Abstract
PURPOSE
Capitalizing on the promise of patient-reported outcomes (PROs), electronic implementations of PROs (ePROs) are expected to play an important role in the development of novel digital health interventions targeting palliative cancer care. We performed a systematic and mapping review of the scientific literature on the current ePRO-based approaches used for palliative cancer care.
METHODS
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guidelines, the conducted review answered the research questions: “What are the current ePRO-based approaches for palliative cancer care; what is their contribution/value in the domain of palliative cancer care; and what are the potential gaps, challenges, and opportunities for further research?” After a screening step, the corpus of included articles indexed in PubMed or the Web of Science underwent full text review, which mapped the articles across 15 predefined axes.
RESULTS
The corpus of 24 mapped studies includes 9 study protocols, 7 technical tools/solutions, 7 pilot/feasibility/acceptability studies, and 1 evaluation study. The review of the corpus revealed (1) an archetype of ePRO-enabled interventions for palliative cancer care, which most commonly use ePROs as study end point assessment instruments rather than integral intervention components; (2) the fact that the literature has not fully embraced the modern definitions that expand the scope of palliative care; (3) the striking shortage of promising ubiquitous computing devices (eg, smart activity trackers); and (4) emerging evidence about the benefits of narrowing down the target cancer population, especially when combined with modern patient-centered intervention design methodologies.
CONCLUSION
Although research on exploiting ePROs for the development of digital palliative cancer care interventions is considerably active and demonstrates several successful cases, there is considerable room for improvement along the directions of the aforementioned findings.
INTRODUCTION
Globally, between 40 and 80 million patients with advanced disease need access to palliative care,1 which focuses on improving pain and symptom management, reducing psychosocial distress, offering spiritual support, and enhancing quality of life (QoL) for patients and their families.2 Initially associated with fatal illnesses (eg, hospice care), especially in the United States, the scope of palliative care has recently become wider. For instance, the latest WHO definition describes palliative care as a health care service that tackles the problems associated with life-threatening illness,2 whereas the Centre to Advance Palliative Care distinguishes palliative care from end-of-life care, hospice care, and bereavement care, because the former is appropriate at any age and at any stage in a serious illness and can be provided alongside curative treatment.3
Context
Key Objective
The current systematic review presents the state of the art of electronic implementations of patient-reported outcome (ePRO)–based digital health interventions and highlights the contribution of such interventions to palliative cancer care. A timely addition to an area of growing research interest, this review identifies the challenges and opportunities in the field of digitally enabled delivery of palliative care and provides concrete recommendations for future research.
Knowledge Generated
The proposed digital health interventions have been evaluated positively by end-users with respect to usability, user acceptance, and satisfaction, with few concerns. Existing findings demonstrate that ePRO interventions could have a significant positive impact on health outcomes. Because of the methodologic diversity of the reviewed studies, the precise impact cannot be fully ascertained as yet.
Relevance
The clinical practice of palliative care can be facilitated or promoted through the use of digital health systems leveraging ePROs throughout the trajectory of cancer.
With this in mind, the World Health Assembly4 resolution called on governments to integrate palliative care into national health systems throughout a patient’s life cycle and throughout the disease trajectory. To achieve these aims, excellent assessment of patient-reported outcomes (PROs) is required.5 PROs are defined as measurements reported directly from the patient about their health status without amendment or interpretation by a physician or anyone else.6 With the increasing availability of handheld and wearable electronic devices, greater Internet connectivity, and digital health, electronic patient-reported outcomes (ePRO) have emerged as a feasible option for improving the quality of assessment, and they are expected to play an important role in the development of new digital health interventions that target the palliation of patients with chronic ailments, critically including cancer, a diverse group of largely chronic diseases that often have a relapsing course.
An indicative example is provided by MyPal,7 a recently funded research program aimed at exploiting ePROs for the development of novel palliative care services for patients with cancer across Europe. Using digital health technologies, MyPal aims to empower patients with cancer and their caregivers by more accurately capturing their symptoms/conditions, communicating them effectively to their health care providers (HCPs) and, ultimately, fostering the time for action through the prompt identification of important deviations in the patient’s health state and QoL.8
Obviously, designing effective digital palliative care interventions for patients with cancer on the basis of ePRO systems relies, among other things, on a thorough appreciation and assessment of the relevant state of the art, representing the motivation and focus of the current systematic and mapping review.9 For this reason, we conducted a systematic and mapping review to address the following research questions: “What are the current ePRO-based approaches for palliative cancer care; what is their contribution/value in the domain of palliative cancer care; and what are the potential gaps, challenges, and opportunities for further research?” Given the emergence of numerous palliative care definitions, the current review opts for adopting the widest in scope, covering the entire care continuum for patients with cancer as well as cancer survivors; the adopted palliative care definition greatly overlaps with supportive care.10
METHODS
This systematic review was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.11,12 The PRISMA checklist is presented in the Data Supplement. The review protocol is outlined in the current section; it has not been registered in any review protocol repository.
Initially, a list of potentially relevant articles was retrieved from online bibliographic sources (Search Strategy), and 3 of the authors independently assessed the eligibility of each article (Inclusion and Exclusion Criteria) on the basis of the information contained in the article title and abstract, whenever possible. The screening was performed with BibReview software.13 Next, the eligible literature corpus was mapped across a set of axes that were defined by the reviewing team (Mapping Axes). The corpus was partitioned on the basis of the main focus of the study by the 3 authors involved in the first phase, and each part was assigned to a pair of authors for full text review and mapping. A stylesheet file with columns matching the mapping axes was designed for the reviewing authors to extract the study data; the completed stylesheet files were subsequently merged and manually harmonized by the 3 main authors. Appropriate measures were taken to mitigate the risk of bias (Risk of Bias and Mitigation Measures).
Search Strategy
Two reference bibliographies, namely PubMed14 and Web of Science,15 were queried. Given that the scope of the review was targeting ePRO systems and, thus, the focus of the study was primarily technical, these 2 databases were considered adequate.
In the retrieval stage, semantically identical (although appropriately formulated) queries were defined and executed for each bibliographic source. These are provided in the Data Supplement. The last search in both sources was performed on June 10, 2019.
Inclusion and Exclusion Criteria
The selection of the corpus of eligible articles for review has relied on the following criteria. No constraints on the publication year of the studies were applied.
Inclusion criteria.
We included articles referring to the implementation and/or deployment of ePRO-based systems (IC1) for the provision of palliative/supportive care (IC2) to patients with cancer (IC3); these had to be journal articles (IC4) written in English (IC5).
Exclusion criteria.
We excluded review, opinion, or editorial articles (EC1), articles referring to the same approach/system (EC2), and articles in which the ePROs were not part of a health care intervention (EC3; eg, studies that collected ePRO data for the sole purpose of statistical analysis).
Mapping Axes
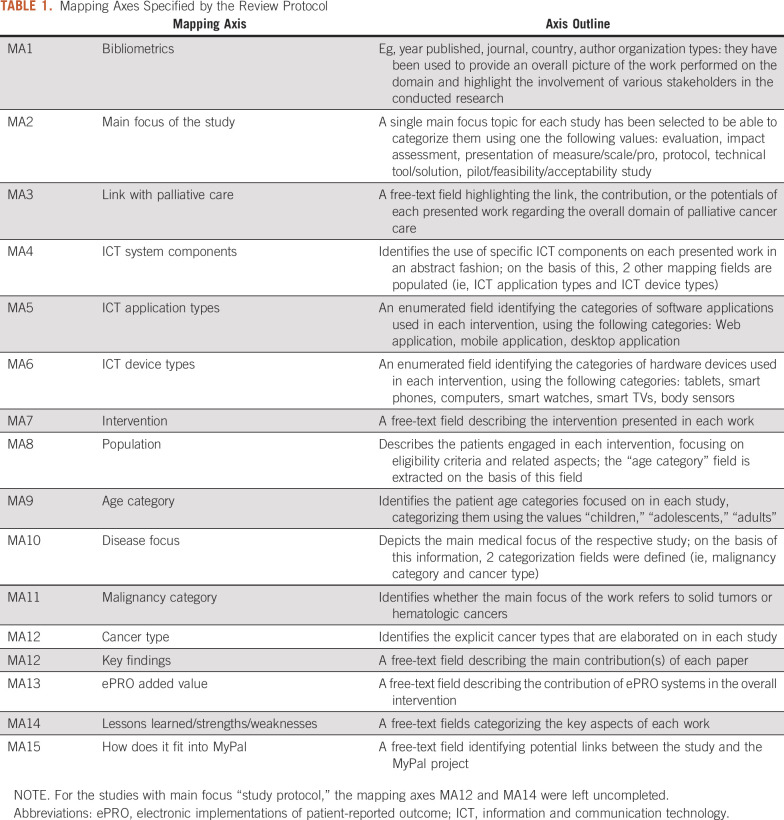
The eligible articles were qualitatively and quantitatively mapped across a number of mapping criteria/axes. These were defined on the basis of the expertise of the authors in digital health systems and palliative care, as well as on the particular focus of the MyPal program; iterative fine-tuning of the mapping axes was performed as the outcomes from the articles were gradually obtained to ensure that the axes were orthogonal (ie, nonoverlapping) to the greatest extent possible. The final list of mapping axes that was used in the study is provided in Table 1.
TABLE 1.
Mapping Axes Specified by the Review Protocol
Risk of Bias and Mitigation Measures
In the context of this review, bias is defined as a systematic error, or deviation from the truth, in results or inferences.16 The main identified sources of bias and the way that the study protocol has mitigated the respective risks are as follows:
Reporting bias is related to the selection of the findings to be presented in a study. Because there is no widely accepted methodology for publishing the results of ePRO interventions, the reviewed studies report results in an arbitrary manner, which could affect the review outcomes. To tackle this source of bias, the review protocol refrained from synthesizing quantitative results reported by the reviewed studies.
Competing interests bias does not exist for the authors of the current review study. However, the authors of some of the reviewed articles are affiliated with industry, which could introduce some level of bias in the reported outcomes. The authors with competing interests with industry were identified; they account for 25% of the reviewed studies.
Evidence selection bias occurs when important research is not identified by the review (eg, research not published because of a lack of statistical significance in the results). To mitigate this risk, 2 widely used reference bibliographic sources (Search Strategy) were used by the review protocol, increasing the search space and, consequently, the chances of articles in this category being published. However, a residual risk remains, which is acceptable in the context of the current work.
RESULTS
This section provides the main findings of this systematic and mapping review. After presenting the outcome of the study selection (Study Selection) and the characteristics of the included studies (Study Characteristics), the remainder of the section summarizes the mapping analysis along a short list of composite mapping axes/directions. The complete data set of the mapping outcome for all the included studies is available in the Data Supplement.
Study Selection
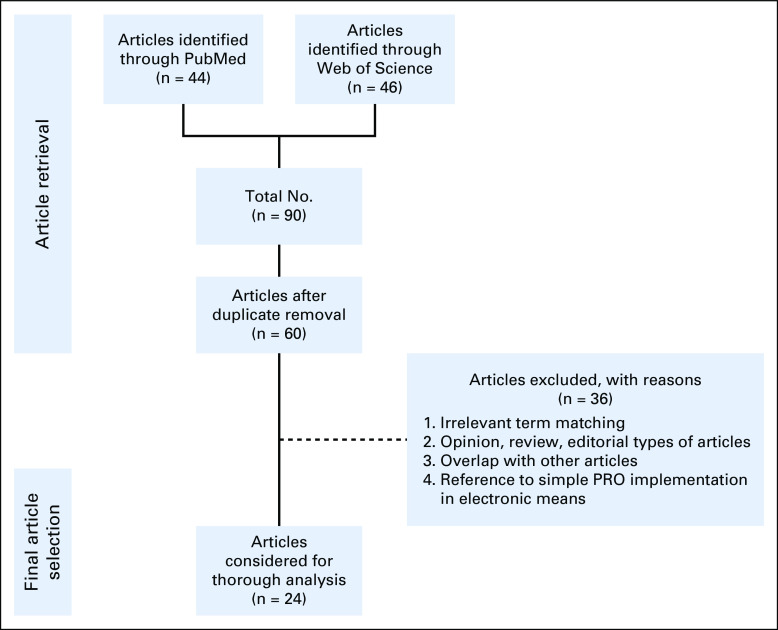
In total, the corpus of included articles consisted of 24 studies.17-40 The PRISMA flow diagram depicting the overall study selection process is presented in Fig 1.
FIG 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the conducted systematic review. PRO, patient-reported outcome.
Study Characteristics
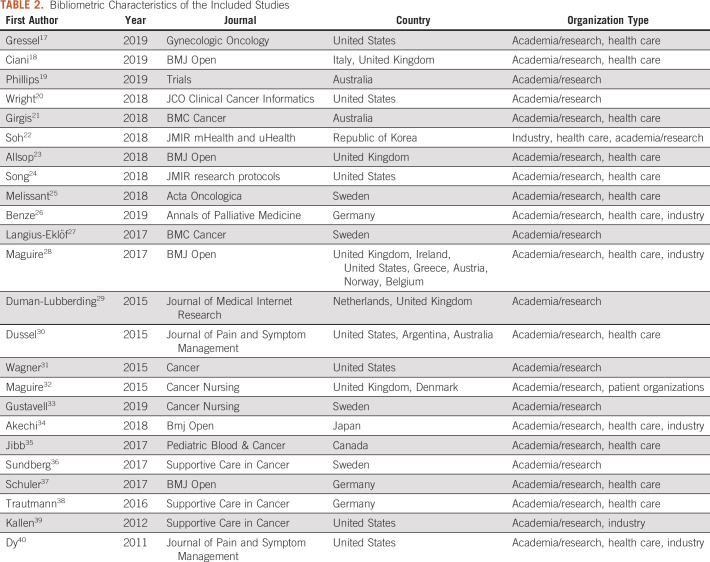
The characteristics of the 24 included studies were extracted from the data that were collected with respect to the bibliometric mapping axis (MA1), and they are presented in Table 2.
TABLE 2.
Bibliometric Characteristics of the Included Studies
Publications timeline.
Figure 2A presents the distribution of the included studies per publication year, demonstrating the gradual building of a critical mass of studies involving ePROs for palliative cancer care. This distribution seems to be compatible with the picture of a research topic in the making.
FIG 2.
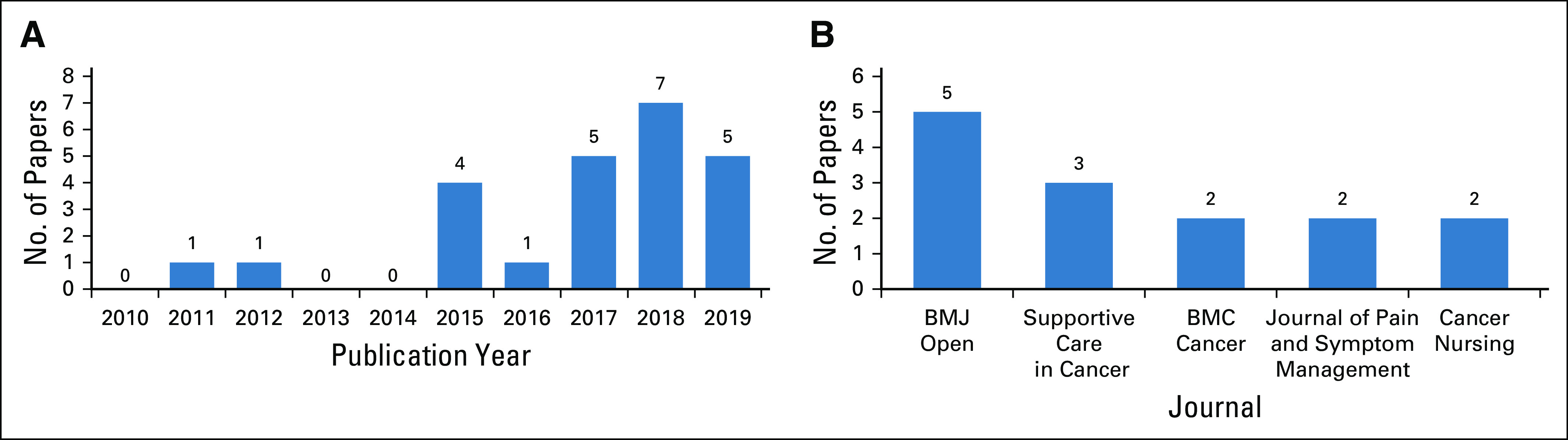
Selected aggregated bibliometric characteristics of the included studies. (A) By publication year. (B) By journal.
Target journals.
A wide range of scientific journals (n = 15) has been targeted by the included studies. In this regard, the most prominent targets were BMJ Open41 (n = 5) and Supportive Care in Cancer42 (n = 3), with the rest of the journals publishing 1 or 2 studies. The distribution of the included studies per publishing journal is presented in Fig 2B.
Geographic distribution.
The geographic distribution of the included studies was extracted at the country level on the basis of the affiliation origin of all the authors of each study; this means that a study could be assigned to multiple countries. In brief, one third of the studies (n = 8) derived from the United States, whereas other countries with strong contributions on the topic included the United Kingdom (n = 5), Sweden (n = 4), Australia (n = 3), and Germany (n = 3). Of note, when combined, the European countries accounted for 83.3% (n = 20) of the included studies. Five studies were international.
Organization types.
The review categorized the potential organization types of the author affiliations for the included studies as academia/research, health care, and industry. From the data in Table 2, one can observe that the academia/research organizations participated in the entirety of the study, followed by health care organizations (n = 15) and then industry (n = 6). During the mapping process, the need to extend the list of potential organization types to include patient organizations (n = 1) emerged. It should also be noted that no policy-making or regulatory organizations were identified as contributors in the included study corpus, indicating a potential gap in the interaction of academia, health care, and industry with the aforementioned organizations.
Study Focus
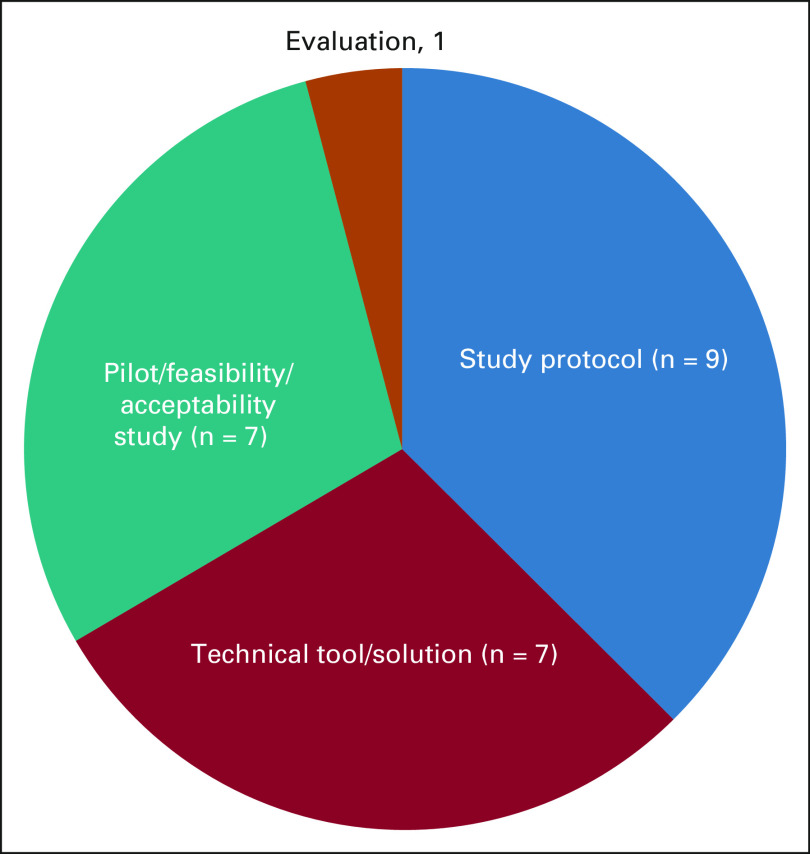
The main focus of each included study (ie, the primary aspect of the ePRO-based intervention that was presented in the articles) has been identified by MA2 and presented in Fig 3. Most of the articles present study protocols (n = 9), whereas technical tools/solutions (n = 7) and pilot/feasibility/acceptability studies (n = 7) are also common.
FIG 3.
Main focus of the included studies.
It should be noted that only the main focus of the respective studies is depicted in this figure and that many of them could be considered to cross-cut these categories. For example, many of the reviewed studies present a feasibility study of a specific technical tool, while also evaluating it in terms of its impact assessment.
Target Populations
Several mapping axes (MA8-MA12) depict the characteristics of the target populations engaged in the respective ePRO-based interventions.
Age category.
The vast majority of the interventions (n = 22) targeted adults, whereas 2 interventions were designed for adolescents. Only 1 intervention targeted children, indicating a significant gap in the literature and also a potential research opportunity.
Disease focus.
Apart from a number of studies that report interventions addressing cancer in general (n = 10), most of the included studies were more focused on the disease of the target population. All 24 studies report on interventions designed for patients with solid tumors, whereas 10 of these studies address hematologic malignancies as well. In more detail, the great majority of the included studies target specific cancer types (eg, prostate cancer [n = 3], lung cancer [n = 2], GI cancer [n = 1], head and neck cancer [n = 1], pancreatic cancer [n = 1], and sarcoma [n = 1], as well as the family of gynecologic cancers, including breast cancer [n = 6]). It should be noted, however, that some interventions focus on more than 1 malignancy and/or cancer type.
Users’ perspective.
The analysis of strengths and weaknesses of the digital health solutions proposed by each study (MA14) provides valuable information about the respective end-users. The proposed digital health solutions have been evaluated positively through a variety of methods by end-users with respect to usability, user acceptance, and satisfaction. System adoption and usage,25,31 as well as attitudes toward recommending20 or reusing the system,26 have often been used. Certain widespread pre-existing (ie, System Usability Scale),39 or adapted measures18,30,34 have also been reported. Finally, interviews with actors (patients, HCPs, caregivers) have been conducted with the intention of exploring facilitators, barriers, and suggestions for improvement.21 Future research should offer recommendations for reliable ways of ePRO-based digital health systems evaluation, thus facilitating comparability among studies.
In rare cases, participants raised serious concerns. One article reported that the use of a system was a “barrier,”35 whereas in another, one half of clinicians doubted the system’s clinical usefulness and viewed it as an addition to their workload.32 However, the majority of reviewed studies presented solutions assisting HCPs go through a large amount of data quickly (ie, by adopting a traffic light system in which only red flags require attention)36 or incorporate self-care components for patients to use on their own at home).20 This transition may not always be smooth, but it has become absolutely paramount, considering the growing number of patients with cancer worldwide requiring supportive care.
One of the most important lessons learned from the study of the user perspective in the reviewed articles is that additional improvement regarding information and communication technology (ICT) components and user interaction is needed.22,25,26,29,32,33,38 The need for improvement with respect to overall symptoms management has also been reported by some studies.32,33,35,36
Digital Health Interventions
Potentially, the primary aspect of the included studies is the digital health interventions that have been proposed for the palliation of patients with cancer. The analysis of MA7 (Intervention) and MA13 (ePRO Added Value) provide insight concerning the goals and the components of the presented interventions.
Intervention goals.
Four main axes have been identified regarding the main goals of the digital health interventions that are discussed in the included studies:
Intervention components.
Most of the reviewed interventions used validated questionnaires to collect information from patients regarding symptom intensity.17,19,20-23,25,28,31,32,35-38 In some cases, alert messages were communicated to health care professionals to warn them in the case of severe symptoms.18,20,21,27,28,31-33,35,36 Furthermore, in many interventions, the patient-reported information was also disseminated to the end users18-25,27,28,33-36,37 for training or guidance on symptom management (eg, by means of training material or even personalized messages). It should be highlighted that none of the reviewed interventions exploited modern user interfaces empowered by computational intelligence, such as conversational agents,43 whereas only one of them (Jibb et al35) adopted a gamification approach.44
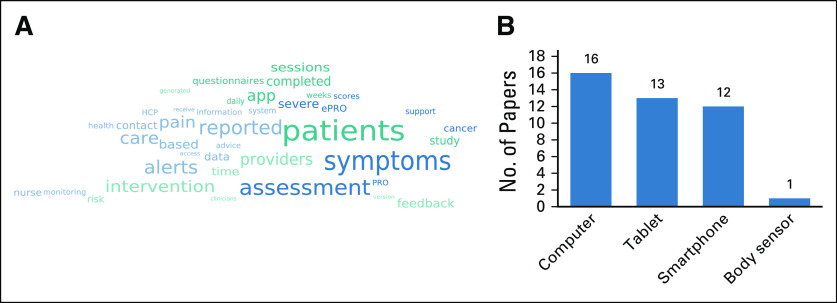
An informative overview of the most prominent themes in the reviewed digital health intervention is provided in Fig 4A. This presents a semantic-aware word cloud that has been extracted from the free-text data of MA7 (Intervention) on the basis of a theoretical word cloud representation model.45 The semantic word cloud has been generated by Cloudy software46 by setting the maximum number of words at 40. The word cloud confirms some of the aforementioned findings that concern the components of the reviewed digital health interventions. For instance, symptom assessment has a prominent place in the word cloud, indicating, as expected, that it is a common building block among the reviewed interventions.
FIG 4.
Digital health interventions associated with the included studies. (A) Semantic-aware word cloud extracted from the free-text description of the intervention (MA7). (B) Types of electronic devices that are used for delivering the interventions (No. of studies); in certain studies, multiple types of devices have been used. ePRO, electronic implementations of patient-reported outcome; HCP, health care provider; PRO, patient-reported outcome.
Digital Health Systems
An equally important aspect of the included studies concerns the digital health systems that have been developed to deliver the proposed palliative care intervention for patients with cancer. Details on the type of used software applications and devices are provided later in the text by the analysis of the data collected for MA4-MA6 (ICT system components, ICT application type, and ICT device type).
Software application types.
Web applications and smartphone applications are almost equally popular among software applications used by the reviewed digital health systems as the main interface with the patients for delivering the associated digital health interventions. They are used by 8 and 7 studies, respectively, whereas 9 studies use both types of applications. No desktop applications have been reported.
Electronic device types.
The types of electronic devices used by the reviewed digital health systems as the primary interface with the patients and their frequencies are presented in Fig 4B. Furthermore, the analysis of the collected mapping data indicates that the use of handheld devices (ie, tablets and smartphones) increases with time (data not shown), owing to their unobtrusiveness and their expanding availability. However, it should also be noted that modern wearable devices, such as smart watches and smart glasses, have not been used in the reviewed studies, indicating a potential research gap, because these devices offer new capabilities for monitoring/tracking the physiologic status of the patient.
Strengths and Weaknesses
The most commonly identified methodologic strengths of the reviewed literature include (1) participatory design (ie, involvement of important actors29 during eHealth system development); (2) multicenter, randomized controlled trials (RCTs) of adequate duration and sample size28 that usually preceded a feasibility or pilot study20; (3) interventions developed and/or performed by a multidisciplinary palliative care team and addressing multiple aspects of palliative care23,32,38; (4) use of validated ePROs measuring symptoms, QoL, and supportive care needs18; and (5) evaluation of the eHealth system regarding usability and user satisfaction.26 The most commonly identified methodologic weaknesses include (1) the exclusion of patients who were not technologically apt21; (2) the monitoring of symptoms not followed by an action (ie, a call from the health care professional)26; and (3) the inability to attribute outcomes changes to specific components of complex multifactorial interventions.34
DISCUSSION
For the most part, the reviewed literature indicates that palliative care tends to be associated with advanced cancer stages, which suggests insufficient digital health research embracing the current definition of palliative care. The aim of future studies should be to assess the applicability and effectiveness of digital health interventions developed for patients with cancer in need of palliation in its wider scope. Specifically, they should aim to facilitate or promote the delivery of palliative care from the point of diagnosis and throughout the continuum of the disease.
The review of the existing literature also disclosed 2 distinct ways that ePROs can be used in the context of digital health studies: (1) as assessment instruments for the end points of a clinical study, which is the most common ePRO use case, and (2) as a building block of a digital health intervention, which is less common but better promotes the integration of ePROs into routine clinical practice. Indeed, the role of ePROs in the former case ends as soon as the assessment of the digital health solution is completed, whereas in the latter case, they constitute an integral part of a real-life digital health product. In a few of the reviewed studies, ePROs served both purposes.
A common pattern emerges from the literature about the structure of digital health interventions for palliative cancer care. In brief, the following workflow is adopted by the archetypical digital health palliative cancer care intervention: (1) ePRO data on disease-related symptoms are collected; (2) the patient gets value from the ePRO data in the context of self-care: this can entail acting on self-care advice provided via the digital health system or the patient is empowered to monitor their own health status and make informed decisions about their care in collaboration with the health care team; (3) the collected ePRO data are presented to the health care professional(s), in real time if possible; and (4) the health care professional(s) review the data and take action, if needed (eg, tailor the treatment/medication to the patient on the basis of symptom severity or bothersomeness, contact the patient, and so forth).
Other common themes identified within the reviewed interventions include (1) the integration of intelligent modules and/or system-initiated actions in the presented digital health systems, mainly in the form of dynamic evidence-based self-care feedback provided after ePRO completion and alert service notifications for health care professionals; and (2) statements that the proposed digital health solutions are not emergency services, in line with the current rationale of palliative care.
One exemplary study is eSMART, a multicenter RCT aiming to evaluate an electronic symptom management system for patients with cancer.28 Appropriate ePROs will be part of the intervention, leveraged to elicit patients’ chemotherapy-related symptoms, leading to evidence-based, self-care feedback and automatic alerts for HCPs. Furthermore, ePROs will also be used for the intervention end points’ evaluation, namely, reduced symptom burden and anxiety and supportive care needs, as well as improved self-care efficacy and QoL. Cost-effectiveness and potential changes to clinical practice will also be considered. Another pragmatic study is LuCApp, an RCT aiming to evaluate a mobile supportive care app for patients with metastatic lung cancer.18 In this case, ePROs are part of the intervention and the evaluation process. Patients report symptom incidence, and HCPs are alerted if severity overcomes specific thresholds. Evaluation is conducted in terms of QoL impact, supportive care needs, caregiver burden, and resource use.
The reviewed studies have not taken advantage of recent advances in wearables (ie, smart watches or smart wristbands), which could nicely complement ePROs. This is surprising, given the share of such devices in the consumer electronics market and their adoption in telehealth care solutions. The designers of future palliative cancer care services could benefit from advancements in wearable technologies by integrating them into the digital health system they intend to develop.
Finally, the published evidence indicates not only that patients with cancer are generally in favor of ePRO-based interventions, but also that ePRO interventions could contribute to improved health outcomes such as an improvement in physical activity20; a reduction in anxiety and drowsiness32; lower levels of fatigue, nausea, insomnia,36 and pain intensity; as well as a significant improvement in emotional and social functioning.35
Nonetheless, the review gives rise to a clear demand for improvements in the designed digital health systems on the basis of patient needs. With this in mind, research has to be narrowed down as much as possible to a target population and digital health solutions must be adapted to the specific user group needs (eg, in terms of usability), thus increasing efficiency. Indeed, most of the reviewed studies have endorsed this logic, as evidenced by their choice of patient populations with specific characteristics (eg, specific age groups and cancer types; see Target Populations).
For future digital health approaches in palliative cancer care, a good understanding of the particular needs of the target population is paramount. This can be acquired with the help of modern participatory design tools, such as surveys and focus groups with representatives from the target patient population and expert palliative cancer care practitioners.
Although the strong adherence to PRISMA guidelines ensures that the review has been conducted on a solid methodologic ground, there is also 1 important limitation: the included studies were methodologically diverse (including unfinished lines of research such as protocols and pilot/feasibility studies), meaning that the full impact of ePRO interventions on health outcomes cannot yet be fully ascertained.
The conducted review revealed that scientific research on the exploitation of ePROs for developing digital palliative cancer care interventions is active and demonstrated a number of successful cases, while also highlighting significant room for research. Using the aforementioned successful cases as a guide, future research efforts should strategically enhance the state of the art in palliative cancer care ePRO systems and digital health services in general to facilitate the information flow between patients and health care experts and eventually close the information loop, which is of paramount importance for the vision of P4 Medicine,47 especially its last 2 dimensions.
ACKNOWLEDGMENT
We acknowledge the contribution of the late Vassilis Koutkias, PhD, in the conceptualization, design, and implementation of the current review.
SUPPORT
Supported by the European Union’s Horizon 2020 Research and Innovation Programme Grant No. 825872. The funder played no role in the systematic review.
AUTHOR CONTRIBUTIONS
Conception and design: Christina Karamanidou, Christos Maramis
Collection and assembly of data: Christina Karamanidou, Pantelis Natsiavas, Lefteris Koumakis, Kostas Marias, Fatima Schera, Michael Schäfer, Christos Maramis
Data analysis and interpretation: Christina Karamanidou, Pantelis Natsiavas, Sheila Payne, Christos Maramis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Knaul FM, Farmer PE, Krakauer EL, et al. Alleviating the access abyss in palliative care and pain relief-an imperative of universal health coverage: The Lancet Commission report. Lancet. 2018;391:1391–1454. doi: 10.1016/S0140-6736(17)32513-8. [DOI] [PubMed] [Google Scholar]
- 2.Sepúlveda C, Marlin A, Yoshida T, et al. Palliative care: The World Health Organization’s global perspective. J Pain Symptom Manage. 2002;24:91–96. doi: 10.1016/s0885-3924(02)00440-2. [DOI] [PubMed] [Google Scholar]
- 3.Get Palliative Care What is palliative care. https://getpalliativecare.org/whatis/
- 4.World Health Assembly 2014. WHA67.19 Strengthening ofpalliative care as a component of comprehensive care throughout the life course. In Sixty-seventh World Health Assembly. Retrieved fromhttps://apps.who.int/iris/bitstream/handle/10665/162863/A67_R19-en.pdf?sequence=1&isAllowed=y
- 5.Bausewein C, Daveson BA, Currow BC, et al. EAPC White paper on outcome measurement in palliative care: improving practice, attaining outcomes and delivering quality services – Recommendations from the European Association for Palliative Care (EAPC) Task Force on Outcome Measurement. Palliat Med. 2016;30:6–22. doi: 10.1177/0269216315589898. [DOI] [PubMed] [Google Scholar]
- 6.LeBlanc TW, Abernethy AP. Patient-reported outcomes in cancer care - hearing the patient voice at greater volume. Nat Rev Clin Oncol. 2017;14:763–772. doi: 10.1038/nrclinonc.2017.153. [DOI] [PubMed] [Google Scholar]
- 7.MyPal Fostering palliative care of adults and children with cancer through advanced patient reported outcome systems. https://mypal-project.eu/
- 8.Maramis C, Karamanidou C, Schera F, et al. Using electronic patient reported outcomes to foster palliative cancer care: The MyPal Approach. IEEE 19th International Conference on Bioinformatics and Bioengineering (BIBE), Athens, Greece, October 28-30; 2019. [Google Scholar]
- 9.Grant MJ, Booth A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 10.Radbruch L, Payne S. White paper on standards and norms for hospice and palliative care in Europe: Part 1. Eur J Palliat Care. 2009;16:278–289. [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, et al. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med 151:W65-W94, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Lamy J-B, Séroussi B, Griffon N, et al. Toward a formalization of the process to select IMIA Yearbook best papers. Methods Inf Med. 2015;54:135–144. doi: 10.3414/ME14-01-0031. [DOI] [PubMed] [Google Scholar]
- 14.PubMed – National Center for Biotechnology Informationhttps://www.ncbi.nlm.nih.gov/pubmed/
- 15.Web of Sciencehttps://clarivate.com/webofsciencegroup/solutions/web-of-science/
- 16.Higgins JPT, Altman DG: Assessing risk of bias in included studies, in Higgins JPT, Green S. (eds): Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series, 2008.
- 17.Gressel GM, Dioun SM, Richley M, et al. Utilizing the Patient Reported Outcomes Measurement Information System (PROMIS®) to increase referral to ancillary support services for severely symptomatic patients with gynecologic cancer. Gynecol Oncol. 2019;152:509–513. doi: 10.1016/j.ygyno.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 18.Ciani O, Cucciniello M, Petracca F, et al. Lung Cancer App (LuCApp) study protocol: A randomised controlled trial to evaluate a mobile supportive care app for patients with metastatic lung cancer. BMJ Open. 2019;9:e025483. doi: 10.1136/bmjopen-2018-025483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips JL, Heneka N, Lovell M, et al. A phase III wait-listed randomised controlled trial of novel targeted inter-professional clinical education intervention to improve cancer patients’ reported pain outcomes (The Cancer Pain Assessment (CPAS) Trial): Study protocol. Trials. 2019;20:62. doi: 10.1186/s13063-018-3152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright AA, Raman N, Staples P, et al. The HOPE Pilot Study: Harnessing patient-reported outcomes and biometric data to enhance cancer care. JCO Clin Cancer Inform. 2018;2:1–12. doi: 10.1200/CCI.17.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girgis A, Durcinoska I, Gerges M, et al. Study protocol for a controlled trial of an eHealth system utilising patient reported outcome measures for personalised treatment and care: PROMPT-Care 2.0. BMC Cancer. 2018;18:845. doi: 10.1186/s12885-018-4729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soh JY, Cha WC, Chang DK, et al. Development and validation of a multidisciplinary mobile care system for patients with advanced gastrointestinal cancer: Interventional observation study. JMIR Mhealth Uhealth. 2018;6:e115. doi: 10.2196/mhealth.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allsop MJ, Wright-Hughes A, Black K, et al. Improving the management of pain from advanced cancer in the community: Study protocol for a pragmatic multicentre randomised controlled trial. BMJ Open. 2018;8:e021965. doi: 10.1136/bmjopen-2018-021965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song L, Dunlap KL, Tan X, et al. Enhancing survivorship care planning for patients with localized prostate cancer using a couple-focused mHealth symptom self-management program: Protocol for a feasibility study. JMIR Res Protoc. 2018;7:e51. doi: 10.2196/resprot.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melissant HC, Verdonck-de Leeuw IM, Lissenberg-Witte BI, et al. ‘Oncokompas’, a Web-based self-management application to support patient activation and optimal supportive care: A feasibility study among breast cancer survivors. Acta Oncol. 2018;57:924–934. doi: 10.1080/0284186X.2018.1438654. [DOI] [PubMed] [Google Scholar]
- 26.Benze G, Nauck F, Alt-Epping B, et al. PROutine: A feasibility study assessing surveillance of electronic patient reported outcomes and adherence via smartphone app in advanced cancer. Ann Palliat Med. 2019;8:104–111. doi: 10.21037/apm.2017.07.05. [DOI] [PubMed] [Google Scholar]
- 27.Langius-Eklöf A, Crafoord M-T, Christiansen M, et al. Effects of an interactive mHealth innovation for early detection of patient-reported symptom distress with focus on participatory care: Protocol for a study based on prospective, randomised, controlled trials in patients with prostate and breast cancer. BMC Cancer. 2017;17:466. doi: 10.1186/s12885-017-3450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maguire R, Fox PA, McCann L, et al. The eSMART study protocol: A randomised controlled trial to evaluate electronic symptom management using the advanced symptom management system (ASyMS) remote technology for patients with cancer. BMJ Open. 2017;7:e015016. doi: 10.1136/bmjopen-2016-015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duman-Lubberding S, van Uden-Kraan CF, Peek N, et al. An eHealth application in head and neck cancer survivorship care: Health care professionals’ perspectives. J Med Internet Res. 2015;17:e235. doi: 10.2196/jmir.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dussel V, Orellana L, Soto N, et al. Feasibility of conducting a palliative care randomized controlled trial in children with advanced cancer: Assessment of the PediQUEST study. J Pain Symptom Manage. 2015;49:1059–1069. doi: 10.1016/j.jpainsymman.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner LI, Schink J, Bass M, et al. Bringing PROMIS to practice: Brief and precise symptom screening in ambulatory cancer care. Cancer. 2015;121:927–934. doi: 10.1002/cncr.29104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maguire R, Ream E, Richardson A, et al. Development of a novel remote patient monitoring system: The advanced symptom management system for radiotherapy to improve the symptom experience of patients with lung cancer receiving radiotherapy. Cancer Nurs. 2015;38:E37–E47. doi: 10.1097/NCC.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 33.Gustavell T, Langius-Eklöf A, Wengström Y, et al. Development and feasibility of an interactive smartphone app for early assessment and management of symptoms following pancreaticoduodenectomy. Cancer Nurs. 2019;42:E1–E10. doi: 10.1097/NCC.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 34.Akechi T, Yamaguchi T, Uchida M, et al. Smartphone problem-solving and behavioural activation therapy to reduce fear of recurrence among patients with breast cancer (SMartphone Intervention to LEssen fear of cancer recurrence: SMILE project): Protocol for a randomised controlled trial. BMJ Open. 2018;8:e024794. doi: 10.1136/bmjopen-2018-024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jibb LA, Stevens BJ, Nathan PC, et al. Implementation and preliminary effectiveness of a real-time pain management smartphone app for adolescents with cancer: A multicenter pilot clinical study. Pediatr Blood Cancer. 2017;64:e26554. doi: 10.1002/pbc.26554. [DOI] [PubMed] [Google Scholar]
- 36.Sundberg K, Wengström Y, Blomberg K, et al. Early detection and management of symptoms using an interactive smartphone application (Interaktor) during radiotherapy for prostate cancer. Support Care Cancer. 2017;25:2195–2204. doi: 10.1007/s00520-017-3625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuler M, Richter S, Ehninger G, et al. A cluster-randomised, controlled proof-of-concept study to explore the feasibility and effect of a patient-directed intervention on quality of life in patients with advanced soft tissue sarcoma. BMJ Open. 2017;7:e014614. doi: 10.1136/bmjopen-2016-014614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trautmann F, Hentschel L, Hornemann B, et al. Electronic real-time assessment of patient-reported outcomes in routine care-first findings and experiences from the implementation in a comprehensive cancer center. Support Care Cancer. 2016;24:3047–3056. doi: 10.1007/s00520-016-3127-0. [DOI] [PubMed] [Google Scholar]
- 39.Kallen MA, Yang D, Haas N. A technical solution to improving palliative and hospice care. Support Care Cancer. 2012;20:167–174. doi: 10.1007/s00520-011-1086-z. [DOI] [PubMed] [Google Scholar]
- 40.Dy SM, Roy J, Ott GE, et al. Tell Us™: a Web-based tool for improving communication among patients, families, and providers in hospice and palliative care through systematic data specification, collection, and use. J Pain Symptom Manage. 2011;42:526–534. doi: 10.1016/j.jpainsymman.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.BMJ Openhttps://bmjopen.bmj.com/pages/
- 42.Springer: Supportive Care in Cancerhttps://www.springer.com/journal/520
- 43.Laranjo L, Dunn AG, Tong HL, et al. Conversational agents in healthcare: A systematic review. J Am Med Inform Assoc. 2018;25:1248–1258. doi: 10.1093/jamia/ocy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson D, Deterding S, Kuhn K-A, et al. Gamification for health and wellbeing: A systematic review of the literature. Internet Interv. 2016;6:89–106. doi: 10.1016/j.invent.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barth L, Fabrikant SI, Kobourov SG, et al. Semantic Word Cloud Representations: Hardness and Approximation Algorithms, in Pardo A, Viola A (eds): LATIN 2014: Theoretical Informatics. Berlin/Heidelberg Germany, Springer, 2014, pp 514-525. [Google Scholar]
- 46.spupyrev: spupyrev/swcvhttps://github.com/spupyrev/swcv
- 47.Hood L, Flores M. A personal view on systems medicine and the emergence of proactive P4 medicine: Predictive, preventive, personalized and participatory. N Biotechnol. 2012;29:613–624. doi: 10.1016/j.nbt.2012.03.004. [DOI] [PubMed] [Google Scholar]