Abstract
The long-term ecological impacts of the Exxon Valdez oil spill (EVOS) are compared to two extensively studied and more recent large spills: Deepwater Horizon (DWH) and the Hebei Spirit oil spill (HSOS). Each of the three spills differed in magnitude and duration of oil released, environmental conditions, ecological communities, response and clean up measures, and ecological recovery. The EVOS began on March 24, 1989 and released 40.8 million liters of Alaska North Slope crude oil into the cold, nearly pristine environment of Prince William Sound, Alaska. EVOS oiled wildlife and rocky intertidal shorelines and exposed early life stages of fish to embryotoxic levels of polycyclic aromatic hydrocarbons (PAH). Long-term impacts following EVOS were observed on seabirds, sea otters, killer whales, and subtidal communities. The DWH spill began on April 10, 2010 and released 507 million liters of light Louisiana crude oil from 1600 m on the ocean floor into the Gulf of Mexico over an 87-day period. The DWH spill exposed a diversity of complex aquatic communities in the deep ocean, offshore pelagic areas, and coastal environments. Large scale persistent ecological effects included impacts to deep ocean corals, failed recruitment of oysters over multiple years, damage to coastal wetlands, and reduced dolphin, sea turtle, and seabird populations. The HSOS began on December 7, 2007 and released approximately 13 million liters of Middle Eastern crude oils into ecologically sensitive areas of the Taean area of western Korea. Environmental conditions and the extensive initial cleanup of HSOS oil stranded on shorelines limited the long-term impacts to changes in composition and abundance of intertidal benthic communities. Comparison of EVOS, DWH, and HSOS show the importance and complexity of the interactions among environment, oil spill dynamics, the affected ecological systems, and response actions.
1. INTRODUCTION
Although the number and total volume of oil spilled annually continues to generally decline, catastrophic releases continue to occur from the production and transport of petroleum (1,2). Three of the largest global oil spills over the last three decades have received intensive scientific study: The March 1989 Exxon Valdez oil spill (EVOS) in Prince William Sound, Alaska; the December 2007 Hebei Spirit oil spill (HSOS) off the western coast of the Republic of Korea; and the 2010 Deepwater Horizon (DWH) release in the northern Gulf of Mexico. In reviewing spills prior to HSOS and DWH, Kingston (3) concluded that oiling from large scale spills can persist for several years, with impacts in salt marshes and mangrove swamps potentially persisting for decades such as in the case of the West Falmouth release (Massachusetts, USA; 4). Environmental recovery from large scale oil spills typically occurs within 2 to 10 years, with long-term impacts generally confined to changes in community structure that persist because of the longevity of the affected species (3, 5). In contrast, spills without extensive releases of oil and limited duration and spatial extent have been generally considered to not have long-term environmental impacts (3).
Enough time has passed to determine longer term impacts of the DWH spill, and to put it in historical context with EVOS and HSOS as two other catastrophic and intensively studied spills over the last three decades. Each of the three spills differed in magnitude and duration of oil released, environmental conditions, ecological communities, response and clean up measures, and ecological recovery. HSOS is less known and unique among the world’s catastrophic oil spills because the massive emergency cleanup responses limited long-term ecological impacts and facilitated more rapid ecosystem recovery than other global spill events. Recent reviews of the ecological impacts of oil major oil spills have focused on either single spills or only in North America (6, 7, 8, 9). Comparing these three large-scale oil spills can provide insights into the complex interactions among environment, spill dynamics, ecological systems, and response that single spill assessments may not provide.
2. EXXON VALDEZ OIL SPILL
2.1. Summary of Spill
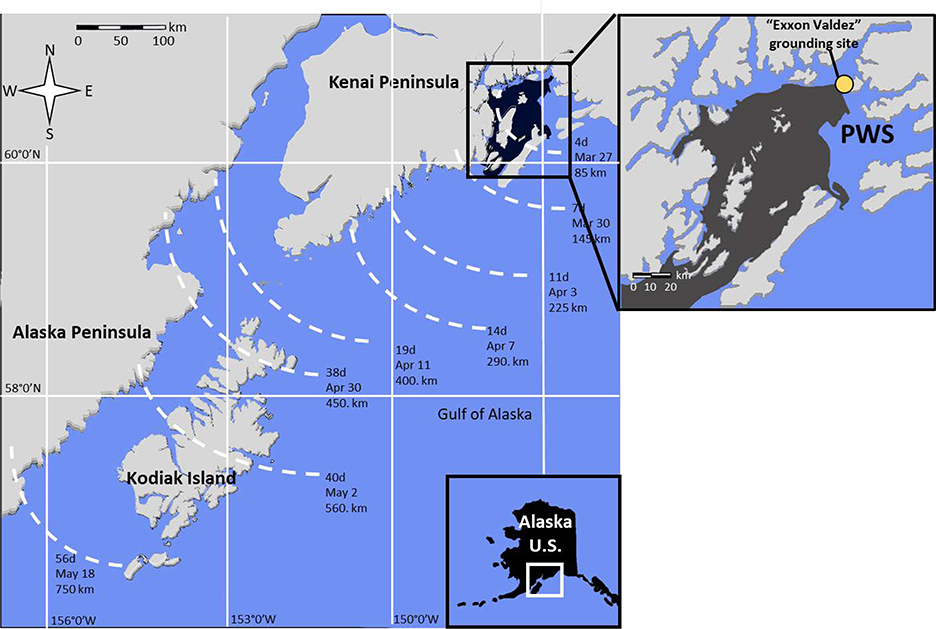
On March 24, 1989 the TV Exxon Valdez left Port Valdez, Alaska and ran aground on Bligh Reef, about 43 km to the southwest in Prince William Sound, AK USA. Prince William Sound is a subarctic semi enclosed sea surrounded by mountains, glaciers, rivers, bays and fjords in south central Alaska. Sea surface temperatures in the affected areas range from 4 to 13 °C. The vessel spilled at least 40.8 million liters (35,500 tonnes) of Alaska North Slope crude oil (10), a medium weight crude with PAHs comprising approximately 1.1% of the unweathered mass. Oil leaked from the vessel into calm seas for 2.5 days before a storm drove the slick towards the southwest where 47% to 56% of it made landfall (11) (Fig. 1). Oil contaminated 738 km of shoreline in Prince William Sound and then exited into the Gulf of Alaska where it oiled another 2100 km of shoreline along the Alaska Peninsula through Shelikof Strait including Cook Inlet and Kodiak Island (12, 13) (Figure 1). By 1991, only 14% of the spilled oil had been recovered during clean-up operations (10), but surface deposits had substantially decreased (14) indicating the importance of storm and tidal action in oil removal (10). However, surveys conducted 12 years after the spill continued to find surface deposits in Prince William Sound (15).
Figure 1.
Map showing the extent of oiling in Prince William Sound (PWS) and the Gulf of Alaska. Darker areas show heavy oiling and dashed lines show the time that oil was first observed and the distance from the Bligh Reef grounding site. Inset: location within State of Alaska, USA. Figure adapted from Peterson et al. (13), Galt et al. (16), Stringer et al. (17), and Iverson and Esler 2010 (18).
2.2. Long-term Ecological Effects and Recovery
EVOS can be viewed historically as a large-scale ecosystem experiment because the absence of substantial existing anthropogenic impacts in the spill area facilitated the identification of adverse effects (19). The identified impacts and associated mechanisms altered the prevailing view of oil spill impacts (13) by highlighting the persistence of oil in contaminated habitats, the effects of environmentally persistent 3–5 ring PAH, and the duration of impacts in affected species. At the time of the spill, conventional reasoning was that impacts would be limited to the narcotic effects of the single ring aromatics (benzene, toluene, ethylbenzene and xylenes; BTEX). Consequently, the rapid dissolution of volatile single ring aromatics led to some initial conclusions of minimal biological impacts in the water column (20). The remote location of Prince William Sound and limited response preparation resulted in marginal oil recovery during the initial phase of the spill when seas were calm. The subsequent storm mixed the oil with sea water before it made landfall. Samples of the stranded oil collected within 11 d of the spill demonstrated that the most volatile aromatics had evaporated from the oil (21). Stranded oil was characterized by only trace levels of single ring aromatics and relatively high concentrations of PAH with two or more alkyl substitutions. However, mortality among pink salmon (Oncorhynchus gorbuscha) embryos incubating in the intertidal zone of contaminated beaches remained elevated for four years after the spill, indicating that subsurface oil was exerting toxic effects (22,23,24).
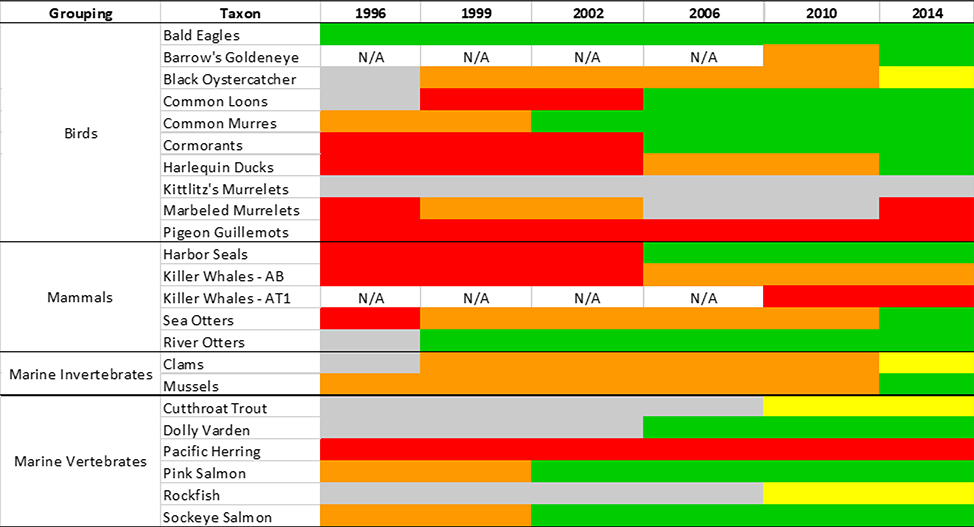
Acute toxic effects from oil ingestion, inhalation, smothering, drowning and hypothermia included mortality of 250,000 seabirds, 2,800 sea otters, 300 harbor seals, 250 bald eagles, up to 22 killer whales, and billions of salmon and herring eggs (13). In some cases, these acute impacts may have led to long-term demographic injury. For example, the AT1 pod of killer whales (Orcinus orca) in Prince William Sound were observed surfacing in the oil slick. The pod suffered high mortality rates following the spill and has had no recruitment since 1989 and is not expected to recover (25). In addition to acute toxicity, PAHs likely exerted embryotoxic effects in fish that included teratogenesis, chromosomal aberrations, and acute mortality through photo-enhanced toxicity (26,23,27,28,29). In 1996 the Exxon Valdez Oil Spill Trustee Council established criteria for categorizing the recovery status of many of the affected species (30). The rate at which species recovered from the spill varied (Fig. 2) and several have remained in unrecovered status. Incongruencies in recovery status in some EVOS impacted taxa (e.g., inconsistent trend between not recovering to recovered status; Fig. 2) highlight the challenge of quantifying impacts and recovery in complex ecosystems.
Figure 2.
Recovery status of taxa evaluated by Exxon Valdez Oil Spill Trustee Council following EVOS. Adapted from EVOSTC (30). Colored bars indicate ecological recovery status in Prince William Sound: not recovering (red); recovering (orange); likely recovered (yellow); recovered (green); status uncertain (gray/NA).
The intertidal zone was impacted by both direct oil and clean-up treatments, reducing fucoid algal cover, and lowering abundance of mollusc and sediment infauna for up to eight years after the spill (31). Reports of lingering oil contamination in mussel beds (32) and evidence of buried oil deposits (33) indicated that despite the rapid recovery of the surface of contaminated beaches substantial reservoirs of oil remained below the surface. Surveys in the early 2000’s indicated that oil still remained buried under the most contaminated beaches (15, 34, 35, 36, 37). Moreover, these surveys indicated that the oil was symmetrically distributed on the beaches with peak concentrations at the mid-tide level (34), suggesting that the buried oil was potentially bioavailable to organisms foraging in subtidal sediments (34, 38). More recent modeling indicated that slightly more oil remained in the upper intertidal areas compared with the lower sections, and that oil remained in the lower sections (39). Surveys conducted in 2015 indicated that the oil had undergone minimal weathering since it made landfall and its distribution in the intertidal has remained generally constant over time (40).
As of 2016, patches of oil, constituting approximately 0.6% of the stranded oil mass, can be found on sheltered beach segments initially classified as heavily oiled (39). Upon landfall, the bulk oil percolated through the highly permeable boulder armoring and became entrained in fine grained sediments located below. Natural breakwaters and headlands protect these beaches from wave exposure (41) and limited hypoxic groundwater flow through the relatively impermeable fine sediments minimizes the potential for microbial degradation of the oil (42). Most of the subsurface oil is currently found in Prince William Sound below 10–20 cm of clean sediments (39). Oil can also be found on the Kenai Peninsula and the Alaska Peninsula (43). Current estimates of oil loss rates from Prince William Sound beaches cannot be distinguished from zero (40) suggesting that oil can remain in these locations for decades.
The mechanisms by which PAH injured species in Prince William Sound involved specific features of their life histories (44). For example, incubation in intertidal sections of streams cutting across contaminated beaches put pink salmon embryos at risk of exposure to PAH in first four years following the spill (23). Interstitial water contaminated by flowing through buried oil deposits transported PAH to the embryos. Laboratory studies demonstrated that this led to increased embryo mortality, decreased growth, and delayed mortality resulted from exposure to environmentally persistent PAH with three or more aromatic rings at aqueous concentrations as low as one part per billion (23). Similarly, pink salmon juveniles emigrating seaward at the time of the spill spent time foraging in nearshore habitats (45). Consumption of bulk oil led to reduced growth rates (45, 46) with concomitant effects on adult returns in 1990 (46, 47).
Other mechanisms involved species with high fidelity to contaminated intertidal habitats such as harlequin ducks (Histrionicus histrionicus) and sea otters (Enhydra lutris). Survival rates among harlequin ducks in affected areas did not recover until 2003 (18) but monitoring of 7-ethoxyresorufin-O-deethylase activity (EROD) activity revealed prolonged exposure to PAHs until 2011 (48). Survival rates among harlequin ducks in affected areas did not recover until 2003 (18) but monitoring of 7-ethoxyresorufin-O-deethylase activity (EROD) activity revealed prolonged exposure to PAH until 2011 (46). Similarly, sea otter abundance around heavily oiled beaches on Knight Island remained depressed for 20 years after the spill despite recovery throughout most of Prince William Sound (44), but direct evidence of exposure was less clear. For both species, delayed recovery may have resulted from their dependence on prey found in intertidal habitats. Evaluations of the spatial overlap between the distribution of contaminated sediments and locations where sea otters had evidently foraged suggested otters may have avoided contaminated sites (49). However, later studies involving otters equipped with time depth recorders revealed a much greater spatial overlap and potential for exposure (50).
Relatively few species could be monitored following EVOS and therefore the impacts observed can be considered as only index of the extent of injury to the ecosystem. Ecological processes such as predator/prey and competitor relationships were likely affected from impacts to specific populations (13). Impacts on ecological processes is one possible explanation for the collapse of Pacific herring (Clupea pallasi) populations following the spill. The population was near its peak biomass at the time of the spill and then collapsed four years later when 60% of the spawning biomass failed to arrive on the spawning grounds (51). The collapse, which affected all adult age classes, has been attributed to poor nutritional condition (52). Disease may have been a secondary factor because 13% −43% of the herring bore external lesions consistent with. viral hemorrhagic septicemia virus and all age classes were affected (53). The role of oil exposure in the collapse is unknown and has been widely debated (52), but the time delay between the spill and the subsequent collapse likely rules out any direct effects of oil exposure. However, the loss of significant numbers of herring predators due to oiling in 1989 (13) would have exacerbated any density dependent effects. Moreover, exposure to weathered oil has been shown to decrease immune function (54) in adult herring and the embryotoxic effects include a long-term reduction in cardiorespiratory performance (55). While the relative roles of population density, oil toxicity, disease, predation and competition are unlikely to be resolved, they highlight the need to better understand the impact of spilled oil on ecological processes in affected environments.
3. HEBEI SPIRIT OIL SPILL
3.1. Summary of Spill
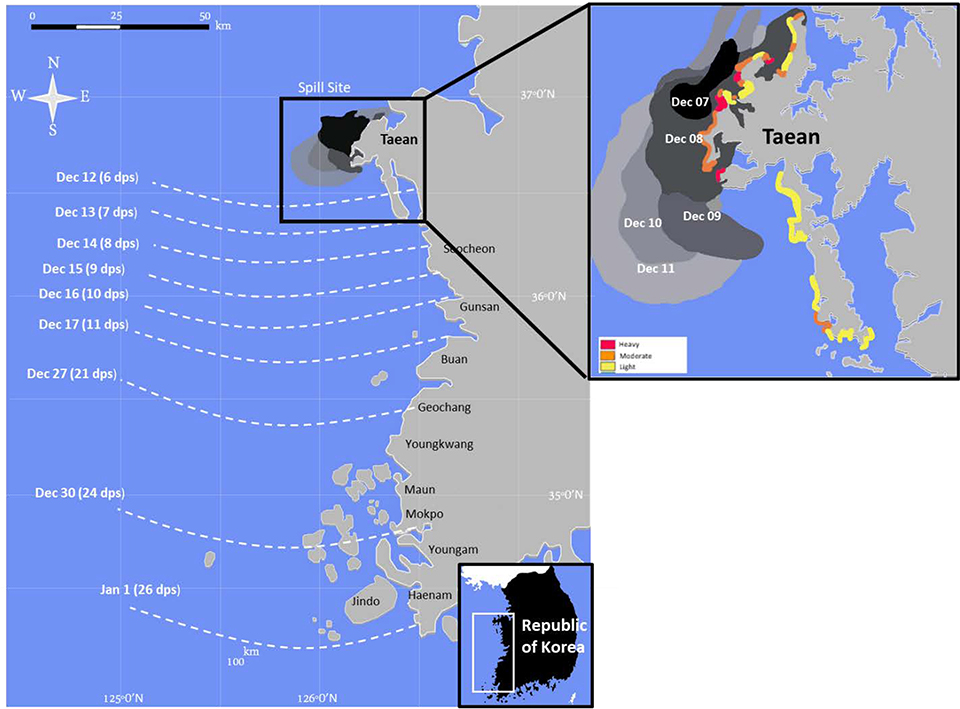
On December 7, 2007, the oil tanker M/V Hebei Spirit was struck by a crane barge 10 km off Taean, on the west coast of the Republic of Korea. The Taean region is an area of multiple rocky coves and sand beaches, with surface temperatures ranging from 8 to 24°C. The collision resulted in three punctures to the tanker, and three different types of Middle East crudes were spilled. Approximately 10,900 tons (~13 million L) of crude oil spilled into the sea from the tanker (56). During a 30-day period, the spilled oil was rapidly transported over several hundred kilometers of coastline by northwesterly winds and currents and resulted in heterogenous shoreline oiling. About 70 km of the Taean shoreline was affected by thick stranded oil in the first days of the spill (Fig. 3). The rapid spread of spilled oil resulted in area-wide impacts to fisheries, marine aquaculture, and beaches along the west coast of Korea, with potential larger scale impacts to the marine ecosystem (57).
Figure 3.
Map showing spreading of spilled oil during the 2007–2008 Hebei Spirit oil spill near Taean, Republic of Korea. Dark shading shows areas of heavy oiling, and dashed lines show the days post spill (dps) and the date oil was first observed. Shoreline oiling shown as heavy (red), moderate (orange), or light (yellow). Figure adapted from Yim et al. (58) and Hong et al. (59).
In contrast to EVOS and DWH (Section 4), extensive initial removal of HSOS oil limited long-term ecological impacts to changes in the composition and abundance of intertidal benthic communities. Environmental conditions, including high tidal energy and wind direction, also affected oil distribution and persistence. Response activities conducted at sea included using oil booms and absorbents, recovery of slick oil at the surface, and application of oil spill dispersants (298 kL). More than 1 million volunteers contributed to the shoreline cleanup operation for one month after the spill (57). Cleanup activities at sea and in the onshore areas of Taean were officially terminated in October 2008. However, and consistent with EVOS, oil persisted in deeper subsurface (>20 cm) of sediments along the most heavily affected intertidal areas of Taean 24 months after the spill (59).
3.2. Long-term Ecological Effects and Recovery
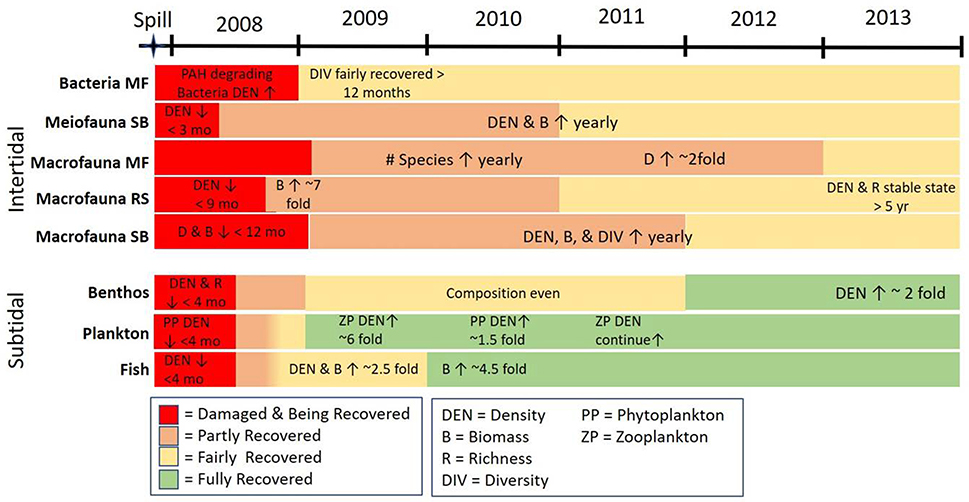
Oil contamination, toxic effects on organisms, and ecosystem injury were intensively investigated following HSOS. Long-term monitoring after the spill focused on residual oiling, exposure to lingering oil and their toxic potentials, and ecosystem recovery of intertidal and subtidal habitats (Fig. 4). Concentrations of total petroleum hydrocarbons (TPH) and PAH in most affected areas rapidly decreased with average concentrations of petroleum hydrocarbons reaching background levels within 7–18 months for seawater, surface sediments, and oyster tissues. Hotspot areas persisted in the intertidal area where residual oils remained in the subsurface layers for longer periods. These hotspot areas included the low energy regions such as the upper intertidal area of mudflats and pocket beaches, and the rocky shores with boulder-armored beaches (56).
Figure 4.
Ecosystem responses and recovery status of subtidal and intertidal habitats and fauna following HSOS. Recovery of ecosystem components considered complete after 2013. Adapted from Yim et al. (57).
Coastal environments impacted by HSOS recovered rapidly following the massive shoreline cleanup activities and favorable environmental conditions including high tidal energy (~9 m of tidal range) (57). While exposure biomarkers such as biliary 1-hydroxypyrene, and EROD were initially very high in resident fishes (e.g. rockfish, Sebastes schlegeli and marbled flounder, Pseudopleuronectes yokohamae), they gradually decreased to background levels within 2–4 years after the spill (60). A positive correlation was observed between mortality rate and PAH concentrations in whole sediment bioassays with the amphipod (Monocorophium uenoi). Except for highly contaminated areas and rocky zones with subsurface oil, surface sediment PAH concentrations in intertidal areas decreased below a toxicity a threshold of 1,200 ng g−1 approximately three years after the spill (61).
Due to the low pre-spill abundance of keystone species such as marine mammals and seabirds in the region, the long-term monitoring focused mainly on macrobenthic communities in intertidal sandflat, mudflat, rocky shore, and subtidal soft bottom habitats (Figure 4). The initial impact and rate of recovery were habitat dependent, where oiling and community structures differed substantially different. A general trend of ecosystem recovery is exemplified in the intertidal sandflat habitat. Following HSOS, sensitive organisms such as mud shrimp (Upogebia major) were almost eradicated, and 12–24 months later the opportunistic species (Felaniella sowerbyi, Batillaria spp., etc.) dominated in large densities. Overall, monitoring results indicated that the macrobenthic communities of intertidal sandflat, mudflat, rocky shore, and subtidal area recovered 65–73 months after the spill. Within 72–96 months after the HSOS, the intertidal and subtidal benthic habitats and were considered to be fully recovered and community structure stablized (57,62).
4. DEEPWATER HORIZON OIL SPILL
4.1. Summary of Spill
The DWH disaster began on April 10, 2010 and released 507 million liters of light Louisiana crude oil from 1600 m on the ocean floor into the Gulf of Mexico over 87 days. The northern Gulf of Mexico includes multiple subsea, pelagic and coastal ecosystems, with deep ocean temperatures of 4°C in the aphotic zone, and warmer surface water temperatures ranging seasonally from 10 to 33°C. Spill response efforts included oil skimming, over 400 in situ burns, and application of approximately 7 million liters of chemical dispersants at the surface and for the first time in history at the well head in the deep ocean. Approximately 180,000 km2 of surface waters and 2000 km of shoreline were oiled, and over 20 million hectares of the Gulf of Mexico were closed to fishing (63,64) (Fig. 5). Following multiple efforts to stop the release of oil during the 87 days of the spill, the well head was capped on July 15, and finally sealed on September 19, 2010 (8, 63).
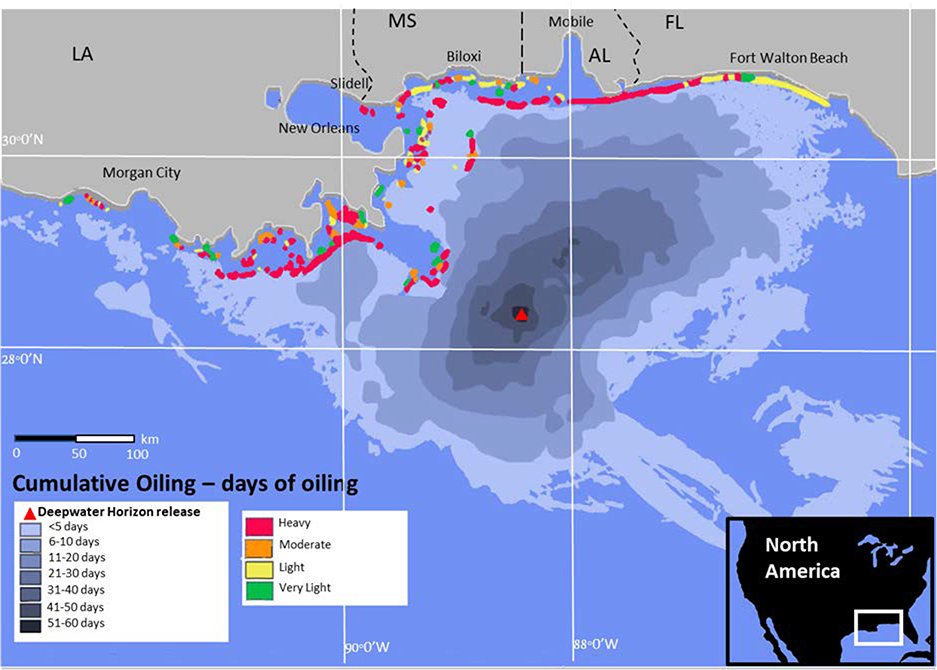
Figure 5.
Cumulative spatial distribution of Deepwater Horizon spill oil in the Gulf of Mexico (days oil present) and maximum shoreline oiling during the spill (May-July 2010). Adapted from Barron 2012 (63).
4.2. Long-term Ecological Effects and Recovery
The DWH spill exposed a diversity of complex aquatic communities in the deep ocean, offshore pelagic areas, and coastal environments (63, 8, 64). Large scale persistent ecological impacts included oiling of deep ocean corals, failed recruitment of oysters over multiple years, damage to coastal wetlands, reduced dolphin, sea turtle, and seabird populations, and likely cascading impacts on menhaden and other coastal species from disruption of predator-prey relationships. Recovery of natural resources in some areas of the Gulf of Mexico may take more than 20 years (65).
4.2.1. Deep Ocean
Evidence of deep-water oil plumes and oil-associated marine snow impacts on deep water communities of meiofauna and megafauna included lower taxonomic richness and abundance at 500 m North and 2000 m South of the release site (66). Mortality and emigration at these two locations were attributed to the higher concentration of hydrocarbons at 500 m N of the well and the trajectory of the subsea plume 2000 m from the well head (66). At one site of deep-water corals located about 11 km Southwest of the well, 86% of coral colonies were impacted with tissue loss, excessive mucus production and retracted polyps (64). The degree of initial impact to these corals directly correlated with their recovery and secondary hydroid colonization (68,69). The marine oil snow pulse that settled on the seafloor was short-lived but had impacts of higher sedimentation rates and lack of bioturbation for up to 3 years (70).
4.2.2. Offshore
Oil released from DWH entered the offshore pelagic zone as both dissolved phase and particulate oil exposing plankton, invertebrates, fish, sea turtles, sea birds and marine mammals during the 87-day spill. Over 10,000 sea birds were affected by the spill, with some estimates exceeding 0.5 million birds (63, 71). Although there were no documented fish kills, sensitive life stages of pelagic fish including tuna were likely exposed as both embryos and larvae (72). Early life stages of Gulf of Mexico fish are sensitive to both embryonic cardiotoxicity and phototoxicity at low part per billion concentrations of total PAH (73,74). Pelagic zooplankton and early life stages of other marine invertebrates were also at risk from phototoxicity in the translucent high solar radiation in the offshore environment of the Gulf of Mexico (75). Impacts to marine mammals and sea turtles were also documented during DWH (63), with exposures occuring within and outside of the offshore pelagic zone.
4.2.3. Coastal Habitats
Approximately 2000 km of Gulf of Mexico shoreline were oiled during the DWH spill, with maximum oiling along the shorelines and bays of Louisiana Shoreline cleaning and natural processes have removed most of the oil from these areas (Figure 5). Sand beach habitat and submersed aquatic vegetation were impacted by a combination of oiling and response actions (65). Monitoring of coastal condition using sediment chemistry, benthic organism surveys and toxicity testing indicated some impacts during the spill but minimal impacts after the well was capped (63). Severe reductions in root and rhizome biomass of Juncus roemerianus (76,77) ultimately changed the shoreline structure from a mixed Spartina alterniflora-J. roemerianus marsh to a predominately S. alterniflora marsh (77). While above ground biomass and below ground biomass of both marsh plants was equivalent in moderately oiled areas to reference marshes 24–30 months post spill, (77), shoreline erosion persisted at least 30 months (78).
Resident marsh species influence secondary production and provide energy transfer from marsh species to estuarine habitats (79,80,81). Fundulus grandis, a marsh-resident fish dominant in Louisiana estuaries (82) is an important component of the marsh food web (83) that has been used as a sentinel species for evaluating marsh health and degradation of environmental quality (84,85). F. grandis reproduction coincided with DWH marsh oiling (86,87), including depositing eggs on vegetation, shell and sediment surfaces (86,88,89). Embryonic F. grandis exposed to sediments from oiled locations in Louisiana caused delayed hatching, reduced hatching success, cardiovascular defects, and pericardial edema (90). However longer-term population level effects were not observed 2–3 years after the spill (82). Ecological impacts in heavily oiled marshes may have been mitigated through selection of less oiled habitat, and long-term adaption to hydrocarbon exposure (91,82).
Marsh transients, such as menhaden, can play an important role in export of production from estuaries to offshore environments (92). For example, Deegan (93) showed that 10–15% of total production in a Louisiana estuary was exported to offshore areas by Gulf menhaden, Brevoortia patronus. High recruitment of Gulf menhaden occurred in 2010 and abundance remained high for two years after DWH (94). Decreased predation by piscivorous seabirds and bottlenose dolphin have been suggested as causes for high menhaden recruitment following DWH (94). With the recovery of marsh habitats and resident populations, long-term ecological disruptions to energy transfer from the marsh to coastal environments appear to have been minimized.
4.2.4. Oysters
Oysters (Crassostrea virginica) were impacted from both oil exposure and freshwater releases from the Mississippi River used to limit oil movement into Louisiana estuaries (65). Post spill densities of oysters declined significantly across all size classes where freshwater was discharged (95). Losses were greatest in seed-sized oysters (95) contributing to a recruitment failure observed in Barataria Bay, Black Bay, Breton Sound and Mississippi sound (65). Minimal impacts on growth and mortality were observed in areas where low salinity lasted only a few months, but still impacted recruitment (96).
4.2.5. Marine Mammals
Mortality of Gulf of Mexico cetaceans were reported during DWH, and sub-populations of dolphins were among the most affected species during the spill. Barataria Bay, Louisiana, one of the most heavily oiled locations of the DWH spill (87) has a resident population of common bottlenose dolphin (Tursiops truncatus) that shows little to no movement outside the bay (97). Dolphins sampled throughout the Barataria Bay after the spill showed pulmonary disease, adrenal gland abnormalities and poor body conditions (97,98). Reproductive success was reported as 20% in Barataria Bay when compared to 83% in Sarasota Bay (97). Direct exposure to oil or chronic health effects due to the exposure could explain low mortality and reproductive success. For example, the adrenal gland abnormalities were reported to be evidence of hypoadrenocorticism and immunotoxicity could leave dolphins susceptible to pathogens such as Brucella sp., pathogens that can cause late term abortions, stillbirths and weak calves (99,97). Long-term impacts on dolphins in Barataria Bay have resulted in a survival rate of only 86.8% (97) when compared to unaffected areas Charleston, SC and Sarasota Bay, Fl which have survival rates at 95.1% and 96.2% respectively (100,101).
5. Interactions AMONG OIL Spill Dynamics and Response, ENVIRONMENT and Ecological Systems
Comparison of EVOS, HSOS, and DWH shows the importance and complexity of the interactions among oil spill dynamics and response actions, environment, and ecological systems. Understanding how they interact to determine ecosystem impacts and recovery are critical to preparing for and responding to future spills.
5.1. Spill Dynamics and Response Actions
The dynamics of a spill, including oil characteristics and composition, spill volume, release rate, trajectories, and the response actions have long been considered the drivers of long-term ecological impacts. The massive response of a one million human volunteers during HSOS demonstrates the importance of extensive and rapid removal of spilled oil in limiting long-term ecological impacts and facilitating ecosystem recovery. During DWH, all spill response options were used in combatting the 180,000 km2 of oiled surface waters and 2000 km of oiled shoreline, including subsea and surface dispersant applications, in situ burning, booming, deflection, and mechanical recovery. In contrast to HSOS and DWH, the EVOS spill response was limited and shoreline washing may have exacerbated ecological damage (102). These three catastrophic spills demonstrate the importance of contingency planning and preparedness for worst case spill scenarios. They also highlight the challenges of associating particular environmental benefits and impacts of specific response actions because of the dynamic nature of oil spills and complex environmental interactions.
5.2. Environment
The three spills occurred in marine environments differing in temperature, prior oil exposure, and affected habitats. Environmental temperature can be one of the most important factors determining the ecological impacts of large-scale spills because of temperature affects oil viscosity control of oil spreading and movement, degradation rates, and ultimately environmental exposures and persistence. Additionally, weather events and oceanographic conditions affected spill response options and oil distribution. The EVOS occurred in the nearly pristine subarctic waters of Prince William Sound, Alaska, and the HSOS occurred in the ecologically sensitive temperate waters of western Korea. In contrast to EVOS and HSOS, DWH released oil in the Gulf of Mexico, a region with warmer surface waters, extensive oil production, and multiple natural oil seeps that can facilitate more rapid biodegradation of petroleum.
Affected shoreline habitats in EVOS and HSOS were relatively similar, consisting of high energy sand beaches and extensive rocky cobble substrates with wide tidal ranges. High wave energy and tidal height can facilitate oil removal as occurred in HSOS, whereas oil trapped in rocky cobble shorelines can persist for decades and provide continued exposure to ecological receptors (35). In both EVOS and HSOS, oil trapped in localized subsurface areas of rocky cobble persisted substantially longer than in surface sediments. In contrast, DWH oiled shoreline habitats consisted of barrier islands composed of lower energy sand beaches and extensive estuarine subtropical wetlands. As was the case for DWH, petroleum can have relatively short residence time in highly mineral sand beaches, whereas oil can be persistent in salt marshes and mangrove swamps (3, 4, 5, 63).
Incident solar radiation and light attenuation in the water column were also likely important environmental factors in all three spills. The potential for phototoxicity in oil spills was recognized following EVOS (27,103), and subsequently validated in multiple resident and model species and oil spills including HSOS and DWH (61, 74,75). Phototoxicity risks will generally be greatest in areas and seasons of high incident solar radiation and lower attenuation of ultraviolet radiation within the water column (28, 75). The complex interplay within regional environments and localized conditions determines oil distribution, fate, persistence, and macroscale and localized ecological impacts.
5.3. Ecological Systems
The complexity, interrelationships, and natural variability within ecological systems makes the assessment of impact and recovery particularly challenging for large scale oil spills. Determining commonalities in how EVOS, HSOS, and DWH impacted ecological systems is additionally challenging because of differences in oil spill dynamics, response actions, and environmental conditions. One example of adverse impacts common in all three spills was a syndrome of embryotoxic effects, including edema and skeletal defects, first observed in Prince William Sound pink salmon and Pacific herring during EVOS. The developmental effects were initially unexplained following EVOS, and were subsequently observed in HSOS and DWH in multiple embryonic fish species (73,104). Research stemming from all three of these spills revealed a pathophysiological pathway by which petrogenic tricyclic PAHs cause a cascade of cardiotoxic effects through impaired cardiac muscle cell calcium cycling and action potential generation leading to malformations (29,55,73). This syndrome of cascading embryonic cardiotoxicity has been now been observed in multiple fish species following exposure to a range of fresh and weathered oils, and has been purported to be a causative factor in impacts to pink salmon and Pacific herring during EVOS (22,23,26,31).
Pacific herring populations in Prince William Sound substantially declined after EVOS and continue to have low abundance and recruitment 30 years after the spill. However, causation from EVOS has never been established despite decades of study. Substantial large-scale impacts from embryotoxicity and phototoxicity appear plausible because of the coincident timing of the spill and herring spawning and development, and part per billion PAH exposures and effects levels. Embryotoxicity observed in EVOS and DWH also points to the importance of life histories. Had the spills occurred when the affected species were in less sensitive developmental stages the impacts may have differed.
In a comprehensive review of oil spills prior to HSOS and DWH, Kingston (3) concluded that environmental recovery from large spills is usually complete within 2 to 10 years, and that longer-term impacts are generally limited to effects on community structure that can persist because of the longevity of the affected species. Long lived keystone species common to the three spills include species of commercial fish, piscivorous birds and marine mammals. Cetaceans were substantially affected during EVOS and DWH, with long-term impacts still apparent. Cetaceans were present during HSOS, but impacts were not apparent. Sea birds were substantially affected during EVOS and DWH, and catastrophic sea bird mortality has been hypothesized to have caused an ecological cascade affecting prey fish and plankton in the Gulf of Mexico. Additional complexities in assessing long-term ecological impacts is the density dependent interaction between fishing pressure and natural mortality in commercial fisheries. For example, the closure of 20 million hectares of the Gulf of Mexico for three months during DWH complicated the subsequent fishery-based assessment of impacts on populations following the spill.
Emerging ecosystem-level modeling studies by Olsen et al. (105) and Ainsworth (106) provide insights into ecosystem shifts resulting from mass mortality events of early life stages of fish and keystone species. For example, modeling of DWH exposures and species life histories revealed that recovery of high-turnover fish populations was generally predicted to occur within 10 years, but some slower-growing populations may take over 30 years to fully recover (106). Similarly, modeling of mass mortality events from single large-scale oil spills in the Nordic and Barents seas predicted population level impacts on multiple fish species, and near permanent reductions in herring populations (105). Together, these studies show that catastrophic oil spills may substantially perturb ecological systems and reset the ecosystem to a new state (105).
6. Conclusions
6.1. Lessons Learned
The long-term ecological impacts of large-scale catastrophic oil spills are less predictable and can be more persistent then has been generally recognized because of the complex interactions among the environment, spill dynamics, the affected ecological systems, and response and restoration actions (105,106). Prior to EVOS, the ecological impacts of oil spills were generally thought to be short-term and controlled by the mono-aromatic and less persistent components of oil (13). Decades of assessment and research following EVOS have shifted paradigms on the persistence and toxic components of oil, and the adverse outcomes of large-scale oil spills. Advances in petroleum analytical chemistry shifted the focus of impact assessments to petrogenic PAH and adoption of geochemical biomarker approaches to source attribution (e.g., 107). Following EVOS, highly weathered oil from multiple spills including HSOS and DWH has been shown to contain substantial concentrations of hydrocarbon and heterocyclic aromatics, and oxidized PAHs (e.g., 108,109). Whereas large-scale oil spills can have long-term ecosystem-level perturbations, smaller oil spills are unlikely to have persistent ecological effects (3,9,105,106).
Advancements in monitoring technology have transformed spill response and impact assessment, from the first uses of satellite imagery in EVOS (17) to sophisticated trajectory analysis and cumulative oil mapping in DWH (110). However, challenges to responding to large scale spills will continue, including determining the specific benefits and risks of various spill counter measures in the context of the dynamic nature of an oil spill and the complexity and interrelationships within the affected environments. Additionally challenging will be assessing the magnitude and persistence of perturbations from baseline conditions and subsequent recovery of the diversity of habitats and ecological receptors.
6.2. Research Needs
Critical research needs include determining baseline ecological conditions, natural variability, and intrinsic sensitivity of potentially affected habitats and species. Determining ecological impacts will be particularly difficult for species like herring that have high variance in population abundance, reproduction and recruitment. Similarly, quantifying oil impacts on ecological processes and ecosystem recovery will remain challenging. Understanding impacts on ecosystem structure and function may account for lost ecological services or changes in species demographics just as much as traditional assessments of injuries and damages. For example, determining the complex interactions and long-term consequences of loss of top predators or other key stone species will be essential for determining how catastrophic oil spills may have ecosystem-level impacts. Assessing and understanding the ecological impacts of subsea oil releases and Arctic oil spills is becoming increasingly important with expanding hydrocarbon extraction and transport. These environments have largely unknown species sensitivity, and the physical factors in the deep ocean and polar environments will uniquely affect hydrocarbon behavior, fate and toxicity (111,112). The emerging application of whole ecosystem modeling to catastrophic oils spills offers an opportunity to understand and predict complex ecological interactions and system perturbations not quantifiable from traditional assessment methods (105).
6.3. Human Dimensions
Beyond the ecological impacts of catastrophic large-scale oil spills are the human dimensions of psychological stress, economic disruption, and long-term health consequences. High levels of initial psychological stress were observed during EVOS and DWH arising from concerns for family health, exposure to oil, and economic impacts to commercial fisheries (113). In contrast to the rapid recovery of affected habitats following HSOS, human health effects persist in local communities and the multitude of cleanup workers (114,115). For example, health assessments following HSOS (2009–2013) showed significantly higher rates of prostate cancer (male), thyroid cancer (female), and leukemia (female) in residents of Taean than national averages (115). Effects on human health and social disruptions to families and communities add an additional and often underappreciated dimension to the long-term impacts of catastrophic oil spills.
ACKNOWLEDGEMENTS
We thank Jeff Short and John Incardona for a review of an early draft of this manuscript, Matt Harwell for advice on manuscript graphics, and two anonymous reviewers for comments that improved this article. The opinions expressed in this work are those of the authors and do not represent the policies or opinions of the U.S. EPA.
REFERENCES
- 1.Jernelov A The threats from oil spills: now, then, and in the future. Ambio. 2010, 39, 353–366; DOI 10.1007/s13280-010-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckle P; Burgherr P; Michaux E Risk of large oil spills: a statistical analysis in the aftermath of Deepwater Horizon. Environ. Sci. Tech 2012, 46, 13002–13008; DOI 10.1021/es3029523. [DOI] [PubMed] [Google Scholar]
- 3.Kingston PF Long-term environmental impact of oil spills. Spill. Sci. Tech. Bull 2002, 7, 53–61; DOI 10.1016/S1353-2561(02)00051-8. [DOI] [Google Scholar]
- 4.Culbertson JB; Valiela I; Pickart M; Peacock EE; Reddy CB Long-term consequences of residual petroleum on salt marsh grass. J. Appl. Ecol 2008, 45, 1284–1292; DOI 10.1111/j.1365-2664.2008.01477.x. [DOI] [Google Scholar]
- 5.Mendelssohn IA; Andersen GL; Baltz DM; Caffey RH; Carman KR; Fleeger JW; Joye SB; Lin Q; Maltby E; Overton EB; Rozas LP Oil impacts on coastal wetlands: implications for the Mississippi river delta ecosystem after the Deepwater Horizon oil spill. BioScience. 2012, 62(6), 562–574; DOI 10.1525/bio.2012.62.6.7. [DOI] [Google Scholar]
- 6.Atlas RM; Hazen TC Oil biodegradation and bioremediation: a tale of the two worst spills in US History. Environ. Sci. Tech 2011, 45(16), 6709–6715; DOI 10.1021/es2013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrons RK Assessing the damage caused by Deepwater Horizon: not just another Exxon Valdez. Mar. Poll. Bull 2013, 71(1–2), 20–22; DOI 10.1016/j.marpolbul.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Beyer J; Trannum HC; Bakke T; Hodson PV; Collier TK Environmental effects of the Deepwater Horizon oil spill: A review. Mar. Poll. Bull 2016, 110, 28–51; DOI 10.1016/j.marpolbul.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Short JW Advances in understanding the fate and effects of oil from accidental spills in the United States beginning with the Exxon Valdez. Arch. Environ. Contam. Toxicol 2017, 73(1); 5–11; DOI 10.1007/s00244-016-0359-4. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe D; Michel J; Hameedi MJ; Payne JR; Galt JA; Watabayashi G; Braddock J; Short J; O’Claire C; Rice S The fate of the oil spilled from the Exxon Valdez. Environ. Sci. Tech 1994, 28(13), pp.560A–568A. [DOI] [PubMed] [Google Scholar]
- 11.Xia Y; Boufadel MC Lessons from the Exxon Valdez oil spill disaster in Alaska. Disaster Adv. 2010, 3(4), pp.270–273. [Google Scholar]
- 12.Maki AW The Exxon Valdez oil spill: initial environmental impact assessment. Part 2. Environ. Sci. Technol 1991, 25(1), 24–29; DOI 10.1021/es00013a001. [DOI] [Google Scholar]
- 13.Peterson CH; Rice SD; Short JW; Esler D; Bodkin JL; Ballachey BE; Irons DB Long-term ecosystem response to the Exxon Valdez oil spill. Science. 2003, 302(5653), 2082–2086; DOI 10.1126/science.1084282. [DOI] [PubMed] [Google Scholar]
- 14.Neff JM; Owens EH; Stoker SW; McCormick DM Shoreline oiling conditions in Prince William Sound following the Exxon Valdez oil spill In Exxon Valdez oil spill: Fate and effects in Alaskan waters; Wells PG, Butler JN, Hughes JS, Eds; ASTM International; 1995; 312–346. [Google Scholar]
- 15.Short JW; Lindeberg MR; Harris PM; Maselko JM; Pella JJ; Rice SD Estimate of oil persisting on the beaches of Prince William Sound 12 years after the Exxon Valdez oil spill. Environ. Sci. Technol 2004, 38(1), 19–25; DOI 10.1021/es0348694. [DOI] [PubMed] [Google Scholar]
- 16.Galt J; Lehr WJ; Payton DL Fate and transport of the Exxon Valdez oil spill. Environ. Sci. Technol 1991, 25(2), 202–209. [Google Scholar]
- 17.Stringer WJ; Dean KG; Guritz RM; Garbeil HM; Groves JE; Ahlnaes K Detection of petroleum spilled from the MV Exxon Valdez. Internat J Remote Sensing 13(5), 799–824. [Google Scholar]
- 18.Iverson SA; Esler D Harlequin Duck population injury and recovery dynamics following the 1989 Exxon Valdez oil spill. Ecol. App 2010, 20(7), 1993–2006; DOI 10.2307/25741363. [DOI] [PubMed] [Google Scholar]
- 19.Rice SD Persistence, toxicity, and long-term environmental impact of the Exxon Valdez Oil Spill. U of St. Thomas L. J 2009, 71(1), 55–67. [Google Scholar]
- 20.Neff JM; Stubblefield WA “Chemical and Toxicological Evaluation of Water Quality Following the Exxon Valdez Oil Spill,” In Exxon Valdez oil spill: Fate and effects in Alaskan waters; Wells PG, Butler JN, Hughes JS, Eds; ASTM International; 1995; 141–177; DOI 10.1520/STP19863S. [DOI] [Google Scholar]
- 21.Short JW; Heintz RA Identification of Exxon Valdez oil in sediments and tissues from Prince William sound and the northwestern Gulf of Alaska based on a PAH weathering model. Environ. Sci. Technol 1997, 31(8), 2375–2384; DOI 10.1021/es960985d. [DOI] [Google Scholar]
- 22.Bue BG; Sharr S; Seeb JE Evidence of damage to pink salmon populations inhabiting Prince William Sound, Alaska, two generations after the Exxon Valdez oil spill. Trans. Amer. Fish. Soc 1998, 127(1), 35–43; DOI 10.1577/1548-8659(1998)127. [DOI] [Google Scholar]
- 23.Heintz R; Short JW; Rice SD Sensitivity of fish embryos to weathered crude oil: Part II. Increased mortality of pink salmon (Onchorhynchus gorbuscha) embryos incubating downstream from weathered Exxon Valdez crude oil. Env. Tox. Chem 1999, 18, 494–503; DOI 10.1002/etc.5620180318. [DOI] [Google Scholar]
- 24.Carls MG; Thomas RE; Lilly MR; Rice SD Mechanism for transport of oil-contaminated groundwater into pink salmon redds. Mar. Ecol. Prog. Ser 2003, 248, 245–255; DOI 10.3354/meps248245. [DOI] [Google Scholar]
- 25.Matkin CO; Saulitis EL; Ellis GM; Olesiuk P; Rice SD Ongoing population-level impacts on killer whales Orcinus orca following the ‘Exxon Valdez’oil spill in Prince William Sound, Alaska. Mar. Ecol. Prog. Ser 2008, 356, 269–281; DOI 10.3354/meps07273. [DOI] [Google Scholar]
- 26.Carls MG; Rice SD; Hose JE Sensitivity of fish embryos to weathered crude oil: Part I. Low‐level exposure during incubation causes malformations, genetic damage, and mortality in larval pacific herring (Clupea pallasi). Environ. Toxicol. Chem 1999, 18(3), 481–493; DOI 10.1102/etc.5620180317. [DOI] [Google Scholar]
- 27.Barron MG; Carls MG; Short JW; Rice SD Photoenhanced toxicity of aqueous phase and chemically dispersed weathered Alaska North Slope crude oil to Pacific herring eggs and larvae. Environ. Toxicol. Chem 2003, 22(3), 650–660; DOI 10.1002/etc.5620220326. [DOI] [PubMed] [Google Scholar]
- 28.Barron MG; Vivian DN; Yee SH; Diamond S Temporal and spatial variation in solar radiation and photoenhanced toxicity risks of spilled oil in Prince William Sound, Alaska. Environ. Toxicol. Chem 2008, 27, 227–236; DOI 10.1897/07-317. [DOI] [PubMed] [Google Scholar]
- 29.Brette F; Machado B; Cros C; Incardona JP; Scholz NL; Block BA Crude oil impairs cardiac excitation-contraction coupling in fish. Science 2014, 343 (6172), 772–776; DOI 10.1126/science.1242747. [DOI] [PubMed] [Google Scholar]
- 30.EVOSTC. Exxon Valdez Oil Spill Restoration Plan 2014 Update Injured Resources and Services. Exxon Valdez Oil Spill Trustee Council, Anchorage AK: 2014, 47 pp. [Google Scholar]
- 31.Peterson CH The “Exxon Valdez” oil spill in Alaska, indirect and chronic effects on the ecosystem. Adv. Mar. Bio 2001, 39, 1–103; DOI 10.1016/S0065-2881(01)39008-9. [DOI] [Google Scholar]
- 32.Carls MG; Babcock MM; Harris PM; Irvine GV; Cusick JA; Rice SD Persistence of oiling in mussel beds after the Exxon Valdez oil spill. Mar. Environ. Res 2001, 51(2), 167–190; DOI 10.1016/S0141-1136(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 33.Hayes MO; Michel J Factors determining the long-term persistence of Exxon Valdez oil in gravel beaches. Mar. Poll. Bull 1999, 38(2), 92–101; DOI 10.1016/S0025-326X(99)00099-5. [DOI] [Google Scholar]
- 34.Short JW; Maselko JM; Lindeberg MR; Harris PM; Rice SD Vertical distribution and probability of encountering intertidal Exxon Valdez oil on shorelines of three embayments within Prince William Sound, Alaska. Environ. Sci. Technol 2006, 40(12), 3723–3729; DOI 10.1021/es0601134. [DOI] [PubMed] [Google Scholar]
- 35.Short JW; Irvine GV; Mann DH; Maselko JM; Pella JJ; Lindeberg MR; Payne JR; Driskell WB; Rice SD Slightly weathered Exxon Valdez oil persists in Gulf of Alaska beach sediments after 16 years. Environ. Sci. Technol 2007, 41(4), 1245–1250; DOI 10.1021/es0620033. [DOI] [PubMed] [Google Scholar]
- 36.Boehm PD; Page DS; Brown JS; Neff JM; Bragg JR; Atlas RM Distribution and weathering of crude oil residues on shorelines 18 years after the Exxon Valdez oil spill. Environ. Sci. Technol 2008, 42, 24; DOI 10.1021/es8022623. [DOI] [PubMed] [Google Scholar]
- 37.Taylor E; Reimer D Oil persistence on beaches in Prince William Sound – A review of SCAT surveys conducted from 1989 to 2002. Mar. Poll. Bull 2008, 56, 458–474; DOI 10.1016/jmarpolbul.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Monson DH; Doak DF; Ballachey BE; Bodkin JL Could residual oil from the Exxon Valdez spill create a long‐term population “sink” for sea otters in Alaska? Ecol. App 2011, 21(8), 2917–2932; DOI 10.1890/11-0152.1. [DOI] [Google Scholar]
- 39.Nixon Z; Michel J A review of distribution and quantity of lingering subsurface oil from the Exxon Valdez oil spill. Deep-Sea Res. PT II. 2018, 147, 20–26; DOI 10.1016/j.dsr2.2017.07.009. [DOI] [Google Scholar]
- 40.Lindeberg MR; Maselko J; Heintz RA; Fugate CJ; Holland L Conditions of persistent oil on beaches in Prince William Sound 26 years after the Exxon Valdez spill. Deep-Sea Res. PT II. 2018, 147, 9–19; DOI 10.1016/j.dsr2.2017.07.011. [DOI] [Google Scholar]
- 41.Nixon Z; Michel J; Hayes MO; Irvine G; Short J Geomorphic factors controlling the persistence of subsurface oil from the Exxon Valdez Oil Spill. J. Coastal Res., Spec. Issue 2013, 69, 115–127; DOI 10.1016/10.2112?SI_69_9. [DOI] [Google Scholar]
- 42.Li H; Boufadel MC Long-term persistence of oil from the Exxon Valdez spill in two-layer beaches. Nat. Geosci 2010, 3, 96–99; DOI 10.1038/ngeo749. [DOI] [Google Scholar]
- 43.Irvine GV; Mann DH; Carls MG; Holland LG; Reddy C; Nelson RK; Aeppli C Lingering oil on boulder-armored beaches in the Gulf of Alaska 23 years after the “Exxon Valdez” oil spill “Exxon Valdez” Oil Spill Restoration Project Final Report (Restoration project 11100112), US Geological Survey, Alaska Science Center, Anchorage, Alaska: 2014, 138pp. [Google Scholar]
- 44.Esler D; Ballachey BE; Matkin C; Cushing D; Kaler R; Bodkin J; Monson D; Esslinger G; Kloecker K Timelines and mechanisms of wildlife population recovery following the Exxon Valdez oil spill. Deep-Sea Res. PT II. 2018, 147, 36–42; DOI 10.1016/j.dsr2.2017.04.007. [DOI] [Google Scholar]
- 45.Wertheimer AC; Celewycz AG Abundance and Growth of Juvenile Pink Salmon in Oiled and Non-Oiled Locations of Western Prince William Sound after the Exxon Valdez Oil Spill. In Proceedings of the Exxon Valdez Oil Spill Symposium held at Anchorage, Alaska; Rice SD; Spies RB; Wolfe DA; Wright BA Eds.; American Fisheries Society, Bethesda, MD, 1996; pp 931. [Google Scholar]
- 46.Willette M Impacts of the Exxon Valdez Oil Spill on the Migration, Growth, and Survival of Juvenile Pink Salmon in Prince William Sound. In Proceedings of the Exxon Valdez Oil Spill Symposium held at Anchorage, Alaska; Rice SD; Spies RB; Wolfe DA; Wright BA Eds.; American Fisheries Society, Bethesda, MD, 1996; pp 931. [Google Scholar]
- 47.Gieger HJ; Blue BG; Sharr S; Wertheimer AC; Willette TM A life history approach to estimating damage to Prince William sound pink salmon caused by the Exxon Valdez oil spill. In Proceedings of the Exxon Valdez Oil Spill Symposium held at Anchorage, Alaska; Rice SD; Spies RB; Wolfe DA; Wright BA Eds.; American Fisheries Society, Bethesda, MD, 1996; pp 931. [Google Scholar]
- 48.Esler D; Ballachey B; Dickson MA; Henderson DR Cessation of oil exposure in harlequin ducks after the Exxon Valdez oil spill: Cytochrome P4501A biomarker evidence. Environ. Toxicol. Chem 2017, 36(5), 1294–1300; DOI 10.1002/etc.3659. [DOI] [PubMed] [Google Scholar]
- 49.Boehm PD; Page DS; Neff JM; Brown JS Are sea otters being exposed to subsurface intertidal oil residues from the Exxon Valdez oil spill? Mar. Pol. Bull 2011, 62(3), 581–590; DOI 10.1016/j.marpolbul.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 50.Bodkin JL; Ballachey BE; Coletti HA; Esslinger GG; Kloecker KA; Rice SD; Reed JA; Monson DH Long-term effects of the “Exxon Valdez” oil spill: sea otter foraging in the intertidal as a pathway of exposure to lingering oil. Mar. Ecol. Prog. Ser 2012, 447, 273–287; DOI 10.3354/meps09523. [DOI] [Google Scholar]
- 51.Marty GD; Hulson PJ; Miller SE; Quinn TJ II, Moffitt SD; Merizon RA Failure of population recovery in relation to disease in Pacific herring. Dis. Aquat. Org 2010, 90(1), 1–4; DOI 10.3354/dao02210. [DOI] [PubMed] [Google Scholar]
- 52.Pearson WH; Deriso RB; Elston RA; Hook SE; Parker KR; Anderson JW Hypotheses concerning the decline and poor recovery of Pacific herring in Prince William Sound, Alaska. Rev. Fish. Biol. Fisher 2012, 22(1), 95–135; DOI 10.1007/s11160-011-9225-7. [DOI] [Google Scholar]
- 53.Meyers TR, Short S, Lipson K, Batts WN, Winton JR, Wilcock J, Brown E Association of viral hemorrhagic septicemia virus with epizootic hemorrhages of the skin in Pacific herring Clupea harengus pallasi from Prince William Sound and Kodiak Island, Alaska, USA. Dis. Aquat. Org 1994, 19(1), 27–37; DOI 10.3354/dao019027. [DOI] [Google Scholar]
- 54.Carls MG; Marty GD; Meyers TR; Thomas RE; Rice SD Expression of viral hemorrhagic septicemia virus in prespawning Pacific herring (Clupea pallasi) exposed to weathered crude oil. Can. J. of Fish. Aquat. Sci 1998, 55(10), 2300–2309; DOI 10.1139/f98-116. [DOI] [Google Scholar]
- 55.Incardona JP; Carls MG; Hollarnd L; Linbo TL; Baldwin DH; Myers MS; Peck KA; Tagal M; Rice SD; Scholz NL Very low embryonic crude oil exposures cause lasting cardiac defects in salmon and herring. Sci Rep. 2015, 5, 13499; DOI 10.1038/srep13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yim UH; Kim M; Ha SY; Kim S; Shim WJ Oil spill environmental forensics: the Hebei Spirit oil spill case. Environ. Sci. Technol 2012, 46, 6431–6437.; DOI 10.1021/es3004156. [DOI] [PubMed] [Google Scholar]
- 57.Yim UH; Khim JS; Kim M; Jung J-H; Shim WJ Environmental impacts and recovery after the Hebei Spirit oil spill in Korea. Arch. Environ. Contam. Toxicol 2017, 73, 47–54; DOI 10.1007/s00244-017-0375-z.50. [DOI] [PubMed] [Google Scholar]
- 58.Yim UH; Ha SY; An JG; Won JH; Han GM; Hong SH; Kim M; Jung JH; Shim WJ Fingerprint and weathering characteristics of stranded oils after the Hebei Spirit oil spill. J. Haz. Mat 2011, 197, 60–69. [DOI] [PubMed] [Google Scholar]
- 59.Hong S; Khim JS; Ryu JS; Kang SG; Shim WJ; Yim UH Environmental and ecological effects and recoveries after five years of the Hebei Spirit oil spill, Taean, Korea. Ocean. Coast. Manag 2014, 102, 522–532; DOI 10.1016/j.ocecoaman.2014.01.006. [DOI] [Google Scholar]
- 60.Jung JH; Kim M; Yim UH; Ha SY; An JG; Won JH; Han GM; Kim NS; Addison RF; Shim WJ Biomarker responses in pelagic and benthic fish over 1 year following the Hebei Spirit oil spill (Taean, Korea). Mar. Pollut. Bull 2012, 62, 1859–1866; DOI 10.1016/j.marpolbul.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 61.Lee CH; Lee JH; Sung CG; Moon SD; Kang SK; Lee JH; Yim UH; Shim WJ; Ha SY Monitoring toxicity of polycyclic aromatic hydrocarbons in intertidal sediments for five years after the Hebei Spirit oil spill in Taean, Republic of Korea. Mar. Pollut. Bull 2013, 76, 241–249; DOI 10.1016/j.marpolbul.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 62.Yu OH; Lee HG; Shim WJ; Kim M; Park HS Initial impacts of the Hebei Spirit oil spill on the sandy beach microbenthic community west coast of Korea. Mar. Pollut. Bull 2013, 70,189–196; DOI 10.1016/j.marpolbul.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 63.Barron MG Ecological impacts of the Deepwater Horizon oil spill: Implications for Immunotoxicity. Toxicol. Path 2012, 40, 315–320; DOI 10.1177/0192623311428474. [DOI] [PubMed] [Google Scholar]
- 64.Nixon Z; Zengel S; Baker M; Steinhoff M; Fricano G; Rouhani S; Michel J Shoreline oiling from the Deepwater Horizon oil spill. Mar. Poll. Bull 2016, 107, 170–178; DOI 10.1016/j.marpolbul.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 65.Baker MC; Steinhoff MA; Fricano GF Integrated effects of the Deepwater Horizon oil spill on nearshore ecosystems. Mar. Ecol. Prog. Ser 2017, 576, 219–234; DOI 10.3354/meps11920. [DOI] [Google Scholar]
- 66.Valentine MM; Benfield MC Characterization of epibenthic and demersal megafauna at Mississippi Canyon 252 shortly after the Deepwater Horizon Oil Spill. Mar. Poll. Bull 2013, 77, 196–209; DOI 10.1016/j.marpolbul.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 67.White HK, Hsing P, Cho W, Shank TM, Cordes EE, Quattrini AM, Nelson RK, Camilli R, Demopoulos AWJ, German CR, Brooks JM, Roberts HH, Shedd W, Reddy CM, and Fisher CR Impact of the Deepwater Horizon oil spill on a deep-water coral community in the Gulf of Mexico. 2012. PNAS 109(50): 20303–20308; DOI 10.1073/pnas.1118029109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsing PY; Fu B; Larcom EA; Berlet SP; Shank TM; Frese Govindarajan A; Lukasiewicz AJ; Dixon PM; Fisher CR Evidence of lasting impact of the Deepwater Horizon oil spill on a deep Gulf of Mexico coral community. Elem. Sci. Anth 2013, 1, 1–12: DOI 10.12952/journal.elementa.000012. [DOI] [Google Scholar]
- 69.Fisher CR; Montagna PA, Sutton TT How did the Deepwater Horizon oil spill impact Deep-Sea Ecosystems? Oceanography. 2016, September, 182–195; DOI 10.2307/24862720. [DOI] [Google Scholar]
- 70.Larsen RA; Brooks GR; Schwing PT; Holmes CW; Carter SR; Hollander DJ High-resolution investigation of event driven sedimentation: Northeastern Gulf of Mexico. Anthropocene. 2018, 24, 40–50; DOI 10.1016/j.ancene.2018.11.002. [DOI] [Google Scholar]
- 71.Haney JC; Geiger HJ; Short JW Bird mortality from the Deepwater Horizon oil spill. II. Carcass sampling and exposure probability in the coastal Gulf of Mexico. Mar. Ecol. Prog. Ser 2014, 513, 239–252; DOI 10.3354/meps10839. [DOI] [Google Scholar]
- 72.Brewton RA; Fulford R; Griffitt RJ Gene expression and growth as indicators of effects of the BP Deepwater Horizon oil spill on spotted seatrout (Cynoscion nebulosus). J. Toxicol. Env. Heal. A 2013, 76 (21), 1198–1209; DOI 10.1080/15287394.2013.848394. [DOI] [PubMed] [Google Scholar]
- 73.Incardona JP; Gardner LD; Linbo TL; Brown TL; Esbaugh AJ; Mager EM; Stieglitz JD; French BL; Labenia JS; Laetz CA; Tagal M; Sloan CA; Elizur A; Benetti DD; Grosell M; Block BA; Scholz NL Deepwater Horizon crude oil impacts the developing hearts of large predatory pelagic fish. PNAS. 2014. 111, E1510–E1518; DOI 10.1073/pnas.1320950111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barron MG Photoenhanced toxicity of petroleum to aquatic invertebrates and fish. Arch. Environ. Contam. Tox. 2017, 73:40–46; DOI 10.1007/s00244-016-0360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bridges KN; Lay CR; Alloy MM; Gielazyn ML; Morris JM; Forth HP; Takeshita R; Travers CL; Oris JT; Roberts AP Estimating incident ultraviolet radiation exposure in the northern Gulf of Mexico during the Deepwater Horizon oil spill. Environ. Tox. Chem 2018, 37(6), 1679–1687; DOI 10.1002/etc.4119. [DOI] [PubMed] [Google Scholar]
- 76.Lin Q; Mendelssohn IA Impacts and recovery of the Deepwater Horizon oil spill on vegetation structure and function of coastal salt marshes in the Northern Gulf of Mexico. Environ. Sci. Technol 2012, 46, 3737–3743; DOI 10.1021/es203552p. [DOI] [PubMed] [Google Scholar]
- 77.Lin Q; Mendelssohn IA; Graham SA; Hou A; Fleeger JW; Deis DR Response of salt marshes to oiling from the Deepwater Horizon spill: implications for plant growth, soil-surface erosion, and shoreline stability. Sci. Total Environ 2016, 557–558, 369–377; DOI 10.1016/j.scitotenv.2016.03.049. [DOI] [PubMed] [Google Scholar]
- 78.Rabalais NN; Turner RE Effects of the Deepwater Horizon oil spill on coastal marshes and associated organisms. Oceanography. 2016, 29(3), 150–159; DOI 10.2307/24862717. [DOI] [Google Scholar]
- 79.Kneib RT The role of tidal marshes in the ecology of estuarine nekton. Oceanogr. Mar. Biol. 1997, 35, 163–220. [Google Scholar]
- 80.Nemerson DM; Able KW Spatial patterns in diet and distribution of juveniles of four fish species in Delaware Bay marsh creeks: factors influencing fish abundance. Mar. Ecol. Prog. Ser 2004, 276, 249–262; DOI 10.3354/meps276249. [DOI] [Google Scholar]
- 81.Hagan SM; Brown SA; Able KW Production of mummichog Fundulus heteroclitus: response in marshes treated for common reed Phragmites australis removal. Wetlands. 2007, 27(1), 54–67; DOI 10.1672/0277-5212(2007)27(54:POMFHR)2.0.CO.2. [DOI] [Google Scholar]
- 82.Able KW; Lopez-Duarte PC; Fodrie FJ; Jensen OP; Martin CW; Roberts BJ; Valenti J; O’Connor K; Halbert SC Fish assemblages in Louisiana salt marshes: effects of the Macondo oil spill. Estuaries Coast. 2014, 38(5), 1385–1398; DOI 10.1007/s12237-014-9890-6. [DOI] [Google Scholar]
- 83.McCann MJ; Able KW; Christian RR; Fodrie FJ; Jensen OP; Johnson JJ; Lopez-Duarte PC; Martin CW; Olin JA; Polito MJ; Roberts BJ; Ziegler SL Key taxa in food web responses to stressors: the Deepwater Horizon oil spill. Front. Ecol. Environ 2017, 15(3), 142–149; DOI 10.1002/fee.1474. [DOI] [Google Scholar]
- 84.Burnett KG; Bain LJ; Baldwin WS; Callard GV; Cohen S; DiGiulio RT; Evans DH; Gomez-Chiarri M; Hahn ME; Hoover CA; Karchner SI; Katoh F; MacLatchy DL; Marshall WS; Meyer JN; Nacci DE; Oleksiak MF, Rees BB; Singer TD; Stegeman JJ; Towle DW; Van Veld PA; Vogelbein WK; Whitehead A; Winn RN; Crawford DL Fundulus as the premier teleost model in environmental biology: Opportunities for new insights using genomics. Comp. Biochem. Physiol 2007, D2, 257–286; DOI 10.1016/j.cbd.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vivian DN; Rakocinski CF; Peterson MS Habitat function of a restored salt marsh: post-larval Gulf Killifish as a sentinel In Estuaries: classification, ecology and human impacts; Jordan S, Ed; Nova Publishers, Inc, Hauppauge: 2012, pp 57–74. [Google Scholar]
- 86.Greeley MS; MacGregor R Annual and semilunar reproductive cycles of the gulf killifish, Fundulus grandis, on the Alabama gulf coast. Copeia. 1983, 3, 711–718; DOI 10.2307/1444337. [DOI] [Google Scholar]
- 87.Michel J; Owens EH; Zengel S; Graham A; Nixon Z; Allard T; Holton W; Reimer PD; Lamarche A; White M; Rutherford N; Childs C; Mauseth G; Challenger G; Taylor E Extent and degree of shoreline oiling: Deepwater Horizon oil spill, Gulf of Mexico, USA. PLoS One. 2013, 8(6): e65087; DOI 10.1371/journal.pone.0065087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Able KW; Hata D Reproductive behavior in the Fundulus heteroclitus-F.grandis complex. Copeia. 1984, 4, 820–825; DOI 10.2307/1445323. [DOI] [Google Scholar]
- 89.Lipicius RN; Subrahmanyam CB Temporal factors influencing killifish abundance and recruitment in Gulf of Mexico salt marshes. Estuar. Coast. Shelf S 1986, 22, 101–114. [Google Scholar]
- 90.Dubansky B; Whitehad A; Miller JT; Rice CD; Galvez F Multitissue molecular, genomic, and developmental effects of the Deepwater Horizon oil spill on resident gulf killifish (Fundulus grandis). Environ. Sci. Tech 2013, 47, 5074–5082; DOI 10.1021/es400458p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meyer JN; Di Giulio RT Heritable adaptation and fitness costs in killifish (Fundulus heteroclitus) inhabiting a polluted estuary. Ecol. App 2003, 13(2), 490–503; DOI 10.1890/1051-0761(2003)013[0490:HAAFCI]2.0.CO;2. [DOI] [Google Scholar]
- 92.Deegan LA Salt marsh ecosystem support of marine transient species In Concepts and Controversies in Tidal Marsh Ecology; Weinstein MP, Kreeger DA, Eds; Kluwer Academic Publishers, Netherlands: 2000; pp 333–365. [Google Scholar]
- 93.Deegan LA Nutrient and energy transport between estuaries and coastal marine ecosystems by fish migration. Can. J. of Fish. Aquat. Sci 1993, 50, 74–79; DOI 10.1139/f93-009. [DOI] [Google Scholar]
- 94.Short JW; Geiger HJ; Haney JC; Voss CM; Vozzo ML; Guillory V; Peterson CH Anomalously high recruitment of the 2010 gulf menhaden (Brevoortia patronus) year class: evidence of indirect effects from the Deepwater Horizon blowout in the Gulf of Mexico. Arch. Environ. Contam. Toxicol 2017, 73, 76–92; DOI 10.1007/s00244-017-0374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Powers SP; Grabowski JH; Roman H; Geggel A; Rouhani S; Oehrig J; Baker M Consequences of large-scale salinity alteration during the Deepwater Horizon oil spill on subtidal oyster populations. Mar. Ecol. Prog. Ser 2017, 576, 175–187; DOI 10.3354/meps12147. [DOI] [Google Scholar]
- 96.La Peyre MK; Eberline BS; Soniat TM; La Peyre JF Differences in extreme low salinity timing and duration differentially affect eastern oyster (Crassostrea virginica) size class growth and mortality in Breton Sound, LA. Estuar. Coast. Shelf S 2013, 135, 146–157; DOI 10.1016/j.ecss.2013.10.001. [DOI] [Google Scholar]
- 97.Lane SM; Smith CR; Mitchell J; Balmer BC; Barry KP; McDonald T; Mori CS; Rosel PE; Rowles TK; Speakman TR; Townsend FI; Tumlin MC; Wells RS; Zolman ES; Schwacke LH Reproductive outcome and survival of common bottlenose dolphins sampled in Barataria Bay, Louisiana, USA, following the Deepwater Horizon oil spill. Proc. R. Soc. B. 2015, 282, 1–9; DOI 10.1098/rspb.2015.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schwacke LH; Smith CR; Townsend FI; Wells RS; Hart LB; Balmer BC; Collier TK; De Guise S; Fry MM; Guillette LJ Jr.; Lamb SV; Lane SM; McFee WE; Place NJ; Tumlin MC; Ylitalo GM; Zolman ES; Rowles TK Health of common bottlenose dolphins (Tursiops truncatus) in Barataria Bay, Louisiana, following the Deepwater Horizon oil spill. Environ. Sci. Technol 2014, 48, 93–103; DOI 10.1021/es403610f. [DOI] [PubMed] [Google Scholar]
- 99.Miller WG; Adams LG; Ficht TA; Cheville NF; Payeur JP; Harley DR; House C; Ridgway SH Brucella-induced abortions and infection in bottlenose dolphins (Tursiops truncatus). J. Zoo. Wildl. Med 1999, 30, 100–110. [PubMed] [Google Scholar]
- 100.Wells RS; Scott MD Estimating bottlenose dolphin population parameters from individual identification and capture-release techniques. In Individual recognition of cetaceans: use of photo-identification and other techniques to estimate population parameters. Incorporation the proceedings of the symposium and workshop on individual recognition and the estimation of cetacean population parameters; Hammond PS; Mizrock SA; Donovan GP, Eds; Report of the International Whaling Commission, Special Issue (12); International Whaling Commission: Cambridge 1990, pp 407–415. [Google Scholar]
- 101.Speakman TR; Lane SM; Schwacke LH; Fair PA; Zolman ES Mark-recapture estimates of seasonal abundance and survivorship for bottlenose dolphins (Tursiops truncatus) near Charleston, South Carolina, USA. J. Cetacean Res. Manag 2010, 11(2), 153–162. [Google Scholar]
- 102.Paine RT; Ruesink JL; Sun A; Soulanille EL; Wonham MJ; Harley CD; Brumbaugh DR; Secord DL Trouble on oiled waters: lessons from the Exxon Valdez oil spill. Annu. Rev. Ecol. Evol. Syst 1996, 27(1), 197–235; DOI 10.1146/annurev.ecolsys.27.1.197. [DOI] [Google Scholar]
- 103.Pelletier MC; Burgess RM; Ho KT; Kuhn A; McKinney RA; Ryba SA Phototoxicity of individual polycyclic aromatic hydrocarbons and petroleum to marine invertebrate larvae and juveniles. Environ. Tox. Chem 1997, 16(10), 2190–2199; DOI 10.1002/etc.5620161029. [DOI] [Google Scholar]
- 104.Jung JH; Moonkoo K; Yim UH; Ha SY; Shim WJ; Chae YS; Kim H; Incardona JP; Linbo TL; Kwon JH Differential toxicokinetics determines the sensitivity of two marine embryonic fish exposed to Iranian heavy crude oil. Environ. Sci. Technol 2015, 49 (22), 13639–13648; DOI 10.1021/acs.est.5b03729. [DOI] [PubMed] [Google Scholar]
- 105.Olsen E; Hansen C; Nilsen I; Perryman H; Vikebe F Ecological effects and ecosystem shifts caused by mass mortality events on early life stages of fish. Front Mar Sci. 2019, 6 (699); DOI 10.3389/fmars.2019.00669. [DOI] [Google Scholar]
- 106.Ainsworth CH; Paris CB; Perlin N; Dornberger LN; Patterson III WF; Cancellor E; Murawski S; Hollander D; Daly K; Romero IC; Coleman F; Perryman H Impacts of the Deepwater Horizon oil spill evaluated using an end-to-end ecosystem model. Plos One. 2018, 13(1), e0190840; DOI 10.1371/journal.pone.0190840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bence AE; Kvenvolden KA; Kennicutt II MC Organic geochemistry applied to environmental assessments of Prince William Sound, Alaska, after the Exxon Valdez oil spill – a review. Org. Geochem. 1996, 24(1), 7–42; DOI 10.1016/0146-6380(96)00010-1. [DOI] [Google Scholar]
- 108.Barron MG; Podrabsky T; Ogle S; Ricker RW Are aromatic hydrocarbons the primary determinant of petroleum toxicity to aquatic organisms? Aquat. Toxicol 1999, 46:253–268; DOI 10.1016/S0166-445X(98)00127-1. [DOI] [Google Scholar]
- 109.Aeppli C; Carmichael CA; Nelson RK; Lemkau KL; Graham WM; Redmond MC; Valentine DL; Reddy CM Oil weathering after the Deepwater Horizon disaster led to the formation of oxygenated residues. Environ. Sci. Technol 2012, 46 (16), 8799–8807; DOI 10.1021/es3015138. [DOI] [PubMed] [Google Scholar]
- 110.Leifer I; Lehr WJ; Simecek-Beatty D; Bradley E; Clark R; Dennison P; Hu Y; Matheson S; Jones CE; Holt B; Reif M; Roberts DA; Svejkovsky J; Swayze G; Wozencraft J State of the art satellite and airborne marine oil spill remote sensing: Application to the BP Deepwater Horizon oil spill. Remote Sensing Environ. 2012. 124, 185–209. [Google Scholar]
- 111.Barron MG; Chiasson SC; Bejarano AC Ecotoxicology of deep ocean spills Chapter 27. Eds: Murawski SA; Ainsworth CH; Gilbert S; Hollander DJ; Paris CB; Schluter M; Wetzel DL 2020, Springer, Cham: pp. 466–479. [Google Scholar]
- 112.Bejarano AC; Gardiner WW; Barron MG; Word JQ Relative sensitivity of Arctic species to physically and chemically dispersed oil determined from three hydrocarbon measures of aquatic toxicity. Mar. Poll. Bull 2017, 122, 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gill DA; Picou S; Ritchie LA The Exxon Valdez and BP oil spills: A comparison on initial social and psychological impacts. Amer. Behav. Sci 2012, 56(1), 3–23; DOI 10.1177/002764211408585. [DOI] [Google Scholar]
- 114.Jung D; Kim JA; Park MS; Yim UH; Choi K Human health and ecological assessment programs for Hebei Spirit oil spill accident of 2007: Status, lessons, and future challenges. Chemosphere, 2017, 173, 180–189; DOI 10.1016/j.chemosphere.2016.12.153. [DOI] [PubMed] [Google Scholar]
- 115.Choi KH; Park MS; Ha M; Hur JI; Cheong HK Cancer Incidence Trend in the Hebei Spirit Oil Spill Area, from 1999 to 2014: An Ecological Study. Int. J. Environ. Res. Public Health 2018, 15,1006; DOI 10.3390/ijerph15051006. [DOI] [PMC free article] [PubMed] [Google Scholar]