Abstract
Whereas the apoptosis cell death pathway typically enables cells to undergo death in an immunologically silent manner, cell death by necroptosis induces cell lysis and release of cellular constituents known to elicit an immune response. Consequently, the origins of necroptosis likely originated in host defense against pathogens, although recently it has emerged that dysregulation of the pathway underlies many human pathologies. The past decade has seen a rapid advance in our understanding of the molecular mechanisms underlying necroptotic cell death, including the implication of the pseudokinase, mixed lineage kinase domain-like protein (MLKL), as the terminal effector in the pathway. Here, I review our current understanding of how MLKL is activated by the upstream receptor interacting protein kinase (RIPK)3, the proposed mechanism(s) by which MLKL kills cells, and recently described layers of regulation that tune MLKL's killing activity.
The programmed necrosis pathway was first described by Holler, Tschopp, and colleagues in 2000. This cell death mechanism relies on the protein kinase, receptor interacting protein kinase (RIPK)1, downstream of Fas, tumor necrosis factor (TNF), or TRAIL receptor activation, in the absence of the proteolytic activity of the apoptosis effector, caspase-8 (Holler et al. 2000). The name necroptosis was coined to describe the pathway in 2005 (Degterev et al. 2005) to convey that, like apoptosis, necroptosis is a form of regulated cell death. In 2009, the protein kinase RIPK3 was implicated in the pathway (Cho et al. 2009; He et al. 2009; Zhang et al. 2009), with the terminal effector pseudokinase, mixed lineage kinase domain-like protein (MLKL), implicated 3 years later (Sun et al. 2012; Zhao et al. 2012). In parallel with these advances, our knowledge of the upstream cues that induce necroptotic signaling has advanced. Stimulation of TNF receptor 1 is the best studied mode of necroptosis induction, with other death receptor ligands, Toll-like receptor (TLR) ligands (e.g., double-stranded RNA, lipopolysaccharide) (He et al. 2011; Kaiser et al. 2013), type I interferons (Thapa et al. 2011; McComb et al. 2014), and, most recently, oxidized low-density lipoprotein (oxLDL) (Karunakaran et al. 2016) known to induce necroptosis in various cellular contexts.
Physiologically, necroptosis signaling has been attributed roles in a wide range of human pathologies. Most of these attributions have arisen from studies of RIPK3-deficient mice (Newton et al. 2004), which, like MLKL-deficient mice (Murphy et al. 2013; Wu et al. 2013), are viable, indicating that necroptosis is dispensable for development and not triggered in the absence of challenge. Recent revelations that RIPK3 performs additional functions beyond necroptosis signaling, such as directing inflammatory cytokine production and apoptotic signaling (Mandal et al. 2014; Newton et al. 2014; Wong et al. 2014; Alvarez-Diaz et al. 2016), have led to reevaluation of whether phenotypes observed in challenged RIPK3-knockout mice represent necroptosis. Because MLKL has only been credibly attributed functions in necroptosis signaling, studies of MLKL-deficient mice have enabled validation of dysregulated necroptosis contributing to the pathologies of several diseases, including kidney ischemic-reperfusion injury (Müller et al. 2017; Pefanis et al. 2019), pathogen clearance (Kitur et al. 2016), sterile sepsis (Newton et al. 2016a; Moerke et al. 2019), dermatitis (Dannappel et al. 2014), and multiorgan inflammation (Rickard et al. 2014a,b). One of the major challenges with ascribing roles for necroptosis in pathologies is that different death modalities can cooccur in diseased tissues, as evidenced by apoptosis and necroptosis cooccurring in mice with hyperactive TNF signaling (Dannappel et al. 2014; Rickard et al. 2014a), and necroptosis and ferroptosis cooccurring following acute kidney injury (Müller et al. 2017). Nevertheless, the recent discovery of a mouse strain harboring an activated MLKL mutant clearly implicates inflammation as an important contributor to necroptotic pathologies, as these animals succumbed to a lethal inflammatory syndrome within 6 days of birth (Hildebrand et al. 2019). Consequently, much interest has arisen in therapeutically targeting the necroptosis pathway. To this end, there has been a flurry of interest in the molecular mechanisms underlying MLKL activity, how MLKL kills cells and regulators of its lytic activity, which are reviewed below.
OVERVIEW OF THE NECROPTOSIS PATHWAY
Following ligation of cell surface receptors, such as TNF receptor 1, in contexts in which RIPK1 ubiquitylation by inhibitors of apoptosis proteins (IAPs) and caspase-8 activity are depleted or inhibited, a large molecular weight platform termed the “necrosome” assembles in the cytoplasm. Although the composition and magnitude of the necrosome complex likely differs depending on cellular context, at the core of this platform are the RIPK1 and RIPK3 protein kinases and the MLKL pseudokinase (Fig. 1). RIPK1 and RIPK3 contain an amino-terminal protein kinase domain and a short module termed the RIP homotypic interacting motif (RHIM) (Sun et al. 2002), responsible for oligomerization, carboxy-terminal to the kinase domain. RIPK1 contains a further protein interaction module, the death domain, at its carboxyl terminus, which is known to mediate oligomerization (Meng et al. 2018) and interaction with other death domain containing proteins, such as TNF receptor 1 (Hsu et al. 1996). Assembly of RIPK1-RIPK3 hetero-oligomers is facilitated by their RHIMs, which in isolation assemble into amyloid structures of indeterminate size (Li et al. 2012; Mompeán et al. 2018; Pham et al. 2019). Via its RHIM, RIPK1 is thought to seed assembly of RIPK3 into fibrils, where it is presumed recruitment of further RIPK3 protomers and their autophosphorylation are key events in necroptosis signal propagation. RIPK3 recruitment to other RIPK3 protomers within this assembly may be favored by allosteric interactions between their kinase domains and activation by phosphorylation of a site in the C-lobe of their kinase domains (Raju et al. 2018). It is from this complex that RIPK3 can interact with, and activate MLKL by phosphorylation to kill cells (described below; Figs. 1 and 2). The kinase activity of RIPK1, which is known only to phosphorylate its own activation loop, is required for necroptotic signaling (Berger et al. 2014; Newton et al. 2014; de Almagro et al. 2017; Newton 2019). Presumably this autophosphorylation leads to an electrostatic repulsion or conformational change that disfavors RIPK3 hetero-oligomer formation to allow RIPK3 to preferentially self-associate within the necrosome complex. Other RHIM proteins, such as ZBP1/DAI and TRIF, are thought to facilitate RIPK3 homo-oligomerization by sequestering RIPK1 or direct interaction with RIPK3, respectively (Kaiser et al. 2013; Lin et al. 2016; Newton et al. 2016b). Although TRIF acts as a cytoplasmic adaptor for TLRs, whether ZBP1/DAI is activated by viral DNA, viral RNA, and/or RNA:protein complexes is incompletely understood. Full comprehension of the mechanism by which RHIM-containing proteins might activate RIPK3 and cues that prompt RIPK3 self-association await more complete structural examination.
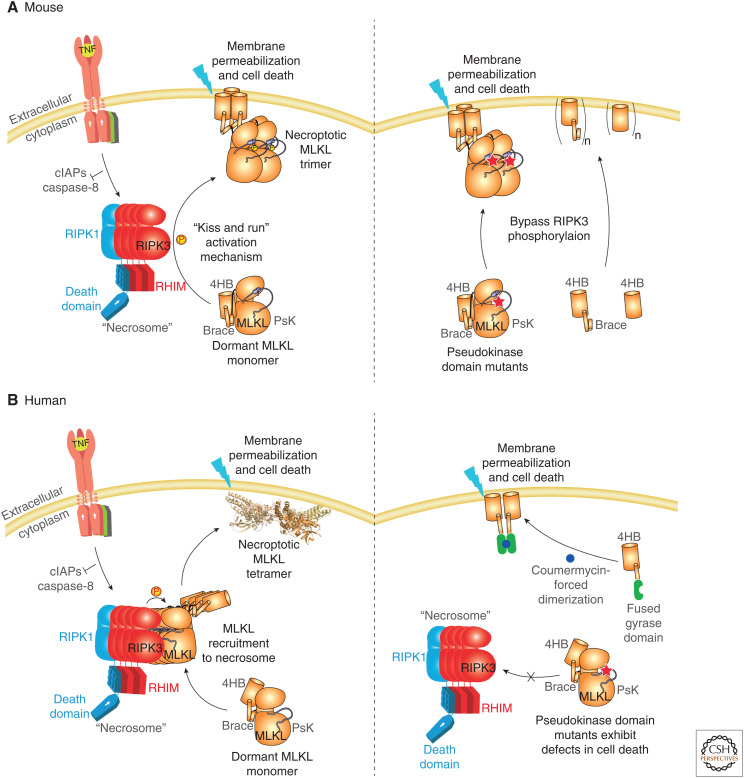
Figure 1.
Simplified schematic overview of the necroptosis signaling pathway. Exogenous stimuli provoke assembly of the receptor interacting protein kinase (RIPK)1:RIPK3 oligomeric complex termed the necrosome, which acts as a platform for recruiting and activating mixed lineage kinase domain-like protein (MLKL). MLKL phosphorylation by RIPK3 induces assembly of killer complexes of unclear stoichiometry, which are trafficked to the plasma membrane where cell permeabilization occurs. Several conflicting models of permeabilization have been proposed (Cai et al. 2014; Chen et al. 2014; Dondelinger et al. 2014; Hildebrand et al. 2014; Su et al. 2014; Wang et al. 2014; Xia et al. 2016; Huang et al. 2017), and the precise mechanism remains to be deduced. (TIR) Toll-interleukin receptor, (4HB) four-helix bundle, (PsKD) pseudokinase domain, (RHIM) RIP homotypic interacting motif.
Figure 2.
Mouse and human necroptosis pathways differ in mechanism. Although mouse mixed lineage kinase domain-like protein (MLKL) can be activated by receptor interacting protein kinase (RIPK)3 via a transient interaction that can be bypassed via mutations in the pseudoactive site or simply expression of the four-helix bundle (4HB) domain (A) (Murphy et al. 2013; Hildebrand et al. 2014), the same is not true of human MLKL (B) (Petrie et al. 2018). Human MLKL relies on recruitment to necrosomal RIPK3 for its activation (left) and it is not possible to bypass this activation step by introducing mutations in the human MLKL pseudoactive site (right). Furthermore, human MLKL 4HB domain expression is insufficient to induce necroptosis; forced dimerization via a fused domain is crucial to enabling the human MLKL 4HB domain to kill cells (Quarato et al. 2016; Tanzer et al. 2016). The concepts presented in this summary figure have been presented individually elsewhere previously (Tanzer et al. 2016; Petrie et al. 2018, 2019a).
MLKL: THE END OF THE LINE
MLKL is composed of an amino-terminal four-helix bundle (4HB) domain, a two-helix “brace” region, and a carboxy-terminal pseudokinase domain (Fig. 3A; Murphy et al. 2013). The 4HB domain functions as the executioner domain by virtue of its membrane permeabilization activity (Cai et al. 2014; Chen et al. 2014; Dondelinger et al. 2014; Hildebrand et al. 2014; Su et al. 2014; Wang et al. 2014; Tanzer et al. 2016). The 4HB domain fold is akin to the HeLo domain, which exerts the membrane permeabilization function of the yeast Het-S protein (Daskalov et al. 2016) and the recently described plant MLKL ortholog, AtMLKL (Mahdi et al. 2019). The brace helices serve two principal functions within MLKL: as key contributors to MLKL oligomerization, and as levers to communicate signals from the pseudokinase domain to the 4HB domain (Su et al. 2014; Quarato et al. 2016; Davies et al. 2018). The carboxy-terminal pseudokinase domain serves a regulatory function by binding and restraining the executioner function of the 4HB domain in the dormant, nonactivated state (Murphy et al. 2013; Hildebrand et al. 2014; Tanzer et al. 2016; Davies et al. 2018; Petrie et al. 2018). Additionally, the pseudokinase domain serves as a signal integrator, in which phosphorylation of the activation loop by RIPK3 serves as a cue for activation. This event is thought to trigger a conformational change within the pseudokinase that promotes 4HB domain exposure, enabling MLKL to oligomerize, translocate to, and permeabilize the plasma membrane (Fig. 2; Petrie et al. 2017, 2019a).
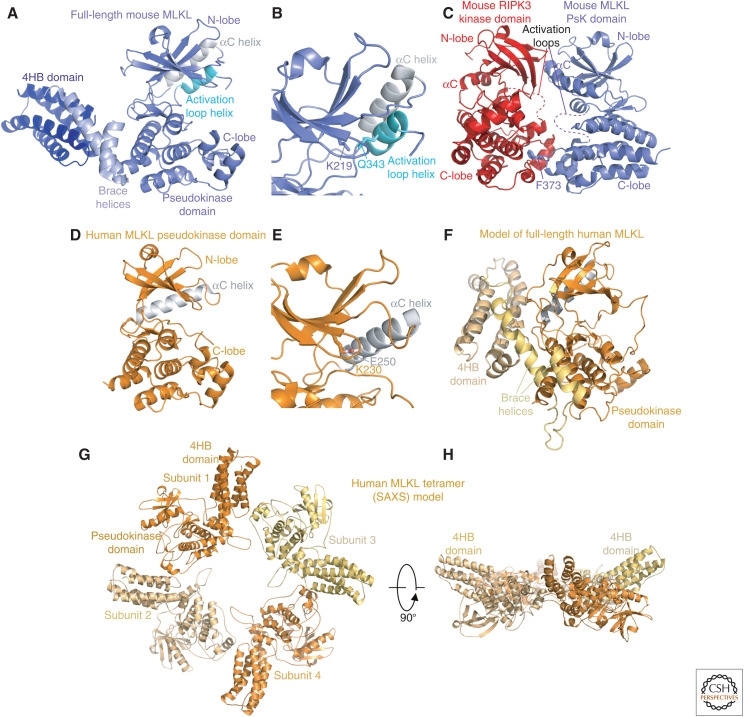
Figure 3.
Structural studies of full-length mixed lineage kinase domain-like protein (MLKL) and the MLKL pseudokinase domain. (A) The structure of full-length mouse MLKL revealed a domain architecture comprising an amino-terminal four-helix bundle (4HB) domain, the connector “brace” helices, and the carboxy-terminal pseudokinase domain (PDB, 4BTF). (B) Zoomed-in view of the pseudokinase domain pseudoactive site within the structure shown in panel A. The atypical interacting residues K219 from the N-lobe β3 strand and the activation loop helix Q343 are shown as sticks. The αC helix is shown in gray in A, B, D, E, and F; the activation loop helix of mouse MLKL in cyan in A and B. (C) The mouse MLKL pseudokinase domain (blue):RIPK3 kinase domain (red) cocrystal structure identified a face-to-face interaction between the domains, with activation loops apposed (PDB, 4M69) (Xie et al. 2013). The MLKL F373 residue (blue sticks) is buried in the RIPK3 C-lobe, and was found to be a crucial determinant of human MLKL binding to RIPK3 in subsequent studies (Petrie et al. 2019b). (D) The human MLKL pseudokinase domain crystal structure revealed a more conventional kinase domain structure with a canonical β3 strand Lys salt bridge with the αC helix Glu (shown in sticks in zoomed image in panel E). This structure was drawn from PDB 4MWI (Murphy et al. 2014), but is comparable with PDB 4M67 solved in parallel (Xie et al. 2013). (F) The structure of full-length human MLKL was modeled using distance restraints derived from cross-linking mass spectrometry data (Petrie et al. 2018), which were consistent with the sequestration of the killer 4HB domain by the suppressor pseudokinase domain within the inactive human MLKL monomer. (G,H) Orthogonal views of the human MLKL tetramer modeled using small-angle X-ray scattering (SAXS) data (Petrie et al. 2018). Throughout the figure, mouse MLKL domains are shown in blue shades, and human MLKL domains in yellow-orange shades.
DIVERGENT MECHANISMS OF MOUSE AND HUMAN MLKL ACTIVATION
The necroptosis pathway is by definition a caspase-independent pathway relying on the core RIPK3-MLKL cassette for execution. Recent advances have illuminated important mechanistic differences between the mouse and human necroptotic signaling pathways (Fig. 2), with marked differences between RIPK3's engagement and phosphorylation of MLKL (Petrie et al. 2018, 2019a). Importantly, human and mouse MLKL are not interchangeable and do not complement necroptotic signaling in cells derived from the opposing species (Tanzer et al. 2016; Davies et al. 2018; Petrie et al. 2018). The crucial factor governing this break in communication is the capacity of MLKL to interact with, and be phosphorylated by, the RIPK3 endogenous to the species in which MLKL is expressed. Consequently, these data support the idea that RIPK3 and MLKL have coevolved and diverged in a species-specific manner.
At the heart of MLKL activation is its phosphorylation by RIPK3 (Sun et al. 2012; Murphy et al. 2013; Tanzer et al. 2015; Rodriguez et al. 2016). It is the RIPK3-mediated phosphorylation of the activation loop of the MLKL pseudokinase domain at T357/S358 in human MLKL and S345 in mouse MLKL that is considered a key hallmark of MLKL activation in both species, and is widely used to monitor MLKL activation experimentally. However, consistent with divergent evolution of the RIPK3-MLKL cassettes between mouse and human cells, recent studies suggest the functions these events serve in signaling are quite distinct between species (Fig. 2).
Mouse MLKL
In the case of mouse MLKL, mutations within the regulatory pseudokinase domain led to stimulus-independent cell death (Murphy et al. 2013; Hildebrand et al. 2014). Mutations within the “pseudoactive site” (the ATP-binding site, such as K219M or Q343A; Fig. 3A,B) or mutation of the adjacent activation loop residue, S345, to a negatively charged residue to mimic RIPK3 phosphorylation, are known to trigger MLKL activation (Murphy et al. 2013). We proposed that this phosphorylation event triggers a conformational change in the pseudokinase domain, representing a molecular switch that converts dormant cytoplasmic MLKL into a killer protein that can form oligomers and translocate to, and permeabilize, the plasma membrane. Indeed, expression of the amino-terminal 4HB domain of mouse MLKL is sufficient to kill cells (Hildebrand et al. 2014), consistent with the role of the carboxy-terminal pseudokinase domain in suppressing the killer activity of the 4HB domain until this molecular switch is toggled by RIPK3 modification. Mutation of the pseudokinase domain within full-length mouse MLKL or expression of the 4HB executioner domain overrides this molecular switch (Murphy et al. 2013; Tanzer et al. 2015; Jacobsen et al. 2016; Rodriguez et al. 2016), because these constructs kill RIPK3-deficient cells in the absence of any further stimulus. It is highly likely that this is not a simple binary switch, however, because mutational studies of other phosphosites in mouse MLKL, including S158 in the interdomain “brace” helices, S228 and S248 in the pseudokinase domain, indicate other phosphorylation events within mouse MLKL likely tune the killing activity (Tanzer et al. 2015).
Human MLKL
In the case of human MLKL, however, activation is markedly more tightly regulated. Early studies suggested that mutation of the pseudokinase activation loop residues, T357 and S358, to mimic RIPK3 phosphorylation did not compromise cell killing by human MLKL, whereas mutation of these sites to Ala abrogated necroptosis signaling (Sun et al. 2012). Subsequent studies suggested that phosphomimetic mutant MLKL could modestly enhance cell death relative to the wild-type counterpart when oligomerized via a fused domain (Wang et al. 2014). Further studies, also in cell lines that do not typically undergo necroptosis and also involving knockdown, rather than knockout, of endogenous MLKL supported the idea that phosphomimetic mutation of T357/S358 in human MLKL could promote stimulus-independent death (Yoon et al. 2016), as observed in studies of mouse MLKL. However, subsequent studies in the human histiocytic lymphoma U937 and adenocarcinoma HT29 cell lines, which are commonly used for studies of the necroptosis signaling pathway, suggested the opposite (Petrie et al. 2018). These cell lines were edited to delete endogenous human MLKL, and wild-type or mutant human MLKL constructs stably introduced and inducibly expressed. In both lines, although wild-type human MLKL could reconstitute the signaling pathway, neither the T357E/S358E phosphomimic nor the T357A/S358A phospho-ablating human MLKL constructs signaled either in the absence or presence of necroptosis stimuli. Furthermore, introduction of constructs harboring mutations within the human MLKL pseudoactive site, such as those observed in colon, lung, and endometrial carcinomas and melanoma specimens, into MLKL−/− U937 cells did not promote MLKL's killing activity, but rather delayed the kinetics of cell death following treatment with a necroptosis stimulus (Petrie et al. 2018). Biophysical data with recombinant proteins suggest the basis for these mutants showing defects in signaling is that they are locked in a monomeric conformation, which hampers assembly into higher order oligomers that are responsible for cell death. The basis for complete abrogation of signaling by human MLKL activation loop mutations was rationalized by binding studies using recombinant proteins. Although wild-type human MLKL robustly bound human RIPK3 kinase domain, no binding was detected for the T357E/S358E human MLKL mutant (Petrie et al. 2018). These data support the idea that contrary to mouse MLKL activation, which can be achieved artificially in the absence of RIPK3, human MLKL relies on recruitment to human RIPK3 in cells as a precursor to its activation (Petrie et al. 2019a).
The role of RIPK3-mediated phosphorylation of human MLKL then becomes a conundrum. Although it is possible that phosphomimetic mutations fail to fully recapitulate the effects of MLKL activation loop phosphorylation, it is clear that human MLKL activation is not triggered by such mutations and this differs from observations using mouse MLKL mutant counterparts. Instead, it is probable that human MLKL phosphorylation serves a different role in activation, such as by promoting higher order MLKL assemblies that are required for killing cells, prompting reorganization of MLKL subunits with such assemblies, and/or promoting disengagement from RIPK3 to facilitate membrane translocation and permeabilization. The crucial role oligomerization plays in human MLKL activation is evident from studies of the 4HB domain or the 4HB + brace domain in human cells. Expression of these portions of human MLKL do not measurably induce necroptosis in cell lines typically used to study the pathway. However, if these domains are fused to an inducible dimerization domain, forced oligomerization can provoke cell death, supporting a key function for MLKL oligomerization in killing human cells (Quarato et al. 2016; Tanzer et al. 2016). The particulars of this choreography remain of enormous interest in the field, because each checkpoint represents a possible target for therapeutic intervention in the pathway.
FROM PROTEIN STRUCTURE TO MECHANISM
MLKL Full-Length and Pseudokinase Domain Structures
Structural studies of MLKL have played a crucial role in advancing our understanding of the mechanism underlying its activation and cell killing. To date, the only full-length protein structure among the necroptosis machinery is the X-ray crystal structure of mouse MLKL (Fig. 3A,B). This structure identified a number of defining features, including the aforementioned amino-terminal 4HB domain (discussed further below), the two helix linker termed the “brace helices,” and the carboxy-terminal pseudokinase domain (Murphy et al. 2013). MLKL was designated a pseudokinase because it has divergent sequences in place of the Mg2+-binding DFG motif at the start of the activation loop (conserved as GFE throughout species), with variable sequences in place of the conventional catalytic loop HRD motif (e.g., HRN in mouse, HGK in human) (Manning et al. 2002; Jacobsen and Murphy 2017). The latter is consistent with a loss of selective pressure to retain a catalytically competent sequence and active site geometry that would be requisite for a catalytic enzyme. Instead, an absence of catalytic activity has allowed sequence divergence among species within these motifs. Intriguingly, MLKL has retained the N-lobe β3 strand lysine of the VAIK motif (K219 in mouse, Fig. 3B; K230 in human, Fig. 3E), which positions ATP for phosphoryl transfer in catalytically active protein kinases. This is the last remnant of a conventional protein kinase among the catalytic motifs in MLKL and, accordingly, biophysical studies have revealed the importance of this Lys in mediating ATP binding (Murphy et al. 2013, 2014, 2017; Petrie et al. 2018). The physiological function of ATP binding in MLKL's regulation has been somewhat of a conundrum because mutation of K219 in mouse MLKL not only abrogated ATP binding but also triggered constitutive necroptosis (Murphy et al. 2013). This was not a consequence of loss of ATP binding, however, but rather a result of perturbing the molecular switch mechanism by disrupting the hydrogen bond between K219 and a residue in the noncanonical activation loop helix, Q343. This helix is unusual and was observed to displace the typical N-lobe regulatory element, the αC helix, which conventionally contributes a Glu to a charged pair with the VAIK Lys. Furthermore, biophysical studies revealed that, contrary to conventional protein kinases, cations competed for nucleotide binding to MLKL (Murphy et al. 2013). Thus, the likelihood that ATP binding could serve a physiological role appeared unlikely.
The structure of the human MLKL pseudokinase domain revealed stark differences to its mouse counterpart (Fig. 3D,E; Xie et al. 2013; Murphy et al. 2014). Unlike the mouse MLKL pseudokinase domain, human MLKL shows a conventional “closed” conformation, in which the αC helix is in the position of a typical active protein kinase with the key αC helix Glu (Glu250) engaged in an ion pair with the N-lobe β3 Lys (K230; Fig. 3D,E). Contrary to the mouse pseudokinase domain structure, a ladder of hydrophobic interactions termed the regulatory “R-spine” (Kornev et al. 2008), which is synonymous with the active conformations of conventional protein kinases, is intact in the human structure. The catalytic “C-spine” is not intact in either structure, but would typically rely on the adenine ring of ATP to complete the ladder. To date, no structures of MLKL bound to ATP or analogs have been reported, so it remains unclear whether such binding might promote toggling of the conformational switch. It also remains unclear whether there may be different functions conferred by ATP binding in mouse versus human MLKL. An intriguing observation from biophysical analyses of mutant proteins was that the determinants of ATP binding have diverged between mouse and human MLKL (Murphy et al. 2014). This supports the concept that without the selective pressure of maintaining active site geometry for catalysis that is imposed on conventional enzymes, the pseudoactive sites of pseudoenzymes have greater liberty to diverge (Ribeiro et al. 2019). In the case of human MLKL, it was observed that ATP binding destabilized a tetrameric form to favor a monomer, and human MLKL mutations that abrogated ATP binding led to delayed kinetics in necroptotic signaling when expressed in MLKL−/− cells (Petrie et al. 2018). Collectively, these data suggest that ATP binding is likely to be synonymous with the capacity of MLKL to adopt different conformers, thereby enabling the pseudokinase domain to perform a molecular switch function, rather than a cue for activation or attenuation of MLKL activation per se.
The pseudokinase domain of MLKL is known to engage the kinase domain of RIPK3, stably in the case of the human system (Sun et al. 2012; Zhao et al. 2012; Petrie et al. 2018), but transiently in the mouse system (Murphy et al. 2013). The only structural information on this interaction to date arise from a cocrystal structure of the mouse MLKL pseudokinase and RIPK3 kinase domains, where the proteins were crystallized in a face-to-face orientation with activation loops apposed (Fig. 3C; Xie et al. 2013). Notably, the pseudokinase domain conformation observed in the full-length mouse MLKL crystal structure (Fig. 3A,B) was preserved in the context of this complex, supporting the idea that the activation loop helix and displaced αC helix are not artifacts arising from crystallization conditions or contacts. Interestingly, recombinant mouse RIPK3 kinase domain and MLKL pseudokinase domains do not form a stable complex when mixed in vitro, but were copurified in complex when coexpressed in insect cells (Xie et al. 2013). This implicates cotranslational binding or the phosphorylation state of either protein as possible determinants of complex formation, which are currently poorly understood. Nonetheless, in this complex, it was observed that F373 of mouse MLKL projects from the αF-αG loop into a cavity adjacent to αG in RIPK3 (Fig. 3C). Although the importance of this interaction to mouse MLKL interaction with RIPK3 has not been examined, recent data suggest a similar mode of interaction occurs in the human system (Petrie et al. 2019b). Ala substitution of the equivalent human MLKL residue, F386, abrogated reconstitution of necroptotic signaling in MLKL−/− U937 cells, suggesting more broadly that this C-lobe:C-lobe interaction underpins RIPK3 engagement by MLKL (Petrie et al. 2019b). Even though this Phe is conserved in human and mouse MLKL, additional interactions must govern the RIPK3–MLKL interaction because mouse RIPK3 was not able to activate human MLKL expressed in Mlkl−/− mouse fibroblasts.
In the structure of full-length mouse MLKL, the killer 4HB domain was crystallized in a conformation extended away from the pseudokinase domain (Fig. 3A). This contrasts a conformation in which the 4HB domain is sequestered by the pseudokinase domain to prevent killing, which was predicted from biochemical studies (Hildebrand et al. 2014). In the absence of an experimental human MLKL full-length structure, structural mass spectrometry was used to deduce the organization of domains in the dormant monomer form (Petrie et al. 2018). In this model, the 4HB domain is closely associated with the pseudokinase domain (Fig. 3F), consistent with the suppressor function of the latter. Using small-angle scattering, it was possible to model the higher order, tetrameric assembly of human MLKL at low resolution (Fig. 3G,H). Coupled with cross-linking mass spectrometry data, this revealed a torus structure where the 4HB domains are directed toward a common face (Petrie et al. 2018), which is consistent with a role for positively charged residues on this surface in lipid association and membrane permeabilization (discussed below). Our current knowledge is insufficient to deduce whether this is the assembly that permeabilizes plasma membranes or whether higher order assembly is a prerequisite for cell killing.
The Killer 4HB Domain
The function of the amino-terminal 4HB domain as the cellular executioner was revealed from truncation and overexpression studies (Chen et al. 2014; Dondelinger et al. 2014; Hildebrand et al. 2014; Wang et al. 2014). The mouse MLKL 4HB domain is a potent killer of cells, as mentioned above, and recombinant protein potently permeabilizes lipid bilayers in vitro (Tanzer et al. 2016). In contrast, the human counterpart shows much less of these activities, and instead relies on forced oligomerization to induce cell death in human cells (Tanzer et al. 2016). The basis for these differences is not yet clear, and because structural studies of MLKL amino-termini have focused on the human 4HB domain, much of our understanding is based on the human system. Small-angle X-ray scattering studies of the mouse MLKL 4HB domain + brace support the idea that mouse MLKL assembles into trimers (Fig. 4A; Davies et al. 2018), unlike the tetramers observed for full-length human MLKL (Fig. 3G,H; Petrie et al. 2018), and that trimerization is centered on the brace helices in mouse MLKL. Validation of this organization and stoichiometry in the context of full-length mouse MLKL awaits further experimentation. Although the residues that facilitate lipid binding and membrane permeabilization have not yet been formally defined for the mouse MLKL 4HB domain, scanning mutagenesis identified two clusters of residues centered on the α1–α2 helix junction and the α4 helix (salmon and magenta sticks, respectively, Fig. 4B) that were required for MLKL to induce cell death (Hildebrand et al. 2014). The most deleterious were alanine substitutions of the α4 helix, E109/E110 and R105/D106 (Fig. 4B), which completely abrogated cell death. Interestingly, forced oligomerization of full-length mouse MLKL harboring these mutations was insufficient to hurdle this signaling checkpoint (Tanzer et al. 2016). Because many of these residues possess negatively charged side chains, it is likely that they serve functions distinct from directly engaging the negatively charged phospholipids of the plasma membrane.
Figure 4.
Comparison of four-helix bundle (4HB) domain structures between mouse and human mixed lineage kinase domain-like protein (MLKL). (A) The mouse MLKL 4HB + brace trimer structures were modeled based on small-angle X-ray scattering (SAXS) studies (Davies et al. 2018). (B) The mouse MLKL 4HB + brace region from the full-length structure (PDB 4BTF). Two sites identified in alanine scanning experiments as key for necroptotic signaling are shown as magenta and salmon sticks (Hildebrand et al. 2014). (C) The nuclear magnetic resonance (NMR) structure of human MLKL 4HB domain with first brace helix (PDB 2MSV). Distinct from the mouse 4HB structure, an additional loop was observed between the α3 and α4 helices (Su et al. 2014). Residues identified as key to necroptotic signaling from studies of mutants in cells are shown in magenta. Those involved in phospholipid binding are shown as red sticks. Most residues were identified by Quarato and colleagues (2016), but K22 and K25 were identified by Su et al. (2014). (D) The human MLKL 4HB domain and first brace helix structure shown in panel C with residues identified as inositol phosphate interactors shown as cyan sticks (Dovey et al. 2018; McNamara et al. 2019).
The solution structure of the human 4HB domain and first brace helix topologically resembled the mouse counterpart, albeit with the additional structural feature of an additional helix in the loop connecting the α3 and α4 helices of the 4HB domain (Su et al. 2014; McNamara et al. 2019), which was not ordered in the mouse crystal structure. Several basic amino acids have been identified as mediators of lipid engagement in nuclear magnetic resonance (NMR) spectroscopy studies (red sticks, Fig. 4C; Su et al. 2014; Quarato et al. 2016). Consistent with a role in lipid binding, Ala substitution of K17/R18 led to delays in necroptotic signaling (Quarato et al. 2016; Petrie et al. 2018), although curiously did not abrogate cell death altogether. This suggests that others of the lipid-binding residues enable lipid engagement to proceed, although in the absence of a lipid bound structure, the precise details remain unknown. Mutational analyses identified D107/E111 (magenta sticks, Fig. 4C) as essential for necroptosis signaling (Petrie et al. 2018). This site is spatially analogous to the aforementioned key residues in mouse MLKL, D105, and E109 (Fig. 4B), which suggests that both human and mouse MLKL rely on nonphospholipid interactions with currently unknown coeffectors to induce necroptotic cell death. This possibility remains of enormous ongoing interest. The recent discovery of inositol phosphates as additional activators of human 4HB domain activation (interaction sites shown as cyan sticks, Fig. 4D) indicates that not all regulators of MLKL activation need be proteins (Dovey et al. 2018; McNamara et al. 2019).
In the absence of a membrane-bound 4HB domain structure, the underlying mechanism of membrane permeabilization remains unclear. The membrane aperture is referred to loosely as a “pore” although the precise composition, including the stoichiometry of MLKL and the inclusion of other components, remain unclear. The aperture size has been estimated as ∼4 nm diameter indirectly (Ros et al. 2017), although whether it is determinate in size remains to be established. One study has proposed the 4HB domain might reorganize in membranes to assemble into ion channels (Xia et al. 2016); however, this remains to be fully explored in cellular contexts and structurally. Earlier studies implicated MLKL in engaging mitochondrial membranes to provoke mitochondrial fission or promote ion channel activity (Cai et al. 2014), although subsequent studies have discounted these possibilities (Murphy et al. 2013; Tait et al. 2013; Moujalled et al. 2014; Remijsen et al. 2014; Wang et al. 2014). Based on studies showing that the 4HB domain can permeabilize membranes in vitro (Dondelinger et al. 2014; Su et al. 2014; Wang et al. 2014; Tanzer et al. 2016; Petrie et al. 2018), it is thought that MLKL kills cells via direct action on the plasma membrane. Such a function has previously been attributed to an ancient 4HB domain initially characterized in yeast termed the HeLo domain (Daskalov et al. 2016). While HeLo domain-containing proteins are abundant in yeast, with similar domains present in plants (Jubic et al. 2019; Mahdi et al. 2019), the only example in animals is that of MLKL. Whether the domain has a common ancestor or arose by convergent evolution is an interesting conundrum.
TUNING NECROPTOSIS SIGNALING
Although recombinant MLKL or the 4HB domain alone can permeabilize artificial membranes, it is evident that there are many layers of regulation in cells that prevent unbridled cell killing that would lead to inflammatory diseases (Fig. 5). MLKL is present in most cell types throughout animals as a dormant protein (Murphy et al. 2013; Wu et al. 2013), and cues are necessary to toggle MLKL to an activated form that kills cells. Many checkpoints have been reported to date, which collectively likely dictate the relatively slow kinetics of necroptotic death. Induction of necroptosis leads to early changes in the plasma membrane, such as phosphatidylserine (PS) exposure, within minutes (Gong et al. 2017b; Zargarian et al. 2017), but only after 3–6 hours (depending on strength of stimulus) is necroptotic death observed (Tanzer et al. 2015; Gong et al. 2017b; Zargarian et al. 2017). Recent efforts have been directed toward understanding whether there are additional steps involved in facilitating MLKL to translocate and assemble into killer complexes and, conversely, whether additional mechanisms exist to suppress activated MLKL and limit its killing capacity. These studies have identified several processes, of which our understanding is only emerging.
Figure 5.
Multiple effectors influence the necroptosis signaling pathway. Numerous auxiliary interactions with mixed lineage kinase domain-like protein (MLKL) have been reported to promote (green arrows, Bigenzahn et al. 2016; Jacobsen et al. 2016; Zhao et al. 2016; Dovey et al. 2018; McNamara et al. 2019; Najafov et al. 2019) or counteract (red arrows, Gong et al. 2017a,b; Reynoso et al. 2017; Yoon et al. 2017; Zargarian et al. 2017; Fan et al. 2019; Petrie et al. 2019b) necroptosis.
Posttranslational Modifications
The most definitive step in the conversion of MLKL from a dormant form to a killer is phosphorylation of the MLKL pseudokinase domain activation loop by RIPK3. The observation of additional posttranslational modifications has led to a picture of MLKL activation as not a binary switch, but rather a graded switch, where multiple signals can be integrated to tune MLKL activity. Other phosphorylation events in human MLKL have been identified in mass spectrometry screens, such as in a cell-cycle-dependent manner in the 4HB domain (S125) and pseudokinase domain (T377) (Dephoure et al. 2008), but the responsible kinases and the impact of these events on signaling is yet to be determined. Most recently, the PS-binding TAM (Tyro3, Axl, Mer) tyrosine kinase family were reported to phosphorylate MLKL at Y376 and proposed to promote MLKL activation by directing MLKL oligomerization (Najafov et al. 2019). Because Y376 is best known for its role as a structural residue in MLKL, the precise mechanism by which its phosphorylation might trigger MLKL oligomerization remains to be completely elucidated. To date, no phosphatases have been reported to dephosphorylate MLKL phosphosites, although it is possible that an “off switch” of this nature exists. Furthermore, MLKL has been shown to undergo ubiquitylation on induction of necroptosis signaling (Lawlor et al. 2015). Whether such a signal consigns MLKL to proteasomal degradation to defuse necroptosis signaling, or whether it serves to regulate MLKL localization and thus positively or negatively impact necroptosis remains of outstanding interest. Recently, RIPK3 was reported to undergo O-GlcNAcylation, which limited its capacity to engage in RIPK1 hetero-oligomers and RIPK3 homo-oligomers (Li et al. 2019) and attenuated necroptotic signaling. Whether similar modifications might occur in MLKL remain to be determined.
Positive Regulators of MLKL Activation
Recent data suggest a role for second messengers, such as inositol phosphates generated by the IPMK, ITPK1, and IPPK metabolic kinases, in direct binding of the human MLKL 4HB domain (Fig. 4D) to perform an auxiliary role in promoting 4HB domain unmasking to kill cells (Fig. 5; Dovey et al. 2018; McNamara et al. 2019). The ubiquity of the “inositol phosphate code,” the relative contributions of each metabolite and their cognate kinases in directing necroptosis, and whether inhibition of IPMK, ITPK1, and/or IPPK could be targeted to block necroptosis therapeutically, remain to be further explored.
Beyond RIPK3, remarkably few proteins have been identified as MLKL interactors, with the cochaperones, HSP90-Cdc37, among the few identified as playing a key role in MLKL activation (Fig. 5; Bigenzahn et al. 2016; Jacobsen et al. 2016; Zhao et al. 2016). These chaperones contribute to activation of most protein kinases, including RIPK1 and RIPK3 (Lewis et al. 2000; Cho et al. 2009), and with their role in regulating MLKL activation, they are established as important coeffectors in the necroptosis pathway. Whether HSP90 exerts its effects on MLKL folding, oligomerization, or translocation to membranes has not been precisely determined, although it is plausible HSP90 impacts each of these activation steps. It is highly likely that additional positive regulators of MLKL activation exist and await discovery.
Negative Regulators of MLKL Activation
Over the past few years, a number of proteins have emerged as candidate attenuators of MLKL activity (Fig. 5, red arrows), although it remains to be determined whether they act directly on MLKL or by proxy. Further, it remains to be established how universally these proteins are involved in necroptosis, because they have been studied only in limited contexts to date. For instance, repulsive guidance molecule-b (RGMb) was implicated in inhibiting necroptosis in kidney tubular cells, and by overexpression was observed to block human MLKL membrane translocation and therefore necroptotic cell death (Liu et al. 2018). Whether this protein functions more broadly in necroptotic signaling, and at endogenous levels, requires further investigation. The putative interactor, thioredoxin-1 (Trx1), was proposed to limit MLKL oxidation and assembly into higher order disulfide cross-linked species (Reynoso et al. 2017), which have been widely used as a readout of MLKL activation without complete knowledge of whether they represent killer assemblies or are a consequence of membrane compromise and a dysregulated redox environment in the cytoplasm.
Recent studies have suggested exocytosis and endocytosis of membrane-associated MLKL can extinguish the necroptotic signal by respectively shedding or degrading activated (phosphorylated) MLKL from membranes (Fig. 5). Exocytosis of phospho-MLKL via the ESCRT machinery in vesicles or exosomes referred to as “necroptotic bodies” was identified by three independent groups recently (Gong et al. 2017a,b; Yoon et al. 2017; Zargarian et al. 2017). Further studies have implicated phospho-MLKL interaction with ALIX/syntenin-1 in the exocytosis process, and interaction with flotillin-1 and flotillin-2 within lipid rafts in endocytosis-mediated lysosomal degradation (Fan et al. 2019). Whether these interactions are directly with phospho-MLKL, or membrane-scaffolded, remains of enormous interest, but they illustrate the breadth of mechanisms used by cells to counteract a death signal and prolong longevity of the cell.
In a similar vein, pathogens have evolved mechanisms to prolong cell longevity by counteracting death pathways, including necroptosis. The best characterized of these are RHIM-targeting pathogens (Pearson and Murphy 2017), which either act to inhibit RIPK3 oligomerization by competition in the case of viral proteins (Upton et al. 2010, 2012) or by cleaving RHIM domains in the case of the enteropathogenic Escherichia coli EspL protease (Pearson et al. 2017). Very recently, an additional mechanism to disarm necroptosis by pathogens was uncovered. MLKL xenologs encoded in the genomes of some poxviruses were identified to function as mimics of cellular MLKL by sequestering RIPK3 in the host cell to prevent activation of cellular MLKL (Fig. 5; Petrie et al. 2019b). One of the curiosities of necroptosis signaling is that some organisms, like carnivores, lack RIPK3 and MLKL, and others like birds and rabbits, lack RIPK3 (Dondelinger et al. 2016; Newton and Manning 2016), suggesting that the pathway was negatively selected against in evolution of some species. In addition, the amino acid sequences of MLKL are highly divergent among species, with identity as low as 68% between human and mouse MLKL, which contributes to their inability to complement signaling in cells derived from the respective other species. The forces driving such divergence have remained unclear. The recent discovery of virally encoded MLKL homolog pseudokinase domains indicates that pathogens have contributed to, if not driven, RIPK3-MLKL evolution. MLKL orthologs encoded by two related rodent poxviruses were found to inhibit necroptosis in human and mouse cells, while an ortholog from swinepox did not (Petrie et al. 2019b). Interestingly, although RIPK3 is the target in mouse and human cells, the mechanism of inhibition differed. In human cells, a highly phosphorylated form of RIPK3 was stabilized but could not activate cellular MLKL; in mouse cells, RIPK3 phosphorylation was attenuated. Curiously, birds and rabbit do not express RIPK3, which raises the question about whether viral MLKL in avian poxes and myxomas have evolved a distinct target and, more generally, whether other non-RIPK3 mediated signals might exist that can trigger MLKL activation. Like animal MLKL sequences, viral MLKL sequences are highly divergent, with exemplars from each poxviral MLKL ortholog family sharing only ∼30% sequence identity. This is consistent with the idea that coevolution of viral MLKL with the host RIPK3-MLKL has led to divergence of both systems from those of other species.
CONCLUDING REMARKS
Our current understanding of how the terminal necroptosis effector, MLKL, is activated and kills cells has emerged from only 7 years of intensive study. Consequently, there are many gaps in our understanding. Precisely how MLKL permeabilizes membranes, whether this is a determinate stoichiometry (like with the pore-forming pyroptosis effector gasdermin proteins) (Ruan et al. 2018; Xia et al. 2019), and whether this is a highly organized structure await further structural studies. It is a curiosity that to date very few MLKL interactors have been identified. Thus, it remains of enormous interest to understand which proteins or metabolites are essential for MLKL activation and the range of mechanisms by which necroptosis can be defused. Finally, obtaining a thorough understanding of differences in the necroptosis pathway between species is essential as endeavors to therapeutically target this pathway advance.
ACKNOWLEDGMENTS
I thank members of my laboratory and colleagues for discussions, Emma Petrie for critical reading of the manuscript, and collaborators who have contributed to many of the studies described herein. I thank the National Health and Medical Research Council of Australia for funding support (1105754, 1124735, 1124737, and 9000433) and the Victorian State Government Operational Infrastructure Support scheme.
Footnotes
Editors: Kim Newton, James M. Murphy, and Edward A. Miao
Additional Perspectives on Cell Survival and Cell Death available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Alvarez-Diaz S, Dillon CP, Lalaoui N, Tanzer MC, Rodriguez DA, Lin A, Lebois M, Hakem R, Josefsson EC, O'Reilly LA, et al. 2016. The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity 45: 513–526. 10.1016/j.immuni.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SB, Kasparcova V, Hoffman S, Swift B, Dare L, Schaeffer M, Capriotti C, Cook M, Finger J, Hughes-Earle A, et al. 2014. Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol 192: 5476–5480. 10.4049/jimmunol.1400499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigenzahn JW, Fauster A, Rebsamen M, Kandasamy RK, Scorzoni S, Vladimer GI, Müller AC, Gstaiger M, Zuber J, Bennett KL, et al. 2016. An inducible retroviral expression system for tandem affinity purification mass-spectrometry-based proteomics identifies mixed lineage kinase domain-like protein (MLKL) as a heat shock protein 90 (HSP90) client. Mol Cell Proteomics 15: 1139–1150. 10.1074/mcp.O115.055350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. 2014. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 16: 55–65. 10.1038/ncb2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P, et al. 2014. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res 24: 105–121. 10.1038/cr.2013.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. 2009. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137: 1112–1123. 10.1016/j.cell.2009.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, et al. 2014. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 513: 90–94. 10.1038/nature13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalov A, Habenstein B, Sabaté R, Berbon M, Martinez D, Chaignepain S, Coulary-Salin B, Hofmann K, Loquet A, Saupe SJ. 2016. Identification of a novel cell death-inducing domain reveals that fungal amyloid-controlled programmed cell death is related to necroptosis. Proc Natl Acad Sci 113: 2720–2725. 10.1073/pnas.1522361113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KA, Tanzer MC, Griffin MDW, Mok YF, Young SN, Qin R, Petrie EJ, Czabotar PE, Silke J, Murphy JM. 2018. The brace helices of MLKL mediate interdomain communication and oligomerisation to regulate cell death by necroptosis. Cell Death Differ 25: 1567–1580. 10.1038/s41418-018-0061-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almagro MC, Goncharov T, Izrael-Tomasevic A, Duttler S, Kist M, Varfolomeev E, Wu X, Lee WP, Murray J, Webster JD, et al. 2017. Coordinated ubiquitination and phosphorylation of RIP1 regulates necroptotic cell death. Cell Death Differ 24: 26–37. 10.1038/cdd.2016.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. 2005. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1: 112–119. 10.1038/nchembio711 [DOI] [PubMed] [Google Scholar]
- Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. 2008. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci 105: 10762–10767. 10.1073/pnas.0805139105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, Hulpiau P, Weber K, Sehon CA, Marquis RW, et al. 2014. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep 7: 971–981. 10.1016/j.celrep.2014.04.026 [DOI] [PubMed] [Google Scholar]
- Dondelinger Y, Hulpiau P, Saeys Y, Bertrand MJM, Vandenabeele P. 2016. An evolutionary perspective on the necroptotic pathway. Trends Cell Biol 26: 721–732. 10.1016/j.tcb.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Dovey CM, Diep J, Clarke BP, Hale AT, McNamara DE, Guo H, Brown NW Jr, Cao JY, Grace CR, Gough PJ, et al. 2018. MLKL requires the inositol phosphate code to execute necroptosis. Mol Cell 70: 936–948.e7. 10.1016/j.molcel.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Guo J, Gao B, Zhang W, Ling L, Xu T, Pan C, Li L, Chen S, Wang H, et al. 2019. Flotillin-mediated endocytosis and ALIX-syntenin-1-mediated exocytosis protect the cell membrane from damage caused by necroptosis. Sci Signal 12: eaaw3423 10.1126/scisignal.aaw3423 [DOI] [PubMed] [Google Scholar]
- Gong YN, Guy C, Crawford JC, Green DR. 2017a. Biological events and molecular signaling following MLKL activation during necroptosis. Cell Cycle 16: 1748–1760. 10.1080/15384101.2017.1371889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong YN, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P, Linkermann A, Green DR. 2017b. ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell 169: 286–300.e16. 10.1016/j.cell.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. 2009. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137: 1100–1111. 10.1016/j.cell.2009.05.021 [DOI] [PubMed] [Google Scholar]
- He S, Liang Y, Shao F, Wang X. 2011. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci 108: 20054–20059. 10.1073/pnas.1116302108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P, Pierotti C, Garnier JM, Dobson RC, Webb AI, et al. 2014. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci 111: 15072–15077. 10.1073/pnas.1408987111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JM, Kauppi M, Majewski IJ, Liu Z, Cox A, Miyake S, Petrie EJ, Silk MA, Li Z, Tanzer MC, et al. 2019. Missense mutations in the MLKL “brace” region lead to lethal neonatal inflammation in mice and are present in dependent frequency in humans. bioRxiv 628370. [Google Scholar]
- Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. 2000. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1: 489–495. 10.1038/82732 [DOI] [PubMed] [Google Scholar]
- Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. 1996. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4: 387–396. 10.1016/S1074-7613(00)80252-6 [DOI] [PubMed] [Google Scholar]
- Huang D, Zheng X, Wang ZA, Chen X, He WT, Zhang Y, Xu JG, Zhao H, Shi W, Wang X, et al. 2017. The MLKL channel in necroptosis is an octamer formed by tetramers in a dyadic process. Mol Cell Biol 37: e00497–e00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen AV, Murphy JM. 2017. The secret life of kinases: insights into non-catalytic signalling functions from pseudokinases. Biochem Soc Trans 45: 665–681. 10.1042/BST20160331 [DOI] [PubMed] [Google Scholar]
- Jacobsen AV, Lowes KN, Tanzer MC, Lucet IS, Hildebrand JM, Petrie EJ, van Delft MF, Liu Z, Conos SA, Zhang JG, et al. 2016. HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis 7: e2051 10.1038/cddis.2015.386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubic LM, Saile S, Furzer OJ, El Kasmi F, Dangl JL. 2019. Help wanted: helper NLRs and plant immune responses. Curr Opin Plant Biol 50: 82–94. 10.1016/j.pbi.2019.03.013 [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. 2013. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 288: 31268–31279. 10.1074/jbc.M113.462341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran D, Geoffrion M, Wei L, Gan W, Richards L, Shangari P, DeKemp EM, Beanlands RA, Perisic L, Maegdefessel L, et al. 2016. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci Adv 2: e1600224 10.1126/sciadv.1600224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitur K, Wachtel S, Brown A, Wickersham M, Paulino F, Peñaloza HF, Soong G, Bueno S, Parker D, Prince A. 2016. Necroptosis promotes Staphylococcus aureus clearance by inhibiting excessive inflammatory signaling. Cell Rep 16: 2219–2230. 10.1016/j.celrep.2016.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornev AP, Taylor SS, Ten Eyck LF. 2008. A helix scaffold for the assembly of active protein kinases. Proc Natl Acad Sci 105: 14377–14382. 10.1073/pnas.0807988105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D'Cruz AA, Hall C, Kaur Spall S, Anderton H, Masters SL, et al. 2015. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun 6: 6282 10.1038/ncomms7282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Devin A, Miller A, Lin Y, Rodriguez Y, Neckers L, Liu ZG. 2000. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-κB activation. J Biol Chem 275: 10519–10526. 10.1074/jbc.275.14.10519 [DOI] [PubMed] [Google Scholar]
- Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, et al. 2012. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150: 339–350. 10.1016/j.cell.2012.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gong W, Wang H, Li T, Attri KS, Lewis RE, Kalil AC, Bhinderwala F, Powers R, Yin G, et al. 2019. O-GlcNAc transferase suppresses inflammation and necroptosis by targeting receptor-interacting serine/threonine-protein kinase 3. Immunity 50: 576–590.E6. 10.1016/j.immuni.2019.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Kumari S, Kim C, Van TM, Wachsmuth L, Polykratis A, Pasparakis M. 2016. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature 540: 124–128. 10.1038/nature20558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Chen B, Wang Y, Meng C, Huang H, Huang XR, Qin J, Mulay SR, Anders HJ, Qiu A, et al. 2018. RGMb protects against acute kidney injury by inhibiting tubular cell necroptosis via an MLKL-dependent mechanism. Proc Natl Acad Sci 115: E1475–E1484. 10.1073/pnas.1716959115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi L, Huang M, Zhang X, Nakano RT, Kopp LB, Saur IML, Jacob F, Kovacova V, Lapin D, Parker JE, et al. 2019. Plant mixed lineage kinase domain-like proteins limit biotrophic pathogen growth. bioRxiv 681015 10.1101/681015 [DOI]
- Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, et al. 2014. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell 56: 481–495. 10.1016/j.molcel.2014.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. 2002. The protein kinase complement of the human genome. Science 298: 1912–1934. 10.1126/science.1075762 [DOI] [PubMed] [Google Scholar]
- McComb S, Cessford E, Alturki NA, Joseph J, Shutinoski B, Startek JB, Gamero AM, Mossman KL, Sad S. 2014. Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proc Natl Acad Sci 111: E3206–E3213. 10.1073/pnas.1407068111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara DE, Dovey CM, Hale AT, Quarato G, Grace CR, Guibao CD, Diep J, Nourse A, Cai CR, Wu H, et al. 2019. Direct activation of human MLKL by a select repertoire of inositol phosphate metabolites. Cell Chem Biol 26: 863–877.E7. 10.1016/j.chembiol.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Liu Z, Li X, Wang H, Jin T, Wu G, Shan B, Christofferson DE, Qi C, Yu Q, et al. 2018. Death-domain dimerization-mediated activation of RIPK1 controls necroptosis and RIPK1-dependent apoptosis. Proc Natl Acad Sci 115: E2001–E2009. 10.1073/pnas.1722013115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerke C, Bleibaum F, Kunzendorf U, Krautwald S. 2019. Combined knockout of RIPK3 and MLKL reveals unexpected outcome in tissue injury and inflammation. Front Cell Dev Biol 7: 19 10.3389/fcell.2019.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mompeán M, Li W, Li J, Laage S, Siemer AB, Bozkurt G, Wu H, McDermott AE. 2018. The structure of the necrosome RIPK1-RIPK3 core, a human hetero-amyloid signaling complex. Cell 173: 1244–1253.e10. 10.1016/j.cell.2018.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moujalled DM, Cook WD, Murphy JM, Vaux DL. 2014. Necroptosis induced by RIPK3 requires MLKL but not Drp1. Cell Death Dis 5: e1086 10.1038/cddis.2014.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Dewitz C, Schmitz J, Schroder AS, Bräsen JH, Stockwell BR, Murphy JM, Kunzendorf U, Krautwald S. 2017. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol Life Sci 74: 3631–3645. 10.1007/s00018-017-2547-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, et al. 2013. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39: 443–453. 10.1016/j.immuni.2013.06.018 [DOI] [PubMed] [Google Scholar]
- Murphy JM, Lucet IS, Hildebrand JM, Tanzer MC, Young SN, Sharma P, Lessene G, Alexander WS, Babon JJ, Silke J, et al. 2014. Insights into the evolution of divergent nucleotide-binding mechanisms among pseudokinases revealed by crystal structures of human and mouse MLKL. Biochem J 457: 369–377. 10.1042/BJ20131270 [DOI] [PubMed] [Google Scholar]
- Murphy JM, Zhang Q, Young SN, Reese ML, Bailey FP, Eyers PA, Ungureanu D, Hammaren H, Silvennoinen O, Varghese LN, et al. 2017. A robust methodology to subclassify pseudokinases based on their nucleotide binding-properties. Biochem J 457: 323–334. 10.1042/BJ20131174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafov A, Mookhtiar AK, Luu HS, Ordureau A, Pan H, Amin PP, Li Y, Lu Q, Yuan J. 2019. TAM kinases promote necroptosis by regulating oligomerization of MLKL. Mol Cell 75: 457–468.E4. 10.1016/j.molcel.2019.05.022 [DOI] [PubMed] [Google Scholar]
- *.Newton K. 2019. Multitasking kinase RIPK1 regulates cell death and inflammation. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a036368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Manning G. 2016. Necroptosis and inflammation. Annu Rev Biochem 85: 743–763. 10.1146/annurev-biochem-060815-014830 [DOI] [PubMed] [Google Scholar]
- Newton K, Sun X, Dixit VM. 2004. Kinase RIP3 is dispensable for normal NF-κBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol 24: 1464–1469. 10.1128/MCB.24.4.1464-1469.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, et al. 2014. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343: 1357–1360. 10.1126/science.1249361 [DOI] [PubMed] [Google Scholar]
- Newton K, Dugger DL, Maltzman A, Greve JM, Hedehus M, Martin-McNulty B, Carano RA, Cao TC, van Bruggen N, Bernstein L, et al. 2016a. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ 23: 1565–1576. 10.1038/cdd.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Wickliffe KE, Maltzman A, Dugger DL, Strasser A, Pham VC, Lill JR, Roose-Girma M, Warming S, Solon M, et al. 2016b. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature 540: 129–133. 10.1038/nature20559 [DOI] [PubMed] [Google Scholar]
- Pearson JS, Murphy JM. 2017. Down the rabbit hole: is necroptosis truly an innate response to infection? Cell Microbiol 19: e12750 10.1111/cmi.12750 [DOI] [PubMed] [Google Scholar]
- Pearson JS, Giogha C, Mühlen S, Nachbur U, Pham CL, Zhang Y, Hildebrand JM, Oates CV, Lung TW, Ingle D, et al. 2017. EspL is a bacterial cysteine protease effector that cleaves RHIM proteins to block necroptosis and inflammation. Nat Microbiol 2: 16258 10.1038/nmicrobiol.2016.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pefanis A, Ierino FL, Murphy JM, Cowan PJ. 2019. Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int 96: 291–301. 10.1016/j.kint.2019.02.009 [DOI] [PubMed] [Google Scholar]
- Petrie EJ, Hildebrand JM, Murphy JM. 2017. Insane in the membrane: a structural perspective of MLKL function in necroptosis. Immunol Cell Biol 95: 152–159. 10.1038/icb.2016.125 [DOI] [PubMed] [Google Scholar]
- Petrie EJ, Sandow JJ, Jacobsen AV, Smith BJ, Griffin MDW, Lucet IS, Dai W, Young SN, Tanzer MC, Wardak A, et al. 2018. Conformational switching of the pseudokinase domain promotes human MLKL tetramerization and cell death by necroptosis. Nat Commun 9: 2422 10.1038/s41467-018-04714-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie EJ, Czabotar PE, Murphy JM. 2019a. The structural basis of necroptotic cell death signaling. Trends Biochem Sci 44: 53–63. 10.1016/j.tibs.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Petrie EJ, Sandow JJ, Lehmann WIL, Liang LY, Coursier D, Young SN, Kersten WJA, Fitzgibbon C, Samson AL, Jacobsen AV, et al. 2019b. Viral MLKL homologs subvert necroptotic cell death by sequestering cellular RIPK3. Cell Rep 28: 3309–3319. [DOI] [PubMed] [Google Scholar]
- Pham CL, Shanmugam N, Strange M, O'Carroll A, Brown JW, Sierecki E, Gambin Y, Steain M, Sunde M. 2019. Viral M45 and necroptosis-associated proteins form heteromeric amyloid assemblies. EMBO Rep 20: e46518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarato G, Guy CS, Grace CR, Llambi F, Nourse A, Rodriguez DA, Wakefield R, Frase S, Moldoveanu T, Green DR. 2016. Sequential engagement of distinct MLKL phosphatidylinositol-binding sites executes necroptosis. Mol Cell 61: 589–601. 10.1016/j.molcel.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju S, Whalen DM, Mengistu M, Swanson C, Quinn JG, Taylor SS, Webster JD, Newton K, Shaw AS. 2018. Kinase domain dimerization drives RIPK3-dependent necroptosis. Sci Signal 11: eaar2188 10.1126/scisignal.aar2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AJM, Das S, Dawson N, Zaru R, Orchard S, Thornton JM, Orengo C, Zeqiraj E, Murphy JM, Eyers PA. 2019. Emerging concepts in pseudoenzyme classification, evolution, and signaling. Sci Signal 12: eaat9797. [DOI] [PubMed] [Google Scholar]
- Remijsen Q, Goossens V, Grootjans S, Van den Haute C, Vanlangenakker N, Dondelinger Y, Roelandt R, Bruggeman I, Goncalves A, Bertrand MJ, et al. 2014. Depletion of RIPK3 or MLKL blocks TNF-driven necroptosis and switches towards a delayed RIPK1 kinase-dependent apoptosis. Cell Death Dis 5: e1004 10.1038/cddis.2013.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynoso E, Liu H, Li L, Yuan AL, Chen S, Wang Z. 2017. Thioredoxin-1 actively maintains the pseudokinase MLKL in a reduced state to suppress disulfide bond-dependent MLKL polymer formation and necroptosis. J Biol Chem 292: 17514–17524. 10.1074/jbc.M117.799353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard JA, Anderton H, Etemadi N, Nachbur U, Darding M, Peltzer N, Lalaoui N, Lawlor KE, Vanyai H, Hall C, et al. 2014a. TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice. eLife 3: e03464 10.7554/eLife.03464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard JA, O'Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers TW, Vince JE, Lawlor KE, Ninnis RL, Anderton H, et al. 2014b. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 157: 1175–1188. 10.1016/j.cell.2014.04.019 [DOI] [PubMed] [Google Scholar]
- Rodriguez DA, Weinlich R, Brown S, Guy C, Fitzgerald P, Dillon CP, Oberst A, Quarato G, Low J, Cripps JG, et al. 2016. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ 23: 76–88. 10.1038/cdd.2015.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros U, Peña-Blanco A, Hänggi K, Kunzendorf U, Krautwald S, Wong WW, García-Sáez AJ. 2017. Necroptosis execution is mediated by plasma membrane nanopores independent of calcium. Cell Rep 19: 175–187. 10.1016/j.celrep.2017.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan J, Xia S, Liu X, Lieberman J, Wu H. 2018. Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557: 62–67. 10.1038/s41586-018-0058-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Quade B, Wang H, Sun L, Wang X, Rizo J. 2014. A plug release mechanism for membrane permeation by MLKL. Structure 22: 1489–1500. 10.1016/j.str.2014.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. 2002. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem 277: 9505–9511. 10.1074/jbc.M109488200 [DOI] [PubMed] [Google Scholar]
- Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, et al. 2012. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148: 213–227. 10.1016/j.cell.2011.11.031 [DOI] [PubMed] [Google Scholar]
- Tait SW, Oberst A, Quarato G, Milasta S, Haller M, Wang R, Karvela M, Ichim G, Yatim N, Albert ML, et al. 2013. Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep 5: 878–885. 10.1016/j.celrep.2013.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer MC, Tripaydonis A, Webb AI, Young SN, Varghese LN, Hall C, Alexander WS, Hildebrand JM, Silke J, Murphy JM. 2015. Necroptosis signalling is tuned by phosphorylation of MLKL residues outside the pseudokinase domain activation loop. Biochem J 471: 255–265. 10.1042/BJ20150678 [DOI] [PubMed] [Google Scholar]
- Tanzer MC, Matti I, Hildebrand JM, Young SN, Wardak A, Tripaydonis A, Petrie EJ, Mildenhall AL, Vaux DL, Vince JE, et al. 2016. Evolutionary divergence of the necroptosis effector MLKL. Cell Death Differ 23: 1185–1197. 10.1038/cdd.2015.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa RJ, Basagoudanavar SH, Nogusa S, Irrinki K, Mallilankaraman K, Slifker MJ, Beg AA, Madesh M, Balachandran S. 2011. NF-κB protects cells from gamma interferon-induced RIP1-dependent necroptosis. Mol Cell Biol 31: 2934–2946. 10.1128/MCB.05445-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. 2010. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7: 302–313. 10.1016/j.chom.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. 2012. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11: 290–297. 10.1016/j.chom.2012.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. 2014. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell 54: 133–146. 10.1016/j.molcel.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Wong WW, Vince JE, Lalaoui N, Lawlor KE, Chau D, Bankovacki A, Anderton H, Metcalf D, O'Reilly L, Jost PJ, et al. 2014. cIAPs and XIAP regulate myelopoiesis through cytokine production in an RIPK1- and RIPK3-dependent manner. Blood 123: 2562–2572. 10.1182/blood-2013-06-510743 [DOI] [PubMed] [Google Scholar]
- Wu J, Huang Z, Ren J, Zhang Z, He P, Li Y, Ma J, Chen W, Zhang Y, Zhou X, et al. 2013. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res 23: 994–1006. 10.1038/cr.2013.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia B, Fang S, Chen X, Hu H, Chen P, Wang H, Gao Z. 2016. MLKL forms cation channels. Cell Res 26: 517–528. 10.1038/cr.2016.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Xia S, Hollingsworth LR IV, Wu H. 2019. Mechanism and regulation of gasdermin-mediated cell death. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a036400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Peng W, Yan C, Wu J, Gong X, Shi Y. 2013. Structural insights into RIP3-mediated necroptotic signaling. Cell Rep 5: 70–78. 10.1016/j.celrep.2013.08.044 [DOI] [PubMed] [Google Scholar]
- Yoon S, Bogdanov K, Kovalenko A, Wallach D. 2016. Necroptosis is preceded by nuclear translocation of the signaling proteins that induce it. Cell Death Differ 23: 253–260. 10.1038/cdd.2015.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Kovalenko A, Bogdanov K, Wallach D. 2017. MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity 47: 51–65.e7. 10.1016/j.immuni.2017.06.001 [DOI] [PubMed] [Google Scholar]
- Zargarian S, Shlomovitz I, Erlich Z, Hourizadeh A, Ofir-Birin Y, Croker BA, Regev-Rudzki N, Edry-Botzer L, Gerlic M. 2017. Phosphatidylserine externalization, “necroptotic bodies” release, and phagocytosis during necroptosis. PLoS Biol 15: e2002711 10.1371/journal.pbio.2002711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. 2009. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325: 332–336. 10.1126/science.1172308 [DOI] [PubMed] [Google Scholar]
- Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG. 2012. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci 109: 5322–5327. 10.1073/pnas.1200012109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XM, Chen Z, Zhao JB, Zhang PP, Pu YF, Jiang SH, Hou JJ, Cui YM, Jia XL, Zhang SQ. 2016. Hsp90 modulates the stability of MLKL and is required for TNF-induced necroptosis. Cell Death Dis 7: e2089 10.1038/cddis.2015.390 [DOI] [PMC free article] [PubMed] [Google Scholar]