Abstract
Degenerative retinal disease is the major cause of sight loss in the developed world and currently there is a lack of effective treatments. As the loss of vision is directly the result of the loss of retinal cells, effective cell replacement through stem-cell-based therapies may have the potential to treat a great number of retinal diseases whatever their underlying etiology. The eye is an ideal organ to develop cell therapies as it is immune privileged, and modern surgical techniques enable precise delivery of cells to the retina. Furthermore, a range of noninvasive diagnostic tests and high-resolution imaging techniques facilitate the evaluation of any therapeutic intervention. In this review, we evaluate the progress to date of current cell therapy strategies for retinal repair, focusing on transplantation of pluripotent stem-cell-derived retinal pigment epithelium (RPE) and photoreceptor cells.
The loss of sight has a profound effect on everyday life and it is predicted that by 2020 blindness will afflict 38.5 million people worldwide (Flaxman et al. 2017). Disorders such as age-related macular dystrophy (AMD) and inherited retinal disease are two major causes of severe visual impairment in the developed world, affecting around 15 million people (Resnikoff et al. 2004; Klein 2007). The economic burden associated with vision loss is substantial, and with increasing longevity and the high prevalence of age-related disorders, this is predicted to rise further (Cruess et al. 2008). For advanced degenerative disease, which is characterized by extensive loss of retinal neurons, there are currently no effective treatments. Several therapeutic strategies are currently being explored including optogenetics, electrical prosthetic implants, and, the subject of this review, cell-based therapies.
Optogenetic approaches for the treatment of outer retinal degeneration utilize the remaining retinal cells and circuitry. In general, these approaches aim to convert the secondary or tertiary neurons that remain in the degenerate retina to light-sensing cells through viral vector–mediated gene delivery of light-sensitive proteins, such as the microbial channel rhodopsins. A number of preclinical studies have demonstrated retinal function can be improved via various optogenetic strategies. However, they also indicate the efficacy of these approaches may currently be limited by the low sensitivity or the slow kinetics of the optogenetic molecules used and by limited spatial resolution and retinal processing when secondary or tertiary neurons, rather than photoreceptors, are used as light sensing cells (for review, see Baker and Flannery 2018; Simunovic et al. 2019). A phase I/II clinical trial is currently underway in patients with advanced retinitis pigmentosa (RP) to determine whether some visual function can be restored using a viral vector to deliver a microbial channel rhodopsin to retinal ganglion cells (RGCs) (NCT02556736). An alternative strategy for the treatment of advanced disease is to replace photoreceptors with photodiode arrays. The implantation of these light-sensitive prosthetic devices (e.g., ArgusII, Alpha-IMS, IMI, IRIS, and EPI-RET 3) that generate and pass electrical stimuli to the remaining retinal circuitry have been tested in several clinical trials (Luo and da Cruz 2014). Because of very limited spatial resolution achieved with the current electrical devices, this approach is only suitable for those rare patients who have complete lack of light perception. Nevertheless, trials have demonstrated that such devices are able to restore light perception and basic shape recognition, demonstrating the plasticity of the brain to interpret new input from the retina. Although optogenetic molecules and electrical prostheses are continuing to be refined, considerable improvements are still needed to recreate the range and sensitivity of light responses achieved by the highly evolved cells and circuits of the human retina.
Attempts to develop cell-based therapies have been boosted in the last two decades by the development of pluripotent stem cell (PSC) technologies that have enabled the generation of potentially unlimited quantities of specific human cells from either embryonic stem cell (ESC) or induced pluripotent stem cell (iPSC) sources. Cell-based therapeutic strategies to restore vision following degenerative retinal disease have focused on the replacement of three key retinal cells, RGCs, the retinal pigment epithelium (RPE), and photoreceptors. With the support of the RPE, photoreceptors convert light to neuronal impulses that, via the inner retinal neurons, are sent to the brain by the projection of RGC axons (see Fig. 1 for schematic of retinal circuitry). Given the indispensable role of each of these cells in the visual process, their loss ultimately results in blindness. Conditions such as glaucoma result in blindness through the loss of RGCs but achieving functional connectivity following transplantation of these cells poses a major challenge because their axons make very long connections to the brain. Although impressive progress has been made with regard to RGC transplantation and integration (for review, see Laha et al. 2017; Miltner and La Torre 2019), replacement of RPE and photoreceptors may be more feasible in the near term. In this review, we explore the current cell-based strategies to restore vision through PSC-derived RPE and photoreceptor transplantation.
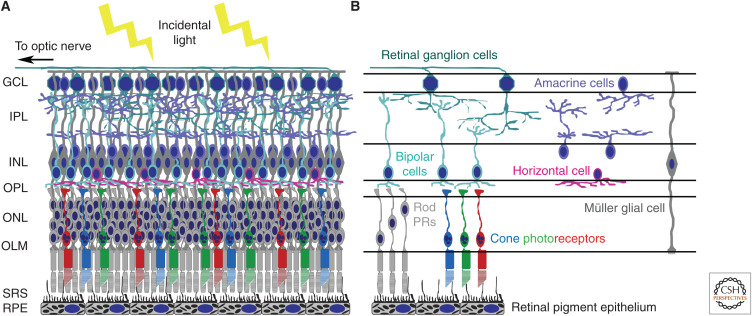
Figure 1.
Structure of the mammalian retina. (A) The retina lines the back of the eye and is a highly organized laminated structure. Supported by the retinal pigment epithelium (RPE) cell monolayer, the neural retina is composed of three layers of cell bodies separated by two plexiform layers that are comprised of the neural processes and synapses. The photoreceptor cell bodies form the outer nuclear layer (ONL), whereas the bipolar, amacrine, horizontal, and Müller glia cells form the inner nuclear layer (INL). The outer plexiform layer (OPL) contains synaptic connections between the photoreceptors, bipolar, and horizontal cells, whereas the inner plexiform layer (IPL) comprises the processes and connections between the retinal ganglion (RGC), bipolar, and amacrine cells. The upper ganglion cell layer (GCL) consists of the RGCs whose axons form the nerve fiber layer and exit the eye through the optic nerve, which projects to the visual structures of the brain. (B) Photoreceptors (PRs), bipolar cells, and ganglion cells form the principal retinal circuit that mediates light stimuli to neural impulses transmitted to the brain. However, the signals are modulated by the other retinal neurons, horizontal and amacrine cells. Müller glial cells span the entire retina providing both structural and metabolic support. The photoreceptors of the mammalian eye can be subdivided in to two different cell types, rods and cones. Rod PRs are the predominant photoreceptor cell type in the human retina and contain the visual pigment rhodopsin. They are sensitive to dim light and are required for scotopic (night) and peripheral vision. In contrast, cone photoreceptors contain cone opsin visual pigment and can be further divided into subtypes dependent on the wavelength of light they detect. Human retinas contain L cones, which detect long wavelengths of light (red light), M cones, which respond to medium wavelengths (green light), and S cones, which detect short wavelengths (blue light). The RPE cells form a monolayer beneath the neural retina, and the proximity of the photoreceptor–RPE complex is essential for both the structure and function of the overlying photoreceptors. Tight junctions between the RPE cells form a physical barrier that prevents the free passage of molecules and ions maintaining the outer blood–retinal barrier. (OLM) Outer limiting membrane, (SRS) subretinal space.
RPE CELL TRANSPLANTATION
The human eye contains approximately 3.5 million RPE cells that form a monolayer between the overlying neural retina and Bruch's membrane (BrM), an acellular structure that separates the RPE from the choroid below. The RPE is essential for maintaining the health of the neural retina and enabling photoreceptor function. Of note, there are relatively few primary disorders of the RPE, given the complexity of this cell and its diverse role (Marmour and Kent 1998), and most of these are recessive monogenetic disorders resulting in deficiency of RPE65, LRAT, MERTK, or Bestrophin (Gu et al. 1997; Veske et al. 1999; D'Cruz et al. 2000; Marmorstein et al. 2000; Thompson et al. 2001; Sun et al. 2002). Despite the relatively few primary RPE dystrophies, there are numerous photoreceptor dystrophies, including Stargardt disease and RP, that result in secondary RPE cell loss. RPE cell dysfunction is also implicated in the pathogenesis of acquired retinal disease such as proliferative vitreoretinopathy (PVR) and AMD (Witmer et al. 2003; Zarbin 2004; Xu et al. 2009), which are characterized by a loss of RPE cells. In such conditions, photoreceptor transplantation alone will fail to rescue visual function and so RPE transplantation has a broader potential role beyond that of treating primary RPE dystrophy.
Preclinical proof-of-concept studies for RPE cell transplantation have been complicated by the lack of appropriate animal models. Although widely used as an animal model for testing RPE transplantation, the Royal College of Surgeons (RCS) rat only provides a model of recessive RP caused by defects in the MerTK gene (D'Cruz et al. 2000). The mutation results in the RPE being unable to phagocytose photoreceptor outer segments and the progressive loss of photoreceptor cells because of a buildup of outer segment debris between the outer nuclear layer (ONL) and the RPE (Bourne et al. 1938; Dowling and Sidman 1962). One limitation of this model for testing RPE transplantation is that because of trophic effects resulting from cell transplantation itself, substantial rescue of photoreceptors can be achieved irrespective of the cell type transplanted (Lawrence et al. 2000; Gamm et al. 2007; Pinilla et al. 2009; Huo et al. 2012). Furthermore, despite the rapid loss of photoreceptors by 2–3 months of age, the RPE monolayer remains intact, even at 1 year (Dowling and Sidman 1962), making this model very dissimilar to most conditions such as to AMD and Stargardt macular dystrophy (SMD), in which the RPE is lost. More conclusive proof-of-concept studies for RPE cell replacement have come from autologous RPE transplants and macular translocation surgeries in AMD patients. These experimental surgeries involve either harvesting a patch of autologous RPE from the periphery of the patient's eye and transplanting it under the macular or repositioning the macular over healthy RPE cells (MacLaren et al. 2007; Treumer et al. 2007; van Romunde et al. 2015; Oshima et al. 2017). Improvements in visual acuity were observed and maintained in some patients. However, because of the complicated surgery involved there has also been a high rate of postoperative complications (MacLaren et al. 2007; Treumer et al. 2007; Parolini et al. 2018; van Romunde et al. 2019). These studies confirm that the replacement of healthy RPE cells, beneath the diseased macular, can, in principle, restore vision.
Clinical Studies Investigating PSC-Derived RPE Transplantation
The RPE was the first human PSC (hPSC)-derived cell type to be transplanted into humans, and since the first trial was initiated in 2011 many more trials have been initiated. This is partly a consequence of the relatively facile differentiation of bone fide RPE cells from PSCs in a highly reproducible manner as well as the ability to generate large-scale quantities of clinical-grade RPE cells (Carr et al. 2009; Idelson et al. 2009; Osakada et al. 2009; Vaajasaari et al. 2011). Trials were also facilitated by the previous development of advanced surgical techniques and imaging modalities, such as optical coherence tomography (OCT), which enable the transplanted RPE cells to be imaged in situ following transplantation.
The first trial using human embryonic stem cell (hESC)-derived RPE was initiated in 2011 by Schwartz and colleagues. In this FDA-approved, phase I/II trial patients with advanced forms of either Stargardt disease or the atrophic form (dry) of AMD received hESC-derived RPE cells transplanted to the subretinal space (Schwartz et al. 2012). The hESC-derived RPE cells were delivered as a cell suspension in escalating dose cohorts of 50,000, 100,000, and 150,000 cells, with each cohort consisting of three patients. The primary aim of the trial was the assessment of safety and tolerability, and no major adverse events were reported. Although minor improvements in best-corrected visual acuity (BCVA) were reported for 6/14 subjects that completed the 12-month follow-up, this was not correlated with the number of cells transplanted and there was no clear evidence of efficacy. A further follow-up at 22 months confirmed no major adverse events (Schwartz et al. 2012, 2015, 2016). A further trial transplanting suspensions of hESC-derived RPE cells into patients with dry AMD and Stargardt disease also reported no serious safety issues after a 12-month follow-up of four patients (Song et al. 2015). More recently, the first European trial using hESC-derived RPE for macular repair in patients with advanced Stargardt disease was reported (Mehat et al. 2018). This involved escalating dose cohorts of 50,000 100,000, 150,000, and 200,000 hESC-derived RPE cells transplanted into a total of 12 patients with detailed retinal sensitivity mapping pre- and posttransplantation. In this study, focal areas of hyperpigmentation were reported for all patients in a dose-dependent manner. No serious adverse events were observed and no uncontrolled proliferation or inflammatory responses were identified. Borderline improvements in visual acuity and retinal sensitivity were unsustained or matched by similar improvements in the untreated eye. In one of the patients that received the highest dose of cells, localized thinning and reduced sensitivity in the area of hyperpigmentation was reported, suggesting the potential for harm with the highest dose. An extended follow-up is necessary to determine whether there are any effects on the progression of degeneration following hESC-derived RPE transplantation in these patients.
An alternative strategy to the use of cell suspensions is the transplantation of hPSC-derived RPE sheets, with or without artificial scaffolds or membranes. Preclinical studies have suggested that RPE cell survival and function are better maintained following the transplantation of sheets of RPE cells, compared with RPE cell suspensions (Tezel and Del Priore 1997; Diniz et al. 2013). Another concern is the rejection of the allogeneic RPE cell graft, because although the subretinal space has a level of immune privilege, healthy RPE cells have a role in maintaining this. Therefore, in diseases that affect the RPE cells and compromise the outer blood–retinal barrier, acute immune rejection of allogenic cells is a possibility. As the majority of adverse responses in the initial hESC-derived RPE safety trial were the result of long-term immunosuppression, possibly exacerbated by the aged patient population, the use of allografts or major human leukocyte antigen (HLA)-matched hPSC-derived tissue would alleviate these concerns. To this end, researchers in Japan developed autologous iPSC-derived RPE cell sheets to determine the feasibility of generating individual autologous grafts for patients. Initial preclinical studies in monkeys, transplanting iPSC-derived RPE cell sheets held together by their extracellular deposits and without the use of immunosuppression, were encouraging (Kamao et al. 2014). In 2014, two patients with exudative (wet) AMD were enrolled in the trial; however, only one received an autologous transplant before the trial was suspended, because of a change in Japan's regenerative medicine law. No serious adverse events were observed throughout the 4-year follow-up. Although the RPE cell sheet appeared intact and the macula showed signs of recovery, no improvement in visual acuity was reported (Mandai et al. 2017b; Takagi et al. 2019). The iPSC line generated from the second patient was found to contain three aberrations and it was decided not to transplant the autologous hPSC-derived RPE cell graft into the second patient, partly as a safety precaution because of the unknown effects of the mutations and partly because of the patient responding to anti-VEGF treatment (Mandai et al. 2017b). It was subsequently concluded that the cost and complexity involved in generating patient-specific iPSC-derived tissue for transplantation was prohibitive for larger scale trials. Therefore, further trials will involve the use of major HLA-matched iPSC lines, available from iPSC banks that have been validated for genomic stability; this may facilitate efficient and affordable generation of HLA-matched hiPSC-derived RPE tissue for transplantation (Nakatsuji et al. 2008; Okita 2011). Although the presence of minor histocompatibility antigens may trigger rejection in humans, preclinical trials in monkeys with major histocompatibility complex (MHC)-matched iPSC-derived RPE sheets without immunosuppression have been encouraging (Sugita et al. 2016).
Other ongoing trials using sheets of hESC-derived RPE cells are investigating the use of artificial scaffolds and support membranes. As changes to BrM can occur as a result of both age and disease, this may reduce its capacity for RPE cell attachment and have adverse effects on both transplanted RPE cell structure and survival (Gullapalli et al. 2004, 2005). The use of hPSC-derived RPE cell sheets with artificial scaffolds or membranes may be able to prevent the transplanted RPE cells coming into contact with the native BrM and maintain the integrity of the cell sheet. Two clinical trials, one in the United Kingdom using polyester membranes and one in the United States using ultrathin parylene membranes, are currently ongoing to investigate the use of nondegradable membranes to immobilize the RPE cells and replace the existing basement membrane (Lu et al. 2012; Carr et al. 2013). The UK group recently published data from their preclinical studies and the 12-month follow-up for the first two patients with severe exudative AMD (da Cruz et al. 2018). This initial safety study also investigated whether the transplanted RPE cell sheet could be sustained with the use of long-term local immunosuppression in the form of an intraocular steroid implant. No severe adverse events were observed in this initial 12-month follow-up and the transplanted RPE sheet was still apparent by OCT imaging, suggesting that, so far, local immunosuppression was sufficient to maintain the allograft. Both patients demonstrated sustained improvements in visual acuity. However, 50% of patients with severe exudative AMD recover vision following surgery to remove membranes, and without enrolling many more patients it is impossible to know whether the improvement was the result of the transplanted RPE cell sheet (da Cruz et al. 2018). Similarly, the U.S. study has published a preliminary report on the first five patients enrolled in the phase 1/2a study to test the safety and potential efficacy of a composite implant in patients with advanced, nonneovascular AMD (Kashani et al. 2018). So far, one out of the five patients had a marginal improvement in visual acuity, with the remaining patients maintaining vision and demonstrating the short-term safety of this approach. Further follow-up and enrollment of up to 20 patients will help determine the continued safety and efficacy of RPE sheet studies. The use of degradable poly(lactic-co-glycolic acid) (PLGA) scaffolds as a temporary substrate to improve RPE cell sheet attachment to the existing BrM is also being investigated, prior to clinical trial (Liu et al. 2014; Song and Bharti 2016).
Combined, these initial safety studies are reassuring and support the potential of PSCs as a source for RPE cell therapy applications. Further follow-up will confirm the long-term safety of this approach, and as more trials investigate the use of various scaffold and membrane materials, as well as iPSC-derived RPE cells, this will help to determine the optimal strategy for RPE transplantation. However, the replacement of RPE cells alone will not be sufficient to improve or reverse visual impairment if the photoreceptor cells have been lost, which is the case in advanced AMD and Stargardt disease. As such, a therapy for end-stage retinal degeneration involving the RPE would also require the transplantation of photoreceptor cells.
PHOTORECEPTOR CELL TRANSPLANTATION
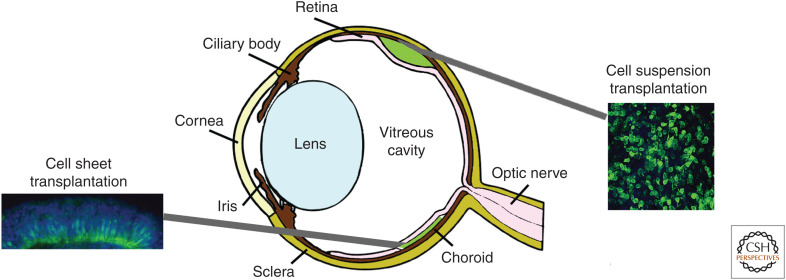
Once photoreceptors are lost, they do not regenerate. However, the rest of the retinal circuitry remains, even in late stages of disease, albeit synaptically remodeled (Marc and Jones 2003; Strettoi et al. 2003; Jones and Marc 2005; Jones et al. 2012). As photoreceptors are afferent neurons, they need only make a single short-range synaptic connection to the host interneurons to restore the visual circuit. There are two main strategies for cell transplantation to the retina, the injection of a suspension of single retinal cells or the transplantation of a sheet of cells into the subretinal space (see Fig. 2).
Figure 2.
Strategies for retinal cell replacement. There are two main strategies for cell transplantation to the retina, the injection of a suspension of single retinal cells and the transplantation of a sheet of cells into the subretinal space (SRS). Both approaches position cells between the host retina and the host retinal pigment epithelium (RPE) cell layer.
The Transplantation of Donor-Derived Cell Suspensions
Early studies in the 1990s, transplanting postnatal photoreceptors to the degenerate retina, reported the survival and maturation of these cells in the subretinal space for up to 3 months (Gouras et al. 1991a,b,c; Kwan et al. 1999). Our own studies in 2006 examined the optimal developmental stage of the transplanted donor population in more detail. We found that whereas the transplantation of GFP+ retinal progenitors resulted in the formation of rosettes in the subretinal space, the transplantation of early GFP+ postnatal cells (∼P1-7) resulted in the presence of GFP+ photoreceptors correctly located within the host ONL, following transplantation in both the developing and adult retina (MacLaren et al. 2006). By utilizing a transgenic Nrl.GFP+/+ mouse, which expresses GFP only in postmitotic rod cells, we demonstrated that this donor population resulted in the correctly located GFP+ photoreceptors within the host retina; these findings have been replicated by a number of other studies (MacLaren et al. 2006; Bartsch et al. 2008; West et al. 2008, 2010; Lakowski et al. 2010). To determine whether photoreceptor precursor transplantation could restore visual function, studies in GNAT1−/− mice, a model of stationary night blindness, were performed. The photoreceptors in this model lack rod α-transducin and are therefore nonfunctional; the photoreceptors, however, remain intact. Following photoreceptor precursor transplantation to the subretinal space, GFP+ photoreceptors were observed in the host expressing rod α-transducin (Pearson et al. 2012). Improved visual function was demonstrated at both the cellular and behavioral level, as assessed with dim light levels, and was indicative of a rod-mediated light response (Pearson et al. 2012). Other studies have also demonstrated the expression of photoreceptor-specific proteins that are lacking in host cells, but present in GFP+-labeled photoreceptors in the recipient ONL following precursor transplantation (MacLaren et al. 2006; Bartsch et al. 2008; Lakowski et al. 2010; Barber et al. 2013).
Until recently, it was thought that transplanted GFP+ photoreceptor precursor cells migrated into the host ONL, where they integrated with the host retinal circuitry and formed outer segments that protruded into the subretinal space (MacLaren et al. 2006; Warre-Cornish et al. 2014). However, this interpretation of the results was revised by two studies in 2016. We and others independently verified that the majority of the GFP+ photoreceptors present in the recipient retina were in fact host cells that had exchanged either protein and/or RNA material with transplanted donor photoreceptors (see Fig. 3; Pearson et al. 2016; Santos-Ferreira et al. 2016a). The mechanism by which this process occurs is still unclear, as is the exact type of material transferred between cells. It is known that this is not a classic cell-fusion process and it appears restricted to the transplanted photoreceptor cell population, as GFP+ fibroblasts, retinal and neural progenitor cells have not resulted in the same transfer of GFP reporter (West et al. 2012; Pearson et al. 2016). Neither has the presence of recombinant GFP, when injected directly into the subretinal space, suggesting it is not the uptake of free protein (Pearson et al. 2016). Material transfer appears to be a transient process that allows for the exchange of a range of proteins and/or RNA expressed by the donor cell population, including rhodopsin, Peripherin-2, and rod α-transducin (MacLaren et al. 2006; Pearson et al. 2012, 2016; Barber et al. 2013). In light of these findings, previously reported improvements in visual responses were most likely achieved by the transfer of functional levels of the phototransduction proteins missing in host photoreceptors, rendering them light-responsive (Pearson et al. 2012; Santos-Ferreira et al. 2015; Neves et al. 2016; Zhu et al. 2016). Further studies have corroborated the findings of cytoplasmic material exchange following photoreceptor precursor transplantation to the intact host retina and demonstrated that it is a developmentally regulated phenomenon (Singh et al. 2016; Decembrini et al. 2017; Ortin-Martinez et al. 2017; Waldron et al. 2018). Importantly, a minority (∼1% ± 0.8%) of GFP+-labeled cells were identified as truly integrated donor-derived cells, by the use of Y chromosome FISH to identify male donor photoreceptors in female wild-type recipient retinas (Pearson et al. 2016; Waldron et al. 2018). Further investigation has demonstrated that the host retinal environment effects the proportion of true cellular integration events, as well as the extent of material exchange. In models with disrupted outer limiting membranes (OLMs), the proportion of true Y+/GFP+ donor photoreceptors in the female host ONL were significantly increased (∼23% ± 3%) compared to models with intact OLMs (∼6% ± 1%) (Waldron et al. 2018). This confirms previous findings that OLM integrity influences transplantation outcome and that it may be possible to improve true levels of integration by manipulation of the host retinal environment (West et al. 2008, 2009; Pearson et al. 2010; Barber et al. 2013).
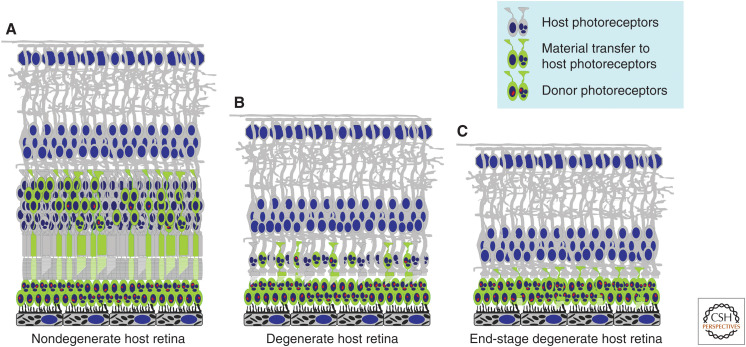
Figure 3.
Schematic representation of photoreceptor cell transplantation into various models of retinal disease. Photoreceptor cell transplantation into (A) nondegenerate retina or into retina at early stages of degeneration can result in transfer of donor RNA and/or protein to host photoreceptors (material transfer) in addition to donor photoreceptor integration. Even in degenerate models that rapidly lose photoreceptors (B), cone photoreceptors can remain for extended periods. Following photoreceptor cell transplantation into these models, the presence of remaining host photoreceptors can be a confounding factor that complicates the interpretation of any therapeutic effects observed, either because of material transfer from the donor photoreceptor population or residual function from surviving host cells. In end-stage retinal disease (C), in which there are few remaining host photoreceptors, it is possible to make rigorous assessments of whether donor photoreceptors make functional connections with the remaining retinal circuitry. However, additional barriers to the integration of donor photoreceptors are present in this stage of retinal disease, such as increased gliotic scarring and inner retinal remodeling.
The Transplantation of Mouse PSC (mPSC)-Derived Retinal Cell Suspensions
Although the majority of investigations have transplanted murine donor-derived photoreceptor precursors, the equivalent developmental stage in humans would require the use of second-trimester fetal retinal tissue, which is ethically challenging, of limited availability, and logistically difficult to source. Despite the reinterpretation of transplantation results because of the discovery of material transfer, the postmitotic photoreceptor precursor is still the optimal donor stage, and the use of PSCs as a source of photoreceptors for cell replacement remains the ideal choice for future clinical translation. Therefore, protocols to derive photoreceptors from mouse and hPSCs have been widely investigated.
Initial studies using mouse ESCs (mESCs) established the stepwise differentiation of rostral neuronal progenitors and RAX/Pax6-positive retinal progenitors using serum-free floating embryoid body (SFEB) cultures (Ikeda et al. 2005; Watanabe et al. 2005). Further defined conditions were developed based on this method for human, mouse, and monkey ESCs and used to generate rhodopsin positive rod photoreceptors in adherent cultures (Osakada et al. 2008). Our own investigations utilized this method for mESCs; however, despite the presence of some photoreceptor markers, the levels of Nrl transcript were relatively low, and following transplantation typical GFP+ photoreceptors present in the ONL were not observed. Even with the reinterpretation of these results as a lack of material transfer to the host photoreceptors, the findings remain the same, that the differentiated retinal cells were not equivalent to donor-derived postnatal retinal cell populations (West et al. 2012).
In 2011, a landmark study by Sasai and colleagues was the first to demonstrate three-dimensional morphological recapitulation of retinal developmental processes from mESCs. This protocol demonstrated the ability of the retinal neuroepithelium to form spontaneously from SFEB cultures and suggests that the physical forces and signaling required for optic vesicle invagination to form the bilayered optic cup are intrinsic to the neuroepithelium (Eiraku et al. 2011, 2012; Sasai et al. 2012). In addition, they reported the interkinetic nuclear migration of dividing retinal progenitors and the production of all retinal cell types in a time-dependent manner, resulting in a laminated retina-like structure. However, these structures lost integrity by 35 days and photoreceptor maturation and segment formation were not reported (Eiraku et al. 2011). We adapted this method to enable neural retinal epithelia to be further cultured without optic cup dissection, thus providing sufficient photoreceptor precursor cells for transplantation studies (Gonzalez-Cordero et al. 2013). In addition, we determined that Rho.GFP+ ESC-derived photoreceptors expressed genes enriched in P4 donor-derived rod precursors by microarray gene expression profiling. Following the transplantation of Rho.GFP+ photoreceptors into GNAT−/− mice, GFP+/rod α-transducin+ photoreceptors were observed in the host ONL. The proportion of true integration events, relative to the material transfer of GFP and rod α-transducin to host photoreceptor cells, was undetermined in this study. However, it is clear that the msESC-derived photoreceptors generated by 3D retinal differentiation methods were equivalent to donor-derived photoreceptor precursors (Gonzalez-Cordero et al. 2013). Further to this, other studies have observed similar results following the transplantation of msESC-derived photoreceptor precursors (Decembrini et al. 2014; Lakowski et al. 2015; Santos-Ferreira et al. 2016b; Waldron et al. 2018).
Transplantation of hPSC-Derived Neuroretinal Cell Suspensions
In parallel to the investigation of differentiation methods for mPSCs, a number of protocols have been established to differentiate hPSCs into retinal cell lineages (Lamba et al. 2006; Osakada et al. 2008, 2009; Meyer et al. 2009, 2011; Nakano et al. 2012). Lamba and colleagues (2006) reported one of the first hPSC-derived retinal differentiation methods, whereby, following a brief suspension culture to stimulate neural induction, cells were plated onto Matrigel and further cultured for 3 weeks to give 80% retinal progenitors, of which 12% were Crx+ photoreceptor precursors. Of note, this protocol generated human cells within a relatively short time frame, compared to normal retinal development and other methods of hPSC differentiation (Osakada et al. 2008; Meyer et al. 2009). Following the transplantation of hPSC-derived retinal cultures, GFP+-labeled cells were observed throughout the host retina (Lamba et al. 2009, 2010). It seems likely that these GFP+-labeled cells were the result of cytoplasmic material transfer rather than true human photoreceptor cell integration. The electroretinography (ERG) recordings from treated animals did not have normal waveforms and appeared to be recording artefacts. Furthermore, recent transplantation studies into nonhuman primates did not result in any reporter-labeled photoreceptors within the host retina (Chao et al. 2017).
Studies by Meyer and colleagues determined that retinal differentiation could be achieved by the endogenous secretion of DKK-1, Noggin, and FGF following a combination of suspension and adherent cell culture conditions. Progression through all the major stages of retinogenesis were required for the complete differentiation of retinal progeny and in a timeframe that reflected normal human development (Meyer et al. 2009, 2011). Furthermore, studies by the Sasai group established a 3D protocol for hPSCs that, as in the mPSC method, self-organized to form stratified neural retina-like structures that contained all retinal cell types (Nakano et al. 2012). In a later study, they developed a stepwise induction-reversal method that resulted in the formation of RPE adjacent to the neural retina, and using this method they determined the presence of a ciliary margin-like growth zone at the RPE-neural retinal boundary (Kuwahara et al. 2015). Although the efficiency with which hPSC-derived neural retinal epithelia were produced was far less than in mPSC cultures, this method improved the long-term maintenance of continuous retinal progenitor cell sheets that could be utilized for transplantation (Kuwahara et al. 2015; Shirai et al. 2016; Iraha et al. 2018; Tu et al. 2019). In light of these pioneering studies, the majority of established retinal differentiation methods since then have been focused on generating 3D neural retinal structures, in a time frame consistent with human retinal development.
Subsequent studies based on the Meyer differentiation method, and incorporating 3D culture techniques, determined the 3D retinogenesis of all retinal cell types from self-organizing optic vesicle-like aggregates. They demonstrated for the first time the long-term (up to 180 days) culture and maturation of human photoreceptor cells that exhibited a few primitive outer segment-like structures (Phillips et al. 2012; Zhong et al. 2014). In addition, perforated-patch recordings determined that a small proportion of hPSC-derived photoreceptors (2/13) were responsive to light (Zhong et al. 2014). Another study has also demonstrated the formation of rudimentary outer segment-like structures by adaptations to the Sasai method (Wahlin et al. 2017). However, it remains to be determined whether more developed outer segment-like structures can be formed without direct contact with the RPE.
All retinal differentiation protocols described thus far have relied on the generation of embryoid bodies or cell clump suspensions to initiate neural differentiation, and the presence of Matrigel or serum. Reichman and colleagues developed a method that bypassed this step and instead allowed optic vesicle-like neuroectoderm to form spontaneously in confluent hiPSC cultures, by the removal of FGF2 and the endogenous presence of Dkk-1 and Noggin (Reichman et al. 2014). Serum-free proneural medium was used to culture the developing vesicles for 2 weeks, before they were isolated from adherent cultures and maintained in suspension as 3D retinal neuroepithelia, similar to other protocols (Nakano et al. 2012; Phillips et al. 2012; Zhong et al. 2014). These neural retina-like structures maintained laminated organization until 21 days in culture, whereby they developed rosettes with the photoreceptors positioned toward the center of the structure. The timing of photoreceptor cell marker expression was similar to that observed by other methods of retinal differentiation and typical of normal human development, with rhodopsin and cone opsin protein present by day 112 of culture (Osakada et al. 2008; Nakano et al. 2012; Phillips et al. 2012; Reichman et al. 2014). The benefits of this method for hPSC-derived differentiation are the ease and scalability with which neural retina-like structures can be generated. We therefore utilized this approach to generate photoreceptors for transplantation studies. Optic vesicle–like structures were isolated by manual dissection at 4–7 weeks of adherent culture and maintained as 3D neural retinal vesicles, similar to other studies (Zhong et al. 2014). Detailed analysis of photoreceptor development in hPSC-derived vesicles found that it recapitulated human fetal development (Gonzalez-Cordero et al. 2017). In addition, lamination of the retinal neuroepithelium was observed, with brush-like protrusions demonstrating primitive and disorganized membranous discs at 28 weeks, similar to those observed in other long-term cultures (Zhong et al. 2014; Wahlin et al. 2017).
Having established rod and cone photoreceptor development in hPSC-derived retinal neuroepithelia, virally labeled M/L opsin cone precursors were selected from 17-week cultures and transplanted into adult mouse models. The hPSC-derived cone precursors survived in the subretinal space for at least 3 weeks, with a minority of GFP+/hNUCLEI+ cells (55 ± 38 cells) present within the Nrl−/− host retina, demonstrating correctly orientated bud-like protrusions positive for M/L cone opsin. Importantly, as well as the entire nucleus labeling positive for hNUCLEI, the size of nuclei was significantly greater than in the host retina, suggesting true incorporation of hPSC-derived photoreceptors. However, a small number of GFP+ cells were negative for the hNUCLEI marker (30 ± 34 cells), positive for a Y chromosome probe (male host), and had murine Nrl−/− typical photoreceptor morphology and nuclear size (Gonzalez-Cordero et al. 2017). These cells could reflect carryover of viral particles used to label the PSC-derived cones, although this is unlikely, as following transplantation into the wild-type host retina, very few GFP+ cells were observed (2 ± 3 cells). As previously mentioned, it has been reported that true murine donor-derived photoreceptor cell integration and material transfer to host photoreceptors are increased in the Nrl−/− retina (Waldron et al. 2018). These findings suggest that hPSC-derived photoreceptor precursors may also be able to engage in material transfer with host murine photoreceptors, following transplantation to the subretinal space. Therefore, the presence of human-specific proteins may not be sufficient to identify hPSC-derived photoreceptor cells following transplantation. Thus, to ascertain whether there is restoration of vision because of the connection of hPSC-derived photoreceptors with the host retina, it is necessary to utilize end-stage models of retinal degeneration, with no remaining endogenous photoreceptors. In addition, the host retinal environment present in late-stage retinal degeneration models may be more in keeping with potential clinical applications that are likely to involve transplantation in regions of the retina with very few remaining photoreceptors.
Rescuing End-Stage Retinal Degeneration
An established model of end-stage retinal degeneration is the PDEβrd1/rd1 mouse model (known as rd1), which is homozygous for a null mutation in the gene encoding the β subunit of rod photoreceptor cyclic GMP phosphodiesterase (Pde6b). This results in rapid rod photoreceptor cell loss by 3 weeks of age and an absence of residual cone photoreceptor function by 12 weeks (Chang et al. 2007; Barber et al. 2013). The rd1 mouse model has been utilized for many decades. However, recently, we reported the presence of an additional mutation in the gpr179 gene, found in C3H/HeN mice, the most commonly used rd1 mouse strain (Nishiguchi et al. 2015). This naturally occurring mutation results in nonfunctional ON-bipolar cells, including rod bipolar cells. Gene supplementation therapy, using AAV vectors, was shown to rescue visual responses in the rd1 model, but only after backcrossing the line to remove the mutation from the C3H/HeN background (Nishiguchi et al. 2015). Further characterization of multiple C3H substrains has determined that the presence of this additional mutation was confined to the C3H/HeN mouse strain (Chang 2015). However, transplantation studies have been performed in this model using P3-4 mouse donor-derived photoreceptors, with the suggestion of improved light-mediated visual responses, as shown by a variety of tests including light/dark chamber tests, pupillary reflex, and cortical imaging (Singh et al. 2013). As the majority of transplanted cells are rod photoreceptors and rod bipolar cell function is abolished in this strain, it would be interesting to know whether functional restoration is occurring through atypical synaptic connections formed with cone bipolar cells. It is known that in late-stage degeneration there is retinal remodeling (Marc and Jones 2003). Alternatively, the presence of any remaining endogenous cone photoreceptors must be considered when interpreting the functional restoration data, even under scotopic light conditions (Cehajic-Kapetanovic et al. 2015).
Further studies using the rd1 mouse model have investigated the transplantation of hPSC-derived photoreceptors into the degenerate retina (Barnea-Cramer et al. 2016; Collin et al. 2019). Both studies demonstrated the survival of hPSC-derived photoreceptors and the expression of photoreceptor-specific proteins, indicative of further photoreceptor maturation in vivo. Transplanted cells resided next to the host inner retina and the presence of some synaptic markers was demonstrated, in close proximity to hPSC-derived photoreceptor processes (Barnea-Cramer et al. 2016; Collin et al. 2019). In addition, improved optomotor responses in transplanted eyes were reported in the Barnea-Cramer study, suggesting some restoration of light-mediated responses. However, the possibility of neurotrophic effects as a result of the transplanted cells preserving residual cones could not be excluded (Barnea-Cramer et al. 2016).
Our own studies investigating PSC-derived photoreceptor transplantation to the degenerate retina have focused on the severely degenerate Aipl1−/− mouse model of Leber congenital amaurosis (LCA4). This model is deficient in the photoreceptor-specific gene encoding aryl hydrocarbon receptor–interacting protein-like 1 (Aipl1), resulting in the rapid degeneration of both rod and cone photoreceptors by 4 weeks of age, because of the destabilization of cGMP PDE (Ramamurthy et al. 2004; Kirschman et al. 2010). Initial studies investigated the transplantation of mESC-derived cone photoreceptor precursors into 8- to 12-wk-old Aipl1−/− mice. Because human daylight vision relies on cone photoreceptor function, we adapted our previously described protocol to enable sufficient numbers of mESC-derived cone photoreceptor precursors to be generated and isolated (Kruczek et al. 2017). Transplanted mESC-derived cones appeared to make contact with host inner retinal neurons and expressed components associated with synaptic transmission alongside phototransduction related proteins, suggesting advanced differentiation and maturation (Kruczek et al. 2017). Similar results were obtained following the transplantation of isolated hPSC-derived cone photoreceptor precursors in this model (Gonzalez-Cordero et al. 2017). Transplanted hPSC-derived cone photoreceptors were able to survive in the degenerate retina, even without immunosuppression, for 3 weeks posttransplantation and formed a distinct layer adjacent to the host inner retina. The presynaptic protein ribeye was present in hPSC-derived cone photoreceptor processes that extended toward host retinal neurons, alongside the protrusion of bud-like structures that contained human mitochondria and the presence of phototransduction-related proteins throughout the cells. A key difference between transplanted mESC-derived and hPSC-derived cone photoreceptors was the increased presence of Peripherin-2-positive buds in the former cells compared to the latter (Gonzalez-Cordero et al. 2017; Kruczek et al. 2017). This most likely reflects the increased time required for human cone cell maturation compared with mouse cells and indicates that time periods posttransplantation need to be set accordingly. Although some hPSC-derived cone photoreceptors were still present 6 weeks posttransplantation, this would most likely be improved and prolonged with the use of immunosuppression. It remains to be determined whether PSC-derived cone photoreceptor precursors are able to functionally mature and connect with the recipient degenerate retina, enabling the restoration of light-mediated responses in this model.
A further rod and cone degenerate model that has been established is the Cpfl 1/Rho−/− mouse, which has no functional photoreceptors and a rapid degeneration of both rods and cones, with one row of photoreceptor cell bodies remaining at 10–12 weeks of age (Santos-Ferreira et al. 2016b). The transplantation of mESC-derived rod photoreceptors into this degenerate model demonstrated the survival of cells and the presence of rod-specific phototransduction proteins, suggesting photoreceptor maturation in vivo. However, reduced contact between transplanted rod photoreceptors and host inner retinal neurons was reported, as well as relatively few examples of presynaptic markers, ribeye and pikachurin, in the mESC-derived photoreceptor cell processes (Santos-Ferreira et al. 2016b). It remains to be determined whether the differences observed in this study compared to our own reflect differential abilities of rod and cone photoreceptor precursors to form presumptive connections with host retinal neurons, or if the models of advanced retinal degeneration used differ in terms of the remodeling of inner retinal circuitry that occurs with rapid photoreceptor degeneration (Santos-Ferreira et al. 2016b; Kruczek et al. 2017). Further studies in late-stage models of retinal degeneration are required to determine whether transplanted photoreceptor precursors are able to restore light-mediated responses to the recipient retina. Furthermore, the use of various degenerate models will establish by what means the host retinal environment influences functional restoration and how this will translate to the human degenerate retina.
Retinal Sheet Transplantation
An alternative strategy to the transplantation of retinal cell suspensions is retinal sheet transplantation, whereby an organized sheet of fetal retina or PSC-derived retinal organoid is dissected and transplanted as a unit into the subretinal space. A potential advantage of this approach is the improved organization of grafted cells, although the presence of other retinal neurons may limit host interneuron connectivity with the grafted photoreceptors. Transplantation of an organized structure, which can include an RPE layer, may also be beneficial to long-term cell survival and maturation, especially at later stages of retinal degeneration (Seiler and Aramant 2012; Seiler et al. 2016; Lorach et al. 2019). Studies involving the use of fetal retinal sheets have previously demonstrated photoreceptor cell survival and maturation in the subretinal space and several clinical trials have been performed with no adverse effects reported (Kaplan et al. 1997; Radtke et al. 1999, 2004, 2008; Humayun et al. 2000; Berger et al. 2003). Because of the limited availability and ethical implications of using human fetal tissue, many groups are now investigating the use of hPSC-derived retinal sheets, using 3D retinal organoid culture methods.
Preclinical studies have been performed with mPSC-derived retinal progenitor cell sheets being transplanted into the end-stage retinal degeneration rd1 mouse model (Assawachananont et al. 2014; Mandai et al. 2017a). Following transplantation, mPSC-derived retinal sheets developed into laminated retina-like structures in the subretinal space, containing both inner and outer retinal cell layers. However, in some regions of the mPSC-derived graft, the inner retinal cells were absent, resulting in direct contact between the host interneurons and the donor photoreceptors. Ultrastructural analysis of the mPSC-derived photoreceptors demonstrated the ability of these cells to mature in vivo forming typical inner and outer segments, either in rosette-like structures or in close contact with the host RPE (Assawachananont et al. 2014). Some indications of light-mediated responses in the retina were detected by ex vivo microelectroretinography (mERG) and RGC recordings, using a multielectrode array (MEA) system, in the vicinity of the retinal graft. In addition, light-mediated behavioral responses were observed in a proportion (9/21) of the treated animals (Mandai et al. 2017a). A further study from the same group has shown similar histological results following the transplantation of hESC-derived retinal sheets, at an equivalent retinal progenitor stage (∼60 days), into an immune-deficient rd1 mouse model (NOG-rd1-2J) (Iraha et al. 2018). The transplanted hESC-derived retinal grafts demonstrated photoreceptor maturation with inner and outer segment formation and an indication of synaptic contact in some areas where the hESC-derived photoreceptors were in direct contact with the host inner retina. However, light-mediated responses were only detected in 3/7 retinas with substantial hESC-derived transplants, by RGC recordings only, compared to all retinas that received mPSC-derived transplants (Iraha et al. 2018). Although in both studies the MEA responses were greatest in animals that received transplants and in the area of the graft, it is not possible to exclude the possibility that the transplants improved the function of residual cone photoreceptors or melanopsin-positive ganglion cells through trophic effects.
In another study, day 50–60 hPSC-derived retinal sheets were transplanted into an immune-deficient end-stage retinal degeneration rat model (SD-Foxn1 Tg(S334ter)3LavRrrc), as well as a laser-induced photoreceptor degeneration monkey model (Tu et al. 2019). Histological analysis confirmed the development of mature photoreceptors in both models and regions of direct hPSC-derived photoreceptor and host bipolar cell interaction, with the hPSC-derived retinal grafts surviving for 10 months in immunocompromised rats and >2 years in the monkey model with cyclosporine immunosuppression. A modest recovery of light perception was suggested by the visual field test, associated with the grafted area, in the induced monkey model of photoreceptor degeneration. However, light-mediated responses could not be confirmed to originate from the grafted tissue by other functional examinations. In addition, SD-Foxn1 Tg(S334ter)3LavRrrc rats had substantial light responses remaining in the host retina, even at 10 months. This resulted in difficulty in conclusively distinguishing the hPSC-derived graft-originated responses from the residual sensitivity of the recipient retina in this model (Tu et al. 2019). Other studies have also utilized this rat model to examine hESC-derived retinal sheet transplantation and reported electrophysiological recordings in the superior colliculus, as well as improvements in visual acuity based on optokinetic head tracking (OKT) (McLelland et al. 2018). However, it has been reported that the use of OKT in a number of degenerative transgenic rat lines may be limited as a therapeutic outcome measure (McGill et al. 2012). These studies highlight the difficulties in confirming that the light-mediated responses originate from the donor photoreceptors rather than residual endogenous photoreceptors and also highlight the need for multiple testing methodologies to establish the restoration of light-mediated responses at multiple levels of visual function.
Similar to the strategies previously mentioned for RPE cell sheets, a number of studies have investigated the use of various scaffold biomaterials with which to transplant retinal progenitor cells, to increase cell survival, improve cell organization, and promote host–graft cell interactions (Young 2005; Redenti et al. 2009; Tucker et al. 2010; Kundu et al. 2014; Lawley et al. 2015; Yao et al. 2015; Park et al. 2019). In addition, 3D micropatterned polymers have been designed that could act as polarized delivery substrates to enable the transplantation of retinal progenitors or photoreceptor precursors in an organized array, potentially improving donor–host connectivity in models of end-stage retinal degeneration (Worthington et al. 2017; Jung et al. 2018; Thompson et al. 2019). If an ideal biodegradable or porous material can be found that can maintain orientated photoreceptor precursors and position them in close apposition to the degenerate host retina, this may provide the advantages of pure photoreceptor cell suspensions with the organization of a retinal sheet.
Considerations for the Clinical Application of Photoreceptor Cell Transplantation
As photoreceptor cell transplantation progresses toward the clinic, there are a number of key considerations. The first is the optimal cell population to transplant that will result in the replacement of functional photoreceptors with the ability to connect with the recipient retinal circuitry. This differs depending on whether retinal cell sheets or retinal cell suspensions are the preferred strategy. For retinal cell sheets, earlier stages of development, ∼day 60 of hPSC-derived retinal neuroepithelia, appear optimal when the majority of cells are retinal progenitors (Shirai et al. 2016). For retinal cell suspensions, despite the reinterpretation of earlier findings because of cytoplasmic material transfer, the optimal cell population is still considered to be postmitotic photoreceptor precursors, once photoreceptor cell fate has been specified but before the development of structures that reduce cell viability upon dissociation (Gust and Reh 2011; Pearson et al. 2016; Waldron et al. 2018). To enable the selection and isolation of a defined population of photoreceptor precursors for clinical use, a number of studies have investigated the presence of cell surface antigens, CD markers (Koso et al. 2009; Lakowski et al. 2011, 2015, 2018; Kaewkhaw et al. 2015; Welby et al. 2017). Furthermore, many studies have demonstrated the transplantation of enriched photoreceptor precursors from donor and PSC sources, using CD73 marker selection, into various retinal models (Eberle et al. 2011, 2012, 2014; Lakowski et al. 2011, 2015, 2018; Santos-Ferreira et al. 2015; Gagliardi et al. 2018). However, the restoration of daylight vision may be of more use to patients; therefore, the isolation and transplantation of cone photoreceptor precursors may be preferable to rods. Although it was initially assumed that cone precursors had a reduced capacity to integrate into the adult rod-rich murine retina, we now know this was because of material transfer to host photoreceptors and not necessarily an indication of true integration (Lakowski et al. 2010; Santos-Ferreira et al. 2015; Smiley et al. 2016; Waldron et al. 2018). It remains to be determined whether there are any differences in the capacity of rod or cone photoreceptor precursors to integrate into the recipient retina; however, CD markers that enable the enrichment of cone photoreceptor precursors have been studied (Welby et al. 2017).
In addition to selecting the desired cell population for transplantation, the removal of proliferative cells may be of equal benefit, as a safety measure to prevent tumor formation that can arise from the incomplete differentiation of unselected cell populations (West et al. 2012). Importantly, cell populations need to be well-defined and -characterized, as the transplantation of unknown populations can have devastating effects in patients (Kuriyan et al. 2017). With advances in transcriptomics, more data is available from developing retinal tissue with which to compare PSC-derived cell populations (Mustafi et al. 2016; Aldiri et al. 2017; Hoshino et al. 2017; Mellough et al. 2019). This helps to refine the stage of development of PSC-derived cells, as well as confirming the differentiation of bona fide photoreceptors that should be able to function in response to light once fully developed.
A second consideration is the requirement for a robust, clinically relevant differentiation method with which to generate the desired retinal cell population. The adaptation of retinal differentiation research methods to more GMP-like processes is ongoing and this has been helped by the increasing availability of defined xeno-free reagents and small molecules (Osakada et al. 2009; Sridhar et al. 2013; Tucker et al. 2013; Wiley et al. 2016; Reichman et al. 2017; Slembrouck-Brec et al. 2018). In addition, several studies have examined methods to scale up production of retinal cell types using bioreactors (DiStefano et al. 2018; Ovando-Roche et al. 2018). Because of the length of manufacturing processes involved, generating sufficiently high yields of defined and pure photoreceptor precursor populations at the same stage of development is challenging and methods have been explored to enable batches of retinal neuroepithelia to be cryopreserved and stored prior to use (Nakano et al. 2012; Reichman et al. 2017; Gagliardi et al. 2018; Slembrouck-Brec et al. 2018). This would enable multiple batches of retinal epithelia to be pooled, as well as the storage of retinal sheets prior to use. However, optimal methods to freeze the final purified photoreceptor precursor cell product have yet to be defined.
Another consideration is the host retinal environment, as the subretinal space may not be immune privileged in some diseased states, and immunosuppression may be required if allografts are used (Streilein et al. 2002). Further studies in degenerate recipient retinas are required to ascertain the long-term survival and function of transplanted photoreceptors. Clinical trials using RPE cells are currently investigating the use of local immunosuppression methods, such as intraocular steroid implants, and the results from these studies will inform further trials for retinal cell therapy (da Cruz et al. 2018). Alternatively, the use of HLA-matched iPSC-derived cells and tissue may negate the need for long-term immune modulation in patients (Kamao et al. 2017). Planned clinical studies using HLA-matched iPSC-derived RPE sheets will again inform future strategies for photoreceptor cell transplantation. However, early trials will most likely utilize both hESC and hiPSC lines, as banks of validated and genetically screened iPSC lines are produced. In addition, further preclinical studies will provide more information as to the extent of photoreceptor restoration in severely degenerate retinas and the optimal delivery method, either cell suspensions or sheets, required for the optimal recovery of light-mediated responses, which may vary with disease phenotype. Finally, a combined approach to replace both the photoreceptors and the supportive RPE cells will be required in certain degenerative retinal diseases, such as AMD and SMD.
CONCLUDING REMARKS
Major advances in the field of regenerative cell therapy in recent years have resulted in the rapid progression of differentiation methods to generate retinal cell types from hPSC sources, as well as the refinement and improvement of techniques and models to detect functional responses from these cells once transplanted. Because of its external location, the advanced imaging techniques, and the functional tests available, the eye remains at the forefront of regenerative medicine strategies. Ongoing clinical trials are establishing the safety and efficacy of RPE cell therapy, and preclinical advances over the last decade have opened the way to trials of hPSC-derived photoreceptor cell transplantation in the next few years. Combined, these strategies may eventually provide the potential to restore sight in many blinding conditions that currently have no therapeutic options.
Footnotes
Editors: Cristina Lo Celso, Kristy Red-Horse, and Fiona M. Watt
Additional Perspectives on Stem Cells: From Biological Principles to Regenerative Medicine available at www.cshperspectives.org
REFERENCES
- Aldiri I, Xu B, Wang L, Chen X, Hiler D, Griffiths L, Valentine M, Shirinifard A, Thiagarajan S, Sablauer A, et al. 2017. The dynamic epigenetic landscape of the retina during development, reprogramming, and tumorigenesis. Neuron 94: 550–568.e10. 10.1016/j.neuron.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assawachananont J, Mandai M, Okamoto S, Yamada C, Eiraku M, Yonemura S, Sasai Y, Takahashi M. 2014. Transplantation of embryonic and induced pluripotent stem cell–derived 3D retinal sheets into retinal degenerative mice. Stem Cell Reports 2: 662–674. 10.1016/j.stemcr.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CK, Flannery JG. 2018. Innovative optogenetic strategies for vision restoration. Front Cell Neurosci 12: 316 10.3389/fncel.2018.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AC, Hippert C, Duran Y, West EL, Bainbridge JW, Warre-Cornish K, Luhmann UF, Lakowski J, Sowden JC, Ali RR, et al. 2013. Repair of the degenerate retina by photoreceptor transplantation. Proc Natl Acad Sci 110: 354–359. 10.1073/pnas.1212677110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Cramer AO, Wang W, Lu SJ, Singh MS, Luo C, Huo H, McClements ME, Barnard AR, MacLaren RE, Lanza R. 2016. Function of human pluripotent stem cell–derived photoreceptor progenitors in blind mice. Sci Rep 6: 29784 10.1038/srep29784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch U, Oriyakhel W, Kenna PF, Linke S, Richard G, Petrowitz B, Humphries P, Farrar GJ, Ader M. 2008. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res 86: 691–700. 10.1016/j.exer.2008.01.018 [DOI] [PubMed] [Google Scholar]
- Berger AS, Tezel TH, Del Priore LV, Kaplan HJ. 2003. Photoreceptor transplantation in retinitis pigmentosa: short-term follow-up. Ophthalmology 110: 383–391. 10.1016/S0161-6420(02)01738-4 [DOI] [PubMed] [Google Scholar]
- Bourne MC, Campbell DA, Tansley K. 1938. Hereditary degeneration of the rat retina. Br J Ophthalmol 22: 613–623. 10.1136/bjo.22.10.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AJ, Vugler A, Lawrence J, Chen LL, Ahmado A, Chen FK, Semo M, Gias C, da Cruz L, Moore HD, et al. 2009. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell–derived RPE cells using a novel human retinal assay. Mol Vis 15: 283–295. [PMC free article] [PubMed] [Google Scholar]
- Carr AJ, Smart MJ, Ramsden CM, Powner MB, da Cruz L, Coffey PJ. 2013. Development of human embryonic stem cell therapies for age-related macular degeneration. Trends Neurosci 36: 385–395. 10.1016/j.tins.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Cehajic-Kapetanovic J, Eleftheriou C, Allen AE, Milosavljevic N, Pienaar A, Bedford R, Davis KE, Bishop PN, Lucas RJ. 2015. Restoration of vision with ectopic expression of human rod opsin. Curr Biol 25: 2111–2122. 10.1016/j.cub.2015.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B. 2015. Survey of the nob5 mutation in C3H substrains. Mol Vis 21: 1101–1105. [PMC free article] [PubMed] [Google Scholar]
- Chang B, Hawes NL, Pardue MT, German AM, Hurd RE, Davisson MT, Nusinowitz S, Rengarajan K, Boyd AP, Sidney SS, et al. 2007. Two mouse retinal degenerations caused by missense mutations in the β-subunit of rod cGMP phosphodiesterase gene. Vision Res 47: 624–633. 10.1016/j.visres.2006.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao JR, Lamba DA, Klesert TR, Torre A, Hoshino A, Taylor RJ, Jayabalu A, Engel AL, Khuu TH, Wang RK, et al. 2017. Transplantation of human embryonic stem cell–derived retinal cells into the subretinal space of a non-human primate. Transl Vis Sci Technol 6: 4 10.1167/tvst.6.3.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin J, Zerti D, Queen R, Santos-Ferreira T, Bauer R, Coxhead J, Hussain R, Steel D, Mellough C, Ader M, et al. 2019. CRX expression in pluripotent stem cell–derived photoreceptors marks a transplantable subpopulation of early cones. Stem Cells 37: 609–622. 10.1002/stem.2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruess AF, Zlateva G, Xu X, Soubrane G, Pauleikhoff D, Lotery A, Mones J, Buggage R, Schaefer C, Knight T, et al. 2008. Economic burden of bilateral neovascular age-related macular degeneration: multi-country observational study. Pharmacoeconomics 26: 57–73. 10.2165/00019053-200826010-00006 [DOI] [PubMed] [Google Scholar]
- da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, Vernon A, Daniels JT, Nommiste B, Hasan SM, et al. 2018. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol 36: 328–337. 10.1038/nbt.4114 [DOI] [PubMed] [Google Scholar]
- D'Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. 2000. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet 9: 645–651. 10.1093/hmg/9.4.645 [DOI] [PubMed] [Google Scholar]
- Decembrini S, Koch U, Radtke F, Moulin A, Arsenijevic Y. 2014. Derivation of traceable and transplantable photoreceptors from mouse embryonic stem cells. Stem Cell Reports 2: 853–865. 10.1016/j.stemcr.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decembrini S, Martin C, Sennlaub F, Chemtob S, Biel M, Samardzija M, Moulin A, Behar-Cohen F, Arsenijevic Y. 2017. Cone genesis tracing by the Chrnb4-EGFP mouse line: evidences of cellular material fusion after cone precursor transplantation. Mol Ther 25: 634–653. 10.1016/j.ymthe.2016.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz B, Thomas P, Thomas B, Ribeiro R, Hu Y, Brant R, Ahuja A, Zhu D, Liu L, Koss M, et al. 2013. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Invest Ophthalmol Vis Sci 54: 5087–5096. 10.1167/iovs.12-11239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano T, Chen HY, Panebianco C, Kaya KD, Brooks MJ, Gieser L, Morgan NY, Pohida T, Swaroop A. 2018. Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors. Stem Cell Reports 10: 300–313. 10.1016/j.stemcr.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE, Sidman RL. 1962. Inherited retinal dystrophy in the rat. J Cell Biol 14: 73–109. 10.1083/jcb.14.1.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle D, Schubert S, Postel K, Corbeil D, Ader M. 2011. Increased integration of transplanted CD73-positive photoreceptor precursors into adult mouse retina. Invest Ophthalmol Vis Sci 52: 6462–6471. 10.1167/iovs.11-7399 [DOI] [PubMed] [Google Scholar]
- Eberle D, Kurth T, Santos-Ferreira T, Wilson J, Corbeil D, Ader M. 2012. Outer segment formation of transplanted photoreceptor precursor cells. PLOS ONE 7: e46305 10.1371/journal.pone.0046305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle D, Santos-Ferreira T, Grahl S, Ader M. 2014. Subretinal transplantation of MACS purified photoreceptor precursor cells into the adult mouse retina. J Vis Exp 84: e50932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. 2011. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472: 51–56. 10.1038/nature09941 [DOI] [PubMed] [Google Scholar]
- Eiraku M, Adachi T, Sasai Y. 2012. Relaxation-expansion model for self-driven retinal morphogenesis: a hypothesis from the perspective of biosystems dynamics at the multi-cellular level. Bioessays 34: 17–25. 10.1002/bies.201100070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, et al. 2017. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health 5: e1221–e1234. 10.1016/S2214-109X(17)30393-5 [DOI] [PubMed] [Google Scholar]
- Gagliardi G, Ben M'Barek K, Chaffiol A, Slembrouck-Brec A, Conart JB, Nanteau C, Rabesandratana O, Sahel JA, Duebel J, Orieux G, et al. 2018. Characterization and transplantation of CD73-positive photoreceptors isolated from human iPSC-derived retinal organoids. Stem Cell Reports 11: 665–680. 10.1016/j.stemcr.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamm DM, Wang S, Lu B, Girman S, Holmes T, Bischoff N, Shearer RL, Sauvé Y, Capowski E, Svendsen CN, et al. 2007. Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS ONE 2: e338 10.1371/journal.pone.0000338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cordero A, West EL, Pearson RA, Duran Y, Carvalho LS, Chu CJ, Naeem A, Blackford SJI, Georgiadis A, Lakowski J, et al. 2013. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol 31: 741–747. 10.1038/nbt.2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cordero A, Kruczek K, Naeem A, Fernando M, Kloc M, Ribeiro J, Goh D, Duran Y, Blackford SJI, Abelleira-Hervas L, et al. 2017. Recapitulation of human retinal development from human pluripotent stem cells generates transplantable populations of cone photoreceptors. Stem Cell Reports 9: 820–837. 10.1016/j.stemcr.2017.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P, Du J, Gelanze M, Kwun R, Kjeldbye H, Lopez R. 1991a. Transplantation of photoreceptors labeled with tritiated thymidine into RCS rats. Invest Ophthalmol Vis Sci 32: 1704–1707. [PubMed] [Google Scholar]
- Gouras P, Du J, Gelanze M, Lopez R, Kwun R, Kjeldbye H, Krebs W. 1991b. Survival and synapse formation of transplanted rat rods. J Neural Transplant Plast 2: 91–100. 10.1155/NP.1991.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P, Du J, Kjeldbye H, Kwun R, Lopez R, Zack DJ. 1991c. Transplanted photoreceptors identified in dystrophic mouse retina by a transgenic reporter gene. Invest Ophthalmol Vis Sci 32: 3167–3174. [PubMed] [Google Scholar]
- Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, et al. 1997. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet 17: 194–197. 10.1038/ng1097-194 [DOI] [PubMed] [Google Scholar]
- Gullapalli VK, Sugino IK, Van Patten Y, Shah S, Zarbin MA. 2004. Retinal pigment epithelium resurfacing of aged submacular human Bruch's membrane. Trans Am Ophthalmol Soc 102: 123–138. [PMC free article] [PubMed] [Google Scholar]
- Gullapalli VK, Sugino IK, Van Patten Y, Shah S, Zarbin MA. 2005. Impaired RPE survival on aged submacular human Bruch's membrane. Exp Eye Res 80: 235–248. 10.1016/j.exer.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Gust J, Reh TA. 2011. Adult donor rod photoreceptors integrate into the mature mouse retina. Invest Ophthalmol Vis Sci 52: 5266–5272. 10.1167/iovs.10-6329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Ratnapriya R, Brooks MJ, Chaitankar V, Wilken MS, Zhang C, Starostik MR, Gieser L, La Torre A, Nishio M, et al. 2017. Molecular anatomy of the developing human retina. Dev Cell 43: 763–779.e4. 10.1016/j.devcel.2017.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humayun MS, de Juan E Jr, del Cerro M, Dagnelie G, Radner W, Sadda SR, del Cerro C. 2000. Human neural retinal transplantation. Invest Ophthalmol Vis Sci 41: 3100–3106. [PubMed] [Google Scholar]
- Huo SJ, Li YC, Xie J, Li Y, Raisman G, Zeng YX, He JR, Weng CH, Yin ZQ. 2012. Transplanted olfactory ensheathing cells reduce retinal degeneration in Royal College of Surgeons rats. Curr Eye Res 37: 749–758. 10.3109/02713683.2012.697972 [DOI] [PubMed] [Google Scholar]
- Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N, Khaner H, Smith Y, Wiser O, Gropp M, et al. 2009. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell 5: 396–408. 10.1016/j.stem.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Ikeda H, Osakada F, Watanabe K, Mizuseki K, Haraguchi T, Miyoshi H, Kamiya D, Honda Y, Sasai N, Yoshimura N, et al. 2005. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci 102: 11331–11336. 10.1073/pnas.0500010102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraha S, Tu HY, Yamasaki S, Kagawa T, Goto M, Takahashi R, Watanabe T, Sugita S, Yonemura S, Sunagawa GA, et al. 2018. Establishment of immunodeficient retinal degeneration model mice and functional maturation of human ESC-derived retinal sheets after transplantation. Stem Cell Reports 10: 1059–1074. 10.1016/j.stemcr.2018.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Marc RE. 2005. Retinal remodeling during retinal degeneration. Exp Eye Res 81: 123–137. 10.1016/j.exer.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Jones BW, Kondo M, Terasaki H, Lin Y, McCall M, Marc RE. 2012. Retinal remodeling. Jpn J Ophthalmol 56: 289–306. 10.1007/s10384-012-0147-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YH, Phillips MJ, Lee J, Xie R, Ludwig AL, Chen G, Zheng Q, Kim TJ, Zhang H, Barney P, et al. 2018. 3D microstructured scaffolds to support photoreceptor polarization and maturation. Adv Mater 30: e1803550 10.1002/adma.201803550 [DOI] [PubMed] [Google Scholar]
- Kaewkhaw R, Kaya KD, Brooks M, Homma K, Zou J, Chaitankar V, Rao M, Swaroop A. 2015. Transcriptome dynamics of developing photoreceptors in three-dimensional retina cultures recapitulates temporal sequence of human cone and rod differentiation revealing cell surface markers and gene networks. Stem Cells 33: 3504–3518. 10.1002/stem.2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, Kiryu J, Takahashi M. 2014. Characterization of human induced pluripotent stem cell–derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports 2: 205–218. 10.1016/j.stemcr.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamao H, Mandai M, Ohashi W, Hirami Y, Kurimoto Y, Kiryu J, Takahashi M. 2017. Evaluation of the surgical device and procedure for extracellular matrix-scaffold-supported human iPSC-derived retinal pigment epithelium cell sheet transplantation. Invest Ophthalmol Vis Sci 58: 211–220. 10.1167/iovs.16-19778 [DOI] [PubMed] [Google Scholar]
- Kaplan HJ, Tezel TH, Berger AS, Wolf ML, Del Priore LV. 1997. Human photoreceptor transplantation in retinitis pigmentosa. A safety study. Arch Ophthalmol 115: 1168–1172. 10.1001/archopht.1997.01100160338012 [DOI] [PubMed] [Google Scholar]
- Kashani AH, Lebkowski JS, Rahhal FM, Avery RL, Salehi-Had H, Dang W, Lin CM, Mitra D, Zhu D, Thomas BB, et al. 2018. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med 10: eaao4097 10.1126/scitranslmed.aao4097 [DOI] [PubMed] [Google Scholar]
- Kirschman LT, Kolandaivelu S, Frederick JM, Dang L, Goldberg AF, Baehr W, Ramamurthy V. 2010. The Leber congenital amaurosis protein, AIPL1, is needed for the viability and functioning of cone photoreceptor cells. Hum Mol Genet 19: 1076–1087. 10.1093/hmg/ddp571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. 2007. Overview of progress in the epidemiology of age-related macular degeneration. Ophthalmic Epidemiol 14: 184–187. 10.1080/09286580701344381 [DOI] [PubMed] [Google Scholar]
- Koso H, Minami C, Tabata Y, Inoue M, Sasaki E, Satoh S, Watanabe S. 2009. CD73, a novel cell surface antigen that characterizes retinal photoreceptor precursor cells. Invest Ophthalmol Vis Sci 50: 5411–5418. 10.1167/iovs.08-3246 [DOI] [PubMed] [Google Scholar]
- Kruczek K, Gonzalez-Cordero A, Goh D, Naeem A, Jonikas M, Blackford SJI, Kloc M, Duran Y, Georgiadis A, Sampson RD, et al. 2017. Differentiation and transplantation of embryonic stem cell–derived cone photoreceptors into a mouse model of end-stage retinal degeneration. Stem Cell Reports 8: 1659–1674. 10.1016/j.stemcr.2017.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu J, Michaelson A, Baranov P, Young MJ, Carrier RL. 2014. Approaches to cell delivery: substrates and scaffolds for cell therapy. Dev Ophthalmol 53: 143–154. 10.1159/000357369 [DOI] [PubMed] [Google Scholar]
- Kuriyan AE, Albini TA, Townsend JH, Rodriguez M, Pandya HK, Leonard RE 2nd, Parrott MB, Rosenfeld PJ, Flynn HW Jr, Goldberg JL. 2017. Vision loss after intravitreal injection of autologous “stem cells” for AMD. N Engl J Med 376: 1047–1053. 10.1056/NEJMoa1609583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara A, Ozone C, Nakano T, Saito K, Eiraku M, Sasai Y. 2015. Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nat Commun 6: 6286 10.1038/ncomms7286 [DOI] [PubMed] [Google Scholar]
- Kwan AS, Wang S, Lund RD. 1999. Photoreceptor layer reconstruction in a rodent model of retinal degeneration. Exp Neurol 159: 21–33. 10.1006/exnr.1999.7157 [DOI] [PubMed] [Google Scholar]
- Laha B, Stafford BK, Huberman AD. 2017. Regenerating optic pathways from the eye to the brain. Science 356: 1031–1034. 10.1126/science.aal5060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski J, Baron M, Bainbridge J, Barber AC, Pearson RA, Ali RR, Sowden JC. 2010. Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells. Hum Mol Genet 19: 4545–4559. 10.1093/hmg/ddq378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski J, Han YT, Pearson RA, Gonzalez-Cordero A, West EL, Gualdoni S, Barber AC, Hubank M, Ali RR, Sowden JC. 2011. Effective transplantation of photoreceptor precursor cells selected via cell surface antigen expression. Stem Cells 29: 1391–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski J, Gonzalez-Cordero A, West EL, Han YT, Welby E, Naeem A, Blackford SJ, Bainbridge JW, Pearson RA, Ali RR, et al. 2015. Transplantation of photoreceptor precursors isolated via a cell surface biomarker panel from embryonic stem cell–derived self-forming retina. Stem Cells 33: 2469–2482. 10.1002/stem.2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski J, Welby E, Budinger D, Di Marco F, Di Foggia V, Bainbridge JWB, Wallace K, Gamm DM, Ali RR, Sowden JC. 2018. Isolation of human photoreceptor precursors via a cell surface marker panel from stem cell–derived retinal organoids and fetal retinae. Stem Cells 36: 709–722. 10.1002/stem.2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Ware CB, Reh TA. 2006. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci 103: 12769–12774. 10.1073/pnas.0601990103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, Gust J, Reh TA. 2009. Transplantation of human embryonic stem cell–derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell 4: 73–79. 10.1016/j.stem.2008.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. 2010. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS ONE 5: e8763 10.1371/journal.pone.0008763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley E, Baranov P, Young M. 2015. Hybrid vitronectin-mimicking polycaprolactone scaffolds for human retinal progenitor cell differentiation and transplantation. J Biomater Appl 29: 894–902. 10.1177/0885328214547751 [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Sauve Y, Keegan DJ, Coffey PJ, Hetherington L, Girman S, Whiteley SJ, Kwan AS, Pheby T, Lund RD. 2000. Schwann cell grafting into the retina of the dystrophic RCS rat limits functional deterioration. Royal College of Surgeons. Invest Ophthalmol Vis Sci 41: 518–528. [PubMed] [Google Scholar]
- Liu Z, Yu N, Holz FG, Yang F, Stanzel BV. 2014. Enhancement of retinal pigment epithelial culture characteristics and subretinal space tolerance of scaffolds with 200 nm fiber topography. Biomaterials 35: 2837–2850. 10.1016/j.biomaterials.2013.12.069 [DOI] [PubMed] [Google Scholar]
- Lorach H, Kang S, Bhuckory MB, Trouillet A, Dalal R, Marmor M, Palanker D. 2019. Transplantation of mature photoreceptors in rodents with retinal degeneration. Transl Vis Sci Technol 8: 30 10.1167/tvst.8.3.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Zhu D, Hinton D, Humayun MS, Tai YC. 2012. Mesh-supported submicron parylene-C membranes for culturing retinal pigment epithelial cells. Biomed Microdevices 14: 659–667. 10.1007/s10544-012-9645-8 [DOI] [PubMed] [Google Scholar]
- Luo YH, da Cruz L. 2014. A review and update on the current status of retinal prostheses (bionic eye). Br Med Bull 109: 31–44. 10.1093/bmb/ldu002 [DOI] [PubMed] [Google Scholar]
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. 2006. Retinal repair by transplantation of photoreceptor precursors. Nature 444: 203–207. 10.1038/nature05161 [DOI] [PubMed] [Google Scholar]
- MacLaren RE, Uppal GS, Balaggan KS, Tufail A, Munro PM, Milliken AB, Ali RR, Rubin GS, Aylward GW, da Cruz L. 2007. Autologous transplantation of the retinal pigment epithelium and choroid in the treatment of neovascular age-related macular degeneration. Ophthalmology 114: 561–570.e2. 10.1016/j.ophtha.2006.06.049 [DOI] [PubMed] [Google Scholar]
- Mandai M, Fujii M, Hashiguchi T, Sunagawa GA, Ito SI, Sun J, Kaneko J, Sho J, Yamada C, Takahashi M. 2017a. iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice. Stem Cell Reports 8: 69–83. 10.1016/j.stemcr.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, et al. 2017b. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med 376: 1038–1046. 10.1056/NEJMoa1608368 [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW. 2003. Retinal remodeling in inherited photoreceptor degenerations. Mol Neurobiol 28: 139–147. 10.1385/MN:28:2:139 [DOI] [PubMed] [Google Scholar]
- Marmorstein AD, Marmorstein LY, Rayborn M, Wang X, Hollyfield JG, Petrukhin K. 2000. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci 97: 12758–12763. 10.1073/pnas.220402097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmour MF, Kent KW. 1998. Dystrophies of the retinal pigment epithelium. In The retinal pigment epithelium (ed. Marmour MF, Wolfensberger TJ). Oxford University Press, Oxford. [Google Scholar]
- McGill TJ, Prusky GT, Douglas RM, Yasumura D, Matthes MT, Lowe RJ, Duncan JL, Yang H, Ahern K, Daniello KM, et al. 2012. Discordant anatomical, electrophysiological, and visual behavioral profiles of retinal degeneration in rat models of retinal degenerative disease. Invest Ophthalmol Vis Sci 53: 6232–6244. 10.1167/iovs.12-9569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland BT, Lin B, Mathur A, Aramant RB, Thomas BB, Nistor G, Keirstead HS, Seiler MJ. 2018. Transplanted hESC-derived retina organoid sheets differentiate, integrate, and improve visual function in retinal degenerate rats. Invest Ophthalmol Vis Sci 59: 2586–2603. 10.1167/iovs.17-23646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehat MS, Sundaram V, Ripamonti C, Robson AG, Smith AJ, Borooah S, Robinson M, Rosenthal AN, Innes W, Weleber RG, et al. 2018. Transplantation of human embryonic stem cell–derived retinal pigment epithelial cells in macular degeneration. Ophthalmology 125: 1765–1775. 10.1016/j.ophtha.2018.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellough CB, Bauer R, Collin J, Dorgau B, Zerti D, Dolan DWP, Jones CM, Izuogu OG, Yu M, Hallam D, et al. 2019. An integrated transcriptional analysis of the developing human retina. Development 146: dev169474 10.1242/dev.169474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, Zhang SC, Gamm DM. 2009. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci 106: 16698–16703. 10.1073/pnas.0905245106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, Pinilla I, Martin JM, Tian S, Stewart R, et al. 2011. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells 29: 1206–1218. 10.1002/stem.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner AM, La Torre A. 2019. Retinal ganglion cell replacement: current status and challenges ahead. Dev Dyn 248: 118–128. 10.1002/dvdy.24672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafi D, Kevany BM, Bai X, Golczak M, Adams MD, Wynshaw-Boris A, Palczewski K. 2016. Transcriptome analysis reveals rod/cone photoreceptor specific signatures across mammalian retinas. Hum Mol Genet 25: 4376–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y. 2012. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10: 771–785. 10.1016/j.stem.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Nakatsuji N, Nakajima F, Tokunaga K. 2008. HLA-haplotype banking and iPS cells. Nat Biotechnol 26: 739–740. 10.1038/nbt0708-739 [DOI] [PubMed] [Google Scholar]
- Neves J, Zhu J, Sousa-Victor P, Konjikusic M, Riley R, Chew S, Qi Y, Jasper H, Lamba DA. 2016. Immune modulation by MANF promotes tissue repair and regenerative success in the retina. Science 353: aaf3646 10.1126/science.aaf3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi KM, Carvalho LS, Rizzi M, Powell K, Holthaus SM, Azam SA, Duran Y, Ribeiro J, Luhmann UF, Bainbridge JW, et al. 2015. Gene therapy restores vision in rd1 mice after removal of a confounding mutation in Gpr179. Nat Commun 6: 6006 10.1038/ncomms7006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K. 2011. iPS cells for transplantation. Curr Opin Organ Transplant 16: 96–100. 10.1097/MOT.0b013e32834252a2 [DOI] [PubMed] [Google Scholar]
- Ortin-Martinez A, Tsai EL, Nickerson PE, Bergeret M, Lu Y, Smiley S, Comanita L, Wallace VA. 2017. A reinterpretation of cell transplantation: GFP transfer from donor to host photoreceptors. Stem Cells 35: 932–939. 10.1002/stem.2552 [DOI] [PubMed] [Google Scholar]
- Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M. 2008. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol 26: 215–224. 10.1038/nbt1384 [DOI] [PubMed] [Google Scholar]
- Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, Sasai Y, Takahashi M. 2009. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci 122: 3169–3179. 10.1242/jcs.050393 [DOI] [PubMed] [Google Scholar]
- Oshima H, Iwase T, Ishikawa K, Yamamoto K, Terasaki H. 2017. Long-term results after limited macular translocation surgery for wet age-related macular degeneration. PLoS ONE 12: e0177241 10.1371/journal.pone.0177241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovando-Roche P, West EL, Branch MJ, Sampson RD, Fernando M, Munro P, Georgiadis A, Rizzi M, Kloc M, Naeem A, et al. 2018. Use of bioreactors for culturing human retinal organoids improves photoreceptor yields. Stem Cell Res Ther 9: 156 10.1186/s13287-018-0907-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Baranov P, Aydin A, Abdelgawad H, Singh D, Niu W, Kurisawa M, Spector M, Young MJ. 2019. In situ cross-linking hydrogel as a vehicle for retinal progenitor cell transplantation. Cell Transplant 28: 596–606. 10.1177/0963689719825614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolini B, Di Salvatore A, Pinackatt SJ, Baldi A, Besozzi G, Finzi A, Cardillo D, Sallam AB, Frisina R. 2018. Long-term results of autologous retinal pigment epithelium and choroid transplantation for the treatment of exudative and atrophic maculopathies. Retina 10.1097/IAE.0000000000002429 [DOI] [PubMed] [Google Scholar]
- Pearson RA, Barber AC, West EL, MacLaren RE, Duran Y, Bainbridge JW, Sowden JC, Ali RR. 2010. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant 19: 487–503. 10.3727/096368909X486057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, West EL, Duran Y, Smith AJ, Chuang JZ, Azam SA, et al. 2012. Restoration of vision after transplantation of photoreceptors. Nature 485: 99–103. 10.1038/nature10997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Gonzalez-Cordero A, West EL, Ribeiro JR, Aghaizu N, Goh D, Sampson RD, Georgiadis A, Waldron PV, Duran Y, et al. 2016. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat Commun 7: 13029 10.1038/ncomms13029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MJ, Wallace KA, Dickerson SJ, Miller MJ, Verhoeven AD, Martin JM, Wright LS, Shen W, Capowski EE, Percin EF, et al. 2012. Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Invest Ophthalmol Vis Sci 53: 2007–2019. 10.1167/iovs.11-9313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinilla I, Cuenca N, Martinez-Navarrete G, Lund RD, Sauvé Y. 2009. Intraretinal processing following photoreceptor rescue by non-retinal cells. Vision Res 49: 2067–2077. 10.1016/j.visres.2009.05.014 [DOI] [PubMed] [Google Scholar]
- Radtke ND, Aramant RB, Seiler M, Petry HM. 1999. Preliminary report: indications of improved visual function after retinal sheet transplantation in retinitis pigmentosa patients. Am J Ophthalmol 128: 384–387. 10.1016/S0002-9394(99)00250-0 [DOI] [PubMed] [Google Scholar]
- Radtke ND, Aramant RB, Seiler MJ, Petry HM, Pidwell D. 2004. Vision change after sheet transplant of fetal retina with retinal pigment epithelium to a patient with retinitis pigmentosa. Arch Ophthalmol 122: 1159–1165. 10.1001/archopht.122.8.1159 [DOI] [PubMed] [Google Scholar]
- Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ. 2008. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol 146: 172–182.e1. 10.1016/j.ajo.2008.04.009 [DOI] [PubMed] [Google Scholar]
- Ramamurthy V, Niemi GA, Reh TA, Hurley JB. 2004. Leber congenital amaurosis linked to AIPL1: a mouse model reveals destabilization of cGMP phosphodiesterase. Proc Natl Acad Sci 101: 13897–13902. 10.1073/pnas.0404197101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redenti S, Neeley WL, Rompani S, Saigal S, Yang J, Klassen H, Langer R, Young MJ. 2009. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials 30: 3405–3414. 10.1016/j.biomaterials.2009.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman S, Terray A, Slembrouck A, Nanteau C, Orieux G, Habeler W, Nandrot EF, Sahel JA, Monville C, Goureau O. 2014. From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc Natl Acad Sci 111: 8518–8523. 10.1073/pnas.1324212111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman S, Slembrouck A, Gagliardi G, Chaffiol A, Terray A, Nanteau C, Potey A, Belle M, Rabesandratana O, Duebel J, et al. 2017. Generation of storable retinal organoids and retinal pigmented epithelium from adherent human iPS cells in xeno-free and feeder-free conditions. Stem Cells 35: 1176–1188. 10.1002/stem.2586 [DOI] [PubMed] [Google Scholar]
- Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. 2004. Global data on visual impairment in the year 2002. Bull World Health Organ 82: 844–851. [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira T, Postel K, Stutzki H, Kurth T, Zeck G, Ader M. 2015. Daylight vision repair by cell transplantation. Stem Cells 33: 79–90. 10.1002/stem.1824 [DOI] [PubMed] [Google Scholar]
- Santos-Ferreira T, Llonch S, Borsch O, Postel K, Haas J, Ader M. 2016a. Retinal transplantation of photoreceptors results in donor–host cytoplasmic exchange. Nat Commun 7: 13028 10.1038/ncomms13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira T, Völkner M, Borsch O, Haas J, Cimalla P, Vasudevan P, Carmeliet P, Corbeil D, Michalakis S, Koch E, et al. 2016b. Stem cell–derived photoreceptor transplants differentially integrate into mouse models of cone-rod dystrophy. Invest Ophthalmol Vis Sci 57: 3509–3520. 10.1167/iovs.16-19087 [DOI] [PubMed] [Google Scholar]
- Sasai Y, Eiraku M, Suga H. 2012. In vitro organogenesis in three dimensions: self-organising stem cells. Development 139: 4111–4121. 10.1242/dev.079590 [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. 2012. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379: 713–720. 10.1016/S0140-6736(12)60028-2 [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, et al. 2015. Human embryonic stem cell–derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385: 509–516. 10.1016/S0140-6736(14)61376-3 [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Tan G, Hosseini H, Nagiel A. 2016. Subretinal transplantation of embryonic stem cell–derived retinal pigment epithelium for the treatment of macular degeneration: an assessment at 4 years. Invest Ophthalmol Vis Sci 57: ORSFc1–ORSFc9. 10.1167/iovs.15-18681 [DOI] [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB. 2012. Cell replacement and visual restoration by retinal sheet transplants. Prog Retin Eye Res 31: 661–687. 10.1016/j.preteyeres.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, McLelland BT, Mathur A, Lin R, Alexander DT, Patel PN, Cummings B, Aramant RB. 2016. Human retinal progenitor sheet transplants in immunodeficient retinal degenerate (RD) rats. Invest Ophth Vis Sci 57: 3737. [Google Scholar]
- Shirai H, Mandai M, Matsushita K, Kuwahara A, Yonemura S, Nakano T, Assawachananont J, Kimura T, Saito K, Terasaki H, et al. 2016. Transplantation of human embryonic stem cell–derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci 113: E81–E90. 10.1073/pnas.1512590113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simunovic MP, Shen W, Lin JY, Protti DA, Lisowski L, Gillies MC. 2019. Optogenetic approaches to vision restoration. Exp Eye Res 178: 15–26. 10.1016/j.exer.2018.09.003 [DOI] [PubMed] [Google Scholar]
- Singh MS, Charbel Issa P, Butler R, Martin C, Lipinski DM, Sekaran S, Barnard AR, MacLaren RE. 2013. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc Natl Acad Sci 110: 1101–1106. 10.1073/pnas.1119416110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MS, Balmer J, Barnard AR, Aslam SA, Moralli D, Green CM, Barnea-Cramer A, Duncan I, MacLaren RE. 2016. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nat Commun 7: 13537 10.1038/ncomms13537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slembrouck-Brec A, Nanteau C, Sahel JA, Goureau O, Reichman S. 2018. Defined xeno-free and feeder-free culture conditions for the generation of human iPSC-derived retinal cell models. J Vis Exp 139: e57795. 10.3791/57795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley S, Nickerson PE, Comanita L, Daftarian N, El-Sehemy A, Tsai EL, Matan-Lithwick S, Yan K, Thurig S, Touahri Y, et al. 2016. Establishment of a cone photoreceptor transplantation platform based on a novel cone-GFP reporter mouse line. Sci Rep 6: 22867 10.1038/srep22867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MJ, Bharti K. 2016. Looking into the future: using induced pluripotent stem cells to build two and three dimensional ocular tissue for cell therapy and disease modeling. Brain Res 1638: 2–14. 10.1016/j.brainres.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WK, Park KM, Kim HJ, Lee JH, Choi J, Chong SY, Shim SH, Del Priore LV, Lanza R. 2015. Treatment of macular degeneration using embryonic stem cell–derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports 4: 860–872. 10.1016/j.stemcr.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar A, Steward MM, Meyer JS. 2013. Nonxenogeneic growth and retinal differentiation of human induced pluripotent stem cells. Stem Cells Transl Med 2: 255–264. 10.5966/sctm.2012-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein JW, Ma N, Wenkel H, Ng TF, Zamiri P. 2002. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vision Res 42: 487–495. 10.1016/S0042-6989(01)00185-7 [DOI] [PubMed] [Google Scholar]
- Strettoi E, Pignatelli V, Rossi C, Porciatti V, Falsini B. 2003. Remodeling of second-order neurons in the retina of rd/rd mutant mice. Vision Res 43: 867–877. 10.1016/S0042-6989(02)00594-1 [DOI] [PubMed] [Google Scholar]
- Sugita S, Iwasaki Y, Makabe K, Kamao H, Mandai M, Shiina T, Ogasawara K, Hirami Y, Kurimoto Y, Takahashi M. 2016. Successful transplantation of retinal pigment epithelial cells from MHC homozygote iPSCs in MHC-matched models. Stem Cell Reports 7: 635–648. 10.1016/j.stemcr.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Tsunenari T, Yau KW, Nathans J. 2002. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci 99: 4008–4013. 10.1073/pnas.052692999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Mandai M, Gocho K, Hirami Y, Yamamoto M, Fujihara M, Sugita S, Kurimoto Y, Takahashi M. 2019. Evaluation of transplanted autologous induced pluripotent stem cell–derived retinal pigment epithelium in exudative age-related macular degeneration. Ophthalmol Retina 3: 850–859. [DOI] [PubMed] [Google Scholar]
- Tezel TH, Del Priore LV. 1997. Reattachment to a substrate prevents apoptosis of human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol 235: 41–47. 10.1007/BF01007836 [DOI] [PubMed] [Google Scholar]
- Thompson DA, Li Y, McHenry CL, Carlson TJ, Ding X, Sieving PA, Apfelstedt-Sylla E, Gal A. 2001. Mutations in the gene encoding lecithin retinol acyltransferase are associated with early-onset severe retinal dystrophy. Nat Genet 28: 123–124. 10.1038/88828 [DOI] [PubMed] [Google Scholar]
- Thompson JR, Worthington KS, Green BJ, Mullin NK, Jiao C, Kaalberg EE, Wiley LA, Han IC, Russell SR, Sohn EH, et al. 2019. Two-photon polymerized poly(caprolactone) retinal cell delivery scaffolds and their systemic and retinal biocompatibility. Acta Biomater 94: 204–218. 10.1016/j.actbio.2019.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treumer F, Bunse A, Klatt C, Roider J. 2007. Autologous retinal pigment epithelium-choroid sheet transplantation in age related macular degeneration: morphological and functional results. Br J Ophthalmol 91: 349–353. 10.1136/bjo.2006.102152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu HY, Watanabe T, Shirai H, Yamasaki S, Kinoshita M, Matsushita K, Hashiguchi T, Onoe H, Matsuyama T, Kuwahara A, et al. 2019. Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine 39: 562–574. 10.1016/j.ebiom.2018.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Redenti SM, Jiang C, Swift JS, Klassen HJ, Smith ME, Wnek GE, Young MJ. 2010. The use of progenitor cell/biodegradable MMP2-PLGA polymer constructs to enhance cellular integration and retinal repopulation. Biomaterials 31: 9–19. 10.1016/j.biomaterials.2009.09.015 [DOI] [PubMed] [Google Scholar]
- Tucker BA, Anfinson KR, Mullins RF, Stone EM, Young MJ. 2013. Use of a synthetic xeno-free culture substrate for induced pluripotent stem cell induction and retinal differentiation. Stem Cells Transl Med 2: 16–24. 10.5966/sctm.2012-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaajasaari H, Ilmarinen T, Juuti-Uusitalo K, Rajala K, Onnela N, Narkilahti S, Suuronen R, Hyttinen J, Uusitalo H, Skottman H. 2011. Toward the defined and xeno-free differentiation of functional human pluripotent stem cell–derived retinal pigment epithelial cells. Mol Vis 17: 558–575. [PMC free article] [PubMed] [Google Scholar]
- van Romunde SH, Polito A, Bertazzi L, Guerriero M, Pertile G. 2015. Long-term results of full macular translocation for choroidal neovascularization in age-related macular degeneration. Ophthalmology 122: 1366–1374. 10.1016/j.ophtha.2015.03.012 [DOI] [PubMed] [Google Scholar]
- van Romunde SHM, Polito A, Peroglio Deiro A, Bertazzi L, Guerriero M, Pertile G. 2019. Morphological changes in the diseased retina on a healthy choroid-retinal pigment epithelial complex after full macular translocation for exudative age-related macular degeneration. Acta Ophthalmol 97: e283–e289. 10.1111/aos.13880 [DOI] [PubMed] [Google Scholar]
- Veske A, Nilsson SE, Narfström K, Gal A. 1999. Retinal dystrophy of Swedish briard/briard–beagle dogs is due to a 4-bp deletion in RPE65. Genomics 57: 57–61. 10.1006/geno.1999.5754 [DOI] [PubMed] [Google Scholar]
- Wahlin KJ, Maruotti JA, Sripathi SR, Ball J, Angueyra JM, Kim C, Grebe R, Li W, Jones BW, Zack DJ. 2017. Photoreceptor outer segment-like structures in long-term 3D retinas from human pluripotent stem cells. Sci Rep 7: 766 10.1038/s41598-017-00774-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron PV, Di Marco F, Kruczek K, Ribeiro J, Graca AB, Hippert C, Aghaizu ND, Kalargyrou AA, Barber AC, Grimaldi G, et al. 2018. Transplanted donor- or stem cell–derived cone photoreceptors can both integrate and undergo material transfer in an environment-dependent manner. Stem Cell Reports 10: 406–421. 10.1016/j.stemcr.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warre-Cornish K, Barber AC, Sowden JC, Ali RR, Pearson RA. 2014. Migration, integration and maturation of photoreceptor precursors following transplantation in the mouse retina. Stem Cells Dev 23: 941–954. 10.1089/scd.2013.0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y. 2005. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci 8: 288–296. 10.1038/nn1402 [DOI] [PubMed] [Google Scholar]
- Welby E, Lakowski J, Di Foggia V, Budinger D, Gonzalez-Cordero A, Lun ATL, Epstein M, Patel A, Cuevas E, Kruczek K, et al. 2017. Isolation and comparative transcriptome analysis of human fetal and iPSC-derived cone photoreceptor cells. Stem Cell Reports 9: 1898–1915. 10.1016/j.stemcr.2017.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EL, Pearson RA, Tschernutter M, Sowden JC, MacLaren RE, Ali RR. 2008. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res 86: 601–611. 10.1016/j.exer.2008.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EL, Pearson RA, MacLaren RE, Sowden JC, Ali RR. 2009. Cell transplantation strategies for retinal repair. Prog Brain Res 175: 3–21. 10.1016/S0079-6123(09)17501-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EL, Pearson RA, Barker SE, Luhmann UF, Maclaren RE, Barber AC, Duran Y, Smith AJ, Sowden JC, Ali RR. 2010. Long-term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation. Stem Cells 28: 1997–2007. 10.1002/stem.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EL, Pearson RA, Duran Y, Gonzalez-Cordero A, MacLaren RE, Smith AJ, Sowden JC, Ali RR. 2012. Manipulation of the recipient retinal environment by ectopic expression of neurotrophic growth factors can improve transplanted photoreceptor integration and survival. Cell Transplant 21: 871–887. 10.3727/096368911X623871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley LA, Burnight ER, DeLuca AP, Anfinson KR, Cranston CM, Kaalberg EE, Penticoff JA, Affatigato LM, Mullins RF, Stone EM, et al. 2016. cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Sci Rep 6: 30742 10.1038/srep30742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. 2003. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 22: 1–29. 10.1016/S1350-9462(02)00043-5 [DOI] [PubMed] [Google Scholar]
- Worthington KS, Wiley LA, Kaalberg EE, Collins MM, Mullins RF, Stone EM, Tucker BA. 2017. Two-photon polymerization for production of human iPSC-derived retinal cell grafts. Acta Biomater 55: 385–395. 10.1016/j.actbio.2017.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV. 2009. Para-inflammation in the aging retina. Prog Retin Eye Res 28: 348–368. 10.1016/j.preteyeres.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Yao J, Ko CW, Baranov PY, Regatieri CV, Redenti S, Tucker BA, Mighty J, Tao SL, Young MJ. 2015. Enhanced differentiation and delivery of mouse retinal progenitor cells using a micropatterned biodegradable thin-film polycaprolactone scaffold. Tissue Eng Part A 21: 1247–1260. 10.1089/ten.tea.2013.0720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MJ. 2005. Stem cells in the mammalian eye: a tool for retinal repair. APMIS 113: 845–857. 10.1111/j.1600-0463.2005.apm_334.x [DOI] [PubMed] [Google Scholar]
- Zarbin MA. 2004. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol 122: 598–614. 10.1001/archopht.122.4.598 [DOI] [PubMed] [Google Scholar]
- Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, et al. 2014. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun 5: 4047 10.1038/ncomms5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Cifuentes H, Reynolds J, Lamba DA. 2017. Immunosuppression via loss of IL2rγ enhances long-term functional integration of hESC-derived photoreceptors in the mouse retina. Cell Stem Cell 20: 374–384.e5 10.1016/j.stem.2016.11.019 [DOI] [PubMed] [Google Scholar]