Abstract
Background
The efficacy and optimal duration of postexposure influenza prophylaxis with oseltamivir are undetermined in hospital settings, where immediate separation from index cases is not feasible.
Methods
In an open-label noninferiority randomized clinical trial in a single-center university hospital, the efficacy of 5-day vs 10-day postexposure prophylaxis with oseltamivir was compared in adult patients exposed to influenza who could not be immediately separated from index influenza cases. Influenza incidence was assessed for 10 days after discontinuing prophylaxis.
Results
Among 222 exposed patients (median age, 75 years; male 119; median Charlson Comorbidity Index, 5), 110 patients were assigned to 5 days of postexposure prophylaxis with oseltamivir, and 112 patients were assigned to the 10-day group. The median duration of exposure to influenza (interquartile range) was 2 (1–3) days. In the intention-to-treat analysis, the incidence of influenza was 2/110 (1.8%) in the 5-day group and 0/112 (0%) in the 10-day group (difference, 1.8 percentage points; 1-sided 95% CI, –1 to 4.9 percentage points; P = .77).
Conclusions
For patients exposed to influenza in a hospital setting and who were not immediately separated from index cases, postexposure prophylaxis with oseltamivir resulted in low incidence of nosocomial influenza transmission. Five-day postexposure prophylaxis was noninferior to 10-day regimen.
ClinicalTrials.gov Registration
Keywords: chemoprophylaxis, infection control, influenza, neuraminidase inhibitor
Hospitalized patients are particularly vulnerable to influenza and its complications because of their age, baseline health status, and admission illness [1, 2]. Vaccination is the most effective strategy for preventing influenza [3, 4], but once a hospitalized patient has been exposed to influenza, vaccination is insufficient for prevention of nosocomial transmission because many people are not vaccinated and because of the suboptimal effectiveness of the vaccine in conjunction with the urgent need for intervention [5]. Prevention of nosocomial transmission, therefore, relies much more on other preventive measures [3, 6, 7]. In a hospital influenza outbreak, that is, when 2 cases of health care–associated laboratory-confirmed influenza are identified within 72 hours of each other in patients from the same ward or unit, postexposure antiviral chemoprophylaxis in conjunction with standard and droplet precautions is recommended for ≥14 days and until 7 days after the last case has been identified [6, 8]. Guidelines and recommendations on the use of postexposure prophylaxis in a nonoutbreak hospital setting do not exist, but when used in community settings, antiviral chemoprophylaxis is recommended for 7 days and up to 10 days after the last exposure to a close contact with influenza [3, 8]. The timing of the last exposure can be determined accurately if the index case and the exposed patient are separated; however, it is less clear when the index case and the exposed patient remain in close contact. In such circumstances, the timing of the last exposure depends on the duration of virus shedding in the index case; this peaks during the first 1‒3 days of clinical illness [9, 10], correlates well with infectiousness [11], and may be reduced but not eliminated by oseltamivir therapy [12]. Accordingly, it cannot be assumed that introduction of oseltamivir treatment in index cases is equivalent to physical separation. In 1 study, in households where isolation of index cases receiving antiviral treatment was not possible, the proportion of secondary cases was effectively reduced with 10-day prophylaxis [13]. In a hospital setting where exposed patients were immediately separated from index cases with influenza, postexposure chemoprophylaxis with oseltamivir for 3 [14] and 5–7 days [15] was similarly effective in reducing nosocomial transmission. However, in a hospital setting where index patients cannot be isolated immediately (eg, for spatial constraints) yet <48 hours have elapsed since the first exposure to influenza, the efficacy and optimal duration of oseltamivir prophylaxis are not known.
In this randomized, open-label study, we aimed to assess (1) the incidence of nosocomial influenza transmission when postexposure prophylaxis with oseltamivir was used for 5 days or 10 days, defining the last exposure as either separation or completion of the first 3 days of oseltamivir therapy in index cases with influenza who could not be isolated immediately, and (2) specifically the noninferiority of 5-day postexposure prophylaxis compared with a 10-day regimen to prevent nosocomial influenza transmission in this epidemiologic setting.
METHODS
Study Design and Participants
Patients aged ≥18 years hospitalized in the University Medical Center Ljubljana Slovenia during the period January 23 to March 20, 2019, who were prescribed oseltamivir prophylaxis because of exposure to influenza of up to 24 hours’ duration, and for whom immediate separation from index cases was not feasible, were eligible for the study. Patients were identified from the hospital pharmacy database. Those hospitalized in intensive care units or hematology departments were excluded. The study was approved by the Medical Ethics Committee of the Ministry of Health of the Republic of Slovenia (No. 0120-15/2019/9) and registered at http://ClinicalTrials.gov, Identifier NCT03899571. Written or verbal informed consent was obtained from study participants or their family members, respectively.
Infection Prevention Measures and Drug Administration
Influenza vaccination for all medical staff was not mandatory but was strongly encouraged and was provided free of charge in the previous autumn season. Cumulative vaccination rates among staff of different professions varied from 15.6% in the surgical department to 30.4% in general medicine to 56.7% in the infectious diseases department. Nonpharmacologic infection prevention measures consisted of (1) isolation or cohorting of patients with influenza if possible, (2) implementation of droplet precautions, and (3) restriction of visits. Patients with laboratory-confirmed influenza were prescribed oseltamivir therapy 75 mg twice daily for 5 days. Exposed patients were randomized 1:1 using a computer-generated randomization schedule to receive oseltamivir prophylaxis 75 mg once daily (or adjusted equivalent in the case of renal failure) for either 5 days or 10 days postexposure.
Case Definitions and Clinical Monitoring
An index case was defined by the presence of acute respiratory illness (ARI) [3] and laboratory confirmation of influenza virus infection. Exposure was defined as staying in the same room for ≥12 hours with an index case. Last exposure to influenza was defined as separation from or completion of the first 3 days of oseltamivir treatment in index cases, whichever was sooner. Postexposure prophylaxis day number 1/5 or 1/10 was defined as the first day after discontinuing the last exposure, regardless of the starting date of prophylaxis and regardless of whether the exposed patient and the index case continued to share the room. Physical examination, including evaluation of respiratory symptoms and body temperature, was performed daily during oseltamivir prophylaxis and up to 10 days after discontinuation of prophylaxis. Exposed patients who had been discharged from the hospital earlier were instructed to seek medical advice in case of a febrile respiratory illness and asked by telephone about respiratory symptoms and fever during oseltamivir prophylaxis and up to 10 days after its discontinuation. Exposed patients who developed ARI were tested for influenza virus infection. The study outcome measure was efficacy of oseltamivir postexposure prophylaxis, defined as the percentage of exposed patients with ARI and laboratory confirmation of influenza virus infection during and up to 10 days after discontinuation of oseltamivir prophylaxis.
Laboratory Detection of Influenza Virus
MagNa Pure Total Nucleic Acid Isolation Kits and a MagNa Pure Compact instrument (Roche Applied Science, Mannheim, Germany) were used in accordance with the manufacturer’s instructions. Thus, tubes with swabs were vortexed vigorously for 30 seconds and 200-μL samples were used for extractions. Nucleic acids were eluted in 100 μL of elution buffer. The respiratory samples were tested for influenzas A and B and respiratory syncytial virus strains A and B using single real-time polymerase chain reaction (RT-PCR) and TaqMan or MGB probes, as described previously [16].
Statistical Analysis
Sample size was based on the assumption that ≤2% of patients receiving 10 days of oseltamivir would develop influenza [15]. We assumed that the primary efficacy outcome would occur with the same frequency in the 2 treatment groups. To obtain 90% statistical power with a 1-sided α equal to .05, 95 patients per group were necessary for establishing the noninferiority of 5-day prophylaxis, compared with 10-day prophylaxis, with a margin of noninferiority equal to a difference of 7 percentage points (p.p.). To account for attrition, the required sample size was estimated to be about 110 patients per group. The intention-to-treat (ITT) population represented the total population that received oseltamivir prophylaxis, regardless of adherence to the study protocol. The per-protocol (PP) population represented the population that received a randomly assigned oseltamivir dosing regimen and completed the follow-up according to the study protocol.
Categorical data were summarized as frequency (%) and numerical data as median (interquartile range [IQR]). Differences between the 5-day and 10-day regimens were tested using the Mann-Whitney test or chi-square test with Yates continuity correction. The difference between the proportions of patients with influenza receiving the 5-day vs the 10-day regimen was estimated using a 1-sided 95% CI based on the normal approximation with continuity correction. The noninferiority of the 5-day regimen was established if the upper limit of the 95% CI did not exceed 7 p.p. The ITT and PP populations were analyzed separately. A P value <.05 was considered significant. R statistical language was used for the analyses (version 3.4.1) [17].
RESULTS
Patients’ Characteristics
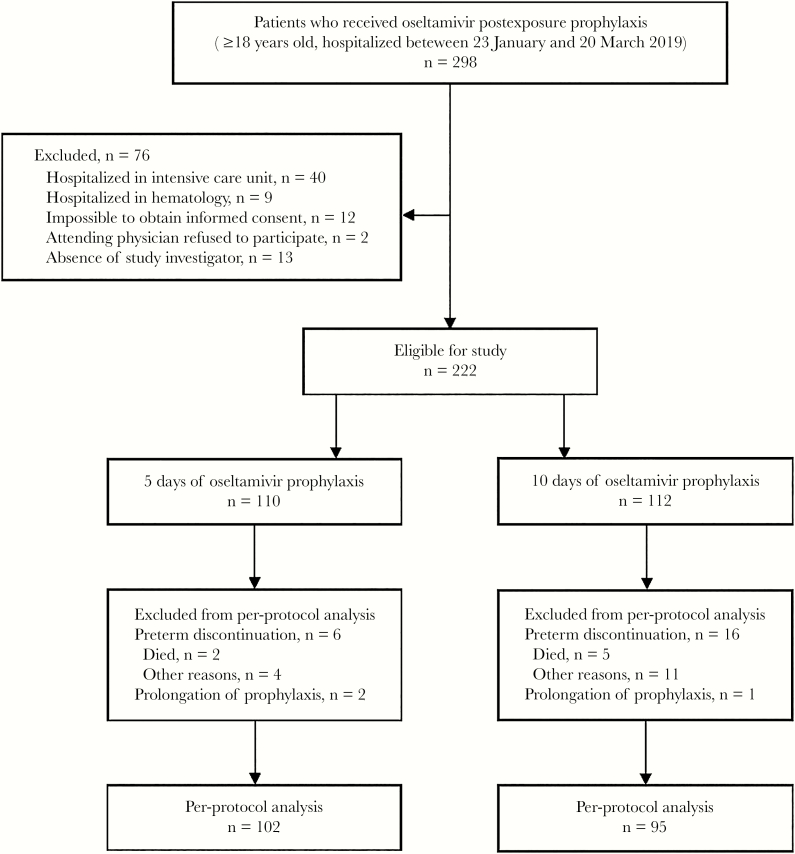
During the 8-week study period, 298 patients were exposed to influenza virus, and of these, 222 (74.5%) were prescribed oseltamivir postexposure prophylaxis and included in the study (Figure 1). The first 5 weeks of our study coincided with the timing of peak influenza activity in the community, that is, between the fourth and eighth weeks of the year 2019 [18]. During this period, significantly more exposed patients were enrolled than during the ninth and 12th weeks of the same year, when influenza activity in the community declined (171/222, 77.0%, vs 51/222, 23.0%; P < .01). Among the 222 patients, 110 (49.5%) were randomized to receive postexposure oseltamivir for 5 days and 112 for 10 days. The 2 groups of exposed patients were balanced regarding basic demographic and clinical characteristics at enrollment; the majority (185/222, 83.3%) had ≥2 risk factors for complications from influenza [3], the most common being age ≥65 years. In the study overall, only 22 (9.9%) patients had been vaccinated against seasonal influenza for the current season (Table 1), and the proportion of vaccinated elderly was also low (20/164, 12.2%). During the study period, influenza hospital outbreak, as defined by Uyeki et al. [3], was not declared.
Figure 1.
Study diagram.
Table 1.
Characteristics of Patients Exposed to Influenza and Prescribed Postexposure Prophylaxis With Oseltamivir for 5 or 10 Days
| ITT Analysis | PP Analysis | |||||
|---|---|---|---|---|---|---|
| Characteristic | 5 d (n = 110) | 10 d (n = 112) | P Value | 5 d (n = 102) | 10 d (n = 95) | P Value |
| Age, y | 77 (63.8–84) | 73 (64–81) | .39 | 77 (63.8–84) | 73 (64–81) | .33 |
| Male sex | 57 (51.8) | 62 (55.4) | .69 | 53 (52.0) | 52 (54.7) | .81 |
| Charlson Comorbidity Index | 5 (3–7) | 5.5 (4–7) | .42 | 5 (3–7) | 6 (4–7) | .38 |
| Vaccinated against influenza | 10 (9.1) | 12 (10.7) | .86 | 10 (9.8) | 9 (9.5) | 1.00 |
| Risk factor for complications from influenza | ||||||
| Age ≥65 y | 82 (74.5) | 82 (73.2) | .94 | 76 (74.5) | 69 (72.6) | .89 |
| Chronic pulmonary disorder | 16 (14.5) | 18 (16.1) | .90 | 16 (15.7) | 16 (16.8) | .98 |
| Chronic cardiovascular disorder | 68 (61.8) | 69 (61.6) | 1.00 | 62 (60.8) | 56 (58.9) | .91 |
| Chronic renal disorder | 18 (16.4) | 20 (17.9) | .91 | 15 (14.7) | 17 (17.9) | .68 |
| Chronic hepatic disorder | 4 (3.6) | 8 (7.1) | .39 | 4 (3.9) | 7 (7.4) | .46 |
| Chronic neurologic disorder | 30 (27.3) | 27 (24.1) | .70 | 27 (26.5) | 22 (23.2) | .71 |
| Metabolic disorder | 49 (44.5) | 42 (37.5) | .35 | 44 (43.1) | 36 (37.9) | .55 |
| Immunosuppression | 14 (12.7) | 12 (10.7) | .80 | 12 (11.8) | 11 (11.6) | 1.00 |
| Days of exposurea | 2 (1–3) | 2 (1–3) | <.01 | 2 (1–3) | 2 (1–3) | <.01 |
| Type of department | .88 | |||||
| Infectious diseases | 32 (29.1) | 35 (31.3) | .79 | 31 (30.4) | 32 (33.7) | |
| General medicine | 63 (57.3) | 65 (58.0) | 58 (56.9) | 52 (54.7) | ||
| Surgical | 15 (13.6) | 12 (10.7) | 13 (12.8) | 11 (11.6) | ||
| Hospitalization, d | 14 (8–23.5) | 15 (10–27.3) | .09 | 14 (8–25.5) | 17 (11–29) | .05 |
| Bedridden patients | 40 (36.4) | 54 (48.2) | .10 | 37 (36.3) | 46 (48.4) | .11 |
| Bedridden index cases | 44/100 (44) | 43/90 (47.8) | .71 | 40/92 (43.5) | 33/75 (44.0) | 1.00 |
Abbreviations: ITT, intention to treat; PP, per protocol.
aDuration of exposure to influenza, that is, sharing a room for ≥12 hours with an index case receiving the first 3 days of oseltamivir therapy.
Epidemiologic and Microbiologic Results
During the study period, 246 patients were prescribed oseltamivir because of laboratory-confirmed influenza, and among these, at least 137/246 (55.7%) could not be isolated or cohorted but were placed in rooms with ≥2 beds and therefore represented index cases. The distance between patients’ beds ranged from 0.4 to 1.2 meters. Index cases were identified for 202/222 (91.0%) exposed patients. As index cases with influenza were admitted to the hospital while symptomatic and an unspecified number of index cases with influenza were not prescribed oseltamivir until infection was laboratory-confirmed, which led to a delay of several hours and up to 1 day before oseltamivir was prescribed, in all exposed patients, prophylaxis was introduced <48 hours since the first exposure, as recommended [6].
The median duration of exposure to influenza was 2 days in both treatment groups, but the distribution of exposure times was longer in the 5-day group than in the 10-day group (P = .01). On average 2 (IQR, 1–3) exposed patients were prescribed prophylaxis per index case. For 87 index cases, a single exposed patient per index case was prescribed prophylaxis; 29 index cases were in contact with 2 exposed patients; 11 with 3 exposed patients; 4 with 4 exposed patients; and 1 index case represented a source for 8 exposed patients. Conversely, 5 patients were exposed to 2 index cases consecutively. The number of exposed patients was higher in the departments of surgery and internal medicine, where patients were hospitalized in rooms with 3–6 beds, than in infectious diseases departments, which had rooms with 2–3 beds (median [IQR], 2 [1–3] vs 1 [1–1], respectively; P < .01). All 137 identified index cases were diagnosed with influenza A. Subtyping of influenza A in 19 index cases identified infection with H1N1 in 12 cases and H3N2 in 7.
Adherence to the Study Protocol
Among 110 exposed patients in the 5-day group, 102 (92.7%) completed the PP regimen, 3 completed 4 days of prophylaxis, 2 completed 3 days of prophylaxis, and 1 each received 1, 6, and 7 days of treatment. Two exposed patients died after completing 1 and 3 days of postexposure prophylaxis, respectively; their deaths were related to other medical diagnoses, and both tested negative for influenza. Among 112 exposed patients in the 10-day group, 95 (84.8%) completed the PP regimen, 1 completed 11 days of prophylactic treatment, and 11 received 1–6 days less prophylactic therapy than the 10 days prescribed. Five patients died from causes unrelated to influenza before completing the prophylaxis.
Among 222 exposed patients, 111 (50%) were discharged from the hospital before completing oseltamivir prophylaxis and were followed up by telephone; 15/27 (55.6%) from surgical wards, 36/67 (53.7%) from infectious diseases wards, and 60/128 (46.9%) from general medicine wards (P < .55). Noncompliance with the study protocol was presumed due to miscommunication between the study physician and the attending physician or between the attending physician and the exposed patient. In none of the patients was prophylaxis discontinued because of an adverse event. In 140 exposed patients, oseltamivir prophylaxis was prescribed concurrently (on the same day) with prescribing oseltamivir for the identified index case. The median total duration of oseltamivir prophylaxis (IQR) was 7 (6–8) days in the 5-day group and 11 (11–12.3) days in the 10-day group (P < .01).
Outcome of Postexposure Prophylaxis With Oseltamivir
Among the 222 exposed patients included in the ITT analysis, 2 (0.9%) developed ARI during their hospital stay, and both had laboratory-confirmed influenza. The proportion of exposed patients who were discharged home before completing oseltamivir prophylaxis differed between the 5-day group and the 10-day group (46/110, 41.8%, vs 65/112, 59.1%; P = .02), but none developed ARI during follow-up. Both patients with breakthrough influenza were from the 5-day group; none were from the 10-day group (2/110, 1.8%, vs 0/112, 0%; difference, 1.8 p.p.; 1-sided 95% CI, –1 to 4.9 p.p.; P = .77). Rates of breakthrough influenza in the PP study population were 2/102 (2.0%) in the 5-day group and 0/95 (0%) in the 10-day group (difference, 2.0 p.p.; 1-sided 95% CI, –1 to 5.3 p.p.; P = .75). The first exposed patient in whom prophylaxis failed to prevent influenza developed an ARI on day 3 of oseltamivir prophylaxis, which was day 9 of hospitalization in the traumatology ward. Both the index case and the exposed patient were bedridden. They were prescribed oseltamivir concurrently and shared a room for 1 further day before they were separated. The second exposed patient with breakthrough influenza also started oseltamivir prophylaxis on the same day as oseltamivir therapy was prescribed for the index case. Both of these patients were bedridden and shared a room for 3 consecutive days before they were separated. The exposed patient developed an ARI on day 6 after starting oseltamivir, which was day 11 after admission to the general medicine ward. Infection with influenza A virus was demonstrated in both exposed patients with breakthrough influenza and their corresponding index cases. The distance between patients’ beds ranged from 0.4 to 1.2 meters, putting exposed patients at risk of influenza transmission even if both the index case and the exposed patient were bedridden. The 2 breakthrough cases occurred in the fifth and seventh weeks of the year 2019, respectively, which was during the peak influenza activity in the community.
DISCUSSION
In this randomized controlled study of postexposure influenza prophylaxis with oseltamivir for prevention of nosocomial transmission in adult patients exposed to treated index cases of influenza and not immediately separated from them, the 5-day regimen was noninferior to the 10-day regimen, and the incidence rate of nosocomial influenza transmission was low with both prophylaxis regimens.
In our study, last exposure to influenza was defined as separation or completion of the first 3 days of oseltamivir treatment in the index case, whichever was sooner, and the start of postexposure prophylaxis was defined only after the last exposure was discontinued. Thus, the median total duration of prophylaxis (time of exposure plus time of postexposure prophylaxis) was actually 7 days in the 5-day group and 11 days in the 10-day group.
The attack rates of nosocomial influenza during an outbreak vary from 9% in acute care hospitals to 50% on epidemic wards [1, 19], possibly reaching 70% in oncology units [20]. However, the probability of a single influenza case triggering an influenza outbreak is not known [3]. In hospital settings, postexposure prophylaxis with oseltamivir for 3–7 days in conjunction with immediate separation of index cases from exposed patients was shown to reduce nosocomial transmission from 13.3%‒21.4% to 0.9%‒1.9% [14, 15]. In households where isolation of index cases treated with antivirals was not possible, the proportion of secondary cases was reduced from 12.6% (26/296) to 1.4% (3/209) with 10-day prophylaxis [13]. In our study in a hospital setting where immediate isolation of index cases was not feasible because of spatial constraints and was delayed on average for 2 days, the incidence rate of influenza transmission was similarly low (0.9%) using postexposure chemoprophylaxis for either 5 or 10 days. Importantly, only a minority (12.2%) of exposed patients in our study population were vaccinated, and the majority (73.9%) of exposed patients were elderly people (≥65 years old), who even when vaccinated have a higher risk of acquiring influenza in comparison with vaccinated younger adults, because older people mount a smaller antibody response to vaccination [21]. These findings in a hospital setting suggest that once the exposed patients are prescribed oseltamivir prophylaxis, a median delay of 2 days in their separation from index cases does not affect the efficacy of oseltamivir in preventing nosocomial transmission.
In our study, the difference in breakthrough influenza in exposed patients receiving postexposure prophylaxis for 2 different durations was not significant: 1.8% with the 5-day regimen and none with the 10-day regimen. Considering the high attack rates of influenza in hospital outbreaks [1, 19, 20], we believe that 7 p.p., which we selected as the noninferiority margin in our study, was acceptable. Furthermore, the occurrence of the 2 breakthrough cases in the 5-day group does not suggest inferiority of the 5-day regimen, because both patients developed influenza before completing the 5-day course. They were 88 and 89 years old and had Charlson Comorbidity Index scores of 5 and 8, respectively. However, the number of patients with high age or Charlson Index score was too small to permit analysis of the association between these parameters and prophylaxis efficacy. We are not aware of any similar studies in hospital settings where isolation of index cases was not possible or where different durations of postexposure prophylaxis were directly compared. Indirect comparison of results from 2 studies in hospital settings where index cases were isolated immediately suggests that the outcomes of 3-day [14] and 5‒7-day [15] regimens of postexposure oseltamivir are similar, with breakthrough influenza rates of 2/212 (0.9%) and 2/102 (1.9%), respectively.
There are several limitations to our study. First, the open-label model raises the possibility of bias in outcomes, representing over- or under-reporting of ARI in the 2 groups. We believe this was unlikely, because patients were not explicitly told which regimen they were assigned to and because ARI is not very likely to be subjected to a placebo effect. In contrast, the prospective study approach with active surveillance of close contacts and objective laboratory confirmation of infection using RT-PCR testing [22] enabled us to reliably detect potential influenza transmissions. The risk of bias in detecting symptoms might have been higher among exposed patients who were discharged home before completing their follow-up period. However, we believe that by actively asking about the presence of respiratory symptoms and fever in patients discharged from the hospital before the end of the follow-up period, the possibility of missing patients with an asymptomatic or very atypical course of influenza would not differ considerably from the frequency of such cases among exposed patients who stayed in the hospital during follow-up. Second, in our study, only the date of therapeutic administration of oseltamivir in index cases but not the exact duration of influenza symptoms was recorded, which could have led to variable infectiousness of index cases. However, this would apply to both treatment regimens and would not have influenced the results. Third, virus sequencing was not performed, and transmission of influenza from visitors, who were permitted only exceptionally, or from health care personnel cannot be excluded in breakthrough cases. However, at the same time, this is the strength of our study because, unless there is a hospital outbreak of influenza, only patients with laboratory-confirmed influenza serve as potential index cases that trigger oseltamivir prophylaxis for exposed patients if health care personnel are advised to stay home when acutely ill and if restriction of visits is implemented.
In conclusion, our results show that in a hospital setting where exposed patients could not be separated immediately from treated index cases, 5-day postexposure prophylaxis with oseltamivir was noninferior to a 10-day regimen in preventing nosocomial transmission of influenza, and the incidence of nosocomial transmission was low with both prophylactic regimens, provided the last exposure was defined as separation or completion of the first 3 days of oseltamivir therapy in index cases. In this regard, the association between duration of exposure and the efficacy of different durations of postexposure oseltamivir prophylaxis requires further assessment.
Acknowledgments
The authors thank Martina Ravnikar and Vesna Bizjak, who provided information from the hospital pharmacy database.
Financial support. This work was supported by the University Medical Center Ljubljana, grant number 20190151. The funding source had no role in the study design; the collection, analysis, and interpretation of data; the writing of the paper; or the decision to submit the paper for publication.
Potential conflicts of interest. The authors declare no conflict of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. D.S. designed the study. L.L., M.V., R.S., and D.S. gathered and interpreted the data. R.B. analyzed the data. M.P. performed microbiologic analyses. F.F.B. and D.S. searched the literature and wrote the manuscript. All authors have seen and approved the manuscript.
References
- 1. Salgado CD, Farr BM, Hall KK, Hayden FG. Influenza in the acute hospital setting. Lancet Infect Dis 2002; 2:145–55. [DOI] [PubMed] [Google Scholar]
- 2. Sayers G, Igoe D, Carr M, et al. High morbidity and mortality associated with an outbreak of influenza A(H3N2) in a psycho-geriatric facility. Epidemiol Infect 2013; 141:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis 2019; 68:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pop-Vicas A, Rahman M, Gozalo PL, et al. Estimating the effect of influenza vaccination on nursing home residents’ morbidity and mortality. J Am Geriatr Soc 2015; 63:1798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okoli GN, Otete HE, Beck CR, Nguyen-Van-Tam JS. Use of neuraminidase inhibitors for rapid containment of influenza: a systematic review and meta-analysis of individual and household transmission studies. PLoS One 2014; 9:e113633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disese Control and Prevention. Influenza antiviral medications: summary for clinicians Available at: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed 19 February 2019.
- 7. Centers for Disese Control and Prevention. Prevention strategies for seasonal influenza in healthcare settings. Guidelines and recommendations Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed 19 February 2020.
- 8. European Centre for Disease Prevention and Control. Expert opinion on neuraminidase inhibitors for the prevention and treatment of influenza Available at: https://www.ecdc.europa.eu/sites/default/files/documents/Scientific-advice-neuraminidase-inhibitors-2017.pdf. Accessed 19 February 2020.
- 9. Lau LL, Cowling BJ, Fang VJ, et al. Viral shedding and clinical illness in naturally acquired influenza virus infections. J Infect Dis 2010; 201:1509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ip DKM, Lau LLH, Chan KH, et al. The dynamic relationship between clinical symptomatology and viral shedding in naturally acquired seasonal and pandemic influenza virus infections. Clin Infect Dis 2016; 62:431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferguson NM, Cummings DA, Cauchemez S, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature 2005; 437:209–14. [DOI] [PubMed] [Google Scholar]
- 12. Fry AM, Goswami D, Nahar K, et al. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis 2014; 14:109–18. [DOI] [PubMed] [Google Scholar]
- 13. Hayden FG, Belshe R, Villanueva C, et al. Management of influenza in households: a prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis. J Infect Dis 2004; 189:440–9. [DOI] [PubMed] [Google Scholar]
- 14. Higa F, Tateyama M, Tomishima M, et al. Role of neuraminidase inhibitor chemoprophylaxis in controlling nosocomial influenza: an observational study. Influenza Other Respir Viruses 2012; 6:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishiguro N, Oyamada R, Nasuhara Y, et al. Three-day regimen of oseltamivir for postexposure prophylaxis of influenza in wards. J Hosp Infect 2016; 94:150–3. [DOI] [PubMed] [Google Scholar]
- 16. Uršič T, Miksić NG, Lusa L, Strle F, Petrovec M. Viral respiratory infections in a nursing home: a six-month prospective study. BMC Infect Dis 2016; 16:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 18. Institute of Public Health of the Republic of Slovenia. Ljubljana 2020 Available online: https://www.nijz.si/sl/tedensko-spremljanje-gripe-in-drugih-akutnih-okuzb-dihal-v-sezoni-20192020. Accessed 28 April 2020.
- 19. Murti M, Whelan M, Friedman L, et al. Influenza outbreaks in Ontario hospitals 2012–2016. Can Commun Dis Rep 2018; 44:201–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson EK, Wood MS, Schaecher EK, et al. Nosocomial outbreak of influenza A H3N2 in an inpatient oncology unit related to health care workers presenting to work while ill. Am J Infect Control 2019; 7:683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006; 24:1159–69. [DOI] [PubMed] [Google Scholar]
- 22. Gooskens J, Swaan CM, Claas EC, Kroes AC. Rapid molecular detection of influenza outbreaks in nursing homes. J Clin Virol 2008; 41:7–12. [DOI] [PubMed] [Google Scholar]