Highlights
-
•
Delivering fresh, safe, high quality and long shelf life lettuce is a key challenge.
-
•
Poor shelf life leads to major crop waste, largely overlooked in breeding programmes.
-
•
Research has identified genetic variation for post-harvest quality.
-
•
We outline multiomic datasets, genetic and genomic resources for lettuce breeding.
Keywords: Food safety, Lactuca sativa, Gene editing, Plant breeding
Abstract
Societal awareness of healthy eating is increasing alongside the market for processed bagged salads, which remain as one of the strongest growing food sectors internationally, including most recently from indoor growing systems. Lettuce represents a significant proportion of this ready-to-eat salad market. However, such products typically have a short shelf life, with decay of post-harvest quality occurring through complex biochemical and physiological changes in leaves and resulting in spoilage, food waste and risks to health. We review the functional and quantitative genetic understanding of lettuce post-harvest quality, revealing that few findings have translated into improved cultivar development. We identify (i) phytonutrient status (for enhanced antioxidant and vitamin status, aroma and flavour) (ii) leaf biophysical, cell wall and water relations traits (for longer shelf life) (iii) leaf surface traits (for enhanced food safety and reduced spoilage) and (iv) chlorophyll, other pigments and developmental senescence traits (for appearance and colour), as key targets for future post-harvest breeding. Lettuce is well-placed for rapid future exploitation to address postharvest quality traits with extensive genomic resources including the recent release of the lettuce genome and the development of innovative breeding technologies. Although technologies such as CRISPR/Cas genome editing are paving the way for accelerated crop improvement, other equally important resources available for lettuce include extensive germplasm collections, bi-parental mapping and wide populations with genotyping for genomic selection strategies and extensive multiomic datasets for candidate gene discovery. We discuss current progress towards post-harvest quality breeding for lettuce and how such resources may be utilised for future crop improvement.
1. The need to breed for post-harvest quality
Lettuce (Lactuca sativa L.) is one of the most widely consumed and economically important vegetables, valued at over $2.7 billion in 2018 in the US alone (USDA, 2019). Thought to descend directly from wild species Lactuca serriola L. (de Vries, 1996), cultivated lettuce is produced either as a compacted whole head, it can be shredded, or harvested at the baby leaf stage. Baby leaf and shredded lettuce is typically sold in bags of pre-washed, ready-to-eat salad mixes, described as ‘minimally processed and value-added’ products, which are becoming increasingly popular due to the added convenience presented to the consumer (Dash et al., 2013). Lettuce is highly versatile and can be grown as a field crop, greenhouse hydroponic or indoor vertical crop. As societal awareness of the importance of healthy eating has increased, so has the market for processed bagged salads, which remains as one of the strongest growing food sectors across the globe (Brug et al., 2008; Stuart, 2011). Production of bagged salads originated at a small-scale in San Francisco Bay Area in the mid-1980′s before progressing to large-scale production towards the end of the decade (Stuart and Woroosz, 2011). Since a rapid growth in the 1990s, which in the US saw a 51.5 % expansion in sales in 1995, the market for prepared salads has been steadily increasing (Stuart, 2011).
In intensively managed lettuce cropping systems, water and agrochemicals are applied systematically to ensure satisfactory crop performance, driven by the high post-harvest quality demanded by retailers (Barrière et al., 2014). Post-harvest quality is largely governed by sensory factors such as appearance, flavour, aroma and texture. However, nutritional value and safety, in terms of the absence of agrochemicals or human pathogenic microorganisms, are other key aspects of product quality (Pentzer and Heinze, 1984). These post-harvest quality factors determine the product shelf life, defined as the length of time a product can be stored before quality declines and it becomes unfit for consumption. A reduction of product quality throughout shelf life is usually first perceived as a decline of visual appearance due to leaf tearing, yellowing and browning, followed by a deterioration of the flavour and aroma profiles caused by the development of fermentative metabolites by microbial contaminants and a loss of leaf firmness, succulence and juiciness, altering texture and mouthfeel (Kevers and Falkowski, 2007).
Crops lacking homogeneity or with visual imperfections can be rejected at harvest and processing stages, with up to 30 % of total salad crop production estimated to be lost before it reaches the retailer (Tesco Plc., 2013). Additionally, processing salad leaves by washing, drying, shredding and packaging can cause mechanical damage through bruising and tearing, leading to discolouration and also providing additional attachment sites for bacteria, increasing the opportunity for microbial spoilage and accelerating leaf decomposition (Gil et al., 2009). This leads to a relatively short shelf life of bagged salads of typically only 5–6 d after purchase and results in large amounts of waste, with 35 % of total salads produced estimated to be wasted by the consumer in the home (Zhang et al., 2007; Tesco Plc., 2013). In 2012, salad waste in the UK equated to 44,000 tonnes and £277 million of losses, with the main cause attributed to the product not being used before it was deemed inedible (WRAP, 2012). Not only is this likely to impact consumer satisfaction and willingness to repeatedly purchase products, but it is highly unsustainable. Worldwide in 2010, wasted food utilised 250 km3 of surface and groundwater, accounted for 1.4 billion hectares of land (30 % of the total Earth’s land suitable for agriculture) and if ranked in terms of whole country greenhouse gas emissions, food waste would be in the top three, beaten only by the USA and China (FAO, 2013). Post-harvest losses are attributable to a considerable amount of this food waste. Mitigating such losses would place a reduced strain on the need to increase crop yields to accommodate the world’s growing population.
Whilst breeding efforts such as those of the Green Revolution have focused on crop yield in an attempt to alleviate the world’s mounting food crisis or for financial gain, nutrient content and quality is becoming a growing concern for food security. Even in developed cultures, where the availability and variety of food is considered plentiful, malnutrition persists and has been linked to the prevalence of chronic diseases such as cardiovascular disease, cancer, obesity and type 2 diabetes, which are the leading causes of death worldwide (IHME, 2017). Lack of fruit and vegetables in the diet has been ranked in the global top 20 risk factors for disability-adjusted life-years, a measure of the number of years of life lost to poor-health, disability or early death (Forouzanfar et al., 2015) and up to 8 million deaths in 2013 were estimated to have been preventable by an increase in fruit and vegetable consumption (Aune et al., 2017). Poor diet-related illnesses also place a huge economic burden on society, costing the UK National Health Service £5.8 billion from 2006 to 2007 alone (Scarborough et al., 2011).
As environmental awareness increases, continued success of the bagged salad industry in a consumer-driven market will require significant efforts to improve post-harvest quality. With the release of genetic resources such as the lettuce reference genome (Reyes-Chin-Wo et al., 2017) providing exciting opportunities for lettuce breeding and improvement, we discuss progress made towards improving post-harvest quality and outline breeding targets for future enhancement of the crop.
2. Key determinants of post-harvest quality
2.1. Appearance
Though the limit of product acceptance is subjective, it is fundamentally based upon appearance and freshness at the point of purchase, including bright-coloured, blemish-free leaves (Rico et al., 2007). Mechanical damage of leaves incurred throughout the harvesting and processing chain, despite careful optimisation of this process, is the major factor influencing post-harvest appearance and presents a trade-off between convenience, safety and product shelf life. Any damage to whole baby leaves during processing, such as bruising, tearing, and the mechanical shredding of whole head lettuce, compromises visual appearance by inducing browning, pinking and leaf senescence (Fig. 1) resulting in immediate rejection by consumers (Rico et al., 2007). Leaf wounding leads to the rupturing of cellular membranes, enabling the mixture of phenolic compounds in the vacuole with enzymes in the cytoplasm (Saltveit, 2016). Enzymatic discolouration then proceeds as a result of polyphenol oxidase (PPO) activity, which catalyses the oxidation of phenols already present in the lettuce leaves, such as chicoric, caftaric and chlorogenic acids, to quinones (Hunter et al., 2017). Caffeic acid derived quinones are pink-pigmented, whilst higher molecular weight phenols, such as chlorogenic acid, produce brown-pigmented quinones (Saltveit, 2018). Additionally, wound signalling leads to an increase in active phenylalanine ammonia-lyase (PAL), the first key enzyme in phenylpropanoid metabolism, which generates additional phenolic metabolites, accelerating browning (García et al., 2017). This wound response, measured by PAL activity, has been shown to transmit to tissues neighbouring the wound site at a rate of 0.5 cm per hour for excised lettuce leaves (Saltveit, 2016). Yellowing or loss of green colour intensity can also reduce visual quality, resulting from chlorophyll breakdown (Toivonen and Brummell, 2008).
Fig. 1.
Decline in visual quality throughout the shelf life of romaine (A to B) and lollo rosso (C to D) lettuce. Adapted from Wagstaff et al. (2010).
2.2. Flavour and aroma
Flavour is a factor governing quality which is often overlooked, yet usually deteriorates before visual shelf life. Although product appearance is the main driver for initially buying minimally processed vegetables, taste is the determining factor leading to recurrent purchasing and aroma, controlled by odorous volatile compounds, and plays a key role in flavour perception (Rico et al., 2007; Kader, 2008). A balance between sweetness and acidity is essential for favourable taste and bitterness instinctively triggers avoidance responses, evolved in mammals to prevent the consumption of toxic foods which often have an intense bitter taste (Bachmanov et al., 2014). Sweetness of lettuce, largely determined by glucose, was found to be the main factor influencing consumer liking, whilst bitterness was strongly negatively correlated (Chadwick et al., 2016). Bitter taste perception is facilitated by a large family of taste receptors (T2Rs), enabling the detection of bitterness at extremely low thresholds of micromolar concentrations and with a prolonged sensation in comparison to other tastes (Chandrashekar et al., 2000; Drewnowski and Gomez-Carneros, 2000). For this reason, debittering of food crops has been a longstanding goal of plant breeding (Drewnowski and Gomez-Carneros, 2000). A number of compounds are responsible for bitterness of lettuce, including flavonoids such as the flavonols and anthocyanins, as well as the terpenoid sesquiterpene lactone (Drewnowski and Gomez-Carneros, 2000; Soares et al., 2013). Flavonols such as quercetin and kaempferol also determine astringency, a drying mouthfeel which can be unpleasant at high concentrations and is important characteristic in determining flavour (Kader, 2008).
Throughout product shelf life, respiration of detached leaves can lead to the breakdown of sugars and organic acids, resulting in a deterioration of flavour (Saltveit, 2016). This effect is exaggerated in wounded tissues, in which respiration is accelerated in order to synthesise compounds required for tissue repair (Saltveit, 2016). Indeed, the respiration rate of baby leaf lettuce has been observed to increase significantly throughout the course of product shelf life (Wagstaff et al., 2007). Low oxygen concentration of tissues can induce anaerobic respiration, producing fermentative metabolites and leading to the development of off-odours by the generation of volatile organic compounds (VOCs), impacting on aroma and flavour (Tudela et al., 2013). Cut iceberg lettuce has been found to produce VOCs including aldehydes, alcohols, terpenes, ketones, acids and sulphurous compounds (Deza-Durand and Petersen, 2011, 2014). However, differences in VOC production have been noted depending on cutting technique and cultivar, suggesting results may not be translatable to intact baby leaves. Analysis of the VOC profiles of whole rocket leaves throughout storage indicated that an increase in sulphur-containing compounds reduced aroma scores, with dimethyl sulphide exhibiting a rotten cabbage-like odour with a low odour tolerance threshold (Spadafora et al., 2016), and was also produced from fresh-cut lettuce (Deza-Durand and Petersen, 2011). An increased incidence of microorganism contaminants can also produce fermentative metabolites, further adjusting the aroma and flavour profile (Kader, 2008).
2.3. Texture
Leaf texture is another important sensory attribute for determining post-harvest quality and consumer acceptance, comprised of firmness, succulence and juiciness. Leaf anatomy including cell wall thickness and strength, cell size and cell:cell adhesion determines firmness, along with leaf turgor determined by water content at point of harvest (Toivonen and Brummell, 2008). Leaves with smaller, tightly packed cells and increased cell wall content give rise to a firm, less juicy leaves. Water loss post-harvest is one of the major factors contributing to the negative perception of leaf texture, resulting in the reduction of leaf turgidity and wilting, impacting on leaf firmness and perceived juiciness (Agüero et al., 2011). The rate of water loss is typically delayed by post-harvest storage at cold temperatures and at low ambient relative humidity, to reduce respiration and transpiration.
2.4. Nutritional value
Aside from the visual quality of lettuce, an additional factor valued by consumers, but often less so by producers, is the nutritional content of the product. Though consisting of approximately 95 % water, lettuce is a source of carotenoids such as lutein, zeaxanthin and β-carotene, vitamins E and C and a variety of non-vitamin secondary metabolites (phytochemicals), thought to reduce the incidence of chronic diseases (Kim et al., 2016). There is significant potential to enhance the nutritional status of lettuce. Metabolite profiling of lettuce has identified a wide variety of phytochemicals; primarily polyphenolic compounds such as chicoric acid (dicaffeoyltartaric acid), chlorogenic acid (caffeoyl quinic acid), anthocyanins, quercetin and kaempferol derivatives (Nicolle et al., 2004; Llorach et al., 2008; Damerum et al., 2015). Such phytonutrients have been recognised for their health benefits, including a reduced incidence of cardiovascular and neurodegenerative diseases, cancer and obesity (Martin, 2013; Cory et al., 2018).
Despite the diverse biochemistries observed amongst phytochemicals, a common feature is their antioxidant activity, which is linked to their health benefits. A major contention with this theory is the bioavailability and bioaccessibility of compounds and whether they are absorbed into the blood plasma and transported to tissues after consumption, or whether they are simply metabolised or transformed by gut microorganisms (Bjørklund and Chirumbolo, 2017). Attributing the health benefits of phytonutrients to their antioxidant activity is oversimplified. One hypothesis is that instead of acting as antioxidants directly, phytonutrients are perceived as toxins, eliciting cellular stress responses which indirectly results in increased antioxidant activity (Bjørklund and Chirumbolo, 2017). For example, quercetin was identified to induce apoptosis of colon cancer cells in vitro, via reactive oxygen species-mediated activation of the Sestrin 2-AMPK-mTOR signalling pathway (Kim et al., 2013). As lettuce is most commonly consumed raw, not only does this increase convenience and therefore likeliness of consumption due to reduced preparation times, but could also be beneficial given that cooking has been reported to interfere with the chemical properties of phytochemicals. Phytochemicals such as chlorogenic acid are suggested to be partially or wholly lost when cooked, making uncooked lettuce an important dietary source (Clifford, 2000).
2.5. Food safety
The biosafety of ready-to-eat leafy salad consumption is becoming increasingly important, with a higher prevalence of foodborne illnesses observed over the last decade than can be attributed to increased consumption (Taban and Halkman, 2011). In the last two decades, there have been numerous reports of foodborne outbreaks resulting in hospitalization and even death, most commonly caused by shiga toxin-producing Escherichia coli strains, Listeria and Salmonella sp. (Critzer and Doyle, 2010). Recently, 58 cases of illness and 2 deaths in the US and Canada were reportedly caused by an E. coli outbreak originating from romaine lettuce (Chokshi, 2018). Such outbreaks also result in huge economic losses, estimated at $7 billion per year in the US, as a consequence of removing products from shelves, notifying customers and the cost of lawsuit damages, aside from the sharp decline in market sales often accompanying such outbreaks which can take years to stabilise (Hussain and Dawson, 2013).
Investigations into the lettuce leaf surface microbiota, termed the phyllosphere, have demonstrated abundance ranging from 105 to 107 cells per g of plant material, with bacteria typically being the most abundant (Williams et al., 2013). Low bacterial diversity has been observed in lettuce phyllosphere, which is largely dominated by the phyla Proteobacteria and Firmicutes, followed by Actinobacteria, of which the overall ratios were seasonally variable (Williams et al., 2013). In addition to the indigenous microflora present on lettuce leaves, the crop can be exposed to human pathogens at various opportunities on the journey from farm to fork pre- and post-harvest, through contaminated irrigation water, improperly treated organic fertilisers, wild animals and mechanical and human handling during processing and packaging (Taban and Halkman, 2011). Fresh and minimally processed products such as lettuce are exposed to additional possibilities for contamination during production and are usually consumed raw, eliminating the potential to kill microorganisms via heating. Although the human pathogen E. coli O157 has been shown to initially decay rapidly following inoculation onto the lettuce leaf surface, this decay has generally been characterised as biphasic, with small populations surviving over long time periods (Munther et al., 2020). Amongst these surviving sub-populations are “persister” cells present in a dormant, low metabolic and nonreplicating state, which have been observed to increase during population decline on drying leaves (Munther et al., 2020). E. coli O157 has been detected from surface-sterilised leaf samples, suggesting it is able to enter into the leaf (Erickson et al., 2010), where it could evade subsequent washing or disinfectant.
The increase in foodborne diseases is observed despite the implementation of enhanced safety measures, such as the Food Safety Modernization Act by the US Food and Drug Administration, which applies science-based decision making to outline standards for growing, harvesting, packing, storing and transporting goods for human consumption (Melotto et al., 2020). The failure to curtail foodborne disease outbreaks by rigorous control and regulation of production procedures alone indicates that a multidisciplinary approach to crop development is required, considering plant genotype, along with plant and microbe genotype x environment interactions.
3. Pre- and Post-harvest factors influencing post-harvest quality of lettuce
Both pre-harvest and post-harvest factors throughout the farm-to-fork journey can compromise post-harvest quality. Pre-harvest, good farm management practices including irrigation scheduling, fertiliser and pesticide application and harvest planning maximises crop quality. Excessive nitrogen application can reduce the nutritional quality (Albornoz, 2016) and overall post-harvest quality, measured as visual quality and texture (Hoque et al., 2010), whilst applying a deficit irrigation can increase nutritional content and reduce browning in fresh-cut lettuce (Oh and Carey, 2010; Luna et al., 2012). Fertilisation regimes can also impact on the mechanical properties of lettuce. For example, calcium application was found to increase leaf strength and stiffness of iceberg lettuce (Newman et al., 2005). Enriching soil with coffee grounds was found to increase carotenoid and chlorophyll content and improved biomass (Cruz et al., 2012). Using appropriate harvesting tools is important to minimise mechanical damage. Cutting lettuce with a blunt blade instead of a sharp blade has been found to increase cellular damage, loss of cell sap and accelerate browning, leading to a significant reduction in shelf life (Bolin et al., 1977). Harvest scheduling can also influence post-harvest quality, with harvesting at the end of the day found to increase shelf life by up to 2 d (Clarkson et al., 2005).
During processing and packaging, the addition of sanitizers to wash water is common practice in Europe and the US in an attempt to reduce microbial spoilage, though the efficacy and safety of such methods is debated (Gil et al., 2009). Allende et al. (2008) compared the effectiveness of washing shredded iceberg lettuce in chlorine and six other commercial sanitisers and found that although washing with sanitiser was initially more effective than just water alone in reducing microbial attachment, similar counts of microorganisms could be recovered from leaves following storage, suggesting washing may have limited benefits by the time the product is actually consumed. There are also safety concerns over the use of chlorine-based chemicals, due to the production of potentially carcinogenic, chlorinated organic compounds such as chloropicrin, haloacetic acids, haloketones and trihalomethanes (Gil et al., 2009). The use of modified atmosphere packaging (MAP), in which the internal atmosphere of packaging is altered to have a reduced O2 concentration, has been found to decrease PAL and PPO enzyme activities and lead to a reduction in cut-edge browning (Luna et al., 2016). Effects are thought to be caused by suppression of respiration, ethylene-induced metabolism, moisture loss and the high CO2 environment is thought to reduce tissue pH, inhibiting enzyme activity (Watada and Qi, 1999). However the effectiveness of MAP was found to be genotype dependent, with its use found to extend the shelf life of certain cultivars and not others (Hayes and Liu, 2008).
Post-harvest, the preservation of low storage temperatures is critical for maintaining post-harvest quality, to reduce respiration rate and consequent deterioration (Watada and Qi, 1999). Lettuce is often vacuum-cooled soon after harvest to remove field heat and surface moisture, processed and package at cold temperatures (<5 °C), before distribution in refrigerated trucks (Ozturk and Ozturk, 2009). This cold chain can be compromised during distribution and retail. Though 0−5 °C is considered the optimal temperature for lettuce storage, retailers often store bagged salads as high as 10 °C due to the use of open refrigerator displays, because consumers are more likely to purchase produce stored on an open shelf (Hall et al., 2013). Aside from impacting on visual quality and flavour, sub-optimal storage temperatures can also facilitate bacterial growth. An increase in the shiga toxin-producing strain E. coli O157:H7 was observed on fresh-cut lettuce stored at 12 °C compared to 5 °C (Luo et al., 2010). Despite this, visual quality was maintained at an acceptable level for consumption, outlining the importance of low temperature storage in maintaining biosafety. Not only is bacterial abundance increased, evidence suggests temperatures above 15 °C induce acid resistance pathways, which facilitates survival in the gastrointestinal tract and increasing pathogenicity (Chua et al., 2008). Relative humidity is another important factor influencing post-harvest quality, with low humidity leading to an acceleration in evapotranspiration and wilting (Agüero et al., 2011). Even at optimal temperatures, storage under low relative humidity (70–72 %) was found to reduce the shelf life of butterhead lettuce by up to 75 % and led to increased pigment degradation than when stored at optimal relative humidity (95–98 %, Agüero et al., 2011). Changes in nutritional quality have also been observed during storage, with a strong decline in ascorbic acid and sucrose concentration of whole baby leaf lettuce within 5 d storage at both 4 °C and 10 °C, however little change was observed in total chlorophyll, carotenoid or polyphenol content (Spinardi and Ferrante, 2012).
4. Targeting plant genetics for improving the post-harvest quality of lettuce
Investigations on improving the post-harvest quality of lettuce thus far have largely focused on washing, packaging and storage (for a review, see Gil, 2016), but there has been little research on breeding for improved post-harvest quality, perhaps reflecting a relatively poor understanding of the genetic basis of these complex traits. Genetic variation for post-harvest traits has been observed, demonstrating the potential for breeding lettuce for improved quality. Numerous investigations have identified significant differences in the nutritional quality of lettuce, particularly between red and green cultivars, with red varieties containing increased densities of phenolic compounds and anthocyanins (Nicolle et al., 2004; Mou, 2005; Llorach et al., 2008; Damerum et al., 2015). Metabolite profiling of 30 leaf and head lettuce cultivars identified leaf varieties containing higher levels of phenolic compounds, whilst head varieties contained higher concentrations of terpenoids and long chain fatty acids (Yang et al., 2018). Metabolomics has also been utilised to identify biomarkers able to predict browning susceptibility, with chlorogenic acid derivatives positively correlated with browning and sinapaldehyde derivatives, precursors in ligning biosynthesis, found to be negatively correlated with browning (García et al., 2019). This suggests that redirecting phenolic acid metabolism towards lignin biosynthesis via the increase of key enzymes such as caffeate O-methyl transferase and ferulate 5-hydroxylase may be a good approach to reducing browning pigmentation.
In an attempt to characterise physiological leaf changes which occur over the course of baby leaf lettuce shelf life, Wagstaff et al. (2007) investigated cellular properties which are altered during the senescence of excised leaves in lettuce types lollo rosso and cos. A number of changes to leaf physiology were observed, including fracturing of the plasma membrane leading to membrane leakage, plastid senescence, formation of plastoglobuli (storage structures protecting photosynthetic electron carriers from oxidative damage) and apparent plasmolysis of cells (Wagstaff et al., 2007). Signs of discolouration were noted, particularly in regions that had been previously damaged during processing, suggesting that for baby leaf production, leaves that are better able to withstand the harvest and processing chain and suffer minimal damage are likely to have an improved post-harvest quality and increased shelf life. Although leaves had an increased percentage plasticity towards the end of shelf life, no major changes to cell wall structure were observed, which suggests more subtle changes to the cell wall were occurring and could perhaps be caused by differences in cell:cell adhesion (Wagstaff et al., 2007). The end of shelf life of bagged baby leaves is often characterised by a phenotype resembling leaf senescence, including blackening, yellowing and waterlogging (Wagstaff et al., 2007). Though symptoms are similar, Wagstaff et al. (2007) reported key differences between developmental senescence and the senescence of excised baby leaf lettuce. Most notably, was the absence of DNA laddering which occurs at the later stages of developmental senescence during programmed cell death, suggesting the death of bagged excised leaves begins as a regulated process but then becomes disorganised (Wagstaff et al., 2007). Given that bagged leaves are in an enclosed atmosphere, with no organ sink for the recycling of nutrients, it is perhaps unsurprising that death occurs in a distinct way to developmental senescence. Analysis of gene expression in detached lettuce leaves over the course of storage revealed an initial increase in genes associated with stress, redox regulation, phenylpropanoid metabolism and ethylene and auxin inducible genes (Ripoll et al., 2019). Later at 7 d, dehydration responsive, WRKY transcription factors and cytokinin-related genes demonstrated increased expression, whilst those involved in primary metabolism, cell cycle regulation and cell wall maintenance were largely downregulated (Ripoll et al., 2019).
Clarkson et al. (2003) measured leaf traits in contrasting baby leaf lollo rosso cultivars observed to be “good” and “poor” at withstanding processing without incurring damage and found leaves from the “good” variety had an increased osmolarity and reduced cell wall plasticity. Shelf life was found to be improved by up to 33 % in leaves which had undergone mechanical touch stress pre-harvest and these leaves had a reduced epidermal cell and leaf area and increased dry weight in comparison to non-stressed plants (Clarkson et al., 2003). This was hypothesised to be caused by an increase in the activity of a group of cell wall modifying enzymes, xyloglucan endotransglucosylases/hydrolases (XTHs), involved in the deposition of the hemicellulose xyloglucan and therefore contributing to the strength of the cell wall (Clarkson et al., 2003). By reducing XTH gene expression in lettuce using an RNA interference (RNAi) ‘knockdown’ approach, shelf life was increased by up to six days in the transgenic lines, which had a reduced leaf area, improved cell wall strength and a thickened membrane in comparison to wild type plants (Wagstaff et al., 2010). Interestingly, XTH gene expression has been found to be influenced by touch stress in Arabidopsis, with the expression of four XTH genes shown to be elevated in response to touch and three demonstrating a reduced expression (Lee et al., 2005). Recently, 10 XTH genes were shown to have reduced expression during post-harvest storage (Ripoll et al., 2019). These studies suggest that pre-harvest leaf physiology traits are important influencers of shelf life and are therefore targets for post-harvest quality improvement.
There is evidence that the phyllosphere and rhizosphere colonisation of the human pathogen E. coli O157 varies depending on lettuce cultivar, likely caused by genotype-specific differences in root exudates and leaf morphology (Quilliam et al., 2012). On the leaf surface, bacteria preferentially reside at the base of trichomes, on the outer rim of stomata and in the cell grooves and colonisation is also affected by epicuticular wax content (Erickson et al., 2010). Internalisation of E. coli O157:H7 into lettuce leaves was increased when inoculated onto the abaxial as opposed to the adaxial leaf surface, suggesting that stomata, which are more abundant on the abaxial leaf surface, may be an important point of entry (Erickson et al., 2010). Indeed, the internalisation rate of E. coli O157:H7 and Salmonella enterica has been found to positively correlate with stomatal aperture and pore area, a trait determined to vary significantly amongst Iceberg, Batavia, Romaine and green and red loose leaf lettuce cultivars (Jacob and Melotto, 2020). Cultivars also differed in the survival and population growth of bacteria in the apoplast following direct infiltration, with a cultivar demonstrating low apoplastic persistence exhibiting higher reactive oxygen species production than a cultivar with higher persistence post-inoculation, likely indicative of a more pronounced hypersensitive pathogen response (Jacob and Melotto, 2020). The ability to characterise such genetic variation could be harnessed in the development of elite cultivars with reduced attachment and internalisation of human pathogens.
4.1. Genetic and genomic resources
Complex traits such as post-harvest quality are quantitative in nature; that is, they are controlled by multiple, distinct and often interacting loci, but are also heavily influenced by the environment. In quantitative genetics, the amount of phenotypic variation that is controlled by the genotype can be measured, based on the inheritance pattern of genetic markers and the associated phenotype of a trait of interest by quantitative trait loci (QTL) mapping (Moose and Mumm, 2008). QTL can be investigated to identify the genes underlying traits and knowledge of the beneficial or antagonistic alleles governing an important phenotype can be used to inform molecular breeding programmes by marker-assisted selection (MAS), in which successful crosses can be identified rapidly by genotyping alone without the need to phenotype thousands of plants, accelerating the breeding process (Moose and Mumm, 2008). In order to detect QTL, resources required include experimental (inbred) or natural (wide) mapping populations, polymorphic molecular markers and a wealth of phenotype and genotype data to enable links between phenotype and underlying genotype to be elucidated.
The first genetic map produced for lettuce consisted of 53 markers, primarily restriction fragment length polymorphism (RFLP) loci, covering 404 cM and 9 linkage groups, constructed from a population of a Crisphead x Butterhead cultivar cross (L. sativa cv. Calmar and L. sativa cv. Kordaat, respectively) of 66 F2 plants (Landry et al., 1987). Advances in molecular marker discovery have since enabled the development of an ultra-dense high resolution molecular marker map, identified using a customised lettuce Affymetrix GeneChip consisting of 6.5 million probes designed from expressed sequence tag (EST) sequences covering 35,628 unigenes (Stoffel et al., 2012). This custom GeneChip was used to analyse polymorphism in a population of 213 F7:8 recombinant inbred lines (RILs) developed from an interspecific cross between L. sativa cv. Salinas and L. serriola acc. US96UC23. This has become the core reference mapping population for lettuce, enabling the construction of a 1585 cM map with 13,943 loci with an average distance of 0.4 cM across nine linkage groups (Truco et al., 2013). This ultra-dense genetic map is publicly available as part of the Compositae Genome Project (CGP) database of resources, utilised worldwide. The CGP began as a collaboration between researchers in the United States at the University of California (Davis), Oregon State University (Corvallis), Indiana University (Bloomington) and the University of Massachusetts (Boston) in 1999, with the intention of developing the genetic and genomic resources and tools for breeding of economically relevant members of the Compositae family and to aid in the understanding of genome evolution and diversification of this family (Michelmore et al., 2003). For lettuce, the project has so far delivered a comprehensive EST database for cultivated and wild accessions, transcriptome assemblies and genetic mapping resources (Table 1). In collaboration with the Lettuce Genome Sequencing Project (LGSP; Michelmore laboratory at University of California, Davis and the Beijing Genomics Institute, China) and supported by the Lettuce Genome Sequencing Consortium (LGSC) comprised of ten seed companies worldwide, a reference assembly of the lettuce genome has now been published (Reyes-Chin-Wo et al., 2017). The 2.7 Gb genome was initially sequenced to 72.5 % coverage using a whole genome shotgun method of Illumina reads and scaffold contiguity was later improved using in vitro proximity ligation, whereby chromatin is reconstituted from high molecular weight DNA, to ∼88 % coverage (Putnam et al., 2016; Reyes-Chin-Wo et al., 2017). This dual approach was necessary due to the large size and highly repetitive nature of the genome, which is thought to have undergone a whole-genome triplication around 66 million years ago (Reyes-Chin-Wo et al., 2017). The final assembly was 2.38 Gb, comprising 11,474 scaffolds with an N50 of 1.8 Mb and 38,919 annotated genes (Reyes-Chin-Wo et al., 2017). The Lettuce Genome Resource (LGR) was developed as a tool for accessing the wealth of genetic and genomic data available, including the L. sativa reference genome assembly, a gene model database and whole genome BLAST tools, which are readily available to the public (http://lgr.genomecenter.ucdavis.edu/; Table 1).
Table 1.
Resources available for lettuce breeding and research.
| Resource | Details | Link |
|---|---|---|
| Sequencing data | Genome assembly. Access to seven genome versions through GBrowse, sequence annotation retrieval, gene model database, with GO term, protein and homolog search databases. Available to download from NCBI, Bioproject PRJNA173551. | http://lgr.genomecenter.ucdavis.edu/Private/Tools/GBrowse.php |
| Transcriptome assembly. Lactuca sativa, 51,860 sequences. | https://www.ncbi.nlm.nih.gov/nuccore/?term=lactuca+sativa+transcriptome+assembly | |
| EST libraries for L. sativa, L. serriola, L. saligna, L. virosa and L. perennis, library information and a contig viewer | http://cgpdb.ucdavis.edu/cgpdb2/ | |
| Genetic mapping resources | Genetic map and >350 F8 RILs for the L. sativa cv. Salinas x L. serriola acc. US96UC23 RIL mapping population. | http://chiplett.ucdavis.edu/public/Data/data.php |
| Collection (CGNSC002) of 470 single seed decent lines including cultivated and wild lettuce (including: L. serriola, L. saligna, L. virosa and L. georgica) available for genome wide association studies. DNA sequencing data to be released. | https://www.wur.nl/ | |
| SNP genotyping Affymetrix GeneChip with >6.5 million features, covering ∼35,000 unigenes. Illumina GoldenGate custom oligo pool assays for SNP genotyping. | http://chiplett.ucdavis.edu/public/Data/data.phphttp://compgenomics.ucdavis.edu/compositae_SNP.php | |
| Germplasm collections | United States Department of Agriculture (USDA). > 1500 Lactuca accessions with worldwide origins. | https://npgsweb.ars-grin.gov/gringlobal/search.aspx |
| Centre for Genomic resources, the Netherlands (CGN). The International Lactuca Database comprising 2401 global Lactuca accessions. Range of phenotypic data, including leaf physiology and metabolomics data available. | https://cgngenis.wur.nl | |
| COMPOSITdb. Lettuce cultivar database containing information on 4501 cultivars. | http://compositdb.ucdavis.edu/ | |
| Warwick Crop Centre Genetic Resources Unit. Holds the UK lettuce collection with 1483 Lactuca accessions. | https://www2.warwick.ac.uk/fac/sci/lifesci/wcc/gru/ | |
| Phenotype data | Morphodb Phenotype Viewer and Ontology Browser. | http://compgenomics.ucdavis.edu/morphodb_index.php |
| Other tools | Genes2Growers website, providing information on molecular markers linked to disease resistance genes. | http://scri.ucdavis.edu/ |
| Genome assembly BLAST on CoGe. | https://genomevolution.org/coge/ | |
| SNP/indel discovery pipeline from ESTs. | http://cgpdb.ucdavis.edu/SNP_Discovery/ |
4.2. Mapping quantitative traits in lettuce
The suite of lettuce resources have been deployed to identify QTL for numerous abiotic and biotic stress, seed and post-harvest quality traits (summarised in Table 2). Zhang et al. (2007) utilised the L. sativa cv. Salinas x L. serriola interspecific RIL population developed by Truco et al. (2007) to identify QTL for shelf life and associated physiological, biophysical and biochemical characteristics in baby leaf lettuce. QTL for nutritional quality have also been mapped using the same population, from which candidate genes were identified, improving the understanding for the genetic basis of this trait (Damerum et al., 2015). For shredded lettuce, QTL for post-harvest discolouration traits including pinking and browning were identified by Atkinson et al. (2013) using an intraspecific population derived from a cross between two cultivars differing significantly for post-harvest discolouration traits. A large-effect QTL on chromosome 4 explaining 40–75 % of the phenotypic variation for the decay of fresh-cut lettuce stored in MAP has been verified in two independent intraspecific mapping populations, over multiple growing seasons (Hayes et al., 2014). Recently, this QTL has been verified in a genome wide association study, in which significant marker-trait associations were identified for shelf life at the same locus (Sthapit Kandel et al., 2020). Single nucleotide polymorphism (SNP) alleles at the QTL site were identified which can reliably predict the rate of deterioration for a range of lettuce cultivar types (Simko et al., 2018). The ability to characterise germplasm at the seedling stage using molecular markers, without having to grow to harvest maturity and undertaking laborious processing, can rapidly accelerate the development of novel improved cultivars. The public availability of a chromosome-scale reference genome assembly for lettuce (Reyes-Chin-Wo et al., 2017) provides a frame for unifying QTL and association studies, by collating multiple QTL experiments onto one physical map. Such an approach could be used to develop a cohesive QTL database for lettuce, as has been achieved for sorghum (Mace et al., 2019).
Table 2.
Summary of QTL studies conducted in lettuce, organised by trait category.
| Trait(s) | Description | Population(s) | Reference(s) |
|---|---|---|---|
| Abiotic stress | |||
| Various | Applied nutrient, drought, salt and competition stress and identified QTL for above ground dry weight | L. sativa cv. Salinas and L. serriola acc. US96UC23 RIL population | Hartman et al., 2014 |
| Drought stress | QTL for root architecture and water acquisition after mild drought stress | L. sativa cv. Salinas and L. serriola acc. US96UC23 F2 population | Johnson et al., 2000 |
| Resource utilisation | Identified significant marker-trait associations for water and nitrate capture below-ground and above-ground accumulation | Collection of cultivars for association analysis | Kerbiriou et al., 2016 |
| Tipburn | QTL for tip burn incidence and severity and other temperature stress-related physiological disorders | L. sativa cvs. “Emperor” x “El Dorado” RIL population | Jenni et al., 2013 |
| QTL for tipburn severity mapped alongside head lettuce morphological traits | Seven RIL populations, including L. sativa cv. Salinas and L. serriola acc. US96UC23 RIL population and crosses between several crisphead, butterhead, batavia and leaf lettuce types | González et al., 2019 | |
| Biotic stress | |||
| Downy mildew | QTL for downy mildew resistance | L. saligna (CGN 5271) x L. sativa cv. “Olof” F2 population | Jeuken and Lindhout, 2002 |
| QTL for downy mildew resistance and QTL fine-mapping reported | Backcross inbred lines (BC4) developed by crossing lines of an L. saligna (CGN 5271) x L. sativa cv. “Olof” F2 population to the L. sativa parent |
Jeuken et al., 2008; Zhang et al., 2009a, 2009b den Boer et al., 2013 |
|
| QTL for downy mildew resistance | L. sativa cvs. “Iceberg” x “Grand Rapids” RIL population | Simko et al., 2013 | |
| QTL for downy mildew resistance | L. sativa cv. Salinas and L. serriola acc. US96UC23 RIL population | Simko et al., 2014 | |
| QTL for downy mildew resistance | L. sativa cvs. “Salinas 88” x “La Brillante” RIL population | Simko et al., 2015 | |
| Lettuce dieback | QTL for lettuce dieback resistance | L. sativa cvs. “Salinas” x “Iceberg” RIL population | Grube et al., 2005 |
| QTL for lettuce dieback resistance | L. sativa cvs. “Salinas” x “Iceberg” RIL population for linkage mapping and a collection of cultivars for association analysis | Simko et al., 2009 | |
| Verticillium dahliae | Identified a QTL for Verticillium dahliae resistance | L. sativa cvs. “Salinas 88” x “La Brillante” RIL population | Hayes et al., 2011 |
| Lettuce drop | QTL for lettuce drop resistance | L. sativa cvs. “Eruption” x “Reine des Glaces” RIL population | Mamo et al., 2019 |
| Bacterial leaf spot | Significant marker-trait associations for bacterial leaf spot resistance | Diversity panel of cultivars representing the major leaf types | Lu et al., 2014 |
| QTL for bacterial leaf spot resistance | L. sativa cvs. “Eruption” x “Reine des Glaces” and “Delsay” x “Reine des Glaces” RIL populations | Sandoya et al., 2019 | |
| Root rot | QTL for race 2 major resistance locus | L. sativa cvs. “VP1013” x “Patriot” F2 population | Aruga et al., 2012 |
| Lettuce aphid | Identified significant marker-trait associations for aphid resistance | Diverse panel of wild and cultivated lettuce accessions, representing all major leaf types and of worldwide origin | Walley et al., 2017 |
| Post-harvest | |||
| Shelf life | Identification of multiple QTL for shelf life and association physiological, biochemical and biophysical traits, trait data collected in two field trials in two geographic locations | L. sativa cv. Salinas and L. serriola acc. US96UC23 RIL population | Zhang et al., 2007 |
| Identification of three QTL for shelf life from five field trials | L. sativa cvs. “Salinas 88” x “La Brillante” RIL population | Hayes et al., 2014 | |
| Significant marker-trait associations identified for shelf life and developmental rate | Diversity panel of cultivars, advanced breeding lines, individuals from RIL populations and plant introductions | Sthapit Kandel et al., 2020 | |
| Discolouration | Identified six QTL for discolouration traits in fresh-cut lettuce in two geographic locations | L. sativa cvs. “Saladin” x “Iceberg” RIL population | Atkinson et al., 2013 |
| Nutrition | QTL for antioxidant and chlorophyll content detected at two different stages of maturity and several production cycles | L. sativa cvs. “Diplomat” x “Margarita” RIL population | Hayashi et al., 2012 |
| Multiple QTL for antioxidant content, total phenolics and individual secondary metabolites, chlorophyll and carotenoids measured in two trials | L. sativa cv. Salinas and L. serriola acc. US96UC23 RIL population | Damerum et al., 2015 | |
| Seed traits | |||
| Seed germination/ longevity | Identified QTL for seed germination under different temperature and light conditions | L. sativa cvs. “Diplomat” x “Margarita” RIL population | Hayashi et al., 2008 |
| QTL for germination under high and low temperature and hormone treatment, seedling quality | L. sativa cv. Salinas and L. serriola acc. US96UC23 RIL population | Argyris et al., 2005, 2008 | |
| QTL for seed longevity following different storage conditions and after seed priming | L. sativa cv. Salinas and L. serriola acc. US96UC23 RIL population | Schwember and Bradford, 2010a | |
| Identified nine significant marker-trait associations for horticultural traits such as leaf undulation, leaf and stem anthocyanin content and seed colour | Diversity panel of homozygous accessions collected worldwide | Kwon et al., 2013a | |
| General horticultural/ domestication traits | QTL detected for 37 fitness-related traits including plant morphology, flowering time, seed output and numerous other domestication traits | L. sativa cv. Salinas and L. serriola acc. US96UC23 RIL population | Hartman et al., 2013 |
| QTL for plant height, fresh and dry weight and relative moisture content identified | A first and second generation of backcrosses, with L. serriola backcrossed to L. sativa cv. Dynamite | Uwimana et al., 2012 | |
| QTL for 10 fitness-related traits including germination, biomass, flowering time and seed output | L. sativa cv. Salinas and L. serriola acc. US96UC23 RIL population | Hartman et al., 2012 | |
| QTL for leaf morphology and rubber content | L. serriola F2 population | Bell et al., 2015 |
4.3. Exploiting wild relatives for crop improvement
Crop domestication often leads to a population bottleneck, meaning only a fraction of the genetic diversity available is being exploited in breeding programmes today. Crop wild relatives (CWR) are considered valuable sources of natural genetic variation, which may be vital to replenishing the gene pool for breeding (McCouch et al., 2013). It was estimated that the capitalisation of CWR is responsible for yield increases valued at $115 billion annually worldwide (Pimentel et al., 1997), due to an increased capacity to survive under unfavourable and unpredictable conditions. In order for CWR to be utilised in non-transgenic breeding, species must be interfertile. The Lactuceae subfamily of the Asteraceae is thought to be comprised of around 100 species and L. serriola and the L. serriola-like species such as L. aculeata, L. altaica, L. azerbaijanica, L. georgica, L. scarioloides and L. dregeana are considered the primary gene pool for domesticated lettuce, though other wild relatives including L. saligna and L. virosa are also sexually compatible, with viability of offspring decreasing respectively (Zohary, 1991; Lebeda et al., 2009). Cultivated lettuce is thought to originate from the Fertile Crescent ∼10,800 years ago (ya), with subsequent dispersal and selection leading to the development of distinct horticultural types including stem lettuce ∼900 ya and romaine, butterhead and crisphead 500 ya (Zhang et al., 2017). Evaluation of nucleotide diversity between wild and cultivated lettuce types identified a greater genetic variation in wild lettuce compared to cultivated, with crisphead lettuce demonstrating the lowest diversity, indicative of a genetic bottleneck (Zhang et al., 2017).
Lactuca species are distributed worldwide, with the highest number of species found in Asia, Africa, Europe and the Americas, respectively, and occupying diverse habitats, from dry warm regions, woodland, rocky slopes, waste land, semiarid cold regions and East African rainforests (Lebeda et al., 2004). The success of Lactuca species in such a wide range of frequently unfavourable habitats suggests they are valuable sources of genetic variation for biotic and abiotic stresses, which may translate into improved post-harvest quality. Wild relatives of lettuce have been identified as important sources of resistance to diseases and pests, improved drought tolerance and increased nutrient content (Lebeda et al., 2014; Damerum et al., 2015). In a recent evaluation of the phytochemical content of 150 cultivated and wild Lactuca accessions, genetic differences were observed in phenolic compounds and vitamin C content, with wild accessions demonstrating increased concentrations (van Treuren et al., 2018). The potential for utilising CWR in breeding highlights the importance of the management and maintenance of seedbanks (Tanksley and Mccouch, 1997). Germplasm collections, such as the International Lactuca Database (ILDB) currently held at the CGN (see Table 1), provide access to wild accessions, however many wild species are massively underrepresented and likely contain duplications and classification inaccuracies (Treuren et al., 2011), emphasising that more needs to be done to maintain such databases. There is hope in the scientific community that the implementation of the Nagoya Protocol, an international treaty recognising the rights of countries over their natural genetic resources, will not negatively impact on the exchange and thus exploitation of such genetic resources (Welch et al., 2013). The domestication of lettuce has led to a number of key modifications which distinguish cultivated lettuce from its inedible wild progenitor, including a reduction of latex, the removal of leaf and stem prickles, leaf bunching and shortening of internodes, changes to leaf texture and shape, de-bittering, delayed bolting and non-shattering of seeds (de Vries, 1997). Therefore, the use of CWR may be limited to expanding the gene pool for lettuce pre-breeding, as opposed to utilising directly in marker-assisted breeding programmes, due to negative impacts of linkage drag (Collard and Mackill, 2008).
5. Genomics-informed breeding for improved post-harvest quality
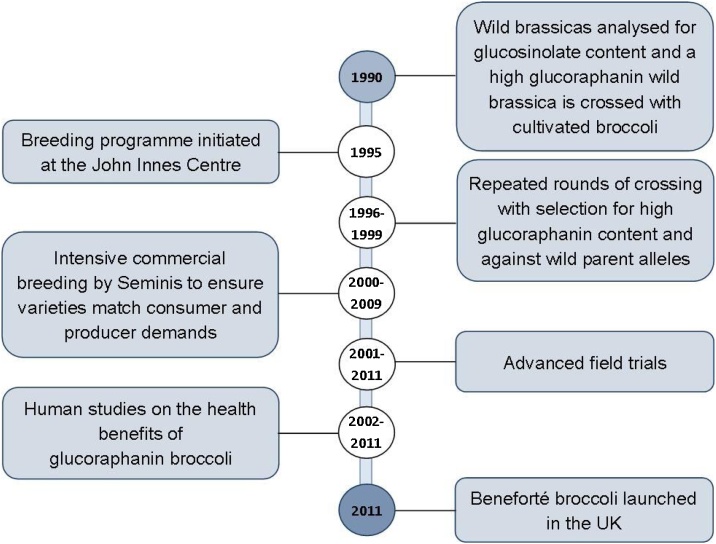
For decades, lettuce research has utilised molecular breeding technologies to identify QTL; but in contrast to the amount of QTL mapping data generated, limited successful applications to marker-assisted breeding (MAB) are described in the literature for post-harvest traits. Whilst marker-assisted selection (MAS) and genomic selection (GS) provides attractive advantages to traditional breeding techniques, by enabling efficient and early selection of germplasm at the seedling stage, reducing the amount of material that needs to be phenotyped and requiring less time to inform selections, it can still take over a decade to produce an improved cultivar (Collard and Mackill, 2008; Scheben et al., 2017). For example, Beneforté, a novel broccoli variety developed from wild allele introgression with increased levels of the phytochemical glucoraphanin, took over 20 years to commercialise (Fig. 2; Traka et al., 2013). This is likely to due to the complex genetic architecture of post-harvest quality traits, often comprised of multiple low-effect QTL which can be difficult to accurately detect using linkage and association mapping approaches (Collard and Mackill, 2008). The power of QTL detection is limited in small mapping populations (<200 individuals) and QTL x environment interactions, which may not be understood in QTL mapping studies typically replicated over a limited number of growing seasons or environments (Collard and Mackill, 2008). This makes it challenging to confidently identify robust QTL worthy of the time and economic investment required for MAS.
Fig. 2.
Timeline for the commercialisation of Beneforté, a broccoli variety with 2-3 higher glucoraphanin content (Traka et al., 2013), developed by exploiting the genetic variation in wild brassica germplasm (adapted from: http://www.beneforte.com/).
An alternative approach to MAB is genomic selection (GS), in which predictive statistical models are employed to make informed breeding selections in the absence of phenotyping, using genome-wide molecular marker data (Crossa et al., 2017). A population, referred to as the training population, is first genotyped and phenotyped and data is utilised to develop a predictive model, which can predict phenotypes based on the genotype alone to provide a genomic estimated breeding value (GEBV; Crossa et al., 2017). Using only genotype data from a collection of breeding material, the GEBV can be calculated and enable the rapid selection of desirable breeding material. The considerably reduced cost of high-throughput molecular marker genotyping technologies which have emerged over the last decade have enabled high density molecular marker genotyping on a mass scale, making GS feasible (Thomson, 2014). Although GS has the potential to accelerate MAB, this approach is still limited to the crop generation time and the existing genetic variation of cultivars, which are often highly inbred, or sexually compatible wild relatives. Whilst utilising wild relatives of crops has the potential to introduce novel sources of variation, this has the added task of ensuring only the beneficial loci are introgressed due to the negative effects of linkage drag (Hospital, 2005). This can require several generations of inbreeding as observed in the development of Beneforté (Fig. 2), even after the desirable trait has been introgressed, to minimise the presence of wild alleles which can negatively impact on crop performance (Jansen, 1996; Collard and Mackill, 2008).
Emerging technologies are aiming to shorten plant generation times, thereby increasing genetic gain by reducing the breeding cycle. One such technology, termed “Speed breeding”, involves using temperature regulation and supplemental LED lighting to extend the photoperiod, which has been implemented to increase the number of generations per year for crops including spring wheat, barley, canola and chickpea (Ghosh et al., 2018). High temperatures and exogenous gibberellic acid treatment have been demonstrated to induce bolting in lettuce (Han et al., 2016) and could be applied in conjunction with environmental controls to achieve speed breeding of lettuce. Additional techniques to accelerate the breeding process include the use of haploids, which can expedite the production of homozygous plants. Haploid lettuce plants can be generated by callus induction of in vitro cultured, haploid proembryos, obtained following cross-pollination with distantly related species in the Asteraceae family (Piosik and Zenkteler, 2016).
The use of innovative breeding technologies, such as genome editing, have the potential to transform the field of plant breeding, reducing new variety development time from 8 to 10 years, with methods such as cross and mutation breeding, to 4–6 years (Chen et al., 2019). Making targeted genome edits to already elite cultivars can allow the development of improved cultivars in a single generation, eliminating the multiple generations of crossing or selfing required for MAB and vastly accelerating novel cultivar development (Scheben et al., 2017). In the last five years, advancements in genome editing technologies including the use of meganucleases, zinc finger nucleases, transcription activator-like effector nucleases and CRISPR/Cas, have made it possible to make rapid, targeted genome modifications at high efficiency and low cost (Bortesi and Fischer, 2015). The potential for multiplex genome editing is also an attractive advantage of this technology, particularly in the case of complex, multigenic traits such as post-harvest quality. Six genes were simultaneously targeted in Arabidopsis, with homozygous mutants obtained in the T3 generation and biallelic quadruple gene mutants have been generated in a single generation in rice (Zhang et al., 2016; Minkenberg et al., 2017). Achieving such results by MAB could take several years of crossing and selfing with countless rounds of germplasm screening.
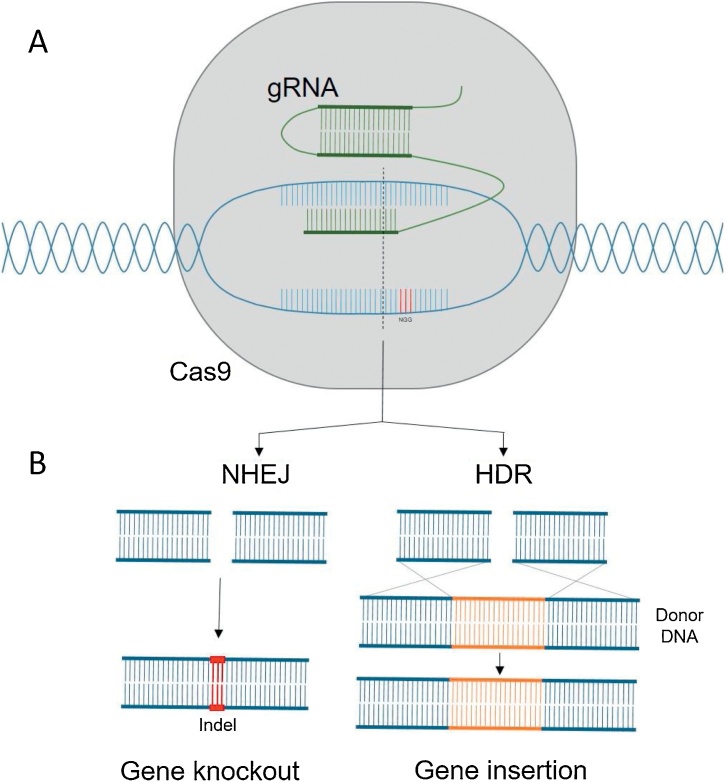
CRISPR/Cas9 technologies (Fig. 3) have thus far been employed in precision plant breeding to target yield, biotic and abiotic stress resistance and quality traits, via gene knockout, knock-in and replacement (Chen et al., 2019). Examples of successful knock-out mediated crop improvements via genome editing to date include rice with low amylose and improved cooking quality (Zhang et al., 2018b), low-gluten wheat (Sánchez-León et al., 2018) enhancement of oleic fatty acids in the oilseed crop Camelina sativa (Jiang et al., 2017) and enriched lycopene content in tomatoes (Li et al., 2018). Despite the reduced efficiency of the homology-directed repair (HDR) pathway, targeted gene replacement has been achieved in tomato to improve fruit shelf life (Yu et al., 2017). In lettuce, whilst there are no reports of gene knock-in, both standard and “DNA-free” genome editing has already been successfully employed to induce gene knockouts, generating lettuce plants which are theoretically indistinguishable from those generated by traditional breeding practices (Woo et al., 2015; Bertier et al., 2018). Deep amplicon sequencing revealed gene editing efficiency was consistent across lettuce cultivars, with over a third of plants regenerated having stable, germline mutations in one or both gene copies in a single generation (Bertier et al., 2018). Editing the upstream open reading frame of a gene encoding a key enzyme in vitamin C biosynthesis was able to increase ascorbate content by ∼150 % (Zhang et al., 2018a). In our own research, we have utilised CRISPR/Cas9 editing to target XTH cell wall remodelling enzymes and have achieved successful knockout of multiple genes simultaneously, using a single construct encoding Cas9 and multiple gRNAs (Damerum, 2017). This demonstrates the utility of CRISPR systems to generate multiple gene knockouts in non-model organisms. Advances including the use of alternative CRISPR systems such as Cas12a which demonstrate higher precision, catalytically inactive “dead” Cas9 endonucleases (dCas9) which can be utilised for gene activation or interference via fusion to transcriptional regulators, and base or methylation editing via fusion of dCas9 to base editing enzymes (such as cytidine deaminase) or epigenetic modulators (such as methylation or deacetylation enzymes) are expanding the potential application of such technologies (Moradpour and Abdulah, 2020). Switching the focus from identifying QTL or associated markers to underlying causal genes has the potential to rapidly increase our understanding of complex quantitative traits.
Fig. 3.
The basic premise of CRISPR/Cas genome editing (A) is the use of RNA-directed nucleases to make targeted double stranded DNA (dsDNA) breaks at specific sites in the genome, as directed by a short guide RNA (gRNA) sequence, which are repaired by endogenous DNA repair mechanisms inherent to all living organisms (Gaj et al., 2013). A dsDNA break is repaired via two major pathways (B): non-homologous end joining (NHEJ) or homology-directed repair (HDR). NHEJ, in which blunt DNA ends are stitched directly back together, is typically error-prone, often resulting in the incorporation or deletion of base pairs and leading to indels and frame shift mutations, which can be exploited for targeted gene knockout (Rodgers and Mcvey, 2016). Alternatively, if an appropriate template is provided, breaks can be repaired via HDR, which occurs by homologous recombination with the template, leading to gene insertion (Rodgers and Mcvey, 2016). “DNA-free” genome editing involves delivering the CRISPR reagents into target cells as pre-assembled ribonucleotide-protein complexes, without exogenous gene or vector backbone integration (Woo et al., 2015).
These studies demonstrate the power of genome editing technologies to transform crop breeding. However, as witnessed for genetic modification technologies, sociopolitical factors and regulatory hurdles are likely to obstruct the application of genome editing to crop improvement. Indeed, reports of human embryo editing for the correction of human diseases (Ma et al., 2017) have generated a huge ethical debate over the use of the technology, with some labelling it as ‘eugenic’, which could have detrimental impacts on its application to crop improvement (Benston, 2017). A major advantage to genome editing over prevailing transgenic technologies is the potential for generating gene knockouts without the presence of transgenes by NHEJ, providing hope that genome-edited crops will not be considered as “Genetically modified organisms” (GMOs) and increasing the likeliness of such crops entering the commercial market (Scheben and Edwards, 2017). In March 2018 the US secretary of Agriculture deemed that the United States Department of Agriculture (USDA) will not regulate genome edited crops as GMOs (Press Release Number: 0070.18) and there have been several examples of genome-edited crops which have by-passed GMO regulation, including corn engineered to contain higher levels of amylopectin (utilised in processed foods and adhesives) and an anti-browning mushroom with reduced polyphenol oxidase expression (Waltz, 2016). Without the same costly and time-consuming legal procedures required to develop genome-edited crops as seen for GMOs, this would open up the market to academics and small-scale breeding companies, which has until now been dominated by major seed companies (Hartung and Schiemann, 2014; Huang et al., 2016).
However, in July 2018 the European Union (EU) Court of Justice ruled that gene-edited crops are to face the same rigorous regulatory hurdles as GMOs, which includes any “organism, with the exception of human beings, in which the genetic material has been altered in a way that does not occur naturally by mating and/or natural recombination”, under the EU directive 2001/18/EC (EC, 2001). This decision has faced much resistance from the scientific community, who have described the ruling as unscientific and detrimental to the future of European farming and have called for a science-based re-evaluation (Urnov et al., 2018). It is clear that future lettuce breeding for complex post-harvest quality traits will require the deployment of a multitude of tools and that innovative breeding technologies could be crucial in accelerating sustainable crop improvement (Fig. 4).
Fig. 4.
Unravelling the genetic basis of complex quantitative traits using a multitude of tools. Those which have been applied to post-harvest quality improvement of lettuce (in the public domain) are highlighted in blue, those which have been applied in lettuce, but not for post-harvest quality traits are in light blue and those which have not been exploited in lettuce are in red. Numbers indicate key references; [1] Landry et al., 1987, [2] https://cgngenis.wur.nl, [3] Stoffel et al., 2012, [4] Simko, 2016, [5] Reyes-Chin-Wo et al., 2017, [6] Zhang et al., 2017, [7] Damerum et al., 2015, [8] see Table 2,[9] Sthapit Kandel et al., 2020, [10] Ripoll et al., 2019, [11] Jeuken and Lindhout, 2004, [12] Michelmore et al., 1991, [13] Damerum, 2017, [14] Su et al., 2020, [15] Wagstaff et al., 2010, [16] Zhang et al., 2018a, [17] Simko et al., 2018, [18] Zhang et al., 2009b (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
6. Conclusions and a look to the future
Lettuce will remain an important leafy green, widely consumed across the world, and largely eaten raw. As such, there are significant challenges associated with delivering fresh, safe and high quality lettuce with a long shelf life, but these also present opportunities to deliver valuable phytonutrients and enhanced human disease-prevention chemicals. Post-harvest quality traits have not yet featured significantly in breeding programmes to date, despite the potential of lettuce. At the same time, concerns around food safety and post-harvest quality have also not been addressed through plant breeding. A significant molecular and germplasm toolbox is now available to make rapid progress in lettuce post-harvest breeding, including a reference genome, multiple germplasm and genetic collections, marker and sequence resources, and lettuce is highly amenable to gene editing. Future lettuce post-harvest breeding should focus on traits for nutrition, post-harvest quality and longevity, delivering sustainable, high quality leaves for the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was funded by the California Leafy Greens Research Board.
References
- Agüero M.V., Ponce A.G., Moreira M.R., Roura S.I. Lettuce quality loss under conditions that favor the wilting phenomenon. Postharvest Biol. Technol. 2011;59:124–131. [Google Scholar]
- Albornoz F. Scientia Horticulturae Crop responses to nitrogen overfertilization : a review. Sci. Hortic. 2016;205:79–83. [Google Scholar]
- Allende A., Selma M.V., López-Gálvez F., Villaescusa R., Gil M.I. Role of commercial sanitizers and washing systems on epiphytic microorganisms and sensory quality of fresh-cut escarole and lettuce. Postharvest Biol. Technol. 2008;49:155–163. [Google Scholar]
- Argyris J., Truco M.J., Ochoa O., Knapp S.J., Still D.W., Lenssen G.M., Schut J.W., Michelmore R.W., Bradford K.J. Quantitative trait loci associated with seed and seedling traits in Lactuca. Theor. Appl. Genet. 2005;111:1365–1376. doi: 10.1007/s00122-005-0066-4. [DOI] [PubMed] [Google Scholar]
- Argyris J., Dahal P., Hayashi E., Still D.W., Bradford K.J. Genetic variation for lettuce seed thermoinhibition is associated with temperature-sensitive expression of abscisic Acid, gibberellin, and ethylene biosynthesis, metabolism, and response genes. Plant Physiol. 2008;148:926–947. doi: 10.1104/pp.108.125807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruga D., Tsuchiya N., Matsumura H., Matsumoto E., Hayashida N. Analysis of RAPD and AFLP markers linked to resistance to Fusarium oxysporum f. Sp. Lactucae race 2 in lettuce (Lactuca sativa L.) Euphytica. 2012;187:1–9. [Google Scholar]
- Atkinson L.D., McHale L.K., Truco M.J., Hilton H.W., Lynn J., Schut J.W., Michelmore R.W., Hand P., Pink D a C. An intra-specific linkage map of lettuce (Lactuca sativa) and genetic analysis of postharvest discolouration traits. Theor. Appl. Genet. 2013;126:2737–2752. doi: 10.1007/s00122-013-2168-8. [DOI] [PubMed] [Google Scholar]
- Aune D., Giovannucci E., Boffetta P., Lars T., Keum N., Norat T., Greenwood D.C., Vatten L.J., Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality–a systematic review and dose- response meta-analysis of prospective studies. Int. J. Epidemiol. 2017 doi: 10.1093/ije/dyw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov A., Bosak N., Lin C., Matsumoto I., Ohmoto M., Reed D., Nelson T. Genetics of taste receptors. Curr. Pharm. Des. 2014;20:2669–2683. doi: 10.2174/13816128113199990566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrière V., Lecompte F., Nicot P.C., Maisonneuve B., Tchamitchian M., Lescourret F. Lettuce cropping with less pesticides. A review. Agron. Sustain. Dev. 2014;34:175–198. [Google Scholar]
- Bell J.L., Burke I.C., Neff M.M. Genetic and biochemical evaluation of natural rubber from Eastern Washington Prickly Lettuce (Lactuca serriola L.) J. Agric. Food Chem. 2015;63:593–602. doi: 10.1021/jf503934v. [DOI] [PubMed] [Google Scholar]
- Benston S. Everything in moderation, even hype: learning from vaccine controversies to strike a balance with CRISPR. J. Med. Ethics (Published. 2017 doi: 10.1136/medethics-2016-103666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertier L.D., Ron M., Huo H., Bradford K.J., Britt A.B., Michelmore R.W. High-resolution analysis of the efficiency, heritability, and editing outcomes of CRISPR/Cas9-induced modifications of NCED4 in lettuce (Lactuca sativa) G3 Genes Genomes Genet. 2018;8:1513–1521. doi: 10.1534/g3.117.300396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørklund G., Chirumbolo S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition. 2017;33:311–321. doi: 10.1016/j.nut.2016.07.018. [DOI] [PubMed] [Google Scholar]
- Bolin H.R., Stafford A.E., King A.D., Huxsoll C.C. Factors affecting the storage stability of shredded lettuce. J. Food Sci. 1977;42:1319–1321. [Google Scholar]
- Bortesi L., Fischer R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 2015;33:41–52. doi: 10.1016/j.biotechadv.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Brug J., Kremers S.P., Lenthe van F., Ball K., Crawford D. Environmental determinants of healthy eating: in need of theory and evidence. Proc. Nutr. Soc. 2008;67:307–316. doi: 10.1017/S0029665108008616. [DOI] [PubMed] [Google Scholar]
- Chadwick M., Gawthrop F., Michelmore R.W., Wagstaff C., Methven L. Perception of bitterness, sweetness and liking of different genotypes of lettuce. Food Chem. 2016;197:66–74. doi: 10.1016/j.foodchem.2015.10.105. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J., Mueller K.L., Hoon M.A., Adler E., Feng L., Guo W., Zuker C.S., Ryba N.J.P., Hughes H. T2Rs Function as Bitter Taste Receptors defined (Lindemann, 1996b). however, several biochem- ical and physiological studies have suggested that bitter transduction in mammalian taste receptor cells is medi. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Chen K., Wang Y., Zhang R., Zhang H., Gao C. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 2019;70:667–697. doi: 10.1146/annurev-arplant-050718-100049. [DOI] [PubMed] [Google Scholar]
- Chokshi N. New York Times; 2018. E. Coli Deaths Linked to Romaine Lettuce, Officials Say. [Google Scholar]
- Chua D., Goh K., Saftner R.A., Bhagwat A.A. Fresh-cut lettuce in modified atmosphere packages stored at improper temperatures supports enterohemorrhagic E. Coli isolates to survive gastric acid challenge. J. Food Sci. 2008;73:M148–153. doi: 10.1111/j.1750-3841.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- Clarkson G.J.J., O’Byrne E.E., Rothwell S.D., Taylor G. Identifying traits to improve postharvest processability in baby leaf salad. Postharvest Biol. Technol. 2003;30:287–298. [Google Scholar]
- Clarkson G.J.J., Rothwell S.D., Taylor G. End of day harvest extends shelf life. Hort. Sci. 2005;40:1431–1435. [Google Scholar]
- Clifford M.N. Chlorogenic acids and other cinnamates – nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000;80:1033–1043. [Google Scholar]
- Collard B.C.Y., Mackill D.J. Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2008;363:557–572. doi: 10.1098/rstb.2007.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front. Nutr. 2018;5:1–9. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critzer F.J., Doyle M.P. Microbial ecology of foodborne pathogens associated with produce. Curr. Opin. Biotechnol. 2010;21:125–130. doi: 10.1016/j.copbio.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Crossa J., Pérez-Rodríguez P., Cuevas J., Montesinos-López O., Jarquín D., de los Campos G., Burgueño J., González-Camacho J.M., Pérez-Elizalde S., Beyene Y. Genomic selection in plant breeding: methods, models, and perspectives. Trends Plant Sci. 2017;22:961–975. doi: 10.1016/j.tplants.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Cruz R., Baptista P., Cunha S., Pereira J.A., Casal S. Carotenoids of lettuce (Lactuca sativa L.) grown on soil enriched with spent coffee grounds. Molecules. 2012;17:1535–1547. doi: 10.3390/molecules17021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damerum A. Lactuca sativa; 2017. Improving the Nutritional Quality and Shelf Life of Baby Leaf Lettuce. [Google Scholar]
- Damerum A., Selmes S.L., Biggi G.F., Clarkson G.J., Rothwell S.D., Truco M.J., Michelmore R.W., Hancock R.D., Shellcock C., Chapman Ma. Elucidating the genetic basis of antioxidant status in lettuce (Lactuca sativa) Hortic. Res. 2015;2:1–13. doi: 10.1038/hortres.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash S.K., Kar A., Gorrepati K. Modified atmosphere packaging of minimally processed fruits and vegetables. Trends in Post Harvest Technol. 2013;1:1–19. [Google Scholar]
- de Vries I.M. Characterization and identification of Lactuca sativa cultivars and wild relatives with SDS-electrophoresis (Lactuca sect. Lactuca, Compositae) Genet. Resour. Crop Evol. 1996;43:193–202. [Google Scholar]
- de Vries I.M. Origin and domestication of Lactuca sativa L. Genet. Resour. Crop Evol. 1997;44:165–174. [Google Scholar]
- den Boer E., Zhang N.W., Pelgrom K., Visser R.G.F., Niks R.E., Jeuken M.J.W. Fine mapping quantitative resistances to downy mildew in lettuce revealed multiple sub-QTLs with plant stage dependent effects reducing or even promoting the infection. Theor. Appl. Genet. 2013;126:2995–3007. doi: 10.1007/s00122-013-2188-4. [DOI] [PubMed] [Google Scholar]
- Deza-Durand K.M., Petersen M.A. The effect of cutting direction on aroma compounds and respiration rate of fresh-cut iceberg lettuce (Lactuca sativa L.) Postharvest Biol. Technol. 2011;61:83–90. [Google Scholar]
- Deza-Durand K.M., Petersen M.A. Volatile compounds of modified atmosphere packaged cut iceberg lettuce: effect of extremely low O2, season, cultivar and storage time. Food Res. Int. 2014;62:254–261. [Google Scholar]
- Drewnowski A., Gomez-Carneros C. Bitter taste, phytonutrients, and the consumer: a review. Am. J. Clin. Nutr. 2000;72:1424–1435. doi: 10.1093/ajcn/72.6.1424. [DOI] [PubMed] [Google Scholar]
- EC . 2001. Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the Deliberate Release Into the Environment of Genetically Modified Organisms and Repealing Council Directive 90/220/EEC. [Google Scholar]
- Erickson M.C., Webb C.C., Diaz-Perez J.C., Phatak S.C., Silvoy J.J., Davey L., Payton A.S., Liao J., Ma L., Doyle M.P. Surface and internalized escherichia coli O157: H7 on field-grown spinach and lettuce treated with spray-contaminated irrigation water. J. Food Prot. 2010;73:1023–1029. doi: 10.4315/0362-028x-73.6.1023. [DOI] [PubMed] [Google Scholar]
- FAO . 2013. Food Wastage Footprint. Impacts on Natural Resources. [Google Scholar]
- Forouzanfar M.H., Alexander L., Anderson H.R., Bachman V.F., Biryukov S., Brauer M., Burnett R., Casey D., Coates M.M., Cohen A. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T., Gersbach C.A., Barbas C.F. ZFN, TALEN and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García C.J., García-Villalba R., Gil M.I., Tomas-Barberan F.A. LC-MS untargeted metabolomics to explain the signal metabolites inducing browning in fresh-cut lettuce. J. Agric. Food Chem. 2017;65:4526–4535. doi: 10.1021/acs.jafc.7b01667. [DOI] [PubMed] [Google Scholar]
- García C.J., Gil M.I., Tomás-Barberán F.A. Targeted metabolomics analysis and identification of biomarkers for predicting browning of fresh-cut lettuce. J. Agric. Food Chem. 2019;67:5908–5917. doi: 10.1021/acs.jafc.9b01539. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Watson A., Gonzalez-Navarro O.E., Ramirez-Gonzalez R.H., Yanes L., Mendoza-Suárez M., Simmonds J., Wells R., Rayner T., Green P. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 2018;13:2944–2963. doi: 10.1038/s41596-018-0072-z. [DOI] [PubMed] [Google Scholar]
- Gil M.I. Preharvest factors and fresh-cut quality of leafy vegetables. Acta Hortic. 2016;1141:57–64. [Google Scholar]
- Gil M.I., Selma M.V., López-Gálvez F., Allende A. Fresh-cut product sanitation and wash water disinfection: problems and solutions. Int. J. Food Microbiol. 2009;134:37–45. doi: 10.1016/j.ijfoodmicro.2009.05.021. [DOI] [PubMed] [Google Scholar]
- González M.M., Bertier M.J.T.L.D., Simko S.J.I., Michelmore R.J.H.R.W. Genetic architecture of tipburn resistance in lettuce. Theor. Appl. Genet. 2019 doi: 10.1007/s00122-019-03349-6. [DOI] [PubMed] [Google Scholar]
- Grube R.C., Wintermantel W.M., Hand P., Aburomia R., Pink D.A.C., Ryder E.J. Genetic analysis and mapping of resistance to lettuce dieback: a soilborne disease caused by tombusviruses. Theor. Appl. Genet. 2005;110:259–268. doi: 10.1007/s00122-004-1825-3. [DOI] [PubMed] [Google Scholar]
- Hall M.K.D., Jobling J.J., Rogers G.S. Influence of storage temperature on the seasonal shelf life of perennial wall rocket and annual garden rocket. Int. J. Veg. Sci. 2013;19:83–95. [Google Scholar]
- Han Y., Chen Z., Lv S., Ning K., Ji X., Liu X., Wang Q., Liu R., Fan S., Zhang X. MADS-box genes and gibberellins regulate bolting in lettuce (Lactuca sativa L.) Front. Plant Sci. 2016;7:1–14. doi: 10.3389/fpls.2016.01889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman Y., Hooftman D a P., Uwimana B., van de Wiel C.C.M., Smulders M.J.M., Visser R.G.F., van Tienderen P.H. Genomic regions in crop-wild hybrids of lettuce are affected differently in different environments: implications for crop breeding. Evol. Appl. 2012;5:629–640. doi: 10.1111/j.1752-4571.2012.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman Y., Hooftman D.A.P., Eric Schranz M., Tienderen P.H. QTL analysis reveals the genetic architecture of domestication traits in Crisphead lettuce. Genet. Resour. Crop Evol. 2013;60:1487–1500. [Google Scholar]
- Hartman Y., Hooftman Da.P., Uwimana B., Schranz M.E., van de Wiel C.C.M., Smulders M.J.M., Visser R.G.F., Michelmore R.W., van Tienderen P.H. Abiotic stress QTL in lettuce crop-wild hybrids: comparing greenhouse and field experiments. Ecol. Evol. 2014;4:2395–2409. doi: 10.1002/ece3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung F., Schiemann J. Precise plant breeding using new genome editing techniques: opportunities, safety and regulation in the EU. Plant J. 2014;78:742–752. doi: 10.1111/tpj.12413. [DOI] [PubMed] [Google Scholar]
- Hayashi E., Aoyama N., Still D.W. Quantitative trait loci associated with lettuce seed germination under different temperature and light environments. Genome. 2008;51 doi: 10.1139/G08-077. 928–497. [DOI] [PubMed] [Google Scholar]
- Hayashi E., You Y., Lewis R., Calderon M.C., Wan G., Still D.W. Mapping QTL, epistasis and genotype × environment interaction of antioxidant activity, chlorophyll content and head formation in domesticated lettuce (Lactuca sativa) Theor. Appl. Genet. 2012;124:1487–1502. doi: 10.1007/s00122-012-1803-0. [DOI] [PubMed] [Google Scholar]
- Hayes R.J., Liu Y. Genetic variation for shelf-life of salad-cut lettuce in modified-atmosphere environments. J. Am. Soc. Hortic. Sci. 2008;133:228–233. [Google Scholar]
- Hayes R.J., McHale L.K., Vallad G.E., Truco M.J., Michelmore R.W., Klosterman S.J., Maruthachalam K., Subbarao K. The inheritance of resistance to Verticillium wilt caused by race 1 isolates of Verticillium dahliae in the lettuce cultivar La Brillante. Theor. Appl. Genet. 2011;123:509–517. doi: 10.1007/s00122-011-1603-y. [DOI] [PubMed] [Google Scholar]
- Hayes R.J., Galeano C.H., Antonise R., Simko I. Inheritance of decay of fresh-cut lettuce in a recombinant inbred line population from ‘Salinas 88’ x ‘La brillante’. J. Am. Soc. Hortic. Sci. 2014;139:388–398. [Google Scholar]
- Hoque M.M., Ajwa H., Othman M., Smith R., Cahn M. Yield and postharvest quality of lettuce in response to nitrogen, phosphorus, and potassium fertilizers. HortScience. 2010;45:1539–1544. [Google Scholar]
- Hospital F. Selection in backcross programmes. Philos. Trans. Biol. Sci. 2005;360:1503–1511. doi: 10.1098/rstb.2005.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Weigel D., Beachy R.N., Li J. A proposed regulatory framework for genome-edited crops. Nat. Genet. 2016;48:109–111. doi: 10.1038/ng.3484. [DOI] [PubMed] [Google Scholar]
- Hunter P.J., Atkinson L.D., Vickers L., Lignou S., Oruna-Concha M.J., Pink D., Hand P., Barker G., Wagstaff C., Monaghan J.M. Oxidative discolouration in whole-head and cut lettuce: biochemical and environmental influences on a complex phenotype and potential breeding strategies to improve shelf-life. Euphytica. 2017;213:1–16. doi: 10.1007/s10681-017-1964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Dawson C. Economic impact of food safety outbreaks on food businesses. Foods. 2013;2:585–589. doi: 10.3390/foods2040585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IHME . 2017. Findings From the Global Burden of Disease Study; p. 2017. [Google Scholar]
- Jacob C., Melotto M. Human pathogen colonization of lettuce dependent upon plant genotype and defense response activation. Front. Plant Sci. 2020;10:1–17. doi: 10.3389/fpls.2019.01769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J.P.A. 1996. Aphid Resistance in Composites. [Google Scholar]
- Jenni S., Truco M.J., Michelmore R.W. Quantitative trait loci associated with tipburn, heat stress-induced physiological disorders, and maturity traits in crisphead lettuce. Theor. Appl. Genet. 2013;126:3065–3079. doi: 10.1007/s00122-013-2193-7. [DOI] [PubMed] [Google Scholar]
- Jeuken M., Lindhout P. Lactuca saligna, a non-host for lettuce downy mildew (Bremia lactucae), harbors a new race-specific Dm gene and three QTLs for resistance. Theor. Appl. Genet. 2002;105:384–391. doi: 10.1007/s00122-002-0943-z. [DOI] [PubMed] [Google Scholar]
- Jeuken M.J.W., Lindhout P. The development of lettuce backcross inbred lines (BILs) for exploitation of the Lactuca saligna (wild lettuce) germplasm. Theor. Appl. Genet. 2004;109:394–401. doi: 10.1007/s00122-004-1643-7. [DOI] [PubMed] [Google Scholar]
- Jeuken M.J.W., Pelgrom K., Stam P., Lindhout P. Efficient QTL detection for nonhost resistance in wild lettuce: backcross inbred lines versus F(2) population. TAG. Theor. Appl. Genet. Theoretis und angewandte Genetik. 2008;116:845–857. doi: 10.1007/s00122-008-0718-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W.Z., Henry I.M., Lynagh P.G., Comai L., Cahoon E.B., Weeks D.P. Significant enhancement of fatty acid composition in seeds of the allohexaploid, Camelina sativa, using CRISPR/Cas9 gene editing. Plant Biotechnol. J. 2017;15:648–657. doi: 10.1111/pbi.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W.C., Jackson L.E., Ochoa O., van Wijk R., Peleman J., St. Clair Da., Michelmore R.W. Lettuce, a shallow-rooted crop, and Lactuca serriola, its wild progenitor, differ at QTL determining root architecture and deep soil water exploitation. Theor. Appl. Genet. 2000;101:1066–1073. [Google Scholar]
- Kader A.A. Flavor quality of fruits and vegetables. J. Sci. Food Agric. 2008;1868:1863–1868. [Google Scholar]
- Kerbiriou P.J., Maliepaard C.A., Stomph T.J., Koper M., Froissart D., Roobeek I., Lammerts Van Bueren E.T., Struik P.C. Genetic control of water and nitrate capture and their use efficiency in lettuce (Lactuca sativa L.) Front. Plant Sci. 2016;7:1–14. doi: 10.3389/fpls.2016.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevers C., Falkowski M. Evolution of antioxidant capacity during storage of selected fruits and vegetables. J. Agric. Food Chem. 2007;55:8596–8603. doi: 10.1021/jf071736j. [DOI] [PubMed] [Google Scholar]
- Kim G.T., Lee S.H., Kim Y.M. Quercetin regulates sestrin 2-AMPK-mTOR signaling pathway and induces apoptosis via increased intracellular ROS in HCT116 Colon Cancer cells. J. Cancer Prev. 2013;18:264–270. doi: 10.15430/JCP.2013.18.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Moon Y., Tou J.C., Mou B., Waterland N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.) J. Food Compos. Anal. 2016;49:19–34. [Google Scholar]
- Kwon S., Simko I., Hellier B., Mou B., Hu J. Genome-wide association of 10 horticultural traits with expressed sequence tag-derived SNP markers in a collection of lettuce lines. Crop J. 2013;1:25–33. [Google Scholar]
- Landry B.S., Kesseli R.V., Farrara B., Michelmore R.W. A genetic map of lettuce (Lactuca sativa L.) with restriction fragment length polymorphism, isozyme, disease resistance and morphological markers. Genetics. 1987;116:331–337. doi: 10.1093/genetics/116.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeda A., Dolezalova I., Ferakova V., Astley D. Geographical distribution of wild Lactuca species (Asteraceae, lactuceae) Bot. Rev. 2004;70:328–356. [Google Scholar]
- Lebeda A., Doležalová I., Křístková E., Kitner M., Petrželová I., Mieslerová B., Novotná A. Wild Lactuca germplasm for lettuce breeding: current status, gaps and challenges. Euphytica. 2009;170:15–34. [Google Scholar]
- Lebeda A., Křístková E., Kitner M., Mieslerová B., Jemelková M., Pink Da.C. Wild Lactuca species, their genetic diversity, resistance to diseases and pests, and exploitation in lettuce breeding. Eur. J. Plant Pathol. 2014;138:597–640. [Google Scholar]
- Lee D., Polisensky D.H., Braam J. Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: a focus on calmodulin-like and XTH genes. New Phytol. 2005;165:429–444. doi: 10.1111/j.1469-8137.2004.01238.x. [DOI] [PubMed] [Google Scholar]
- Li X., Wang Y., Chen S., Tian H., Fu D., Zhu B., Luo Y., Zhu H. Lycopene is enriched in tomato fruit by CRISPR/Cas9-mediated multiplex genome editing. Front. Plant Sci. 2018;9:1–12. doi: 10.3389/fpls.2018.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorach R., Martínez-Sánchez A., Tomás-Barberán F.A., Gil M.I., Ferreres F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008;108:1028–1038. doi: 10.1016/j.foodchem.2007.11.032. [DOI] [PubMed] [Google Scholar]
- Lu H., Hu J., Kwon S.J. Association analysis of bacterial leaf spot resistance and SNP markers derived from expressed sequence tags (ESTs) in lettuce (Lactuca sativa L.) Mol. Breed. 2014;34:997–1006. [Google Scholar]
- Luna M.C., Tudela J.A., Martínez-Sánchez A., Allende A., Marín A., Gil M.I. Long-term deficit and excess of irrigation influences quality and browning related enzymes and phenolic metabolism of fresh-cut iceberg lettuce (Lactuca sativa L.) Postharvest Biol. Technol. 2012;73:37–45. [Google Scholar]
- Luna M.C., Tudela J.A., Tomás-Barberán F.A., Gil M.I. Modified atmosphere (MA) prevents browning of fresh-cut romaine lettuce through multi-target effects related to phenolic metabolism. Postharvest Biol. Technol. 2016;119:84–93. [Google Scholar]
- Luo Y., He Q., McEvoy J.L. Effect of storage temperature and duration on the behavior of Escherichia coli O157:H7 on packaged fresh-cut salad containing romaine and iceberg lettuce. J. Food Sci. 2010;75:390–397. doi: 10.1111/j.1750-3841.2010.01722.x. [DOI] [PubMed] [Google Scholar]
- Ma H., Marti-Gutierrez N., Park S.-W., Wu J., Lee Y., Suzuki K., Koski A., Ji D., Hayama T., Ahmed R. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548:413–419. doi: 10.1038/nature23305. [DOI] [PubMed] [Google Scholar]
- Mace E., Innes D., Hunt C., Wang X., Tao Y., Baxter J., Hassall M., Hathorn A., Jordan D. The Sorghum QTL Atlas: a powerful tool for trait dissection, comparative genomics and crop improvement. Theor. Appl. Genet. 2019;132:751–766. doi: 10.1007/s00122-018-3212-5. [DOI] [PubMed] [Google Scholar]
- Mamo B.E., Hayes R.J., Truco M.J., Puri K.D., Michelmore R.W., Subbarao K.V., Simko I. The genetics of resistance to lettuce drop (Sclerotinia spp.) in lettuce in a recombinant inbred line population from Reine des Glaces × Eruption. Theor. Appl. Genet. 2019;132:2439–2460. doi: 10.1007/s00122-019-03365-6. [DOI] [PubMed] [Google Scholar]
- Martin C. The interface between plant metabolic engineering and human health. Curr. Opin. Biotechnol. 2013;24:344–353. doi: 10.1016/j.copbio.2012.11.005. [DOI] [PubMed] [Google Scholar]
- McCouch S., Baute G.J., Bradeen J., Bramel P., Bretting P.K., Buckler E., Burke J.M., Charest D., Cloutier S., Cole G. Agriculture: feeding the future. Nature. 2013;499:23–24. doi: 10.1038/499023a. [DOI] [PubMed] [Google Scholar]
- Melotto M., Brandl M.T., Jacob C., Jay-Russell M.T., Micallef S.A., Warburton M.L., Van Deynze A. Breeding crops for enhanced food safety. Front. Plant Sci. 2020;11:1–14. doi: 10.3389/fpls.2020.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelmore R.W., Paran I., Kesseli R.V. Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc. Natl. Acad. Sci. U.S.A. 1991;88:9828–9832. doi: 10.1073/pnas.88.21.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkenberg B., Xie K., Yang Y. Discovery of rice essential genes by characterizing a CRISPR-edited mutation of closely related rice MAP kinase genes. Plant J. 2017;89:636–648. doi: 10.1111/tpj.13399. [DOI] [PubMed] [Google Scholar]
- Moose S.P., Mumm R.H. Molecular plant breeding as the foundation for 21st century crop improvement. Plant Physiol. 2008;147:969–977. doi: 10.1104/pp.108.118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradpour M., Abdulah S.N.A. CRISPR/dCas9 platforms in plants: strategies and applications beyond genome editing. Plant Biotechnol. J. 2020;18:32–44. doi: 10.1111/pbi.13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou B. Genetic variation of beta-carotene and lutein contents in lettuce. J. Am. Soc. Hortic. Sci. 2005;130:870–876. [Google Scholar]
- Munther D.S., Carter M.Q., Aldric C.V., Ivanek R., Brandl M.T. Formation of escherichia coli O157: H7 persister cells in the lettuce phyllosphere and application of differential equation models to predict their prevalence on lettuce plants in the field. Appl. Environ. Microbiol. 2020;86:1–15. doi: 10.1128/AEM.01602-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J.M., Hilton H.W., Clifford S.C., Smith A.C. The mechanical properties of lettuce: a comparison of some agronomic and postharvest effects. J. Mater. Sci. 2005;40:1101–1104. [Google Scholar]
- Nicolle C., Carnat A., Fraisse D., Lamaison J., Rock E., Michel E., Amouroux P., Remesy C. Characterisation and variation of antioxidant micronutrients in lettuce (Lactuca sativa folium) J. Sci. Food Agric. 2004;84:2061–2069. [Google Scholar]
- Oh M., Carey E.E. Regulated water deficits improve phytochemical concentration in lettuce. J. Am. Soc. Hortic. Sci. 2010;135:223–229. [Google Scholar]
- Ozturk H.M., Ozturk H.M.K. Effect of pressure on the vacuum cooling of iceberg lettuce. Int. J. Refrig. 2009;32:402–410. [Google Scholar]
- Pentzer W.T., Heinze P.H. Postharvest physiology of fruits and vegetables. Annu. Rev. Plant Physiol. 1984;5:205–224. [Google Scholar]
- Pimentel D., Wilson C., McCullum C., Huang R., Dwen P., Flack J., Tran Q., Saltman T., Cliff B. Economic and environmental benefits of biodiversity. Bio. Sci. 1997;47:747–757. [Google Scholar]
- Piosik Zenkteler E., Zenkteler M. Development of haploid embryos and plants of Lactuca sativa induced by distant pollination with Helianthus annuus and H. Tuberosus. Euphytica. 2016;208:439–451. [Google Scholar]
- Putnam N.H., Connell B.O., Stites J.C., Rice B.J., Hartley P.D., Sugnet C.W., Haussler D., Rokhsar D.S. Chromosome-scale shotgun assembly using an in vitro method for long-range linkage. Genome Res. 2016;26:342–350. doi: 10.1101/gr.193474.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilliam R.S., Williams A.P., Jones D.L. Lettuce cultivar mediates both phyllosphere and rhizosphere activity of escherichia coli o157:H7. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Chin-Wo S., Wang Z., Yang X., Kozik A., Arikit S., Song C., Xia L., Froenicke L., Lavelle D.O., Truco M.-J. Genome assembly with in vitro proximity ligation data and whole-genome triplication in lettuce. Nat. Commun. 2017;8:14953. doi: 10.1038/ncomms14953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico D., Martín-Diana a.B., Barat J.M., Barry-Ryan C. Extending and measuring the quality of fresh-cut fruit and vegetables: a review. Trends Food Sci. Technol. 2007;18:373–386. [Google Scholar]
- Ripoll J., Charles F., Vidal V., Laurent S., Klopp C., Lauri F., Sallanon H., Roux D. Transcriptomic view of detached lettuce leaves during storage: a crosstalk between wounding, dehydration and senescence. Postharvest Biol. Technol. 2019;152:73–88. [Google Scholar]
- Rodgers K., Mcvey M. Error-prone repair of DNA double-strand breaks. J. Cell. Physiol. 2016;231:15–24. doi: 10.1002/jcp.25053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltveit M.E. The three responses of plant tissue to wounding. Acta. Hortic. 2016;1141:13–20. [Google Scholar]
- Saltveit M. Pinking of lettuce. Postharvest Biol. Technol. 2018;145:41–52. [Google Scholar]
- Sánchez-León S., Gil-Humanes J., Ozuna C.V., Giménez M.J., Sousa C., Voytas D.F., Barro F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018;16:902–910. doi: 10.1111/pbi.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoya G.V., Maisonneuve B., Truco M.J., Bull C.T., Simko I., Trent M., Hayes R.J., Michelmore R.W. Genetic analysis of resistance to bacterial leaf spot in the heirloom lettuce cultivar Reine des Glaces. Mol. Breed. 2019;39 [Google Scholar]
- Scarborough P., Bhatnagar P., Wickramasinghe K.K., Allender S., Foster C., Rayner M. The economic burden of ill health due to diet, physical inactivity, smoking, alcohol and obesity in the UK: an update to 2006-07 NHS costs. J. Public Health (Bangkok) 2011;33:527–535. doi: 10.1093/pubmed/fdr033. [DOI] [PubMed] [Google Scholar]
- Scheben A., Edwards D. Genome editors take on crops. Science. 2017;355:1122–1123. doi: 10.1126/science.aal4680. [DOI] [PubMed] [Google Scholar]
- Scheben A., Wolter F., Batley J., Puchta H., Edwards D. Towards CRISPR/Cas crops - bringing together genomics and genome editing. New Phytol. 2017 doi: 10.1111/nph.14702. [DOI] [PubMed] [Google Scholar]
- Schwember A.R., Bradford K.J. Quantitative trait loci associated with longevity of lettuce seeds under conventional and controlled deterioration storage conditions. J. Exp. Bot. 2010;61:4423–4436. doi: 10.1093/jxb/erq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simko I. High-resolution DNA melting analysis in plant research. Trends Plant Sci. 2016;21:528–537. doi: 10.1016/j.tplants.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Simko I., Pechenick Da, McHale L.K., Truco M.J., Ochoa O.E., Michelmore R.W., Scheffler B.E. Association mapping and marker-assisted selection of the lettuce dieback resistance gene Tvr1. BMC Plant Biol. 2009;9:135. doi: 10.1186/1471-2229-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simko I., Atallah A.J., Ochoa O.E., Antonise R., Galeano C.H., Truco M.J., Michelmore R.W. Identification of QTLs conferring resistance to downy mildew in legacy cultivars of lettuce. Sci. Rep. 2013;3:2875. doi: 10.1038/srep02875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simko I., Rauscher G., Sideman R.G., McCreight J.D., Hayes R.J. Evaluation and QTL mapping of resistance to powdery mildew in lettuce. Plant Pathol. 2014;63:344–353. [Google Scholar]
- Simko I., Ochoa O., Pel Ma, Tsuchida C., Font i Forcada C., Hayes R.J., Truco M.-J., Antonise R., Galeano C.H., Michelmore R. Resistance to downy mildew in lettuce cv. La Brillante is conferred by Dm50 gene and multiple QTLs. Phytopathology. 2015 doi: 10.1094/PHYTO-02-15-0057-R. [DOI] [PubMed] [Google Scholar]
- Simko I., Hayes R.J., Truco M.-J., Michelmore R.W., Antonise R., Massoudi M. Molecular markers reliably predict post-harvest deterioration of fresh-cut lettuce in modified atmosphere packaging. Hortic. Res. 2018;5 doi: 10.1038/s41438-018-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares S., Kohl S., Thalmann S., Mateus N., Meyerhof W., Freitas V.De. Different phenolic compounds activate distinct human bitter taste receptors. J. Agric. Food Chem. 2013;61:1525–1533. doi: 10.1021/jf304198k. [DOI] [PubMed] [Google Scholar]
- Spadafora N.D., Amaro A., Pereira M., Müller C.T., Pintado M. Multi-trait analysis of post-harvest storage in rocket salad (Diplotaxis tenuifolia) links sensorial, volatile and nutritional data. Food Chem. 2016;211:114–123. doi: 10.1016/j.foodchem.2016.04.107. [DOI] [PubMed] [Google Scholar]
- Spinardi A., Ferrante A. Effect of storage temperature on quality changes of minimally processed baby lettuce. J. Food, Agric. Environ. 2012;10:38–42. [Google Scholar]
- Sthapit Kandel J., Peng H., Hayes R.J., Mou B., Simko I. Genome-wide association mapping reveals loci for shelf life and developmental rate of lettuce. Theor. Appl. Genet. 2020 doi: 10.1007/s00122-020-03568-2. [DOI] [PubMed] [Google Scholar]
- Stoffel K., van Leeuwen H., Kozik A., Caldwell D., Ashrafi H., Cui X., Tan X., Hill T., Reyes-Chin-Wo S., Truco M.-J. Development and application of a 6.5 million feature Affymetrix Genechip® for massively parallel discovery of single position polymorphisms in lettuce (Lactuca spp.) BMC Genomics. 2012;13 doi: 10.1186/1471-2164-13-185. 185–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart D. ‘Nature’ is not guilty: foodborne illness and the industrial bagged salad. Sociol. Ruralis. 2011;51:158–174. [Google Scholar]
- Stuart D., Woroosz M.R. The myth of efficiency: technology and ethics in industrial food production. J. Agric. Environ. Ethics. 2011;26:231–256. [Google Scholar]
- Su W., Tao R., Liu W., Yu C., Yue Z., He S., Lavelle D., Zhang W., Zhang L., An G. Characterization of four polymorphic genes controlling red leaf colour in lettuce that have undergone disruptive selection since domestication. Plant Biotechnol. J. 2020;18:479–490. doi: 10.1111/pbi.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taban M.B., Halkman A.K. Do leafy green vegetables and their ready-to-eat [RTE] salads carry a risk of foodborne pathogens? Anaerobe. 2011;17:286–287. doi: 10.1016/j.anaerobe.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Tanksley S.D., Mccouch S.R. Potential from the wild seed banks and molecular maps: unlocking genetic seed banks and molecular maps: unlocking genetic potential from the wild the narrow genetic base of crop plants. Science. 1997;277:1063–1066. doi: 10.1126/science.277.5329.1063. [DOI] [PubMed] [Google Scholar]
- Tesco Plc. 2013. Tesco and Society: Using Our Scale for Good. 2013-14 Half Year Update. [Google Scholar]
- Thomson M.J. High-throughput SNP genotyping to accelerate crop improvement. Plant Breed. Biotechnol. 2014;2:195–212. [Google Scholar]
- Toivonen P.M.A., Brummell D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008;48:1–14. [Google Scholar]
- Traka M.H., Saha S., Huseby S., Kopriva S., Walley P.G., Barker G.C., Moore J., Mero G., van den Bosch F., Constant H. Genetic regulation of glucoraphanin accumulation in Beneforté broccoli. New Phytol. 2013;198:1085–1095. doi: 10.1111/nph.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treuren R., Coquin P., Lohwasser U. Genetic resources collections of leafy vegetables (lettuce, spinach, chicory, artichoke, asparagus, lamb’s lettuce, rhubarb and rocket salad): composition and gaps. Genet. Resour. Crop Evol. 2011;59:981–997. [Google Scholar]
- Truco M.J., Antonise R., Lavelle D., Ochoa O., Kozik A., Witsenboer H., Fort S.B., Jeuken M.J.W., Kesseli R.V., Lindhout P. A high-density, integrated genetic linkage map of lettuce (Lactuca spp.) Theor. Appl. Genet. 2007;115:735–746. doi: 10.1007/s00122-007-0599-9. [DOI] [PubMed] [Google Scholar]
- Truco M.J., Ashrafi H., Kozik A., van Leeuwen H., Bowers J., Reyes Chin Wo S., Stoffel K., Xu H., Hill T., Van Deynze A. An ultra high-density, transcript-based, genetic map of lettuce. G3: Genes Genomes Genet. 2013;3:617–631. doi: 10.1534/g3.112.004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudela J.A., Marín A., Martínez-Sánchez A., Luna M.C., Gil M.I. Preharvest and postharvest factors related to off-odours of fresh-cut iceberg lettuce. Postharvest Biol. Technol. 2013;86:463–471. [Google Scholar]
- Urnov F.D., Ronald P.C., Carroll D. A call for science-based review of the European court’s decision on gene-edited crops. Nat. Biotechnol. 2018;36:800–802. doi: 10.1038/nbt.4252. [DOI] [PubMed] [Google Scholar]
- USDA . 2019. Vegetables 2018 Summary. [Google Scholar]
- Uwimana B., Smulders M.J.M., Hooftman D.A.P., Hartman Y., van Tienderen P.H., Jansen J., McHale L.K., Michelmore R.W., Visser R.G.F., van de Wiel C.C.M. Crop to wild introgression in lettuce: following the fate of crop genome segments in backcross populations. BMC Plant Biol. 2012;12:1–17. doi: 10.1186/1471-2229-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Treuren R., van Eekelen H.D.L.M., Wehrens R., de Vos R.C.H. Metabolite variation in the lettuce gene pool: towards healthier crop varieties and food. Metabolomics. 2018;14:1–14. doi: 10.1007/s11306-018-1443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff C., Clarkson G.J.J., Rothwell S.D., Page A., Taylor G., Dixon M.S. Characterisation of cell death in bagged baby salad leaves. Postharvest Biol. Technol. 2007;46:150–159. [Google Scholar]
- Wagstaff C., Clarkson GJJ Zhang F., Rothwell S.D., Fry S.C., Taylor G., Dixon M.S. Modification of cell wall properties in lettuce improves shelf life. J. Exp. Bot. 2010;61:1239–1248. doi: 10.1093/jxb/erq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley P.G., Hough G., Moore J.D., Carder J., Elliott M., Mead A., Jones J., Teakle G., Barker G., Buchanan-Wollaston V. Towards new sources of resistance to the currant-lettuce aphid (Nasonovia ribisnigri) Mol. Breed. 2017;37:1–18. doi: 10.1007/s11032-016-0606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz E. CRISPR-edited crops free to enter market, skip regulation. Nat. Biotechnol. 2016;34 doi: 10.1038/nbt0616-582. 582–582. [DOI] [PubMed] [Google Scholar]
- Watada A.E., Qi L. Quality of fresh-cut produce. Postharvest Biol. Technol. 1999;15:201–205. [Google Scholar]
- Welch E.W., Shin E., Long J. Potential effects of the Nagoya Protocol on the exchange of non-plant genetic resources for scientific research: actors, paths, and consequences. Ecol. Econ. 2013;86:136–147. [Google Scholar]
- Williams T.R., Moyne A.L., Harris L.J., Marco M.L. Season, irrigation, leaf age, and Escherichia coli inoculation influence the bacterial diversity in the lettuce phyllosphere. PLoS One. 2013;8:1–14. doi: 10.1371/journal.pone.0068642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J.W., Kim J., Kwon S., II, Corvalán C., Cho S.W., Kim H., Kim S.-G., Kim S.-T., Choe S., Kim J.-S. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 2015;33:1162–1164. doi: 10.1038/nbt.3389. [DOI] [PubMed] [Google Scholar]
- WRAP . 2012. Household Food and Drink Waste in the UK. 95. [Google Scholar]
- Yang X., Wei S., Liu B., Guo D., Zheng B., Feng L., Liu Y., Tomás-Barberán F.A., Luo L., Huang D. A novel integrated non-targeted metabolomic analysis reveals significant metabolite variations between different lettuce (Lactuca sativa. L) varieties. Hortic. Res. 2018;5:1–14. doi: 10.1038/s41438-018-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q.H., Wang B., Li N., Tang Y., Yang S., Yang T., Xu J., Guo C., Yan P., Wang Q. CRISPR/Cas9-induced targeted mutagenesis and gene replacement to generate long-shelf life tomato lines. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-12262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.Z., Wagstaff C., Rae A.M., Sihota A.K., Keevil C.W., Rothwell S.D., Clarkson G.J.J., Michelmore R.W., Truco M.J., Dixon M.S. QTLs for shelf life in lettuce co-locate with those for leaf biophysical properties but not with those for leaf developmental traits. J. Exp. Bot. 2007;58:1433–1449. doi: 10.1093/jxb/erm006. [DOI] [PubMed] [Google Scholar]
- Zhang N.W., Lindhout P., Niks R.E., Jeuken M.J.W. Genetic dissection of Lactuca saligna nonhost resistance to downy mildew at various lettuce developmental stages. Plant Pathol. 2009;58:923–932. [Google Scholar]
- Zhang N.W., Pelgrom K., Niks R.E., Visser R.G.F., Jeuken M.J.W. Three combined quantitative trait loci from nonhost lactuca saligna are sufficient to provide complete resistance of lettuce against bremia lactucae. Mol. Plant-microbe Interact. 2009;22:1160–1168. doi: 10.1094/MPMI-22-9-1160. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Mao Y., Ha S., Liu W., Botella J.R., Zhu J.K. A multiplex CRISPR/Cas9 platform for fast and efficient editing of multiple genes in Arabidopsis. Plant Cell Rep. 2016;35:1519–1533. doi: 10.1007/s00299-015-1900-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Su W., Tao R., Zhang W., Chen J., Wu P., Yan C., Jia Y., Larkin R.M., Lavelle D. RNA sequencing provides insights into the evolution of lettuce and the regulation of flavonoid biosynthesis. Nat. Commun. 2017;8:1–12. doi: 10.1038/s41467-017-02445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Si X., Ji X., Fan R., Liu J., Chen K., Wang D., Gao C. Genome editing of upstream open reading frames enables translational control in plants. Nat. Biotechnol. 2018;36:894–900. doi: 10.1038/nbt.4202. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhang H., Botella J., Zhu J. Generation of new glutinous rice by CRISPR/Cas9-targeted mutagenesis of the Waxy gene in elite rice varieties. J. Integr. Plant Biol. 2018;60:369–375. doi: 10.1111/jipb.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary D. The wild genetic resources of cultivated lettuce (Lactuca saliva L.) Euphytica. 1991;53:31–35. [Google Scholar]