Abstract
Background
Indoor air pollution (IAP) is an emerging issue for both human and veterinary patients under the concept of ‘One Health’. The association between IAP and respiratory disease in companion animals has been reported.
Objectives
The present study investigated the relationship between quantifiable indoor air quality and clinical characteristics of naturally acquired bronchial/lung disease in pet dogs and cats.
Methods
A total of 36 clinical cases (20 dogs and 16 cats) with naturally acquired bronchial/lung disease were prospectively recruited. Lower airway samples were collected and analysed, and clinical signs and the information from pulmonary function testing were examined. Indoor air quality was estimated by the average concentration of particles measuring ≤2.5 μm (PM2.5, μg/m3) and volatile organic compounds (VOC, ppm) in the animals’ domestic microenvironments.
Results
Exposure to IAP was not found to be correlated with the severity of clinical signs, pulmonary function changes or bronchoalveolar lavage fluid cytology in cats with bronchial/lung disease. However, a hypercellular response in canine lower airways was found to be associated with poor indoor air quality, including unacceptable indoor PM2.5 levels (>35 μg/m3) or increases in VOC concentration (>1 ppm) in places most commonly frequented by the dogs in the home.
Conclusions
Poor indoor air quality may exacerbate airway disease in pets and should not be ignored in modern society.
Keywords: bronchial/lung respiratory disease, bronchoalveolar lavage, indoor air pollution, one health, pet dog and cat, PM2.5 and VOC
Pet dogs and cats with naturally acquired bronchial/lung disease are also likely to expose to unacceptable level of particles measuring ≤2.5 μm or volatile organic compounds in their homes. A hypercellular response in canine lower airways was found to be associated with poor indoor air quality, implying that exposure to indoor air pollution (IAP) could stimulate the mucosa and lead to further pathological changes in dogs. Possible threat of IAP is an emerging ‘One Health’ issue that affects both pets and their owners in modern society.

1. INTRODUCTION
Air pollution is a serious global problem, and the role of indoor air pollution (IAP) on respiratory illness has been thoroughly investigated in the human health field over the past decades (Viegi et al., 2004; World Health Organization, 2010). The sources of indoor pollutants are variable, such as environmental tobacco smoke (ETS), other combustion products, volatile organic compounds (VOC) and various biological allergens (Hulin, Simoni, Viegi, & Annesi‐Maesano, 2012; Viegi et al., 2004). Many air pollutants are quantified by the size of particulate matter, such as particles measuring ≤2.5 μm (PM2.5), which are able to deposit in small airways and alveoli (Ivester, Couëtil, & Zimmerman, 2014). These particulate pollutants contribute to the adverse effect on the airways and lungs by oxidative stress, inflammation and an inappropriate immune‐modulating response (Nel, Diaz‐Sanchez, & Li, 2001), and the concentration of PM2.5 has been used to assess air pollution exposure in the microenvironment of dogs and cats (Lin, Lo, Wu, Chang, & Wang, 2018; Montrose et al., 2015). While the source of PM 2.5 can be from ambient air or indoor pollutants, VOCs almost always originate from products used in the house (Nurmatov, Tagiyeva, Semple, Devereux, & Sheikh, 2015; Viegi et al., 2004). Although studies concerning VOCs and respiratory health are relatively scarce, significant associations between indoor VOC concentrations and asthma‐like symptoms have been reported among humans (Hulin et al., 2012).
The phenomenon of the exacerbation of pre‐existing airway disease by exposure to air pollutants has been noticed since the 1950s (Sunyer, 2001). An increase in air pollution levels has been shown to be related to a decrease in lung function, higher rates of hospitalization or emergency admission, and an increase in mortality in human patients with chronic respiratory disease (Hulin et al., 2012; Sunyer, 2001). Interest in indoor pollution has been increasing in modern society, as the concentrations of air pollutants can be higher indoors than outdoors in confined environments (Hulin et al., 2012; Viegi et al., 2004). The human elderly and children with chronic obstructive pulmonary disease or asthma, as well as companion animals with airway/lung disease, stay indoors most of the time and are thus likely at risk for disease exacerbation from IAP exposure. This possible threat of IAP is an emerging issue for both human and veterinary patients under the scope of the ‘One Health’ concept.
The way in which air pollutants or particulate matter affect respiratory clinical signs, pulmonary function, health care burden, and mortality has been extensively evaluated in humans with asthma or chronic obstructive pulmonary disease (Beasley, Semprini, & Mitchell, 2015; Hulin et al., 2012; Viegi et al., 2004). However, there are few reports regarding the effect of IAP on small animal patients with lower airway or lung (LA/L) disease (Yamaya, Sugiya, & Watari, 2015). Therefore, the objective of this study was to investigate the relationship between quantifiable indoor air quality and the clinical characteristics of naturally acquired bronchial/lung disease in pet dogs and cats.
2. MATERIALS AND METHODS
Client‐owned dogs and cats that attended the National Taiwan University Veterinary Hospital for respiratory problems were prospectively screened for recruitment in the present study. The owners of dogs and cats that would receive lower airway sampling as a part of diagnostic procedures according to each individual animal's condition were asked for their permission to have their pet participate in this study. The inclusion criteria included a confirmed diagnosis of LA/L disease by the analysis of lower airway samples (e.g. bronchoalveolar lavage [BAL] fluid with or without bronchoscopic examination), informed consent from the pet owners, willingness to fill out a written questionnaire regarding the existence of well‐known or regionally prevalent indoor air pollutants in the pet's home and a quantifiable measurement of the indoor air quality in the pet's household. Animals less than 1‐year old, animals that had been living in the present home for <2 months at enrolment and animals with an outdoor lifestyle were excluded from the study.
Lower airway samples were aseptically collected with or without bronchoscopic guidance under general anaesthesia. Samples were processed and analysed as in previous studies (Hawkins, DeNicola, & Kuehn, 1990; Lin, Wu, Lee, & Liu, 2015; Nafe, DeClue, & Reinero, 2011). The final diagnosis of each case was given based on cytology and microbiological cultures of lower airway samples as well as other clinical information (history, physical examination, thoracic radiography, bronchoscopic findings, pulmonary function testing or other necessary testing).
The frequency or magnitude of clinical signs associated with the LA/L disease of each animal was assessed by veterinarians and recorded with clinical scores for subsequent statistical analysis. A 12‐point clinical scoring system described in previous feline lower airway disease studies (Lin et al., 2015) was used in cats, and a 6‐point Likert scale for quantifying cough frequency, cough magnitude, activity/appetite affected by cough and sleep/rest quality affected by cough was used in dogs.
Pulmonary function testing was performed in dogs with the spirometry system (Amis & Kurpershoek, 1986; Rozanski & Hoffman, 1999) and in cats with barometric whole body plethysmography (Lin et al., 2015). The presence or absence of an obstructive tidal breathing pattern, tachypnea and other abnormalities were recorded.
Indoor air quality was estimated in the animals’ domestic microenvironments with a method similar to that used in a previous study (Lin et al., 2018). In brief, PM2.5 concentration (μg/m3) was measured by a portable air quality monitor (TES‐5322, TES Electrical Electronic Corp) in accordance with the manufacturer's instructions. The air was sampled at the height of the animal's head (breathing zone), and the VOC (ppm), environmental temperature (°C) and environmental humidity (%) were measured and recorded at the same time. The average PM2.5 concentration in the animal's breathing zone was estimated by recording readings from the five places most commonly frequented by the animal in the home. After taking the five readings, the air quality monitor was then left in one of these five places (where the animal stays most of the time) to record 24‐hr changes.
Statistical analyses were performed using commercial software (SAS 9.4 and SPSS 19.0.0). Non‐parametric statistics were used for the analyses of the relatively small number of cases, and data were presented as a median with a range. The percentages of specific cell types, total nucleated cell count (TNCC) in the BAL fluid, age and clinical scores between animals living in households with acceptable and unacceptable PM2.5 level (Lin et al., 2018) were compared using Wilcoxon two‐sample test. The correlations between PM2.5 or VOC concentration and clinical data with continuous variables (the components of the cellular fraction of the BAL fluid, clinical scores for the severity of clinical signs) were analysed by Spearman's correlation. The associations between acceptable/unacceptable PM2.5 level and categorical data of clinical characteristics (abnormalities of pulmonary function, disease subgroups, gender and household conditions) were examined with Fisher's exact test. Statistical significance was defined as p < .05.
3. RESULTS
A total of 36 clinical cases (20 dogs and 16 cats) with naturally acquired LA/L disease were included in the present study. The median age of all dogs and cats was 9.3 years (range, 4.0–14.0) and 6.0 years (range, 2.0–12.0), respectively.
3.1. Lower airway cytology versus PM2.5 level
Lower airway samples were available in all 36 cases (35 by BAL and one by bronchoscopic‐guided bronchial brush). The PM2.5 level at the five places most commonly frequented by the animal in the home was successfully obtained in all 36 cases, whereas a 24‐hr recording was only available in 30 pet households and with reported problems from some pet owners. The average PM2.5 level in the breathing zone at five separate locations in the home was used for subsequent analyses. The differential cell counts in the BAL fluid of dogs documented a median 54.5% macrophages (range: 5.6%–87.1%), 12.4% neutrophils (range: 2.8–57.1), 1.1% eosinophils (range: 0.0–9.9) and 22.7% lymphocytes (range: 4.5–91.0). For BAL fluid of cats, it documented a median 31.7% macrophages (range: 2.7–63.9), 14.1% neutrophils (range: 1.4–82.1), 33.6% eosinophils (range: 0.7–74.6) and 5.6% lymphocytes (range: 2.1–42.4). A correlation between average PM2.5 and various cell populations or the presence of mucus or anthracosis in the lower airway sample was found in neither pet dogs nor cats. However, the TNCC was significantly higher in dogs living in households with PM2.5 >35 μg/m3 than in those living in households with acceptable levels of PM2.5 (median 622 cells/μL, range, 147–1407 vs. median 344 cells/μL, range, 175–591, p = .023) (Figure 1).
Figure 1.
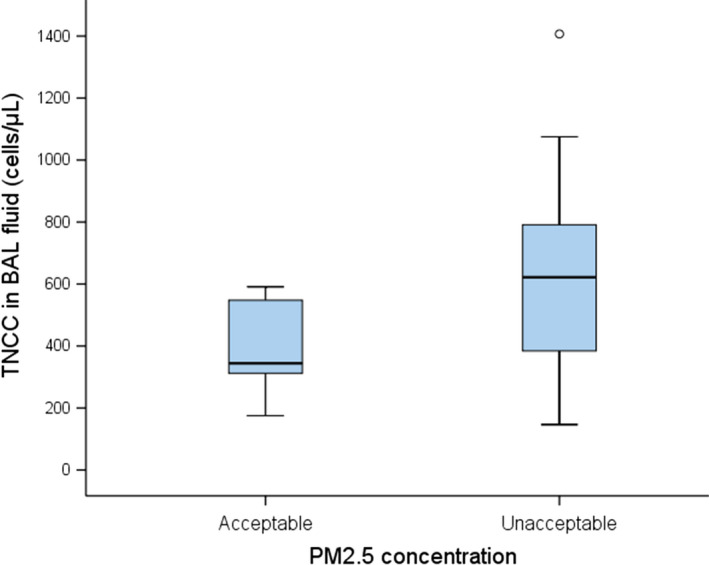
TNCC (cells/μL) in BAL fluid from nine dogs living in households with acceptable levels of PM2.5 and 10 dogs living in households with unacceptable PM2.5 levels (>35 μg/m3). BAL, bronchoalveolar lavage; TNCC, total nucleated cell count
3.2. Clinical signs versus PM2.5 level
Coughing was the most frequently reported clinical sign and was present in 19/20 canine cases and 13/16 feline cases. No statistically significant correlation was found between PM2.5 levels and the severity of clinical signs, thoracic auscultation or radiographic findings.
3.3. Pulmonary function versus PM2.5 level
Pulmonary function testing was performed in 19/20 dogs and all 16 cats. A lower airway obstructive pattern with variable severity was identified in 10/19 dogs and 11/16 cats. No statistically significant correlation was found between PM2.5 levels and the presence of an obstructive tidal breathing pattern.
3.4. Disease subgroups versus PM2.5 level
Among the 20 dogs, diagnoses included inflammatory airway disease in 17, bronchomalacia in 16, tracheal collapse in 12, lower respiratory tract infection in five and neoplasia in one. Among the 16 cats, diagnoses included inflammatory airway disease in 14 (seven asthma, three chronic bronchitis, four mixed‐type inflammation), lower respiratory tract infection in two, pneumonitis in one and neoplasia in one. A correlation between PM2.5 levels and disease subgroups (e.g. presence/absence of inflammatory airway disease or lower respiratory tract infection) was found in neither pet dogs nor cats.
3.5. Age, gender and household conditions versus PM2.5 level
Age and gender were not statistically different between dogs or cats living in households with PM2.5 >35 μg/m3 and in those living in households with acceptable levels of PM2.5. The proportion of existence of tobacco smoking, incense burning, cooking behaviour and room area setting (window closed vs. open most of time and flooring type) between pet households with unacceptable and acceptable levels of PM2.5 were not significantly different (Table 1). The PM2.5 concentrations from 24‐hr recording in 30 pet households showed that the peak value seemed more likely to occur during 8 a.m.–10 p.m., which represents a time period with more human activities relative to the sleep period of the pet owners (10 p.m.–8 a.m.) (Figure 2).
Table 1.
The proportion of existence of well‐known indoor air pollutants and room area setting in pet households with different levels of PM2.5 and VOC
| Household conditions | Dogs | Cats | ||||||
|---|---|---|---|---|---|---|---|---|
|
PM2.5 >35μg/m3 (n = 11) |
PM2.5 ≤35 μg/m3 (n = 9) |
VOC + (n = 4) |
VOC − (n = 16) |
PM2.5 >35μg/m3 (n = 11) |
PM2.5 ≤35 μg/m3 (n = 5) |
VOC + (n = 1) |
VOC − (n = 12) |
|
| Tobacco smoking | 36.4% (4/11) | 44.4% (4/9) | 25.0% (1/4) | 43.8% (7/16) | 45.5% (5/11) | 0.0% (0/5) | 0.0% (0/1) | 33.3% (4/12) |
| Incense burning | 27.3% (3/11) | 11.1% (1/9) | 75.0% (3/4) | 6.3% (1/16) | 9.1% (1/11) | 20.0% (1/5) | 100.0% (1/1) | 8.3% (1/12) |
| Cooking behaviour | 81.8% (9/11) | 77.8% (7/9) | 100.0% (4/4) | 75.0% (12/16) | 72.7% (8/11) | 100% (5/5) | 100.0% (1/1) | 91.7% (11/12) |
| Windows closed most of time | 18.2% (2/11) | 11.1% (1/9) | 50.0% (2/4) | 6.3% (1/16) | 9.1% (1/11) | 40.0% (2/5) | 0.0% (0/1) | 25.0% (3/12) |
| Wooden flooring | 9.1% (1/11) | 33.3% (3/9) | 75.0% (3/4) | 6.3% (1/16) | 18.2% (2/11) | 20.0 (1/5) | 100.0% (1/1) | 8.3% (1/12) |
Bold indicates statistically significant p value.
Abbreviation: VOC, volatile organic compounds.
Figure 2.
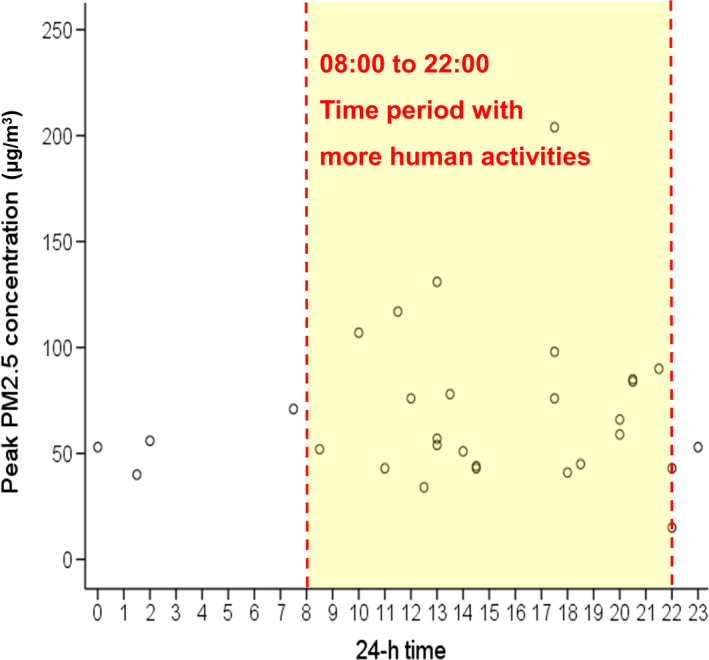
Scatterplot showed that peak PM2.5 concentrations (μg/m3) from 24‐hr recording were more commonly present during 8 a.m.–10 p.m., which represents a time period with more human activities relative to the sleep period of the pet owners (10 p.m.–8 a.m.)
3.6. VOC in pet households
The VOC concentration in the breathing zone at five separate locations in the home was successfully measured in 33 pet households and found to be 0 ppm in 28. VOC was only detected in a single cat household with the reading of 3.2 ppm. In the households of four dogs, the VOC concentration was found to be 2.8–3.6 ppm. In these five households with high level VOC, the proportion of existence of incense burning (80% vs. 7.1%, p = .002) and the room area setting with wooden flooring (80% vs. 7.1%, p = .002) were significantly higher than in households without detectable VOC. Other factors such as window closed setting, the existence of tobacco smoking and cooking behaviour were not significantly different between households with or without VOC. The TNCC in BAL fluid was weakly to moderately correlated with the average VOC concentration in the breathing zone at five separate locations in dog households (p = .038, r s = 0.48) (Figure 3). No statistical correlation was found between the VOC concentration and the severity of clinical signs, various cell populations, the presence of mucus or anthracosis in BAL fluid, and the presence of an obstructive tidal breathing pattern or disease subgroups in pet dogs.
Figure 3.
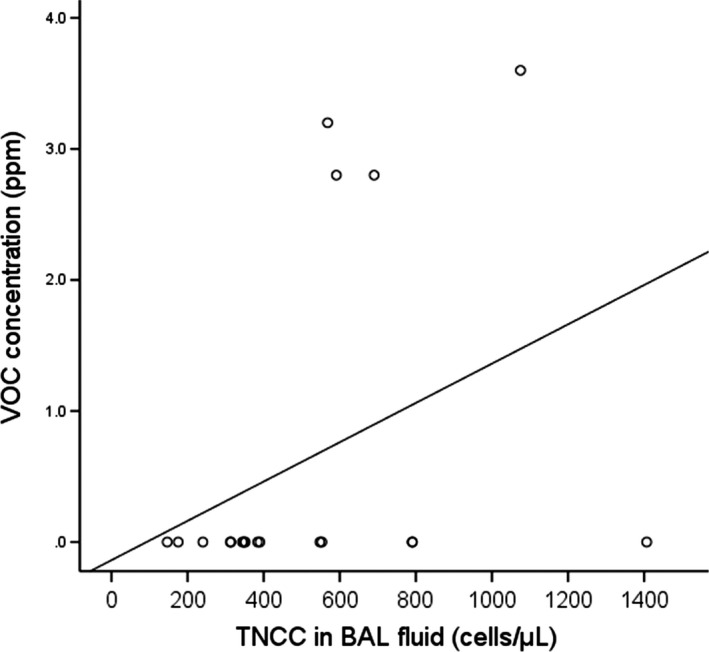
Scatterplot showing a weak to moderate correlation between the VOC concentration (ppm) and TNCC (cells/μL) in BAL fluid from 19 dogs (p = .038, r s = 0.48). BAL, bronchoalveolar lavage; TNCC, total nucleated cell count; VOC, volatile organic compound
4. DISCUSSION
This study showed that poor air quality was associated with a hypercellular response in the mucosa of the lower airways in dogs with naturally acquired bronchial/lung disease. An inflammatory response, oxidative stress and airway remodelling can be induced by short‐term or long‐term exposure to airborne particulate matter (Dye et al., 2001; Goldizen, Sly, & Knibbs, 2016). In human medicine, some studies have implicated the role of IAP in worsening the clinical condition of LA/L diseases, such as asthma and chronic obstructive pulmonary disease (Beasley et al., 2015; Hulin et al., 2012). It is worth noting that the airway response to air pollution may not be consistent across all individuals. Whereas exposure to air pollutants increased neutrophilic inflammation in healthy human airways, short‐term exposure to the same air pollutants elicited airway hyperresponsiveness in asthmatic patients who were on regular inhaled steroids to control inflammation (Nordenhäll et al., 2000, 2001). As a result, the actual clinical response to IAP in dogs or cats with naturally acquired LA/L disease was uncertain, and the data available from our study provided some evidence of this.
A previous study investigating IAP in dogs and cats with and without various kinds of respiratory disease has provided some evidence about whether IAP is a potential threat to the overall respiratory health of dogs and cats (Lin et al., 2018). Instead of focusing on all respiratory problems, the present study only recruited dogs and cats with LA/L disease based on lower airway sample analysis—the study question here was different from the previous study and intended to observe the effect of IAP on a specific subcategory of respiratory disease. In the previous study, the association between IAP and respiratory disease was not found and thought to be complicated in dogs, perhaps because of the frequent presence of multiple respiratory abnormalities (Lin et al., 2018). The same reason may also explain why the present study failed to detect a correlation between IAP and many clinical findings of LA/L disease, except for the increase in TNCC in BAL fluid. Inflammatory airway disease was the most common diagnosis in this study, but concurrent tracheobronchomalacia was also very common in these clinical cases, as found in some previous studies (Hawkins, Clay, Bradley, & Davidian, 2010; Johnson & Pollard, 2010). The interaction of multiple abnormalities may affect various aspects of a dog's clinical signs, underlying inflammatory response and overall pulmonary functional phenotype, leading to the difficulty in observing an effect associated with IAP.
The increase in TNCC in BAL fluid may be a non‐specific response in various lower airway diseases (Hawkins et al., 1990; Ybarra, Johnson, Drazenovich, Johnson, & Vernau, 2012). Nevertheless, it has been found that exposure to ambient air pollutants elicited significant proliferation of alveolar macrophages in BAL fluid, as well as other epithelial and endothelial pathology in healthy street dogs living in a polluted city (Calderón‐Garcidueñas et al., 2001). A significant increase in macrophages and lymphocytes in BAL fluid was also reported in a group of Yorkshire Terriers living in heavy‐smoking households (Roza & Viegas, 2007). In a recent study, the TNCC in BAL fluid was found to be increased in ETS‐exposed dogs with naturally acquired chronic bronchitis (Yamaya et al., 2015). Therefore, the hypercellular response in the BAL fluid of our dogs implies that exposure to IAP could stimulate the mucosa of the canine lower airway, potentially leading to further pathological changes.
A significant association between IAP and the presence of respiratory disease in cats has been reported in a previous study (Lin et al., 2018). However, a correlation between IAP and clinical severity was not found among cats with naturally acquired bronchial/lung disease in this study. It is possible that IAP exposure could induce the occurrence of respiratory disease in cats, but the subsequent severity of the LA/L disease may not be directly affected by the presence of IAP. Further prospective studies in cats are warranted to examine this hypothesis.
Inflammatory airway disease was the most common diagnosis in our study cats, and the percentage of eosinophils ranged from 0.7% to 74.6% in the BAL fluid. Indoor biological allergens such as house dust mites or moulds have been reported to have adverse effects on asthmatic humans by allergic sensitization (Hulin et al., 2012; Viegi et al., 2004). It cannot been known if some of these cats have higher eosinophil counts through exposure to indoor biological allergens, but it should be brought in mind that various sources of IAP can exist in the indoor environment.
The level of PM2.5 concentration in the animal's breathing zone from places most commonly frequented by animals in their homes could not be simply predicted by the presence/absence of a specific human activity associated with IAP such as ETS. It has been noted that IAP defined by questionnaire methodology may fail to show the relevance between ETS and health consequence (Hawkins et al., 2010; Lin et al., 2018), most likely because of the variability of human behaviour and house ventilation. Nevertheless, the highest PM 2.5 concentration from 24‐hr recording mostly occurred in the period with more human activities relative to the sleep period of pet owners, implying that human activities were possibly responsible for the elevation of PM2.5 in the animal's breathing zone.
The effect of VOC on the development or aggravation of some chronic airway diseases is less consistent and less significant than those of other indoor pollutants in human studies (Nurmatov et al., 2015). In dogs examined herein, the correlation between the VOC and TNCC was also not statistically strong enough to offer a definitive conclusion. However, the increased use of VOC‐containing residential materials and the confined space of modern buildings have contributed to the risk of VOC exposure (Hulin et al., 2012; Nurmatov et al., 2015). In our study, the existence of incense burning and the room setting of wooden flooring were more frequently seen in the five households with high level VOC than in households without detectable VOC. The adhesives used in wooden flooring or combustion products from incense burning might be the source of high VOC in these households (Hulin et al., 2012; Viegi et al., 2004; World Health Organization, 2010). Although only a few pet households had VOC in the animal's breathing zone, this still reminds us of the possible IAP exposure for pet dogs and cats living with their owners in modern society.
There were some limitations in this study. First, the small case numbers could have led to insufficient statistical power to detect a difference. However, the invasive procedure to obtain lower airway samples was not allowed by all pet owners. Collaboration across countries to get a larger sample size might be a future direction to draw a more solid conclusion on this emerging issue. Second, the PM2.5 concentration in the five places most commonly frequented by the animal in the home, instead of the 24‐hr PM2.5 recording, was used for analyses. This single sampling time might not have detected the dynamic changes of the PM2.5 level with time. Third, selection bias may exist, as many patients with LA/L disease were excluded if their owners were not willing to pursue lower airway diagnostic procedures. In addition, the constellation of disorders that comprised the diseased groups was heterogeneous. Although these problems are probably inevitable given the limited sample size and the difficulty in obtaining BAL from household pets, one should be aware that the results may be biased.
5. CONCLUSIONS
In conclusion, this study showed that poor air quality (PM2.5 level >35 μg/m3 or VOC concentration >1 ppm) was associated with a hypercellular response in the mucosa of the lower airways in dogs with naturally acquired bronchial/lung disease. Exposure to IAP was not found to be correlated with the severity of clinical signs, pulmonary function changes or BAL fluid cytology in cats with bronchial/lung disease, although poor air quality was associated with the presence of feline respiratory disease in a preceding case–control study. Further investigation is required to evaluate whether the hypercellular response to IAP in BAL fluid in dogs plays a role in ongoing pathological change and whether IAP has a role in the pathogenesis of LA/L disease in cats. The possible threat of IAP is an emerging ‘One Health’ issue that affects both pets and their owners in modern society.
CONFLICT OF INTEREST
There is no conflict of interest in this study.
ETHICAL STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received.
ACKNOWLEDGEMENTS
The authors acknowledge statistical assistance provided by the Department of Medical Research in National Taiwan University Hospital.
Lin C‐H, Lo P‐Y, Wu H‐D. An observational study of the role of indoor air pollution in pets with naturally acquired bronchial/lung disease. Vet Med Sci. 2020;6:314–320. 10.1002/vms3.231
Funding information
This study was supported by Ministry of Science and Technology, Taiwan (MOST 105‐2313‐B‐002‐055 and MOST 106‐2311‐B‐002‐029).
REFERENCES
- Amis, T. C. , & Kurpershoek, C. (1986). Tidal breathing flow‐volume loop analysis for clinical assessment of airway obstruction in conscious dogs. American Journal of Veterinary Research, 47, 1002–1006. [PubMed] [Google Scholar]
- Beasley, R. , Semprini, A. , & Mitchell, E. A. (2015). Risk factors for asthma: Is prevention possible? Lancet, 386, 1075–1085. 10.1016/S0140-6736(15)00156-7 [DOI] [PubMed] [Google Scholar]
- Calderón‐Garcidueñas, L. , Mora‐Tiscareño, A. , Fordham, L. A. , Chung, C. J. , García, R. , Osnaya, N. , … Devlin, R. B. (2001). Canines as sentinel species for assessing chronic exposures to air pollutants: Part 1. Respiratory Pathology. Toxicological Sciences, 61, 342–355. 10.1093/toxsci/61.2.342 [DOI] [PubMed] [Google Scholar]
- Dye, J. A. , Lehmann, J. R. , McGee, J. K. , Winsett, D. W. , Ledbetter, A. D. , Everitt, J. I. , … Costa, D. L. (2001). Acute pulmonary toxicity of particulate matter filter extracts in rats: Coherence with epidemiologic studies in Utah Valley residents. Environmental Health Perspectives, 109(Suppl 3), 395–403. 10.1289/ehp.01109s3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldizen, F. C. , Sly, P. D. , & Knibbs, L. D. (2016). Respiratory effects of air pollution on children. Pediatric Pulmonology, 51, 94–108. 10.1002/ppul.23262 [DOI] [PubMed] [Google Scholar]
- Hawkins, E. C. , Clay, L. D. , Bradley, J. M. , & Davidian, M. (2010). Demographic and historical findings, including exposure to environmental tobacco smoke, in dogs with chronic cough. Journal of Veterinary Internal Medicine, 4, 825–831. 10.1111/j.1939-1676.2010.0530.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins, E. C. , DeNicola, D. B. , & Kuehn, N. F. (1990). Bronchoalveolar lavage in the evaluation of pulmonary disease in the dog and cat. State of the art. Journal of Veterinary Internal Medicine, 4, 267–274. 10.1111/j.1939-1676.1990.tb03120.x [DOI] [PubMed] [Google Scholar]
- Hulin, M. , Simoni, M. , Viegi, G. , & Annesi‐Maesano, I. (2012). Respiratory health and indoor air pollutants based on quantitative exposure assessments. The European Respiratory Journal, 40, 1033–1045. 10.1183/09031936.00159011 [DOI] [PubMed] [Google Scholar]
- Ivester, K. M. , Couëtil, L. L. , & Zimmerman, N. J. (2014). Investigating the link between particulate exposure and airway inflammation in the horse. Journal of Veterinary Internal Medicine, 28, 1653–1665. 10.1111/jvim.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, L. R. , & Pollard, R. E. (2010). Tracheal collapse and bronchomalacia in dogs: 58 cases (7/2001‐1/2008). Journal of Veterinary Internal Medicine, 24, 298–305. 10.1111/j.1939-1676.2009.0451.x [DOI] [PubMed] [Google Scholar]
- Lin, C. H. , Lo, P. Y. , Wu, H. D. , Chang, C. , & Wang, L. C. (2018). Association between indoor air pollution and respiratory disease in companion dogs and cats. Journal of Veterinary Internal Medicine, 32, 1259–1267. 10.1111/jvim.15143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C. H. , Wu, H. D. , Lee, J. J. , & Liu, C. H. (2015). Functional phenotype and its correlation with therapeutic response and inflammatory type of bronchoalveolar lavage fluid in feline lower airway disease. Journal of Veterinary Internal Medicine, 29, 88–96. 10.1111/jvim.12494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrose, L. , Noonan, C. W. , Cho, Y. H. , Lee, J. , Harley, J. , O'Hara, T. , … Ward, T. (2015). Evaluating the effect of ambient particulate pollution on DNA methylation in Alaskan sled dogs: Potential applications for a sentinel model of human health. Science of the Total Environment, 512, 489–494. 10.1016/j.scitotenv.2014.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafe, L. A. , DeClue, A. E. , & Reinero, C. R. (2011). Storage alters feline bronchoalveolar lavage fluid cytological analysis. Journal of Feline Medicine and Surgery, 13, 94–100. 10.1016/j.jfms.2010.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel, A. E. , Diaz‐Sanchez, D. , & Li, N. (2001). The role of particulate pollutants in pulmonary inflammation and asthma: Evidence for the involvement of organic chemicals and oxidative stress. Current Opinion in Pulmonary Medicine, 7, 20–26. 10.1097/00063198-200101000-00004 [DOI] [PubMed] [Google Scholar]
- Nordenhäll, C. , Pourazar, J. , Blomberg, A. , Levin, J. O. , Sandström, T. , & Adelroth, E. (2000). Airway inflammation following exposure to diesel exhaust: A study of time kinetics using induced sputum. The European Respiratory Journal, 15, 1046–1051. 10.1034/j.1399-3003.2000.01512.x [DOI] [PubMed] [Google Scholar]
- Nordenhäll, C. , Pourazar, J. , Ledin, M. C. , Levin, J. O. , Sandström, T. , & Adelroth, E. (2001). Diesel exhaust enhances airway responsiveness in asthmatic subjects. The European Respiratory Journal, 17, 909–915. 10.1183/09031936.01.17509090 [DOI] [PubMed] [Google Scholar]
- Nurmatov, U. B. , Tagiyeva, N. , Semple, S. , Devereux, G. , & Sheikh, A. (2015). Volatile organic compounds and risk of asthma and allergy: A systematic review. The European Respiratory Review, 24, 92–101. 10.1183/09059180.00000714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza, M. R. , & Viegas, C. A. A. (2007). The dog as a passive smoker: Effects of exposure to environmental cigarette smoke on domestic dogs. Nicotine and Tobacco Research, 9, 1171–1176. 10.1080/14622200701648391 [DOI] [PubMed] [Google Scholar]
- Rozanski, E. A. , & Hoffman, A. M. (1999). Pulmonary function testing in small animals. Clinical Techniques in Small Animal Practice, 14, 237–241. 10.1016/S1096-2867(99)80017-6 [DOI] [PubMed] [Google Scholar]
- Sunyer, J. (2001). Urban air pollution and chronic obstructive pulmonary disease: A review. European Respiratory Journal, 17, 1024–1033. 10.1183/09031936.01.17510240 [DOI] [PubMed] [Google Scholar]
- Viegi, G. , Simoni, M. , Scognamiglio, A. , Baldacci, S. , Pistelli, F. , Carrozzi, L. , & Annesi‐Maesano, I. (2004). Indoor air pollution and airway disease. The International Journal of Tuberculosis and Lung Disease, 8, 1401–1415. [PubMed] [Google Scholar]
- World Health Organization (2010). WHO guidelines for indoor air quality: Selected pollutants. WHO Guidelines. Retrieved from http://apps.who.int/iris/handle/10665/260127?locale-attribute=en& [PubMed] [Google Scholar]
- Yamaya, Y. , Sugiya, H. , & Watari, T. (2015). Methylation of free‐floating deoxyribonucleic acid fragments in the bronchoalveolar lavage fluid of dogs with chronic bronchitis exposed to environmental tobacco smoke. Irish Veterinary Journal, 68, 7 10.1186/s13620-015-0035-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybarra, W. L. , Johnson, L. R. , Drazenovich, T. L. , Johnson, E. G. , & Vernau, W. (2012). Interpretation of multisegment bronchoalveolar lavage in cats (1/2001‐1/2011). Journal of Veterinary Internal Medicine, 26, 1281–1287. 10.1111/j.1939-1676.2012.01016.x [DOI] [PubMed] [Google Scholar]


