Abstract
Introduction
The severe acute respiratory syndrome-coronavirus 2 outbreak spread rapidly in Italy and the lack of intensive care unit (ICU) beds soon became evident, forcing the application of noninvasive respiratory support (NRS) outside the ICU, raising concerns over staff contamination. We aimed to analyse the safety of the hospital staff and the feasibility and outcomes of NRS applied to patients outside the ICU.
Methods
In this observational study, data from 670 consecutive patients with confirmed coronavirus disease 2019 referred to pulmonology units in nine hospitals between March 1 and May 10, 2020 were analysed. Data collected included medication, mode and usage of NRS (i.e. high-flow nasal cannula (HFNC), continuous positive airway pressure (CPAP), noninvasive ventilation (NIV)), length of stay in hospital, endotracheal intubation (ETI) and deaths.
Results
42 (11.1%) healthcare workers tested positive for infection, but only three of them required hospitalisation. Data are reported for all patients (69.3% male), whose mean±sd age was 68±13 years. The arterial oxygen tension/inspiratory oxygen fraction ratio at baseline was 152±79, and the majority (49.3%) of patients were treated with CPAP. The overall unadjusted 30-day mortality rate was 26.9%, with 16%, 30% and 30% for HFNC, CPAP and NIV, respectively, while the total ETI rate was 27%, with 29%, 25% and 28%, respectively; the relative probability of death was not related to the NRS used after adjustment for confounders. ETI and length of stay were not different among the groups. Mortality rate increased with age and comorbidity class progression.
Conclusions
The application of NRS outside the ICU is feasible and associated with favourable outcomes. Nonetheless, it was associated with a risk of staff contamination.
Short abstract
In patients with SARS-CoV-2 infection and acute respiratory failure, this study demonstrates that the utilisation of noninvasive respiratory support delivered outside the ICU was feasible and effective, but associated with a risk of staff contamination https://bit.ly/33mrJTU
Introduction
On February 20, 2020, coronavirus disease 2019 (COVID-19) hit the northern part of Italy severely. It was reported that in Lombardy, the most populated region of the country, >1500 patients required intensive care unit (ICU) admission over only 4 weeks, greatly exceeding the actual capacity [1]. In the same period, the number of hospital admissions was 7285 [2]. ∼35% of these patients experienced acute respiratory failure (ARF) requiring any form of respiratory support. A mathematical model of the occupation of intensive care resources in Italy predicted saturation of the theoretically available beds in the national territory by mid-April 2020 [3].
Under these circumstances, despite extraordinary efforts aimed at increasing the availability of ICU resources, the Italian Societies of Respiratory Medicine proposed a protocol to provide ventilatory support outside the ICU in dedicated respiratory COVID units, reinforced by a higher number of nurses and noninvasive monitoring [4]. This recommendation was somehow in contrast to most of the available guidelines, which contraindicated using noninvasive respiratory support (NRS) in these patients due to the major concerns over using bioaerosol-producing techniques, because of possible contamination of the hospital staff [5].
This “emergency” situation gave us the unique opportunity to challenge the hypothesis that NRS should not be used outside the ICU during pandemics. We have analysed the feasibility and safety, in terms of staff contamination, of NRS applied to severely ill patients outside the ICU. In addition, patients’ characteristics and clinical outcomes were analysed.
Methods
The study was conducted in four out of five hospitals in the Area Vasta Emilia network and in five hospitals in the neighbouring regions, serving a population of ∼8 million people. Institutional review boards reviewed the protocol and authorised prospective data collection. Informed consent was waived. A confirmed case of COVID-19 was defined as a patient with a positive result on high-throughput sequencing or real-time reverse transcriptase-PCR assay of nasal and pharyngeal swab specimens. Data were collected from registries of the respiratory disease units coordinators at the nine hospitals identifying all of the patients receiving NRS outside the ICU.
Excluding standard oxygen administration, patients were treated with three different types of NRS, namely high-flow nasal cannula (HFNC), continuous positive airway pressure (CPAP) or noninvasive ventilation (NIV), which also represented the three different groups in the analysis.
The triage of patients was performed according to the Italian Respiratory Societies joint guidelines based on severity. In particular, the following categories were proposed: 1) green (arterial oxygen saturation (SaO2) >94%, respiratory rate (RR) <20 breaths·min–1); 2) yellow (SaO2 <94%, RR >20 breaths·min–1 but responds to oxygen 10–15 L·min–1); 3) orange (SaO2 <94%, RR >20 breaths·min–1 but poor response to oxygen 10–15 L·min–1 and requiring CPAP/NIV with very high inspiratory oxygen fraction (FiO2)); 4) red (SaO2 <94%, RR >20 breaths·min–1 but poor response to oxygen 10–15 L·min–1, CPAP/NIV with very high FiO2 or presenting respiratory distress with arterial oxygen tension (PaO2)/FiO2 <200 and requiring endotracheal intubation (ETI) and intensive care). Patients belonging to these latter two categories were therefore considered eligible for NRS in dedicated respiratory COVID areas (see later) set up for the isolation of confirmed cases and ARF treatment.
These patients were not “usually” treated outside the ICU, but given the “emergency” situation, the lack of ICU beds and only once multiorgan disfunction was excluded, they were eligible for an NRS trial.
The transfer of severely ill patients to the ICU for intubation, with compromised haemodynamic parameters, low PaO2/FiO2 or “not responding to NRS”, was discussed with the intensivists, based on prognosis, and obviously was only possible if beds were available.
Although not specifically mentioned in the guidelines, HFNC was also used in these two categories, during breaks in ventilation or as a standalone support.
The use of helmet CPAP devices was suggested as first-line treatment, mainly for safety reasons. Clearly, this technique requires a sufficient supply of helmet interfaces (which ran out quite rapidly) and a high flow of oxygen (which exceeded the oxygen capacity in some hospitals), so that NIV and HFNC were used as alternatives; the first when it was necessary to “save” oxygen, and the second when CPAP availability ended.
The respiratory COVID areas consisted mainly of two different units, both present in all of the hospitals. The first one, formerly a respiratory ward, was an ad hoc dedicated respiratory monitoring unit consisting of specialised monitored areas with an active full-day shift run by a fixed group of pulmonologists and with a “reinforced” nurse–patient ratio varying from 1:4 to 1:6, depending on the hospital. The second unit, called respiratory intermediate care units, consisted of a fixed medical team. These had a monitoring system similar to that of the respiratory monitoring units, together with the availability of ICU ventilators and a nurse–patient ratio from 1:2 to 1:4, where more severely affected patients were usually treated.
Patients were monitored continuously with electrocardiogram trace, noninvasive blood pressure, arterial oxygen saturation and RR. Intensive care medicine doctors were eventually available around the clock at the request of the ward teams. Great care was taken to keep a distance of >1.5 m between each bed and to provide natural ventilation and airflow of ≥160 L·s–1 per patient.
Concerning staff protection, first of all, courses were quickly organised for staff in the correct use of personal protective equipment (PPE), dressing and undressing. Filtering facepiece class 3 or 2 masks, double nonsterile gloves, long-sleeved water-resistant gowns, goggles or face shields were mandatory in the presence of aerosol-producing procedures.
NIV was delivered mainly by dedicated single-circuit NIV platforms provided with an oxygen blender and ad hoc filters placed in the single tube circuit before the non-rebreathing devices to minimise bioaerosol dispersion, or by ICU ventilators. HFNC was delivered using standard devices (Nasal High Flow Therapy; Fisher and Paykel Healthcare, East Tamaki, New Zealand), while helmet CPAP-dedicated devices, designed for pandemics, were simply activated by connecting them to the oxygen source available in the hospital with blender systems applied to obtain adequate values of delivered FiO2 (Intersurgical, Mirandola, Italy and Dimar, Medolla, Italy).
Data collection
Data were collected prospectively from registries of the respiratory disease units identifying all of the patients receiving NRS outside the ICU.
Variables recorded for each patient were obtained for the period from March 1 until May 10, 2020 and included the following: demographics (age, sex), comorbidities (type and number), respiratory condition at admission (RR, PaO2/FiO2 ratio), medications (type of drugs prescribed), mode and usage of the NRS (ventilatory settings for NIV and CPAP, and flow rate for HFNC), and stay in hospital (days). The number of patients who died, either in the respiratory unit or in the hospital, and the patients who received ETI within the same time frame were recorded. Patients who were still hospitalised at the time of data analysis were excluded.
The health status of the staff working in the respiratory unit was closely monitored. All staff with fever or respiratory symptoms underwent chest radiography, and nasal and pharyngeal swab specimens were taken. Serology for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies and pharyngeal swabs were also periodically performed for all staff.
Statistical analysis
No statistical sample size assessment was performed a priori, and sample size was the number of patients treated during the study period in the participant centres. Baseline characteristics of patients treated with HFNC, CPAP and NIV were compared. Across the treatment subgroups, continuous variables were expressed as means±sd and were compared using the Kruskal–Wallis test and one-way ANOVA test, while categorical variables were expressed as numbers and percentages and were compared using the Chi-squared test or Fisher's exact test. Percentages of available data for the overall study population were based on the total number of patients included in the study, while the distribution of available data over the treatment subgroups was based on the available data for that variable, and the percentages were calculated using the number of available data for that subgroup. The fraction of infected professional healthcare workers was presented as numerical and percentage values. The association between ventilatory treatment and clinical outcomes was calculated using a logistic regression model. The 30-day mortality rate was calculated adjusted for baseline confounders (age, PaO2/FiO2 ratio, steroid usage and number of comorbidities).
Patients were grouped by age and PaO2/FiO2 ratio. Age groups were defined as follows: 21–40 years, 41–50 years, 51–60 years, 61–70 years, 71–80 years, 81–90 years and 91–100 years. PaO2/FiO2 ratio groups were defined as follows: <50 mmHg, 51–100 mmHg, 101–150 mmHg, 151–200 mmHg, 201–250 mmHg and 251–300 mmHg. For age subgroups, patients were further stratified according to the presence of comorbidities, as follows: patients with two or fewer comorbidities and patients with more than two comorbidities. In addition, a dedicated composite index including 30-day mortality and intubation rate was considered.
The main clinical outcomes were presented across the age and comorbidity subgroups and across PaO2/FiO2 ratio strata. Continuous variables were expressed as mean±sd and compared with the Kruskal–Wallis test and one-way ANOVA test while Chi-squared or Fisher's exact test was used to compare categorical variables between median age and comorbidity subgroups as appropriate. A two-sided test of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS (version 25.0; IBM, Armonk, NY, USA) and GraphPad Prism (version 8.4.1; GraphPad Software, La Jolla, CA, USA), unless otherwise indicated.
Results
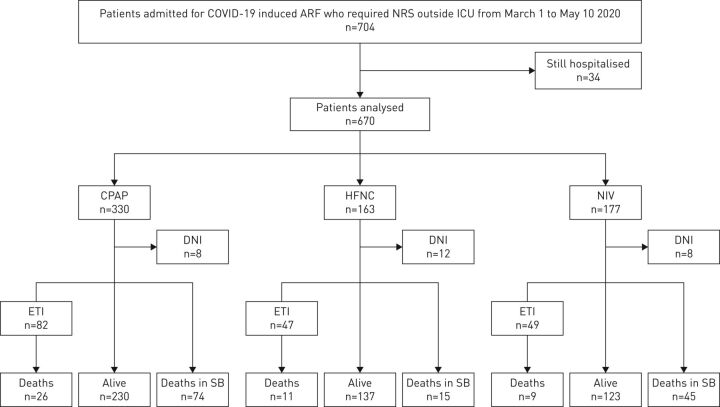
704 patients were considered, and of these 670 patients were included and their data analysed. Table 1 lists the patients’ characteristics. CPAP, as applied by helmet, was used on the majority of patients (supplementary table S1). 28 (4.2%) out of 670 had a do-not-intubate (DNI) order. Figure 1 illustrates the patients’ allocation to NRS and clinical outcome. 180 patients had died at 30 days. 20 out of the 28 DNI patients died (9% of the total number of deaths). In total, 114 patients died on spontaneous breathing without an expressed written DNI order.
TABLE 1.
Demographic and clinical characteristics of the study population and according to cohort
| Total | Cohort | p-value | |||
| HFNC | CPAP | NIV | |||
| Patients | 670 (100) | 163 (24.3) | 330 (49.3) | 177 (26.4) | |
| Age years | 68.3±13.3 | 65.7±14.7 | 70.3±12.1 | 66.8±13.5 | <0.001 |
| Male | 464 (69.3) | 114 (69.9) | 223 (67.6) | 127 (71.8) | ns (0.6) |
| SOFA score | 3.3±1.7 | 2.5±0.9 | 3.3±1.7 | 4±1.9 | <0.0001 |
| PaO2/FiO2 mmHg | 152±79 | 166±65 | 151±90 | 138±66 | <0.01 |
| Respiratory rate breaths·min−1 | 28±7 | 25±5 | 28±7 | 31±7 | <0.0001 |
| Comorbidities | |||||
| Hypertension | 311 (51.2)# | 74 (47)¶ | 153 (55)+ | 84 (49)§ | ns (0.15) |
| Dyslipidaemia | 84 (15.8)ƒ | 20 (12.6)¶ | 53 (19.2)+ | 11 (10.5)## | ns (0.06) |
| Diabetes mellitus | 125 (20.6)# | 32 (20.1)¶ | 60 (21.7)+ | 33 (19.1)§ | ns (0.82) |
| Chronic cardiovascular disease | 105 (17.3)# | 29 (18.2)¶ | 54 (19.6)+ | 22 (12.7)§ | ns (0.16) |
| Obesity | 108 (17.8)# | 33 (20.8)¶ | 31 (11.3)+ | 44 (25.3)§ | <0.001 |
| Chronic kidney disease | 34 (6.4)ƒ | 12 (7.6)¶ | 17 (6.2)+ | 5 (4.8)## | ns (0.59) |
| COPD | 46 (7.6)# | 9 (5.7)¶ | 12 (4.4)+ | 25 (14.5)§ | <0.001 |
| Chronic hepatic disease | 3 (0.6)ƒ | 1 (0.6)¶ | 2 (0.7)+ | 0 (0)## | ns (0.71) |
| Cancer | 53 (10)ƒ | 17 (10.7)¶ | 28 (10.1)+ | 8 (7.6)## | ns (0.72) |
| Treatment | |||||
| Hydroxychloroquine | 263 (82)¶¶ | 128 (91)++ | 108 (72)§§ | 27 (90)ƒƒ | <0.0001 |
| Lopinavir/ritonavir | 74 (23.1)¶¶ | 58 (41)++ | 10 (7)§§ | 6 (20)ƒƒ | <0.0001 |
| Darunavir/cobicistat | 47 (15)¶¶ | 37 (26)++ | 9 (6)§§ | 1 (3)ƒƒ | <0.0001 |
| Remdesivir | 2 (0.6)¶¶ | 1 (0.8)++ | 1 (0.7)§§ | 0 (0)ƒƒ | ns (0.89) |
| Tocilizumab | 161 (34)### | 47 (34)++ | 65 (28)¶¶¶ | 49 (50)+++ | <0.001 |
| Methylprednisolone | 275 (69)§§§ | 105 (75)++ | 113 (75)§§ | 57 (58)+++ | <0.01 |
| Prophylactic LMWH | 90 (35)ƒƒƒ | 63 (45)++ | 20 (21)#### | 7 (23)ƒƒ | <0.001 |
| Therapeutic LMWH | 130 (50)ƒƒƒ | 69 (49)++ | 48 (49)#### | 13 (43)ƒƒ | ns (0.78) |
Data are presented as n (%) or mean±sd, unless otherwise stated. Statistical significance was set at p<0.05. HFNC: high-flow nasal cannula; CPAP: continuous positive airway pressure; NIV: noninvasive mechanical ventilation; SOFA: subsequent organ failure assessment; PaO2: arterial oxygen tension; FiO2: inspiratory oxygen fraction; LMWH: low molecular-weight heparin. #: n=608; ¶: n=159; +: n=276; §: n=173; ƒ: n=532; ##: n=105; ¶¶: n=321; ++: n=140; §§: n=151; ƒƒ: n=30; ###: n=475; ¶¶¶: n=229; +++: n=98; §§§: n=400; ƒƒƒ: n=97; ####: n=259.
FIGURE 1.
Patient allocation to noninvasive respiratory support (NRS) and related clinical outcomes. COVID-19: coronavirus disease 2019; ARF: acute hypoxic respiratory failure; ICU: intensive care unit; CPAP: continuous positive airway pressure; DNI: do-not-intubate order; ETI: endotracheal intubation; SB: spontaneous breathing; HFNC: high-flow nasal cannula; NIV: noninvasive mechanical ventilation.
Most (69.3%) of the study patients were male. Hypertension, diabetes, dyslipidaemia, obesity and chronic cardiovascular disorders were the comorbidities most represented, evenly distributed among the groups, with the exception of obesity, which was more prevalent in the NIV group. Hydroxychloroquine, methylprednisolone, low molecular-weight heparin and tocilizumab were the drugs most used for treatment. The frequency distributions of age and PaO2/FiO2 ratio in the whole study population are shown in supplementary figure S1.
As shown in table 2, 379 healthcare workers, including doctors, nurses and healthcare assistants had been taking care of patients receiving NRS. 42 (11.1%) of them tested positive for SARS-CoV-2 infection, of which nine showed symptoms of mild or moderate disease and three required hospitalisation. All infected workers recovered well. The overall rate of workers infected, in personnel not specifically involved in the care of COVID-19 patients, in the nine hospitals was 3.8±1.9%.
TABLE 2.
Fraction of active professional healthcare workers and percentage of infection
| At work | Infected | |
| Physician | 108 | 8 (7.4) |
| Nurse | 210 | 29 (13.8) |
| Healthcare assistant | 45 | 5 (11) |
| Physiotherapist | 16 | 0 (0) |
| Total | 379 | 42 (11.1) |
Data are presented as n or n (%).
Table 3 illustrates the 30-day mortality rate in the overall population and in the three NRS subgroups with crude values and values adjusted for age, baseline PaO2/FiO2 ratio, number of comorbidities and steroid usage. The three modes of NRS had a similar impact on mortality outcome, as well as on intubation rate and length of stay.
TABLE 3.
Clinical outcomes and relative probability for the whole population and according to ventilatory support
| Total | HFNC | OR (95% CI) | p-value | CPAP | OR (95% CI) | p-value | NIV | OR (95%CI) | p-value | |
| Patients | 670 (100) | 163 (24.3) | 330 (49.3) | 177 (26.4) | ||||||
| 30-day mortality | ||||||||||
| Crude | 180 (26.9) | 26 (15.9) | 0.43 (0.3–0.7) | <0.01 | 100 (30.3) | 1.4 (0.9–2) | 0.05 | 54 (30.5) | 1.3 (0.5–1.9) | 0.20 |
| Adjusted# | 0.52 (0.2–1.2) | 0.10 | 1.7 (0.8–4.3) | 0.11 | 1.1 (0.3–3.7) | 0.88 | ||||
| ETI | ||||||||||
| Crude | 178 (26.6) | 47 (28.8) | 1.1 (0.8–1.7) | 0.45 | 82 (24.8) | 0.8 (0.6–1.2) | 0.32 | 49 (27.7) | 1.1 (0.7–1.6) | 0.80 |
| Adjusted# | 1.5 (0.6–4.1) | 0.39 | 0.9 (0.5–1.7) | 0.76 | 1.2 (0.5–3.3) | 0.65 | ||||
| 30-day mortality/ETI | ||||||||||
| Crude | 312 (46.5) | 62 (38) | 0.6 (0.4–0.9) | 0.01 | 156 (47.3) | 1.06 (0.8–1.4) | 0.7 | 94 (53) | 1.4 (1.2) | 0.04 |
| Adjusted# | 0.89 (0.4–2.1) | 0.79 | 0.9 (0.5–1.7) | 0.76 | 1.1 (0.5–2.7) | 0.78 | ||||
| Length of hospital stay days | 20.3±13.2 | 19.2±13.3 | 0.91 (0.4–1.13) | 0.87 | 19.8±12.1 | 0.95 (0.5–1.14) | 0.82 | 21.5±15.1 | 1.2 (0.6–1.5) | 0.47 |
Data are presented as n (%) or mean±sd, unless otherwise stated. Clinical outcomes and relative probability from fitting a logistic regression model for the whole study population and according to ventilatory support. Statistical significance was set at p<0.05. HFNC: high-flow nasal cannula; CPAP: continuous positive airway pressure; NIV: noninvasive mechanical ventilation; ETI: endotracheal intubation. #: adjusted for age, baseline arterial oxygen tension/inspiratory oxygen fraction ratio, number of comorbidities and steroid usage.
In table 4, outcome measures stratified by age class and number of comorbidities are reported in the overall study population. Notably, the mortality rate increased with age class progression and was significantly lower in younger (median ≤69 years) compared to older patients (>69 years) (12.5% versus 41.2%, difference 28.7%; p<0.0001). Mortality rate was higher in highly comorbid patients compared with patients with fewer than two comorbidities (34.4% versus 19.6%, difference 14.8%; p=0.0006). The percentage of patients subjected to ETI was higher among younger patients and in patients with lower comorbidities compared to older and highly comorbid patients, although not reaching statistical significance in the latter comparison (34.3% versus 18.8%, difference 15.5%; p<0.001 and 29% versus 20.4%, difference 8.6%; p=0.06, respectively). No difference in length of hospital stay was present among the age and comorbidity classes (19.6 versus 20.6 days, p=0.3 and 21.9 versus 22.1 days, p=0.9).
TABLE 4.
Clinical outcomes for the whole study population and stratified according to both age and comorbidity class
| All | Patients by age years | |||||||
| 21–40 | 41–50 | 51–60 | 61–70 | 71–80 | 81–90 | 91–100 | ||
| Overall | ||||||||
| Patients | 670 (100) | 19 (2.8) | 46 (6.8) | 106 (15.8) | 176 (26.3) | 195 (29.1) | 112 (16.7) | 16 (2.3) |
| Died at 30 days | 180 (26.9) | 0 (0) | 7 (15.2) | 6 (5.7) | 31 (17.6) | 71 (36.4) | 55 (49.1) | 10 (62.3) |
| ETI | 178 (26.6) | 7 (36.8) | 12 (26.1) | 36 (34) | 65 (36.9) | 48 (24.6) | 10 (8.9) | 0 (0) |
| Length of hospital stay days | 20.3±13.3 | 16.8±9.1 | 19.1±10 | 20.6±11.6 | 22.7±14.7 | 21±14.1 | 16.6±12.4 | 13.7±7.2 |
| Patients with ≤2 comorbidities# | ||||||||
| Patients | 423 | 19 (4.5) | 33 (7.8) | 75 (17.7) | 113 (26.7) | 118 (27.9) | 58 (13.7) | 7 (1.7) |
| Died at 30 days | 83 (19.6) | 0 (0) | 5 (15.2) | 6 (8.0) | 18 (15.9) | 40 (33.9) | 12 (20.7) | 2 (28.6) |
| ETI | 123 (29.1) | 7 (36.8) | 8 (24.2) | 29 (38.7) | 46 (40.7) | 27 (22.9) | 6 (10.3) | 0 (0) |
| Length of hospital stay days | 21.9±15 | 16.8±9.1 | 19.9±11.1 | 21.5±12.4 | 24.3±15.6 | 21.8±14.7 | 15.8±11.3 | 20.3±5.9 |
| Patients with >2 comorbidities# | ||||||||
| Patients | 122 | 0 (0) | 4 (3.3) | 13 (10.7) | 33 (27.0) | 47 (38.5) | 23 (18.9) | 2 (1.6) |
| Died at 30 days | 42 (34.4) | 2 (50.0) | 0 (0) | 10 (30.3) | 18 (38.3) | 11 (47.8) | 1 (50.0) | |
| ETI | 25 (20.4) | 1 (25.0) | 3 (23.1) | 14 (42.4) | 6 (12.7) | 1 (4.4) | 0 (0) | |
| Length of hospital stay days | 22.1±15.6 | 21.7±8.8 | 21.2±10.9 | 23.1±16.1 | 21.3±14.7 | 23.3±10.8 | 21.5±7.8 | |
Data are presented as n (%), mean±sd, or n. ETI: endotracheal intubation. #: comorbidity status for those with outcome data was available for 545 patients.
Outcome measures stratified by PaO2/FiO2 ratio classes and according to NRS are reported in table 5. Patients with a PaO2/FiO2 ratio <50 presented a higher 30-day mortality rate and a higher rate of ETI (p<0.001 and p<0.001, respectively).
TABLE 5.
Clinical outcomes and do-not-intubate (DNI) status according to arterial oxygen tension (PaO2)/inspiratory oxygen fraction (FiO2) class
| Patients by PaO2/FiO2 ratio | ||||||
| <50 | 51–100 | 101–150 | 151–200 | 201–250 | 251–300 | |
| Patients | 11 (1.6) | 222 (33.1) | 173 (25.8) | 127 (18.9) | 69 (10.3) | 68 (10.1) |
| Died at 30 days | 5 (45.5) | 77 (34.7) | 42 (24.2) | 33 (26) | 14 (20.3) | 9 (13.2) |
| ETI | 7 (63.6) | 76 (34.2) | 42 (24.3) | 32 (25.2) | 16 (23.2) | 5 (7.4) |
| Length of hospital stay days | 15.2±10.1 | 20.7±14.6 | 21.5±14 | 20.8±12.5 | 16.1±9.8 | 17.7±9.8 |
| DNI | 0 (0) | 6 (2.7) | 8 (4.6) | 4 (3.1) | 5 (7.2) | 5 (7.4) |
Data are presented as n (%) or mean±sd. ETI: endotracheal intubation.
NRS settings are shown in supplementary table S1. NIV was used as much as the patients could tolerate and in a small percentage of cases (43 (24%) out of 177), HFNC was applied during the intervals. Patients with bilateral posterior infiltrates were also usually placed in the prone position for few hours a day, in all three NRS groups, with a schedule dependent on their tolerance.
Discussion
This study showed that using NRS devices is feasible in patients with ARF due to SARS-CoV-2 infection treated outside ICUs, in newly developed dedicated COVID respiratory monitoring units, formerly respiratory wards, and in respiratory intermediate care units.
Despite using the recommended PPE, an 11.1% contamination rate was observed among healthcare workers treating the infected patients.
After adjusting for potential confounders, 30-day mortality rates using HFNC, CPAP and NIV were not significantly different.
One of the major concerns of using bioaerosol-generating devices is that healthcare workers are at high risk of contracting the infection and therefore most international guidelines recommend being cautious or even contraindicate their use [6–8]. Nevertheless, the World Health Organization (WHO) advocate using CPAP or NIV for the management of respiratory failure in COVID-19 patients, provided that appropriate PPE is worn by the personnel [9].
Several studies have found that the maximum exhaled air dispersion via different oxygen administration and ventilatory support strategies is minimal for CPAP through an oronasal mask or NIV through a helmet equipped with an inflatable neck cushion, and is much less when compared with any kind of oxygen delivery system [10].
Interestingly enough, so far, studies have been conducted in negative-pressure hospital rooms with at least six air changes per hour (minimum number of air changes recommended by WHO is 12 per hour). When these rooms were not available, as was the case for most of our patients, alternative hospital areas including rooms with natural ventilation (expressed as the product of room volume and air change rate) of ≥160 L·s–1 per patient were routinely employed, in keeping with the WHO statement [11]. Indeed, according to the Italian recommendations [4], the large majority of our study population received CPAP (by helmet or face mask), mask-NIV and HFNC with a medical mask over the nasal prongs.
Taking all these precautions into account and using all of the appropriate protection, the number of health workers who tested positive at serology or pharyngeal swab was still quite high (11.1%); however, those who became ill (12 out of 379, i.e. 3.2% of the staff involved) were in line with the 3.5% of healthcare workers requiring hospital admission in China [12], the only study so far that has reported this outcome during the COVID-19 outbreak. One may claim that our staff could have been infected in the community rather than by exposure to NRS; however, in the nine hospitals in this study, the overall rate of infection of health workers, in personnel not specifically involved in the care of COVID-19 patients was higher. The dramatic and rapidly increasing wave of the pandemic obliged us to treat a high number of severely hypoxic patients with NRS outside the ICU. These patients are usually admitted to “protected” environments. For example, the European Respiratory Society/American Thoracic Society guidelines [13] suggested using NIV in de novo respiratory failure only when managed by an experienced clinical team, and closely monitored in the ICU. Concerning the first point, all of the units involved had extensive experience in NRS use over a long period, and the nurse–patient ratio was “unusually” high for a ward, since during the outbreak, nursing staff levels were reinforced in the locations where the acutely ill patients were admitted. In addition, fully equipped noninvasive monitoring systems were available.
This is by far the largest report on the use of NRS outside the ICU; however, our COVID-dedicated respiratory units cannot be considered equivalent to the “usual” respiratory wards. Previous studies conducted in ICUs where NRS use was reported [1, 14–21] account for 188 patients treated with NIV and 61 by HFNC, without showing their characteristics and severity or the outcomes (in all but one study). Interestingly, this latter study [19] showed a very high mortality rate both with NIV and HFNC (80% and 52%, respectively). Indeed, only a few patients were treated in the respiratory ward or unit, namely 80 and 33 patients using NIV and HFNC, respectively, with a poor survival rate [22, 23].
Despite the fact that comparison among studies is extremely difficult due to the potential heterogeneity of patients included and/or to differing local hospital organisation, the failure rate (i.e. mortality and/or ETI) was much lower in our population, even when adjusted for potential confounders (see table 3), and it was comparable to what was observed (26%) in a large Italian study performed in the ICU in patients mostly intubated and with a PaO2/FiO2 ratio similar to ours [1]. In addition, in a recent two-period retrospective case–control study, Oranger et al. [24] demonstrated that CPAP could avoid intubation at 7 days and at 14 days, particularly in COVID patients with a previous DNI decision.
The mortality rate was similar with all of the NRS modes used after adjustment for confounders; however, it has to be noted that HFNC was usually applied in less-sick patients compared with NIV and CPAP, and this may reflect the attitude of the clinicians to start these latter two modes in patients where they judged that applying a relatively high level of external positive end expiratory pressure (PEEP) was more appropriate.
It has been suggested that using any form of NRS might unduly delay the start of ETI; however, it should be noted that 28 patients received a DNI order (20 of them died), and that an ICU bed was not promptly available at the time of deterioration, as reported in a specific small subset of the patient population. It may also be argued that “only” <5% of patients signed a DNI order.
In Italy, the very large majority of the population are not sufficiently aware of the new advanced directive law [25], or they do not want to complete in advance any document in this respect. Therefore, most of our patients arrived at hospital without any DNI or do-not-resuscitate directives. The reasons for not proceeding to ETI in the absence of a written DNI order might be explained by presumed lack of benefit from ETI or mechanical ventilation based on clinical judgement, sudden death or verbal refusal from the patient at the time of clinical deterioration. However, the majority of patients received “full treatment” when needed.
Despite the fact that this retrospective analysis in a large population indicates that NRS may help to treat severely affected COVID-19 patients outside the ICU, in newly dedicated respiratory areas with experienced staff, it also presents three main limitations. First, the design was retrospective, like most of the studies published during this period. Second, the decision to start one of the NRS modes was left to the attending physicians and mainly relied on the actual availability of equipment, so that the proportion of devices used was not evenly distributed. Third, as in most real-life studies dealing with the COVID-19 pandemic [1], missing data may be quite relevant; however, the critical nature of the situation did not always allow detailed information to be collected.
To conclude, this is the first observational, large multicentre study showing that the application of noninvasive respiratory devices outside the ICU is feasible, but is associated with a risk of staff contamination; however, the retrospective study design precludes drawing firm conclusions about its effectiveness, despite the fact that the mortality and intubation rates compare favourably with those of previous reports.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary table S1. ERJ-02130-2020.Table (171.9KB, pdf)
Supplementary figure S1. Frequency distribution of age classes and PaO2/FIO2 ratio in the whole population. ERJ-02130-2020.Figure (826.1KB, jpg)
Shareable PDF
Footnotes
This article has supplementary material available from erj.ersjournals.com
Conflict of interest: C. Franco has nothing to disclose.
Conflict of interest: N. Faccialongo has nothing to disclose.
Conflict of interest: R. Tonelli has nothing to disclose.
Conflict of interest: R. Dongilli has nothing to disclose.
Conflict of interest: A. Vianello has nothing to disclose.
Conflict of interest: L. Pisani has nothing to disclose.
Conflict of interest: R. Scala has nothing to disclose.
Conflict of interest: M. Malerba has nothing to disclose.
Conflict of interest: A. Carlucci has nothing to disclose.
Conflict of interest: E.A. Negri has nothing to disclose.
Conflict of interest: G. Spoladore has nothing to disclose.
Conflict of interest: G. Arcaro has nothing to disclose.
Conflict of interest: P.A. Tillio has nothing to disclose.
Conflict of interest: C. Lastoria has nothing to disclose.
Conflict of interest: G. Schifino has nothing to disclose.
Conflict of interest: L. Tabbì has nothing to disclose.
Conflict of interest: L. Guidelli has nothing to disclose.
Conflict of interest: G. Guaraldi has nothing to disclose.
Conflict of interest: V.M. Ranieri has nothing to disclose.
Conflict of interest: E. Clini has nothing to disclose.
Conflict of interest: S. Nava has nothing to disclose.
References
- 1.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323: 1574–1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Official bulletin of Lombardy Region, date March 18, 2020. www.open.online/2020/03/18/coronavirus-bollettino-regione-lombardia-18-marzo/ Date last accessed: March 18, 2020.
- 3.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet 2020; 395: 1225–1228. doi: 10.1016/S0140-6736(20)30627-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Italian Thoracic Society (ITS-AIPO), Italian Respiratory Society (IRS-SIP). Managing the Respiratory Care of Patients with COVID-19. Available from: www.aiponet.it and www.sipirs.it Date last accessed: May 22, 2020.
- 5.Wang X, Zhou O, He Y, et al. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur Respir J 2020; 55: 2000544. doi: 10.1183/13993003.00544-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faculty of Intensive Care Medicine, Intensive Care Society, Association of Anaesthetists, et al. Critical Care Preparation and Management in the COVID-19 Pandemic www.icmanaesthesiacovid-19.org Date last accessed: May 22, 2020.
- 7.ANZICS COVID-19 Working Group. ANZICS COVID-19 Guidelines (version 1; March 16, 2020) www.anzics.com.au/wp-content/uploads/2020/03/ANZICS-COVID-19-Guidelines-Version-1.pdf. Date last accessed: May 22, 2020
- 8.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med 2020; 46: 854–887. doi: 10.1007/s00134-020-06022-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease is Suspected. Interim guidance. 13 March 2020 https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf Date last accessed: May 22, 2020.
- 10.Ferioli M, Cisternino C, Leo V, et al. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. Eur Respir Rev 2020; 29: 200068. doi: 10.1183/16000617.0068-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Infection Prevention and Control During Health Care When COVID-19 is Suspected or Confirmed. Interim guidance https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC-2020.4 Date last accessed: May 22, 2020.
- 12.Zhan M, Qin Y, Xue X, et al. Death from Covid-19 of 23 health care workers in China. N Engl J Med 2020; 382: 2267–2268. doi: 10.1056/NEJMc2005696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017; 50: 1602426. doi: 10.1183/13993003.02426-2016 [DOI] [PubMed] [Google Scholar]
- 14.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020; 323: 1612–1614. doi: 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med 2020; 201: 1430–1434. doi: 10.1164/rccm.202003-0736LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368: m1091. doi: 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180: 934–943. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region – case series. N Engl J Med 2020; 382: 2012–2022. doi: 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oranger M, Gonzalez-Bermejo J, Dacosta-Noble P, et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: a two-period retrospective case–control study. Eur Respir J 2020; 56: 2001692. doi: 10.1183/13993003.01692-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciliberti R, Gorini I, Gazzaniga V, et al. The Italian law on informed consent and advance directives: new rules of conduct for the autonomy of doctors and patients in end-of-life care. J Crit Care 2018; 48: 178–182. doi: 10.1016/j.jcrc.2018.08.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary table S1. ERJ-02130-2020.Table (171.9KB, pdf)
Supplementary figure S1. Frequency distribution of age classes and PaO2/FIO2 ratio in the whole population. ERJ-02130-2020.Figure (826.1KB, jpg)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02130-2020.Shareable (329.4KB, pdf)