene, a VDAC oligomerization blocker, resulted in significantly lower bacterial growth in MAI-infected cells, as did suppression of VDAC1 RNA expression. VDAC1 was associated with two mmpL4 lipoproteins known to be involved in the biosynthesis and export of cell wall lipid constituents, as well as with α- and β-subunits of ATP synthase. Furthermore, immunofluorescence staining showed that VDAC1 was always colocalized with MAI-containing phagosomes, most likely related to the fact that the phagosome membrane originates from the host cell plasma membrane.
The same group used a metal mixture proteome to mimic the phagosome environment and examine the characteristics of the MAI secretome (63). They found that MAI secreted unique proteins when it was incubated in the proteome. These proteins were believed to be presynthesized and activated by exposure to the metal mixture based on the lack of change in gene expression of genes encoding the proteins. Furthermore, upon infection of macrophages with MAI, the same proteins were secreted into the cytoplasm.
Treatment of NTM infections is a topic in itself, with many known and new mechanisms continually being discovered. One recent study identified the role of the antimicrobial peptide lactoferrin in MAI autophagy. Silva and colleagues determined that the D-enantiomer of lactoferrin peptide significantly inhibited intracellular growth of MAI (64). When combined with ethambutol, all lactoferrin peptides had an inhibitory effect, suggesting the possibility of a synergistic effect. The internalization of lactoferrin peptides increased proinflammatory cytokines, specifically TNF-α and IL-6; however, the antimicrobial effects of lactoferrin were not dependent on these cytokines. Rather, D-lactoferrin peptides resulted in structural alterations of MAI-infected macrophages leading to increased lysosomal content and increased numbers of autophagic vesicles.
Role of Apoptosis and the Inflammasome in the Host Response to NTM
Induction of apoptosis or cell death is an effective strategy used by macrophages to eliminate intracellular pathogens that escape phagosomal degradation and autophagy. Inflammasome activation by a variety of PAMPs and damage-associated molecular peptides promotes proinflammatory signaling, leading to different types of cell death. Caspases are proteases that play an important role in the initiation and regulation of apoptosis, as well as in the formation of inflammasomes, which are part of the innate immune system (65). Caspases can generally be divided into two groups based on function: proapoptotic caspases, which are responsible for initiating and regulating apoptosis, and proinflammatory caspases, which regulate the production of inflammatory cytokines and their downstream effects (66). Because of the importance of apoptosis and inflammasome formation in controlling infection, both mechanisms have been studied in various NTM.
Studies have shown that NLR recognition of microbial peptides leads to inflammasome activation via caspase-1 activation. Inflammasome activation in MAB was studied by Lee and colleagues in human monocyte-derived macrophages (67). They found that NLRP3 (Nod-like receptor pyrin domain containing protein 3) activation occurred by dectin-1/Syk (spleen tyrosine kinase)–dependent signaling via intracellular calcium influx and involvement of p62/SQSTM1 (p62), a cytosolic scaffolding protein, in signaling pathways. Both dectin-1 and TLR2 were required for mRNA expression of IL-1β (necessary for triggering the innate immune response), human cathelicidin hCAP-18/LL-37 (an antimicrobial peptide), and DEB4 (β-defensin 4). MAB infection led to an increase in IL-1β levels by caspase 1-activation, likely due to p62 expression, which was necessary for NLRP3 inflammasome activation. Pretreatment with caspase-1 inhibitor or silencing of p62 resulted in decreased levels of activated caspase-1 p10 subunit and mature IL-1β release, and knockdown of NLRP3 or ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) led to significantly decreased IL-1β RNA and protein levels. Furthermore, potassium efflux was necessary for caspase-1 activation and production of pro–IL-1β.
Chen and colleagues found that inflammasome activation restricted bacterial growth in M. kansasii infection, which aligns with what is known about inflammasome activation and host defense (68). Restriction of M. kansasii growth was related to caspase-1–activated secretion of IL-1β, as macrophages treated with either a caspase-1 inhibitor or IL-1β–neutralizing antibody exhibited an increased number of colony-forming units of bacteria. Furthermore, pretreatment with the antioxidant n-acetylcysteine led to decreased levels of IL-1β secretion, suggesting that ROS also contribute to activation of the NLRP3/ASC inflammasome.
The role of secretion systems in the activation of inflammasomes has been studied mostly in MTB and M. marinum models, as both organisms contain highly conserved type VII secretion system (known as ESX in mycobacteria) genes. Specifically, ESX-1 and ESX-5 play a significant role in the virulence of MTB, including activation of cell death, secretion of IL-1β and inflammasome activation, and secretion of proteins from a duplicate area of the MTB genome that is responsible for inflammasome activation but not cell death induction (69, 70). Carlsson and colleagues showed that, similar to MTB, ESX-1 was required for granuloma formation in mice infected with M. marinum; these lesions were not noted in mice infected with ESX-1–deficient M. marinum (71). Furthermore, the presence of ESX-1 led to increased levels of TNF-α and IL-1β, and was necessary for NLRP3/ASC inflammasome complex activation. Interestingly, ASC knockout mice had decreased levels of the inflammatory phenotype, suggesting that defective inflammasome activation led to less disease but without significantly different bacterial colony-forming unit counts. Thus, in this study, inflammasome activation increased inflammation without limiting bacterial growth.
Recently, Laencina and colleagues discovered several genes within the ESX-4 locus in MAB ssp massiliense that confer some virulence to MAB (72). Specifically, they found five mutants in the eccB4 gene that were involved in blockage of phagosome acidification and increased intracellular survival. Moreover, the presence of functional eccB4 led to increased signs of phagosomal lysis, suggesting that the ESX-4 secretion system is used by MAB to damage the phagosomal membrane and create communication between the cytosol and contents of the phagosome (72). The role of type VII secretion systems is generally understudied in NTM, but studies in MTB have shown that these systems play an important role in controlling the virulence of the pathogen (73).
Summary and Future Directions
NTM-LD is increasing globally and is often seen in immunocompetent individuals. Macrophages, as the cornerstone of the immune system, have adapted several mechanisms to phagocytose intracellular pathogens, thereby helping to orchestrate an appropriate host response. In the realm of NTM, recent studies have highlighted several mechanisms involved in intracellular bacterial survival, ranging from the recognition of extracellular and intracellular PAMPs to the manipulation of autophagy and phagosome maturation, inhibition or control of apotosis, and alteration of inflammasome activation. The goal is to be able to manipulate these cellular mechanisms to develop novel therapies to fight infection. With the rising prevalence of NTM infection and its concomitant health burdens, more research is being conducted into the molecular mechanisms underlying NTM disease. Further work is needed, however, to better understand the effect of secretomes in NTM infection and what role, if any, they may play in the propagation of infection. Similarly, little is known about the metabolic alterations in NTM during host–cell interactions, and the domains of metabolomics and cell energetics warrant further study.
Footnotes
Supported by VA Merit Award I01 BX001786 (R.T.S.) and U.S. National Institutes of Health grants R01 HL144478 (R.T.S.) and T32 HL116271 (Z.P.).
Originally Published in Press as DOI: 10.1165/rcmb.2019-0241TR on March 11, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.van Ingen J, Boeree MJ, Dekhuijzen PN, van Soolingen D. Environmental sources of rapid growing nontuberculous mycobacteria causing disease in humans. Clin Microbiol Infect. 2009;15:888–893. doi: 10.1111/j.1469-0691.2009.03013.x. [DOI] [PubMed] [Google Scholar]
- 2.Adelman MH, Addrizzo-Harris DJ. Management of nontuberculous mycobacterial pulmonary disease. Curr Opin Pulm Med. 2018;24:212–219. doi: 10.1097/MCP.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 3.Abubakar I, Gupta RK, Rangaka MX, Lipman M. Update in tuberculosis and nontuberculous mycobacteria 2017. Am J Respir Crit Care Med. 2018;197:1248–1253. doi: 10.1164/rccm.201801-0106UP. [DOI] [PubMed] [Google Scholar]
- 4.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185:881–886. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. managed care health plan, 2008-2015. Ann Am Thorac Soc. 2020;17:178–185. doi: 10.1513/AnnalsATS.201804-236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brode SK, Marchand-Austin A, Jamieson FB, Marras TK. Pulmonary versus nonpulmonary nontuberculous mycobacteria, Ontario, Canada. Emerg Infect Dis. 2017;23:1898–1901. doi: 10.3201/eid2311.170959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ringshausen FC, Wagner D, de Roux A, Diel R, Hohmann D, Hickstein L, et al. Prevalence of nontuberculous mycobacterial pulmonary disease, Germany, 2009-2014. Emerg Infect Dis. 2016;22:1102–1105. doi: 10.3201/eid2206.151642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santin M, Barrabeig I, Malchair P, Gonzalez-Luquero L, Benitez MA, Sabria J, et al. Pulmonary infections with nontuberculous mycobacteria, Catalonia, Spain, 1994-2014. Emerg Infect Dis. 2018;24:1091–1094. doi: 10.3201/eid2406.172095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adjemian J, Olivier KN, Seitz AE, Falkinham JO, III, Holland SM, Prevots DR. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med. 2012;186:553–558. doi: 10.1164/rccm.201205-0913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaulding AB, Lai YL, Zelazny AM, Olivier KN, Kadri SS, Prevots DR, et al. Geographic distribution of nontuberculous mycobacterial species identified among clinical isolates in the United States, 2009-2013. Ann Am Thorac Soc. 2017;14:1655–1661. doi: 10.1513/AnnalsATS.201611-860OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falkinham JO., III Ecology of nontuberculous mycobacteria: where do human infections come from? Semin Respir Crit Care Med. 2013;34:95–102. doi: 10.1055/s-0033-1333568. [DOI] [PubMed] [Google Scholar]
- 12.Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:87–94. doi: 10.1055/s-0033-1333567. [DOI] [PubMed] [Google Scholar]
- 13.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [Published erratum appears in Am J Respir Crit Care Med 175:744–745.] [DOI] [PubMed] [Google Scholar]
- 14.Aksamit TR, O’Donnell AE, Barker A, Olivier KN, Winthrop KL, Daniels MLA, et al. Bronchiectasis Research Registry Consortium. Adult patients with bronchiectasis: a first look at the US bronchiectasis research registry. Chest. 2017;151:982–992. doi: 10.1016/j.chest.2016.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McShane PJ, Glassroth J. Pulmonary disease due to nontuberculous mycobacteria: current state and new insights. Chest. 2015;148:1517–1527. doi: 10.1378/chest.15-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowler CJ, Olivier KN, Leung JM, Smith CC, Huth AG, Root H, et al. Abnormal nasal nitric oxide production, ciliary beat frequency, and toll-like receptor response in pulmonary nontuberculous mycobacterial disease epithelium. Am J Respir Crit Care Med. 2013;187:1374–1381. doi: 10.1164/rccm.201212-2197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appelberg R. Pathogenesis of Mycobacterium avium infection: typical responses to an atypical mycobacterium? Immunol Res. 2006;35:179–190. doi: 10.1385/IR:35:3:179. [DOI] [PubMed] [Google Scholar]
- 18.Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. 2015;36:13–34. doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015;36:91–99. doi: 10.1016/j.ccm.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saleeb P, Olivier KN. Pulmonary nontuberculous mycobacterial disease: new insights into risk factors for susceptibility, epidemiology, and approaches to management in immunocompetent and immunocompromised patients. Curr Infect Dis Rep. 2010;12:198–203. doi: 10.1007/s11908-010-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorman SE, Holland SM. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 2000;11:321–333. doi: 10.1016/s1359-6101(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 22.Wu UI, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis. 2015;15:968–980. doi: 10.1016/S1473-3099(15)00089-4. [DOI] [PubMed] [Google Scholar]
- 23.Bogunovic D, Byun M, Durfee LA, Abhyankar A, Sanal O, Mansouri D, et al. Mycobacterial disease and impaired IFN-γ immunity in humans with inherited ISG15 deficiency. Science. 2012;337:1684–1688. doi: 10.1126/science.1224026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365:127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winthrop KL, Chang E, Yamashita S, Iademarco MF, LoBue PA. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-alpha therapy. Emerg Infect Dis. 2009;15:1556–1561. doi: 10.3201/eid1510.090310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saiga H, Shimada Y, Takeda K. Innate immune effectors in mycobacterial infection. Clin Dev Immunol. 2011;2011:347594. doi: 10.1155/2011/347594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awuh JA, Flo TH. Molecular basis of mycobacterial survival in macrophages. Cell Mol Life Sci. 2017;74:1625–1648. doi: 10.1007/s00018-016-2422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orme IM, Ordway DJ. Host response to nontuberculous mycobacterial infections of current clinical importance. Infect Immun. 2014;82:3516–3522. doi: 10.1128/IAI.01606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev. 2015;264:182–203. doi: 10.1111/imr.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halaas O, Steigedal M, Haug M, Awuh JA, Ryan L, Brech A, et al. Intracellular Mycobacterium avium intersect transferrin in the Rab11(+) recycling endocytic pathway and avoid lipocalin 2 trafficking to the lysosomal pathway. J Infect Dis. 2010;201:783–792. doi: 10.1086/650493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pagán AJ, Ramakrishnan L. Immunity and immunopathology in the tuberculous granuloma. Cold Spring Harb Perspect Med. 2014;5:a018499. doi: 10.1101/cshperspect.a018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nathan C. Macrophages’ choice: take it in or keep it out. Immunity. 2016;45:710–711. doi: 10.1016/j.immuni.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Regev D, Surolia R, Karki S, Zolak J, Montes-Worboys A, Oliva O, et al. Heme oxygenase-1 promotes granuloma development and protects against dissemination of mycobacteria. Lab Invest. 2012;92:1541–1552. doi: 10.1038/labinvest.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surolia R, Karki S, Wang Z, Kulkarni T, Li FJ, Vohra S, et al. Attenuated heme oxygenase-1 responses predispose the elderly to pulmonary nontuberculous mycobacterial infections. Am J Physiol Lung Cell Mol Physiol. 2016;311:L928–L940. doi: 10.1152/ajplung.00397.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernut A, Nguyen-Chi M, Halloum I, Herrmann J-L, Lutfalla G, Kremer L. Mycobacterium abscessus-induced granuloma formation is strictly dependent on TNF signaling and neutrophil trafficking. PLoS Pathog. 2016;12:e1005986. doi: 10.1371/journal.ppat.1005986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubois V, Viljoen A, Laencina L, Le Moigne V, Bernut A, Dubar F, et al. MmpL8 MAB controls Mycobacterium abscessus virulence and production of a previously unknown glycolipid family. Proc Natl Acad Sci USA. 2018;115:E10147–E10156. doi: 10.1073/pnas.1812984115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumura Y, Kitabatake M, Ouji-Sageshima N, Yasui S, Mochida N, Nakano R, et al. Persimmon-derived tannin has bacteriostatic and anti-inflammatory activity in a murine model of Mycobacterium avium complex (MAC) disease. PLoS One. 2017;12:e0183489. doi: 10.1371/journal.pone.0183489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehrt S, Rhee K, Schnappinger D. Mycobacterial genes essential for the pathogen’s survival in the host. Immunol Rev. 2015;264:319–326. doi: 10.1111/imr.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 40.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 41.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 42.Fremond CM, Yeremeev V, Nicolle DM, Jacobs M, Quesniaux VF, Ryffel B. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114:1790–1799. doi: 10.1172/JCI21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng CG, Scanga CA, Collazo-Custodio CM, Cheever AW, Hieny S, Caspar P, et al. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J Immunol. 2003;171:4758–4764. doi: 10.4049/jimmunol.171.9.4758. [DOI] [PubMed] [Google Scholar]
- 44.Shin DM, Yang CS, Yuk JM, Lee JY, Kim KH, Shin SJ, et al. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol. 2008;10:1608–1621. doi: 10.1111/j.1462-5822.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- 45.Roux AL, Ray A, Pawlik A, Medjahed H, Etienne G, Rottman M, et al. Overexpression of proinflammatory TLR-2-signalling lipoproteins in hypervirulent mycobacterial variants. Cell Microbiol. 2011;13:692–704. doi: 10.1111/j.1462-5822.2010.01565.x. [DOI] [PubMed] [Google Scholar]
- 46.Ogier-Denis E, Mkaddem SB, Vandewalle A. NOX enzymes and Toll-like receptor signaling. Semin Immunopathol. 2008;30:291–300. doi: 10.1007/s00281-008-0120-9. [DOI] [PubMed] [Google Scholar]
- 47.Awuh JA, Haug M, Mildenberger J, Marstad A, Do CP, Louet C, et al. Keap1 regulates inflammatory signaling in Mycobacterium avium-infected human macrophages. Proc Natl Acad Sci USA. 2015;112:E4272–E4280. doi: 10.1073/pnas.1423449112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee KI, Choi HG, Son YJ, Whang J, Kim K, Jeon HS, et al. Mycobacterium avium MAV2052 protein induces apoptosis in murine macrophage cells through toll-like receptor 4. Apoptosis. 2016;21:459–472. doi: 10.1007/s10495-016-1220-y. [DOI] [PubMed] [Google Scholar]
- 49.Whang J, Back YW, Lee KI, Fujiwara N, Paik S, Choi CH, et al. Mycobacterium abscessus glycopeptidolipids inhibit macrophage apoptosis and bacterial spreading by targeting mitochondrial cyclophilin D. Cell Death Dis. 2017;8:e3012. doi: 10.1038/cddis.2017.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung JY, Madan-Lala R, Georgieva M, Rengarajan J, Sohaskey CD, Bange FC, et al. The intracellular environment of human macrophages that produce nitric oxide promotes growth of mycobacteria. Infect Immun. 2013;81:3198–3209. doi: 10.1128/IAI.00611-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maiuri MC, Kroemer G. Therapeutic modulation of autophagy: which disease comes first? Cell Death Differ. 2019;26:680–689. doi: 10.1038/s41418-019-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byrd TF, Lyons CR. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect Immun. 1999;67:4700–4707. doi: 10.1128/iai.67.9.4700-4707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howard ST, Rhoades E, Recht J, Pang X, Alsup A, Kolter R, et al. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology. 2006;152:1581–1590. doi: 10.1099/mic.0.28625-0. [DOI] [PubMed] [Google Scholar]
- 55.Roux AL, Viljoen A, Bah A, Simeone R, Bernut A, Laencina L, et al. The distinct fate of smooth and rough Mycobacterium abscessus variants inside macrophages. Open Biol. 2016;6:160185. doi: 10.1098/rsob.160185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Chastellier C, Thilo L. Cholesterol depletion in Mycobacterium avium-infected macrophages overcomes the block in phagosome maturation and leads to the reversible sequestration of viable mycobacteria in phagolysosome-derived autophagic vacuoles. Cell Microbiol. 2006;8:242–256. doi: 10.1111/j.1462-5822.2005.00617.x. [DOI] [PubMed] [Google Scholar]
- 57.Kim SW, Subhadra B, Whang J, Back YW, Bae HS, Kim HJ, et al. Clinical Mycobacterium abscessus strain inhibits autophagy flux and promotes its growth in murine macrophages Pathog Dis 201775ftx107. [DOI] [PubMed] [Google Scholar]
- 58.Early J, Fischer K, Bermudez LE. Mycobacterium avium uses apoptotic macrophages as tools for spreading. Microb Pathog. 2011;50:132–139. doi: 10.1016/j.micpath.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gidon A, Åsberg SE, Louet C, Ryan L, Haug M, Flo TH. Persistent mycobacteria evade an antibacterial program mediated by phagolysosomal TLR7/8/MyD88 in human primary macrophages. PLoS Pathog. 2017;13:e1006551. doi: 10.1371/journal.ppat.1006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ribeiro GM, Matsumoto CK, Real F, Teixeira D, Duarte RS, Mortara RA, et al. Increased survival and proliferation of the epidemic strain Mycobacterium abscessus subsp. massiliense CRM0019 in alveolar epithelial cells. BMC Microbiol. 2017;17:195. doi: 10.1186/s12866-017-1102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caire-Brändli I, Papadopoulos A, Malaga W, Marais D, Canaan S, Thilo L, et al. Reversible lipid accumulation and associated division arrest of Mycobacterium avium in lipoprotein-induced foamy macrophages may resemble key events during latency and reactivation of tuberculosis. Infect Immun. 2014;82:476–490. doi: 10.1128/IAI.01196-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Danelishvili L, Chinison JJJ, Pham T, Gupta R, Bermudez LE. The voltage-dependent anion channels (VDAC) of Mycobacterium avium phagosome are associated with bacterial survival and lipid export in macrophages. Sci Rep. 2017;7:7007. doi: 10.1038/s41598-017-06700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chinison JJ, Danelishvili L, Gupta R, Rose SJ, Babrak LM, Bermudez LE. Identification of Mycobacterium avium subsp. hominissuis secreted proteins using an in vitro system mimicking the phagosomal environment. BMC Microbiol. 2016;16:270. doi: 10.1186/s12866-016-0889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silva T, Moreira AC, Nazmi K, Moniz T, Vale N, Rangel M, et al. Lactoferricin peptides increase macrophages’ capacity to kill Mycobacterium avium. mSphere. 2017;2:e00301-17. doi: 10.1128/mSphere.00301-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 67.Lee HM, Yuk JM, Kim KH, Jang J, Kang G, Park JB, et al. Mycobacterium abscessus activates the NLRP3 inflammasome via Dectin-1-Syk and p62/SQSTM1. Immunol Cell Biol. 2012;90:601–610. doi: 10.1038/icb.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen CC, Tsai SH, Lu CC, Hu ST, Wu TS, Huang TT, et al. Activation of an NLRP3 inflammasome restricts Mycobacterium kansasii infection. PLoS One. 2012;7:e36292. doi: 10.1371/journal.pone.0036292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdallah AM, Bestebroer J, Savage ND, de Punder K, van Zon M, Wilson L, et al. Mycobacterial secretion systems ESX-1 and ESX-5 play distinct roles in host cell death and inflammasome activation. J Immunol. 2011;187:4744–4753. doi: 10.4049/jimmunol.1101457. [DOI] [PubMed] [Google Scholar]
- 70.Shah S, Cannon JR, Fenselau C, Briken V. A duplicated ESAT-6 region of ESX-5 is involved in protein export and virulence of mycobacteria. Infect Immun. 2015;83:4349–4361. doi: 10.1128/IAI.00827-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carlsson F, Kim J, Dumitru C, Barck KH, Carano RA, Sun M, et al. Host-detrimental role of Esx-1-mediated inflammasome activation in mycobacterial infection. PLoS Pathog. 2010;6:e1000895. doi: 10.1371/journal.ppat.1000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laencina L, Dubois V, Le Moigne V, Viljoen A, Majlessi L, Pritchard J, et al. Identification of genes required for Mycobacterium abscessus growth in vivo with a prominent role of the ESX-4 locus. Proc Natl Acad Sci USA. 2018;115:E1002–E1011. doi: 10.1073/pnas.1713195115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gröschel MI, Sayes F, Simeone R, Majlessi L, Brosch R. ESX secretion systems: mycobacterial evolution to counter host immunity. Nat Rev Microbiol. 2016;14:677–691. doi: 10.1038/nrmicro.2016.131. [DOI] [PubMed] [Google Scholar]