To the Editor:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a major worldwide health threat in just a few weeks (1). ICU admission and the recourse to extracorporeal organ support, such as continuous renal replacement therapy (CRRT) or venovenous extracorporeal membrane oxygenation (VV-ECMO) may be needed in the most severe forms of the disease (2). Because SARS-CoV-2 viremia has been reported in some cases (3), it has been hypothesized that this small virus (average size of 125 nm) (4) could pass through polymethylpentene ECMO membranes or acrylonitrile/sodium methallylsulfonate CRRT membranes. In this study, we investigated whether SARS-CoV-2 RNA was detected in the dialysis effluent fluid or in the condensate collected from the ECMO membrane exhalation port (gas outlet) when the virus was present in the lower respiratory tract and the plasma.
Methods
We evaluated consecutive patients admitted to three university ICUs in Paris who had severe SARS-CoV-2 infection and required CRRT (hemodiafiltration or hemofiltration), VV-ECMO, or both. Samples were obtained from respiratory tract, plasma, the dialysis effluent fluid, and from 5 to 10 ml of condensate collected from the ECMO membrane gas outlet within 48 hours after ECMO initiation. Real-time RT-PCR targeting the E (envelope) gene of SARS-CoV-2 was performed as previously described (5). The cycle threshold (CT) values of RT-PCR were used as indicators of the RNA viral load in samples: the lower the CT, the higher the RNA viral load. The estimated probability (95% confidence interval [CI]) and the binomial probability of SARS-CoV-2 in the gas outlet and the dialysis fluid were reported, respectively. Ethical approval was applied to our local ethics committee (CER Sorbonne University, N°2020–CER-2020–32).
Results
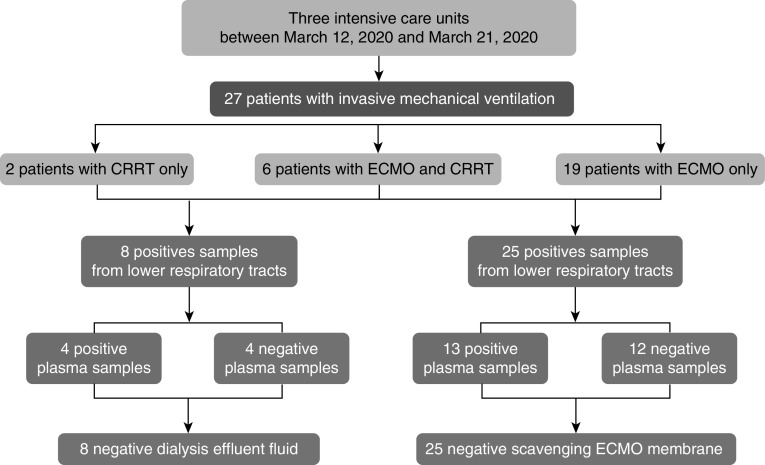
All 27 patients were on mechanical ventilation, and 25/27 were supported by VV-ECMO (20 patients with Quadrox oxygenator [Getinge] and 5 patients with Oxymedos oxygenator [Xenios]). In addition, 8/27 patients received CRRT (Prismaflex, Baxter). CRRT was administered using hemofiltration in four patients and hemodiafiltration in four patients. Main findings are presented in Figure 1. SARS-CoV-2 RNA was detected in all samples from patients’ lower respiratory tract (median CT, 28; 25–75% interquartile range, 22–31) and in the plasma of 13/27 of them (median CT, 29; interquartile range, 29–30). However, SARS-CoV-2 RNA was not detected in the membrane oxygenator gas outlet condensate, whether plasma RNA was positive (n = 13/25) or negative (n = 12/25). Similarly, SARS-CoV-2 RNA was not present in the dialysis effluent of the eight patients on CRRT whether plasma PCR was positive (n = 4/8) or negative (n = 4/8). Therefore, the estimated probability of a positive SARS-CoV-2 RNA in the membrane oxygenator gas outlet condensate and in the dialysis fluid were 0.0 (95% CI, 0.00–0.14) and 0.0 (95% CI, 0.00–0.37), respectively. On the basis of binomial probabilities of our results, the prevalence of a positive SARS-CoV-2 RNA in the ECMO gas outlet and in the dialysis fluid will likely be lower than 11% and 31%, respectively. Individual data for SARS-CoV-2 RNA detection in lower respiratory tracts, plasma, dialysis fluid, and ECMO membrane are given in Table 1 with the CT values resulting from PCR for lower respiratory tracts (column 2) and plasma (column 3).
Figure 1.
Detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in dialysis effluent fluid or in the exhalation port of the extracorporeal membrane oxygenation membrane according to plasma detection of the viral RNA. CRRT = continuous renal replacement therapy; ECMO = extracorporeal membrane oxygenation.
Table 1.
Detection of SARS-CoV-2 RNA in the Condensate Collected from the ECMO Membrane Gas Outlet and in the Dialysis Effluent Fluid
| Lower Respiratory Tracts RT-PCR (CT) | Plasma RT-PCR (CT) | Dialysis RT-PCR (CT) | ECMO Membrane Gas Outlet RT-PCR (CT) | |
|---|---|---|---|---|
| Patient 1 | 32 | Undetectable | No CRRT | Undetectable |
| Patient 2 | 13 | 29 | Undetectable | Undetectable |
| Patient 3 | Positive* | 33 | Undetectable | Undetectable |
| Patient 4 | 28 | Undetectable | Undetectable | No ECMO |
| Patient 5 | Positive* | Undetectable | Undetectable | No ECMO |
| Patient 6 | 29 | 30 | No CRRT | Undetectable |
| Patient 7 | 35 | Undetectable | No CRRT | Undetectable |
| Patient 8 | 21 | Undetectable | No CRRT | Undetectable |
| Patient 9 | 24 | 28 | No CRRT | Undetectable |
| Patient 10 | 24 | Undetectable | Undetectable | Undetectable |
| Patient 11 | 19 | 30 | Undetectable | Undetectable |
| Patient 12 | 26 | 30 | Undetectable | Undetectable |
| Patient 13 | 29 | Undetectable | No CRRT | Undetectable |
| Patient 14 | 36 | Undetectable | No CRRT | Undetectable |
| Patient 15 | 33 | Undetectable | No CRRT | Undetectable |
| Patient 16 | Positive* | 50 | No CRRT | Undetectable |
| Patient 17 | Positive* | Undetectable | No CRRT | Undetectable |
| Patient 18 | 18 | 29 | No CRRT | Undetectable |
| Patient 19 | 18 | 30 | No CRRT | Undetectable |
| Patient 20 | 27 | Undetectable | No CRRT | Undetectable |
| Patient 21 | 15 | 28 | No CRRT | Undetectable |
| Patient 22 | 30 | Undetectable | No CRRT | Undetectable |
| Patient 23 | 30 | 29 | Undetectable | Undetectable |
| Patient 24 | 29 | Undetectable | No CRRT | Undetectable |
| Patient 25 | 23 | 29 | No CRRT | Undetectable |
| Patient 26 | 33 | Undetectable | No CRRT | Undetectable |
| Patient 27 | 32 | 28 | No CRRT | Undetectable |
Definition of abbreviations: CRRT = continuous renal replacement therapy; CT = cycle threshold; ECMO = extracorporeal membrane oxygenation; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Patient tested positive before being transferred in our center; no CT was provided.
Discussion
To the best of our knowledge, this is the first study that investigated the risks for SARS-CoV-2 dissemination through membranes used for extra corporeal organ support in critically ill patients. Though a recent report revealed that SARS-CoV-2 is almost always present in the lower respiratory tract, sometimes in the feces but never in urine samples (3), our findings are reassuring regarding the risk of contamination for ICU professionals when treating patients on VV-ECMO or CRRT. Specifically, our findings do not support the routine use of a viral filter on the exhaust of the commonly used polymethylpentene-based ECMO membrane lungs. Prevention and education of healthcare workers should therefore remain focused on limiting the risks of virus spreading during invasive respiratory procedures, such as high-flow oxygenation, mouth care, intubation, or microbiological sampling of nasopharyngeal, tracheal, or bronchioalveolar secretions. The number of patients with CRRT (n = 8) is limited, but the fact that SARS-CoV-2 PCR was negative in all dialysis effluent is somehow reassuring. Lastly, we cannot rule out that longer ECMO runs could progressively lead to membrane alteration, plasma leakage, and ultimately SARS-CoV-2 aerosolization. However, we purposely chose to investigate the risk of virus spreading within 48 hours after ECMO and CRRT initiation as the viral load—if present in the plasma—is expected to progressively decline afterward. Though our findings may not alter practices, they may contribute to address legitimate interrogations raised by caregivers and reinforce adhesion and trust into infection control measure policies, which is likely to play a major role against the outbreak spreading.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202004-1339LE on January 11, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
Contributor Information
Collaborators: for the Groupe de Recherche Clinique RESPIRE
References
- 1.MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. doi: 10.1001/jama.2020.2342. [online ahead of print] 19 Feb 2020; DOI: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 2.Ramanathan K, Antognini D, Combes A, Paden M, Zakhary B, Ogino M, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8:518–526. doi: 10.1016/S2213-2600(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher D, Heymann D. Q&A: the novel coronavirus outbreak causing COVID-19. BMC Med. 2020;18:57. doi: 10.1186/s12916-020-01533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]