In their letter, R. Cherian and co-workers take issue with our interpretation of the respiratory physiology of coronavirus disease 2019 (COVID-19), arguing that it is based merely on “small cohort studies”, and instead declaring that “a high proportion of mechanically ventilated COVID-19 patients exhibit near-normal lung compliance”. Yet the low respiratory compliance of COVID-19 patients has now been extensively demonstrated by studies totalling more than 800 COVID-19 patients [1–7], including a direct comparison with non-COVID-19 acute respiratory distress syndrome (ARDS) patients that revealed no difference in respiratory compliance [7]. In contrast, the three case series cited by R. Cherian and co-workers in support of their claim comprise cohorts of, respectively, 16, 10 and 26 patients [8–10]. Furthermore, even these case series report average respiratory compliance in COVID-19 of 40–45 mL·cmH2O−1, which is in fact abnormal and far from “near-normal compliance” [11, 12].
Short abstract
Phenotyping of COVID-19-related ARDS should be done using careful, data-driven approaches https://bit.ly/3eX65Yu
From the authors:
In their letter, R. Cherian and co-workers take issue with our interpretation of the respiratory physiology of coronavirus disease 2019 (COVID-19), arguing that it is based merely on “small cohort studies”, and instead declaring that “a high proportion of mechanically ventilated COVID-19 patients exhibit near-normal lung compliance”. Yet the low respiratory compliance of COVID-19 patients has now been extensively demonstrated by studies totalling more than 800 COVID-19 patients [1–7], including a direct comparison with non-COVID-19 acute respiratory distress syndrome (ARDS) patients that revealed no difference in respiratory compliance [7]. In contrast, the three case series cited by R. Cherian and co-workers in support of their claim comprise cohorts of, respectively, 16, 10 and 26 patients [8–10]. Furthermore, even these case series report average respiratory compliance in COVID-19 of 40–45 mL·cmH2O−1, which is in fact abnormal and far from “near-normal compliance” [11, 12]. As an informative comparison, the ANZICS (Australian and New Zealand Intensive Care Society) cohort of ARDS patients used to derive the Berlin definition of ARDS had an average respiratory compliance of 40±15 mL·cmH2O−1 [13]. We thus find no evidence in the authors' citations (or elsewhere) to support their empirical claim that many or most COVID-19 patients present with “normal” or “near-normal” respiratory compliance.
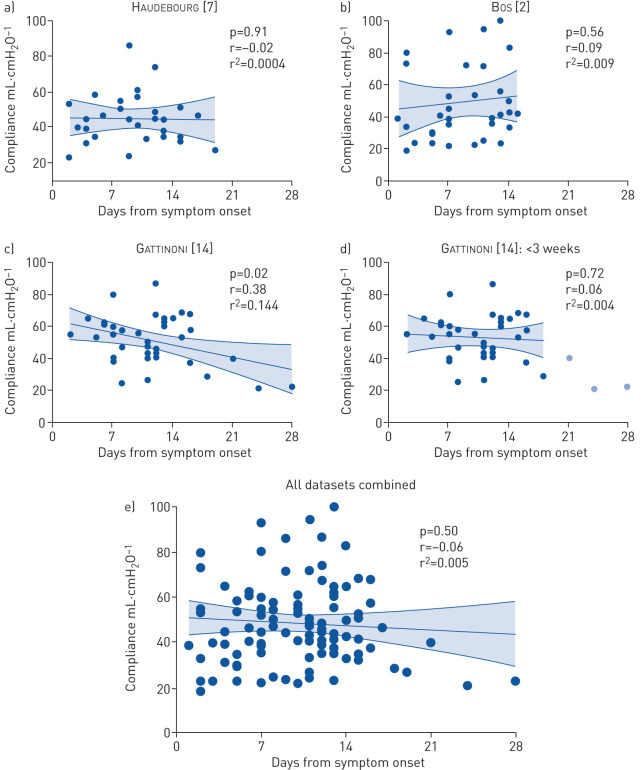
R. Cherian and co-workers also assume a temporal progression from “early” COVID-19 physiology (characterised by normal respiratory compliance) to “late” physiology (characterised by impaired respiratory compliance). Yet three published studies comprising nearly 350 mechanically ventilated COVID-19 patients have reported serial measurements of respiratory compliance [1, 3, 6], and none has shown any temporal trend towards decreased compliance in the days following initiation of mechanical ventilation. Furthermore, a recent report from Haudebourg et al. [7] demonstrated no correlation between duration of symptoms and respiratory compliance in COVID-19 patients (figure 1a). We have since validated this observation using our own clinical data (figure 1b). As shown in figure 1c, Gattinoni et al. [14] recently published their own data countering these findings. Importantly, when data from all three cohorts are combined and analysed together, no temporal trend is present (p=0.50, r2=0.005; figure 1e). Closer inspection reveals that the purported correlation in the cohort of Gattinoniet al. [14] is entirely attributable to two patients with low respiratory compliance and >3 weeks of symptoms, a duration of disease irrelevant to considerations of acute pathogenesis and rarely encountered in the literature and not at all by Haudebourg et al. [7] or ourselves (figure 1a and b). As shown in figure 1d, if analysis is restricted to the 35 patients with symptoms shorter than 3 weeks, there is no evidence of a temporal correlation (p=0.72, r2=0.004), and the results align with the meta-analysis (figure 1e).
FIGURE 1.
Respiratory compliance and duration of symptoms in COVID-19: a lesson in the risks of drawing premature inferences. a) Haudebourg et al. [7] observed no correlation between duration of symptoms and respiratory compliance in patients with COVID-19 receiving mechanical ventilation. b) We validated this lack of association with a separate cohort [2]. c) In contrast, Gattinoni et al. [14] recently reported a negative association between duration of symptoms and respiratory compliance. d) However, this purported association was attributable entirely to three patients who had been symptomatic for 3–4 weeks (a duration of questionable relevance to discussions of the pathophysiology of acute respiratory failure). e) When all datasets were combined (including the temporal outliers from Gattinoni et al. [14]), no evidence of a correlation was found. This illustrates the danger of drawing premature inferences from underpowered cohorts, which are prone to over-fitting and spurious correlations.
Taken together, the two central observations undergirding the proposed pathophysiological framework of R. Cherian and co-workers (normal respiratory mechanics in COVID-19 and their temporal deterioration) are unsupported by a burgeoning evidence base. Furthermore, these analyses are valuable working demonstrations of the numerous pitfalls we cautioned against in our editorial [15]: 1) the risks of drawing premature pathophysiological conclusions from underpowered case series and then making possibly erroneous therapeutic recommendations; 2) the instability of statistical inferences using small, single-variable data sources; and 3) the predictable correction of initial human intuitions when more data emerge. A final under-appreciated and unmeasurable pitfall of premature phenotyping raised in our editorial, and one that the multitude of publications addressing these purported phenotypes are substantiating, is the cost to research resources caused by high-profile yet unsupported speculation. This factor is all the more pertinent in the face of an unforgiving pandemic in which clinical intensive care unit workload is highly demanding and clinical research is a zero-sum game. Furthermore, clinicians have even less time than usual to critically evaluate scientific literature. Therefore, it is incumbent as clinician-scientists that, whilst our data gathering may be agile and creative, its interpretation should be cautious and deliberate.
While we agree with R. Cherian and co-workers regarding the potential pathophysiological importance of endothelial injury in COVID-19, the data at hand are simply insufficient to declare if this aspect of pathogenesis is a central mediator of disease progression and lung injury in COVID-19. For example, it is worth noting that, while endothelial injury has been described in post mortem histopathological evaluations, it is not ubiquitous; epithelial injury and diffuse alveolar damage, however, are [16, 17]. Our editorial did not take a position on the pathophysiology of COVID-19, nor do we dispute the need to identify more homogenous biological pathways. Frankly, we do not believe in “typical ARDS”, as the syndrome encompasses diverse aetiological pathways with only partially intersecting clinical and histopathological “bottlenecks”. We are merely arguing that the currently postulated phenotypes are unconvincing, and insufficient to justify a widespread change in clinical management (as proposed by the correspondence).
In his response to our editorial [15], R. Rajendram reveals a curious misinterpretation: “Thus, while the net effect of the ARDSNet protocol is beneficial at the level of the study population, theoretically it may harm select patients […] contrary to the opinions of the Surviving Sepsis Campaign [18], and Bos et al. [15], the ARDSNet protocol is not a panacea.” Putting aside the wishful thinking of a supportive intervention functioning as a “panacea” for a condition with persistent mortality of 30–40%, R. Rajendram (along with R. Cherian and co-workers) seems to think that we dispute the heterogeneity of ARDS, and advocate for a “one-size-fits-all” approach to its clinical management. Quite the opposite: we strongly believe that ARDS represents a pathophysiologically heterogenous syndrome and have argued the same for COVID-19-related ARDS [19]. Until well-defined biological subgroups are identified, the ceiling of effective interventions is likely to remain supportive. We also strongly suspect there are likely to be considerable biological differences between COVID-19 and non-COVID-19 ARDS [20].
Where we differ with our correspondents, we suspect, is in our lack of confidence that clinicians can identify meaningful subphenotypes using underpowered cohorts and bedside intuitions and then recommend effective interventions without testing them in a scientific study. This was the central point of our editorial and is illustrated with two examples in this response (the “normal compliance” of COVID-19 and its purported temporal worsening). As a contrast, the correspondents may consider recent pre-COVID-19 research identifying hypoinflammatory and hyperinflammatory subphenotypes in ARDS (to which we have contributed) [8, 21]. These ARDS subphenotypes were derived using unsupervised clustering of more than 3000 rigorously adjudicated and extensively characterised patients [22]. The ARDS subphenotypes have been consistently validated across multiple cohorts and research groups [23–25]. In contrast, the high-compliance “L” phenotype, for the reasons already detailed, seems inherently unstable. Whereas it was initially described as constituting 70–80% of COVID-19 ARDS cases [21], it now is defined as a rarely encountered extreme of a one-dimensional physiological continuum. In comparison, the previously identified ARDS subphenotypes represent distinct clinical “clusters” of patients, informed by measurements across organ systems and physiological domains in secondary analyses of well-curated cohorts of patients [22]. Yet despite this robustness, we would recommend that any therapeutic interventions for which benefits have been observed in these hyperinflammatory and hypoinflammatory phenotypes require testing in prospective trials before they are implemented into clinical practice, as they were derived using secondary analyses.
The objective of our editorial [15] was to challenge the subclassification of patients with COVID-19 that frequently occurred in the early weeks of the pandemic, based on “discussions” and “close observations”, before they became entrenched dogmas. An unintended consequence of such a challenge may be that it evokes negative emotions with the reader, especially in these troubling and polarising times. We were, therefore, saddened to learn that our editorial caused irritation among L. Gattinoni and co-workers. While we vehemently disagree that “the observations of Bos et al. [15] are expressed with a tone that goes beyond healthy and reasonable scientific debate”, we acknowledge that our essay was interpreted as such by L. Gattinoni and co-workers, and that is regrettable. We would like to clarify that the particular quoted sentences from our editorial that prompted the irritation and concern of L. Gattinoni and co-workers were aimed at premature phenotyping in general. It is an unfortunate misunderstanding that they assumed we were speaking directly and exclusively about them. For the reasons already outlined, however, we stand by our editorial.
L. Gattinoni and co-workers state that “the ‘L’ and ‘H’ [phenotypes] were not intended to be tightly descriptive nor mutually exclusive ‘bins’ into which each patient falls,” yet this is what is usually implied by disease “subphenotypes” or “endotypes” [26]. As described in our editorial [15], for phenotypes to be purposeful, they should be discrete, robust, generalisable, easily identifiable and, ideally, have an actionable intervention. Seemingly, almost none of these conditions are met in the current case. As an illustration, the problem with loosely defined phenotypes, as described by the correspondents, emerges when we try to precisely identify, at the bedside, who the patients are in whom they “hoped to help prevent use of high positive end-expiratory pressure when there is no benefit and, equally important, to avoid maintaining low pressures when higher pressures can be beneficial.” It is difficult to conceive how these phenotypes would be identifiable using quantifiable variables and when precisely to intervene, given that L. Gattinoni and co-workers themselves concede that these phenotypes are temporally dynamic, neither mutually exclusive nor discrete, and that “usually, there is overlap”.
We agree entirely with L. Gattinoni and co-workers that ventilator management should be individualised to each patient's physiology, and have never argued otherwise. In the theoretical “limit case” of a patient with normal lung compliance and minimal lung recruitability, we would similarly discourage use of high levels of positive end-expiratory pressure, as surely would most practising intensivists. We merely disagree with the conclusions of L. Gattinoni and co-workers regarding the prevalence of these theoretical patients based on data from 16 patients [21], as well as their subsequent recommendations to deviate from safe ventilatory practice for COVID-19 patients based on this limited data [27]. As catalogued already, the available data show that this purported “phenotype” is rarely encountered in COVID-19 ARDS.
Unexpectedly, L. Gattinoni and co-workers request evidence from us that their efforts at phenotyping have caused harm. Basic scientific convention, however, mandates that before they implore the field to deviate from usual practice, the burden is rather on them to demonstrate the benefits and safety of their proposed phenotyping scheme and linked interventions, using robust scientific studies. Thankfully for our patients, that is how best medical science works: primum non nocere (first, do no harm). Putting aside the complete absence of efficacy data, the validity of the physiological basis for their proposed interventions for these phenotypes has also been recently questioned [28, 29].
We hope our response clarifies for the correspondents and readers that we in no way dispute the underlying heterogeneity of ARDS, nor the uniqueness of COVID-19, nor the need for patient-tailored therapy; indeed, much of our research is focused on attaining this. We merely insist that phenotyping be done using careful, data-driven approaches. To paraphrase R. Rajendram, rather than strengthening a house of cards, we should instead aspire to build a foundation out of sturdier, more lasting materials: in this case agile, yet robust, scientific studies using a responsible, data-informed approach. At this stage of the pandemic, sufficient data points exist to equip us to advance from anecdote-based intuitions to evidence-informed science.
Shareable PDF
Footnotes
Conflict of interest: L.D.J. Bos reports grants from the Dutch Lung Foundation (young investigator grant, Dirkje Postma Award and a public–private partnership grant), and personal fees from Bayer (for consultancy), outside the submitted work.
Conflict of interest: P. Sinha has nothing to disclose.
Conflict of interest: R.P. Dickson has nothing to disclose.
References
- 1.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. COVID-19 in critically ill patients in the Seattle region – case series. N Engl J Med 2020; 382: 2012–2022. doi: 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos LDJ, Paulus F, Vlaar APJ, et al. Subphenotyping ARDS in COVID-19 patients: consequences for ventilator management. Ann Am Thorac Soc 2020; in press [ 10.1513/AnnalsATS.202004-376RL]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med 2020; 201: 1560–1564. doi: 10.1164/rccm.202004-1163LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auld SC, Caridi-Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med 2020; in press [ 10.1097/CCM.0000000000004457]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020; 395: 1763–1770. doi: 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenck EJ, Hoffman K, Goyal P, et al. Respiratory mechanics and gas exchange in COVID-19 associated respiratory failure. Ann Am Thorac Soc 2020; in press [ 10.1513/AnnalsATS.202005-427RL]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haudebourg AF, Perier F, Tuffet S, et al. Respiratory mechanics of COVID-19- versus non-COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 202: 287–290. doi: 10.1164/rccm.202004-1226LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gattinoni L, Coppola S, Cressoni M, et al. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 201: 1299–1300. doi: 10.1164/rccm.202003-0817LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauri T, Spinelli E, Scotti E, et al. Potential for lung recruitment and ventilation–perfusion mismatch in patients with the acute respiratory distress syndrome from coronavirus disease 2019. Crit Care Med 2020; 48: 1129–1134. doi: 10.1097/CCM.0000000000004386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rello J, Storti E, Belliato M, et al. Clinical phenotypes of SARS-CoV-2: implications for clinicians and researchers. Eur Respir J 2020; 55: 2001028. doi: 10.1183/13993003.01028-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galetke W, Feier C, Muth T, et al. Reference values for dynamic and static pulmonary compliance in men. Respir Med 2007; 101: 1783–1789. doi: 10.1016/j.rmed.2007.02.015 [DOI] [PubMed] [Google Scholar]
- 12.Dohi S, Gold MI. Pulmonary mechanics during general anaesthesia. The influence of mechanical irritation on the airway. Br J Anaesth 1979; 51: 205–214. doi: 10.1093/bja/51.3.205 [DOI] [PubMed] [Google Scholar]
- 13.ARDS Definition Task Force , Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 14.Gattinoni L, Coppola S, Cressoni M, et al. Reply to: Hedenstierna et al, Haouzi et al, Maley et al, Fowler et al, Bhatia and Mohammed, Bos, & Koumbourlis and Motoyama. Am J Respir Crit Care Med 2020; in press [ 10.1164/rccm.202004-1052LE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bos LDJ, Sinha P, Dickson RP. The perils of premature phenotyping in COVID-19: a call for caution. Eur Respir J 2020; 56: 2001768. doi: 10.1183/13993003.01768-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaller T, Hirschbühl K, Burkhardt K, et al. Postmortem examination of patients with COVID-19. JAMA 2020; 323: 2518–2520. doi: 10.1001/jama.2020.8907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med 2020; 383: 120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med 2020; 48: e440–e469. doi: 10.1097/CCM.0000000000004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bos LDJ. COVID-19 related acute respiratory distress syndrome: not so atypical. Am J Respir Crit Care Med 2020; in press [ 10.1164/rccm.202004-1423LE]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med 2020; in press [ 10.1001/jamainternmed.2020.3313]. [DOI] [PubMed] [Google Scholar]
- 21.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020; 46: 1099–1102. doi: 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014; 2: 611–620. doi: 10.1016/S2213-2600(14)70097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Famous KR, Delucchi K, Ware LB, et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med 2017; 195: 331–338. doi: 10.1164/rccm.201603-0645OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bos LD, Schouten LR, van Vught LA, et al. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax 2017; 72: 876–883. doi: 10.1136/thoraxjnl-2016-209719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delucchi K, Famous KR, Ware LB, et al. Stability of ARDS subphenotypes over time in two randomised controlled trials. Thorax 2018; 73: 439–445. doi: 10.1136/thoraxjnl-2017-211090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prescott HC, Calfee CS, Thompson BT, et al. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med 2016; 194: 147–155. doi: 10.1164/rccm.201512-2544CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA 2020; 323: 2329–2330. doi: 10.1001/jama.2020.6825 [DOI] [PubMed] [Google Scholar]
- 28.Fan E, Beitler JR, Brochard L, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med 2020; 8: 816–821.doi: 10.1016/S2213-2600(20)30304-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care 2020; 10: 78. doi: 10.1186/s13613-020-00692-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02756-2020.Shareable (150.7KB, pdf)