Abstract
Small mammals such as rodents can to carry zoonotic pathogens. Currently, there is impaired knowledge on zoonotic pathogens in rodents and insectivores in the Netherlands. This limits opportunities for preventive measures and complicates risk‐assessments for zoonotic transmission to humans. Leptospira spp. and Toxoplasma gondii are present on a list of prioritized emerging pathogens in the Netherlands and were therefore the focus of this study. Both pathogens have the ability to survive under moist environmental conditions. In total, a group of 379 small mammals (rodents & insectivores) were tested on pathogenic Leptospira spp., and 312 on T. gondii. Rodents and insectivores were trapped at various sites, but mostly on pig and dairy farms throughout the country. Over five percent of the animals (5.3%, n = 379) tested positive for Leptospira DNA, and five of the animals (1.6%, n = 312) tested were positive for T. gondii DNA. The animals positive for T.gondii were all brown rats and the ones for Leptospira spp. were various species. Our results show that insectivores and rodents might be used as an indicator for the environmental contamination and/or the contamination in wildlife for Leptospira spp.
Keywords: leptospirosis, mice, pathogen–host relationship, rats, reservoir, zoonoses
Over five percent of the rodents and insectivores (5.3%, n = 379) tested positive for Leptospira DNA and five of the animals (n = 312) tested were positive for Toxoplasma gondii DNA. Leptospira spp. and T. gondii are present in the Dutch population of wild small mammals, indicating the importance of the studies for these infectious agents. Both rodent ánd insectivore species might be considered as potential sources for human leptospirosis in The Netherlands and as an indicator for the environmental contamination and the contamination in, and of wildlife.

1. INTRODUCTION
Rodents and insectivores can be potential hosts for numerous zoonotic pathogens (Meerburg, Singleton, & Kijlstra, 2009). Thus, it is essential to monitor the pathogen presence in these small mammal populations. There is impaired knowledge (e.g. prevalence, geographic distribution, rodent species that are host) on rodent borne diseases in the Netherlands, which limits opportunities for preventive measures and complicates the assessment of risk of zoonotic transmission to humans. In order to increase the understanding on rodent‐borne pathogens in the Netherlands, we set up a study to assess the pathogen presence in common rodent and insectivore species from the Netherlands. Two important pathogens were selected from a list of prioritized emerging pathogens relevant for the Netherlands; (I) Leptospira spp., and (II) Toxoplasma gondii. Both pathogens are able to infect a wide range of species (Acha & Szyfres, 2003; Bharti et al., 2003; Levett, 2001; Newell et al., 2010; Opsteegh, 2011). The spirochaetal bacteria Leptospira spp. causes leptospirosis, which is an acute febrile disease in humans occurring worldwide (Bharti et al., 2003). The global burden of human leptospirosis is estimated on more than 60,000 deaths and over 1 million of severe leptospirosis cases in studies led by the World Health Organization (Costa et al., 2015; Torgerson et al., 2015; WHO, 2011). The bacterium is generally transmitted via direct or indirect contact with spirochetes secreted in the environment via the urine of infected reservoir animals (Hartskeerl, Collares‐Pereira, & Ellis, 2011). Hosts can be divided into reservoir and accidental hosts. Reservoir hosts are animal species which do not show symptoms after infection, and act as infection reservoir by lifelong shedding of leptospires in their urine and via parent–offspring transmission (Foley & Straub, 2017; Mwachui, Crump, Hartskeerl, Zinsstag, & Hattendorf, 2015). Accidental hosts shed only for a relative short period leptospires in their urine after infection, and these hosts develop severe or even lethal disease after infection (a.o. humans) (Fraga, Carvalho, Isaac, & Barbosa, 2015; Mwachui et al., 2015) Shedded leptospires have the ability to survive for prolonged periods of time in moist environments (Levett, 2001). Moreover, contaminated water is a serious risk for infection (Haake et al., 2002). One of the most important wildlife reservoir hosts are rodents (Faine, 1994; Terpstra, 1989). In the Netherlands brown rats (Rattus norvegicus) and voles (Microtus arvalis) are the most important reservoir species and infection sources for human leptospirosis (Fernandes et al., 2016; Guernier et al., 2016; Himsworth, Parsons, Jardine, & Patrick, 2013; Obiegala et al., 2017; Zilber et al., 2016; Zuerner, 2015). A publication from Hartskeerl and Terpstra (1996) from the Netherlands showed that other rodents species and some insectivores can also be reservoir hosts (hedgehogs (Erinaceus europeanus), muskrats (Ondatra zibethicus), house shrews (Crocidura russula) and house mice (Mus musculus)). Nevertheless, there is hardly scientific information available on the presence of Leptospira spp. in rodents and insectivores in the Netherlands. In 1934 a report was published on Leptospirosis in the Netherlands, mentioning a prevalence of Leptospira spp. in brown rats of 11%–56% (n = 60), emphasizing the differences between test‐locations (Schüffner, 1934). Research from 1992 on muskrats in the Netherlands found 7% (n = 327) positive on Leptospira interrogans (Steinen, Schuurman, Gravekamp, Korver, & Terpstra, 1992). More recently, a study on Leptospira spp. in brown rats found a prevalence of 42% (n = 150), with prevalences varying between geographical areas within the Netherlands (range of 33%–57%) (Maas et al., 2018). The National Institute for Public Health and the Environment in the Netherlands (RIVM) tested 189 mice on Leptospira spp. from between 2007 and 2015, and found 45.5% of Apodemus sylvaticus (N = 55), 73.3% of the tested Microtus arvalis (n = 60) and 41.8% of the tested Myodus glareolus (n = 74) mice positive for Leptospira spp. (Uiterwijk et al., 2016).
Besides potential carriage of Leptospira spp., rodents and insectivores can also be infected with T. gondii, a protozoan parasite (Dubey, 2014, 2016; Kim & Weiss, 2008; Krijger, Cornelissen, Wisselink, & Meerburg, 2014; Robert‐Gangneux & Dardé, 2012). Rodents have been suggested to be reservoirs of infection for cats, pigs and dogs (Dubey & Frenkel, 1998; Kijlstra et al., 2008). Felid species are the only hosts that are able to shed T. gondii oocysts in the environment (Dubey, 2016) (Nicolle & Manceaux, 1908). However, the parasite is present in a wide range of warm blooded animals, first as tachyzoites and later as bradyzoites, also called tissue cysts (Dubey, 2016). When a cat consumes an infected intermediate host, the parasite can complete its lifecycle (Dubey, Miller, & Frenkel, 1970). There are a couple publications on T. gondii in rodent and insectivore species in the Netherlands. In 2012 250 small mammals were tested and found 4% positive for T. gondii (Meerburg, Craeye, Dierick, & Kijlstra, 2012). Another study form the Netherlands found 11.9% of the rodents and insectivores (n = 101) positive for T. gondii (Kijlstra et al., 2008). Research from 2014 on common moles (insectivore) from the Netherlands found a prevalence of 2.3% (n = 86)(Krijger et al., 2014). It is interesting to see that the prevalence varies per species and even per location.
Because rodents can be host to both zoonotic pathogens Leptospira spp. (definitive host) and T. gondii (intermediate host), and since the current status of its prevalence in the Netherlands remains unknown, rodents and insectivores from several geographically spread areas in the Netherlands were tested on the presence of those two zoonotic pathogens.
2. METHODS
Rodent trapping was conducted from November 2016 until January 2017 on 10 conventional pig farms and one cow farm, distributed over four provinces in the Netherlands; Limburg, Noord‐Brabant, Gelderland and Overijssel (Figure 1) by professional and certified rodent management companies. Each farmer was surveyed and asked about the presence of cats and/or stray cats on their farm. All locations were visited and screened for rodent tracks. Snap‐traps were then placed accordingly by a certified pest‐manager. Traps were placed one week in pre‐bait position, after which they were placed and used for 1 month. Traps were checked upon daily to ensure a maximum period between capture and storage of 24 hr. Trapped animals were stored in separate seal bags at −18°C.
Figure 1.
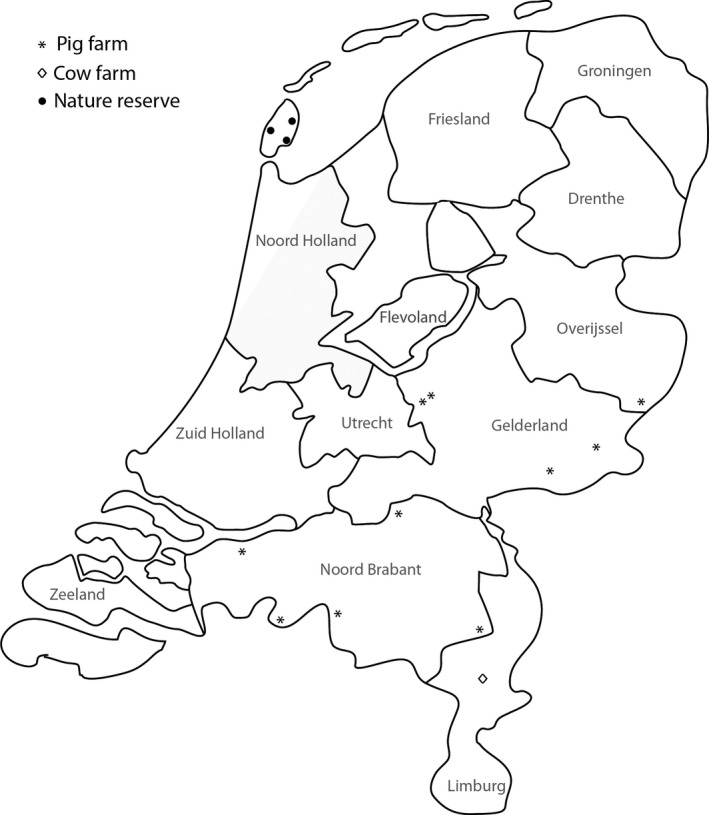
Map of The Netherlands showing rodent trapping locations. * is a pig farm, ◊ is a cow farm and ● a nature reserve
In October 2018, rodents were trapped on three locations on recreational areas in nature reserves on the island Texel (province of Noord Holland, Figure 1) by a rodent manager using the EKO1000 traps, and by use of the rodenator (Meyer Industries). Trapped animals were stored in separate seal bags at −18°C.
All rodents were thawed at 4°C 24 hr before dissection. During dissection at Wageningen Bioveterinary Research (WBVR) each animal was identified to species level and sexed and of each rodent randomly one kidney and the brains were collected. Samples were stored at −20°C until further analysis. All rodent samples were tested for Leptospira spp. (n = 379), whereas the samples from rodents trapped on pig farms and Texel were besides Leptospira spp. also tested on T. gondii (n = 312).
2.1. Leptospira spp. diagnostics
From each kidney sample a small transversal slice (≤25 mg) was cut (Figure 2) and treated for DNA extraction (QIAamp DNA Mini Kit, QIAGEN). All tissues were processed for DNA extraction according to the manufacturer's protocol with some modifications; tissues were digested by using 360 μl of buffer ATL (QIAGEN) and 40 μl of proteinase K (QIAGEN), mixed and incubated for 3 hr at 56°C, were heated at 70°C for 10 min after adding AL buffer, after which ethanol was added. All DNA samples were stored at −20°C until further testing by PCR.
Figure 2.
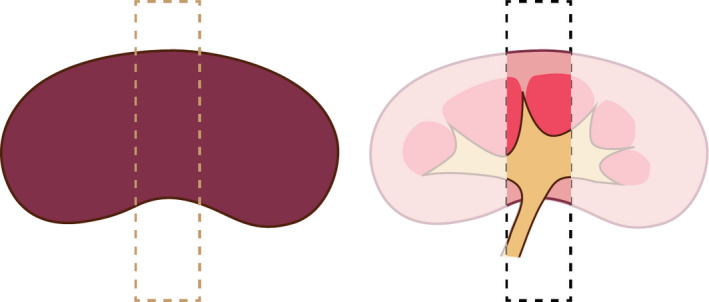
Schematic overview of the transversal slice of the kidney
2.2. Quantitative polymerase chain reaction (RT‐qPCR) for Leptospira spp. detection and speciation
Each DNA sample was diluted (1:10) with UltraPure DNase/RNase‐free distilled water (Invitrogen) and tested in triplicate. The SYBR Green real‐time qPCR targetting secY gene was used (Ahmed, Engelberts, Boer, Ahmed, & Hartskeerl, 2009). Reactions of in total 25 μl were set up with 10 μl sample to be examined, 12.5 μl of SYBR Green Supermix (Bio‐Rad) of 2× stock reagent (100 mM KCl, 40 mM Tris‐HCI, pH 8.4, 0.4 mM of each dNTP, 50 units/ml iTaq DNA polymerase, 6 mM MgCl2, 20 nM fluoresein and stabilizers), 1 μl SecYIVF (400 nM) as forward primer and 1 μl SecYIV (400 nM) as reverse primer, 0.5 μl UltraPure DNase/RNase‐free distilled water (Invitrogen). For the negative control 10 μl sterile UltraPure water was used as template. A Bio‐Rad CFX96 real‐time PCR fast detection system was used to perform the reactions by a first cycle of 5 min of activation at 95°C with subsequent dissociation steps consisting of: 95°C/5 s; 54°C/5 s; 72°C/15 s for 40 cycles. The programme finished with 95°C/1 min and a cooling at 20°C/1 min and the dissociation was measured stepwise, every 0.5°C.
Amplicon specificity was checked by conducting a melting curve analysis which was also used to determine the Leptospira species; a sample was classified positive when Ct value ≤ 35 cycles and Tm between 78.5–84.5°C. Samples were tested in triplicate and classified as positive when ≥2 runs resulted positive. A retest in trifold was conducted on samples that gave only one amplification curve. Samples were classified as positive if the repeat run resulted in ≥1 positive reaction and if the amplification melting curve was conform to set values.
2.3. Toxoplasma gondii diagnostics
The brain tissue was thawed at 4°C. Samples were homogenized for 30 s by an ultra turrax homogenizer after adding 1 ml DPBS. DNA was extracted from 250 μl of the homogenized brain tissue with the DNeasy Blood & Tissue kit (Qiagen GMBH). The manufacturers’ protocol was slightly adjusted; 50–100 pc glass homogenizer beads were added to each sample and the samples were mixed by vortexing for 10 min at 220 g to facilitate lysis. Hereafter, lysis buffer was added and samples were then incubated for 2.5 hr at 56°C, after which another vortexing cycle of 10 min at 220 g took place. During the addition of ethanol, we added 1.5 µl HCl 35%, and used only 50 µl AE buffer to elute the DNA. DNA samples were stored at −20°C until tested by Real‐Time PCR. Of each sample 5 µl DNA was tested by a RT‐qPCR using SYBR Green (Applied Biosystems) in an ABI 7500 Real‐Time PCR system (PE Applied Biosystems). A standard reaction mixture contained 12.5 µl of SYBR Select Master Mix, 1 µl (10 µM) of the primers, 5 µl of DNA template and 5.5 µl PCR grade water. The primers (529‐F: AGG AGA GAT ATC AGG ACT GTA G and 529‐R: GCG TCG TCT CGT CTA GAT CG) are complementary to the 529‐bp repeat element (GenBank AF146527). The cycling profile involved an initial PCR activation step at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and primer annealing and extension at 60°C for 60 s. Following amplification, a melt curve analysis was performed to verify the specificity of the amplified products by their specific melting temperatures (Tm). For quantification of the amount of T. gondii DNA in the samples, a standard curve of DNA extracted from cultured tachyzoites from the T. gondii RH strain was used. Data acquisition and analysis of the results were performed using the 7500 System SDS Software (Applied Biosystems). Samples with Ct‐value < 37.5 and Tm‐value between 81.9 and 83.5°C were considered as positive.
2.4. Statistical analysis
To compare frequency between sex the Chi‐square test was used, to analyse between provinces a one‐way ANOVA was used, for further analyses descriptive statistics were used. Results were considered statistically significant with a p‐value of p < .05. Statistical analyses were performed using SPSS, version 23 (IBM SPSS Statistics Inc).
3. RESULTS
In total 379 rodents and insectivores were trapped, 351 on livestock farms (Limburg, Brabant, Gelderland and Overijssel), and 28 in nature reserves (Noord‐Holland). The trapped animals consisted out of three insectivore and seven different rodent species. About half of the number of animals were black rats (Rattus rattus, 49.6%), second predominant species was the house mouse (Mus musculus, 22.2%). All trapped animals were tested for pathogenic Leptospira spp. Twenty were found positive (Leptospira species Interrogans (n = 15) and Kirschneri (n = 5)) thus showing an overall incidence of 5.3% (Table 1). The prevalence of Leptospira spp. among wild rodents and insectivores differs significantly per province (p = .006), with Gelderland being the province with the highest incidence (Table 2). There was no significant association between rodent sex and Leptospira spp. infection (p = .85).
Table 1.
Infection percentage of rodent species with Leptospira and Toxoplasma gondii
| Mammal species | Rodent or insectivore | No. positive/total (%) | |
|---|---|---|---|
| T. gondii | Leptospira | ||
| Wood mouse (Apodemus sylvaticus) | Rodent | 0/19 (0) | 2/19 (10.5)a |
| Harvest mouse (Micromys minutus) | Rodent | 0/1 (0) | 0/1 (0) |
| Common vole (Microtus arvalis) | Rodent | 0/8 (0) | 2/8 (25.0)b |
| Common house mouse (Mus musculus) | Rodent | 0/84 (0) | 5/84 (6.0)c |
| Muskrat (Ondatra zibethicus) | Rodent | 0/1 (0) | 1/1 (100)a |
| Brown rat (Rattus norvegicus) | Rodent | 5/36 (13.8) | 5/66 (7.6)a |
| Black rat (Rattus rattus) | Rodent | 0/151 (0) | 1/188 (0.5)b |
| Greater white‐toothed shrew (Crocidura russula) | Insectivore | 0/2 (0) | 0/2 (0) |
| Common shrew (Sorex araneus) | Insectivore | 0/9 (0) | 4/9 (44.4)c |
| Crowned shrew (Sorex coronatus) | Insectivore | 0/1 (0) | 0/1 (0) |
| Total | 5/312 (1.6%) | 20/379 (5.3%) | |
Species Leptospira interrogans.
Species Leptospira kirschneri.
Both species Leptospira interrogans (Mus musculus n = 4, Sorex araneus n = 3) and kirschneri (Mus musculus n = 1, Sorex araneus n = 1).
Table 2.
Leptospira infection percentage of the tested small mammals per province
| Province | No. tested animals | No. positive | Prevalence |
|---|---|---|---|
| Limburg | 219 | 4 | 1.8% |
| Noord‐Brabant | 66 | 7 | 10.6% |
| Overijssel | 40 | 5 | 12.5% |
| Gelderland | 26 | 4 | 15.4% |
| Noord Holland | 28a | 0 | 0% |
| Total | 379 | 20 | 5.3% |
On Texel (Noord Holland), only brown rats were trapped (n = 28).
Five animals were found positive for T. gondii (1.6%, Table 1), of which three female and two male rats. All five were brown rats from Texel (Noord Holland). With 28 brown rats (17 females, 11 males) trapped on Texel, the prevalence of this group of rodents from this specific island comes to 17.9%.
4. DISCUSSION
Although research is conducted, still little is known about the presence and risks of zoonotic pathogens carried by rodents and/or insectivores zoonoses in the Netherlands. This knowledge gap limits opportunities for preventive measures and confounds the approximation of the potential transmission to humans. Until now, there is still little known and published about the presence of Leptospira spp. in rodents and insectivores in the Netherlands and other European countries. Therefore we tested rodents and insectivores from several geographically spread areas in the Netherlands on presence of those two zoonotic pathogens. In total, 5.3% of the animals (n = 379) tested positive for Leptospira DNA, and 1.6% of the animals (n = 312) tested were positive for T. gondii DNA. Our results show that insectivores and rodents might be used as an indicator for the environmental contamination and/or the contamination in wildlife for Leptospira spp.
Most studies focus on Rattus norvegicus only, because these animal carriers are recognized as important infection sources for humans (Aviat et al., 2009; Runge et al., 2013) and are often present near shores of lakes, canals and rivers. In this way, they pose a serious threat for surface water contamination. A study from France (Aviat et al., 2009) found 34.7% of the trapped brown rats (n = 36) positive for pathogenic Leptospira spp., and a study in Germany found 21% of the 586 brown rats positive (Runge et al., 2013). A recent study from the Netherlands reported an infection range of 33%–57% in brown rats (Maas et al., 2018). It is known that the infection rate among rats is highly variable in time and place (Kuiken, 1990; Kuiken, van Dijk, Terpstra, & Bokhout, 1991), which is also underpinned by the recent study in the Netherlands (Maas et al., 2018). We found a lower infection percentage in the small mammals tested (5.3%) than these European studies. This difference could be due to multiple factors, such as difference in diagnosis methods used, or trapping year, or season. Although the majority of publications use serological methods, it is important to use molecular detection, like in this study. A serious disadvantage of using serological methods for diagnosis is that it only detects the pathogens presence when there are sufficient levels of anti‐Leptospira spp. antibodies present (Ahmed, Grobusch, Klatser, & Hartskeerl, 2012; Musso & La Scola, 2013). Using serological assays could therefore might lead to incorrect results. However, the main reasons for the difference in infection percentages found is that studies mentioned above focus on R. norvegicus only, in contrast to our study which includes more animal species. Another important reason for the difference in infection percentages is the location where the mammals were trapped. The studies above all researched mammals trapped nearby water. The animals from our study are from farms and nature reserve areas and not on locations linked to water or water rich spaces such as rivers, canals or recreation lakes.
Although brown rats are considered the most important hosts spreading the bacterium to humans, almost every mammal might be reckoned as potential bearer and disseminator of Leptospira spp. (Hartskeerl, 2006; Mwachui et al., 2015). Therefore, this study was set up to test more animal species than brown rats only. In this study it is indicated that, even though with a lower abundance, pathogenic Leptospira spp. are also widely distributed in other small mammals; the prevalence of Leptospira spp. in the tested rodents and insectivores ranged between 1% and 15%, with an average of 5.3%. This is confirmed by literature from European countries which report on the occurrence of Leptospira spp. in small rodents and shrews. A study on Leptospira spp. in small rodents from Croatia tested 7% of the rodents positive (n = 227) (Turk et al., 2003). Research from Germany on small mammals found an incidence of 5.7% (n = 736) (Obiegala et al., 2017), which is in line with our findings. Another study from Germany (Obiegala et al., 2016) found an overall infection percentage of 9.7% (n = 2,961). A Swiss study from 2002 found leptospiral DNA in 12.6% of 190 small mammals (Adler, Vonstein, Deplazes, Stieger, & Frei, 2002). Czech research showed 11.6% of the trapped small mammals (n = 429) positive for pathogenic Leptospira spp., with infection ranges varying from 0% to 20% between species (Treml, Pejcoch, & Holesovska, 2002). We found both L. interrogans and L. kirschneri in the rodents population tested. It is remarkable that L. kirschneri was found in the Rattus rattus (black rat). This black rat is worldwide associated with Icterohaemorrhagiae infections which belong to L. interrogans (Kuiken, 1990) although it harbours also L. kirschneri in Brazil and Mayotte (Desvars et al., 2012; Moreno et al., 2016). It can be concluded that besides seasonal, geographic and temporal factors, the host species also plays a role in the infection rate.
When looking at T. gondii in the trapped animals, all rodents and insectivores caught on the pig and cow farms tested negative for this parasite. This is not in line with the expectations since previous studies conducted on farms in the Netherlands found rodents as well as insectivore species carrying T. gondii; rodents and insectivores trapped on organic farms in the Netherlands in 2004 gave an infection rate with T. gondii of 4% (n = 250) among species; house mice (9.0%), common voles (4.2%) and white‐toothed shrews (2.0%) (Meerburg et al., 2012). Research from 2008 in the Netherlands on rodents from pig‐farms, found a prevalence of 11.9% (n = 101) in rodents and insectivores (Kijlstra et al., 2008). Prevalences differed among animal species, in descending order: 14.3% of Apodemus sylvaticus (n = 7) tested positive for T. gondii, 13.6% of the Crocidura russula (n = 22), 10.3% of the Rattus norvegicus (n = 39) and 6.5% of the trapped Mus musculus (n = 31) (Kijlstra et al., 2008). As well in the study from Meerburg et al. (2012) as in the study by Kijlstra et al. (2008), it was noted that cats were present on the participating farms. Being the definitive host for T. gondii, cats could become infected by predation of infected intermediate hosts such as wildlife, or via ingestion of oocysts from the environment (Afonso, Thulliez, & Gilot‐Fromont, 2006; Afonso, Thulliez, Pontier, & Gilot‐Fromont, 2007; Hejlíček & Literak, 1998). In this study, however, all farms were free of cats, which might explain the absence of T. gondii in the small mammals tested. This is in contrast to the situation on the island Texel (NL) where there is a problem with stray cats (News, 2018; Spek, 2015). The presence of wild cats on this island (≈460 km2) could explain the relatively high prevalence of 17.9% among the trapped rodents (brown rats) from Texel.
Our study had some limitations, as the rodents and insectivores came from five provinces, while there are 12 provinces in the Netherlands. A suggestion for further research would be to collect (more) rodents and insectivores from over the whole country, including all provinces to get insight in high and low frequency areas. Another ‘limitation’ of the study is that the samples were tested using primers which could not detect mixed infections (Moseley et al., 2018), leading to a conclusion of the presence of maximal one Leptospira species per infected animal, whereas the animal could potentially be infected with multiple Leptospira species. For future research, the primers for testing mixed infections should be tested and if they work as described, they should be used.
In conclusion, the results of this study indicate that Leptospira spp. and T. gondii are present in the population of wild small mammals in the Netherlands, indicating the importance of the studies for these infectious agents. The presence of Leptospira spp. in rodents and insectivores living around farms, could lead to transmission of the bacterium to human food (livestock) of humans itself.
The presence of T. gondii in small rodents present around farms could be a risk factor as rodents tend to visit barns. Theoretically production animals such as pigs could then get acquire infection, leading to potential risk for human infection as the infected meat ends on our table, potentially raw or undercooked (Guo et al., 2015; Kijlstra & Jongert, 2009). Another very important risk factor for T. gondii is the presence of (stray) cats. A suggestion for further research would be to study the prevalence of T. gondii in (stray) cats in the Netherlands. For Leptospira spp. it is an interesting and important finding that not only brown rats, but both rodent and insectivore species are carriers, and therewith could be considered as potential sources for human leptospirosis in the Netherlands. Consequently, rodents and insectivores could be good indicator species for monitoring of the presence of these zoonotic pathogens in the environment.
CONFLICT OF INTEREST
No conflict of interest exist.
ACKNOWLEDGEMENTS
The authors acknowledge the considerable support provided by Mr. H. H. J. Verlinden for his help with trapping the pest‐species. Furthermore, thanks to Henk Wisselink for his participation in the design of the study. We thank Conny van Solt for her participation in the laboratory work. The authors sincerely thank all farmers, civilians and pest managers for their cooperation.
Krijger IM, Ahmed AAA, Goris MGA, Cornelissen JBWJ, Groot Koerkamp PWG, Meerburg BG. Wild rodents and insectivores as carriers of pathogenic Leptospira and Toxoplasma gondii in The Netherlands. Vet Med Sci. 2020;6:623–630. 10.1002/vms3.255
REFERENCES
- Acha, P. N. , & Szyfres, B. (2003). Zoonoses and communicable diseases common to man and animals (Vol. 580). Washington, DC: Pan American Health Org. [Google Scholar]
- Adler, H. , Vonstein, S. , Deplazes, P. , Stieger, C. , & Frei, R. (2002). Prevalence of Leptospira spp. in various species of small mammals caught in an inner‐city area in Switzerland. Epidemiology & Infection, 128(1), 107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso, E. , Thulliez, P. , & Gilot‐Fromont, E. (2006). Transmission of Toxoplasma gondii in an urban population of domestic cats (Felis catus). International Journal for Parasitology, 36(13), 1373–1382. 10.1016/j.ijpara.2006.07.010 [DOI] [PubMed] [Google Scholar]
- Afonso, E. , Thulliez, P. , Pontier, D. , & Gilot‐Fromont, E. (2007). Toxoplasmosis in prey species and consequences for prevalence in feral cats: Not all prey species are equal. Parasitology, 134(14), 1963–1971. 10.1017/S0031182007003320 [DOI] [PubMed] [Google Scholar]
- Ahmed, A. , Engelberts, M. F. M. , Boer, K. R. , Ahmed, N. , & Hartskeerl, R. A. (2009). Development and validation of a real‐time PCR for detection of pathogenic Leptospira species in clinical materials. PLoS ONE, 4(9), e7093 10.1371/journal.pone.0007093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, A. , Grobusch, M. P. , Klatser, P. R. , & Hartskeerl, R. A. (2012). Molecular approaches in the detection and characterization of Leptospira. Journal of Bacteriology & Parasitology, 3(2). 10.4172/2155-9597.1000133 [DOI] [Google Scholar]
- Aviat, F. , Blanchard, B. , Michel, V. , Blanchet, B. , Branger, C. , Hars, J. , … Andre‐Fontaine, G. (2009). Leptospira exposure in the human environment in France: A survey in feral rodents and in fresh water. Comparative Immunology, Microbiology and Infectious Diseases, 32(6), 463–476. 10.1016/j.cimid.2008.05.004 [DOI] [PubMed] [Google Scholar]
- Bharti, A. R. , Nally, J. E. , Ricaldi, J. N. , Matthias, M. A. , Diaz, M. M. , Lovett, M. A. , … Vinetz, J. M. (2003). Leptospirosis: A zoonotic disease of global importance. The Lancet Infectious Diseases, 3(12), 757–771. 10.1016/S1473-3099(03)00830-2 [DOI] [PubMed] [Google Scholar]
- Costa, F. , Hagan, J. E. , Calcagno, J. , Kane, M. , Torgerson, P. , Martinez‐Silveira, M. S. , … Ko, A. I. (2015). Global morbidity and mortality of Leptospirosis: A systematic review. PLOS Neglected Tropical Diseases, 9(9), e0003898 10.1371/journal.pntd.0003898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvars, A. , Naze, F. , Vourc'h, G. , Cardinale, E. , Picardeau, M. , Michault, A. , & Bourhy, P. (2012). Similarities in Leptospira serogroup and species distribution in animals and humans in the Indian Ocean island of Mayotte. The American Journal of Tropical Medicine and Hygiene, 87(1), 134–140. 10.4269/ajtmh.2012.12-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey, J. P. (2014). The history and life cycle of Toxoplasma gondii In Weiss L. M. & Kim K. (Eds.), Toxoplasma gondii the model apicomplexan – perspectives and methods (pp. 1–17). Cambridge, MA: Academic Press. [Google Scholar]
- Dubey, J. P. (2016). Toxoplasmosis of animals and humans. Beltsville, MD: CRC Press. [Google Scholar]
- Dubey, J. , & Frenkel, J. K. (1998). Toxoplasmosis of rats: A review, with considerations of their value as an animal model and their possible role in epidemiology. Veterinary Parasitology, 77(1), 1–32. 10.1016/S0304-4017(97)00227-6 [DOI] [PubMed] [Google Scholar]
- Dubey, J. , Miller, N. , & Frenkel, J. (1970). Toxoplasma gondii life cycle in cats. Journal of the American Veterinary Medical Association, 157(11), 1767–1770. [PubMed] [Google Scholar]
- Faine, S. (1994). Leptospira and leptospirosis. Boca Raton: CRC Press Inc. [Google Scholar]
- Fernandes, L. G. , Siqueira, G. H. , Teixeira, A. R. , Silva, L. P. , Figueredo, J. M. , Cosate, M. R. , … Nascimento, A. L. (2016). Leptospira spp.: Novel insights into host–pathogen interactions. Veterinary Immunology and Immunopathology, 176, 50–57. [DOI] [PubMed] [Google Scholar]
- Foley, J. , & Straub, M. H. (2017). Leptospira spp. interpretation of equine laboratory diagnostics, 203. [Google Scholar]
- Fraga, T. R. , Carvalho, E. , Isaac, L. , & Barbosa, A. S. (2015). Leptospira and Leptospirosis In Tang Y.-W., Sussman M., Liu D., Poxton I., & Schwartzman J. (Eds.), Molecular medical microbiology (pp. 1973–1990). London, UK: Academic Press. [Google Scholar]
- Guernier, V. , Lagadec, E. , Cordonin, C. , Le Minter, G. , Gomard, Y. , Pagès, F. , … Dellagi, K. (2016). Human leptospirosis on Reunion Island, Indian Ocean: Are rodents the (only) ones to blame? PLOS Neglected Tropical Diseases, 10(6), e0004733 10.1371/journal.pntd.0004733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, M. , Dubey, J. P. , Hill, D. , Buchanan, R. L. , Gamble, H. R. , Jones, J. L. , & Pradhan, A. K. (2015). Prevalence and risk factors for Toxoplasma gondii infection in meat animals and meat products destined for human consumption. Journal of Food Protection, 78(2), 457–476. 10.4315/0362-028X.JFP-14-328 [DOI] [PubMed] [Google Scholar]
- Haake, D. A. , Dundoo, M. , Cader, R. , Kubak, B. M. , Hartskeerl, R. A. , Sejvar, J. J. , & Ashford, D. A. (2002). Leptospirosis, water sports, and chemoprophylaxis. Clinical Infectious Diseases, 34(9), e40–e43. 10.1086/339942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartskeerl, R. (2006). Leptospirosis: Current status and future trends. Indian Journal of Medical Microbiology, 24(4), 309 10.4103/0255-0857.29404 [DOI] [PubMed] [Google Scholar]
- Hartskeerl, R. , Collares‐Pereira, M. , & Ellis, W. (2011). Emergence, control and re‐emerging leptospirosis: Dynamics of infection in the changing world. Clinical Microbiology and Infection, 17(4), 494–501. 10.1111/j.1469-0691.2011.03474.x [DOI] [PubMed] [Google Scholar]
- Hartskeerl, R. , & Terpstra, W. (1996). Leptospirosis in wild animals. Veterinary Quarterly, 18(sup3), 149–150. 10.1080/01652176.1996.9694722 [DOI] [PubMed] [Google Scholar]
- Hejlíček, K. , & Literak, I. (1998). Long‐term study of Toxoplasma gondii prevalence in small mammals (Insectivora and Rodentia). Folia Zoologica, 47(2), 93–101. [Google Scholar]
- Himsworth, C. G. , Parsons, K. L. , Jardine, C. , & Patrick, D. M. (2013). Rats, cities, people, and pathogens: A systematic review and narrative synthesis of literature regarding the ecology of rat‐associated zoonoses in urban centers. Vector‐Borne and Zoonotic Diseases, 13(6), 349–359. 10.1089/vbz.2012.1195 [DOI] [PubMed] [Google Scholar]
- Kijlstra, A. , & Jongert, E. (2009). Toxoplasma‐safe meat: Close to reality? Trends in Parasitology, 25(1), 18–22. 10.1016/j.pt.2008.09.008 [DOI] [PubMed] [Google Scholar]
- Kijlstra, A. , Meerburg, B. , Cornelissen, J. , De Craeye, S. , Vereijken, P. , & Jongert, E. (2008). The role of rodents and shrews in the transmission of Toxoplasma gondii to pigs. Veterinary Parasitology, 156(3–4), 183–190. 10.1016/j.vetpar.2008.05.030 [DOI] [PubMed] [Google Scholar]
- Kim, K. , & Weiss, L. M. (2008). Toxoplasma: The next 100 years. Microbes and Infection, 10(9), 978–984. 10.1016/j.micinf.2008.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijger, I. M. , Cornelissen, J. B. , Wisselink, H. J. , & Meerburg, B. G. (2014). Prevalence of Toxoplasma gondii in common moles (Talpa europaea). Acta Veterinaria Scandinavica, 56(1), 48 10.1186/s13028-014-0048-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken, T. (1990). Infectierisico door contact met knaagdieren en insecteneters Leptospirose gevaar voor de mens. Zoogdier, 1(2), 3–10. [Google Scholar]
- Kuiken, T. , van Dijk, J. E. , Terpstra, W. J. , & Bokhout, B. A. (1991). The role of the common vole (Microtus arvalis) in the epidemiology of bovine infection with Leptospira interrogans serovar hardjo. Veterinary Microbiology, 28(4), 353–361. 10.1016/0378-1135(91)90070-V [DOI] [PubMed] [Google Scholar]
- Levett, P. N. (2001). Leptospirosis. Clinical Microbiology Reviews, 14(2), 296–326. 10.1128/CMR.14.2.296-326.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas, M. , De Vries, A. , Reusken, C. , Buijs, J. , Goris, M. , Hartskeerl, R. , … Pijnacker, R. (2018). Prevalence of Leptospira spp. and Seoul hantavirus in brown rats (Rattus norvegicus) in four regions in the Netherlands, 2011–2015. Infection Ecology & Epidemiology, 8(1), 1490135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerburg, B. G. , De Craeye, S. , Dierick, K. , & Kijlstra, A. (2012). Neospora caninum and Toxoplasma gondii in brain tissue of feral rodents and insectivores caught on farms in the Netherlands. Veterinary Parasitology, 184(2), 317–320. 10.1016/j.vetpar.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Meerburg, B. G. , Singleton, G. R. , & Kijlstra, A. (2009). Rodent‐borne diseases and their risks for public health. Critical Reviews in Microbiology, 35(3), 221–270. 10.1080/10408410902989837 [DOI] [PubMed] [Google Scholar]
- Moreno, L. Z. , Kremer, F. S. , Miraglia, F. , Loureiro, A. P. , Eslabao, M. R. , Dellagostin, O. A. , … Moreno, A. M. (2016). Comparative genomic analysis of Brazilian Leptospira kirschneri serogroup Pomona serovar Mozdok. Memórias do Instituto Oswaldo Cruz, 111(8), 539–541. 10.1590/0074-02760160174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley, M. , Rahelinirina, S. , Rajerison, M. , Garin, B. , Piertney, S. , & Telfer, S. (2018). Mixed Leptospira infections in a diverse reservoir host community, Madagascar, 2013–2015. Emerging Infectious Diseases, 24(6), 1138–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso, D. , & La Scola, B. (2013). Laboratory diagnosis of leptospirosis: A challenge. Journal of Microbiology, Immunology and Infection, 46(4), 245–252. 10.1016/j.jmii.2013.03.001 [DOI] [PubMed] [Google Scholar]
- Mwachui, M. A. , Crump, L. , Hartskeerl, R. , Zinsstag, J. , & Hattendorf, J. (2015). Environmental and behavioural determinants of leptospirosis transmission: A systematic review. PLOS Neglected Tropical Diseases, 9(9), e0003843 10.1371/journal.pntd.0003843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell, D. G. , Koopmans, M. , Verhoef, L. , Duizer, E. , Aidara‐Kane, A. , Sprong, H. , … Kruse, H. (2010). Food‐borne diseases — The challenges of 20 years ago still persist while new ones continue to emerge. International Journal of Food Microbiology, 139, S3–S15. 10.1016/j.ijfoodmicro.2010.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- News, N. (Producer). (2018). Natuurdrama op Texel: Wilde katten roven nesten leeg en verslinden jongen vogels. Retrieved from https://www.texelsecourant.nl/nieuws/natuur/32282/wilde-katten-blijven-bedreiging?redir [Google Scholar]
- Nicolle, C. , & Manceaux, L. (1908). Sur une infection à corps de Leishman (ou organismes voisins) du gondi. Comptes Rendus De L'académie Des Sciences, 147, 763–766. [Google Scholar]
- Obiegala, A. , Albrecht, C. , Dafalla, M. , Drewes, S. , Oltersdorf, C. , Turni, H. , … Pfeffer, M. (2017). Leptospira spp. in small mammals from areas with low and high human hantavirus incidences in south‐west Germany. Vector‐Borne and Zoonotic Diseases, 17(5), 312–318. [DOI] [PubMed] [Google Scholar]
- Obiegala, A. , Woll, D. , Karnath, C. , Silaghi, C. , Schex, S. , Eßbauer, S. , & Pfeffer, M. (2016). Prevalence and genotype allocation of pathogenic Leptospira species in small mammals from various habitat types in Germany. PLOS Neglected Tropical Diseases, 10(3), e0004501 10.1371/journal.pntd.0004501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opsteegh, M. (2011). Toxoplasma gondii in animal reservoirs and the environment. Retrieved from: Utrecht University. http://dspace.library.uu.nl/handle/1874/204414. [Google Scholar]
- Robert‐Gangneux, F. , & Dardé, M.‐L. (2012). Epidemiology of and diagnostic strategies for toxoplasmosis. Clinical Microbiology Reviews, 25(2), 264–296. 10.1128/CMR.05013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge, M. , von Keyserlingk, M. , Braune, S. , Becker, D. , Plenge‐Bönig, A. , Freise, J. F. , … Esther, A. (2013). Distribution of rodenticide resistance and zoonotic pathogens in Norway rats in Lower Saxony and Hamburg. Germany. Pest Management Science, 69(3), 403–408. 10.1002/ps.3369 [DOI] [PubMed] [Google Scholar]
- Schüffner, W. (1934). Recent work on leptospirosis. Transactions of the Royal Society of Tropical Medicine and Hygiene, 28(1), 7–31. 10.1016/S0035-9203(34)90094-8 [DOI] [Google Scholar]
- Spek, E. V. D. (2015). Loslopende katten worden in beeld gebracht in nationaal Park Duinen. Retrieved from https://www.boswachtersblog.nl/texel/2015/12/07/loslopende-katten-worden-in-beeld-gebracht-in-nationaal-park-duinen-van-texel/. [Google Scholar]
- Steinen, A. C. M. , Schuurman, J. L. , Gravekamp, C. , Korver, H. , & Terpstra, W. J. (1992). Muskrats as carriers of pathogenic leptospires in The Netherlands. Antonie Van Leeuwenhoek, 61(1), 43–50. 10.1007/BF00572121 [DOI] [PubMed] [Google Scholar]
- Terpstra, W. J. (1989). Ziekte van Weil, melkerskoorts en andere leptospirosen, 1981–1987. [PubMed] [Google Scholar]
- Torgerson, P. R. , Hagan, J. E. , Costa, F. , Calcagno, J. , Kane, M. , Martinez‐Silveira, M. S. , … Abela‐Ridder, B. (2015). Global burden of leptospirosis: Estimated in terms of disability adjusted life years. PLoS Neglected Tropical Diseases, 9(10), e0004122 10.1371/journal.pntd.0004122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treml, F. , Pejcoch, M. , & Holesovska, Z. (2002). Small mammals‐natural reservoir of pathogenic leptospires. Veterinarni Medicina‐Praha, 47(10/11), 309–314. [Google Scholar]
- Turk, N. , Milas, Z. , Margaletic, J. , Staresina, V. , Slavica, A. , Riquelme‐sertour, N. , … Postic, D. (2003). Molecular characterization of Leptospira spp. strains isolated from small rodents in Croatia. Epidemiology and Infection, 130(1), 159–166. 10.1017/S0950268802008026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uiterwijk, M. , Keur, I. , Friesema, I. , Valkenburgh, S. , Holtslag, M. , van Pelt, W. , … Maassen, K. (2016). Staat van Zoönosen 2016. Rijksinstituut voor Volksgezondheid en. Milieu RIVM.
- WHO (2011). Report of the second meeting of the Leptospirosis Burden Epidemiology Reference Group (p. 37). Geneva: World Health Organisation. [Google Scholar]
- Zilber, A.‐L. , Belli, P. , Grezel, D. , Artois, M. , Kodjo, A. , & Djelouadji, Z. (2016). Comparison of mucosal, subcutaneous and intraperitoneal routes of rat Leptospira infection. PLOS Neglected Tropical Diseases, 10(3), e0004569 10.1371/journal.pntd.0004569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuerner, R. L. (2015). Host response to Leptospira infection In Ahmed R., Akira S., Aktories K., Casadevall A., Compans R. W., Galan J. E., Garcia-Sastre A., Malissen B., Rappuoli R. (Eds.), Leptospira and Leptospirosis (pp. 223–250). Berlin, Heidelberg: Springer. [Google Scholar]


