Abstract
Obesity has been identified as a risk factor for developing breast cancer in post‐menopausal period in humans and has been suspected to be associated with a worse prognosis also in the bitch. The aims of this study were to investigate the association between body condition score (BCS) and the prognosis of canine mammary carcinomas (CMCs) and the relationships between adiponectin expression and tumour behaviour. Seventy‐three bitches with tubular, tubulopapillary, solid or complex carcinomas were included in the present study. For each dog, evaluation of BCS was conducted using a nine‐point BCS system and the study population was divided into normal weight (4–5/9 points; n = 42), overweight (6–7/9 points; n = 19) and obese (8–9/9 points; n = 12). Type of diet (commercial, homemade or mixed) was recorded. After surgical excision, histological type, tumour size and nodal status were assessed and adiponectin expression was determined and quantified by immunohistochemistry and morphometric analysis. CMC histotype was not correlated with BCS, while a positive correlation between BCS and histological grade (p < .01) was observed. Overweight and obese bitches combined showed a shorter cancer‐specific survival than normal weighted bitches (p < .01). Bitches fed with a homemade diet had a higher BCS than dogs fed with a commercial one, although no relationship was observed between diet and cancer‐specific survival. Thirty‐six CMCs scored positive for adiponectin expression (49%), but no correlation was found between the hormone expression and either CMC characteristics or prognosis. In conclusion, a higher BCS seems to be related with a higher prevalence of more aggressive CMCs and negatively affects the survival time in bitches with these mammary tumours.
Keywords: adiponectin, body condition score, canine mammary tumour, cancer-specific survival, immunohistochemistry, obesity
Body condition score correlated with the histological grade of canine mammary carcinoma (CMC). Overweight bitches showed a shorter cancer‐specific survival. No correlation was found between adiponectin expression and either CMC characteristics or prognosis.
1. INTRODUCTION
Obesity is defined as an abnormal and excessive accumulation of adipose tissue in the body (German, 2006) and is also a well‐recognized risk factor for the development of post‐menopausal breast cancer in women. In particular, body mass index (BMI), distribution of body fat and time periods of weight gain are closely associated with tumour incidence (Cleary, Grossmann, & Ray, 2010; Han et al., 2006). Obesity is also associated with a poor prognosis of human breast cancer, namely higher rates of recurrence and mortality in pre‐ and post‐menopausal women. (Abrahamson et al., 2006; Berclaz et al., 2004; Carmichael, 2006).
Overweight became a significant health issue in both human and veterinary practice (German, 2006; James, Leach, Kalamara, & Shayeghi, 2001). The incidence of obesity is dramatically increasing in dogs and current estimates suggest that almost two‐thirds of dogs are obese (Crane, 1991; Lund, Armstrong, Kirk, Kolar, & Klausner, 1999; McGreevy et al., 2005). Findings concerning the association between obesity and the onset of canine mammary tumours (CMTs) are contradictory. Although the relationship between obesity and canine mammary neoplasms is not clearly known, it is speculated that overweight causes increased secretion of aromatase enzyme, insulin and IGF‐1 protein, influencing the carcinogenesis process (Cleary et al., 2010).
Among the various adipokines and inflammatory mediators involved in breast tumour development, adiponectin has been the subject of several studies in humans (Grossmann et al., 2010; Jardé et al., 2008; Kelesidis, Kelesidis, & Mantzoros, 2006), rodents (Denzel et al., 2009) and dogs (Lim et al., 2015a). Adiponectin is an adipose tissue‐secreted protein, negatively correlated with BMI, which has shown a strong apoptotic and anti‐proliferative activity in human breast cancer cell lines (Dieudonne et al., 2006). A decreased plasma adiponectin level and its decreased expression in mammary tumours are significantly associated with a higher incidence of breast cancer and a worse prognosis (Mantzoros et al., 2004).
The aims of this study were to investigate the relationships between body condition score (BCS), diet, and adiponectin expression with tumour size, the histological types and grades of CMCs and lymphatic invasion, and to assess their prognostic value in terms of cancer‐specific survival.
2. MATERIALS AND METHODS
2.1. Animals and procedures
This study was performed at the Veterinary Teaching Hospital of the University of Pisa, between January 2010 and February 2016. One hundred and fifty‐seven bitches referred for mammary gland nodules at the physical examination were evaluated. Of these, 73 bitches bearing malignant tumours and that, at the time of diagnosis, did not show any co‐morbidities that could significantly influence cancer‐specific survival at 24 months after mastectomy, were included in the study. For each dog, an accurate physical examination was conducted and a complete anamnesis was recorded, including the type of habitual diet over the past 2 years. Weight assessment was conducted using a nine‐point BCS evaluation system and the study population was divided into three groups based on the BCS: normal weight (BCS 4–5/9 points), overweight (BCS 6–7/9 points) and obese (BCS 8–9/9 points) (Laflamme, 1997). Staging was performed by abdominal ultrasound and a complete radiographic survey of the thorax (left lateral, right lateral and ventrodorsal views). A complete blood count, a biochemical analysis and a coagulation test (prothrombin time and partial thromboplastin time) were performed to verify the presence of co‐morbidities and to evaluate patient suitability for mammary surgery. Normal mammary gland tissues were collected during routine necropsies with the owners’ consent from four bitches who died due to causes unrelated with mammary tumours. This study was approved by the Ethical Committee (OBA) of the University of Pisa.
2.2. Tissue processing and histopathology
Representative samples of tumour and lymph node tissues collected after mastectomy and normal canine mammary tissues, were fixed in 10% neutral buffered formalin, routinely processed and embedded in paraffin and sectioned at 4 µm for histopathological examination. Hematoxylin and eosin (HE) stained sections were used to classify mammary tumours according to the classification proposed by Goldschmidt, Peña, Rasotto, and Zappulli (2011) by two experienced pathologists (AP and FM) who were blind to clinical data. Three/four sections per nodules were investigated to determine the histotype and grade of mammary tumours sampled. When dogs were presented with multiple tumours, the tumour with the most aggressive clinical and histopathological features (larger size, infiltrative growth and undifferentiated histology) was selected as reference lesion (Sorenmo, Worley, & Goldschmidt, 2011). Tumour grading was performed according the Elton Ellis criteria (Elton & Ellis, 2002). Mitotic index (number of mitoses/400x magnification), presence of lymphatic invasion and necrosis were also recorded for each tumour.
2.3. Immunohistochemistry and morphometrical evaluation
Adiponectin expression was investigated by immunohistochemistry. Four‐µm thick sections were dewaxed in xylene, hydrated throughout a graded series of ethanol and rehydrated in deionised water. Antigen retrieval was performed with citrate buffer pH 6.0 in a microwave oven for 5 min at 750 W and 13 min at 35°C and cooled at room temperature. After rinsing in 0.05% Tween TRIS‐Buffered Saline solution (TBST) at pH 7.6, endogenous peroxidase activity was exhausted by incubation of the sections with Peroxidase blocking solution (Dako, Glostrup, Denmark) for 10 min at room temperature and then two TBST washes were performed. Non‐specific reactions were blocked by incubating each section with two drops of Ultra V‐block (Thermo Scientific), for 5 min and a mouse monoclonal anti‐adiponectin primary antibody (19F1, Abcam Inc; diluted 1:100) was incubated for 1 hr in a moist chamber. After two washes with TBST, this phase was followed by incubation with a biotinylated goat polyvalent IgG secondary antibody (Thermo Scientific; diluted 1:200) for 10 min. After washing with TBST, streptavidin‐peroxidase complex (Thermo Scientific) was incubated for 30 min. Peroxidase activity was revealed by incubation for 10 min in 3,3′‐diaminobenzidine tetrahydrochloride (ImmPACT DAB Peroxidase Substrate Kit, Vector Labs Inc.) and blocked with deionized water. Finally, sections were counterstained with Mayer's haematoxylin, dried and covered with cover slips.
Negative controls were performed by replacing the primary antibody with species matched unrelated monoclonal antibodies and canine adipose tissue was used as internal positive control in each experiment.
For the morphometrical evaluation, five images, representative of adjacent and non‐overlapping fields, were acquired from each neoplastic lesion, using a semi‐automated image analysis system (LAS 4.10, Leica). Counts and intensity of adiponectin stained cells were assessed at 400× magnification and the percentage of positive neoplastic cells recorded. As previously proposed (Lim et al., 2015a), tumour samples with no or weak cytoplasmic staining of <10% tumour cells were classified as negative, while those with weak to strong cytoplasmic staining of >10% tumour cells were classified as positive.
2.4. Cancer‐specific survival study
A telephone survey was conducted to assess the cancer‐specific survival at 24 months after mastectomy. Owners were contacted to provide information on the health status of their animals (every 6 months) for the study period. The definitive diagnosis of the cause of death was made at post‐mortem examination by a single experienced pathologist who was not aware of the patients' clinical data (AP).
2.5. Statistical analysis
Statistical analysis was performed using the statistical package SPSS Advanced Statistic 21 (SPSS Inc, Chicago). Chi‐squared test to assess the correlation between the various parameters. Statistical significance was based on a 5% (.05) significance level. Cancer‐specific survival analysis was performed using the Kaplan–Meier method and the Tarone–Ware test was used to investigate the relationship between different parameters and cancer‐specific survival. Bitches that died for causes not related to the neoplastic mammary disease were excluded from the investigation about the overall survival.
3. RESULTS
The mean age of examined bitches was 9.7 ± 2.5 years (range: 4–16 years), 59% (43/73) of which were purebred and 41% (30/73) mix‐breed. The most represented breeds were Miniature Poodle (n = 9), English Setter, Yorkshire Terrier (n = 5), Beagle, German Shepherd (n = 4), Dachshund, Jack Russell Terrier (n = 3). Following, with two cases for each breed, we found English Cocker Spaniel, Maltese, Miniature Pinscher, Brittany Spaniel and Pointer. Sixty‐nine dogs were intact females and only four bitches were spayed at the time of mammary gland tumour diagnosis. Fifty‐seven/seventy‐three (79%) of bitches presented multiple mammary tumours and the median number of nodules per dog was 2. Lymph nodes invasion, histopathologically confirmed, was detected in 18/73 bitches, while no subject showed the presence of distant metastases.
Forty‐two bitches had normal BCS (57.5%), while bitches considered overweight and obese were 19 (26%) and 12 (16.5%), respectively. For normal weight, overweight and obese groups, the mean age at diagnosis was 9.7 ± 2.7 years, 10.2 ± 1.9 years and 9.4 ± 2.8 years, respectively. The control group (normal mammary gland tissues) was composed of a bitch with a normal BCS, two overweight bitches and an obese bitch (mean age was 9.8 ± 2.2 years). Lymph node metastases were observed in 8/42 (19%) bitches with normal BCS, while in subjects considered overweight and obese the presence of lymph node invasion was detected in 10/31 animals (32.2%; p > .05).
Concerning the diet, 21 (29%) animals ate commercial food, 23 (31%) followed a homemade diet and the remaining 29 (40%) dogs followed a mixed diet (predominantly commercial food with a homemade food supplementation).
The histologic types found were simple tubular CMCs (n = 24, 33%), tubulopapillary CMCs (n = 13, 18%), simple solid CMCs (n = 9, 12%) and complex CMCs (n = 27, 37%). Tumoural lesions were classified as grade I (n = 58, 79%), grade II (n = 11, 15%) and grade III (n = 4, 6%).
Adiponectin expression was characterized by the presence of a cytoplasm brown staining of different intensity. No cytoplasmic staining of adiponectin was recognized in normal epithelial cells in normal mammary gland tissue (Figure 1a), while scattered adiponectin‐positive cells were observed in lobular hyperplasia detected in mammary tissue adjacent to malignant mammary tumours (Figure 1b). Of the CMCs examined, 36/73 (49%) scored positive (Figure 1c‐d), while 37 (51%) were negative. In adiponectin‐positive complex CMCs, some myoepithelial cells scored positive (Figure 1d).
Figure 1.
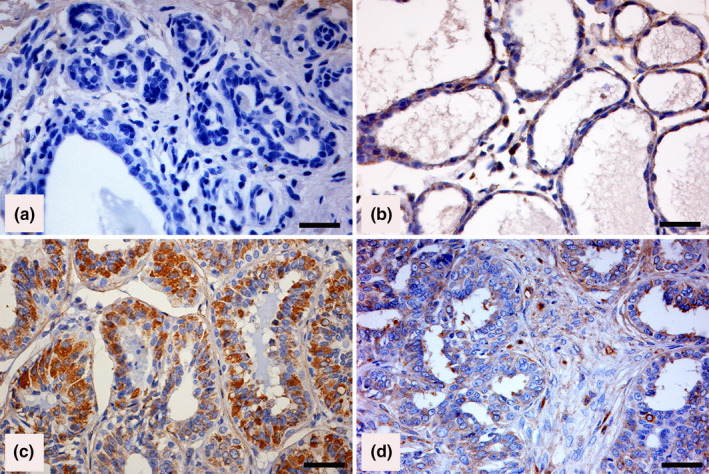
Canine mammary tissues. Immunohistochemical staining of adiponectin (Horseradish peroxidase and haematoxylin counterstain; Bar =50 µm). (a) Adiponectin‐negative labelling in normal mammary tissue. (b) Weak immunostaining in dysplatic cells in a lobular hyperplasia adjacent to a malignant mammary neoplasm. (c) Strong staining in more than 75% of neoplastic cells in an adiponectin‐positive tubular simple carcinoma. (d) Strong staining in most neoplastic cells and in myoepithelial cells in an adiponectin‐positive complex mammary carcinoma
Adiponectin expression did not significantly correlate with the tumour size, histotype, grade of CMCs or the presence of lymphatic invasion.
In our study, dogs that were still alive at 24 months after the surgical intervention were 44 (60%), 25 (34%) were dead from mammary cancer and 4 (6%) were dead from other causes. None of the patients was lost to follow‐up.
When bitches were classified by BCS, no differences in relative frequencies of the histological type of the carcinomas were observed. However, in grade I CMCs 38/58 (65.5%) bitches had a normal BCS, while there was a significantly higher proportion of histological grade II and III when BCS was ≥6 (11/15 73.3%; p < .01). Mean cancer‐specific survival was longer in dogs with normal BCS (690 days; 95% CI 651‐729 days) compared with mean cancer‐specific survival of overweight and obese bitches combined (546 days; 95% CI 460‐633 days) (Figure 2; p < .01).
Figure 2.
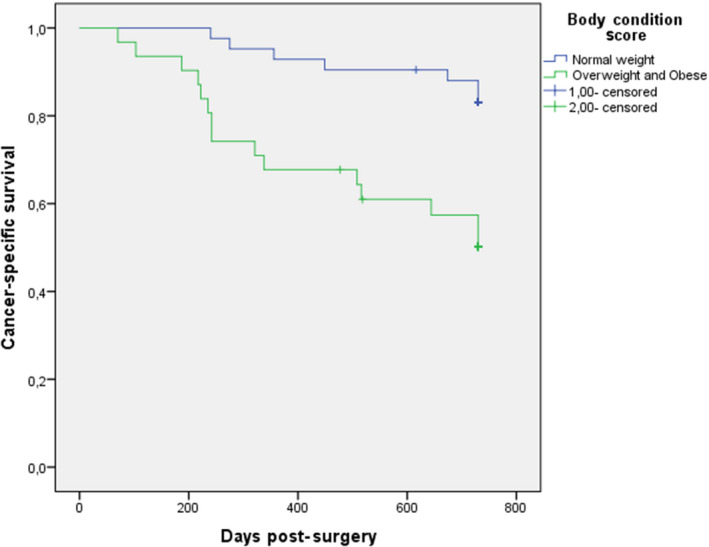
Cancer‐specific survival curves (a) for the group of 73 bitches bearing mammary carcinomas subdivided depending on BCS (normal: blue line, overweight and obese: green line; p < .01)
Bitches fed with a homemade diet had a higher BCS than dogs fed with a commercial one (p < .05), although no relationship was observed between diet and cancer‐specific survival (Figure 3).
Figure 3.
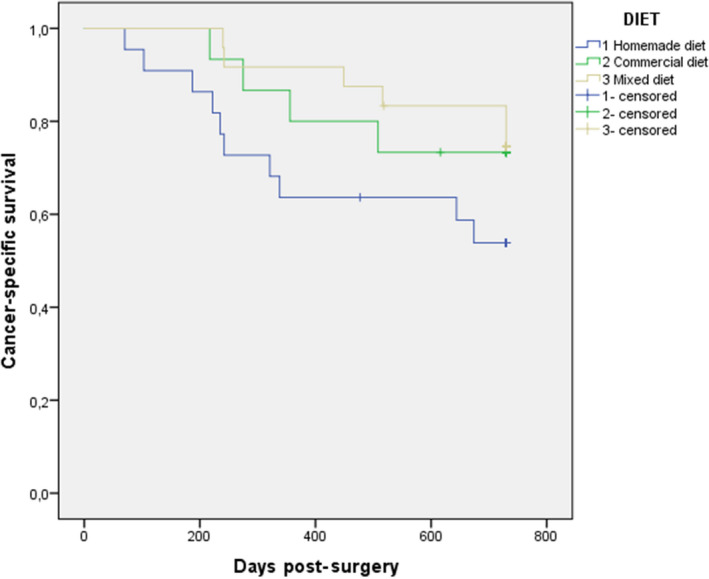
Cancer‐specific survival curve (a) for the group of 73 bitches bearing mammary carcinomas subdivided depending on the type of diet (homemade diet: blue line, commercial diet: green line and mixed diet: yellow line; p > .05)
Finally, the expression of adiponectin in the cytoplasm of epithelial cells did not significantly correlate with the BCS and cancer‐specific survival.
4. DISCUSSION
Some studies observed a higher prevalence of CMT and a worse prognosis in overweight and obese dogs (Alenza, Rutteman, Pena, Beynen, & Cuesta, 1998; Lim et al., 2015b) while others did not reveal any particular association (Marconato et al., 2009; Sonnenschein, Glickman, Goldschmidt, & McKee, 1991).
In this study, we did not observe any relationship between BCS and histotypes, while grade II and III CMCs were detected more often in overweight and obese bitches. This finding was in agreement with that of Lim et al. (2015b), who observed a higher proportion of grade III tumours in the overweight and obese groups. In our study, overweight and obese dogs had a lower cancer‐specific survival at 24 months after mastectomy than normal weight dogs. Shofer, Sonnenschein, Goldschmidt, Laster, and Glickman (1989) observed that dogs considered lean 1 year prior to diagnosis had a poorer survival compared with normal weight or overweight dogs (Shofer et al., 1989). The above‐mentioned results contrast with those reported by Philibert et al. (2003) who did not observe any difference in long‐term survival after mastectomy with respect to BMI: 10 months in obese dogs (BMI > 1) versus 14 months in normal weight dogs (BMI < 1) (Philibert et al., 2003). In human medicine, large cohort survival studies are numerous and reveal how obese patients have a significantly lower cancer‐specific survival and a positive association with breast cancer recurrence and death (Bastarrachea, Hortobagyi, Smith, Kau, & Buzdar, 1994; Kroenke, Chen, Rosner, & Holmes, 2005; Loi et al., 2005; Senie, 1994; Whiteman et al., 2005). Concerning canine species, the contrasting results reported in literature may be related to differences in sample population, therefore, further studies are required to reveal if the relationship between BMI and survival time is comparable to that observed in women.
The biological antagonist of leptin, adiponectin, displays pro‐apoptotic activity in various breast cancer cell lines (Dieudonne et al., 2006) and, in vivo studies, acts as inhibitor of angiogenesis (Brakenhielm et al., 2004).
By immunohistochemistry, 49% of CMCs were positive for adiponectin, similarly to the results observed in a previous study: Lim et al. (2015a) found that 36/70 (51%) of CMTs examined scored positive for adiponectin expression.
In the study of Lim et al. (2015a), overweight or obese female dogs had a lower proportion of adiponectin‐positive MCs. Furthermore, a positive adiponectin expression was associated with a lower proportion of grade III tumours and a decreased lymphatic invasion (Lim et al., 2015a). In contrast to the previous study, we could not describe the relationships between adiponectin expression with any of the parameters evaluated (tumour size, histological type, histological grade, linfatic invasion, BCS and cancer‐specific survival). Adiponectin is known to be negatively related to BCS (Dieudonne et al., 2006) and positively related to prognosis (Mantzoros et al., 2004). Namely, the lower the BCS and the better the prognosis, the greater the tendency of this protein expression in tumours, which was evidenced in the study, since most bitches were of normal weight, had less aggressive carcinoma and were alive after 24 months of follow‐up (60%). In our study, bitches fed with homemade diet had a significantly higher BCS than dogs fed with a commercial one, suggesting how important is the type of diet in the occurrence of obesity. In most cases, homemade diets were not based on the indications of a nutritionist and contained high levels of carbohydrate and saturated fatty acids. Conversely, commercial diets were often quality products, perfectly balanced and did not exceed the nutritional requirements of the dog (Mao, Xia, Chen, & Yu, 2013). According to Alenza et al. (1998), a homemade diet significantly increases the incidence of CMTs, especially if characterized by a high intake of red meat (in particular beef and pork) and low intake of chicken. Although homemade diet was significantly related to overweight and obesity, no association was observed between diet and cancer‐specific survival in the present study.
In conclusion, in bitches higher BCS at the time of CMC diagnosis was correlated to a higher histological grade and a shorter cancer‐specific survival, suggesting its useful role as prognostic factor. Adiponectin expression does not appear to be related to CMC characteristics or BCS. Further studies are needed to determine if adiponectin expression and diet may have a prognostic value in the canine mammary gland carcinoma.
CONFLICT OF INTEREST
None of the authors of this article has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the study.
Tesi M, Millanta F, Poli A, et al. Role of body condition score and adiponectin expression in the progression of canine mammary carcinomas. Vet Med Sci. 2020;6:265–271. 10.1002/vms3.238
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.238
REFERENCES
- Abrahamson, P. E. , Gammon, M. D. , Lund, M. J. , Flagg, E. W. , Porter, P. L. , Stevens, J. , … Coates, R. J. (2006). General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiology, Biomarkers & Prevention, 15, 1871–1877. 10.1158/1055-9965.EPI-06-0356 [DOI] [PubMed] [Google Scholar]
- Alenza, D. P. , Rutteman, G. R. , Pena, L. , Beynen, A. C. , & Cuesta, P. (1998). Relation between habitual diet and canine mammary tumors in a case‐control study. Journal of Veterinary Internal Medicine, 12, 132–139. 10.1111/j.1939-1676.1998.tb02108.x [DOI] [PubMed] [Google Scholar]
- Bastarrachea, J. , Hortobagyi, G. N. , Smith, T. L. , Kau, S. W. , & Buzdar, A. U. (1994). Obesity as an adverse prognostic factor for patients receiving adjuvant chemotherapy for breast cancer. Annals of Internal Medicine, 120, 18–25. 10.7326/0003-4819-120-1-199401010-00004 [DOI] [PubMed] [Google Scholar]
- Berclaz, G. , Li, S. , Price, K. N. , Coates, A. S. , Castiglione‐Gertsch, M. , Rudenstam, C. M. , … Goldhirsch, A. (2004). Body mass index as a prognostic feature in operable breast cancer: The International Breast Cancer Study Group experience. Annals of Oncology, 15, 875–884. 10.1093/annonc/mdh222 [DOI] [PubMed] [Google Scholar]
- Brakenhielm, E. , Veitonmaki, N. , Cao, R. , Kihara, S. , Matsuzawa, Y. , Zhivotovsky, B. , … Cao, Y. (2004). Adiponectin‐induced antiangiogenesis and antitumor activity involve caspase‐mediated endothelial cell apoptosis. Proceeding of the National Academy of Sciences USA, 101(8), 2476–2481. 10.1073/pnas.0308671100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael, A. R. (2006). Obesity and prognosis of breast cancer. Obesity Reviews, 7, 333–340. 10.1111/j.1467-789X.2006.00261.x [DOI] [PubMed] [Google Scholar]
- Cleary, M. P. , Grossmann, M. E. , & Ray, A. (2010). Effect of obesity on breast cancer development. Veterinary Pathology, 47(2), 202–213. 10.1177/0300985809357753 [DOI] [PubMed] [Google Scholar]
- Crane, S. W. (1991). Occurrence and management of obesity in companion animals. Journal of Small Animal Practice, 32, 275–282. 10.1111/j.1748-5827.1991.tb00930.x [DOI] [Google Scholar]
- Denzel, M. S. , Hebbard, L. W. , Shostak, G. , Shapiro, L. , Cardiff, R. D. , & Ranscht, B. (2009). Adiponectin deficiency limits tumor vascularization in the MMTV‐PyV‐mT mouse model of mammary cancer. Clinical Cancer Research, 15(10), 3256–3264. 10.1158/1078-0432.CCR-08-2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudonne, M. N. , Bussiere, M. , Dos Santos, E. , Leneveu, M. C. , Giudicelli, Y. , & Pecquery, R. (2006). Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochemical and Biophysical Research Communications, 345, 271–279. 10.1016/j.bbrc.2006.04.076 [DOI] [PubMed] [Google Scholar]
- Elston, C. W. , & Ellis, I. O. (2002). Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long‐term follow‐up. Histopathology, 19, 403–410. 10.1046/j.1365-2559.2002.14892.x [DOI] [PubMed] [Google Scholar]
- German, A. J. (2006). The growing problem of obesity in dogs and cats. Journal of Nutrition, 136, 1940S–1946S. 10.1093/jn/136.7.1940S [DOI] [PubMed] [Google Scholar]
- Goldschmidt, M. , Peña, L. , Rasotto, R. , & Zappulli, V. (2011). Classification and grading of canine mammary tumors. Veterinary Pathology, 48(1), 117–131. 10.1177/0300985810393258 [DOI] [PubMed] [Google Scholar]
- Grossmann, M. E. , Ray, A. , Nkhata, K. J. , Malakhov, D. A. , Rogozina, O. P. , Dogan, S. , & Cleary, M. P. (2010). Obesity and breast cancer: Status of leptin and adiponectin in pathological processes. Cancer Metastasis Reviews, 29(4), 641–653. 10.1007/s10555-010-9252-1 [DOI] [PubMed] [Google Scholar]
- Han, D. , Nie, J. , Bonner, M. R. , McCann, S. E. , Muti, P. , Trevisan, M. , … Freudenheim, J. L. (2006). Lifetime adult weight gain, central adiposity, and the risk of pre‐ and postmenopausal breast cancer in the Western New York exposures and breast cancer study. International Journal of Cancer, 119, 2931–2937. 10.1002/ijc.22236 [DOI] [PubMed] [Google Scholar]
- James, P. T. , Leach, R. , Kalamara, E. , & Shayeghi, M. (2001). The worldwide obesity epidemic. Obesity Research & Clinical Practice, 9(4), 228–233. 10.1038/oby.2001.123 [DOI] [PubMed] [Google Scholar]
- Jardé, T. , Caldefie‐Chezet, F. , Damez, M. , Mishellany, F. , Perrone, D. , Penault‐Llorca, F. , … Vasson, M. P. (2008). Adiponectin and leptin expression in primary ductal breast cancer and in adjacent healthy epithelial and myoepithelial tissue. Histopathology, 53(4), 484–487. 10.1111/j.1365-2559.2008.03121.x [DOI] [PubMed] [Google Scholar]
- Kelesidis, I. , Kelesidis, T. , & Mantzoros, C. S. (2006). Adiponectin and cancer: A systematic review. British Journal of Cancer, 94(9), 1221–1225. 10.1038/sj.bjc.6603051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke, C. H. , Chen, W. Y. , Rosner, B. , & Holmes, M. D. (2005). Weight, weight gain, and survival after breast cancer diagnosis. Journal of Clinical Oncology, 23, 1370–1378. 10.1200/jco.2005.01.079 [DOI] [PubMed] [Google Scholar]
- Laflamme, D. (1997). Development and validation of a body condition score system for dogs. Canine Practice, 22, 10–15. [Google Scholar]
- Lim, H. Y. , Im, K. S. , Kim, N. H. , Kim, H. W. , Shin, J. I. , & Sur, K. H. (2015a). Obesity, expression of adipocytokines, and macrophage infiltration in canine mammary tumors. Veterinary Journal, 203, 326–331. 10.1016/j.tvjl.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Lim, H. Y. , Im, K. S. , Kim, N. H. , Kim, H. W. , Shin, J. I. , Yhee, J. Y. , & Sur, J. H. (2015b). Effects of obesity and obesity‐related molecules on canine mammary gland tumors. Veterinary Pathology, 52, 1045–1051. 10.1177/0300985815579994 [DOI] [PubMed] [Google Scholar]
- Loi, S. , Milne, R. L. , Friedlander, M. L. , McCredie, M. R. , Giles, G. G. , Hopper, J. L. , & Phillips, K. A. (2005). Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiology, Biomarkers & Prevention, 14, 1686–1691. 10.1158/1055-9965.EPI-05-0042 [DOI] [PubMed] [Google Scholar]
- Lund, E. M. , Armstrong, P. J. , Kirk, C. K. , Kolar, L. M. , & Klausner, J. S. (1999). Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. Journal of American Veterinary Medical Association, 214, 1336–1341. [PubMed] [Google Scholar]
- Mantzoros, C. , Petridou, E. , Dessypris, N. , Chavelas, C. , Dalamaga, M. , Alexe, D. M. , … Trichopoulos, D. (2004). Adiponectin and breast cancer risk. Journal of Clinical Endocrinology and Metabolism, 89, 1102–1107. 10.1210/jc.2003-031804 [DOI] [PubMed] [Google Scholar]
- Mao, J. , Xia, Z. , Chen, J. , & Yu, J. (2013). Prevalence and risk factors for canine obesity surveyed in veterinary practices in Beijing, China. Preventive Veterinary Medicine, 112, 438–442. 10.1016/j.prevetmed.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Marconato, L. , Romanelli, G. , Stefanello, D. , Giacoboni, C. , Bonfanti, U. , Bettini, G. , … Zini, E. (2009). Prognostic factors for dogs with mammary inflammatory carcinoma: 43 cases (2003–2008). Journal of American Veterinary Medical Association, 235, 967–972. 10.2460/javma.235.8.967 [DOI] [PubMed] [Google Scholar]
- McGreevy, P. D. , Thomson, P. C. , Pride, C. , Fawcett, A. , Grassi, T. , & Jones, B. (2005). Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Veterinary Record, 156, 696–707. 10.1136/vr.156.22.695 [DOI] [PubMed] [Google Scholar]
- Peña, L. , De Andrés, P. J. , Clemente, M. , Cuesta, P. , & Pérez‐Alenza, M. D. (2013). Prognostic value of histological grading in noninflammatory canine mammary carcinomas ia a prospective study with two‐year follow‐up: Relationship with clinical and histological characteristics. Veterinary Pathology, 50(1), 94–105. [DOI] [PubMed] [Google Scholar]
- Philibert, J. C. , Snyder, P. W. , Glickman, N. , Glickman, L. T. , Knapp, D. W. , & Waters, D. J. (2003). Influence of host factors on survival in dogs with malignant mammary gland tumors. Journal of Veterinary Internal Medicine, 17, 102–106. 10.1111/j.1939-1676.2003.tb01330.x [DOI] [PubMed] [Google Scholar]
- Senie, R. T. (1994). Breast cancer prognosis: Role of obesity? Journal of Surgical Oncolgy, 57, 30 10.1002/jso.2930570109 [DOI] [PubMed] [Google Scholar]
- Shofer, F. S. , Sonnenschein, E. G. , Goldschmidt, M. H. , Laster, L. L. , & Glickman, L. T. (1989). Histopathologic and dietary prognostic factors for canine mammary carcinoma. Breast Cancer Research and Treatment, 13, 49–60. 10.1007/BF01806550 [DOI] [PubMed] [Google Scholar]
- Sonnenschein, E. G. , Glickman, L. T. , Goldschmidt, M. H. , & McKee, L. J. (1991). Body conformation, diet, and risk of breast cancer in pet dogs: A case‐control study. American Journal of Epidemiology, 133, 694–703. 10.1093/oxfordjournals.aje.a115944 [DOI] [PubMed] [Google Scholar]
- Sorenmo, K. U. , Worley, D. R. , & Goldschmidt, M. H. (2011). Tumors of the mammary gland In Withrow S. J., Vail D. M., & Rodney L. P. (Eds.), Small animal clinical oncology (5th Ed., pp. 538–556). St Louis, MO: Saunders Elsevier. [Google Scholar]
- Whiteman, M. K. , Hillis, S. D. , Curtis, K. M. , McDonald, J. A. , Wingo, P. A. , & Marchbanks, P. A. (2005). Body mass and mortality after breast cancer diagnosis. Cancer Epidemiology, Biomarkers & Prevention, 14, 2009–2014. 10.1158/1055-9965.EPI-05-0106 [DOI] [PubMed] [Google Scholar]


