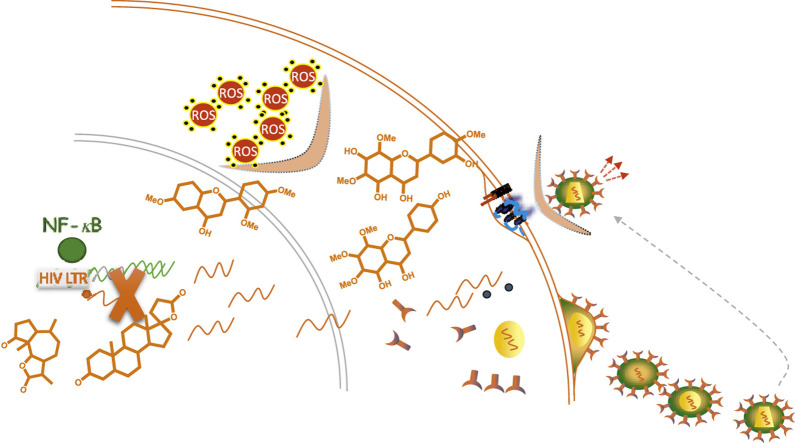
Abstract
Ethno–pharmacological relevance
The genus Artemisia spp. is well known for its anti–infectious properties and its high content in anti–infectious compounds, like the well–known sweet wormwood (Artemisia annua L.). Another Artemisia species, Artemisia campestris subsp. glutinosa (Besser) Batt., field wormwood, has been traditionally used as medicinal plant in the Mediterranean region.
Aim of the study
The aim of this study is to investigate the anti–HIV activity of field wormwood, to identify the compounds responsible for this activity and their structure and mechanism of action.
Materials and methods
Antiviral activity of isolated compounds and extracts was evaluated in HIV–1 infections of lymphoblastoid cells. We also evaluated the mechanism of action of isolated compounds. Viral entry was studied comparing the inhibitory effect of isolated compounds on wild type HIV–1 and VSV pseudotyped HIV–1. To assess the viral transcriptional effect, plasmids encoding luciferase reporter genes under the control of the whole genome of HIV–1 or NF–κB or Sp1 transcription factors were transfected in the presence of the compounds under evaluation. Finally, antioxidant activity was assessed by quantitation of reduced and total glutathione in treated cell cultures.
Results
Ethanolic and aqueous extracts of Artemisia campestris subsp. glutinosa (Besser) Batt. subsp. glutinosa displayed anti–HIV activity in vitro, although ethanolic extract was more powerful (IC50 14.62 μg/mL). Bio–guided ethanolic extract fractionation leads to the isolation and characterization of two terpenes, damsin and canrenone, and four flavonoids, 6, 2′, 4′–trimethoxyflavone, acerosin, cardamonin and xanthomicrol. All the isolated compounds inhibited HIV–1 replication in vitro with IC50 values between the middle nanomolar and the low micromolar range. Their anti–HIV mechanism of action is due to the bloking of viral entry and/or transcription inhibition, without correlation with the antioxidant activity, through interference with the cellular transcription factors NF–κB and Sp1, which are targets that are not currently reached by antiretroviral therapy.
Conclusion
We describe here the anti–HIV activity of field wormwood, Artemisia campestris subsp. glutinosa (Besser) Batt., and the isolation and study of the mechanism of action of two terpenes and four flavonoids, responsible, at least in part, for its activity, through the inhibition of two different cellular targets affecting the HIV replication cycle. The activity of these compounds in cellular targets could explain why plant extracts can be used in the treatment of different diseases. Besides, the presence of several compounds with dual and different mechanisms of action could prove useful in the treatment of HIV–1 infection, since it could aid to overcome drug resistances and simplify drug therapy. This work is a further step in understanding the anti–infectious activity of wormwood species and their use in treating infectious diseases.
Keywords: Artemisia, Field wormwood, Natural products, Antiretrovirals, HIV, NF–κB
Graphical abstract
Highlights
-
•
Artemisia campestris subsp. glutinosa (Besser) Batt. ethanolic and aqueous extracts inhibit HIV–1 infection in vitro .
-
•
Two terpenes, damsin and canrenone have been isolated from the active anti–HIV fractions.
-
•
Four flavonoids, 6,2’,4’–trimethoxyflavone, acerosin, cardamonin and xanthomicrol were isolated from the active fractions.
-
•
Acerosin and xanthomicrol inhibit viral entry.
-
•
Damsin, canrenone, 6, 2’, 4’–trimethoxyflavone and xanthomicrol, and also acerosin, show activity in HIV–1 transcription.
-
•
Flavonoids displayed also an antioxidant effect which showed no correlation with the transcriptional activity.
-
•
Isolated compounds acted through multiple targets, suggesting their potential involvement in other diseases.
Abbreviatures
- ACT
Artemisinin combination therapies
- AE
Aqueous extract
- Aqueous-EE
Aqueous fraction of EE
- ART
Antiretroviral therapy
- CC50
Cytotoxic concentration 50%
- CI95%
Confidence interval 95%
- DCM
Dichloromethane
- DCM-EE
Dichloromethane fraction of EE
- EC50ox
EC50 antioxidant
- EC50trans
EC50 transcription
- EE
Ethanol extract
- EIMS
Electron impact mass spectrometry
- Emax
Maximum effect
- Ethanol-EE.
Ethanol fraction of EE
- GSH
Reduced glutathione
- GSSG
Oxidized glutathione
- HCV
Hepatitis C virus
- HE
Hexane EE
- HIV-1
Human immunodeficiency virus 1
- HIV-VSV
Pseudotyped recombinant HIV
- HPV
Human papilloma virus
- HSV
Herpesvirus
- IC50
Inhibitory concentration 50%
- LTR
Long terminal repeats
- OPT
o– Phthalaldehyde
- RI
Redox index
- RLUs
Relative luminescence units
- ROS
Reactive oxygen species
- TCA
Trichloroacetic acid
- TLC
Thin layer chromatography
- TMS
Tetramethylsilane
- VSV
Vesicular stomatitis virus
1. Introduction
Viral infections can be treated by means of pharmacological interventions, although a cure is not always achieved. Hepatitis C virus (HCV) infections are successfully treated from a pharmacological point of view, clearing the virus from the human body. However, other viral infections as herpesvirus (HSV) or human papilloma virus (HPV) can be treated but not cured with drug therapy alone, due to the establishment of viral reservoirs (Massanella and Richman, 2016). In this context, human immunodeficiency virus type 1 (HIV–1) infection is effectively controlled by antiretroviral therapy (ART), although it is unable to eliminate the infection. The main reason of viral persistence is the presence of latent viral reservoirs, mainly in infected resting CD4+ T cells, which are not susceptible to ART. New approaches are under study to eliminate these reservoirs, such as the “shock and kill” strategy (Deeks, 2012), but none of them has shown effectiveness in clinical trials yet. Furthermore, only 24.5 million of the 37.9 million people infected with HIV were on ART in 2019 (UNAIDS, AIDS info accessed April 2020), and it seems unlikely that an effective vaccine will be obtained in coming years.
Artemisia genus comprises several species and some are among the most used plants in traditional Chinese medicine for the treatment of infectious diseases produced by fungi, bacteria and viruses (Abad et al., 2012; Abad. et al., 2012; Bora and Sharma, 2011; Pandey and Singh, 2017; Taleghani et al., 2020; Tan et al., 1998). The best–known compound isolated from an Artemisia species (A. annua) is an endoperoxide sesquiterpene lactone sesquiterpene, artemisinin, an antimalarial drug used in artemisinin combination therapies (ACT) which is active against the intraerythrocytic forms of Plasmodium falciparum by preventing the detoxification of hemodigestion products (Talman et al., 2019). Novel derivatives have been tested for their activity against malaria or other infectious diseases (for a review see Efferth, 2018; Liu et al., 2019; Taleghani et al., 2020).
Other species of Artemisia genus, known as wormwoods, have been used traditionally as medicinal plants (Guibourt, 1862). The pharmacological importance of Artemisia campestris, known as field wormwood, was revised by Al–Sanafi in 2015 (Al–Sanafi, 2015). Traditionally, A. campestris was used as antiseptic (Redwood, 1857), and for the treatment of pleurisy, a pathological condition caused by viral or bacterial infections (Breverton, 2011). Moreover, North American indigenous people use A. campestris extracts to treat the common cold, caused by rhinovirus or coronavirus (Palmer, 1975). However, neither the mechanism of action nor the compounds responsible for this activity were described. In the Mediterranean region, this species is commonly used as an anthelmintic drug, as well as for the treatment of cutaneous, respiratory and digestive disorders. Several reviews on taxonomical aspects, cytogeography, biological activities and bioactive compounds of A. campestris have been published (Dib et al., 2017a; Dib & El Alaoui–Faris, 2019; Dib et al., 2017b; Dib et al., 2017c; Aniya et al., 2000; Jabri et al., 2018; Kadi et al., 2019; Memmi et al., 2007) which describe how the aqueous extracts and essential oils, rich in flavonoids and terpenes, are responsible for the biological activity. Antioxidant and anti-inflammatory activities have been evaluated through several in vitro and in vivo methods by measuring glutathione levels, superoxide dismutase activity, protein oxidation and nitric oxide products and in carrageenan-induced rat paw edema (Ghlissi et al., 2016; Sefi et al., 2010, 2012), identifying some of the targets of this species in human pathologies.
In this paper we have studied the potential activity of A. campestris in HIV–1 replication in vitro. Hence, the antiviral activity against HIV–1 of A. campestris extracts, the isolation of the active compounds, two terpenes and four flavonoids, and the identification of their targets in the HIV replication cycle are reported in this study.
2. Material and methods
2.1. Plant material
The aerial parts of Artemisia campestris subsp. glutinosa (Besser) Batt. were collected by Dr. José Antonio Molina in October 1998 in Castejón del Puente, Huesca, Spain (N 40° 57′ 0″, E 0° 10′ 12″) and identified in the Herbarium of the Department of Pharmacology, Pharmacognosy and Botany, Faculty of Pharmacy, Complutense University of Madrid (MAF 153.628). The plant name has been checked on http://www.theplantlist.org (data accessed April 22nd, 2020).
2.2. General experimental procedures
UV spectra were recorded on a Shimadzu 160A spectrophotometer. IR spectra were acquired with a Perkin–Elmer FT–1725X spectrophotometer. 1H NMR and 13C NMR spectra were measured on a BRUCKER Advance DRX–700 and Advance DRX–500 spectrometer, using TMS as internal standard. EIMS were obtained on a Hewlett Packard 5930 spectrometer.
The following adsorbents were used for purification: gel filtration chromatography, Carlo Erba (SDS ®) (40/60 μm; 20/45 μm); analytical thin layer chromatography (TLC) Al, Merck Si gel 60 F254. TLC chromatograms were visualized under UV light at 254 and 366 nm and/or sprayed with Naturstoffreagents–PEG. Known compounds were isolated and identified by comparing their spectral data with those in the literature.
2.3. Extraction and isolation
The air–dried aerial parts of Artemisia campestris subsp. glutinosa (Besser) Batt. (1.0 kg) were repeatedly extracted at room temperature with ethanol (126 g) and water (146 g). Both extracts were subjected to antiviral evaluation and ethanol extract (EE) was selected as the most active one. EE was further extracted with hexane, dichloromethane, ethanol and water. Dichloromethane fraction of EE (DCM–EE) was selected as the most active one and subjected to column (3 x 25 cm) chromatography in silica gel (40–60 μm) medium pressure in n–hexane (12.47 L), toluene and ethyl acetate (24.93 L) in an increasing polarity gradient. Fractions obtained (I–XVIII) were pooled according to the results of the chromatographic analysis by TLC with mobile phase n–hexane, ethyl acetate (8:2 and 4:6) and the results of antiviral activity. Further purification of the active fractions on silica gel columns yielded two terpenes and four flavonoids.
2.4. Cell culture reagents and drugs
MT–2 cells were cultured in RPMI–1640 medium and 293T cells in DMEM medium, both containing 10% (v/v) fetal bovine serum, 2 mM L–glutamine, penicillin (50 IU/mL) and streptomycin (50 μg/mL) (all Whittaker M.A. Bio–Products). Cells were maintained at 37 °C in a 5% CO2 humidified atmosphere and split twice a week. The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: T–20 (Enfuvirtide). PMA was obtained from Merck.
2.5. Plasmids and viral supernatants
The plasmid pNL4.3–luc was generated by cloning the luciferase gene in the HIV–1 proviral clone pNL4.3, and the plasmid pNL4.3–Ren was generated cloning the renilla gene in the nef site of pNL4.3 (Garcia–Perez et al., 2007). pNL4–3.Luc.R–.E– was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: pNL4–3.Luc.R–.E– from Dr. Nathaniel Landau, and pcDNA–VSV, encoding the G protein of vesicular stomatitis virus (VSV), from Dr. Arenzana–Seisdedos from Pasteur Institute (Oberlin et al., 1996). The 3–enh–κB–ConA–luc plasmid carries a luciferase gene under the control of three synthetic copies of the κB consensus of the immunoglobulin k–chain promoter cloned into the BamHI site located upstream from the conalbumin transcription start site (Arenzana–Seisdedos et al., 1993). The Sp1–luc plasmid (kindly donated by Dr. Solís–Herruzo, Hospital 12 de Octubre, Madrid, Spain) contains two consensus sequences for Sp1 cloned into the p19LUC vector.
Infectious supernatants were obtained by calcium phosphate transfection of 293T of plasmids pNL4.3 Ren (wild type HIV) or by cotransfection of pNL4–3.Luc.R–E–, a full–length HIV DNA that does not express the HIV envelope, and pcDNA–VSV, DNA for VSV G glycoprotein cloned in the pcDNA3.1 plasmid (HIV–VSV). All the infectious supernatants obtained were titrated in target cells by two different methods, p24–gag protein quantification by ELISA (ELECSYS system, Roche) and Renilla–luciferase activity determination following manufacturer instructions (Promega). Briefly, cell cultures were lysed with 100 μl of the lysis buffer, and relative luminescence units (RLUs) were obtained in a luminometer (Berthold Detection Systems) after adding substrate to cell extracts. All the supernatants were stored at −80 °C until use.
2.6. Evaluation of anti–HIV activity
Extracts, fractions and isolated compounds were dissolved in DMSO (Merck), at 10 mg/mL or 10 mM, aliquoted and stored at −20 °C.
NL4.3–Ren infectious supernatants (100.000 RLUs per well or 20 ng gag–p24 per well) were used to infect MT–2 cells in the presence or absence of different concentrations of compounds. Infected cultures were maintained in a 5% CO2 humidified atmosphere for 48 h. Afterwards, anti–HIV activity was quantified by measuring Renilla–luciferase activity following the manufacturer instructions (Promega). Briefly, cell cultures were lysed with 100 μl of the lysis buffer, and RLUs were obtained in a luminometer (Berthold Detection Systems) after adding substrate to cell extracts. All the experiments were controlled with cells treated with the same DMSO concentration of extracts or compounds. HIV–1 replication inhibition was evaluated by measuring the luminescence activity or RLUs, with 100% being the infection of non–treated cells. IC50 was calculated with a non–linear regression formula using Prism v8.0 (GraphPad Software).
Cell viability was measured in treated mock–infected cells in parallel under the same conditions as in the antiviral assay by the CellTiter Glo (Promega) assay system. Cell viability is expressed as percentage of viable cells compared to a non–treated (DMSO same concentration as compound) control (100%). CC50 was calculated with a non–linear regression formula using Prism v8.0 (GraphPad Software).
2.7. Viral entry inhibition
To evaluate viral entry we have compared MT–2 infections performed with recombinant wild type HIV (NL4.3–Ren) (100.000 RLUs per well or 20 ng p24 per well) and a VSV–pseudotyped recombinant HIV (HIV–VSV) (100.000 RLUs per well or 20 ng p24 per well), which enter the target cells through a receptor independent mechanism. The methodology is similar to the antiviral activity evaluation. Infections were performed with both viruses (HIV and VSV–HIV) in parallel and, after 48 h, RLUs obtained on a luminometer. Enfuvirtide was used as a reference control of viral entry inhibition. IC50 was calculated with a non–linear regression, dose response formula, and ANOVA analysis was performed to calculate the significant differences (p value) between HIV IC50 and VSV–HIV IC50, both using Prism v8.0 (GraphPad Software).
2.8. Transcriptional activity
MT–2 cells were maintained in culture without stimuli and collected prior to the assay in RPMI without serum and antibiotics. MT–2 cells were then suspended in 350 μL of RPMI without supplements and electroporated using an Easyject plus Electroporator (Equibio) at 260V, 1500 mF and maximum resistance with 1 μg/106 cells of a luciferase plasmid under the control of the whole genome of HIV–1 (pNL4.3–Luc), NF–κB (3–enh–κB–ConA–luc) or Sp1 (pSp1–Luc). Afterwards, MT–2 cells were seeded in 24 well microplates and treated with different concentrations of compounds, using PMA as a reference control of HIV–1 transactivation, and left in culture in complete RPMI at 37 °C. 48 h later, cultures were lysed with luciferase buffer (luciferase assay system buffer, Promega) and luciferase activity (RLUs) measured in a luminometer (Berthold Detection Systems).
2.9. Glutathione determination
Total glutathione, reduced glutathione (GSH) and oxidized glutathione (GSSG) levels were determined in THP–1 cells extracts following the Hissin and Hill method (Hissin and Hilf, 1976). Briefly, cell culture was lysed with phosphate–EDTA (0.1 M sodium phosphate and 0.005 M EDTA) buffer (pH = 8) at 100 mg/mL concentration, adding 10 μL/mL of HClO4 (60%). Then, cell homogenates were spun at 10.000 rpm (6000g) for 10 min at 4 °C and supernatants were maintained at 4 °C until reduced glutathione (GSH) and oxidized glutathione (GSSG) were determined. Fluorometric measurement of the interaction of thiols with o–Phtaldehyde (OPT) in the presence of compounds was compared to a standard curve produced by measuring increasing concentrations of GSH. GSH standards (0, 10, 50, 75 and 100 mM) were prepared from a 1 mM stock solution in 10% (w/v) trichloroacetic acid (TCA), and measured using OPT. Fluorescence was measured in a FLX 800 fluorimeter (Bio–Tek Instruments, Winooski, Vermont, USA) at λexc = 350 nm and λem = 420 nm. The redox index (RI), a parameter that indicates the antioxidant status of the tissue, was expressed as follows: RI = GSH/(GSH + GSSG). Results are expressed as EC50 or concentration that produces 50% of the maximum antioxidant effect, being the maximum 1 (100% of glutathione is in its reduced form). Furthermore, we have also calculated the Emax reached by each compound to evaluate the antioxidant efficacy. Both parameters were calculated using Prism v8.0 (GraphPad Software) using non–linear regression, dose–response curves.
2.10. Statistical analysis
Non-linear regression was used to calculate IC50, EC50 and CC50. One–way ANOVA statistical analysis (post–test Bonferroni multiple comparison test, *p < 0.05; **p < 0.01) was performed to evaluate the significant differences among values. Antioxidant and transcriptional activity correlation analysis was performed using the non–parametric Spearman analysis and the r coefficient was calculated. All the analyses were performed using Prism v8.0 (GraphPad Software).
3. Results and discussion
3.1. Extraction, isolation and characterization of compounds
Artemisia campestris subsp. glutinosa (Besser) Batt. was extensively extracted with ethanol and water. The obtained extracts were evaporated and submitted to chemical complexity (Figs. S1, S2 and S3) and anti–HIV and cytotoxic activity evaluation (Table 1 ). IC50s and CC50s values obtained from infection and cytotoxicity curves of the extracts were calculated and ethanolic extract (EE) showed the lowest IC50 (28.57 μg/mL). Further extraction of EE with hexane, dichloromethane, ethanol and water yielded four extracts with different composition and antiviral activity. Dichloromethane extract of EE (DCM–EE) was selected as the most powerful fraction (IC50 23.06 μg/mL). However, the hexane EE, ethanol EE and the water EE also displayed anti–HIV activity with IC50 values of 63.17, 42.68 and 98.96 μg/mL, respectively (Table 1), suggesting the presence of more than one type of active compounds in the EE.
Table 1.
Anti–HIV evaluation of the extracts obtained from A. campestris. MT–2 cells were infected with a recombinant HIV–1 (NL4.3–Ren. 100.000 RLUs or 20 ng p24/well) in the presence of different concentrations of the extracts. Results were obtained 48 h later in a luminometer using the Renilla assay system. IC50 and CC50 were calculated using Prism v8.0 (GraphPad Software). Hexane EE: hexane extract from ethanolic extract. DCM EE: Dichloromethane extract from ethanolic extract. Ethanol EE: Ethanol extract from ethanolic extract. Aqueous EE: Aqueous extract from ethanolic extract. IC50: Inhibitory concentration 50%; CI95%: Confidence interval 95%.
| IC50 μg/mL HIV (CI95%; R2) | |
|---|---|
| Ethanol extract (EE) | 28.57 (5.19–790.8; 0.8021) |
| Aqueous extract (AE) | 88.17 (30.17–713.3; 0.8353) |
| Hexane EE | 63.17 (19.22–649.2; 0.8261) |
| DCM EE | 23.06 (11.38–54.68; 0.9863) |
| Ethanol EE | 42.68 (13.37–84.58; 0.7337) |
| Aqueous EE | 98.86 (48.90–275.4; 0.9201) |
DCM–EE, selected as the most active fraction, was then subjected to repeated column chromatography obtaining 18 fractions (I–XVIII) which were submitted again to antiviral activity evaluation (Table 2 ).
Table 2.
Anti–HIV evaluation of the fractions obtained from A. campestris. MT–2 cells were infected with a recombinant HIV–1 (NL4.3–Ren. 100.000 RLUs or 20 ng p24/well) in the presence of different concentrations of the 18 fractions. Results were obtained 48 h later in a luminometer using the Renilla assay system. IC50 were calculated using Prism v8.0 (GraphPad Software). IC50: Inhibitory concentration 50%; CI95%: Confidence interval 95%.
| Fraction | IC50 μg/mL |
|---|---|
| 1 | >50 |
| 2 | >50 |
| 3 | 29.3 (21.65–39.52; 0.7849 |
| 4 | 23.5 (16.50–33.36; 0.8272) |
| 5 | 7.6 (5.397–10.80; 0.6226) |
| 6 | 12.9 (8.40–9.70; 0.6927) |
| 7 | 24.7 (14.51–42.20; 0.6444) |
| 8 | >25<50 |
| 9 | >50 |
| 10 | 8.0 (4.58–14.10; 0.7053) |
| 11 | 4.9 (3.09–7.8; 0.7296) |
| 12 | 7.7 (4.24–14.15; 0.6695) |
| 13 | 8.2 (5.01–13.56; 0.7467) |
| 14 | 20.5 (6.60–63.64; 0.5242) |
| 15 | 1.5 (1.03–2.17; 0.7382) |
| 16 | 7.9 (4.89–12.73; 0.7495) |
| 17 | 12.0 (7.88–18.41; 0.7999) |
| 18 | 20.1 (14.75–27.24; 0.8385) |
Again, we found that several fractions showed anti–HIV activity, but some of them displayed lower IC50 values. Further purification of the most active fractions, 10–12 and 15–16, led to the identification of 6 compounds: two terpenes, damsin and canrenone, and 4 flavonoids, cardamonin, 6,2′,4′–trimethoxyflavone, acerosin and xanthomicrol. Structural data are reported in supplementary material.
Although terpenes and sterols are well known in natural chemistry, this is the first time damsin and canrenone have been identified in the Artemisia genus and, more specifically, in the A. campestris species. Damsin is a pseudoguaianolide sesquiterpene lactone, which was identified in different plant species of the genus Ambrosia, Partheniumm, Chyrsanthemum, Plagiochila and Hymenoclea (Abu–Shady and Soine, 1954; Aponte et al., 2010; Goldsby and Burke, 1987; Herz et al., 1981; Saeed et al., 2015; Villagomez et al., 2013). Canrenone is a steroidal triterpene lactone never isolated before from any plant species although its structure is described elsewhere (Cashman and Peña, 1989).
On the other hand, flavonoids are almost ubiquitous compounds, also found in Artemisia spp. In fact, cardamonin was first identified in A. absinthium L. (Hatziieremia et al., 2006) and acerosin was isolated from Artemisia afra Jacq. ex Willd (Wollenweber and Mann, 1989). However, 6,2′,4′–trimethoxyflavone and xanthimicrol were never isolated from Artemisia spp.
The chemical structure of artemisinin, a sesquiterpene lactone, and other related antimalarial compounds isolated from A. annua, is related to damsin, although the former is a cyclohexane lactone rather than a cyclopentane lactone (Abad et al., 2014). Moreover, the α-methylene-γ-lactone fragment characteristic of sesquiterpene lactones has been previously identified as responsible for the anti-inflammatory and anti-tumor activities (Chaturvedi, 2011) due to its interaction with specific biological nucleophiles, such as the cysteine sulfhydryl groups of target proteins (Hwang et al., 2006). Furthermore, there are references in the literature indicating that sesquiterpene lactones with a α-methylene-γ-lactone fragment inhibits influenza A virus replication (Zhang et al., 2018).
On the other side, canrenone only presents the γ-lactone fragment. If we compare it to damsin, the main differences are the absence of the methylidene group in the lactonic ring and the presence of a steroidal fragment (cyclopentane perhydro phenanthrene). This has two main implications. First, the absence of the methylidene group (exocyclic bond) prevents the conjugation with the carbonyl function of the lactonic ring, reducing its binding capacity and causing a reduction in its biological activity (Gach et al., 2015). Second, the steric effect caused by the steroidal ring of canrenone can cause a decrease in its activity by preventing the compound binding (Cheng and Yuan, 2006). Interestingly, canrenone is thought to be the main metabolite of the aldosterone antagonist spironolactone, and both structures are terpene steroids. In fact, canrenone is widely known as a diuretic due to its antagonism of mineralocorticoids receptors in the kidney (potassium canreronate) (Armanini et al., 2014).
Regarding the four flavonoids isolated, they are widely described in the literature but all of them share different rates of hydroxyl groups methylation as a structural characteristic, with cardamonin as the less methoxylated and trymethoxyflavone, with no free hydroxyl groups, as the most methoxylated flavonoid. As a matter of fact, their antiviral activity could be related to the hydroxylation rate of the rings (Ahmad et al., 2015). For example, flavonols were reported to be more active in comparison to flavones against the herpes simplex type I virus (Dillard and German, 2000). Moreover, the mild selective anti-HIV activity of myricetin relative to quercetin as a result of the presence of one additional hydroxyl group at the 5′ position (Mahmood et al., 1993). Although 6,2′,4′-trimethoxyflavone, acerosin and xanthomicrol have the same number of methoxy groups they have a different number of hydroxyl groups: acerosin 3, xanthomicrol 2 and trimethoxyflavone 0. These observations suggest potential differences in antiviral and antioxidant activities. Regarding cardamonin, previous literature reported the anti-HIV activity of a related chalcone, 2-methoxy-3-methyl-4,6-dihydroxy-5-(3′-hydroxy) cinnamoylbenzaldehyde (Wu et al., 2003). However, the structural differences between both chalcones could lead to a diverse biological activity, since cardamonin lacks the hydroxyl group in the C position.
3.2. Cytotoxic activity and antiviral activity
As the previous screening of extracts and fractions showed, the presence of structurally different compounds with anti–HIV–1 activity in A. campestris EE would yield puzzling results in the EE target identification, due to the concurrent interference with several different targets. Therefore, we have tried to study the mechanism of action of the isolated compounds (See Fig. 1 ).
Fig. 1.
Chemical structure of the compounds isolated from A. campestris: damsin, canrenone, cardamonin, acerosin, trimethoxyflavone and xanthomicrol.
We have first evaluated the inhibitory activity of isolated compounds in HIV–1 infection of MT–2 cells, a lymphoblastoid cell line (Fig. 2 and Table 3 ). We found that terpenes damsin and canrenone showed IC50 of 0.84 μM and 18.87 μM, respectively. Damsin was toxic to MT–2 cells, but only at concentrations higher than 20 μM. On the other hand, canrenone showed only a slight toxicity at 100 μM. The diverse antiviral activity between the two terpenes is not surprising since, as stated above, canrenone could be less active due to the lack of the reactive methylidene group in the lactonic ring of damsin and, also, to the steric effect produced by the presence of the steroidal ring.
Fig. 2.
In vitro evaluation of the anti–HIV activity and cytotoxicity of isolated compounds from A. campestris. MT–2 cells were infected with an X4 recombinant HIV (NL4.3–Ren. 100.000 RLUs or 20 ng p24/well) or with a VSV pseudotyped HIV (VSV–HIV. 100.000 RLUs or 20 ng p24/well) in the presence of different concentrations of compounds or fusion inhibitor enfuvirtide for 48 h. The same concentration of vehicle (DMSO or water) was used as a non–treated control (100%). The cell culture was then lysed and relative luminescence units (RLUs) were measured in a luminometer. Cell viability was evaluated in mock infected cells in parallel using the CellTiter Glo reagent (Promega). Results were analyzed using Prism v8.0 (GraphPad Software), non–linear regression and dose response curves. IC50: inhibitory concentration 50%. CC50: cytotoxic concentration 50%. CI95%: confidence interval 95%.
Table 3.
IC50s and CC50s calculated for the isolated compounds from Artemisia campestris in the HIV infection assay. MT–2 cells were infected with either recombinant wild type HIV (NL4.3–Ren. 100.000 RLUs or 20 ng p24/well) or VSV pseudotyped recombinant wild type HIV (VSV–HIV. 100.000 RLUs or 20 ng p24/well)) and treated with serial dilutions of damsin, canrenone, cardamonin, acerosin, trimethoxyflavone or xanthomicrol. The HIV–1 fusion inhibitor enfuvirtide was used as control. Results were obtained using the renilla–luciferase assay system (Promega) following the manufacturer instructions after 48 h in culture, measuring the RLUs in a luminometer. 100% was the RLUs obtained by untreated controls. Cell viability was determined in parallel in mock infected cells using CellTiter Glo reagent following the manufacturer instructions (Promega). IC50, CC50 and p values were calculated using Prism v8.0 (GraphPad Software) using non–linear regression, dose–response curves, and one–way Anova analysis. IC50: Inhibitory Concentration 50%. CC50: Cytotoxic Concentration 50%. CI95%: Confidence interval 95%.
| IC50 μM HIV (CI95%; R2) | IC50 μM VSV–HIV (CI95%; R2) | CC50 μM (CI95%, R2) | p = | |
|---|---|---|---|---|
| Enfuvirtide | 0.015 (0.009–0.022; 0.9798) | > 1 | – | 0.0021*** |
| Damsin | 0.84 (0.55–1.28; 0.9806) | 0.83 (0.56–1.22; 0.9829) | >20<100 | 0.9434 |
| Canrenone | 18.87 (10.73–33.17) | 14.22 (10.54–19.19) | ≈100 | 0.3115 |
| Cardamonin | 70.58 (43.68–122.4;0.6144) | 71.50 (36.35–167.9; 0.6270) | >100 | 0.9741 |
| Acerosin | 2.70 (1.69–4.22; 0.5819) | 10.57 (7.13–15.65; 0.8728) | ≈50 | <0.0001**** |
| Trimethoxyflavone | 28.41 (16.58–49.18; 0.7568) | 35.58 (19.42–67.80; 0.7200) | >100 | 0.5632 |
| Xanthomicrol | 17.43 (12.07–25.14; 0.6674) | 34.36 (22.85–52.37; 0.8372) | >100 | 0.0151* |
It was also tested the anti–HIV–1 activity of the four flavonoids isolated and it was found that all of them showed significant anti–HIV–1 inhibitory activity between the low and high micromolar range. The flavone acerosin was the most potent flavonoid, with an IC50 of 2.7 μM while the less potent HIV–1 inhibitor was the chalcone cardamonin with an IC50 of 70.6 μM. Xanthomicrol and trimethoxiflavone were both middle range HIV–1 inhibitors (17.4 and 28.4 μM, respectively). None of the flavonoids showed cytotoxicity at the concentrations tested, excepting acerosin, with a CC50 around 50 μM (Fig. 2 and Table 3).
Sesquiterpene lactones have previously shown anti–HIV activity (Mohammed et al., 2014; Zhang et al., 2005), although their mechanism of action remains elusive, while isolated flavonoids have shown previously mixed results. Regarding flavonoids, we did not find literature about the antiviral activity of acerosin, xanthomicrol and 6,2′,4′–trimethoxyflavone, although other trimethoxyflavones, as 5,6,7– trimethoxyflavone, showed activity against poliovirus and herpesvirus (Hayashi et al., 1997) or 4′,5–dihydroxy–3,3′,7–trimethoxyflavone, with selective activity against picornavirus (Ishitsuka et al., 1982). Moreover, four different hydroxymethoxyflavones were found to inhibit HIV–1 replication, although their mechanism of inhibition was not determined (Kongkum et al., 2012). Cardamonin, a chalcone commonly isolated from different plants, has proven to have antifungal (Lopez et al., 2011), antibacterial (Aderogba et al., 2012) and anti–HIV protease (Tewtrakul et al., 2003) activities. Additionally, cardamonin inhibited dengue–2 virus NS4 protease (Kiat et al., 2006) and was also found to inhibit NF–κB activation in HUVEC cells, although it did not alter the grow of Hantaan virus (HTNV) infection (Yu et al., 2014). Taking into consideration the diverse hydroxylation rate among flavonoids, differences were also found in their biological activity. Therefore, as it was hypothesized before, a higher hydroxylation rate would result in a higher activity. This hypothesis could not be confirmed with the chalcone. However, cardamonin lacks the hydroxyl group in the C position present in other bioactive chalcones (Wu et al., 2003), which could be the reason for its low anti-HIV activity.
The multi–infectious activity of these compounds and the uses traditionally reported by plant extracts in several diseases suggest a common target in the inhibition of microorganisms. Since every microorganism encompasses its own and different proteins, we hypothesized here that this target, or targets, could be cellular processes indispensable for the microorganism replication, especially when dealing with viruses, which are obligate intracellular parasites. In fact, some antiretroviral drugs act on cellular targets essential for viral replication, such maraviroc, an inhibitor of CCR5 cellular receptor that inhibits HIV–1 entry. CCR5 antagonism is now under study for other viral infections, such as HCV infection, and also for other diseases, as allogenic stem cell transplant and some cancers (Blackard et al., 2019; Huang et al., 2020; Khandelwal et al., 2020). Therefore, drugs targeting cellular proteins could show activity against several viral infections, since cellular targets can be involved in the defence of the host against several viruses. Among the cellular targets of pharmacological interest in the HIV replication cycle, cellular receptors, as CCR5, CXCR4 or CD4 receptors, or transcription factors involved in HIV transcription, as NF–κB or Sp1, are especially important for the completion of the viral cycle. Therefore, we have studied the effect of isolated compounds in these targets.
CD4 or CXCR4/CCR5 are the main receptors HIV uses to enter the cell. A direct antagonistic effect or their decreased expression could inhibit HIV–1 infection. To study the effect of isolated compounds in viral entry, a VSV pseudotyped recombinant HIV (HIV–VSV) was used to infect MT–2 cells in the presence of different concentrations of isolated compounds and compared to wild type HIV–1 infections. VSV pseudotyped viruses are recombinant HIV expressing the VSV G glycoprotein that enters the target cells independently of CD4, CXCR4 and/or CCR5 receptors. As shown in Fig. 2 and Table 3, damsin and canrenone inhibited HIV and VSV pseudotyped HIV with similar IC50 values (0.84/0.83 μM, and 18.87/14.22 μM for damsin and canrenone, respectively) suggesting that viral entry is not their target in the HIV replication cycle. On the other hand, mixed results were obtained with flavonoids. While the chalcone cardamonin showed also similar IC50 values in both, VSV–HIV and HIV infections, trimethoxyflavone displayed a VSV–HIV IC50 slightly higher than its HIV IC50. However, we found no statistical differences between the HIV IC50 and the VSV–HIV IC50 (p = 0.5632. Table 3), ruling out an effect on viral entry. Regarding xanthomicrol, and especially, acerosin, we found statistical differences with p values of 0.0151 and <0.0001, respectively, displaying IC50s higher in VSV–HIV infections, suggesting that HIV–1 entry is impaired by the treatment with both flavonoids.
The second cellular target involved in HIV replication is viral transcription. HIV–1 is a retrovirus that integrates its genetic material into the host cell genome. Cellular transcriptional activity is then responsible for the viral RNA expression as well as for the completion of a successful viral replication cycle. Viral transcription is controlled by long terminal repeat (LTR) sequences, viral promoter regions present in the proviral form of the integrated provirus, which show binding sites in response to several stimuli. One of the most important viral transcription activators is the transcription factor NF–κB, although others are also involved, such as Sp1 or NFAT.
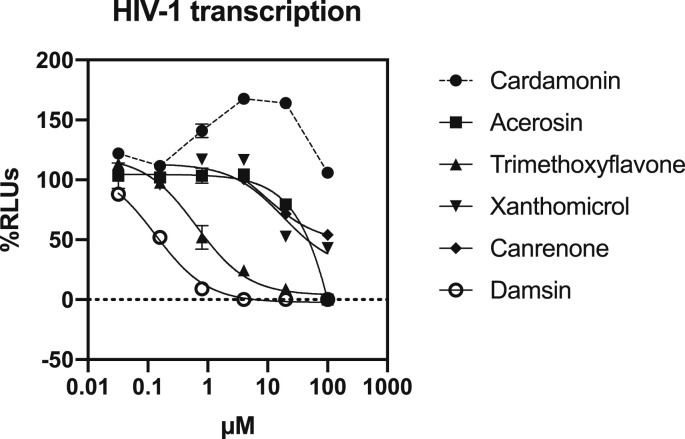
To evaluate the effect on viral transcription, we first used a plasmid encoding a luciferase reporter gene whose expression depends on the whole HIV genome (NL4.3–Luc). This plasmid was transfected in MT–2 cells treated or not with serial concentrations of isolated compounds. This allowed us to easily measure the effect of compounds specifically on HIV transcription, without any potential pre–transcription target interference (Fig. 3 ).
Fig. 3.
Effect of isolated compounds on HIV transcription. MT–2 cells were transfected with a pNL4.3–Luc plasmid (1 ng/106 cells) and treated or not with serial dilutions of compounds for 48 h. The same concentration of vehicle (DMSO) was used as a non–treated control (100%). The cell culture was then lysed and relative luminescence units (RLUs) were measured in a luminometer. Results were analyzed using Prism v8.0 (GraphPad Software), non–linear regression and dose response curves. IC50: inhibitory concentration 50%. CI95%: confidence interval 95%.
As shown in Fig. 3 and Table 4 , damsin inhibited HIV transcription with an IC50 value slightly inferior to the one shown in the antiviral experiments (IC50 transcription of 0.13 vs IC50 infection of 0.84 μM). In the same way, canrenone inhibited HIV transcription with an IC50 of 9.2 μM, which is half of the IC50 in HIV infection IC50 (18.9 μM). These results suggest that HIV transcription is the main target of isolated terpenes in the HIV replication cycle. Again, with flavonoids, we obtained mixed results. Cardamonin was not active at all in viral transcription, showing no activity even at the highest concentration tested (100 μM). Although its activity in viral infection was only achieved at high concentrations (IC50 infection of 70.6 μM), there must be another pretranscriptional target for this compound not yet identified, since viral entry was not affected by cardamonin treatment either. The other flavonoids, trimethoxyflavone, acerosin and xanthomicrol were all active as HIV transcriptional inhibitors. However, the activity is quite diverse among them. Acerosin showed an IC50 at least 20 times higher in transcription than in viral infection (IC50 transcription > 20 μM vs IC50 infection of 2.7 μM, respectively), suggesting an additional mechanism of action, which makes sense regarding the inhibition of viral entry exerted by this compound. Xanthomicrol displayed very similar IC50s in both assays (IC50 transcription of 18 μM vs IC50 infection of 17.4 μM). Thus, its activity in viral transcription could be its main target, although a slight inhibition of viral entry was also detected, while trimethoxyflavone viral transcription IC50 was almost 45–fold lower than the viral infection IC50. This effect was also displayed by terpenes, although to a lesser extent, and highlights their potent transcriptional activity, suggesting that transcription is their main target in HIV replication cycle.
Table 4.
IC50s calculated for the isolated compounds from Artemisia campestris. in the viral transcription assay. MT–2 cells were transfected with a luciferase plasmid (pNL.43–Luc.1 ng/106 cells) under the control of the whole genome of the wild type HIV and treated with serial dilutions of damsin, canrenone, cardamonin, acerosin, trimethoxyflavone and xanthomicrol. Results were obtained using the luciferase assay system following the manufacturer instructions after 48 h in culture measuring the RLUs in a luminometer. 100% was the RLUs obtained by untreated controls. IC50 was calculated using Prism v8.0 (GraphPad Software) using non–linear regression, dose–response curves. CI95%: Confidence interval 95%.
| IC50 μM HIV (CI95%; R2) | |
|---|---|
| Damsin | 0.13 (0.097–0.18; 0.9926) |
| Canrenone | 9.18 (6.74–12.49; 0.9932) |
| Cardamonin | >100 |
| Acerosin | >20 <100 |
| Trimethoxyflavone | 0.64 (0.4451–0.9336; 0.9897) |
| Xanthomicrol | 18.06 (4.101–216.5; 0.8110) |
HIV–integrated provirus depends on several cellular and viral factors to initiate transcription, being the viral protein Tat and the cellular transcription factor NF–kB by far the most significant ones. Sp1 is another important transcriptional factor with consensus sequences in the long terminal repeats (LTR) of HIV. We, therefore, evaluated the effect of isolated compounds on NF–kβ and Sp1 activity through transfection of luciferase plasmids in MT–2 cells under the control of both transcription factors (Fig. 4 ).
Fig. 4.
Effect of isolated compounds on NF–κB and Sp1 activity. MT–2 cells were transfected with a pNL4.3–Luc, NF–κB–Luc or Sp1–Luc plasmid (1 ng/106 cells) and treated or not with compounds for 48 h. PMA 100 nM was used as control. The same concentration of vehicle (DMSO) was used as a non–treated control (100%). The cell culture was then lysed and relative luminescence units (RLUs) were measured in a luminometer. A one–way ANOVA statistical analysis (post–test Bonferroni multiple comparison test) was performed (*p < 0.05; **p < 0.01).
To this purpose, we selected one active concentration of each compound and compared the effect on transcription factors with the effect on viral transcription to assure that selected concentrations were also active in HIV transcription. As shown in Fig. 4, damsin (4 μM) completely inhibits HIV transcription and NF–κB and Sp1 activity (p values < 0.0001, 0.0036 and <0.0001, respectively) when compared to the untreated control. Canrenone was slightly less active, although it showed a more powerful activity as a NF–κB inhibitor than as a Sp1 inhibitor (p values < 0.0001, 0.0066 and 0.0002, respectively). Thus, the main target of damsin, and to a lesser extend canrenone, is NF–κB, and to a lesser extent Sp1. Regarding flavonoids, cardamonin was not tested since it was not active in viral transcription (Fig. 3). When fixed concentrations of 20 μM were used, we found that the weak effect of acerosin in viral transcription was confirmed with the lack of activity in the inhibition of transcription factors. Moreover, acerosin was able to induce the activation of Sp1 and for that reason, theoretically, it could activate viral replication. However, acerosin showed first an IC50 in the inhibition of viral infection of 2.7 μM, ruling out a potential pro–replicative effect. Besides, acerosin was one of the compounds that inhibited viral entry. Thus, this inhibitory activity could be responsible of the HIV infection inhibition, although its weak activity on viral transcription could aid to the final effect. The effect of xanthomicrol, although active as HIV transcriptional inhibitor, was only mediated through a weak inhibition of NF–κB with no effect on Sp1. This effect was expected, since xanthomicrol is the least powerful transcription inhibitor, excepting cardamonin, and showed a slight activity in viral entry. Finally, the most potent flavonoid, trimethoxyflavone confirmed its activity by the almost complete inhibition of HIV transcription and both transcription factors, NF–κB and Sp1 (p values < 0.0001, 0.0044 and <0.0001, respectively).
Although we show here that NF–κB could be involved in the anti–HIV effect of some flavonoids, especially the trimethoxyflavone, the mechanism of inhibition of viral infection is not well understood. Some authors have linked antioxidant activity to NF–κB inhibition (Xiao et al., 2006) which could lead to HIV–1 inhibition. The involvement of oxidation in T–cell activation was first described in the 1980s by several groups, including the inhibition of PMA induced proliferation by antioxidants (Novogrodsky et al., 1982). Reactive oxygen species (ROS) play a dual role in lymphocytes, the main target of HIV–1. They are needed for their activation but also promote apoptosis and cell death. Lymphocyte activation is crucial for the establishment of HIV infection and can be induced through several stimuli. In fact, T cell receptor (TCR) triggered by antigens activates an intracellular signalling cascade leading to transcriptional activation, including NF–κB activation. Moreover, oxidative stimuli in the cytosol are associated with activation and nuclear translocation of NF–κB, while reductive activation by thioredoxin is required for NF–κB binding to DNA (Chandrasekaran and Taylor, 2008). In this process, ROS play an important role, either produced by T cells themselves or by the exposure to exogenous oxidants (Benhar et al., 2016). Besides, NF–κB transcription factor can be activated by several stimuli including infection but also inflammation and oxidative stress (Pahl, 1999).
In this sense, low reduced glutathione (GSH) levels in HIV + patients were found to induce oxidative stress and increase viral replication (Schreck et al., 1991). Furthermore, the decrease of GSH levels seems to be related to HIV–1 LTR sensitization through the enhanced DNA binding ability of NF–κB (Yamaguchi et al., 2002). Actually, antioxidants and low GSH levels could promote viral latency and thus, the maintenance of HIV–1 reservoirs in T cells (Kalebic et al., 1991). However, some controversy has been found, since the role of oxidation in T cell activation varies when cell or environmental conditions change (Simeoni and Bogeski, 2015).
Flavonoids are widely known antioxidants and for this reason, this activity could be related to the viral transcription inhibition. Therefore, we have also tried to evaluate the antioxidant effect of all the isolated compounds, including terpenes and flavonoids, to assess if the effect on the cellular redox state is related to the transcriptional effect. Consequently, we have measured the levels of intracellular GSH and total glutathione after the treatment with different concentrations of compounds and EC50 values and maximum efficacy (Emax) have been calculated for each compound (Fig. 5 and Table 5 ).
Fig. 5.
Effect of the isolated compounds from Artemisia campestris in the antioxidant assay. Total, reduced (GSH) and oxidized glutathione (GSSG) levels were determined in THP–1 cells extracts. Fluorometric measurement of the interaction of thiols with OPT (o–Phtaldehyde) in the presence of serial dilutions of damsin, canrenone, cardamonin, acerosin, trimethoxyflavone and xanthomicrol were compared to a standard curve produced by measuring increasing concentrations of GSH. Fluorescence was measured at λexc = 350 nm and λem = 420 nm. The redox index (RI), a parameter that indicates the antioxidant status of the tissue, was expressed as follows: RI = GSH/(GSH + GSSG). Results are expressed as EC50 or concentration that produce the 50% of the maximum antioxidant effect, being the maximum 1 (maximum of the glutathione is in the reduced form or GSH). Besides, we have also calculated the Emax reached by each compound to evaluate the antioxidant efficacy (Emax). Both parameters were calculated using Prism v8.0 (GraphPad Software) using non–linear regression, dose–response curves.
Table 5.
EC50 calculated for the isolated compounds from Artemisia campestris. in the glutathione assay. Total, reduced (GSH) and oxidized glutathione (GSSG) levels were determined in THP–1 cells extracts. Fluorimetric measurement of the interaction of thiols with OPT (o–Phtaldehyde) in the presence of serial dilutions of damsin, canrenone, cardamonin, acerosin, trimethoxyflavone, xanthomicrol and resveratrol (control) were compared to a standard curve produced by measuring increasing concentrations of GSH. Fluorescence was measured at λexc = 350 nm and λem = 420 nm. The redox index (RI), a parameter that indicates the antioxidant status of the tissue, was expressed as follows: RI = GSH/(GSH + GSSG). Results are expressed as EC50 (concentration that produce the 50% of the maximum antioxidant effect), being the maximum 1 (all of the glutathione is in the reduced form). Besides, we have also calculated the Emax reached by each compound to evaluate antioxidant efficacy (Emax). Both parameters were calculated using Prism v8.0 (GraphPad Software) using non–linear regression, dose–response curves.
| EC50 μM HIV (CI95%; R2) | Emax (CI95%) | |
|---|---|---|
| Damsin | > 100 | – |
| Canrenone | > 100 | – |
| Resveratrol | 1.85 (1.56–2.19; 0.9923) | 0.94 (0.92–0.96) |
| Cardamonin | 5.39 (1.03–28.28; 0.8782) | 0.84 (0.55–1.13) |
| Acerosin | 4.58 (2.76–7.61; 0.9636) | 0.95 (0.88–1.02) |
| Trimethoxyflavone | 3.79 (0.42–34.13; 0.776) | 0.74 (0.47–1.00) |
| Xanthomicrol | 4.59 (2.03–10.36; 0.9209) | 0.93 (0.81–1.04) |
All the flavonoids showed antioxidant activity and, as expected, terpenes did not. The most effective antioxidants were acerosin and xanthomicrol (Emax 0.95 and 0.93, respectively). The high methoxylation rate and the lack of hydroxyl groups of trimethoxiflavone could explain its lower efficacy as antioxidant (Emax 0.74), although it was still higher than terpenes, damsin and canrenone. EC50 was very similar among all the flavonoids, with trimethoxyflavone as the most potent antioxidant (EC50 3.79 μM). It could be hypothesized that a high methoxylation rate could increase cell penetrability and due to this fact, a lower flavonoid concentration would be needed. On the other hand, the lack of hydroxyl groups could diminish its antioxidant activity once inside.
In summary, acerosin and xanthomicrol were the most effective antioxidants and trimethoxiflavone the most powerful one. Although it is difficult to correlate these results with the viral transcriptional inhibition, we found that trimethoxyflavone was the most powerful HIV–transcription inhibitor and one of the most powerful antioxidants, although its efficacy was lower than the efficacy of acerosin or xanthomicrol. Cardamonin was the less powerful antioxidant but it showed a good antioxidant efficacy. However, cardamonin did not show any transcriptional activity. Acerosin and xanthomicrol were medium transcriptional inhibitors, and showed the highest antioxidant effect, although with slightly lower concentrations than trimethoyflavone.
To analyse the potential correlation between the antioxidant effect and the transcriptional inhibition of the isolated compounds we have performed a Spearman correlation analysis, and the r value has been calculated for EC50 transcription (EC50 trans), EC50 antioxidant (EC50 ox) and antioxidant efficacy (Emax) for all the isolated compounds, including terpenes (Fig. 6 A), and only for the flavonoids with proven antioxidant activity (Fig. 6B).
Fig. 6.
Calculated correlation between the antioxidant (EC50ox and Emax) and the transcriptional activity (IC50trans). A non–parametric Spearman correlation analysis was performed using Prism v8.0 (GraphPad Software)and correlation coefficient r was calculated for A: All the isolated compounds and B: Only the flavonoids with antioxidant activity.
Surprisingly, we could not find correlation between transcriptional and antioxidant activities (IC50 trans and IC50 ox), even when the flavonoids alone were analyzed. Moreover, we found a negative correlation even if terpenes were included or not (Spearman r of −0.67 and −0.40, respectively). However, when we compare the effectiveness as antioxidants (Emax) with the transcriptional activity (EC50 trans), a positive r was obtained, even when terpenes were included (Spearman r of 0.60 and 0.40, respectively). However, in this last case, the correlation is inverse. A higher Emax indicates a higher efficacy as antioxidant and it correlates with a higher IC50 which indicates less potency as transcriptional inhibitor. Consequently, the correlation is, in fact, negative. Therefore, the more antioxidant the activity is, the less transcriptional. This rules out a potential involvement of the antioxidant activity in the transcriptional inhibition achieved with these compounds. Despite this fact, the presence of flavonoids with preferred transcriptional activity, as the trimethoxyflavone, and flavonoids with preferred antioxidant activity, as acerosin, highlights the multitarget activity of A. campestris composition. However, more research would be needed to address this issue.
In summary, aqueous and ethanolic extracts of Artemisia campestris subsp. glutinosa (Besser) Batt. displayed anti–HIV activity, with EE as the most powerful inhibitor. Two terpenes and four flavonoids were isolated from this active extract: a pseudoguainolide sesquiterpene lactone, damsin, a steroid triterpene, canrenone, a chalcone, cardamonin, and three methoxylated flavones, acerosin, 6,2′,4′–trimethoxyflavone and xanthomicrol. All the isolated compounds showed anti–HIV replication activity in vitro with IC50s in the medium nanomolar to low-medium micromolar range. Acerosin and xanthomicrol inhibit viral entry and all of them, excepting cardamonin, showed activity in HIV transcription, mainly though NF–κB inhibition. Antioxidant activity was showed exclusively by flavonoids, although a negative correlation with transcriptional activity was found. However, the antioxidant activity would probably add other benefits to the treatment of the AIDS pathology.
4. Conclusions and future perspectives
In this work, we have shown the anti–HIV–1 activity of A. campestris subsp. glutinosa (Besser) Batt. and its components, through the inhibition of multiple cellular targets affecting the HIV replication cycle. The activity of these compounds in cellular targets could explain why plant extracts can be used in the treatment of several infections. Besides, multitarget activity is receiving increased attention in the field of drug discovery and the presence of several compounds with dual and different mechanisms of action could be of interest in pathologies like HIV–1 infection, treated with a mixture of drugs directed at different targets, since it could aid in overcoming drug resistances and simplify drug therapy.
Author contributions
A.T.L performed phytochemical analysis, isolation of compounds, biological experiments and contributed to the writing of this manuscript and to the figures preparation. M.L.R. performed phytochemical analysis. B.P. conceived and supervised the study, provided the plant materials and helped to the manuscript writing. G.J.A., A.M.J. and A.J. helped to the manuscript preparation and to the statistical analysis of the data. B. M. performed biological experiments. B.L.M. conceived and supervised the study, performed the experiments and the statistical analysis and wrote and edited the manuscript and the figures. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The writing assistance of Ms Brooke–Turner and Ms Luisa López is gratefully acknowledged. We would like to thank Dr. Molina for collecting the plant samples.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jep.2020.113163.
Funding
This project was supported by the Spanish Agency for International Cooperation and Development (AECID) (D/020523/08) and the Universidad Complutense de Madrid UCM–Santander (PR87/19–22685). This work was partially supported by Instituto de Salud Carlos III and co–funded by European Regional Development Fund (ERDF) “A way to build Europe” (projects AIDS Research Network RD16CIII/0002/0001 and RD16CIII/0002/0001 to JA).
Appendix B. Supplementary data
The following is the Supplementary data to this article:
1H and 13C NMR, HMQC, HSQC, HMBC, COSY and MS spectra for extracts and isolated compounds.
References
- Abad M.J., Bedoya L.M., Apaza L., Bermejo P. The artemisia L. Genus: a review of bioactive essential oils. Molecules. 2012;17(3):2542–2566. doi: 10.3390/molecules17032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abad M.J., Bedoya L.M., Apaza L., Bermejo P. Chapter 2 – the artemisia L. Genus: a review of bioactive sesquiterpene lactones. In: Atta ur R., editor. Studies in Natural Products Chemistry. Elsevier; 2012. pp. 43–65. [DOI] [Google Scholar]
- Abad M.J., Bedoya L.M., Apaza L., Bermejo P. Pharmacological potentials of artemisinin and related sesquiterpene lactones: recent advances and trends. In: Aftab T., Ferreira J.F.S., Khan M.M.A., Naeem M., editors. Artemisia Annua – Pharmacology and Biotechnology. Springer Berlin Heidelberg; Berlin, Heidelberg: 2014. pp. 75–93. [DOI] [Google Scholar]
- Abu–Shady H., Soine T.O. The chemistry of Ambrosia maritima L. II. Hydrogenation, oxidation, and dehydrogenation of ambrosin and damsin. J Am Pharm Assoc Am Pharm Assoc. 1954;43(6 1):365–369. doi: 10.1002/jps.3030430614. [DOI] [PubMed] [Google Scholar]
- Aderogba M.A., Kgatle D.T., McGaw L.J., Eloff J.N. Isolation of antioxidant constituents from Combretum apiculatum subsp. apiculatum. South Afr. J. Bot. 2012;79:125–131. doi: 10.1016/j.sajb.2011.10.004. [DOI] [Google Scholar]
- Ahmad A., Kaleem M., Ahmed Z., Shafiq H. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections-A review. Food Res. Int. 2015;77(2):221–235. doi: 10.1016/j.foodres.2015.06.021. [DOI] [Google Scholar]
- Al–Sanafi A.E. The pharmacological importance of Artemisia campestris–a review. Asian J. Pharmaceut. Res. 2015;5(2):88–92. [Google Scholar]
- Aniya Y., Shimabukuro M., Shimoji M., Kohatsu M., Gyamfi M.A., Miyagi C., Kunii D., Takayama F., Egashira T. Antioxidant and hepatoprotective actions of the medicinal herb Artemisia campestris from the Okinawa Islands. Biol. Pharm. Bull. 2000;23(3):309–312. doi: 10.1248/bpb.23.309. [DOI] [PubMed] [Google Scholar]
- Aponte J.C., Yang H., Vaisberg A.J., Castillo D., Malaga E., Verastegui M., Casson L.K., Stivers N., Bates P.J., Rojas R., Fernandez I., Lewis W.H., Sarasara C., Sauvain M., Gilman R.H., Hammond G.B. Cytotoxic and anti–infective sesquiterpenes present in Plagiochila disticha (Plagiochilaceae) and Ambrosia peruviana (Asteraceae) Planta Med. 2010;76(7):705–707. doi: 10.1055/s–0029–1240681. [DOI] [PubMed] [Google Scholar]
- Arenzana–Seisdedos F., Fernandez B., Dominguez I., Jacque J.M., Thomas D., Diaz–Meco M.T., Moscat J., Virelizier J.L. Phosphatidylcholine hydrolysis activates NF–kappa B and increases human immunodeficiency virus replication in human monocytes and T lymphocytes. J. Virol. 1993;67(11):6596–6604. doi: 10.1128/jvi.67.11.6596-6604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanini D., Sabbadin C., Dona G., Clari G., Bordin L. Aldosterone receptor blockers spironolactone and canrenone: two multivalent drugs. Expet Opin. Pharmacother. 2014;15(7):909–912. doi: 10.1517/14656566.2014.896901. [DOI] [PubMed] [Google Scholar]
- Benhar M., Shytaj I.L., Stamler J.S., Savarino A. Dual targeting of the thioredoxin and glutathione systems in cancer and HIV. J. Clin. Invest. 2016;126(5):1630–1639. doi: 10.1172/JCI85339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverton T. Quercus publishing plc; London: 2011. Breverton's Complete Herbal, Based on Culpeper's the English Physician and Complete Herbal of 1653, Field Southernwood. [Google Scholar]
- Blackard J.T., Kong L., Rouster S.D., Karns R., Horn P.S., Kottilil S., Shata M.T., Sherman K.E. CCR5 receptor antagonism inhibits hepatitis C virus (HCV) replication in vitro. PloS One. 2019;14(10) doi: 10.1371/journal.pone.0224523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora K.S., Sharma A. The genus Artemisia: a comprehensive review. Pharm. Biol. 2011;49(1):101–109. doi: 10.3109/13880209.2010.497815. [DOI] [PubMed] [Google Scholar]
- Cashman J.R., Peña S. Canrenone formation via general–base–catalyzed elimination of 7alpha–(methylthio)spironolactone S–oxide. Chem. Res. Toxicol. 1989;2:109–113. doi: 10.1021/tx00008a007. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran V., Taylor E.W. Molecular modeling of the oxidized form of nuclear factor–kappa B suggests a mechanism for redox regulation of DNA binding and transcriptional activation. J. Mol. Graph. Model. 2008;26(5):861–867. doi: 10.1016/j.jmgm.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Chaturvedi D. Research Signpost; Trivandrum: 2011. Sesquiterpene Lactones: Structural Diversity and Their Biological Activities. Research Signpost; pp. 313–334. Kerala. [Google Scholar]
- Cheng Y.Y., Yuan H. Quantitative study of electrostatic and steric effects on physicochemical property and biological activity. J. Mol. Graph. Model. 2006;24(4):219‐226. doi: 10.1016/j.jmgm.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Deeks S.G. HIV: shock and kill. Nature. 2012;487(7408):439–440. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- Dib I., Angenot L., Mihamou A., Ziyyat A., Tits M. Artemisia campestris L.: ethnomedicinal, phytochemical and pharmacological review. J. Herb. Med. 2017;7:1–10. doi: 10.1016/j.hermed.2016.10.005. [DOI] [Google Scholar]
- Dib I., El Alaoui–Faris F.E. Artemisia campestris L.: review on taxonomical aspects, cytogeography, biological activities and bioactive compounds. Biomed. Pharmacother. 2019;109:1884–1906. doi: 10.1016/j.biopha.2018.10.149. [DOI] [PubMed] [Google Scholar]
- Dib I., Fauconnier M.L., Sindic M., Belmekki F., Assaidi A., Berrabah M., Mekhfi H., Aziz M., Legssyer A., Bnouham M., Ziyyat A. Chemical composition, vasorelaxant, antioxidant and antiplatelet effects of essential oil of Artemisia campestris L. from Oriental Morocco. BMC Compl. Alternative Med. 2017;17(1):82. doi: 10.1186/s12906–017–1598–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib I., Tits M., Angenot L., Wauters J.N., Assaidi A., Mekhfi H., Aziz M., Bnouham M., Legssyer A., Frederich M., Ziyyat A. Antihypertensive and vasorelaxant effects of aqueous extract of Artemisia campestris L. from Eastern Morocco. J. Ethnopharmacol. 2017;206:224–235. doi: 10.1016/j.jep.2017.05.036. [DOI] [PubMed] [Google Scholar]
- Dillard C.J., German J.B. Phytochemicals: nutraceuticals and human health. J. Sci. Food Agric. 2000;80(12):1744–1756. doi: 10.1002/1097-0010(20000915)80:12<1744::AID-JSFA725>3.0.CO;2-W. [DOI] [Google Scholar]
- Efferth T. Beyond malaria: the inhibition of viruses by artemisinin–type compounds. Biotechnol. Adv. 2018;36(6):1730–1737. doi: 10.1016/j.biotechadv.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Gach K., Długosz A., Janecka A. The role of oxidative stress in anticancer activity of sesquiterpene lactones. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015;388:477–486. doi: 10.1007/s00210-015-1096-3. [DOI] [PubMed] [Google Scholar]
- Garcia–Perez J., Sanchez–Palomino S., Perez–Olmeda M., Fernandez B., Alcami J. A new strategy based on recombinant viruses as a tool for assessing drug susceptibility of human immunodeficiency virus type 1. J. Med. Virol. 2007;79(2):127–137. doi: 10.1002/jmv.20770. [DOI] [PubMed] [Google Scholar]
- Ghlissi Z., Sayari N., Kallel R., Bougatef A., Sahnoun Z. Antioxidant, antibacterial, anti–inflammatory and wound healing effects of Artemisia campestris aqueous extract in rat. Biomed. Pharmacother. 2016;84:115–122. doi: 10.1016/j.biopha.2016.09.018. [DOI] [PubMed] [Google Scholar]
- Goldsby G., Burke B. Sesquiterpene lactones and a sesquiterpene diol from jamaican ambrosia peruviana. Phytochemistry. 1987;26(4):1059–1063. doi: 10.1016/s0031–9422(00)82350–x. [DOI] [Google Scholar]
- Guibourt N.J.B.G. 1862. Historia natural de las drogas simples, Carlos Bailly–Bailliere; p. 29. [Google Scholar]
- Hatziieremia S., Gray A.I., Ferro V.A., Paul A., Plevin R. The effects of cardamonin on lipopolysaccharide–induced inflammatory protein production and MAP kinase and NFkappaB signalling pathways in monocytes/macrophages. Br. J. Pharmacol. 2006;149(2):188–198. doi: 10.1038/sj.bjp.0706856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Hayashi T., Otsuka H., Takeda Y. Antiviral activity of 5,6,7–trimethoxyflavone and its potentiation of the antiherpes activity of acyclovir. J. Antimicrob. Chemother. 1997;39(6):821–824. doi: 10.1093/jac/39.6.821. [DOI] [PubMed] [Google Scholar]
- Herz W., Gage D., Kumar N. Damsinic acid and ambrosanolides from vegetative ambrosia hispida. Phytochemistry. 1981;20(7):1601–1604. doi: 10.1016/s0031–9422(00)98540–6. [DOI] [Google Scholar]
- Hissin P.J., Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976;74(1):214–226. doi: 10.1016/0003–2697(76)90326–2. [DOI] [PubMed] [Google Scholar]
- Huang H., Zepp M., Georges R.B., Jarahian M., Kazemi M., Eyol E., Berger M.R. The CCR5 antagonist maraviroc causes remission of pancreatic cancer liver metastasis in nude rats based on cell cycle inhibition and apoptosis induction. Canc. Lett. 2020;474:82–93. doi: 10.1016/j.canlet.2020.01.009. [DOI] [PubMed] [Google Scholar]
- Hwang D.R., Wu Y.S., Chang C.W., Lien T.W., Chen W.C., Tan U.K., Hsieh H.P. Synthesis and anti-viral activity of a series of sesquiterpene lactones and analogues in the subgenomic HCV replicon system. Bioorg. Med. Chem. 2006;14(1):83–91. doi: 10.1016/j.bmc.2005.07.055. [DOI] [PubMed] [Google Scholar]
- Ishitsuka H., Ohsawa C., Ohiwa T., Umeda I., Suhara Y. Antipicornavirus flavone ro 09–0179. Antimicrob. Agents Chemother. 1982;22(4):611–616. doi: 10.1128/aac.22.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabri M.A., Tounsi H., Abdellaoui A., Marzouki L., Sebai H. Protective effects of Artemisia campestris extract against gastric acid reflux–induced esophageal mucosa injuries. Pathophysiology. 2018;25(1):63–69. doi: 10.1016/j.pathophys.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Kadi I., Ouinten M., Gourine N., Yousfi M. Synergistic antinociceptive activity of combined aqueous extracts of Artemisia campestris and Artemisia herba–alba in several acute pain models. Nat. Prod. Res. 2019;33(6):875–878. doi: 10.1080/14786419.2017.1410802. [DOI] [PubMed] [Google Scholar]
- Kalebic T., Kinter A., Poli G., Anderson M.E., Meister A., Fauci A.S. Suppression of human immunodeficiency virus expression in chronically infected monocytic cells by glutathione, glutathione ester, and N–acetylcysteine. Proc. Natl. Acad. Sci. U. S. A. 1991;88(3):986–990. doi: 10.1073/pnas.88.3.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal P., Fukuda T., Teusink–Cross A., Kashuba A.D.M., Lane A., Mehta P.A., Marsh R.A., Jordan M.B., Grimley M.S., Myers K.C., Nelson A.S., El–Bietar J., Chandra S., Bleesing J.J., Krupski M.C., Davies S.M. CCR5 inhibitor as novel acute graft versus host disease prophylaxis in children and young adults undergoing allogeneic stem cell transplant: results of the phase II study. Bone Marrow Transplant. 2020 doi: 10.1038/s41409–020–0888–3. [DOI] [PubMed] [Google Scholar]
- Kiat T.S., Pippen R., Yusof R., Ibrahim H., Khalid N., Rahman N.A. Inhibitory activity of cyclohexenyl chalcone derivatives and flavonoids of fingerroot, Boesenbergia rotunda (L.), towards dengue–2 virus NS3 protease. Bioorg. Med. Chem. Lett. 2006;16(12):3337–3340. doi: 10.1016/j.bmcl.2005.12.075. [DOI] [PubMed] [Google Scholar]
- Kongkum N., Tuchinda P., Pohmakotr M., Reutrakul V., Piyachaturawat P., Jariyawat S., Suksen K., Yoosook C., Kasisit J., Napaswad C. DNA topoisomerase IIalpha inhibitory and anti–HIV–1 flavones from leaves and twigs of Gardenia carinata. Fitoterapia. 2012;83(2):368–372. doi: 10.1016/j.fitote.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Liu X., Cao J., Huang G., Zhao Q., Shen J. Biological activities of artemisinin derivatives beyond malaria. Curr. Top. Med. Chem. 2019;19(3):205–222. doi: 10.2174/1568026619666190122144217. [DOI] [PubMed] [Google Scholar]
- Lopez S.N., Furlan R.L., Zacchino S.A. Detection of antifungal compounds in Polygonum ferrugineum Wedd. extracts by bioassay–guided fractionation. Some evidences of their mode of action. J. Ethnopharmacol. 2011;138(2):633–636. doi: 10.1016/j.jep.2011.09.038. [DOI] [PubMed] [Google Scholar]
- Mahmood N., Pizza C., Aquino R., De Tommasi N., Piacente S., Colman S., Hay A.J. Inhibition of HIV infection by flavanoids. Antivir. Res. 1993;22(2–3):189–199. doi: 10.1016/0166-3542(93)90095-z. [DOI] [PubMed] [Google Scholar]
- Massanella M., Richman D.D. Measuring the latent reservoir in vivo. J. Clin. Invest. 2016;126(2):464–472. doi: 10.1172/jci80567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmi A., Sansa G., Rjeibi I., El Ayeb M., Srairi–Abid N., Bellasfer Z., Fekhih A. Use of medicinal plants against scorpionic and ophidian venoms. Arch. Inst. Pasteur. Tunis. 2007;84(1–4):49–55. [PubMed] [Google Scholar]
- Mohammed M.M., Christensen L.P., Colla P.L. Isolation and anti–HIV–1 activity of a new sesquiterpene lactone from Calocephalus brownii F. Muell. Nat. Prod. Res. 2014;28(4):221–229. doi: 10.1080/14786419.2012.755970. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Ravid A., Rubin A.L., Stenzel K.H. Hydroxyl radical scavengers inhibit lymphocyte mitogenesis. Proc. Natl. Acad. Sci. U. S. A. 1982;79(4):1171–1174. doi: 10.1073/pnas.79.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin E., Amara A., Bachelerie F., Bessia C., Virelizier J.L., Arenzana–Seisdedos F., Schwartz O., Heard J.M., Clark–Lewis I., Legler D.F., Loetscher M., Baggiolini M., Moser B. The CXC chemokine SDF–1 is the ligand for LESTR/fusin and prevents infection by T–cell–line–adapted HIV–1. Nature. 1996;382(6594):833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- Pahl H.L. Activators and target genes of Rel/NF–kappaB transcription factors. Oncogene. 1999;18(49):6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Palmer G. Shuswap Indian ethnobotany. Syesis. 1975;8 29–5. [Google Scholar]
- Pandey A.K., Singh P. The genus artemisia: a 2012–2017 literature review on chemical composition, antimicrobial, insecticidal and antioxidant activities of essential oils. Medicine (Baltim.) 2017;4(3) doi: 10.3390/medicines4030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwood T.A. third ed. Longman and Co.; London: 1857. Supplement to the Pharmacopoeia. [Google Scholar]
- Saeed M., Jacob S., Sandjo L.P., Sugimoto Y., Khalid H.E., Opatz T., Thines E., Efferth T. Cytotoxicity of the sesquiterpene lactones neoambrosin and damsin from ambrosia maritima against multidrug–resistant cancer cells. Front. Pharmacol. 2015;6:267. doi: 10.3389/fphar.2015.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF–kappa B transcription factor and HIV–1. EMBO J. 1991;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefi M., Fetoui H., Makni M., Zeghal N. Mitigating effects of antioxidant properties of Artemisia campestris leaf extract on hyperlipidemia, advanced glycation end products and oxidative stress in alloxan–induced diabetic rats. Food Chem. Toxicol. 2010;48(7):1986–1993. doi: 10.1016/j.fct.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Sefi M., Fetoui H., Soudani N., Chtourou Y., Makni M., Zeghal N. Artemisia campestris leaf extract alleviates early diabetic nephropathy in rats by inhibiting protein oxidation and nitric oxide end products. Pathol. Res. Pract. 2012;208(3):157–162. doi: 10.1016/j.prp.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Simeoni L., Bogeski I. Redox regulation of T–cell receptor signaling. Biol. Chem. 2015;396(5):555–569. doi: 10.1515/hsz–2014–0312. [DOI] [PubMed] [Google Scholar]
- Taleghani A., Emami S.A., Tayarani–Najaran Z. Artemisia: a promising plant for the treatment of cancer. Bioorg. Med. Chem. 2020;28(1):115180. doi: 10.1016/j.bmc.2019.115180. [DOI] [PubMed] [Google Scholar]
- Talman A.M., Clain J., Duval R., Menard R., Ariey F. Artemisinin bioactivity and resistance in malaria parasites. Trends Parasitol. 2019;35(12):953–963. doi: 10.1016/j.pt.2019.09.005. [DOI] [PubMed] [Google Scholar]
- Tan R.X., Zheng W.F., Tang H.Q. Biologically active substances from the genus Artemisia. Planta Med. 1998;64(4):295–302. doi: 10.1055/s–2006–957438. [DOI] [PubMed] [Google Scholar]
- Tewtrakul S., Puripattanavong J., Panphadung T. HIV–1 protease inhibitory substances from the rhizomes of Boesenbergia pandurata Holtt. Songklanakarin J. Sci. Technol. 2003;25(4):503–508. [Google Scholar]
- Villagomez R., Rodrigo G.C., Collado I.G., Calzado M.A., Munoz E., Akesson B., Sterner O., Almanza G.R., Duan R.D. Multiple anticancer effects of damsin and coronopilin isolated from Ambrosia arborescens on cell cultures. Anticancer Res. 2013;33(9):3799–3805. [PubMed] [Google Scholar]
- Wollenweber E., Mann K. Exudate flavonoids in three essential oil plants from the Ciskei (South Africa) Fitoterapia. 1989;60(3):249–251. [Google Scholar]
- Wu J.H., Wang X.H., Yi Y.H., Lee K. Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. Bioorg. Med. Chem. Lett. 2003;13:1813–1815. doi: 10.1016/S0960-894X(03)00197-5. [DOI] [PubMed] [Google Scholar]
- Xiao L., Zhao L., Li T., Hartle D.K., Aruoma O.I., Taylor E.W. Activity of the dietary antioxidant ergothioneine in a virus gene–based assay for inhibitors of HIV transcription. Biofactors. 2006;27(1–4):157–165. doi: 10.1002/biof.5520270114. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Katoh I., Kurata S. Azidothymidine causes functional and structural destruction of mitochondria, glutathione deficiency and HIV–1 promoter sensitization. Eur. J. Biochem. 2002;269(11):2782–2788. doi: 10.1046/j.1432–1033.2002.02954.x. [DOI] [PubMed] [Google Scholar]
- Yu H., Jiang W., Du H., Xing Y., Bai G., Zhang Y., Li Y., Jiang H., Zhang Y., Wang J., Wang P., Bai X. Involvement of the Akt/NF–kappaB pathways in the HTNV–mediated increase of IL–6, CCL5, ICAM–1, and VCAM–1 in HUVECs. PloS One. 2014;9(4) doi: 10.1371/journal.pone.0093810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.J., Nguyen V.H., Nguyen M.C., Soejarto D.D., Pezzuto J.M., Fong H.H., Tan G.T. Sesquiterpenes and butenolides, natural anti–HIV constituents from Litsea verticillata. Planta Med. 2005;71(5):452–457. doi: 10.1055/s–2005–864142. [DOI] [PubMed] [Google Scholar]
- Zhang X., He J., Huang W., Huang H., Zhang Z., Wang J., Yang L., Wang G., Wang Y., Lia Y. Antiviral activity of the sesquiterpene lactones from centipeda minima against influenza A virus in vitro. Nat Prod Commun. 2018;13(2):115–119. doi: 10.1177/1934578X1801300201. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1H and 13C NMR, HMQC, HSQC, HMBC, COSY and MS spectra for extracts and isolated compounds.