Abstract
Patients with coronavirus disease (COVID-19) are described as exhibiting oxygen levels incompatible with life without dyspnea. The pairing—dubbed happy hypoxia but more precisely termed silent hypoxemia—is especially bewildering to physicians and is considered as defying basic biology. This combination has attracted extensive coverage in media but has not been discussed in medical journals. It is possible that coronavirus has an idiosyncratic action on receptors involved in chemosensitivity to oxygen, but well-established pathophysiological mechanisms can account for most, if not all, cases of silent hypoxemia. These mechanisms include the way dyspnea and the respiratory centers respond to low levels of oxygen, the way the prevailing carbon dioxide tension (PaCO2) blunts the brain’s response to hypoxia, effects of disease and age on control of breathing, inaccuracy of pulse oximetry at low oxygen saturations, and temperature-induced shifts in the oxygen dissociation curve. Without knowledge of these mechanisms, physicians caring for patients with hypoxemia free of dyspnea are operating in the dark, placing vulnerable patients with COVID-19 at considerable risk. In conclusion, features of COVID-19 that physicians find baffling become less strange when viewed in light of long-established principles of respiratory physiology; an understanding of these mechanisms will enhance patient care if the much-anticipated second wave emerges.
Keywords: COVID-19, control of breathing, dyspnea, hypoxemia, pulse oximetry
Case Report Vignettes
Patient MD, a 64-year-old man, tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and coronavirus disease (COVID-19) was diagnosed. While the patient was receiving 6 L/min oxygen by nasal cannula, his oxygen saturation as measured by pulse oximetry (SpO2) was 68%, and arterial blood gas revealed oxygen tension (PaO2) of 37 mm Hg, carbon dioxide tension (PaCO2) of 41 mm Hg, and oxygen saturation (SaO2) of 75%. On questioning, he consistently denied any difficulty with breathing. On examination, he was comfortable and not using accessory muscles of respiration. Comorbidities included diabetes mellitus, hypertension, coronary artery disease and bypass surgery, left carotid endarterectomy, and renal transplantation.
Patient RM, a 74-year-old man, tested positive for SARS-CoV-2, and COVID-19 was diagnosed. While he was receiving 15 L/min oxygen by reservoir mask, his SpO2 was 62%, and arterial blood gas revealed a PaO2 of 36 mm Hg, a PaCO2 of 34 mm Hg, and an SaO2 of 69%. On questioning, he consistently denied any difficulty with breathing (including while drinking). On examination, he was comfortable and not using accessory muscles of respiration. He did not have any comorbidities.
Patient EF, a 58-year-old man, tested positive for SARS-CoV-2, and COVID-19 was diagnosed. While receiving a high-flow nasal cannula, his SpO2 was 76%, and arterial blood gas revealed a PaO2 of 45 mm Hg, a PaCO2 of 38 mm Hg, and an SaO2 of 83%. On questioning, he consistently denied any difficulty with breathing. On examination, he was comfortable and using his cell phone. He had no known comorbidities.
Background
The Wall Street Journal considers it a medical mystery as to why “large numbers of Covid-19 patients arrive at hospitals with blood-oxygen levels so low they should be unconscious or on the verge of organ failure. Instead they are awake, talking—not struggling to breathe” (1). Science judges the lack of patient discomfort at extraordinarily low blood-oxygen concentrations as defying basic biology (2). Writing in The New York Times, Dr. Levitan, with 30 years of emergency medicine experience, notes “A vast majority of Covid pneumonia patients I met had remarkably low oxygen saturations at triage—seemingly incompatible with life—but they were using their cellphones . . . they had relatively minimal apparent distress, despite dangerously low oxygen levels” (3). Despite this extensive coverage in the news media, the topic has not been addressed in medical journals.
Several factors explain why oxygen readings and lack of dyspnea in patients with COVID-19 are baffling to physicians, including the effect of hypoxia on the respiratory centers, the effect of PaCO2 on the ventilatory response to hypoxia, the hypoxia threshold that precipitates dyspnea, the limited accuracy of SpO2 below 80%, shifts in the oxygen dissociation curve, the tolerance of low oxygen levels, and the definition of hypoxemia.
Dyspnea and Control of Breathing
Viral infection of the respiratory system typically provokes inflammation and stimulation of sensory receptors, inducing transmission of afferent impulses to the respiratory centers (4). If the virus involves the alveoli, it may produce hypoxemia (5). The presence of dyspnea would be no physiological surprise in either situation. Surprise would arise only if sensory afferents or hypoxemia elicited significant stimulation of the respiratory centers and the patient did not develop dyspnea (6).
Unpleasant breathing can be recognized only by a patient; it is purely a subjective symptom (6). Caregivers commonly equate physical signs—tachypnea, tachycardia, and facial expression—with dyspnea. This is wrong. Patients vary widely in behavioral responses to discomfort. As with pain, physical signs may overestimate or underestimate patient discomfort (7).
The respiratory centers are exquisitely sensitive to CO2 (7). Small increases in PaCO2 rapidly evoke large increases in minute ventilation (e); an increase in PaCO2 of 10 mm Hg produces an amount of respiratory discomfort that cannot be tolerated for even a few minutes (8). Abnormal lung mechanics also provoke dyspnea but considerably less than hypercapnia does (7).
Hypoxemia produces dyspnea through the stimulation of the carotid bodies, which send signals to the medulla oblongata (9). The resulting increase in respiratory center output is transmitted down to the phrenic nerves and diaphragm, causing increased e (10). Heightened medullary center activity is concurrently transmitted up to the cerebral cortex. It is this cortical projection (corollary discharge) that produces the unpleasant sensation of dyspnea (7).
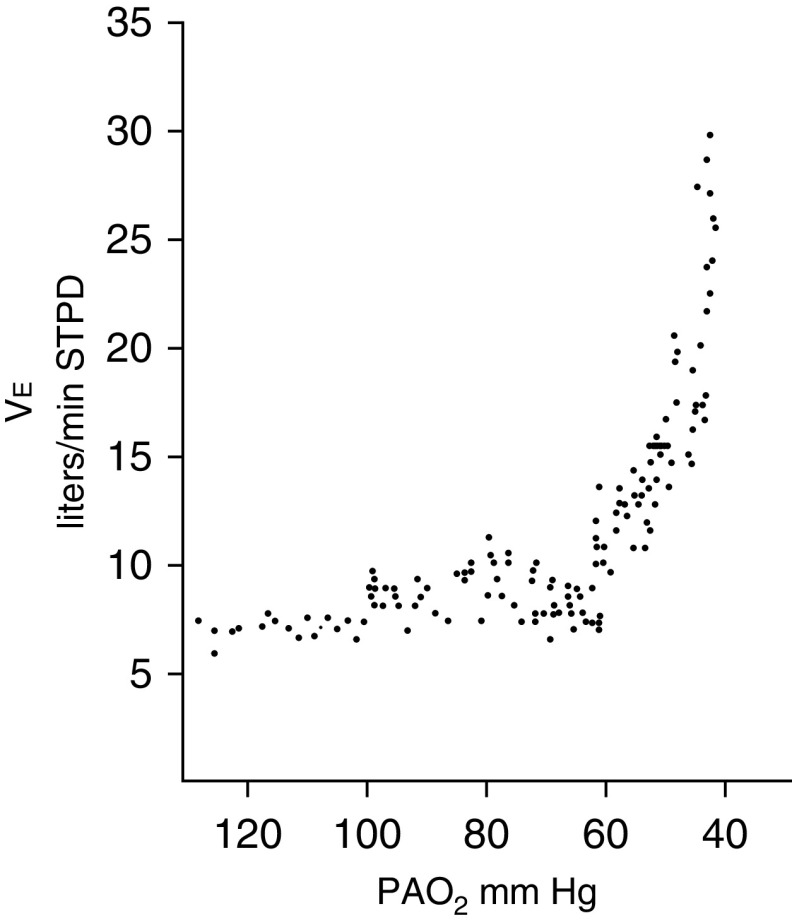
The ventilatory response to hypoxia is characterized as a hyperbolic curve (11). e is unchanged as PaO2 drops from 90 to 60 mm Hg; further decreases in PaO2 provoke an exponential increase in e (Figure 1). Moosavi and colleagues (12) observed that the amount of hypoxia required to induce the ventilatory response to hypoxia is equivalent to that required to induce dyspnea. A fall in end-tidal Po2 to less than 60 mm Hg elicited a strong increase in dyspnea in only half of subjects (12). The ventilatory and dyspnea responses to hypoxia are heavily influenced by the prevailing PaCO2. Severe hypoxia elicits an effective increase in ventilation only when background PaCO2 exceeds 39 mm Hg (12, 13).
Figure 1.
The ventilatory response to progressive isocapnic hypoxia in a healthy subject. Little change in e is noted until alveolar oxygen tension (PaO2) falls to 60 mm Hg, and thereafter the response is very steep. Each data point represents the mean value for PaO2 and e for three successive breaths. Adapted by permission from Reference 11. STPD = standard temperature and pressure dry.
We undertook an informal poll of 58 hospitalists, emergency physicians, and intensivists, inquiring whether they had seen patients who might be regarded as having silent hypoxemia or “happy hypoxia” (the term used by newspapers). Of 37 respondents, 15 did not provide useful data. Nineteen patients had arterial blood gas measurements; of these, 16 patients had a PaO2 of less than 60 mm Hg and communicated to a physician that they were not experiencing difficulty with breathing. Seven of the 16 patients had PaCO2 concentrations above 39 mm Hg (range, 41–49), which, combined with PaO2 of less than 60 mm Hg, would be expected to induce dyspnea; we considered these patients to have probable silent hypoxemia (see above vignette for patient MD). Nine patients had PaCO2 concentrations below 39 mm Hg (range, 29–37), which can blunt the respiratory centers; we do not categorize these patients as having silent hypoxemia (see patient RM and EF vignettes).
A disproportionate number of patients with COVID-19 are elderly and have diabetes (14). Both factors blunt the response of the respiratory control system to hypoxia. The ventilatory response to hypoxia is decreased by 50% in people older than 65 years (15, 16). Given that the dyspnea response to hypoxia parallels the ventilatory response (12), it is likely that older patients with COVID-19 are more prone to silent hypoxemia. All but two of our seven patients with probable silent hypoxemia were 64 years or older (age range, 59–85 yr). The ventilatory response to hypoxia is decreased by more than 50% in diabetes (17, 18). Individuals with diabetes also have a 1.8-fold impaired ability to perceive respiratory sensations (19). A further confounding factor is the broad range in respiratory drive between individuals (20). The chemical drive to breathe (in response to hypercapnia and hypoxia) exhibits as much as 300–600% variation between one subject and the next (20–23). This wide variability in respiratory drive is another factor that explains why some patients with hypoxia do not develop dyspnea.
Hypoxemia as a Threat to Life
Physicians are fearful of hypoxemia, and many view saturations between 80% and 85% as life threatening. We served as volunteers in an experiment probing the effect of hypoxemia on breathing patterns; our pulse oximeter displayed an SpO2 of 80% for over an hour, and we were not able to sense differences between an SpO2 of 80% and an SpO2 of 90% (24). In investigations on control of breathing and oximeter accuracy, subjects experience an SpO2 of 75% (12), or briefly 45% (25), without serious harm. Tourists on drives to the top of Mount Evans near Denver experience oxygen saturations of 65% for prolonged periods; many are comfortable, whereas some sense dyspnea (25).
Pulse Oximetry
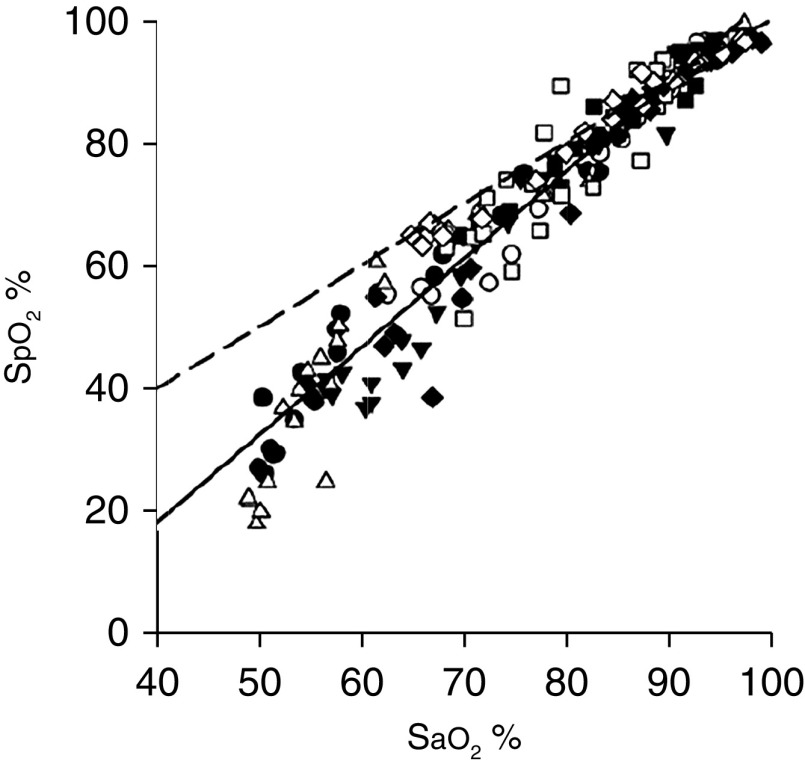
Pulse oximetry estimates SaO2 by illuminating the skin and measuring changes in light absorption of oxyhemoglobin and reduced Hb (26). Pulse oximetry–estimated saturation (SpO2) can differ from true SaO2 (measured with a CO-oximeter) by as much as ±4% (5). Pulse oximetry is considerably less accurate at SaO2 of less than 80%, partly because of the challenge in obtaining human calibration data (and guarding of information through trade secrets and patent protection). SpO2 underestimated true SaO2 by 7% in all three patients in the above vignettes. In subjects exposed to profound hypoxemia in a hypobaric chamber, there was a resulting PaO2 of 21.6–27.8 mm Hg (27). The mean difference and limits of the agreement between pulse oximetry SpO2 and true SaO2 were −5.8 ± 16%; when SpO2 was displayed as less than 40%, 80% of simultaneous SaO2 values were 10% higher (some were 30% higher) (28) (Figure 2).
Figure 2.
Scatterplot of the relationship between estimated oxygen saturation from pulse oximetry (SpO2) and SaO2 from blood gas analysis in healthy subjects exposed to profound hypoxemia in a hypobaric chamber (PaO2, 21.6–27.8 mm Hg). Each subject is represented by a different symbol. The dashed line is the line of identity, and the solid line is the regression line. Adapted by permission from Reference 28.
Pulse oximetry is less reliable in critically ill patients than in healthy volunteers. In critically ill patients, the 95% limit of agreement between SpO2 and SaO2 was ±4.02%, and the difference between SpO2 and SaO2 over time was not reproducible (in magnitude or direction) (29). Pulse oximetry is less accurate in black than in white patients (2.45 times less accurate at detecting ≥4% difference between SpO2 and SaO2) (30). Claims that patients with COVID-19 had oxygenation levels incompatible with life may have arisen because caregivers are not aware that pulse oximeters are inherently inaccurate at low saturations and further impacted by critical illness and skin pigmentation.
Shifts in Oxygen Dissociation Curve
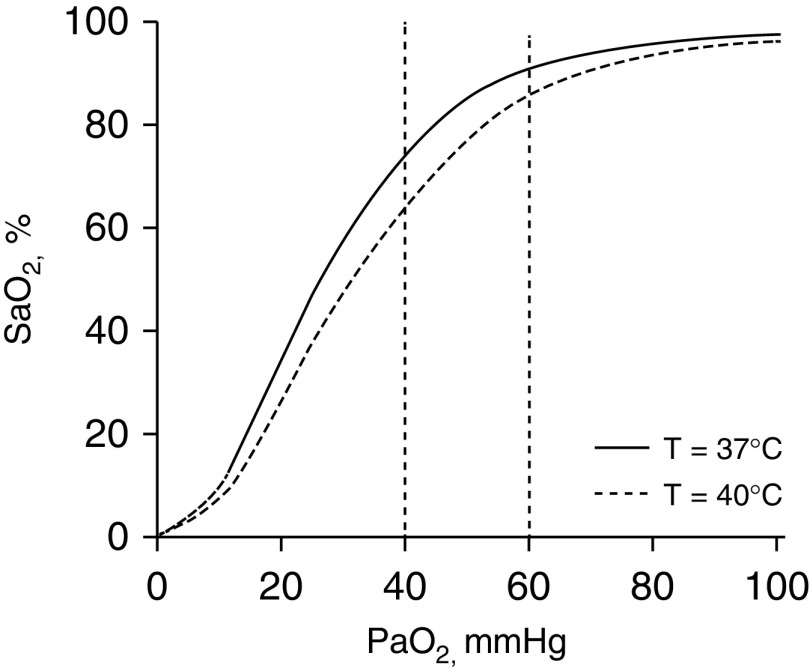
A shift in the oxygen dissociation curve is another confounding factor. Fever, prominent with COVID-19, causes the curve to shift to the right; any given PaO2 will be associated with a lower SaO2 (Figure 3). At a temperature of 37°C, a PaO2 of 60 mm Hg (at normal pH and PaCO2) will be accompanied by an SaO2 of 91.1%. Temperature elevation to 40°C will produce an SaO2 of 85.8% (5.3% decrease) (31). Respective numbers for a PaO2 of 40 mm Hg are an SaO2 of 74.1% at a temperature of 37°C and an SaO2 of 64.2% at a temperature of 40°C (9.9% decrease) (31). These shifts produce substantial desaturations without change in chemoreceptor stimulation (because carotid bodies respond only to PaO2 and not SaO2) (9)—another factor contributing to silent hypoxemia.
Figure 3.
Relationship between arterial oxygen tension (PaO2) and percentage saturation of hemoglobin with oxygen (SaO2) at temperature 37°C (continuous line) and 40°C (dashed line), with a constant pH 7.40 and Pco2 of 40 mm Hg (generated with digital subroutine of Kelman [31]). At a PaO2 of 60 mm Hg, SaO2 is 91.1% at 37°C and decreases to 85.8% at 40°C. At a PaO2 of 40 mm Hg, SaO2 is 74.1% at 37°C and decreases to 64.2% at 40°C.
Mechanism of Silent Hypoxemia
Given that patients with COVID-19 exhibit several unusual findings, it is possible the virus has an idiosyncratic effect on the respiratory control system.
ACE 2 (angiotensin-converting enzyme 2), the cell receptor of SARS-CoV-2, the virus responsible for COVID-19, is expressed in the carotid body, the site at which chemoreceptors sense oxygen (32). ACE2 receptors are also expressed in nasal mucosa. Anosmia-hyposmia occurs in two-thirds of patients with COVID-19 (33), and the olfactory bulb provides a passage along which certain coronaviruses enter the brain (34). Whether SARS-CoV-2 gains access to the brain through the olfactory bulb and contributes to the association between anosmia-hyposmia and dyspnea (33) and whether ACE2 receptors play a role in the depressed dyspnea response in COVID-19 remain to be determined.
Science (2) links silent hypoxemia with the development of thrombi within the pulmonary vasculature. Increased thrombogenesis has been noted in patients with COVID-19 (35). Thrombi within the pulmonary vasculature can cause severe hypoxemia, and dyspnea is related to pulmonary vascular obstruction and its consequences (36). Dyspnea can also arise from the release of histamine or stimulation of juxtacapillary receptors within the pulmonary vasculature. No biological mechanism exists, however, whereby thrombi in the pulmonary vasculature cause blunting of dyspnea (producing silent hypoxemia).
Definition of Hypoxemia
Stedman’s Medical Dictionary defines hypoxemia as “subnormal oxygenation of arterial blood, short of anoxia” (37). Clinicians, however, need to be mindful of the inverse relationship between PaO2 and age; a PaO2 of 66 mm Hg can be normal in an 80-year-old person (38, 39). In the 1990s, hypoxemia was commonly viewed as low PaO2, and FiO2 was excluded from consideration (40, 41). Pierson, for example, specified that a mechanically ventilated patient with acute respiratory distress syndrome receiving 100% oxygen and a PaO2 of 80 mm Hg should not be labeled hypoxemic (42).
There is, of course, no pure essentialist definition of hypoxemia—merely a usage (40). To arrive at a present-day nominalist definition of hypoxemia, it appears that few physicians view hypoxemia in the same manner as Pierson. In our informal poll of physicians caring for patients with COVID-19, we specified “I am NOT looking for oxygen requirements, like the number of liters being delivered.” Yet 77.3% of the respondents provided considerable detail on the amount of supplemental oxygen, and 36.4% viewed an SpO2 of 90% or higher as compatible with hypoxemia. Although a more detailed investigation is necessary, it appears that physicians today commonly define hypoxemia in terms of the amount of oxygen being supplied to a patient.
Judging severity of hypoxemia on the basis of supplemental oxygen is inherently problematic because FiO2 is impossible to estimate unless a patient is intubated or breathing room air. With a nasal cannula at 2 L/min, FiO2 ranges between 24% and 35% (43). To minimize risk of hypoxemia, physicians frequently prescribe oxygen at an amount far exceeding physiological needs. Given the flatness of the upper oxygen dissociation curve, a pulse oximetry reading of 95% can signify a PaO2 of anywhere between 60 and 200 mm Hg (26, 44)—values that signify markedly different amounts of gas-exchange impairment, especially in a patient receiving a high FiO2.
Given that hypoxemia is at the very heart of the most severe cases of COVID-19, one wonders if the lack of a widely accepted definition of hypoxemia contributes to some of the confusion and counterclaims associated with the disease.
In conclusion, COVID-19 has engendered many surprises, but features that baffle physicians are less strange when contemplated through the lens of long-established principles of respiratory physiology (45).
Acknowledgments
Acknowledgment
The authors thank Dr. Marina Saad for the construction of the oxygen dissociation curve based on the digital subroutine of Kelman.
Footnotes
Supported by the National Institute of Nursing Research (grant R01-NR016055) and Merit Review Award from the Veterans Administration Research (1 I01 RX002803-01A1).
Originally Published in Press as DOI: 10.1164/rccm.202006-2157CP on June 15, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Toy S, Roland D.Some doctors pull back on using ventilators to treat Covid-19 The Wall Street Journal 2020 May 11
- 2.Couzin-Frankel J. The mystery of the pandemic’s ‘happy hypoxia’. Science. 2020;368:455–456. doi: 10.1126/science.368.6490.455. [DOI] [PubMed] [Google Scholar]
- 3.Levitan R.The infection that’s silently killing coronavirus patients The New York Times 2020 Apr 20
- 4.Preas HL, II, Jubran A, Vandivier RW, Reda D, Godin PJ, Banks SM, et al. Effect of endotoxin on ventilation and breath variability: role of cyclooxygenase pathway. Am J Respir Crit Care Med. 2001;164:620–626. doi: 10.1164/ajrccm.164.4.2003031. [DOI] [PubMed] [Google Scholar]
- 5.Tobin MJ. Basing respiratory management of COVID-19 on physiological principles. Am J Respir Crit Care Med. 2020;201:1319–1320. doi: 10.1164/rccm.202004-1076ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobin MJ. Dyspnea: pathophysiologic basis, clinical presentation, and management. Arch Intern Med. 1990;150:1604–1613. doi: 10.1001/archinte.150.8.1604. [DOI] [PubMed] [Google Scholar]
- 7.Banzett RB, Similowski T, Brown R.Addressing respiratory discomfort in the ventilated patient Tobin MJ.editor. Principles and practice of mechanical ventilation3rd edNew York: McGraw-Hill Inc.20121265–1280. [Google Scholar]
- 8.Banzett RB, Lansing RW, Evans KC, Shea SA. Stimulus-response characteristics of CO2-induced air hunger in normal subjects. Respir Physiol. 1996;103:19–31. doi: 10.1016/0034-5687(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 9.Tobin MJ, Laghi F, Jubran A. Ventilatory failure, ventilator support, and ventilator weaning. Compr Physiol. 2012;2:2871–2921. doi: 10.1002/cphy.c110030. [DOI] [PubMed] [Google Scholar]
- 10.Tobin MJ, Gardner WN.Monitoring of the control of ventilation Tobin MJ.editor. Principles and practice of intensive care monitoringNew York: McGraw-Hill, Inc.1998415–464. [Google Scholar]
- 11.Weil JV, Byrne-Quinn E, Sodal IE, Friesen WO, Underhill B, Filley GF, et al. Hypoxic ventilatory drive in normal man. J Clin Invest. 1970;49:1061–1072. doi: 10.1172/JCI106322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moosavi SH, Golestanian E, Binks AP, Lansing RW, Brown R, Banzett RB. Hypoxic and hypercapnic drives to breathe generate equivalent levels of air hunger in humans. J Appl Physiol (1985) 2003;94:141–154. doi: 10.1152/japplphysiol.00594.2002. [DOI] [PubMed] [Google Scholar]
- 13.Mohan R, Duffin J. The effect of hypoxia on the ventilatory response to carbon dioxide in man. Respir Physiol. 1997;108:101–115. doi: 10.1016/s0034-5687(97)00024-8. [DOI] [PubMed] [Google Scholar]
- 14.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. and the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kronenberg RS, Drage CW. Attenuation of the ventilatory and heart rate responses to hypoxia and hypercapnia with aging in normal men. J Clin Invest. 1973;52:1812–1819. doi: 10.1172/JCI107363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson DD, Pack AI, Silage DA, Fishman AP. Effects of aging on ventilatory and occlusion pressure responses to hypoxia and hypercapnia. Am Rev Respir Dis. 1981;124:387–391. doi: 10.1164/arrd.1981.124.4.387. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura M, Miyamoto K, Suzuki A, Yamamoto H, Tsuji M, Kishi F, et al. Ventilatory and heart rate responses to hypoxia and hypercapnia in patients with diabetes mellitus. Thorax. 1989;44:251–257. doi: 10.1136/thx.44.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisbrod CJ, Eastwood PR, O’Driscoll G, Green DJ. Abnormal ventilatory responses to hypoxia in Type 2 diabetes. Diabet Med. 2005;22:563–568. doi: 10.1111/j.1464-5491.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- 19.O’Donnell CR, Friedman LS, Russomanno JH, Rose RM. Diminished perception of inspiratory-resistive loads in insulin-dependent diabetics. N Engl J Med. 1988;319:1369–1373. doi: 10.1056/NEJM198811243192102. [DOI] [PubMed] [Google Scholar]
- 20.Tobin MJ, Mador MJ, Guenther SM, Lodato RF, Sackner MA. Variability of resting respiratory drive and timing in healthy subjects. J Appl Physiol (1985) 1988;65:309–317. doi: 10.1152/jappl.1988.65.1.309. [DOI] [PubMed] [Google Scholar]
- 21.Swenson ER, Duncan TB, Goldberg SV, Ramirez G, Ahmad S, Schoene RB. Diuretic effect of acute hypoxia in humans: relationship to hypoxic ventilatory responsiveness and renal hormones. J Appl Physiol (1985) 1995;78:377–383. doi: 10.1152/jappl.1995.78.2.377. [DOI] [PubMed] [Google Scholar]
- 22.Matsuzawa Y, Fujimoto K, Kobayashi T, Namushi NR, Harada K, Kohno H, et al. Blunted hypoxic ventilatory drive in subjects susceptible to high-altitude pulmonary edema. J Appl Physiol (1985) 1989;66:1152–1157. doi: 10.1152/jappl.1989.66.3.1152. [DOI] [PubMed] [Google Scholar]
- 23.McGurk SP, Blanksby BA, Anderson MJ. The relationship of hypercapnic ventilatory responses to age, gender and athleticism. Sports Med. 1995;19:173–183. doi: 10.2165/00007256-199519030-00003. [DOI] [PubMed] [Google Scholar]
- 24.Jubran A, Tobin MJ. Effect of isocapnic hypoxia on variational activity of breathing. Am J Respir Crit Care Med. 2000;162:1202–1209. doi: 10.1164/ajrccm.162.4.9907003. [DOI] [PubMed] [Google Scholar]
- 25.Bickler PE, Feiner JR, Lipnick MS, Batchelder P, MacLeod DB, Severinghaus JW. Effects of acute, profound hypoxia on healthy humans: implications for safety of tests evaluating pulse oximetry or tissue oximetry performance. Anesth Analg. 2017;124:146–153. doi: 10.1213/ANE.0000000000001421. [DOI] [PubMed] [Google Scholar]
- 26.Jubran A.Pulse oximetry Tobin MJ.editor. Principles and practice of intensive care monitoringNew York: McGraw-Hill, Inc.1998261–287. [Google Scholar]
- 27.Ottestad W, Hansen TA, Pradhan G, Stepanek J, Høiseth LØ, Kåsin JI. Acute hypoxia in a simulated high-altitude airdrop scenario due to oxygen system failure. J Appl Physiol (1985) 2017;123:1443–1450. doi: 10.1152/japplphysiol.00169.2017. [DOI] [PubMed] [Google Scholar]
- 28.Ottestad W, Kåsin JI, Høiseth LØ. Arterial oxygen saturation, pulse oximetry, and cerebral and tissue oximetry in hypobaric hypoxia. Aerosp Med Hum Perform. 2018;89:1045–1049. doi: 10.3357/AMHP.5173.2018. [DOI] [PubMed] [Google Scholar]
- 29.Van de Louw A, Cracco C, Cerf C, Harf A, Duvaldestin P, Lemaire F, et al. Accuracy of pulse oximetry in the intensive care unit. Intensive Care Med. 2001;27:1606–1613. doi: 10.1007/s001340101064. [DOI] [PubMed] [Google Scholar]
- 30.Jubran A, Tobin MJ. Reliability of pulse oximetry in titrating supplemental oxygen therapy in ventilator-dependent patients. Chest. 1990;97:1420–1425. doi: 10.1378/chest.97.6.1420. [DOI] [PubMed] [Google Scholar]
- 31.Kelman GR. Digital computer subroutine for the conversion of oxygen tension into saturation. J Appl Physiol. 1966;21:1375–1376. doi: 10.1152/jappl.1966.21.4.1375. [DOI] [PubMed] [Google Scholar]
- 32.Fung ML. Expressions of angiotensin and cytokine receptors in the paracrine signaling of the carotid body in hypoxia and sleep apnea. Respir Physiol Neurobiol. 2015;209:6–12. doi: 10.1016/j.resp.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Sedaghat AR, Gengler I, Speth MM. Olfactory dysfunction: a highly prevalent symptom of COVID-19 with public health significance. Otolaryngol Head Neck Surg. 2020;5:194599820926464. doi: 10.1177/0194599820926464. [DOI] [PubMed] [Google Scholar]
- 34.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wichmann D, Sperhake J-P, Lütgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study Ann Intern Med[online ahead of print] 6 May 2020; DOI: 10.7326/M20-2003 [DOI] [PMC free article] [PubMed]
- 36.Sanchez O, Caumont-Prim A, Riant E, Plantier L, Dres M, Louis B, et al. Pathophysiology of dyspnoea in acute pulmonary embolism: a cross-sectional evaluation. Respirology. 2017;22:771–777. doi: 10.1111/resp.12961. [DOI] [PubMed] [Google Scholar]
- 37.Stedman, Lathrop T.Stedman’s medical dictionary25th edBaltimore: Williams & Wilkins; 1990756 [Google Scholar]
- 38.Sorbini CA, Grassi V, Solinas E, Muiesan G. Arterial oxygen tension in relation to age in healthy subjects. Respiration. 1968;25:3–13. doi: 10.1159/000192549. [DOI] [PubMed] [Google Scholar]
- 39.Malmberg P, Hedenström H, Fridriksson HV. Reference values for gas exchange during exercise in healthy nonsmoking and smoking men. Bull Eur Physiopathol Respir. 1987;23:131–138. [PubMed] [Google Scholar]
- 40.West JB.Pulmonary pathophysiology.Baltimore: Williams and Wilkins; 1977158 [Google Scholar]
- 41.Murray JF.The normal lung2nd edPhiladelphia: WB Saunders; 1986253 [Google Scholar]
- 42.Pierson DJ.Pathophysiology and clinical effects of chronic hypoxia Respir Care 20004539–51.[Discussion pp, 51–53.] [PubMed] [Google Scholar]
- 43.Bazuaye EA, Stone TN, Corris PA, Gibson GJ. Variability of inspired oxygen concentration with nasal cannulas. Thorax. 1992;47:609–611. doi: 10.1136/thx.47.8.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:599–602. doi: 10.1152/jappl.1979.46.3.599. [DOI] [PubMed] [Google Scholar]
- 45.Tobin MJ. Why physiology is critical to the practice of medicine: a 40-year personal perspective. Clin Chest Med. 2019;40:243–257. doi: 10.1016/j.ccm.2019.02.012. [DOI] [PubMed] [Google Scholar]