Abstract
Heart development is a complex process and begins with the long-range migration of cardiac progenitor cells during gastrulation. This culminates in the formation of a simple contractile tube with multiple layers, which undergoes remodeling into a four-chambered heart. During this morphogenesis, additional cell populations become incorporated. It is important to unravel the underlying genetic and cellular mechanisms to be able to identify the embryonic origin of diseases, including congenital malformations, which impair cardiac function and may affect life expectancy or quality. Owing to the evolutionary conservation of development, observations made in nonamniote and amniote vertebrate species allow us to extrapolate to human. This review will focus on the contributions made to a better understanding of heart development through studying avian embryos—mainly the chicken but also quail embryos. We will illustrate the classic and recent approaches used in the avian system, give an overview of the important discoveries made, and summarize the early stages of cardiac development up to the establishment of the four-chambered heart.
EXPERIMENTAL APPROACHES USED IN CHICKEN EMBRYOS
Studies in avian models have made significant contributions to our understanding of embryo development, including cardiac development. Both chicken (Gallus gallus) and Japanese quail (Coturnix japonica) embryos are easily accessible from blastula stages onward. The chick is more commonly used as the eggs are larger and in ovo manipulations—including tissue grafting, ablation, or injection—are performed through a small window cut into the shell, which is then sealed before reincubating the egg for further embryo development (Bronner-Fraser 2011). The complex events of heart development and their timing in the chick embryo are well documented and have been mapped onto the Hamburger–Hamilton stages (Hamburger and Hamilton 1951; Martinsen 2005). In addition, quail embryonic stages (Ainsworth et al. 2010) are extremely similar in size and morphology to those in chick. This enables cross-species grafting of quail donor tissue into a chick host to create chimeric embryos for the purpose of fate mapping (Le Douarin 1973). This classic technique uses antibody staining to trace quail cells and has provided fundamental insights, for example, into the contribution of neural crest cells (NCCs) to outflow tract (OFT) remodeling (Fig. 1; Kirby et al. 1983). The more recent availability of transgenic chick embryos, where all cells are labeled with GFP, has facilitated the straightforward detection of tissue grafted from chick to chick using fluorescence imaging (McGrew et al. 2008). Additional fluorescent reporter lines are now available (summarized in Davey et al. 2018) and will enable even more sophisticated lineage analyses. For example, a Cytbow line uses random recombination between loxP sites to generate clones of cells indelibly labeled with either YFP, RFP, or membrane-blue FP. Although delivery of cre-recombinase remains a challenge, cell labeling using fluorescent marks has the potential to expand on previous clonal analyses, which used infection with replication-incompetent retrovirus (RCAS) (Mikawa et al. 1992; Cheng et al. 1999; Wei and Mikawa 2000).
Figure 1.
Experimental approaches in avian embryos. Schematic overview of experimental approaches commonly used in avian species, namely grafting, microinjection, and electroporation. Grafting has, for example, identified a subpopulation of neural crest cells from the neural folds near somites 1–4 that contribute to the outflow tract. Various microinjection techniques have been described, including the delivery of anatagomirs into the myocardial wall to block microRNA function. This approach allows analysis of up to 3 days postinjection (∼HH27), which covers different phases of cardiac remodeling. Finally, chick embryo electroporation is widely used and allows the targeted delivery of plasmid or viral constructs designed to interfere with cellular processes. (M) mesencephalic, (C) cardiac, (V) vagal, (T) trunk.
The use of cultured primordial germ cells (PGCs) has improved the efficacy of avian transgenesis and should facilitate the generation of genome-edited transgenic birds (van de Lavoir et al. 2006; Macdonald et al. 2010) via modification of the PGC genome using CRISPR-Cas9 (Oishi et al. 2016). Different methods have been used to deliver the CRISPR-Cas9 ribonucleoprotein complex. Examples include electroporation of inducible or constitutively expressed vectors encoding Cas9 and guide RNA (Véron et al. 2015; Gandhi et al. 2017). Electroporation of the preassembled CRISPR-Cas9 complex, as shown in cells (Schumann et al. 2015), should also be possible in embryos and will reduce off-target effects. Furthermore, tools for CRISPR-Cas9 genome engineering are continuously being optimized for the use in chicken (Williams et al. 2018). The quail is also an attractive option for transgenesis as its generation time is shorter (Poynter and Lansford 2008) and quail transgenic lines have been generated and used for live-cell imaging, for example, vasculogenesis (Sato et al. 2010; Davey et al. 2018). Furthermore, adenoviral delivery of CRISPR-Cas9 directly into the quail blastoderm generates chimeras (Lee et al. 2019), which produce carriers of the targeted mutation; alternatively, quail PGCs can be transfected and are available for germline transmission (Shin et al. 2008; Serralbo et al. 2019).
Transient transgenesis avoids the generation and maintenance of transgenic lines and is an extremely useful alternative, which to date has provided many insights into signaling pathways and gene regulatory networks governing cardiac development. This approach uses microinjection of vectors followed by electroporation, either in ovo or in embryos cultured ex ovo (Itasaki et al. 1999; Chapman et al. 2001; Sauka-Spengler and Barembaum 2008). A commonly used ex ovo electroporation set-up is visualized in Figure 1. Combining fluorescent reporters with cardiogenic enhancers followed by electroporation, live imaging, and quantitative analysis of cell migration trajectories can further increase the depth with which cell lineages and their behavior can be resolved (Dormann and Weijer 2006; Song et al. 2014; Zamir et al. 2017).
To investigate the function of genes, their activity can be manipulated. The targeted injection and electroporation of vectors typically enhances gene expression (Fig. 1). In addition, constitutively active or dominant negative forms of the protein of interest can be expressed, for example, of transcriptional regulators, transmembrane receptors, or intracellular signaling components (Song et al. 2014). The knockdown of gene activity can be achieved by antisense morpholinos or RNAi oligos, which can be electroporated or delivered using pluronic gel (Sauka-Spengler and Barembaum 2008; Rutland et al. 2009). This suite of tools also includes cholesterol-modified antisense-inhibitors of microRNA function, so-called AntagomiRs, which can be delivered effectively into the myocardial wall by targeted microinjection (Fig. 1; Wittig et al. 2019). The local application of growth factors, pharmacological agonists or antagonists, on beads or filter paper, provide additional opportunities for functional interference, for example, to study signaling pathways, and similar approaches can be used to examine cardiac toxicity of drugs or chemicals. Microsurgery at later stages of heart development is possible after first removing the extraembryonic membranes (Spurlin and Lwigale 2013). Approaches include atrial ligation, for example, to assess the role of blood flow on aortic arch morphogenesis (Hu et al. 2009), electrocardiogram (ECG) measurements (Shi et al. 2013), or cryoinjury to examine regenerative processes (Table 1; Palmquist-Gomes et al. 2016) .
Table 1.
Experimental approaches used in avian embryos
| Method (title) | Intention | References |
|---|---|---|
| Grafting of quail donor tissue into a chick host | The fate of quail cells can be mapped in chimeric embryos | Le Douarin 1973 |
| Ex ovo culture on filter paper ring in dish, multiwell dish, or imaging chamber | Microinjection and electroporation of plasmid vectors, oligonucleotides, application of growth factors, drugs, or chemicals—live imaging | Chapman et al. 2001; Sato et al. 2010; Song et al. 2014; Rozbicki et al. 2015 |
| Cardiac injections of AntagomiRs as a novel tool for knockdown (KD) of microRNA (miRNA) during heart development | Best suitable between HH13–HH18. Depending on the stage, specific targeting of a subsection of the heart is possible to manipulate expression of genes or KD miRNA function. Also applicable for morpholinos, plasmid constructs, and others | Wittig et al. 2019 |
| A technique to increase accessibility to late-stage chick embryos for in ovo manipulations | This approach allows accessibility of later stage chicken embryos (HH27–HH34); they are viable and can be used for manipulation | Spurlin and Lwigale 2013 |
| An ex-ovo chicken embryo culture system suitable for imaging and microsurgery applications | The method describes a shell-less way to culture chicken embryos ex ovo for up to 14 days and allows for various imaging and microsurgical applications, for example, left atrial ligation | Yalcin et al. 2010 |
| A chick embryo cryoinjury model for the study of embryonic organ development and repair | To examine regenerative and reparative capabilities of embryonic tissues during organogenesis | Palmquist-Gomes et al. 2016 |
| Alterations in pulse wave propagation reflect the degree of outflow tract (OFT) banding in HH18 chicken embryos | Electrocardiogram (ECG) measurements of chick hearts were conducted to study hemodynamic changes that are linked to the cardiac cycle; the latter can be manipulated by various methods as OFT ligation | Shi et al. 2013 |
EARLY EVENTS IN CARDIAC DEVELOPMENT
Cardiac Progenitor Cell Migration, Fate Specification, and Formation of the Linear Heart Tube
Gastrulation is a major event in early development when embryos undergo a dramatic transformation in shape to generate the three germ layers. This morphogenetic process has been studied extensively in chicken embryos and detailed reviews and book chapters are available. Because these early stages are easily accessible, the origin of cardiogenic cells in the blastula and gastrula have been mapped, and their specification toward the cardiac fate is well understood. Just before the start of gastrulation, the blastula—a flat concentric disc—consists of two layers: the epiblast, which generates all of the embryo, and the hypoblast beneath. Dye labeling was used to map the presumptive heart territory in the epiblast (Hatada and Stern 1994). Cocultures of tissue explants showed that posterior epiblast cells respond to an activin transforming growth factor β (TGF-β) signal from the hypoblast to induce cardiac myogenesis (Yatskievych et al. 1997). Interestingly the TGF-β superfamily members bone morphogenetic protein 2 (BMP2) and BMP4 are inhibitory for cardiomyogenesis at this pregastrula stage (Ladd et al. 1998), although they are important slightly later (see below).
The origin of cardiac mesoderm in primitive-streak gastrula stage embryos and their subsequent location in the lateral plate mesoderm, the so-called first heart field (FHF) (Fig. 2), has been determined using a variety of methods including quail-chick chimeras, or labeling with dyes or carbon particles. These studies revealed that cardiac mesoderm comes from the anterior midprimitive streak between HH3 and HH3+, where at these early stages it is arranged in a rostrocaudal sequence that reflects the organization of the straight heart tube (Garcia-Martinez and Schoenwolf 1993; Redkar et al. 2001; Wittig and Münsterberg 2016, and references therein). A few hours later, by midgastrula stages HH4+, the contribution to the heart from the primitive streak has ceased (Psychoyos and Stern 1996). Lineage tracing with RCAS indicates that at primitive streak stages the premyocardial and pre-endocardial cells are distinct subpopulations and it has been suggested that they segregate before arriving in the primitive streak (Wei and Mikawa 2000). Given the rapid advances in single-cell sequencing technologies it is likely that the first appearance of discrete progenitors will be reexamined before long; this may also reveal novel and potentially unique markers for subpopulations.
Figure 2.
From heart fields to early looping. The major steps of early heart formation are illustrated here in cartoon form. The bilateral cardiogenic mesoderm comprises progenitors of the first heart field (green) and second heart field (red), which are organized mediolaterally (HH5). Together they contribute to outflow tract and right ventricle (red) and left ventricle and atria (green). During fusion, which is closely associated with foregut morphogenesis (see text), the mediolateral organization converts into an anteroposterior organization generating the early heart tube by HH10. At that stage, the heart is still linear and shortly after undergoes bulging and dextral (rightward) bending, which initiates the C-shape (HH11–HH13). Subsequent remodeling leads to compartmentalization of the heart.
After completing an epithelial-to-mesenchymal transition (EMT) and emerging from the primitive streak, cardiac progenitor cells (CPCs) undergo long-range migration. Fluorescent labeling of CPCs at HH3 to HH3+, followed by live cell tracking, showed that CPCs move laterally and anteriorly before converging toward the anterior intestinal portal (AIP)—the forming foregut (Yue et al. 2008; Song et al. 2014). Interestingly, the AIP has been identified as a putative heart organizer; it shares a molecular signature with other organizer tissues and can induce cardiac and ventricular identity in noncardiac mesoderm (Anderson et al. 2016). In gastrula stage embryos, the migration trajectories of CPCs are affected by exposure to BMP2/4 and wingless-type MMTV-integration site family member (WNT) 3a, with both signaling pathways converging on Sma and Mad-related Protein (SMAD) 1 and promoting its activity (Yue et al. 2008; Song et al. 2014).
The identification of the source and nature of signals involved in specification of cardiac fate in the gastrula stage embryo was facilitated by combining quail and chick tissues in explant cultures. These experiments used cocultures of candidate-inducing tissues with candidate-responding tissues (Schultheiss et al. 1995), or the implantation of beads soaked in growth factors to test their activity (Andrée et al. 1998). This showed that cardiac fate specification, indicated by expression of the transcription factor Nk2 homeobox 5 (NKX2.5), is mediated by BMP2 from anterior lateral endoderm (Schultheiss et al. 1997). In vivo, NKX2.5 is first detected by in situ hybridization at HH5 in the lateral plate mesoderm. In chick, BMP-responsive cis-regulatory elements of NKX2.5—comprising GATA-binding protein (GATA) and SMAD-binding sites—have been identified (Lee et al. 2004). Additional work identified fibroblast growth factor 8 (FGF-8) together with inhibitors of the canonical WNT pathway as important signals for cardiac fate acquisition (Marvin et al. 2001; Alsan and Schultheiss 2002). How these signaling networks integrate to regulate both the migration of CPCs from the primitive streak toward the FHF and their concomitant fate specification is unclear at present.
By HH7 a number of transcription factors, including Islet-1 (ISL1), GATA2/4, and NKX2.5, are expressed in the lateral splanchnic mesoderm where cardiogenic progenitors are located. At this stage, the bilateral heart-forming regions are separated by the foregut endoderm in the midline, and posteriorly they extend to the level of the first somite (Fig. 2). In quail, a contiguous arc of cardiogenic cells was detected as early as HH4 (Cui et al. 2009), before formation of the AIP. Fate maps using quail-chick grafts revealed a distinct population of cells medially adjacent to the HH7 cardiac crescent, which express endothelial markers and contribute to the endocardium (Milgrom-Hoffman et al. 2011). In addition, tracking cells expressing a fluorescent reporter under the control of a mouse NKX2.5 enhancer identified a population of positive cells from outside the FHF, which also contributes to the hemogenic epithelium of the endocardium (Zamir et al. 2017).
DiI labeling of cardiac mesoderm at HH7–8 combined with microincisions to probe tissue stress followed by time-lapse imaging and computational modeling, showed that the bilateral primordia converge to form a linear heart tube by HH9+, which shortly after begins to bulge (Fig. 2). This process is mediated by convergent extension and treatment with pharmacological inhibitors showed that cellular rearrangements require cell proliferation and Rho-associated coiled-coil-containing protein kinase (ROCK)-dependent myosin contractions (Aleksandrova et al. 2015; Hosseini et al. 2017; Kidokoro et al. 2018). Cells converge toward the midline and epithelialize and form a contractile tube with endocardial and myocardial cell layers separated by an acellular layer of cardiac jelly (CJ) (HH9). The jelly is composed of extracellular matrix (ECM) components including hyaluronan (HA), versican (VCAN), and collagens, which are synthesized by cells of the myocardium in response to BMP2 and WNT6 signals (Person et al. 2005). Through direct labeling, it has been shown that ECM components such as fibronectin and fibrillin-2 translocate into the forming heart tube (Aleksandrova et al. 2012). Furthermore, heart tube formation is tightly coordinated with foregut morphogenesis and is accompanied by large-scale tissue deformations and folding of the endoderm (Cui et al. 2009; Varner and Taber 2012). Computational modeling illustrates how differential growth rates in endoderm and mesoderm can drive these dramatic morphological changes (Hosseini et al. 2017).
After formation of the early heart tube, the bilateral symmetry is broken by rightward looping beginning at HH10 leading to a C-shape (Fig. 2). At this time, the dorsal mesocardium, which connects the heart tube to the foregut and secures it in the pericardial cavity, breaks down. This allows the anterior and posterior ends of the tube—the arterial and venous poles, respectively—to converge, leading to the so-called S-shape (HH14–18). In a final phase, the position of the OFT relative to the atria is rearranged and looping is complete by HH24 (Männer 2000, 2004, additional references in Wittig and Münsterberg 2016). This process was first described in chick and depends on extrinsic and intrinsic mechanisms including mechanical constraints, asymmetric cell growth, and proliferation and ingression of precursor cells at the posterior end of the tube. Recent work also implicates the EMT inducer paired-related homeobox (PRX) 1, which is regulated by left–right asymmetry signals expressed in the lateral plate, in the initiation of the looping process (Ocaña et al. 2017). Importantly, any disturbances can lead to various laterality defects described in a recent review, which also provides detailed comparisons of the process in fish, chick, and mice (Desgrange et al. 2018, and additional references therein).
ADDITIONAL CELL POPULATIONS CONTRIBUTING TO THE HEART
Additional Heart Fields
Another seminal discovery was the identification of a secondary heart field (SHF) and an anterior heart field (AHF), which comprise populations of cells in the pharyngeal mesoderm that make an essential contribution to the OFT (Mjaatvedt et al. 2001; Waldo et al. 2001; Yutzey and Kirby 2002). The origin of AHF and SHF progenitors has been mapped to the primitive streak. Dye labeling showed that AHF precursors are located immediately adjacent and anterior to CPCs that form the FHF and SHF, respectively. By HH7 the cardiogenic mesoderm is organized such that FHF progenitors, contributing to the atria and left ventricle (LV), are located lateral to SHF progenitors, which generate the right ventricle (RV) and OFT (Fig. 2; Abu-Issa and Kirby 2008). This mediolateral organization converts into an anteroposterior organization during the fusion process that generates the primitive heart tube described above (Hosseini et al. 2017; Kidokoro et al. 2018). AHF cells located in the cranial paraxial mesoderm (CPM) at HH8 are found in pharyngeal mesoderm of the first branchial arch and the OFT by HH15. CPCs emerging from the primitive streak slightly posteriorly express the transcription factor ISL1 when located in the splanchnic mesoderm (HH8) and are later found in OFT and endo- and myocardium of the heart tube (HH12) (Camp et al. 2012). This is consistent with classic quail–chick chimera experiments showing that CPM-derived cells contribute to angioblasts in the OFT (Noden 1991) and to OFT myocardium and endocardium (Tirosh-Finkel et al. 2006).
Interestingly, the CPM also contributes to head muscles (Noden 1991) and expression analyses revealed a close relationship between the heart and craniofacial muscle programs with overlapping molecular signatures in splanchnic lateral plate mesoderm and CPM. In particular, the transcription factors T-box 1 (TBX1) and paired-like homeodomain 2 (PITX2) are involved in both cardiogenesis and craniofacial myogenesis (for review, see Tzahor 2009). In the CPM, cardiac differentiation is blocked by neural tube-derived canonical WNT signals. This inhibition can be mimicked by electroporation of vectors encoding WNT ligands; conversely, WNT antagonists promote cardiogenesis (Marvin et al. 2001; Nathan et al. 2008). Activation of WNT signaling at this stage in development led to inhibition of both cardiac and skeletal muscle differentiation markers, indicating a genetic program that is at least partially overlapping (Nathan et al. 2008). Although work from the same group, using overexpression in chick embryos, also showed that ISL1 is involved in specification of cardiac versus skeletal myogenic progenitors (Harel et al. 2009).
Fluorescent dye injections have been used to map the origin of pacemaker cells. In an HH8 embryo, these progenitors are found outside the NKX2.5 and ISL1-positive regions, posterior to the FHF and SHF in lateral plate mesoderm adjacent to somite 3. This region has been referred to as a tertiary heart field (Bressan et al. 2013). Pacemaker fate is specified by WNT8c mediated canonical WNT signals and inhibited by Crescent, a WNT antagonist, which promotes cardiogenesis more anteriorly (Marvin et al. 2001).
Formation of the Epicardial Cell Layer
The epicardium is the outermost layer enveloping the heart, it forms relatively late in heart development from the proepicardial organ (PEO). The PEO is a transient structure and first detected at HH14 by electron microscopy (EM) as a group of cells located at the back of the heart between the sinus venosus and the liver bud (Männer 2000). From this site, the proepicardial cells migrate via a cellular bridge to colonize the myocardium of the looping heart. In addition, proepicardial cells integrate with pacemaker myocardial cells to mediate the remodeling and functional maturation of the cardiac pacemaker region into the sinoatrial node (SAN) (Bressan et al. 2018). In chick, the PEO develops asymmetrically on the right side from coelomic somatic lateral plate mesoderm (Schlueter and Brand 2013). This process depends on left–right signals that determine sidedness in the early embryo, in particular FGF-8 and snail family transcriptional repressor (SNAI) 1. The latter targets Twist-family basic helix–loop–helix transcription factor (TWIST) 1 to regulate mobilization of cells form somatic mesoderm (Schlueter and Brand 2013). A residual PEO initially forms on the left but is lost by apoptosis (Schlueter and Brand 2009). The PEO can be identified by strong expression of Wilms Tumor Protein (WT) 1 and TBX18 and is induced by the liver bud (Ishii et al. 2007). Although the PEO generates the majority of the epicardium, transplantations showed that epicardium covering the distal OFT originates from elsewhere (Pérez-Pomares et al. 2003). Proepicardial identity and the growth of the epicardium are mediated by BMP signaling. BMP triggers PE cell migration outward over the heart; this also requires the transcription factor TBX5 (Hatcher et al. 2004; Schlueter et al. 2006; Ishii et al. 2010). By HH17–18, villous protrusions of the PE reach the atrioventricular canal (AVC) and migrate further by HH20 when they surpass the inner curvature of the heart. By stage HH21–23, the first cells start penetrating the dorsal myocardium of the ventricles. Formation of the epicardium is completed by HH27, but quail chick chimeras determined that subsequently the epicardium contributes to various cell populations until HH31 (day 7 of development). These include the AV-endocardial cushions, the endothelial and smooth muscle cells of the coronary vessels and perivascular and intramyocardial fibroblasts (Männer 1999). A recent comparative review discusses the evolutionary origin of the proepicardium and the potential contribution of single-cell sequencing approaches, which may lead to a better understanding of the lineage relationships of epicardial cell types (Simões and Riley 2018).
CARDIAC DEVELOPMENT POST-HEART TUBE FORMATION
Following heart tube formation, extensive remodeling is necessary to achieve the four-chambered anatomy of the heart. This complex morphogenesis has been studied in detail in chicken embryos and several processes have to take place including (1) cushion formation and valve development, (2) septation and chamber separation, (3) trabeculation and compaction, and (4) the formation of the cardiac conduction system (Martinsen 2005).
Cushion Formation and Valve Development
The first signs of CJ deposition at the OFT and AVC can be observed just after initiation of looping. This marks the beginning of endocardial cushion formation. EMT triggers the migration of endocardial cells into the CJ where they proliferate and further contribute to ECM synthesis expanding the cushions. In chick embryos, EMT takes place between stages HH14 and HH19 (Mercado-Pimentel and Runyan 2007).
Cushion formation occurs at two main locations: the AVC and the OFT. The AVC cushions emerge at stage HH16; they soon get populated by mesenchymal cells originating from the endocardium, which have undergone myocardium-induced EMT causing the endothelial cells to loosen cell–cell adhesions to become migratory to invade the ECM (Harris and Black 2010). In vivo labeling experiments in chick have shown that these early AVC cushions—a dorsal and ventral endocardial cushion, will fuse and form the septum intermedium (SIM) (Fig. 3), which later contributes to the aortic leaflet of the mitral valve and the posterior inferior and septal leaflets of the tricuspid valve (de la Cruz et al. 1977; de la Cruz and Markwald 1998). Further contributions to the mitral and tricuspid valve come from lateral cushions, which develop on both sides of the heart later in development starting at around HH26 in the chick embryo (Fig. 3; Moreno-Rodriguez and Krug 2015).
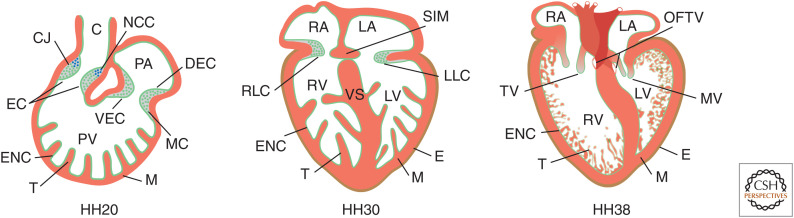
Figure 3.
Formation of cushions and septa. Schematic illustrations of sectioned hearts that depict important steps of cardiac cushion maturation to septa and valves in the chicken embryo. By HH20, the heart consists of a primitive atrium and a primitive ventricle; endocardial cushions have formed in the outflow tract (OFT) and at the atrioventricular canal (AVC) junction. Mesenchymal cells have entered the cushions, originating from epithelial-to-mesenchymal transition (EMT) induced by the endocardium. By HH30, the dorsal endocardial cushion and ventral endocardial cushion have fused to form the septum intermedium (SIM), which is joined with the interatrial septum. More cushions have developed, namely, the left and right lateral cushions, which contribute to the formation of the tricuspid and mitral valves later in development. Growth of the ventricular septum (VS) toward the SIM has not finished by this stage but is completed by HH38 separating the ventricles. By then, all valves have matured. (C) conus, (CJ) cardiac jelly, neural crest cell (NCC), (DEC) dorsal endocardial cushion, (EC) endocardial cushions, (ENC) endocardium, (E) epicardium, (LA) left atrium, (LLC) left lateral cushion, (LV) left ventricle, (M) myocardium, (MV) mitral valve, (OFTV) outflow tract valves, (PA) primitive atrium, (PV) primitive ventricle, (RA) right atrium, (RLC) right lateral cushion, (RV) right ventricle, (T) trabeculae, (TV) tricuspid valve, (VEC) ventral endocardial cushion.
The second site of cushion formation is the OFT, which initiates slightly later than the AVC cushions at around HH18 (Person et al. 2005). By HH21 pairs of distinctive cushions can be observed within OFT (Fig. 3), located proximally (left and right) and distally (dorsal and right ventral). In fact, microscopy of chicken heart sections showed that those cushions are not completely separate units. The left proximal cushion stretches from the ventricular opening to the top of the OFT where it is connected to the arterial segment and becomes the distal dorsal cushion (Qayyum et al. 2001). The third distal OFT cushion forms left to the distal right ventral cushion at around HH25–26. These five OFT cushions contribute to a temporary aorticopulmonary septum that is replaced later and to the semilunar valves, which prevent blood flow back into the heart (Martinsen 2005; Snarr et al. 2008). Importantly, OFT cushion maturation depends not only on EMT-derived mesenchyme but also on cardiac NCC, which migrate into the distal OFT (Kirby and Waldo 1995) as early as stage HH18. The NCCs are essential for the formation of the interatrial septum (IAS). Overall, it takes up to stage HH36 (10 days) to complete formation of valves and septa in the chicken heart (Fig. 3; Martinsen 2005; Person et al. 2005). Interestingly, quail and chick transplantation experiments showed that the developmental potential of NCC is already defined by HH8–10. Quail NCC were grafted from mesencephalic, cardiac, or truncal regions into the cardiac NCC region of host chick embryos (Fig. 1). Grafts of noncardiac NCC lead to severe OFT defects as these cells were not able to contribute to its proper development, suggesting that by this stage fate is restricted (Kirby and Waldo 1995). Interestingly, NCCs also contribute to ventricular cardiomyocytes, as recently revealed using RCAS-mediated lineage analysis in chick. This contribution had previously been overlooked but was also confirmed in mouse by Wnt1-cre lineage analysis. Furthermore, the NCC molecular program was up-regulated in the regenerating zebrafish myocardium, thus highlighting its potential importance for heart repair (Tang et al. 2019).
Atrial Chamber Separation
Early on in heart development the four chambers are not separated from each other and the necessary septa for their division arise during remodeling of the heart. The IAS arises approximately at HH16 in the chicken heart. It originates as septum primum from the cephalodorsal wall (Quiring 1933; Hendrix and Morse 1977). In contrast to higher vertebrates, which have a septum secundum, there is only the septum primum in avian species (Morse et al. 1984). However, a common characteristic among avian and mammalian development is that the IAS does not completely close until after birth to allow for interatrial blood flow. This is necessary for optimal oxygen supply, which is later ensured by the pulmonary system. In the chick embryo, the fusion of the IAS with the ventral and dorsal cushions of the AVC occurs by HH24. During this fusion, a secondary foramen-like structure for interatrial blood flow arises in the form of multiple perforations in the middle of the IAS. This structure stays open until 2 days after hatching when it closes (Martinsen 2005). The dynamic changes of blood flow, which begin during atrial septation, can be measured easily in chicken embryos (for review, see Kowalski et al. 2014). Unidirectional blood flow is already established before cushion formation in the primitive heart tube at HH12 (Hu and Clark 1989). With the appearance of the AVC cushions the backflow of blood is minimized, as they contract together with the myocardium and thus reduce the opening to the atrium (Boselli et al. 2015). Subsequently the AVC cushions become cellularized and lose their flexibility, most likely in preparation to fusion. This loss in flexibility reduces their valve-like function, but myocardial contraction velocity decreases as well as the associated suction effect. As atrial contraction becomes the dominant factor for pumping, AV blood flow velocity increases (Butcher et al. 2007). Optical coherence tomography and ultrasound imaging of avian embryos were used together with modeling to determine the pumping mechanisms that lead to pulsating blood flow. This implicated both peristaltic and impedance pumping mechanisms (Butcher et al. 2007; Kozlovsky et al. 2016). Better understanding of the biomechanics underlying blood flow has important clinical implications, for example, for surgery after aortic dissection or myocardial infarct (for review, see Furst 2015).
Ventricular Trabeculation and Separation
Trabeculae are temporary structures that form at around HH16–17 in the chicken embryo where they initiate at the level of the greater curvature of the looped primitive ventricle (Sedmera et al. 2000). During the course of development, they undergo a process called compaction, which reduces their surface area and ultimately contribute their mass to the myocardial wall. Their function during development is to mediate nutrient and oxygen exchange before coronary vascularization, routing of blood flow, increasing the myocardial mass, and facilitation of intraventricular conduction (Martinsen 2005). Trabeculae are a part of the myocardium, which can be separated into two distinctive layers: a highly proliferative layer—the compact myocardium, which is closer to the epicardium and a mitotically inactive layer on the inside closer to the endocardium (MacGrogan et al. 2018). Studies on the chicken embryo have been instrumental for our understanding of trabeculae morphogenesis during development, characterized in detail by an extensive series of EM images (Sedmera et al. 1997). Soon after initiation of trabeculation at HH16–17, a primitive ventricular septum (VS) can be observed by HH19–20. This structure grows until HH27 before fusing with the SIM. Ventricular septation is complete by HH34 (8 days) (Waldo et al. 1998); at this stage, myocardial proliferation and compaction increase. Proliferation of the compact myocardium and the concomitant compaction process, which continue until embryonic day 14, result in the ingression of the basal portions of the trabeculae into the ventricular wall. This leads to an increase in its thickness and a multilayered organization, which is required for contraction of the adult heart (Martinsen 2005). Trabeculae development is affected by hemodynamic changes in the heart (Sedmera et al. 1999). Left atrial ligation drastically impacted blood flow, which resulted in faster compacting trabeculae in the LV, a pressure overload and increased cardiomyocyte proliferation in the RV. Furthermore, a higher incidence of septation defects was observed in the ligated hearts. More recent studies regarding trabeculae development and function were conducted in mouse using various genetic tools (reviewed by MacGrogan et al. 2018); however, our understanding of their importance remains incomplete.
Formation of the Cardiac Conduction System
Voltage-sensitive dyes have been used to detect action potentials and pacemaking activity as early as the 7–8 somite stage chick embryos (HH9) (Kamino et al. 1981). However, at around HH11–12, the first sign of a more specialized conduction system can be observed as the faster conduction segments of the primitive atrium and ventricle are now separated by a more slowly conducting AV canal (Gourdie et al. 2003). The AV-ring forms shortly after at HH14–15, it is centrally located in the heart and required for the later conduction cascade that originates from the SAN. Electrical impulse is conducted across the atrial chambers to the AV-node and the AV-ring, then along the AV-bundle to finally arrive at the Purkinje fibers in the ventricle completing the signal cascade (Martinsen 2005). Most important for this specialized conduction system is the Purkinje fiber network, which develops in response to vessel-derived endothelin between days 10–20 and spreads across the subendocardium of the left and RVs in the adult chicken heart. Linage tracing in chick has shown that Purkinje fibers originate from myocytes (Cheng et al. 1999). During their conversion from cardiomyocytes and terminal differentiation, Purkinje fibers down-regulate cardiac transcription factors and up-regulate a set of genes associated with skeletal muscle, including the transcription factor myoblast determination protein 1 (MYOD) (Takebayashi-Suzuki et al. 2001). They also express the gap junction protein Connexin 40 (CX40) (Gourdie et al. 1993). Interestingly CX40 is not expressed in the slow AV conduction system and specifically labels the fast conduction system present in atrioventricular bundles, the left and right bundle branches, and the peripheral ventricular conduction system (Christoffels and Moorman 2009).
SIMILARITIES AND DIFFERENCES BETWEEN CHICK AND HUMAN HEARTS
The chick and human hearts are both four-chambered consisting of the right atrium (RA) and ventricle (RV), and the left atrium (LA) and ventricle (LV) (Fig. 4). Chamber separation in these amniote species ensures separation of oxygen-deprived blood (RA/RV) and oxygenated blood (LA/LV). From the RV, the pulmonary artery transports oxygen-poor blood to the lungs, and from the LV the aorta delivers reoxygenated blood to the body. Heart valves ensure the direction of blood circulation and there are four valves in both species. Within the heart the tricuspid valve separates the RA and RV, and the mitral valve is located between the LA and LV. In addition, two valves are situated in the OFT to regulate blood flow where it exits from the heart: the pulmonary valve for pulmonary blood flow from the RV and the aortic valve for aortic blood flow from the LV. Thus, the fundamental circuitry is very similar between chick and human (Lo et al. 2010). But there are some physiological and anatomical differences, which is not unexpected. These differences include the average body temperature and heart rate, which are both higher in chicken. Furthermore, the ventricles have more muscle mass relative to chamber size, and the LV is significantly bigger compared with the RV allowing for up to five times higher systemic pressure (Dzialowski and Crossley 2015). Outside the heart, the details of pharyngeal arch remodeling during embryogenesis vary between species (for details, see Poelmann et al. 2017). As a result, the right branch of the aortic arch remains in the chick; therefore, the ascending aorta bends toward the right before it descends. Conversely in human the ascending aortic arch bends toward the left side (Fig. 4; Lo et al. 2010; Poelmann et al. 2017).
Figure 4.
Chick versus human heart. Shown are illustrations of the chick and human heart to depict their strong similarity and a few species-specific differences. Among these differences are a smaller right ventricle (RV) in the chick, different directions of the ascending aorta, right in chick and left in human, and a thicker myocardium in the chick to facilitate higher cardiac load together with higher heart rates. (LA) left atrium, (LV) left ventricle, (RA) right atrium.
Implication for Congenital Heart Defects
Despite the differences in final form and physiology, there are many examples in which studies in avian embryos have contributed fundamental insights into developmental mechanisms as illustrated throughout this chapter. These, in turn, have been instrumental to enhance our understanding of the origin of congenital heart defects (CHDs). In particular, work from the Kirby laboratory (Kirby et al. 1983) has informed on the importance of the cardiac NCC, as well as the SHF (Waldo et al. 2001), for common OFT malformations such as persistent truncus arteriosus, transposition of the great vessels, and double-outlet RV. Removal of the cardiac NCC in chick can also lead to anomalies of the aortic arch or cardiac inflow. All of these heart defects have also been seen in human and they are often associated with structural defects in other organs to which NCCs contribute (for review, see Kirby and Waldo 1990).
CONCLUDING REMARKS
The chicken embryo has made seminal contributions to our current understanding of heart development and approaches used in avian often work hand-in-hand with genetic manipulations that are more easily performed in the mouse model. In particular, microsurgery is straightforward in avian embryos, which represents a distinct advantage. Multiple populations of cells, which together build the heart, have been discovered and their origin mapped. This includes the FHF and SHF, the cardiac neural crest, the PEO, and pacemaker cells. Furthermore, in vivo functional interference experiments have determined some of the genetic pathways and signals that control their differentiation. With new genetic tools, deep sequencing, and genomics approaches as well as further advances in imaging becoming available, there is no doubt that studies in chick embryos will continue to reveal novel insights into cardiac development and the mechanisms that contribute to congenital heart malformations, to cardiovascular conditions, and to cardiac repair.
ACKNOWLEDGMENTS
J.G.W. was funded by a studentship from the British Heart Foundation (BHF FS/15/41/31564). Research in A.M.’s laboratory was supported by a BHF Project Grant (PG/15/77/3176).
Footnotes
Editors: Benoit G. Bruneau and Paul R. Riley
Additional Perspectives on Heart Development and Disease available at www.cshperspectives.org
REFERENCES
- Abu-Issa R, Kirby ML. 2008. Patterning of the heart field in the chick. Dev Biol 319: 223–233. 10.1016/j.ydbio.2008.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth SJ, Stanley RL, Evans DJ. 2010. Developmental stages of the Japanese quail. J Anat 216: 3–15. 10.1111/j.1469-7580.2009.01173.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrova A, Czirók A, Szabó A, Filla MB, Hossain MJ, Whelan PF, Lansford R, Rongish BJ. 2012. Convective tissue movements play a major role in avian endocardial morphogenesis. Dev Biol 363: 348–361. 10.1016/j.ydbio.2011.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrova A, Czirok A, Kosa E, Galkin O, Cheuvront TJ, Rongish BJ. 2015. The endoderm and myocardium join forces to drive early heart tube assembly. Dev Biol 404: 40–54. 10.1016/j.ydbio.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsan BH, Schultheiss TM. 2002. Regulation of avian cardiogenesis by Fgf8 signaling. Development 129: 1935–1943. [DOI] [PubMed] [Google Scholar]
- Anderson C, Khan MAF, Wong F, Solovieva T, Oliveira NMM, Baldock RA, Tickle C, Burt DW, Stern CD. 2016. A strategy to discover new organizers identifies a putative heart organizer. Nat Commun 7: 12656 10.1038/ncomms12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrée B, Duprez D, Vorbusch B, Arnold HH, Brand T. 1998. BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech Dev 70: 119–131. 10.1016/S0925-4773(97)00186-X [DOI] [PubMed] [Google Scholar]
- Boselli F, Freund JB, Vermot J. 2015. Blood flow mechanics in cardiovascular development. Cell Mol Life Sci 72: 2545–2559. 10.1007/s00018-015-1885-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan M, Liu G, Mikawa T. 2013. Early mesodermal cues assign avian cardiac pacemaker fate potential in a tertiary heart field. Science 340: 744–748. 10.1126/science.1232877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan M, Henley T, Louie JD, Liu G, Christodoulou D, Bai X, Taylor J, Seidman CE, Seidman JG, Mikawa T. 2018. Dynamic cellular integration drives functional assembly of the heart's pacemaker complex. Cell Rep 23: 2283–2291. 10.1016/j.celrep.2018.04.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M, ed. 2011. Avian embryology. Elsevier, New York. [Google Scholar]
- Butcher JT, McQuinn TC, Sedmera D, Turner D, Markwald RR. 2007. Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition. Circ Res 100: 1503–1511. 10.1161/CIRCRESAHA.107.148684 [DOI] [PubMed] [Google Scholar]
- Camp E, Dietrich S, Münsterberg A. 2012. Fate mapping identifies the origin of SHF/AHF progenitors in the chick primitive streak. PLoS ONE 7: e51948 10.1371/journal.pone.0051948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. 2001. Improved method for chick whole-embryo culture using a filter paper carrier. Dev Dyn 220: 284–289. [DOI] [PubMed] [Google Scholar]
- Cheng G, Litchenberg WH, Cole GJ, Mikawa T, Thompson RP, Gourdie RG. 1999. Development of the cardiac conduction system involves recruitment within a multipotent cardiomyogenic lineage. Development 126: 5041–5049. [DOI] [PubMed] [Google Scholar]
- Christoffels VM, Moorman AF. 2009. Development of the cardiac conduction system: why are some regions of the heart more arrhythmogenic than others? Circ Arrhythm Electrophysiol 2: 195–207. 10.1161/CIRCEP.108.829341 [DOI] [PubMed] [Google Scholar]
- Cui C, Cheuvront TJ, Lansford RD, Moreno-Rodriguez RA, Schultheiss TM, Rongish BJ. 2009. Dynamic positional fate map of the primary heart-forming region. Dev Biol 332: 212–222. 10.1016/j.ydbio.2009.05.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MG, Balic A, Rainger J, Sang HM, McGrew MJ. 2018. Illuminating the chicken model through genetic modification. Int J Dev Biol 62: 257–264. 10.1387/ijdb.170323mm [DOI] [PubMed] [Google Scholar]
- de la Cruz MV, Markwald RR. 1998. Embryological development of the ventricular inlets. Septation and atrioventricular valve apparatus. In Living morphogenesis of the heart (ed. de la Cruz MV, Markwald RR), pp. 131–155. Birkhäuser, Boston. [Google Scholar]
- de la Cruz MV, Sanchez Gomez C, Arteaga MM, Arguello C. 1977. Experimental study of the development of the truncus and the conus in the chick embryo. J Anat 123: 661–686. [PMC free article] [PubMed] [Google Scholar]
- Desgrange A, Le Garrec JF, Meilhac SM. 2018. Left–right asymmetry in heart development and disease: forming the right loop. Development 145: dev162776 10.1242/dev.162776 [DOI] [PubMed] [Google Scholar]
- Dormann D, Weijer CJ. 2006. Imaging of cell migration. EMBO J 25: 3480–3493. 10.1038/sj.emboj.7601227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzialowski EM, Crossley DA. 2015. The cardiovascular system. In Sturkie's avian physiology, 6th ed. (ed. Scanes CG), pp. 193–283. Academic, San Diego. [Google Scholar]
- Furst B. 2015. The heart: pressure-propulsion pump or organ of impedance? J Cardiothorac Vasc Anesth 29: 1688–1701. 10.1053/j.jvca.2015.02.022 [DOI] [PubMed] [Google Scholar]
- Gandhi S, Piacentino ML, Vieceli FM, Bronner ME. 2017. Optimization of CRISPR/Cas9 genome editing for loss-of-function in the early chick embryo. Dev Biol 432: 86–97. 10.1016/j.ydbio.2017.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Martinez V, Schoenwolf GC. 1993. Primitive-streak origin of the cardiovascular system in avian embryos. Dev Biol 159: 706–719. 10.1006/dbio.1993.1276 [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Green CR, Severs NJ, Anderson RH, Thompson RP. 1993. Evidence for a distinct gap-junctional phenotype in ventricular conduction tissues of the developing and mature avian heart. Circ Res 72: 278–289. 10.1161/01.RES.72.2.278 [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Harris BS, Bond J, Justus C, Hewett KW, O'Brien TX, Thompson RP, Sedmera D. 2003. Development of the cardiac pacemaking and conduction system. Birth Defects Res C Embryo Today 69: 46–57. 10.1002/bdrc.10008 [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. 1951. A series of normal stages in the development of the chick embryo. J Morphol 88: 49–92. 10.1002/jmor.1050880104 [DOI] [PubMed] [Google Scholar]
- Harel I, Nathan E, Tirosh-Finkel L, Zigdon H, Guimarães-Camboa N, Evans SM, Tzahor E. 2009. Distinct origins and genetic programs of head muscle satellite cells. Dev Cell 16: 822–832. 10.1016/j.devcel.2009.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris IS, Black BL. 2010. Development of the endocardium. Pediatr Cardiol 31: 391–399. 10.1007/s00246-010-9642-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatada Y, Stern CD. 1994. A fate map of the epiblast of the early chick embryo. Development 120: 2879–2889. [DOI] [PubMed] [Google Scholar]
- Hatcher CJ, Diman NY, Kim MS, Pennisi D, Song Y, Goldstein MM, Mikawa T, Basson CT. 2004. A role for Tbx5 in proepicardial cell migration during cardiogenesis. Physiol Genomics 18: 129–140. 10.1152/physiolgenomics.00060.2004 [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Morse DE. 1977. Atrial septation. I: Scanning electron microscopy in the chick. Dev Biol 57: 345–363. 10.1016/0012-1606(77)90220-2 [DOI] [PubMed] [Google Scholar]
- Hosseini HS, Garcia KE, Taber LA. 2017. A new hypothesis for foregut and heart tube formation based on differential growth and actomyosin contraction. Development 144: 2381–2391. 10.1242/dev.145193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N, Clark EB. 1989. Hemodynamics of the stage 12 to stage 29 chick embryo. Circ Res 65: 1665–1670. 10.1161/01.RES.65.6.1665 [DOI] [PubMed] [Google Scholar]
- Hu N, Christensen DA, Agrawal AK, Beaumont C, Clark EB, Hawkins JA. 2009. Dependence of aortic arch morphogenesis on intracardiac blood flow in the left atrial ligated chick embryo. Anat Rec (Hoboken) 292: 652–660. 10.1002/ar.20885 [DOI] [PubMed] [Google Scholar]
- Ishii Y, Langberg JD, Hurtado R, Lee S, Mikawa T. 2007. Induction of proepicardial marker gene expression by the liver bud. Development 134: 3627–3637. 10.1242/dev.005280 [DOI] [PubMed] [Google Scholar]
- Ishii Y, Garriock RJ, Navetta AM, Coughlin LE, Mikawa T. 2010. BMP signals promote proepicardial protrusion necessary for recruitment of coronary vessel and epicardial progenitors to the heart. Dev Cell 19: 307–316. 10.1016/j.devcel.2010.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itasaki N, Bel-Vialar S, Krumlauf R. 1999. “Shocking” developments in chick embryology: electroporation and in ovo gene expression. Nat Cell Biol 1: E203–E207. 10.1038/70231 [DOI] [PubMed] [Google Scholar]
- Kamino K, Hirota A, Fujii S. 1981. Localization of pacemaking activity in early embryonic heart monitored using voltage-sensitive dye. Nature 290: 595–597. 10.1038/290595a0 [DOI] [PubMed] [Google Scholar]
- Kidokoro H, Yonei-Tamura S, Tamura K, Schoenwolf GC, Saijoh Y. 2018. The heart tube forms and elongates through dynamic cell rearrangement coordinated with foregut extension. Development 145: dev152488 10.1242/dev.152488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ML, Waldo KL. 1990. Role of neural crest in congenital heart disease. Circulation 82: 332–340. 10.1161/01.CIR.82.2.332 [DOI] [PubMed] [Google Scholar]
- Kirby ML, Waldo KL. 1995. Neural crest and cardiovascular patterning. Circ Res 77: 211–215. 10.1161/01.RES.77.2.211 [DOI] [PubMed] [Google Scholar]
- Kirby ML, Gale TF, Stewart DE. 1983. Neural crest cells contribute to normal aorticopulmonary septation. Science 220: 1059–1061. 10.1126/science.6844926 [DOI] [PubMed] [Google Scholar]
- Kowalski WJ, Pekkan K, Tinney JP, Keller BB. 2014. Investigating developmental cardiovascular biomechanics and the origins of congenital heart defects. Front Physiol 5: 408–424. 10.3389/fphys.2014.004082014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovsky P, Bryson-Richardson RJ, Jaffa AJ, Rosenfeld M, Elad D. 2016. The driving mechanism for unidirectional blood flow in the tubular embryonic heart. Ann Biomed Eng 44: 3069–3083. 10.1007/s10439-016-1620-8 [DOI] [PubMed] [Google Scholar]
- Ladd AN, Yatskievych TA, Antin PB. 1998. Regulation of avian cardiac myogenesis by activin/TGFβ and bone morphogenetic proteins. Dev Biol 204: 407–419. 10.1006/dbio.1998.9094 [DOI] [PubMed] [Google Scholar]
- Le Douarin N. 1973. A biological cell labeling technique and its use in experimental embryology. Dev Biol 30: 217–222. 10.1016/0012-1606(73)90061-4 [DOI] [PubMed] [Google Scholar]
- Lee KH, Evans S, Ruan TY, Lassar AB. 2004. SMAD-mediated modulation of YY1 activity regulates the BMP response and cardiac-specific expression of a GATA4/5/6-dependent chick Nkx2.5 enhancer. Development 131: 4709–4723. 10.1242/dev.01344 [DOI] [PubMed] [Google Scholar]
- Lee J, Ma J, Lee K. 2019. Direct delivery of adenoviral CRISPR/Cas9 vector into the blastoderm for generation of targeted gene knockout in quail. Proc Natl Acad Sci 116: 13288–13292. 10.1073/pnas.1903230116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CW, Yu Q, Shen Y, Leatherbury L, Francis R, Zhao X-Q, Zhang Z, Wessels A, Huang G-Y, Chatterjee B. 2010. Exploring the genetic basis for congenital heart disease with mouse ENU mutagenesis. In Heart development and regeneration (eds. Rosenthal N, Harvey RP), pp. 753–778. Academic, Boston. [Google Scholar]
- Macdonald J, Glover JD, Taylor L, Sang HM, McGrew MJ. 2010. Characterisation and germline transmission of cultured avian primordial germ cells. PLoS ONE 5: e15518 10.1371/journal.pone.0015518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGrogan D, Münch J, de la Pompa JL. 2018. Notch and interacting signalling pathways in cardiac development, disease, and regeneration. Nat Rev Cardiol 15: 685–704. 10.1038/s41569-018-0100-2 [DOI] [PubMed] [Google Scholar]
- Männer J. 1999. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec 255: 212–226. [DOI] [PubMed] [Google Scholar]
- Männer J. 2000. Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat Rec 259: 248–262. [DOI] [PubMed] [Google Scholar]
- Männer J. 2004. On rotation, torsion, lateralization, and handedness of the embryonic heart loop: new insights from a simulation model for the heart loop of chick embryos. Anat Rec A Discov Mol Cell Evol Biol 278: 481–492. 10.1002/ar.a.20036 [DOI] [PubMed] [Google Scholar]
- Martinsen BJ. 2005. Reference guide to the stages of chick heart embryology. Dev Dyn 233: 1217–1237. 10.1002/dvdy.20468 [DOI] [PubMed] [Google Scholar]
- Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. 2001. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev 15: 316–327. 10.1101/gad.855501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew MJ, Sherman A, Lillico SG, Ellard FM, Radcliffe PA, Gilhooley HJ, Mitrophanous KA, Cambray N, Wilson V, Sang H. 2008. Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development 135: 2289–2299. 10.1242/dev.022020 [DOI] [PubMed] [Google Scholar]
- Mercado-Pimentel ME, Runyan RB. 2007. Multiple transforming growth factor-β isoforms and receptors function during epithelial–mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs 185: 146–156. 10.1159/000101315 [DOI] [PubMed] [Google Scholar]
- Mikawa T, Borisov A, Brown AM, Fischman DA. 1992. Clonal analysis of cardiac morphogenesis in the chicken embryo using a replication-defective retrovirus. I: Formation of the ventricular myocardium. Dev Dyn 193: 11–23. 10.1002/aja.1001930104 [DOI] [PubMed] [Google Scholar]
- Milgrom-Hoffman M, Harrelson Z, Ferrara N, Zelzer E, Evans SM, Tzahor E. 2011. The heart endocardium is derived from vascular endothelial progenitors. Development 138: 4777–4787. 10.1242/dev.061192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR. 2001. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol 238: 97–109. 10.1006/dbio.2001.0409 [DOI] [PubMed] [Google Scholar]
- Moreno-Rodriguez R, Krug EL. 2015. Cardiovascular development. Elsevier, New York. [Google Scholar]
- Morse DE, Rogers CS, McCann PS. 1984. Atrial septation in the chick and rat: a review. J Submicrosc Cytol 16: 259–272. [PubMed] [Google Scholar]
- Nathan E, Monovich A, Tirosh-Finkel L, Harrelson Z, Rousso T, Rinon A, Harel I, Evans SM, Tzahor E. 2008. The contribution of Islet1-expressing splanchnic mesoderm cells to distinct branchiomeric muscles reveals significant heterogeneity in head muscle development. Development 135: 647–657. 10.1242/dev.007989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM. 1991. Origins and patterning of avian outflow tract endocardium. Development 111: 867–876. [DOI] [PubMed] [Google Scholar]
- Ocaña OH, Coskun H, Minguillón C, Murawala P, Tanaka EM, Galcerán J, Muñoz-Chápuli R, Nieto MA. 2017. A right-handed signalling pathway drives heart looping in vertebrates. Nature 549: 86–90. 10.1038/nature23454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Yoshii K, Miyahara D, Kagami H, Tagami T. 2016. Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci Rep 6: 23980 10.1038/srep23980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmquist-Gomes P, Guadix JA, Pérez-Pomares JM. 2016. A chick embryo cryoinjury model for the study of embryonic organ development and repair. Differentiation 91: 72–77. 10.1016/j.diff.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Pérez-Pomares JM, Phelps A, Sedmerova M, Wessels A. 2003. Epicardial-like cells on the distal arterial end of the cardiac outflow tract do not derive from the proepicardium but are derivatives of the cephalic pericardium. Dev Dyn 227: 56–68. 10.1002/dvdy.10284 [DOI] [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. 2005. Cell biology of cardiac cushion development. Int Rev Cytol 243: 287–335. 10.1016/S0074-7696(05)43005-3 [DOI] [PubMed] [Google Scholar]
- Poelmann RE, Gittenberger-de Groot AC, Biermans MWM, Dolfing AI, Jagessar A, van Hattum S, Hoogenboom A, Wisse LJ, Vicente-Steijn R, de Bakker MAG, et al. 2017. Outflow tract septation and the aortic arch system in reptiles: lessons for understanding the mammalian heart. Evodevo 8: 9 10.1186/s13227-017-0072-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynter G, Lansford R. 2008. Generating transgenic quail using lentiviruses. Methods Cell Biol 87: 281–293. 10.1016/S0091-679X(08)00215-X [DOI] [PubMed] [Google Scholar]
- Psychoyos D, Stern CD. 1996. Fates and migratory routes of primitive streak cells in the chick embryo. Development 122: 1523–1534. [DOI] [PubMed] [Google Scholar]
- Qayyum SR, Webb S, Anderson RH, Verbeek FJ, Brown NA, Richardson MK. 2001. Septation and valvar formation in the outflow tract of the embryonic chick heart. Anat Rec 264: 273–283. 10.1002/ar.1162 [DOI] [PubMed] [Google Scholar]
- Quiring DP. 1933. The development of the sino-atrial region of the chick heart. J Morphol 55: 81–118. 10.1002/jmor.1050550106 [DOI] [Google Scholar]
- Redkar A, Montgomery M, Litvin J. 2001. Fate map of early avian cardiac progenitor cells. Development 128: 2269–2279. [DOI] [PubMed] [Google Scholar]
- Rozbicki E, Chuai M, Karjalainen AI, Song F, Sang HM, Martin R, Knölker HJ, MacDonald MP, Weijer CJ. 2015. Myosin-II-mediated cell shape changes and cell intercalation contribute to primitive streak formation. Nat Cell Biol 17: 397–408. 10.1038/ncb3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutland C, Warner L, Thorpe A, Alibhai A, Robinson T, Shaw B, Layfield R, Brook JD, Loughna S. 2009. Knockdown of α myosin heavy chain disrupts the cytoskeleton and leads to multiple defects during chick cardiogenesis. J Anat 214: 905–915. 10.1111/j.1469-7580.2009.01079.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Poynter G, Huss D, Filla MB, Czirok A, Rongish BJ, Little CD, Fraser SE, Lansford R. 2010. Dynamic analysis of vascular morphogenesis using transgenic quail embryos. PLoS ONE 5: e12674 10.1371/journal.pone.0012674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauka-Spengler T, Barembaum M. 2008. Gain- and loss-of-function approaches in the chick embryo. Methods Cell Biol 87: 237–256. 10.1016/S0091-679X(08)00212-4 [DOI] [PubMed] [Google Scholar]
- Schlueter J, Brand T. 2009. A right-sided pathway involving FGF8/Snai1 controls asymmetric development of the proepicardium in the chick embryo. Proc Natl Acad Sci 106: 7485–7490. 10.1073/pnas.0811944106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlueter J, Brand T. 2013. Subpopulation of proepicardial cells is derived from the somatic mesoderm in the chick embryo. Circ Res 113: 1128–1137. 10.1161/CIRCRESAHA.113.301347 [DOI] [PubMed] [Google Scholar]
- Schlueter J, Männer J, Brand T. 2006. BMP is an important regulator of proepicardial identity in the chick embryo. Dev Biol 295: 546–558. 10.1016/j.ydbio.2006.03.036 [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, Xydas S, Lassar AB. 1995. Induction of avian cardiac myogenesis by anterior endoderm. Development 121: 4203–4214. [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, Burch JB, Lassar AB. 1997. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev 11: 451–462. 10.1101/gad.11.4.451 [DOI] [PubMed] [Google Scholar]
- Schumann K, Lin S, Boyer E, Simeonov DR, Subramaniam M, Gate RE, Haliburton GE, Ye CJ, Bluestone JA, Doudna JA, et al. 2015. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc Natl Acad Sci 112: 10437–10442. 10.1073/pnas.1512503112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmera D, Pexieder T, Hu N, Clark EB. 1997. Developmental changes in the myocardial architecture of the chick. Anat Rec 248: 421–432. [DOI] [PubMed] [Google Scholar]
- Sedmera D, Pexieder T, Rychterova V, Hu N, Clark EB. 1999. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat Rec 254: 238–252. [DOI] [PubMed] [Google Scholar]
- Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. 2000. Developmental patterning of the myocardium. Anat Rec 258: 319–337. [DOI] [PubMed] [Google Scholar]
- Serralbo O, Véron N, Cooper C, Dejardin M-J, Doran T, Marcelle C. 2019. Generation of transgenic quails by in vivo transfection of primordial germ cells. bioRxiv 10.1101/625665 [DOI] [Google Scholar]
- Shi L, Goenezen S, Haller S, Hinds MT, Thornburg KL, Rugonyi S. 2013. Alterations in pulse wave propagation reflect the degree of outflow tract banding in HH18 chicken embryos. Am J Physiol Heart Circ Physiol 305: H386–H396. 10.1152/ajpheart.00100.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SS, Kim TM, Kim SY, Kim TW, Seo HW, Lee SK, Kwon SC, Lee GS, Kim H, Lim JM, et al. 2008. Generation of transgenic quail through germ cell-mediated germline transmission. FASEB J 22: 2435–2444. 10.1096/fj.07-101485 [DOI] [PubMed] [Google Scholar]
- Simões FC, Riley PR. 2018. The ontogeny, activation and function of the epicardium during heart development and regeneration. Development 145: dev155994 10.1242/dev.155994 [DOI] [PubMed] [Google Scholar]
- Snarr BS, Kern CB, Wessels A. 2008. Origin and fate of cardiac mesenchyme. Dev Dyn 237: 2804–2819. 10.1002/dvdy.21725 [DOI] [PubMed] [Google Scholar]
- Song J, McColl J, Camp E, Kennerley N, Mok GF, McCormick D, Grocott T, Wheeler GN, Münsterberg AE. 2014. Smad1 transcription factor integrates BMP2 and Wnt3a signals in migrating cardiac progenitor cells. Proc Natl Acad Sci 111: 7337–7342. 10.1073/pnas.1321764111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurlin J III, Lwigale P. 2013. A technique to increase accessibility to late-stage chick embryos for in ovo manipulations. Dev Dyn 242: 148–154. 10.1002/dvdy.23907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi-Suzuki K, Pauliks LB, Eltsefon Y, Mikawa T. 2001. Purkinje fibers of the avian heart express a myogenic transcription factor program distinct from cardiac and skeletal muscle. Dev Biol 234: 390–401. 10.1006/dbio.2001.0270 [DOI] [PubMed] [Google Scholar]
- Tang W, Martik ML, Li Y, Bronner ME. 2019. Cardiac neural crest contributes to cardiomyocytes in amniotes and heart regeneration in zebrafish. eLife 8: e47929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh-Finkel L, Elhanany H, Rinon A, Tzahor E. 2006. Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract. Development 133: 1943–1953. 10.1242/dev.02365 [DOI] [PubMed] [Google Scholar]
- Tzahor E. 2009. Heart and craniofacial muscle development: a new developmental theme of distinct myogenic fields. Dev Biol 327: 273–279. 10.1016/j.ydbio.2008.12.035 [DOI] [PubMed] [Google Scholar]
- van de Lavoir MC, Diamond JH, Leighton PA, Mather-Love C, Heyer BS, Bradshaw R, Kerchner A, Hooi LT, Gessaro TM, Swanberg SE, et al. 2006. Germline transmission of genetically modified primordial germ cells. Nature 441: 766–769. 10.1038/nature04831 [DOI] [PubMed] [Google Scholar]
- Varner VD, Taber LA. 2012. Not just inductive: a crucial mechanical role for the endoderm during heart tube assembly. Development 139: 1680–1690. 10.1242/dev.073486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véron N, Qu Z, Kipen PA, Hirst CE, Marcelle C. 2015. CRISPR mediated somatic cell genome engineering in the chicken. Dev Biol 407: 68–74. 10.1016/j.ydbio.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Waldo K, Miyagawa-Tomita S, Kumiski D, Kirby ML. 1998. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure. Dev Biol 196: 129–144. 10.1006/dbio.1998.8860 [DOI] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. 2001. Conotruncal myocardium arises from a secondary heart field. Development 128: 3179–3188. [DOI] [PubMed] [Google Scholar]
- Wei Y, Mikawa T. 2000. Fate diversity of primitive streak cells during heart field formation in ovo. Dev Dyn 219: 505–513. [DOI] [PubMed] [Google Scholar]
- Williams RM, Senanayake U, Artibani M, Taylor G, Wells D, Ahmed AA, Sauka-Spengler T. 2018. Genome and epigenome engineering CRISPR toolkit for in vivo modulation of cis-regulatory interactions and gene expression in the chicken embryo. Development 145: dev160333 10.1242/dev.160333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig J, Münsterberg A. 2016. The early stages of heart development: insights from chicken embryos. J Cardiovasc Dev Dis 3: E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig JG, Billmeier M, Lozano-Velasco E, García MR, Münsterberg AE. 2019. Cardiac injections of AntagomiRs as a novel tool for knockdown of miRNAs during heart development. Dev Biol 445: 163–169. 10.1016/j.ydbio.2018.11.008 [DOI] [PubMed] [Google Scholar]
- Yalcin HC, Shekhar A, Rane AA, Butcher JT. 2010. An ex-ovo chicken embryo culture system suitable for imaging and microsurgery applications. J Vis Exp 44: 2154 10.3791/2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatskievych TA, Ladd AN, Antin PB. 1997. Induction of cardiac myogenesis in avian pregastrula epiblast: the role of the hypoblast and activin. Development 124: 2561–2570. [DOI] [PubMed] [Google Scholar]
- Yue Q, Wagstaff L, Yang X, Weijer C, Münsterberg A. 2008. Wnt3a-mediated chemorepulsion controls movement patterns of cardiac progenitors and requires RhoA function. Development 135: 1029–1037. 10.1242/dev.015321 [DOI] [PubMed] [Google Scholar]
- Yutzey KE, Kirby ML. 2002. Wherefore heart thou? Embryonic origins of cardiogenic mesoderm. Dev Dyn 223: 307–320. 10.1002/dvdy.10068 [DOI] [PubMed] [Google Scholar]
- Zamir L, Singh R, Nathan E, Patrick R, Yifa O, Yahalom-Ronen Y, Arraf AA, Schultheiss TM, Suo S, Han JJ, et al. 2017. Nkx2.5 marks angioblasts that contribute to hemogenic endothelium of the endocardium and dorsal aorta. eLife 6: e20994 10.7554/eLife.20994 [DOI] [PMC free article] [PubMed] [Google Scholar]