Objective
The coronavirus disease 2019 (COVID-19) pandemic caused significant mortalities in the adult population in New York City. Pregnant women at late gestational age positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) showed mild symptoms compared with nonpregnant adults. Rare cases of newborn babies testing positive for the SARS-CoV-2 virus within the first 24 hours have been reported.1 , 2 Miscarriages in second trimesters were also reported related to maternal COVID-19.3 Placental pathology has been studied in small case series with controversial results, and vertical transmission was reported in some cases.4, 5, 6 We sought to examine the placental pathology and to localize the SARS-CoV-2 viral particles within the placental tissue.
Study Design
The case series study was approved by the institutional review board. The placentas were submitted to routine pathology examination based on the institutional criteria of maternal and fetal conditions established previously. All placentas were fixed in formalin overnight, and a standard 4 sections were taken for light microscopy. Routine pathology examination was performed based on the current Amsterdam placental examination guideline. Clinical and pathologic features were collected and statistically analyzed using various programs of the R package. SARS-CoV-2 in situ hybridization (ISH) was performed by using an RNAscope reagent kit (Advanced Cell Diagnostics, Inc, Newark, CA) containing a specific probe against viral M-spike RNA and a Leica Bond III automated instrument (Leica Biosystems Nussloch GmbH, Nußloch, Germany) following the manufacturer’s instruction. Immunostaining for CD68 and CD42b was performed to highlight the tissue macrophages and platelet aggregates using the Leica Bond III automated instrument for routine clinical application as described previously.
Results
We examined 364 consecutive placentas from the mothers tested in our facilities since the universal testing policy was adopted in March 2020, including 74 positive and 290 negative for SARS-CoV-2 by nasopharyngeal swab polymerase chain reaction (PCR) method as previously described.1 Pathologic examination of the placentas after delivery was performed and the clinical characteristics of the mothers were similar to those described previously.1 The placental pathologic findings and clinical characteristics are listed in the Table. There were no histopathologic features within the placentas specific to maternal SARS-CoV-2 infection. The clinical diagnoses of preeclampsia and category 2 fetal heart tracing were shown to be negatively associated with COVID-19 (Table ). The odds ratio of specific placental and clinical features from COVID-19–positive and COVID-19–negative mothers were calculated using the generalized linear model of the R package (Poisson model) (Figure 1 ), and the placental weight and gestational age distributions were plotted using the conditional plot of the R package (Figure 2 ). SARS-CoV-2 viral particles were tested within the placental tissue by automated ISH using a specific COVID-19 probe. SARS-CoV-2 viral particles can be demonstrated within the syncytiotrophoblasts associated with or without placental infarcts (Figure 3 ). There were increased maternal macrophages and platelet aggregates (thrombosis) surrounding the infarcts positive for CD68 and CD42b (Figure 3). SARS-CoV-2 viral RNA signals were also detected within the atrophic endometrial glandular epithelium (Figure 3, bottom panel) and subchorionic plate (Langhan’s fibrinoid) (Figure 4 ). No viral signals were identified within any other maternal, fetal, or placental cell types. A total of 53 placentas from COVID-19–positive mothers were tested, and there were 2 placentas positive for SARS-CoV-2 by ISH (2 of 53, 3.8%). No SARS-CoV-2–positive placentas were identified by ISH in 10 placentas from PCR-negative mothers. Notably, 1 placenta positive for SARS-CoV-2 by ISH was delivered by cesarean delivery at 35 weeks and 6 days because of placental previa associated with placental infarcts, and the newborn baby tested positive by swab PCR method at 24 hours, 48 hours, and 7 days. The neonate was asymptomatic and discharged home. The other positive placenta was from a mother with 40 weeks’ gestation associated with no significant clinical and pathologic features, and the baby tested negative for SARS-CoV-2 by swab PCR method within the first 24 hours. ISH test result for this placenta showed the presence of viral particles only within the endometrial glands but not in the syncytiotrophoblasts. All other neonates tested negative by nasopharyngeal swab PCR method.
Table.
Baseline characteristics of clinical and pathologic findings of placentas from COVID-19–positive and COVID-19–negative mothers
| COVID-19 (placental pathology) | Negative (n=290) |
Positive (n=74) |
P value |
|---|---|---|---|
| Vasculopathy | .729 | ||
| Classic type | 119 (41.0) | 25 (33.8) | |
| Mixed type | 25 (8.6) | 7 (9.5) | |
| Mural hypertrophy | 14 (4.8) | 4 (5.4) | |
| No vasculopathy | 132 (45.5) | 38 (51.4) | |
| Weight | 427.5 (366.0–526.0) | 437.0 (390.0–519.0) | .492 |
| Gestational age | 39.0 (38.0–40.0) | 39.0 (38.0–40.0) | .705 |
| Infarcts | 1.000 | ||
| 0 | 265 (91.4) | 67 (90.5) | |
| –1 | 25 (8.6) | 7 (9.5) | |
| Chorioamnionitis | .981 | ||
| 0 | 99 (34.1) | 26 (35.1) | |
| –1 | 191 (65.9) | 48 (64.9) | |
| Meconium | .459 | ||
| 0 | 180 (62.1) | 50 (67.6) | |
| –1 | 110 (37.9) | 24 (32.4) | |
| Thrombosis | 1.000 | ||
| 0 | 217 (74.8) | 56 (75.7) | |
| –1 | 73 (25.2) | 18 (24.3) | |
| Villitis | 1.000 | ||
| 0 | 225 (77.6) | 57 (77.0) | |
| –1 | 65 (22.4) | 17 (23.0) | |
| Abruption | .965 | ||
| 0 | 281 (96.9) | 71 (95.9) | |
| –1 | 9 (3.1) | 3 (4.1) | |
| Cord issues | .143 | ||
| 0 | 271 (93.4) | 73 (98.6) | |
| –1 | 19 (6.6) | 1 (1.4) | |
| MFI | .555 | ||
| 0 | 275 (94.8) | 72 (97.3) | |
| –1 | 15 (5.2) | 2 (2.7) | |
| Others | .946 | ||
| 0 | 258 (89.0) | 65 (87.8) | |
| –1 | 32 (11.0) | 9 (12.2) | |
| Cord coiling | 3.0 (2.0–5.0) | 3.0 (2.0–4.0) | .428 |
| Avascular villi | .614 | ||
| 0 | 277 (95.5) | 69 (93.2) | |
| –1 | 13 (4.5) | 5 (6.8) |
| COVID-19 (clinical features) | Negative | Positive | P value |
|---|---|---|---|
| Delivery | .048a | ||
| Cesarean | 117 (40.3) | 20 (27.0) | |
| Vaginal | 173 (59.7) | 54 (73.0) | |
| Preeclampsia | .003a | ||
| 0 | 240 (82.8) | 72 (97.3) | |
| –1 | 50 (17.2) | 2 (2.7) | |
| Gestational diabetes mellitus | .449 | ||
| 0 | 264 (91.0) | 70 (94.6) | |
| –1 | 26 (9.0) | 4 (5.4) | |
| Category 2 | .000a | ||
| 0 | 226 (77.9) | 74 (100.0) | |
| –1 | 64 (22.1) | 0 (0.0) | |
| Intrauterine growth restriction | .632 | ||
| 0 | 276 (95.2) | 72 (97.3) | |
| –1 | 14 (4.8) | 2 (2.7) | |
| Lymphopenia | .495 | ||
| 0 | 143 (70.1) | 52 (75.4) | |
| –1 | 61 (29.9) | 17 (24.6) |
Data are presented as number (percentage) or mean (odds ratio), and 0 and −1 denote absence and presence, respectively, of the specific feature. Cord issues include marginal or velamentous insertion, 2-vessel cord, and true or false knots. Other issues include polyhydramnios, maternal history of cholestasis, cancers, autoimmune diseases, thyroid diseases, inflammatory bowel diseases, placental increta or percreta, previa, twins, and MFI (massive perivillous fibrinoid deposit). Lymphopenia denoted the lymphocyte percentage below the reference range (15.5%) at time of admission before delivery (reference, 15.5%–47.1%).
COVID-19, coronavirus disease 2019; MFI, maternal floor infarction.
Zhang. SARS-CoV-2 in placenta and vertical transmission. AJOG MFM 2020.
P < .05 indicates statistically significant difference.
Figure 1.
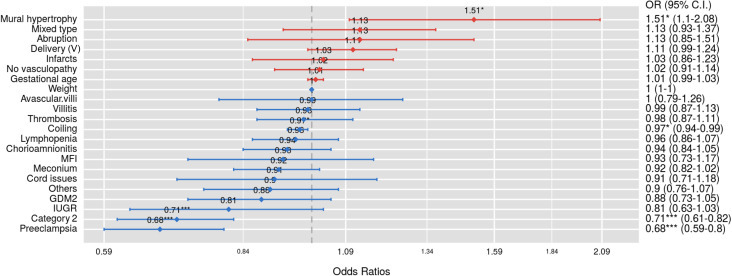
Summary of odds ratios
Clinical and pathologic findings of the 364 placentas from COVID-19–positive and COVID-19–negative mothers generated by using the generalized linear model of R package using the same data in the Table. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
CI, confidence interval; COVID-19, coronavirus disease 2019; GDM2, gestational diabetes mellitus; IUGR, intrauterine growth restriction; MFI, maternal floor infarction; OR, odds ratio; V, vaginal.
Zhang. SARS-CoV-2 in placenta and vertical transmission. AJOG MFM 2020.
Figure 2.
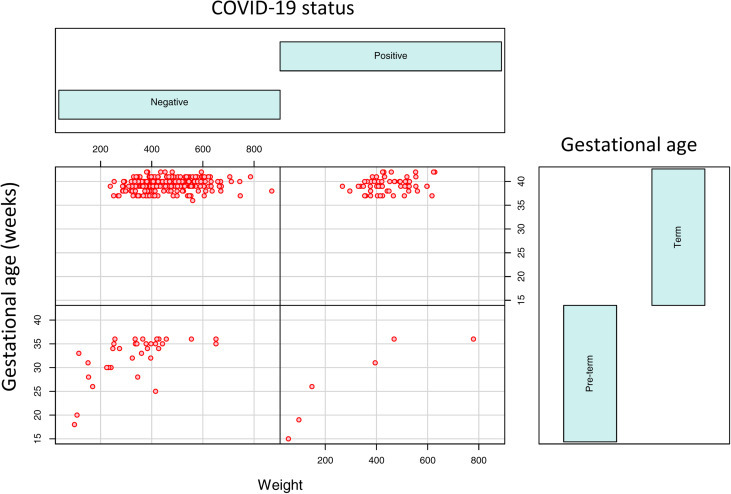
Conditioning plot of placental weight and gestational age
Gestational age and placental weight distributions from the 364 COVID-19–negative and COVID-19–positive mothers delivered preterm (before 37 weeks) and term (37 weeks or later) using the R package.
COVID-19, coronavirus disease 2019.
Zhang. SARS-CoV-2 in placenta and vertical transmission. AJOG MFM 2020.
Figure 3.
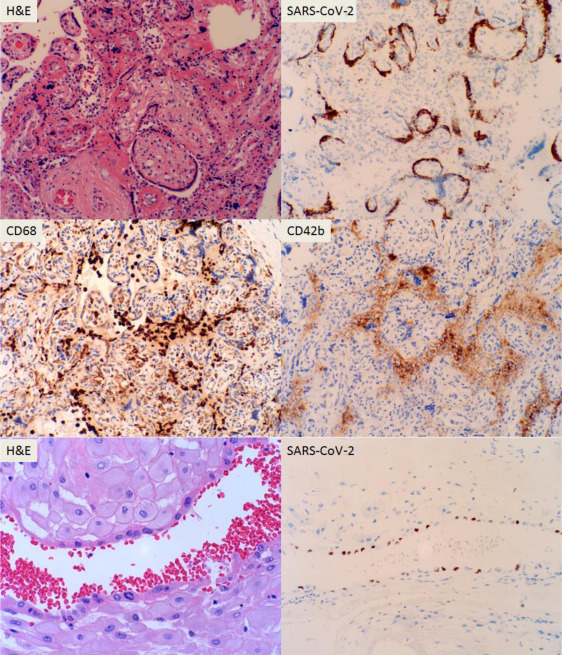
H&E stains of placental villous tissue with placental infarcts and immunostaining for CD42b and CD68 for platelet aggregates and macrophages of 36-week placenta with ISH against specific SARS-CoV-2
The bottom panel indicates endometrial glands from the second positive patient by ISH (200× magnification).
H&E, hematoxylin and eosin; ISH, in situ hybridization; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Zhang. SARS-CoV-2 in placenta and vertical transmission. AJOG MFM 2020.
Figure 4.
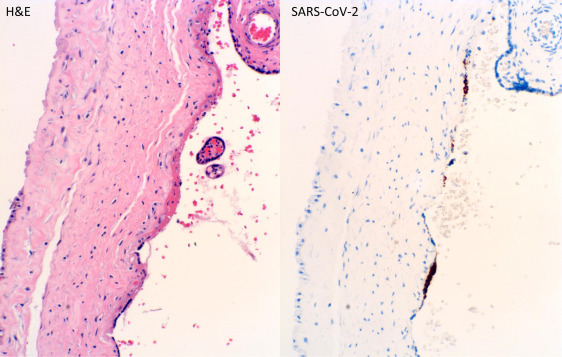
H&E stain of placental subchorionic plate (Langhan’s fibrinoid) with positive ISH signals (400× magnification)
H&E, hematoxylin and eosin; ISH, in situ hybridization; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Zhang. SARS-CoV-2 in placenta and vertical transmission. AJOG MFM 2020.
Conclusion
This study showed that SARS-CoV-2 viral particles are uncommon in placentas from PCR-positive mothers at late gestation. There appears to be largely no relationship between the maternal COVID-19 status and placental pathology. Neonatal testing for SARS-CoV-2 by swab PCR method also showed rare positive cases from COVID-19–positive mothers. The negative association between the COVID-19 status of the mothers and preeclampsia and category 2 fetal heart tracing clinically raised the possibility of disruption or decoupling of oxygen sensing within the maternal tissue by the SARS-CoV-2 viral infection, but the mechanism of viral pathogenesis remains unclear. The presence of SARS-CoV-2 in placenta suggests a high probability of vertical transmission in utero through fetoplacental circulation. Testing blood of the newborn instead of nasopharyngeal swab will likely yield more information of potential vertical transmission. Currently, no clinical testing capability is available for testing SARS-CoV-2 viral load (titer) within the blood of either the mother or the fetus, and such information will likely be important in understanding the transient maternal and fetal viremic conditions. The presence of SARS-CoV-2 viral particles within the atrophic endometrial glands raised the question of potential effects of SARS-CoV-2 infection at early gestation and requires further study. Strictly, the endometrial glands are maternal tissues, and the presence of viral particles in endometrial glands should not be counted as transmission to fetal tissue, although the endometrial tissue is attached to the delivered placenta. The viral signals at the subchorionic plate (Langhan’s stria, fibrinoid) and surrounding areas of infarcts with thrombosis raised the question of potential localization of SARS-CoV-2 in platelets, but a limited number of ISH tests showed no SARS-CoV-2 virus within the subcutaneous microthrombi and the bone marrow of patients with COVID-19 (not shown). Localization of SARS-CoV-2 within the tissue using an ISH test will likely provide critical information for better understanding of viral pathogenesis.
Footnotes
This paper is part of a supplement that represents a collection of COVID-related articles selected for publication by the editors of AJOG MFM without additional financial support.
The authors report no conflict of interest.
References
- 1.Sutton D., Fuchs K., D’Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L., Li Q., Zheng D. Clinical characteristics of pregnant women with COVID-19 in Wuhan, China. N Engl J Med. 2020;382:e100. doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baud D., Greub G., Favre G. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020;323:2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patanè L., Morotti D., Giunta M.R. Vertical transmission of COVID-19: SARS-CoV-2 RNA on the fetal side of the placenta in pregnancies with COVID-19 positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100145. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penfield C.A., Brubaker S.G., Limaye M.A. Detection of SARS-CoV-2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100133. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


