To the Editor:
Primary ciliary dyskinesia (PCD), a rare, genetically heterogeneous disease associated with mutations in >45 different genes, (1) is clinically characterized by neonatal respiratory distress, organ laterality defects, persistent rhinosinusitis, chronic bronchitis, and, ultimately, bronchiectasis. Historically, PCD has been difficult to diagnose because no single test identifies all cases. Published in 2018, the American Thoracic Society Clinical Practice Guideline recommended several PCD diagnostic tools, including extended genetic panel testing, transmission electron microscopy (TEM) assessment of the ciliary axoneme, and nasal nitric oxide measurement (nNO) (2). Although the mechanism is unknown, nNO levels are reproducibly reduced in most cases of PCD. When compared with a reference standard of TEM and/or genetic testing, nNO levels <77 nl/min are >95% sensitive and specific in identifying PCD in cooperative patients 5 years and older, who have compatible clinical phenotypes, after excluding cystic fibrosis (2, 3).
Recent editorials have misinterpreted the American Thoracic Society guideline recommendations, stating that nNO levels >77 nl/min exclude PCD (4). Before publication of the guideline, it was well established that limited individuals with certain PCD-associated genes (e.g., RSPH1, GAS8, RPGR, or CCNO) had nNO results >77 nl/min. Ten additional PCD-causing genes (e.g., CCDC103, CFAP221, DNAH9, FOXJ1, GAS2L2, LRRC56, NEK10, SPEF2, STK36, or TTC12) associated with nNO values >77 nl/min have been discovered since guideline development. Nonetheless, the proportion of affected individuals with higher nNO levels still accounts for <5% of known PCD cases (1). Thus, nNO concentrations >77 nl/min do not exclude the diagnosis of PCD, analogous to a normal sweat chloride measurement not excluding cystic fibrosis.
Moreover, a reduced nNO level alone is not diagnostic for PCD. When using established, standardized protocols (5), meta-analysis shows that low nNO measurements are “comparable to that of TEM and/or genetic testing”; however, the guideline states that “patients should still progress to further corroborative PCD diagnostic studies” (2). This recommendation is underscored by recent observations that some patients with primary immunodeficiency, a heterogeneous group of disorders sharing clinical features with PCD, can also have reduced nNO levels (6). In these cases, misdiagnosis may have serious consequences. Several individuals with chronic suppurative respiratory disease and low nNO values, evaluated in the Genetic Disorders of Mucociliary Clearance Consortium, were ultimately diagnosed with primary immunodeficiency, and some developed secondary malignancies or required stem cell transplantation. Though relatively uncommon, the risk of life-threatening complications in clinically overlapping diseases should give pause to using nNO levels as a single diagnostic test for PCD.
Given this, repeatedly low nNO values with a compatible phenotype can be presumptively diagnostic for PCD, but additional studies are absolutely required for corroboration. In patients who have persistently low nNO levels, if TEM or genetic testing fail to confirm the diagnosis of PCD, an immunology consultation should be considered while initiating PCD therapies. This approach is shown in a revised diagnostic algorithm (Figure 1), which has been reviewed and agreed upon by the entire guideline committee. Subsequent iterations of the guideline will reflect these changes and strive to further improve diagnostic accuracy in patients with PCD.
Figure 1.
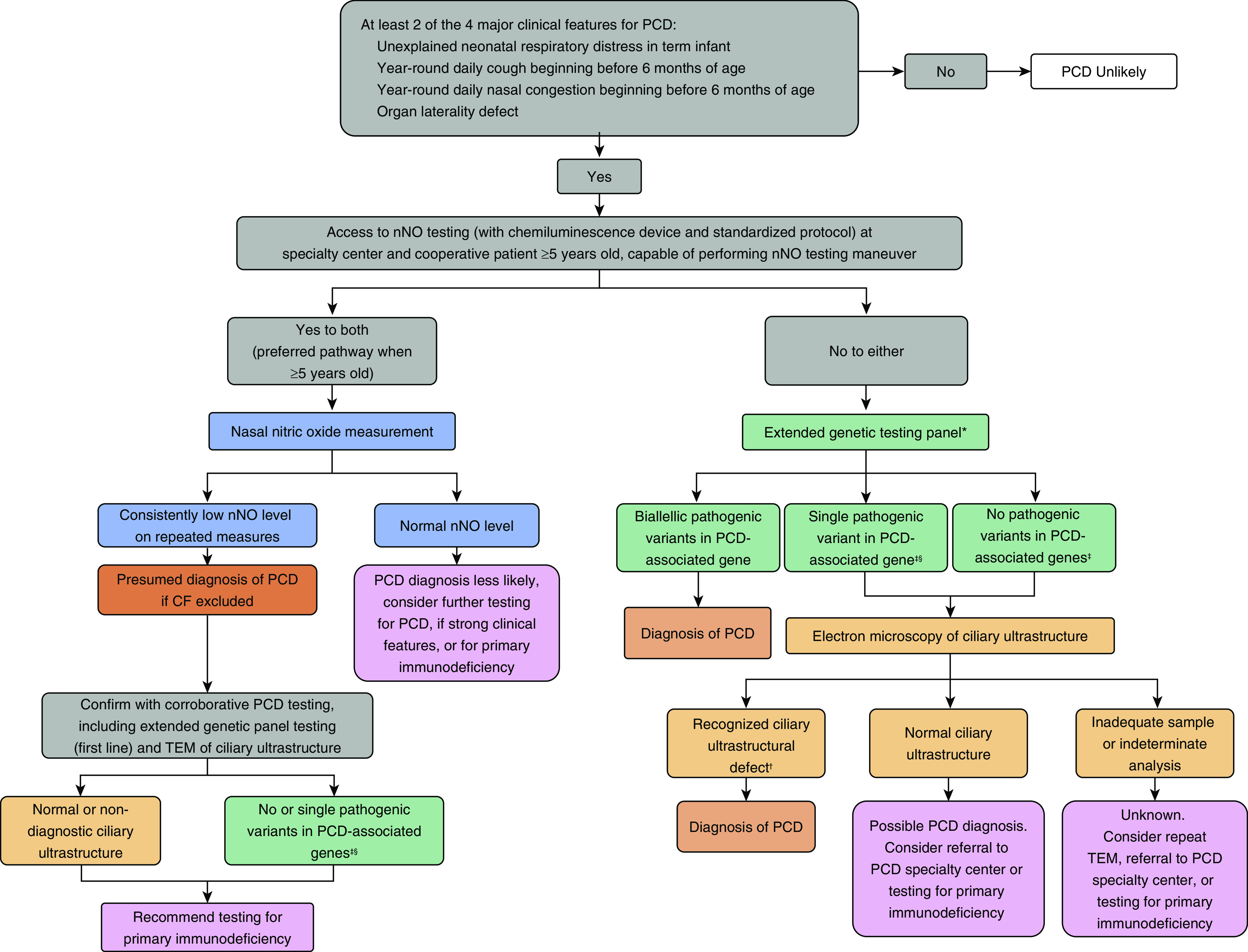
Suggested diagnostic algorithm for evaluating a patient with suspected PCD. *Genetic panels testing for mutations in >12 disease-associated PCD genes, including deletion/duplication analysis. †Known disease-associated TEM ultrastructural defects include outer dynein arm defects, outer dynein arm plus inner dynein arm (IDA) defects, IDA defect with microtubular disorganization, and absent central pair, identified using established criteria (1). Of note, the presence of IDA defects alone is rarely diagnostic for PCD. ‡In genes associated with autosomal recessive trait. §Or presence of variants of unknown significance. Adapted from Reference 2. CF = cystic fibrosis; nNO = nasal nitric oxide; PCD = primary ciliary dyskinesia; TEM = transmission electron microscopy.
Footnotes
Author Contributions: All authors assisted with drafting of this letter and agree with the contents.
Originally Published in Press as DOI: 10.1164/rccm.202003-0835LE on April 24, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Zariwala MA, Knowles MR, Leigh MW. et al. Primary ciliary dyskinesia. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, editors; GeneReviews. Seattle, WA: University of Washington; 1993. [PubMed] [Google Scholar]
- 2.Shapiro AJ, Davis SD, Polineni D, Manion M, Rosenfeld M, Dell SD, et al. American Thoracic Society Assembly on Pediatrics. Diagnosis of primary ciliary dyskinesia: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2018;197:e24–e39. doi: 10.1164/rccm.201805-0819ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leigh MW, Hazucha MJ, Chawla KK, Baker BR, Shapiro AJ, Brown DE, et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc. 2013;10:574–581. doi: 10.1513/AnnalsATS.201305-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas JS, Rubbo B, Jackson CL, Hirst RA, Hogg C, O’Callaghan C, et al. Response. Chest. 2019;156:1033–1034. doi: 10.1016/j.chest.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro AJ, Dell SD, Gaston B, O’Connor M, Marozkina N, Manion M, et al. Nasal nitric oxide measurement in primary ciliary dyskinesia: a technical paper on standardized testing protocols. Ann Am Thorac Soc. 2020;17:e1–e12. doi: 10.1513/AnnalsATS.201904-347OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zysman-Colman ZN, Kaspy KR, Alizadehfar R, NyKamp KR, Zariwala MA, Knowles MR, et al. Nasal nitric oxide in primary immunodeficiency and primary ciliary dyskinesia: helping to distinguish between clinically similar diseases. J Clin Immunol. 2019;39:216–224. doi: 10.1007/s10875-019-00613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


