Abstract
Background
Epstein-Barr virus (EBV) is implicated in the progression of chronic obstructive pulmonary disease. We aimed to determine whether EBV correlates with bronchiectasis severity, exacerbations, and progression.
Methods
We collected induced sputum in healthy controls and spontaneous sputum at 3–6-month intervals and onset of exacerbations in bronchiectasis patients between March 2017 and October 2018. EBV DNA was detected with quantitative polymerase chain reaction.
Results
We collected 442 sputum samples from 108 bronchiectasis patients and 50 induced sputum samples from 50 healthy controls. When stable, bronchiectasis patients yielded higher detection rates of EBV DNA (48.1% vs 20.0%; P = .001), but not viral loads (mean log10 load, 4.45 vs 4.76; P = .266), compared with controls; 64.9% of patients yielded consistent detection status between 2 consecutive stable visits. Neither detection rate (40.8% vs 48.1%; P = .393) nor load (mean log10 load, 4.34 vs 4.45; P = .580) differed between the onset of exacerbations and stable visits, nor between exacerbations and convalescence. Neither detection status nor viral loads correlated with bronchiectasis severity. EBV loads correlated negatively with sputum interleukin-1β (P = .002), CXC motif chemokine-8 (P = .008), and tumor necrosis factor–α levels (P = .005). Patients initially detected with, or repeatedly detected with, EBV DNA had significantly faster lung function decline and shorter time to next exacerbations (both P < .05) than those without. Detection of EBV DNA was unrelated to influenza virus and opportunistic bacteria (all P > .05). The EBV strains detected in bronchiectasis patients were phylogenetically homologous.
Conclusions
Patients with detection of EBV DNA have a shorter time to bronchiectasis exacerbations. EBV may contribute to bronchiectasis progression.
Keywords: airway inflammation, chronic airway disease, chronic viral infection, exacerbation, human herpes virus–4, lung function
The role of chronic viral infections in bronchiectasis is unclear. In this prospective study, we summarize the association between viral loads or detection rate and the clinical status as well as inflammatory response of bronchiectasis.
Bronchiectasis is a debilitating chronic airway inflammatory disease characterized by pathogen infections, chronic airway inflammation, and airway destruction, which provide the niche for bacterial, viral, and fungal infections [1–3]. Acute viral infections are responsible for aggravating airway inflammation that frequently triggers exacerbations [4]. Consistent with findings in chronic obstructive pulmonary disease (COPD), CD8+ lymphocyte infiltrates the bronchiectatic airways [5, 6], suggesting that chronic viral infection correlates with chronic airway inflammation.
Epstein-Barr virus (EBV) is a ubiquitous virus associated with chronic infection of B-memory lymphocytes and airway epithelial cells [7, 8]. The lower respiratory tract is an important reservoir of EBV [8], which could be readily detected in COPD. The considerable detection rate of EBV deoxynecluotide acid (DNA) in COPD patients (46%–48%) [9], coupled with the link between chronic EBV infection and disease progression [9, 10], have hinted at the role of EBV in bronchiectatic airways where host–defense responses are defective.
We hypothesized that EBV predisposes to the inflammatory responses and progression in bronchiectasis. Our primary objectives were to elucidate the association between EBV and the severity, exacerbations, and progression of bronchiectasis. We also determined the changes in viral loads during exacerbations and risk factors for the detection of EBV DNA. Furthermore, we evaluated the phylogenetic homogeneity of the EBV strains in our bronchiectasis patient cohort. Our findings might indicate the roles of EBV in bronchiectasis and highlight prophylactic and therapeutic targets.
METHODS
Study Design and Subjects
In this single-center prospective observational study, we enrolled symptomatic bronchiectasis patients (chronic cough, daily sputum production) from the First Affiliated Hospital of Guangzhou Medical University between March 2017 and October 2018. Eligible patients, aged 18–75 years, underwent chest high-resolution computed tomography (HRCT) within the previous 12 months. Bronchiectasis was diagnosed based on international guidelines [11]. Patients with active tuberculosis, traction bronchiectasis, COPD as the primary etiology of bronchiectasis, malignancy, and severe systemic diseases were excluded.
Healthy subjects included patients’ companions, medical staff, and those who underwent health check-up, had normal chest x-ray and spirometry, and had no respiratory symptoms, severe systemic diseases, or antibiotic use within 4 weeks.
The Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University gave approval (medical ethics 2012 [the 29th]). All participants provided written informed consent.
Procedures
We enrolled bronchiectasis patients who remained exacerbation-free for >4 weeks. At initial visits, patients were asked for clinical history, exacerbation frequency, and concomitant medications. Bronchiectasis etiology, modified Reiff score, and spirometry were evaluated [12–14]. Disease severity was calculated with 2 integrated metrics, including the Bronchiectasis Severity Index (BSI) [15] and E-FACED score [16]. Spontaneous sputum was collected for assays of EBV and other respiratory viruses (Supplementary Data), bacterial culture, and inflammatory biomarker assays in bronchiectasis patients [17].
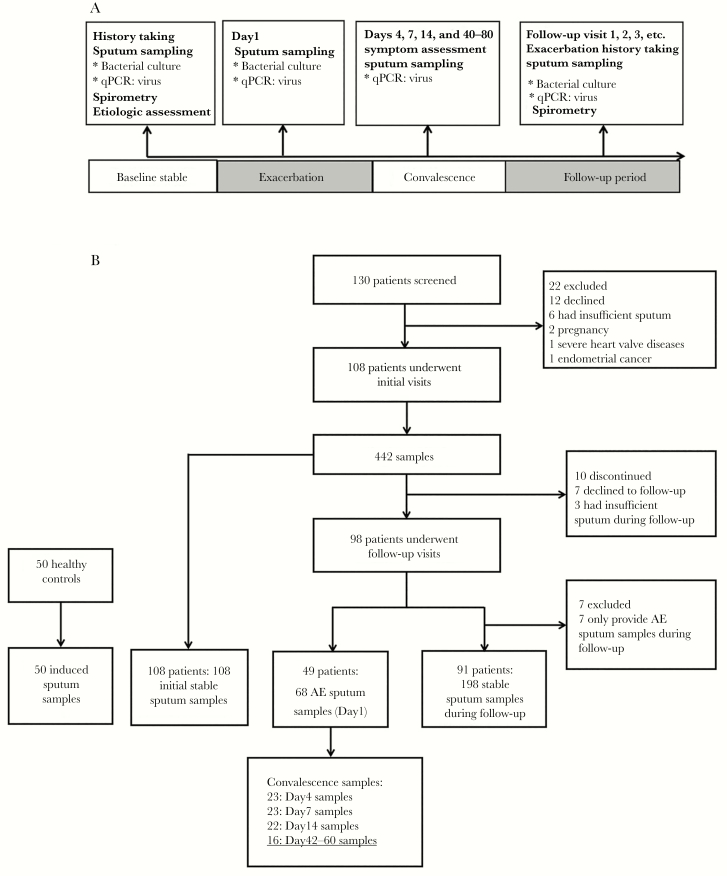
We routinely followed up patients every 3–6 months, during which patients contacted investigators in case of significant symptom worsening (within 2–5 days) and an exacerbation (AE) visit would be conducted within 48 hours. Following an international consensus, an AE was defined as continuous (≥48 hours) significant deterioration of 3 or more symptoms (increased cough frequency, sputum volume and/or consistency, sputum purulence, breathlessness and/or exercise tolerance, fatigue and/or malaise, and hemoptysis) that required changes in treatment [18]. Spontaneous sputum was sampled at stable visits, at AE visit (day 1, before antibiotic administration) and on-treatment days (including days 4, 7, and 14), and postexacerbation stable visit (days 40–80) for a subset of AE events (collectively termed AE convalescence follow-up visits) [19]. The study design is illustrated in Figure 1.
Figure 1.
Flowchart of study participant recruitment and sampling schemes. The 18 sputum specimens that are underlined within the figure were collected during stable-state visits; hence, they were also included in the 198 stable samples of 91 bronchiectasis patients. Abbreviations: AE, acute exacerbation; qPCR, quantitative polymerase chain reaction.
Healthy subjects attended a single visit, during which history taking, 3% hypertonic saline sputum induction (in healthy subjects only), chest x-ray, and spirometry were performed.
Details of sputum collection (including quality control) are described in the Supplementary Data. We extracted viral nucleic acids using the TaKaRa MiniBEST Viral RNA/DNA Extraction Kit (Takara Bio Inc., San Jose, USA) from sputum homogenized with 0.1% dithiothreitol. TaqMan real-time quantitative polymerase chain reaction (qPCR) was performed to detect 18 common respiratory viruses (including EBV) with the ABI Prism 7500 Real-time qPCR System (Thermo Fisher Scientific Inc., Waltham, USA). Viral detection kits were purchased from Guangzhou HuYanSuo Medical Technology Co. Ltd. (Guangzhou, China). The cycle threshold of 40 was considered positive. Sputum samples with positive EBV testing results were chosen for the measurement of copy numbers. The target sequence of the gp58 gene with known concentration (serial dilutions), which was extracted from the plasmid vector, was used for performing the standard curve evaluation. The mean computed tomography value was transformed to the copy number according to the standard curve. For EBV, detection was defined as EBV DNA detected at any visit during follow-up, whereas repeated detection denoted EBV DNA detected on at least 2 occasions at least 3 months apart within 1 year, which was extrapolated from the definition of bacterial isolation or colonization [17].
Sputum was centrifuged (20 000g) at 4°C for 120 minutes to prepare for the sputum sol phase (supernatant). Sputum CXC motif chemokine–8 (CXCL-8), interleukin (IL)-1β, tumor necrosis factor–alpha (TNF-α), and interferon-γ (IFN-γ) were detected with multiplex immunoassay kits (Biorad, Hercules, USA) [17].
Statistics Analysis
The detection rate of EBV DNA in sputum in Chinese people is unknown. Assuming a detection rate of 46% in stable bronchiectasis [9] and 16% in healthy subjects (pilot study with 18 subjects), considering a dropout rate of 20%, we estimated that we needed to recruit 100 bronchiectasis patients and 50 healthy controls based on 2-sided significance of .05 and a power of 90%.
The EBV load was presented as copy numbers and was subjected to logarithmic transformation. The EBV detection rate and loads were analyzed using the initial stable visit samples and samples during onset of AEs. Sputa collected at the initial visits and follow-up were included as “any stable” visit samples, whereas sputa collected during AE visits were included as “any AE” samples. Sputa collected during stable visits and onset of AEs from the same bronchiectasis patient were defined as “paired stable” and “paired AE” samples.
Data were presented as mean ± SD or median (interquartile range [IQR]) for continuous variables depending on the normality (assessed with the Kolmogorov-Smirnov test) and analyzed using independent and paired t tests or Mann-Whitney U tests and Wilcoxon tests where appropriate. Counts (percentages) were presented for categorical variables and analyzed with the chi-square or Fisher exact test. We determined the correlation between the load of EBV and clinical parameters with Pearson’s correlation analysis. Changes in EBV load throughout the course of AE were analyzed with the Wilcoxon test and stratified by the presence of common cold (frequently caused by respiratory viruses). Generalized estimating equations (GEEs) with the logit link were used to explore the effects of EBV DNA detection on the odds of experiencing AEs compared with stable visits. A linear regression structure was used for EBV load comparison between AE visits and stable visits, taking into account the repeated observations from individual participants. The trends of changes among the 3 groups (ie, lung function decline) were analyzed. Linear mixed-effect models were applied to compare lung function decline between patients detected and those not detected with EBV DNA and between patients with and without repeated detection, with the exacerbation frequency being treated as the covariate in the model. The risks of AEs during follow-up were compared with the Kaplan-Meier survival model with the Gehan-Breslow-Wilcoxon test. The odds ratio (OR) of EBV DNA detection was estimated with logistic regression models, and data with P < .20 were entered into multivariate analysis using a backward selection algorithm. P < .05 was considered statistically significant. We constructed the phylogenetic tree among 25 bronchiectasis patients whose EBV was successfully sequenced based on the latent membrane protein–1 (LMP-1) sequences by using MEGA 7.0 (http://www.megasoftware.net/) and calculated the amino acid similarity with BioEdit 7.0.1 (GenBank accession number: MK944375-944399). Statistical analyses were performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA) or Graphpad Prism, version 5.0 (Graphpad Inc., San Diego, CA, USA).
RESULTS
Subject Recruitment and Clinical Characteristics
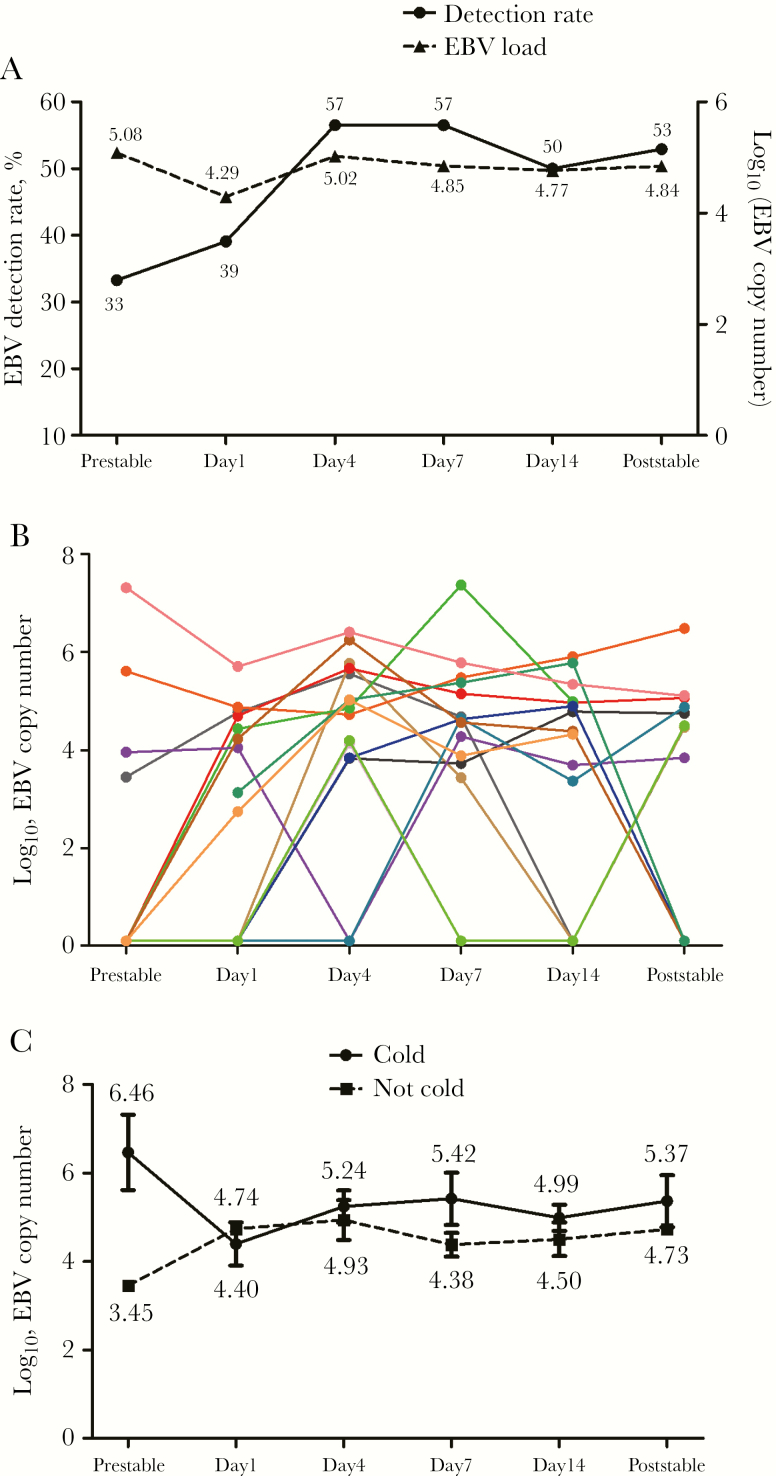
Of 130 bronchiectasis patients screened, 108 completed the initial stable visits. Ninety-eight patients completed follow-up until October 2018, of whom 91 patients with >1 stable visit sample were included for EBV DNA detection analysis. Forty-nine patients provided 68 AE samples, of whom 19 completed 23 AE convalescence follow-up visits.
Overall, we collected a median (IQR) of 3 (3–5) sputum samples per patient (Figure 1). The mean age was 46.8 years, and 60.2% were females among bronchiectasis patients. The median follow-up (IQR) was 13.0 (9.3–15.0) months. Baseline characteristics of the AE cohort were similar to the whole bronchiectasis patient cohort. Bronchiectasis patients tended to be older than controls. Bronchiectasis patients also had significantly lower body mass index, there were fewer current smokers, and they had poorer lung function compared with controls (Table 1).
Table 1.
Demographic and Clinical Characteristics of Participants
| Parameters | Whole Cohort (n = 108) | AE Cohort (n = 49) | Healthy Subjects (n = 50) | P Value |
|---|---|---|---|---|
| Age, median (IQR), y | 49.5 (36.0–59.0) | 50.0 (36.0–59.5) | 41.5 (29.0–55.5) | .165 |
| Body mass index, mean (SD), kg/m2 | 20.4 (3.3) | 19.6 (3.3) | 22.9 (3.4) | <.001 |
| Sex, female, No. (%) | 65 (60.2) | 34 (69.5) | 33 (66.0) | .502 |
| Smoking status, No. (%) | ||||
| Never-smokers | 100 (92.6) | 46 (93.9) | 44 (88.0) | <.001 |
| Ex-smokers | 8 (7.4) | 3 (6.1) | 0 (0.0) | |
| Current smokers | 0 (0.0) | 0 (0.0) | 6 (12.0) | |
| FEV1 % predicted, median (IQR) | 52.9 (41.0–70.1) | 52.5 (40.0–69.2) | 94.3 (87.7–102.6) | <.001 |
| Exacerbation frequency within 1 y, median (IQR) | 2.0 (1.0–2.5) | 2.0 (2.0–3.0)a | NA | NA |
| Bronchiectasis Severity Index | 7 (4–9) | 8 (4–10) | NA | NA |
| Mild, No. (%) | 32 (29.6) | 14 (28.6) | ||
| Moderate, No. (%) | 50 (46.3) | 20 (40.8) | NA | NA |
| Severe, No. (%) | 26 (24.1) | 15 (30.6) | ||
| E-FACED score, median (IQR) | 2.5 (1.0–4.0) | 2.0 (1.0–4.0) | NA | NA |
| Mild, No. (%) | 73 (67.6) | 33 (67.3) | ||
| Moderate, No. (%) | 34 (31.5) | 16 (32.7) | NA | NA |
| Severe, No. (%) | 1 (0.9) | 0 (0) | ||
| Etiology, No. (%) | ||||
| Postinfective | 27 (25.0) | 15 (30.6) | ||
| Idiopathic | 26 (24.1) | 11 (22.4) | NA | NA |
| Post-tuberculous | 17 (15.7) | 7 (14.3) | ||
| Primary immunodeficiency | 11 (10.2) | 5 (10.2) | ||
| Othersb | 27 (25.0) | 11 (22.4) | ||
| Medications, No. (%) | ||||
| Inhaled corticosteroids | 28 (25.9) | 14 (28.6) | NA | NA |
| Low-dose macrolides | 13 (12.0) | 8 (16.3) | ||
| Vaccination status, No. (%) | ||||
| Influenza vaccination within the last 12 mo | 7 (6.5) | 5 (10.2) | 0 (0.0) | .383 |
| Pneumococcal vaccination within the last 5 y | 4 (3.7) | 3 (6.1) | 0 (0.0) | .723 |
Data were presented as mean (SD) or median (IQR) for continuous variables depending on normality (assessed with the Kolmogorov-Smirnov test) and analyzed using independent and paired t tests or Mann-Whitney U tests and Wilcoxon tests where appropriate. P values in the last column denote the tests that compare 3 groups of the whole cohort, AE cohort, and controls. Patients with the primary diagnosis of COPD as the underlying etiology were excluded from this study. The high rates of never smokers are consistent with our previously published studies. No test for nontuberculous Mycobacteria was conducted during the study.
Abbreviations: AE, adverse event; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; IQR, interquartile range.
a P < .05: AE cohort compared with those without an AE.
bOther known causes, including Kartagener’s syndrome (n = 8, 7.4%), asthma (n = 8, 7.4%), gastroesophageal reflux (n = 3, 2.8%), diffuse panbronchiolitis (n = 3, 2.8%), connective tissue disease (n = 2, 1.9%), cystic fibrosis transmembrane regulator–related disease (n = 1, 0.9%), congenital airway defects (n = 1, 0.9%).
The Detection Rate of EBV DNA and Viral Load
At initial stable visits, EBV DNA was detected more frequently in bronchiectasis than in controls (48.1% vs 20.0%; P = .001). However, the detection rate did not differ between pooled stable and pooled AE samples (P = .810) or between paired stable and paired AE samples (P = .761).
The EBV loads did not differ significantly among the whole cohort, AE cohort, and controls even after adjustment for age (P = .393). Similar findings were found in both paired samples and pooled samples (Supplementary Figure 1). EBV DNA was detected more commonly in winter than in summer (39.0% vs 61.5%; P = .036), whereas viral loads were comparable. EBV loads did not differ significantly among healthy controls, inhaled steroid users, and nonusers (P = .458).
Furthermore, 36 (39.6%) patients yielded repeated detection of EBV DNA and tended to have higher EBV loads than those without (P = .073). Patients with EBV detected at stable visits had increased risks (OR, 21.33; 95% CI, 6.44–70.63) for repeated detection in subsequent visits.
EBV Detection Status Remained Consistent Over Time
For 91 patients with ≥2 stable visit samples, 64.9% yielded consistent EBV detection status in 2 consecutive visits (24.2% repeatedly detected; 40.7% consistently negative). EBV detected at initial stable visits was prone to repeated detection (relative risk [RR], 2.94; 95% CI, 1.40–6.20). The RR for being consistently EBV negative was 1.58 (95% CI, 1.17–2.13). Similar EBV transition patterns were found from the initial stable visit to the post-AE stable visit (Supplementary Table 2).
Among 59 patients with ≥3 stable visit samples, EBV DNA detection status remained unchanged in 40.0% during 3 consecutive stable visits (23.0% repeatedly detected; 17.0% continuously negative). EBV was detected at 2 subsequent visits in 35.1% of patients with EBV detected at the initial stable visit, whereas 45.5% with EBV-negative sputum yielded negative findings at 2 subsequent visits (Supplementary Figure 2). The EBV load was constant during 3 (P = .268) and 4 consecutive stable visits (P = .610) (Supplementary Figure 3).
Association Between EBV and AEs
Detection of EBV DNA was not associated with the onset of AE (OR, 0.91; 95% CI, 0.54–1.54), based on the pooled stable visit and AE visit samples at symptom onset (within 2.0 [IQR, 2.0–3.3] days), which yielded no substantial difference in viral loads (P = .498).
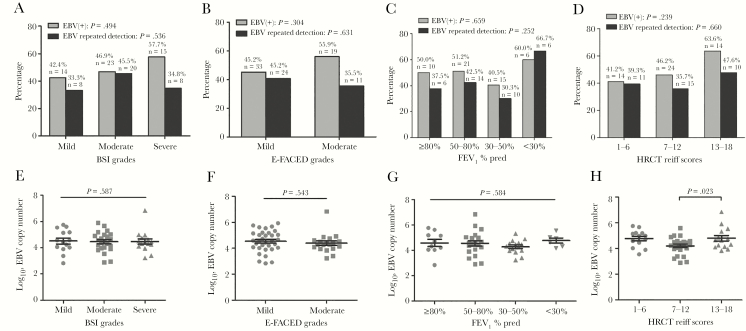
Nineteen patients yielded 23 AE convalescence pairs of samples (120 sputum samples), with 6.0 (IQR, 5.0–6.0) samples per patient. The detection rates were 33.3%, 39.1%, 50%, and 52.9% for pre–stable visit, onset of AE, day 14, and post-AE stable visit (median [IQR], 45 [40–54] days after day 1), respectively. The corresponding mean log10 load of EBV was 5.08, 4.29, 4.77, and 4.84, respectively. The viral loads on day 1 were significantly lower than those on day 4 (P = .011) and day 7 (P = .002). The EBV detection rate and load varied insignificantly throughout the natural course of AE, irrespective of the presence of common cold (Figure 2).
Figure 2.
Changes in the detection rate and load of EBV at stable visits and during the natural course of AEs and convalescence. A, Changes in the EBV detection rate and EBV load (log10 load) during the natural course of AEs and convalescence. B, Change in the EBV load at stable visits and during the natural course of AEs and convalescence within the same bronchiectasis patient. C, Change in the EBV load at stable visits and during the natural course of AEs and convalescence in patients with and without cold symptoms. Data are presented as percentage and mean (SD). The numbers appearing at different time points indicate the log10 load for EBV in sputum. The colors in Figure 2B represent different individual bronchiectasis patients. Day 1: within 2 (IQR, 2.0–3.3) days of the onset of AE symptoms; Poststable: median 45 (IQR, 40–54) days from the onset of AE symptoms. Abbreviations: AE, adverse event; EBV, Epstein-Barr virus; IQR, interquartile range.
Association Between EBV and Bronchiectasis Severity
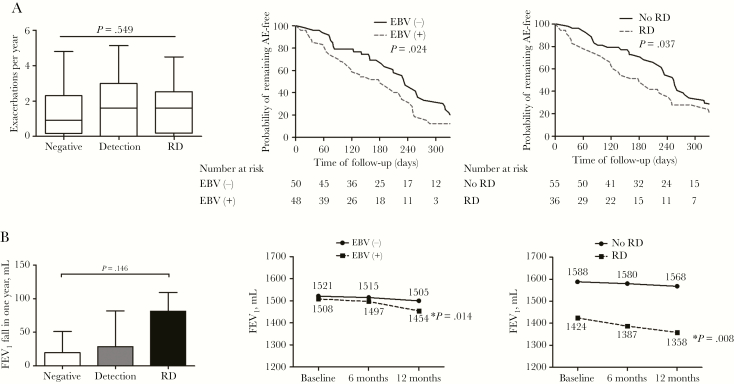
Next, we analyzed the association between EBV and disease severity in the whole cohort (n = 108) for the initial stable visit samples. Higher HRCT scores (≥13 points) were associated with higher EBV loads (4.80 vs 4.21; P = .023) compared with lower HRCT scores (7–12 points). However, the detection rate of EBV DNA and viral load differed unremarkably when stratified by lung function impairment and disease severity, including BSI and E-FACED scores. Repeated detection of EBV DNA was not associated with bronchiectasis severity (Figure 3).
Figure 3.
The rates of detection and repeated detection of EBV DNA (A–D) and EBV loads (E–H) among different severities of bronchiectasis. “ ” indicates the P value for the comparison among 3 groups; “
” indicates the P value for the comparison among 3 groups; “ ” indicates the P value for the comparison between 2 groups. The sputum samples collected during the first stable visits, but not all of the pooled samples, were included for the analyses presented herein. Only 1 patient was graded as having severe bronchiectasis based on E-FACED score. Hence, only patients with mild and moderate bronchiectasis were analyzed and presented. Abbreviation: EBV, Epstein-Barr virus.
” indicates the P value for the comparison between 2 groups. The sputum samples collected during the first stable visits, but not all of the pooled samples, were included for the analyses presented herein. Only 1 patient was graded as having severe bronchiectasis based on E-FACED score. Hence, only patients with mild and moderate bronchiectasis were analyzed and presented. Abbreviation: EBV, Epstein-Barr virus.
Bacterial isolation and colonization (especially Pseudomonas aeruginosa) reportedly augment airway inflammation. One hundred seventy-three stable visit samples and 55 AE samples were available for sputum cytokine detection. EBV DNA detected was not associated with higher levels of sputum IL-1β, CXCL-8, or IFN-γ in stable visits, except for TNF-α. Moreover, EBV DNA detection was not associated with heightened airway inflammation during AE. Interestingly, EBV loads correlated negatively with sputum IL-1β (r = –.33; P = .002), CXCL-8 (r = –.30; P = .008), and TNF-α levels (r = –.31; P = .005), regardless of stable visits or AE visits (Supplementary Figure 4).
EBV and Disease Progression
Patients followed up for >6 months (n = 91, only 9.3% had concomitant asthma) were included. Overall, the median frequency of AEs (IQR) was 1.5 (0–2.4) per patient-year. This figure was 0.9 (0.2–2.3), 1.6 (0–3.0), and 1.6 (0.2–2.5), respectively (P = .587), for patients with consistently negative EBV, detection of EBV DNA, and repeated detection of EBV DNA. The mean forced expiratory volume in a 1-second (FEV1) decline was 49.3 mL/y among 70 patients who completed spirometry. The mean FEV1 decline was 19.9 mL/y, 28.7 mL/y, and 81.4 mL/y (age-adjusted P = .493) for patients with consistently negative EBV, detection of EBV DNA, and repeated detection of EBV DNA, respectively.
After taking into account the exacerbation frequency during longitudinal follow-up, the annual FEV1 decline differed significantly between patients with and without repeated detection (P = .038). However, FEV1 decline was comparable between patients detected and those not detected with EBV DNA (P = .240). There was no significant interaction between the exacerbation frequency and the detection status of EBV (P = .529 and .114, respectively) for the above comparisons.
EBV DNA detected at initial visits was associated with a significantly shorter time to the next AE compared with negative EBV (median, 160 vs 230 days; P = .024), as was repeated detection compared with no repeated detection (median, 139 vs 227 days; P = .025). Meanwhile, FEV1 decline from baseline to follow-up was significantly faster in patients with detection and repeated detection of EBV DNA (Figure 4). EBV loads did not correlate with FEV1 decline (r = .066; P = .696) (Supplementary Figure 5).
Figure 4.
The association between EBV detection status and longitudinal clinical outomes. Negative: EBV not detected during follow-up visits; Detection: EBV detected at 1 visit during follow-up visits; Repeated detection: EBV detected in at least 2 visits during follow-up within 1 year. “ ” indicates the P value for the comparison among the 3 groups; “
” indicates the P value for the comparison among the 3 groups; “ ” indicates the P value for the comparison between 2 groups. EBV (-): EBV not detected at the initial stable visit; EBV (+): EBV detected at the initial stable visit. aWithin-group P value with respect to the baseline level. Abbreviation: EBV, Epstein-Barr virus; RD, Repeated detection.
” indicates the P value for the comparison between 2 groups. EBV (-): EBV not detected at the initial stable visit; EBV (+): EBV detected at the initial stable visit. aWithin-group P value with respect to the baseline level. Abbreviation: EBV, Epstein-Barr virus; RD, Repeated detection.
Risk Factors Associated With EBV DNA Detection
Multivariate analysis (among 108 patients with initial stable visit samples) revealed that having greater daily sputum volume (OR, 2.03; 95% CI, 1.20–3.42), more bronchiectatic lobes (OR, 1.35; 95% CI, 1.03–1.78), and treatment with long-acting muscarinic antagonists (OR, 4.16; 95% CI, 1.24–13.92) were risk factors for EBV DNA detection. Nonetheless, low-dose macrolides were protective for EBV DNA detection (OR, 0.19; 95% CI, 0.05–0.77) (Supplementary Table 3). See the Supplementary Data regarding the independent variables finally included in the regression model. Gender, age, body mass index, detection of respiratory viruses, and bacterial infection or colonization were not associated with EBV DNA detection (Supplementary Table 4). P. aeruginosa infection was also unrelated to EBV loads (Supplementary Figure 6).
EBV Strains Were Phylogenetically Homologous
Finally, we sequenced EBV strains based on nucleic acid sequence of LMP-1, a canonical component of EBV. The EBV strains were highly homologous within our bronchiectasis patient cohort, with amino acid similarity of 75.4%–100%. Moreover, these EBV strains were homologous (similarity, 74.5%–100%) compared with other EBV strains detected in Chinese patients (with diseases other than bronchiectasis) at regions outside Guangdong province (Supplementary Figure 7, Supplementary Table 5).
DISCUSSION
This is the first prospective study to explore the association between EBV and bronchiectasis. EBV DNA was readily detected in sputum and was repeatedly detected in some bronchiectasis patients. Patients with detection and repeated detection of EBV DNA yielded significantly shorter time to the next AE and greater lung function decline than patients without, suggesting that EBV might be associated with bronchiectasis progression.
EBV can be readily detected in blood in >90% of the world’s population [7, 20, 21]. Hence, serological assessment was not conducted further herein. Compared with blood, EBV DNA detection in sputum would more directly reflect the pathogenesis of bronchiectasis because EBV mainly infects lymphocytes and epithelial cells. Importantly, in some bronchiectasis patients, EBV DNA could be detected in sputum but not in saliva or detected with a substantially higher load in sputum than in saliva (Supplementary Table 1). Our observations and previous findings [9, 20] suggest that the high loads of EBV in sputum were not due to salivary contamination.
We have demonstrated a higher detection rate of EBV DNA (48.1%) but not viral loads (log10 load, 4.47) in bronchiectasis compared with healthy subjects. Compared with COPD patients, smokers without COPD yielded a substantially lower detection rate but similar loads of EBV [9]. Similarly, EBV DNA was detected in the sputum of 46% of COPD patients (mean log10 load, 4.71) [9] and in the lung tissues of >60% of patients with idiopathic pulmonary fibrosis (IPF) [10]. EBV can either be eliminated in immunocompetent individuals or persist (latent infections) owing to a delicate balance with host immunity [7, 22]. Patients with bronchiectasis, COPD, and IPF frequently have immune dysfunction, which provides a favorable environment for EBV [3, 23].
Detection of EBV was not associated with the onset of AE. Neither the detection rate of EBV DNA nor the viral load decreased significantly during AE. Overall, the EBV loads were constant despite a temporary nonsignificant reduction at AE onset. This was true when viewing the difference between stable-state and AE onset. Mechanisms underlying the greater PCR positivity at AE onset are unclear given the small sample size. Consistently, the log10 load was lower during AEs (from 4.71 to 4.08) in COPD [9]. Moreover, the EBV load progressively recovered during convalescence and, around day 4 postexacerbation, approached pre-AE stable visit levels. Hence, EBV was not eradicated from airways throughout AE. Interestingly, EBV load was negatively correlated with airway inflammation (possibly because of competition with other pathogenic microorganisms), partly explaining why EBV loads decreased during AE when inflammation was heightened.
Patients with detection and repeated detection of EBV DNA yielded faster lung function decline and a shorter time to the next AE. The precise mechanism remains unknown; however, the greater number of bronchiectatic lobes might have accounted for this finding. EBV LMP-1 positivity was associated with more rapid disease progression in IPF [10, 24], possibly because EBV LMP-1 expression could elicit an altered inflammatory response and impairment of tissue repair that resulted in lung fibrosis [24].
We noted that there was a trade-off between the EBV loads and airway inflammatory responses. The precise mechanisms remain unclear, but the defective host defense and heightened airway inflammation as a consequence of airway surface secretory IgA deficiency have been associated with latent EBV infection. Latent EBV infection has also been associated with CD4+ and CD8+ lymphocyte infiltration and airway remodeling [25].
Treatment with low-dose macrolides protected from, whereas inhaled corticosteroids predisposed to, EBV detection. Inhaled corticosteroids may attenuate airway inflammation, which may help partially elucidate the increased risk of EBV detection. Macrolides suppress inflammation by strengthening epithelial defense, reducing airway hypersecretion, and accelerating lymphocyte apoptosis [26]. Moreover, macrolides reportedly inhibited the growth of EBV-transformed B lymphocytes, providing further hints on the protective effects of EBV in bronchiectasis [27]. Alternatively, patients with more prominent airway inflammation were less likely to have EBV detection, probably because of macrolide therapy. In fact, long-acting muscarinic antagonists reportedly increased the risk of EBV detection by attenuating airway inflammation [28].
Our phylogenetic analyses suggested that EBV was largely homologous among the bronchiectasis population, at least in the Chinese population. Our findings call for greater awareness toward the universal latent viral infection that drives bronchiectasis progression.
Our findings are clinically relevant because EBV might drive the progression of bronchiectasis. Inclusion of EBV detection in clinical assessment may help indicate host immunity. Nevertheless, our study recruited patients from a single center. Comparison of EBV loads in spontaneous vs induced sputum is not ideal because shedding of epithelial cells differs between bronchiectasis and health. The EBV loads might also have been confounded by socioeconomic status, which was not thoroughly taken into account for adjustment. The duration of our study was relatively short, and hence an extended follow-up investigation would be needed to fully unravel the association between EBV DNA detection and the clinical outcomes of bronchiectasis. Furthermore, no sputum lymphocyte count was conducted to provide further evidence of EBV infection. We cannot address why EBV detection status switched during follow-up, nor differentiate latent from active EBV infection. The EBV loads might have been affected by inhaled steroid use. It remains unclear if the higher EBV detection rates and viral loads were surrogates of epithelial cell turnover. Mechanistic studies revealing how EBV is acquired are needed.
In conclusion, the results of this study indicate that patients with detection of EBV DNA have a shorter time to bronchiectasis exacerbations and that EBV may contribute to the progression of bronchiectasis.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported by the National Natural Science Foundation (No. 81870003), Guangdong Natural Science Foundation (No. 2019A1515011634), and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme 2017 to Prof. Guan; as well as the Impact and Mechanisms of Physical, Chemical and Biological Interventions on the Development and Outcome of Acute Lung Injury (No. 81490534), National Key Technology R&D Program (No. 2018YFC1311902), and Guangdong Science and Technology Foundation (No. 2019B030316028) to Prof. Zhong.
Potential conflicts of interest. All authors have no potential conflicts of interest with any companies/organizations whose products or services may be discussed in this article. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Chen CL, Guan WJ, and Zhong NS participated in study design; Chen CL performed laboratory experiments, data analysis, and interpretation; Chen CL, Li HM, Yuan JJ, Huang Y, Guan WJ, Han XR, Chen RC, and Zhong NS recruited patients; Chen CL, Li HM, Yuan JJ, and Huang Y performed follow-up; Chen CL, Guan WJ, de la Rosa-Carrillo D, and Martinez-Garcia MA drafted the manuscript; de la Rosa-Carrillo D, Martinez-Garcia MA, Guan WJ, and Zhong NS were responsible for study conception and provided critical review of the manuscript. de la Rosa-Carrillo D, Martinez-Garcia MA, Chen CL, Li HM, Yuan JJ, Huang Y, Guan WJ, Han XR, Chen RC, and Zhong NS approved the final draft for publication. Guan WJ was the guarantor of the study.
References
- 1. Amalakuhan B, Maselli DJ, Martinez-Garcia MA. Update in bronchiectasis 2014. Am J Respir Crit Care Med 2015; 192:1155–61. [DOI] [PubMed] [Google Scholar]
- 2. Nicotra MB, Rivera M, Dale AM, et al. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest 1995; 108:955–61. [DOI] [PubMed] [Google Scholar]
- 3. Boyton RJ, Altmann DM. Bronchiectasis: current concepts in pathogenesis, immunology, and microbiology. Annu Rev Pathol 2016; 11:523–54. [DOI] [PubMed] [Google Scholar]
- 4. Gao YH, Guan WJ, Xu G, et al. The role of viral infection in pulmonary exacerbations of bronchiectasis in adults: a prospective study. Chest 2015; 147:1635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saetta M, Baraldo S, Corbino L, et al. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160:711–7. [DOI] [PubMed] [Google Scholar]
- 6. Silva JR, Jones JA, Cole PJ, Poulter LW. The immunological component of the cellular inflammatory infiltrate in bronchiectasis. Thorax 1989; 44:668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol 2001; 1:75–82. [DOI] [PubMed] [Google Scholar]
- 8. Lung ML, Lam WK, So SY, et al. Evidence that respiratory tract is major reservoir for Epstein-Barr virus. Lancet 1985; 1:889–92. [DOI] [PubMed] [Google Scholar]
- 9. McManus TE, Marley AM, Baxter N, et al. High levels of Epstein-Barr virus in COPD. Eur Respir J 2008; 31:1221–6. [DOI] [PubMed] [Google Scholar]
- 10. Kelly BG, Lok SS, Hasleton PS, et al. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002; 166:510–3. [DOI] [PubMed] [Google Scholar]
- 11. Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50:1700629. [DOI] [PubMed] [Google Scholar]
- 12. Guan WJ, Gao YH, Xu G, et al. Aetiology of bronchiectasis in Guangzhou, Southern China. Respirology 2015; 20:739–48. [DOI] [PubMed] [Google Scholar]
- 13. Reiff DB, Wells AU, Carr DH, et al. CT findings in bronchiectasis: limited value in distinguishing between idiopathic and specific types. Am J Roentgenol 1995; 165:261–7. [DOI] [PubMed] [Google Scholar]
- 14. Miller MR, Hankinson J, Brusasco V, et al. ; ATS/ERS Task Force Standardisation of spirometry. Eur Respir J 2005; 26:319–38 [DOI] [PubMed] [Google Scholar]
- 15. Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martinez-Garcia MA, Athanazio RA, Girón R, et al. Predicting high risk of exacerbations in bronchiectasis: the E-FACED score. Int J Chron Obstruct Pulmon Dis 2017; 12:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guan WJ, Gao YH, Xu G, et al. Sputum bacteriology in steady-state bronchiectasis in Guangzhou, China. Int J Tuberc Lung Dis 2015; 19:610–9. [DOI] [PubMed] [Google Scholar]
- 18. Hill AT, Haworth CS, Aliberti S, et al. ; EMBARC/BRR definitions working group. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J 2017; 49:1700051. [DOI] [PubMed] [Google Scholar]
- 19. George SN, Garcha DS, Mackay AJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J 2014; 44:87–96. [DOI] [PubMed] [Google Scholar]
- 20. Grant WB. A possible role for Epstein-Barr virus infection in COPD? Eur Respir J 2008; 32:1412–3; author reply 1413. [DOI] [PubMed] [Google Scholar]
- 21. Smatti MK, Al-Sadeq DW, Ali NH, et al. Epstein-Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: an update. Front Oncol 2018; 8:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ressing ME, van Gent M, Gram AM, et al. Immune evasion by Epstein-Barr virus. Curr Top Microbiol Immunol 2015; 391:355–81. [DOI] [PubMed] [Google Scholar]
- 23. Chalmers JD, Hill AT. Mechanisms of immune dysfunction and bacterial persistence in non-cystic fibrosis bronchiectasis. Mol Immunol 2013; 55:27–34. [DOI] [PubMed] [Google Scholar]
- 24. Heukels P, Moor CC, von der Thüsen JH, et al. Inflammation and immunity in IPF pathogenesis and treatment. Respir Med 2019; 147:79–91. [DOI] [PubMed] [Google Scholar]
- 25. Polosukhin VV, Cates JM, Lawson WE, et al. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011; 184:317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev 2010; 23:590–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Majewski M, Korecka M, Kossev P, et al. The immunosuppressive macrolide RAD inhibits growth of human Epstein-Barr virus-transformed B lymphocytes in vitro and in vivo: a potential approach to prevention and treatment of posttransplant lymphoproliferative disorders. Proc Natl Acad Sci U S A 2000; 97:4285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trevethick M, Clarke N, Strawbridge M, Yeadon M. Inhaled muscarinic antagonists for COPD—does an anti-inflammatory mechanism really play a role? Curr Opin Pharmacol 2009; 9:250–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.