Abstract
The aim of this study is to evaluate the effectiveness of rectal ozone (O3) in COVID-19 patients with severe pneumonia admitted at Hospital Universitario Santa Cristina, Madrid. In a before-and-after study, four patients admitted with severe bilateral pneumonia due to COVID-19 were treated with rectal ozone and confirmed with (+) RT-PCR for SARS-CoV-2 and evaluated afterwards. The analyzed outcome variables were as follows: (a) clinical improvement (O2 saturation and O2 supply); (b) biochemical improvement (fibrinogen, D-dimer, urea, ferritin, LDH, IL-6, and CRP); (c) radiological improvement. The treatment protocol consisted of 5 sessions (1 session/day) of intra-rectal ozone, applied in a volume of 100 mL and a concentration of 35 μg/mL. The Protocol was previously approved by the Hospital’s Health Care Ethics Committee (CEAS) (Report 15/4/2020) for compassionate use in the face of this exceptional pandemic situation, and prior informed consent was obtained from the patient/legal representative. The patients improved oxygen saturation, as observed by the lower number of desaturations and the lower supply of O2. Biomarkers of inflammation decreased (fibrinogen, D-dimer, urea, ferritin, LDH, IL-6, and CRP). Finally, the radiological signs of bilateral viral pneumonitis improved between 1 and 2 grades based on Taylor’s radiological scale. Rectal ozone decreases O2 supply and improves O2 saturation, decreases inflammation biomarkers, and improves Taylor’s radiological grade in patients with severe COVID-19 pneumonia. Rectal ozone is a safe, effective, cheap, and simple alternative capable of acting on the SARS-CoV-2 virus, and it is presented as an adjunctive therapeutic option to consider in the management of severe bilateral COVID-19 pneumonia.
Keywords: Ozone, Ozone therapy, Pneumonia, COVID-19, SARS-CoV-2
Introduction
The World Health Organization (WHO) on March 11th, 2020 has declared that we are in an exceptional situation of pandemic due to the new SARS-CoV-2 or COVID-19 virus (https://www.who.int/es/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19%2D%2D-11-march-2020).
Management of SARS-CoV-2 virus disease or COVID-19 disease has no proven effective treatments to date. In fact, the Spanish Ministry of Health, in the Technical document entitled “Clinical Management of COVID-19: Hospital Care” states that there is currently no evidence from controlled clinical trials to recommend a specific treatment for the SARS-CoV-2 infection in patients with suspected or confirmed COVID-19. However, this information could change rapidly due to the results of several ongoing clinical trials (https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Protocolo_manejo_clinico_ah_COVID-19.pdf).
On the other hand, the pandemic situation means that there is a real risk of saturation of the health system with the need to reorganize it and a probable shortage of material and human resources is expected (https://www.eldiario.es/sociedad/coronavirus-sobrecarga-sanidad-Comunidad-Madrid_0_1004050083.html).
Currently, about 80 clinical trials are being carried out that seek to define the best therapy for the management of SARS-CoV-2 infection, of which only 3 are dedicated to the study of ozone on this disease and its potential therapeutic use [1]. None of the clinical trials consider rectal ozone as a therapeutic option for the management of COVID-19 infection.
The current treatment for COVID-19 is supporting, and respiratory failure due to acute respiratory distress syndrome (ARDS) is the main cause of mortality. A subgroup of patients with severe COVID-19 could develop a hyperinflammation or “cytokine storm” syndrome [2]. Early identification and treatment of hyperinflammation using all existing therapies with acceptable safety profiles is of paramount importance in order to reduce mortality [3].
Several studies (from Cuba, Italy, Germany, Russia, and Spain) and years of experience have shown that ozone (O3) is capable of modulating inflammation and pain, in addition to having demonstrated a bactericidal, fungicidal, virucidal, and antiparasitic effect [4, 5]. These antimicrobial properties have made ozone recognized as a disinfectant so effective that it is used in many water purification plants worldwide [4]. In this context, in a recent review, Fernández-Cuadros et al. reasonably considered that ozone has a place in the management of the present SARS-CoV-2 pandemic, due to its virucidal, immunomodulatory, stimulating cellular and humoral immunity properties, and as a facilitator of O2 transport in hypoxemic tissues [6].
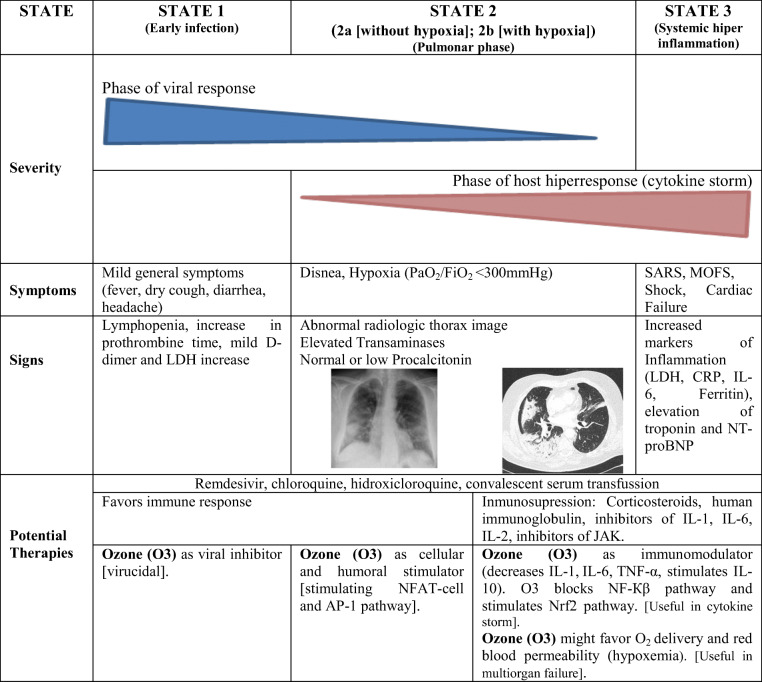
In the SARS-CoV-2 infection, three evolutionary stages are recognized (early infection (stage 1), normoxic and hypoxic lung phase (Stage 2a and b), and systemic hyperinflammation or cytokine storm (stage 3)), with characteristic signs and clinical symptoms [6]. In this scenario, Fernández-Cuadros et al. consider that at least 4 biological properties of O3 could allow its use as adjuvant therapy in the different phases of SARS-CoV-2 infection. Ozone could inactivate the virus by direct (O3) or indirect oxidation (ROS (reactive oxygen species) and LOPs (lipid oxidative products)) and could stimulate the cellular and humoral immune system being useful in the early COVID-19 infection phase (stage 1 and 2a). Ozone improves gas exchange, reduces inflammation, and modulates the antioxidant system, making it useful in the hyper inflammation or cytokine storm phase, and in the hypoxemia and / or multi-organ failure phase (stage 2b and stage 3) [6] (Table 1).
Table 1.
Severity of SARS-CoV-2 infection by stages, signs, symptoms, potential therapies, and ozone therapy proposal according to properties/evolution of COVID-19 disease[6]
SARS severe acute respiratory syndrome, MOFS multiorganic failure syndrome, CRP C-reactive protein, LDH lactate dehydrogenase, NT-proBNP N-terminal pro-brain natriuretic peptide, IL interleukin, NFAT cell nuclear factor activated T-cell, JAK Janus kinase, NF-κβ nuclear factor-κβ. AP-1 activated protein-1, Nrf2 nuclear erythroid factor 2
The objective of this article is to show the preliminary results on the effectiveness of rectal O3 in a small series of COVID-19 patients with severe bilateral pneumonia admitted at the Santa Cristina University Hospital in Madrid, Spain.
Material and Methods
A prospective quasi-experimental before-and-after study was performed. The study included 4 severe COVID-19 patients admitted at the Santa Cristina University Hospital, with clinical symptoms and RT-PCR (reverse transcriptase polymerase chain reaction) positive for SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). The study was conducted from April to May 2020, and the Hospital’s Health Care Ethics Committee (CEAS Report 15/4/2020) authorized the study and ozone treatment for compassionate use.
Inclusion criteria were the following: (1) man or woman, from 18 to 87 years old; (2) with positive detection of new coronavirus nucleic acid (RT-PCR SARS-CoV-2; (3) diagnosed with moderate-severe pneumonia (SpO2 < 93% or PaO2/FiO2 < 300 mmHg), with fever or moderate/severe respiratory symptoms; (4) confirmation of lung lesions with chest X-ray (according to Taylor’s scale) [7]; (5) hospitalized patients with fever and moderate or severe respiratory symptoms; (6) the participant/legal representative must be willing to be given informed consent to participate in the trial.
Exclusion criteria were the following: (1) pregnancy or lactation; (2) G-6PD (glucose 6-phosphate dehydrogenase) deficiency (favism) rare in Spain; (3) Patients who have not participated in other clinical studies.
In the initial evaluation, the objectives of the treatment, the procedure, the indications, and the contraindications were explained to the patients and/or legal representative; the initial biochemical evaluation (leucocytes and lymphocytes count, ferritin, D-Dimer, fibrinogen, procalcitonin, CRP, and IL-6) and the initial radiography of the chest were performed; and informed consent was signed.
The proposed technique, according to the Madrid International Ozone Therapy Declaration, was to administer intra rectally a volume of 100 mL of rectal ozone, at a concentration of 35 μg/mL for 5 to 10 days, according to the severity of the patients.
The supplies needed to perform the technique were as follows: (a) OZONOSAN α-PLUS ® (Ozone Generator); (b) rectal probe, and (c) two silicone syringes of 50-mL capacity.
For administration, the patient was placed in the supine or lateral decubitus position with the lower limbs flexed, depending on their collaboration, two 50-mL syringes of Ozone were loaded with the corresponding concentration (35 μg/mL), and were slowly injected rectally through a 14-French rectal tube, after lubrication with medical gel-type solution. The insufflation time will be a few minutes, at an administration rate of 1 mL/s.
After five to ten sessions of the ozone protocol (O3), the final evaluation was performed; clinical and biochemical analysis and chest radiographies were performed and evaluated; and adverse effects (if any) were recorded.
Since COVID-19 produces an acute and severe respiratory infection, with the lungs being the main target organs affected, chest radiography was the instrument used to grade severity and to confirm diagnosis. Taylor has proposed a severity scale for severe acute respiratory infection, ranging from 1 to 5 degrees. Grade 1 is considered normal. Grade 2 shows patchy atelectasis or hyper inflammation or thickening of the bronchial wall. Grade 3 includes focal alveolar consolidation but without involving more than one segment or lobe. Grade 4 shows multifocal consolidation and grade 5 includes diffuse alveolar consolidation [7].
Statistical analysis was performed using SPSS® version 20.0. Frequencies and percentages were used to evaluate qualitative variables, while for the evaluation of quantitative variables, means and standard deviation were used. The Mann-Whitney U test was the tool used to evaluate a change before-and-after treatment in quantitative variables. The level of significance was 95% (p < 0.05).
Results
Mean age of patients were 66.5 ± 25.7 years (range from 37 to 87 years). The male to female ratio was 1:1. The mean number of sessions was 6.75 ± 2.3 (range from 5 to 10 sessions) (Table 2).
Table 2.
Baseline characteristics of severe COVID-19 patients (n = 4)
| Variables | Value |
|---|---|
| Age (years) | 66.25 |
| Male (%) | 50 |
| Ratio male/female | 1:1 |
| Number of ozone sessions | 6.75 |
| O2 Saturation (%) | 89.0 |
| Taylor’s radiological grade | 4 |
| Leucocytes (10 × 3 μL) | 6.67 |
| Lymphocytes (10 × 3 μL) | 1.65 |
| Fibrinogen (mg/dL) | 550.7 |
| D-Dimer (ng/mL) | 1965 |
| Urea (mg/dL) | 47.2 |
| Ferritin (ng/mL) | 555.8 |
| LDH (U/L) | 253 |
| Procalcitonin (ng/mL) | 0.05 |
| CRP (mg/dL) | 1.1 |
| IL-6 (pg/mL) | 43.7 |
LDH lactate dehydrogenase, CRP C-reactive protein, IL interleukin
We present the preliminary results of a small series of patients admitted with severe COVID-19 pneumonia, confirmed with (+) RT-PCR (reverse transcriptase polymerase chain reaction) of SARS-CoV-2, in addition to compatible clinical and radiological signs (bilateral pneumonitis), who received standard pharmacological treatment (hydroxychloroquine, lopinavir/ritonavir, and azithromycin), corticotherapy in descending regimen and oxygen therapy, and monoclonal antibodies (anakinra (anti IL-1) in one patient and tocilizumab (anti IL-6) in two patients), who despite this, persisted with dyspnea, requiring high flow O2 supply. After 5 sessions of rectal ozone treatment (100 mL of ozone at 35 μg/mL), clinical, biochemical, and radiological improvement was observed. After rectal insufflation, no side effect was observed, except slight meteorism and a feeling of bloating, which subsided spontaneously (Table 3).
Table 3.
Change of outcome variables after ozone protocol (n = 4)
| Variables | Before | After | p |
|---|---|---|---|
| Clinical variable | |||
| O2 saturation (%) | 89.0 | 97.5 | 0.002 |
| Biochemical variable | |||
| Leucocytes (10 × 3 μL) | 6.67 | 5.89 | 0.32 |
| Lymphocytes (10 × 3 μL) | 1.65 | 1.6 | 0.49 |
| Fibrinogen (mg/dL) | 550.7 | 430,5 | 0.25 |
| D-Dimer (ng/mL) | 1965 | 585.5 | 0.17 |
| Urea (mg/dL) | 47.2 | 47,7 | 0.8 |
| Ferritin (ng/mL) | 555.8 | 343.6 | 0.09 |
| LDH (U/L) | 253 | 210 | 0.27 |
| Procalcitonin (ng/mL) | 0.05 | 0.047 | 0.71 |
| CRP (mg/dL) | 1.1 | 0.85 | 0.26 |
| IL-6 (pg/mL) | 43.7 | 8.2 | 0.33 |
| Radiological variable | |||
| Taylor’s radiological scale | 4 | 3 | 0.07 |
LDH lactate dehydrogenase, CRP C-reactive protein, IL interleukin, p Mann-Whitney U test
In an overall view, clinical variables (O2 saturation and O2 supply) improved in all patients. Patients did not desaturate (O2 saturation < 93%) O2 saturation changed from 89 to 97.5% and even reduced O2 supply (Table 3).
In an overall view, biochemical variables of inflammation and leucocyte and lymphocyte count improved in all patients. Leucocytes decreased from 6.67 × 103 to 5.89 × 103 mL; lymphocytes diminished from 1.65 × 103 to 1.6 × 103 mL. Procalcitonin decreased from 0.05 to 0.047 mg/mL. Fibrinogen ameliorated from 550.7 to 430.5 mg/mL. D-Dimer lowered from 1965 to 585.5 U/L. Urea improved slightly its value from 42.2 to 42.7 mg/L. Ferritin decreased its value from 555.8 to 343.6 mg/L. LDL ameliorated from 253 to 210 U/L. CRP diminished values from 1.1 to 0.85 mg/mL. IL-6 improved from 43.7 to 8.2 pg/mL (Table 3, Figs. 1 and 2).
Fig. 1.
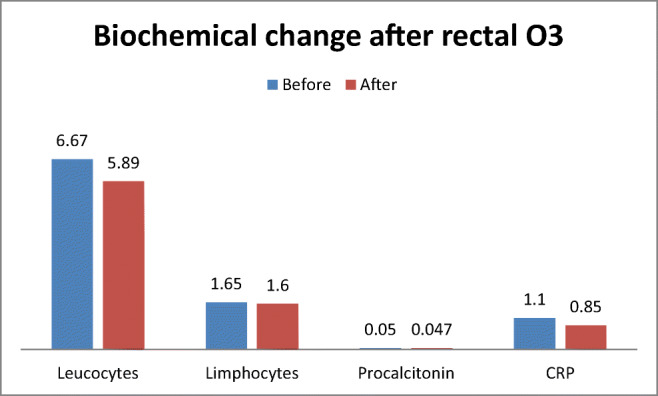
Change of leucocytes, lymphocytes, procalcitonin, and C-reactive protein after ozone protocol. O3 ozone, CRP C-reactive protein
Fig. 2.
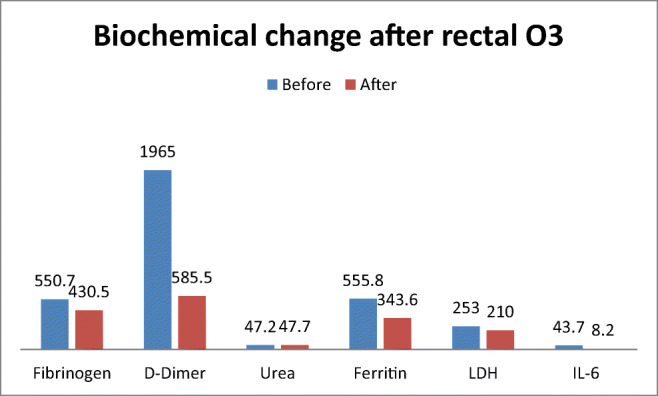
Change of fibrinogen, D-dimer, urea, ferritin, LDH, and IL-6 after rectal ozone. O3 ozone, LDH lactate dehydrogenase, IL-6 interleukin 6
According to Taylor’s scale, patients improved from a 4 to a 3 scale (Table 3).
A resume of the four treated patients is presented according to clinical biochemical and radiological variables.
First case
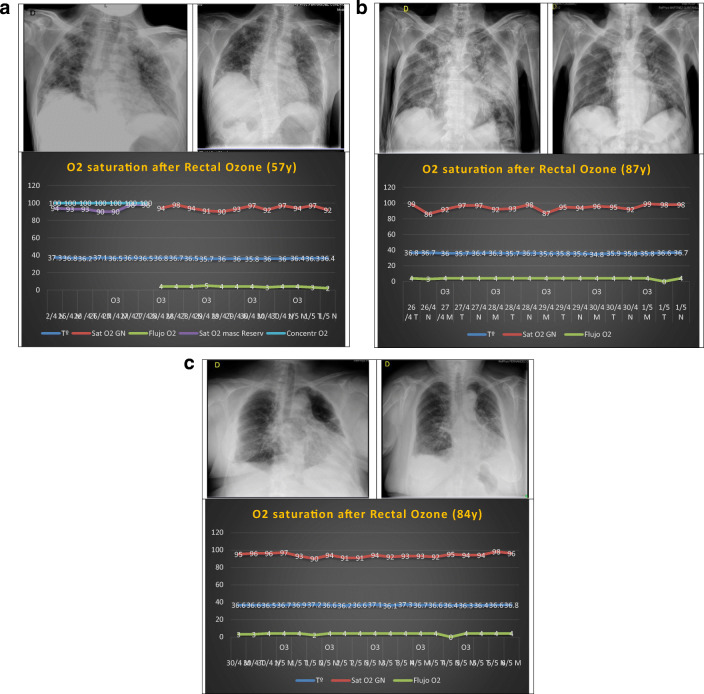
Fifty-seven-year-old man, who after standard treatment already completed (hydroxychloroquine, lopinavir/ritonavir, and azithromycin), one cycle of descending corticosteroid therapy (methylprednisolone), and 26 days of hospital admission, persisted with ventilatory support (reservoir mask at a concentration of 100%) saturating 90%. The radiological diagnosis he presented was grade 5 bilateral viral pneumonitis (according to the Taylor’s scale) [7], and he had biochemical signs of inflammation. Given the clinical severity, compassionate treatment with rectal O3 was authorized. After 5 sessions, evident clinical improvement was observed, decreasing dyspnea and respiratory rate, starting to use O2 through nasal glasses (4 L flow), and presenting increasingly better saturation levels. Inflammation markers decreased (fibrinogen from 582 to 496 mg/dL, D-dimer from 626 to 347 ng/mL; urea from 54 to 39 mg/dL, LDH from 281 to 263 U/L, ferritin from 645 to 343 ng/mL, IL-6 from 22.91 to 6.81 pg/mL, and CRP from 0.6 to 0.2 mg/dL) and the radiological grade improved from 5 to 3 (according to the Taylor’s scale) [7] (Fig. 3a).
Fig. 3.
a First case: 57-year-old male with severe COVID-19 pneumonia, radiological grade 5 (Taylor scale) before treatment improved to grade 3 after it. O2 saturation and O2 supply after 5 rectal ozone sessions are observed. b Second case: 87-year-old male with severe COVID-19 pneumonia, radiological grade 5 (Taylor scale) before treatment improved to grade 3 after it. O2 saturation and O2 supply after 5 rectal ozone sessions are observed. c Third case: 84-year-old female with severe COVID-19 pneumonia, radiological grade 4 (Taylor scale) before treatment improved to grade 3 after it. O2 saturation and O2 supply after 5 rectal ozone sessions is observed. T° temperature, Y years, GN nasal glasses, Sat saturation, Masc Reserv reservoir mask, Concentr concentration
Second case
Eighty-seven-year-old man, who after standard treatment already completed (hydroxychloroquine, azithromycin, meropenem due to bacterial infection and two cycles of descending corticosteroid therapy (methylprednisolone)), including tocilizumab (IL-6 inhibitor), persisted with dyspnea after 31 days of admission, requiring O2 by nasal glasses (4 L flow) and frequent episodes of desaturation of up to 87%. The radiological diagnosis that he showed was bilateral viral pneumonitis grade 5 (according to the Taylor’s scale) [7], and he had elevated inflammation markers. Given the clinical severity, compassionate treatment with rectal O3 was authorized. After 5 sessions, evident clinical improvement was observed, reducing dyspnea and respiratory rate, ceasing to present desaturation episodes. With the exception of IL-6, all other inflammation markers ameliorated (fibrinogen from 760 to 386 mg/dL, D-dimer from 4844 to 1496 ng/mL; urea from 59 to 57 mg/dL, LDH from 276 to 233 U/L, ferritin from 1222 to 343 ng/mL, IL-6 from 10.53 to 12.02 pg/mL, and CRP from 18.8 to 1.6 mg/dL) and the radiological grade decreased from 5 to 3 (according to the Taylor’s scale) [7] (Fig. 3b).
Third case
Eighty-four-year-old woman, who after standard treatment already completed (hydroxychloroquine, lopinavir/ritonavir, ciprofloxacin due to bacterial infection, linezolid due to MRSA [+] in sputum, fluconazole due to candida [+] in sputum, amikacin due to Klebsiella [+] in sputum, trimethoprim/sulfamethoxazole due to E. coli BLEE [+], and a cycle of corticotherapy (methylprednisolone) with descending regimen), after 39 days of admission, persisted with dyspnea, requiring O2 through nasal glasses (4 L flow) and fluctuating episodes of desaturation up to 90%. The radiological diagnosis that she showed was bilateral viral pneumonitis grade 4 (according to the Taylor’s scale) [7], and elevated inflammation markers. Given the clinical severity, compassionate treatment with rectal O3 was authorized. After 5 sessions, evident clinical improvement was observed, decreasing dyspnea and respiratory rate, without presenting episodes of desaturation, saturating up to 98% with O2 (4 L flow). Inflammation markers, except for urea, decreased significantly (fibrinogen from 619 to 590 mg/dL, D-dimer from 2303 to 398 ng/mL, urea from 49 to 61 mg/dL, LDH from 327 to 195 U/L, ferritin from 302 to 152 ng/mL, IL-6 from 136.1 to 9.28 pg/mL, and CRP from 2.3 to 1.6 mg/dL). Likewise, the patient presented radiological improvement, going from grade 4 to 3 (according to the Taylor’s scale) [7] (Fig. 3c).
Fourth case
Thirty-seven-year-old woman, who after standard treatment already completed (hydroxychloroquine, azithromycin, a cycle of corticotherapy (methylprednisolone) with descending regimen; and anakinra (anti IL-1)), after 10 days of admission, persisted with dyspnea, and fluctuating episodes of desaturation up to 90%. The radiological diagnosis that she showed was unilateral viral pneumonitis grade 3 (according to the Taylor’s scale) [7], and elevated IL-6 markers. Given the clinical symptoms, compassionate treatment with rectal O3 was authorized. After 5 sessions, evident clinical improvement was observed, decreasing dyspnea and respiratory rate, without presenting episodes of desaturation, saturating up to 99% without O2. Inflammation markers were on normal range. IL-6 was the only variable that decreased after ozone protocol, the other variables increased slightly (fibrinogen from 242 to 250 mg/dL, D-dimer from 87 to 101 ng/mL; urea from 27 to 26 mg/dL, LDH from 128 to 149 U/L, ferritin from 54.5 to 60.5 ng/mL, IL-6 from 5.32 to 5.02 pg/mL, and CRP was 0 mg/dL and remained in 0 mg/dL). Likewise, the patient presented radiological improvement, going from grade 3 to 2 (according to the Taylor’s scale) [7].
Discussion
To the best of authors’ knowledge, this is the first report in the literature on the effectiveness of rectal ozone in patients with severe COVID-19 pneumonia treated in this new SARS-CoV-2 pandemic. Rectal ozone improved clinical, biochemical, and radiological symptoms in the preliminary results in a small series of patients.
To date, from more than 80 studies that try to find effective therapeutic alternatives for the management of SARS-CoV-2 pandemic and COVID-19 infection, only three studies consider ozone, and all of them apply to autohemotherapy as biologically effective therapy [1]. As far as we are concerned, there are no studies that postulate rectal ozone as a useful alternative in the management of COVID-19 pneumonia [6]. The importance of the study subsides in the unpublished and innovative protocol.
Our study group has previously identified up to 4 properties that would be biologically useful to cope with the complications derived from this COVID-19 infection (viral replication, oxidative stress, hyperinflammation or cytokine storm and hypoxia) [6]. The study protocol of these properties has been recently published, given the pandemic situation, due to the relevance of the study for the management of SARS-CoV-2 infection and pneumonia due to COVID-19 [6] (Table 1).
The clinical improvement observed in the small series of patients confirms that the properties that we had reasonably postulated are effective in managing the complications of this infection (SARS-CoV-2) [6], and they come in line with ozone properties suggested by Martinez-Sanchez [8], Conti [9], and Marini [10].
Our observations come in line with reports on clinical cases observed in China, Italy, and Spain [11–15]. All cases were treated by ozone autohemotherapy, and none of cases were treated by rectal ozone therapy.
In Italy, improvement has been reported in 39 patients in a first report, and after 71 patients (in a second report), treated with ozone via autohemotherapy in the management of patients with COVID-19 pneumonia [11, 12]. The authors have reported improvement in the general clinical situation, temperature normalization, decreased CRP, improved O2 saturation and decreased O2 support [11, 12].
A report of a clinical case in China showed in a 49-year-old patient with severe pneumonia and admitted to the ICU (intensive care unit), after 5 sessions of ozone by autohemotherapy, clinical improvement evident from the outset, the effect of ozone therapy on tissue oxygenation (evaluated by arterial blood gas) lasted approximately 9 h, and the patient was successfully extubated and transferred to Internal Medicine Ward. A significant decrease in D-dimer, fibrinogen, and CRP was observed, in addition to a decrease in IL-6 and negative PCR of SARS-CoV-2 obtained by nasal swab [13].
In China, in another preliminary report from two severe COVID-19 patients treated by 7 sessions of autohemotherapy, Zheng et al. have stated that both patients remitted clinical symptoms, and abnormal laboratory and radiological signs. Moreover, patients were discharged with negative RT-PCR testing for SARS-CoV-2. These two cases were matched and compared with two other subjects that were not treated by ozone. Since all four patients followed the Chinese Guidelines for COVID-19 management, it is expected that ozone therapy is responsible for the good effects observed in the ozone autohemotherapy cases [14].
In Spain, Dr. Alberto Hernández has published the case of a 49-year-old patient with severe pneumonia who needed mechanical ventilation with an ICU admission, and in whom ozone (autohemotherapy) was prescribed as a last resort. The patient showed immediate improvement, to the point that after 2 sessions he did not require ICU admission, and after 5 sessions the need for O2 supply decreased markedly [15]. Other clinical cases treated by the same author are pending publication. Hernández et al. have emphasized that the clinical improvement is due to the immunomodulatory, oxygenating and antioxidant role of ozone via autohemotherapy in this patient [15].
As a summary, the experience in patients with COVID-19 treated in Italy, China, and Spain refers to ozone applied under the autohemotherapy technique [11–15]. To date, we are not aware of the existence of any study that has treated patients with COVID-19 with rectal ozone. The number of sessions in autohemotherapy (5–7 sessions) [11–15] was similar to rectal ozone sessions applied in this study (6.75 sessions on average).
The Ethics Committee of Santa Cristina Hospital (CEAS) that approved our study, although it recognized that there is controversy about ozone therapy, because there are no national laws or regulations that specifically refer to ozone therapy, but, taking into account the principles of bioethics (non-maleficence, beneficence, justice, and autonomy of the patient) and considering that the proposed technique (rectal ozone therapy) is cheap, simple to apply, and does not require excessive human or material resources, they considered reasonable its use in patients diagnosed with COVID-19, taking into account the 4 biological properties proposed by Fernández-Cuadros et al., that could act on the pathophysiology of COVID-19 disease and on the SARS-CoV-2 virus [6] (Table 1). In addition, ozone therapy is part of the Hospital Services portfolio, and has been used for 10 years in the Rehabilitation and Physical Medicine Department for the management of musculoskeletal pain.
Applying translational medicine, our study group has observed that ozone is able to decrease inflammation biomarkers, such as CRP, ESR (erythrocyte sedimentation rate), and uric acid, markers that play an important role in knee osteoarthritis [5]. Therefore, we hypothesized and afterwards demonstrated that ozone decreased CRP in patients with COVID-19, as it was observed in knee osteoarthritis, although the route of application was different (intra-articular in the first case, rectal in the present study) [5].
Fernández-Cuadros et al., in a recent review on the fundamentals of ozone therapy, have established that ozone is a multi-target drug, being able to decrease inflammatory cytokines (IL-1β, IL-6, IFN-γ, TNF-α), to stimulate anti-inflammatory cytokines (IL-4, IL-10), to stimulate the release of nitric oxide (vasodilator) and stem cells [16]. In addition, in a very recent review, Fernández-Cuadros et al. have observed that O3 is capable of inhibiting the inflammasome pathway (NF-κβ pathway) [17], a pathway that would play a major role in stimulating hyperinflammation or cytokine storm [2]. These findings would explain why inflammatory variables such as ferritin, IL-6, and CRP decreased in our COVID-19 patients treated with rectal ozone.
From an analysis of patients with SARS-CoV-2 new pandemic (Wuhan, China), it was observed that those patients with a higher viral load had a higher hyperinflammatory state, with an elevation of inflammatory cytokines (IL-2, IL-6, IL-7, IL-10, GCSF, INF-γ, TNF-α, MCP-1, and MIP-1). In this series, the patients who died presented high levels of inflammation markers such as ferritin, IL-6, and ESR, suggesting that the severe inflammatory reaction could contribute to the severity and mortality of the disease [18].
In this scenario of cytokine storm or hyperinflammation and given the high risk of mortality, it is necessary to reduce this severe inflammatory response, either with the use of corticosteroids [6], biological drugs (anti IL-1, anti-IL-6, anti-TNF-α, IFN-β1, JAK inhibitors) [6], human immunoglobulin [6], extracorporeal cytokine clearance therapy [18], or through the use of ozone (due to its “ideal” cytokine-inducing or immunomodulatory effect) [4–6, 15–17]. For this reason, we have opted for ozone therapy in this study as compassionate use, since previous therapies had failed (corticosteroids, anakinra and tocilizumab).
Infection with SARS-CoV-2 or COVID-19 can produce a state of hyperinflammation or cytokine storm, as occurs in malaria, dengue, Ebola hemorrhagic fever, and in bacterial sepsis. In fact, it was Rowen who used this route (rectal ozone) for the first time in the management of 5 patients infected with Ebola (60% mortality), and after 5 treatment sessions, significant improvement was achieved without any deaths [19]. We have postulated that this benefit could be extrapolated to patients with COVID-19 [6], a hypothesis that has been demonstrated with the presentation of this small series of patients.
COVID-19 produces an acute and severe respiratory infection, with the lungs being the main target organs affected. In this sense, it is important to grade severity using validated scales and diagnostic elements. Taylor has proposed a severity scale for severe acute respiratory infection, ranging from 1 to 5 degrees [7]. This scale is a valid tool to describe the overall severity in patients with acute and severe respiratory infection. Furthermore, this scale allows an overall description of the respiratory characteristics to be provided and will allow comparisons over time in affected patients, in order to assess the evolution of the disease. It will also allow comparisons between different populations [9]. In our study, this scale has allowed us to see an improvement between 1 and 2 degrees, in patients treated with rectal ozone.
A postmortem histopathological analysis has established that phase 3 (hyperinflammation or cytokine storm) of COVID-19 infection produces alveolar edema, hyalinosis, and fibrin deposition with infiltration of immune cells at the pulmonary level [18]. The fact that rectal ozone has decreased edema and alveolar infiltrate, verified by radiological tests (Taylor’s scale), added to the decrease in inflammation markers (LDH, ferritin, fibrinogen, D-dimer, IL-6, and CRP) and to the improvement in pulmonary oxygenation (O2 saturation), suggests that ozone is an alternative to consider in the management of severe acute respiratory distress syndrome caused by the SARS-CoV-2 or COVID-19 virus.
A limitation of this small series of patients is the small sample analyzed. The fact that the improvement observed in the variables analyzed was nominal, but not statistical; it does not mean that there is not a benefit from this intervention. A greater sample is necessary to get a statistical improvement, although the clinical benefit was already observed in this preliminary study.
A one-group before-and-after study lacks of randomization and control group. A quasi-experimental study by definition is used to establish the causality (the effect of an independent variable over a dependent variable) in situations where researchers are not able to randomly assign groups to the subjects for various reasons [20]. One of those reasons is sample size and another reason is ethical purposes. In a pretest-posttest analysis, the effect of the intervention (rectal ozone treatment) is expected to be the change in a before-and-after evaluation [20]. Quasi-experimental studies are used because they are more practical and feasible to conduct. This design is preferred when sample size is small or the availability of control group because of ethical reasons is not possible [20]. This design is more suitable for a real natural world setting (rehabilitation setting) than true experimental designs. This design allows the researchers to evaluate the impact of a quasi-independent variable (rectal ozone) under naturally occurring conditions (natural history of COVID-19 disease) [20].However, neither the small sample size nor the lack of control group did not influence on the results observed in the current study.
The strength of the study is that the patients have been evaluated taking into account clinical (O2 saturation and O2 supply), laboratory (systemic inflammation markers) and radiological characteristics, using instruments (pulse oximeter), laboratory equipment and radiological scales (Taylor’s scale) that are clinically validated.
In a cost/effectiveness analysis, Ozone is an anti-inflammatory technique capable of decreasing several markers of inflammation (as it was observed in this study), and ozone is cheaper and safer if compared to biological treatments (monoclonal antibodies) [9]. This analysis makes this treatment option a valid alternative for low-middle-income countries, where patients have to pay for their medical bills.
Finally, our study group, given the effectiveness observed in these first cases treated in the world with rectal ozone, and taking into account the safety, simplicity of the technique and the low cost, has submitted to the AEMPS (Spanish Agency for Medicines and Medical Devices) request to conduct a clinical trial, in order to confirm these promising results, in a much larger sample of patients.
Conclusion
Rectal ozone decreases O2 supply and improves O2 saturation, decreases inflammation biomarkers, and improves Taylor’s radiological grade in patients with severe COVID-19 pneumonia. Rectal ozone is a safe, effective, cheap, and simple alternative capable of acting on the SARS-CoV-2 virus, and it is presented as an adjunctive therapeutic option to consider in the management of severe bilateral COVID-19 pneumonia.
Acknowledgments
The librarian Saturnino Díaz Trujillo is acknowledged for the bibliographic search for the preparation of this study.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study protocol has been approved by the Ethical Committee of the Hospital (April 15th, 2020).
Informed Consent
For the treatment used in this research article [1], informed consent was obtained from the patient/legal representative included in the study and [2] the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethical Committee of the Hospital.
Footnotes
This article is part of the Topical Collection on Covid-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Coronavirus disease (COVID-2019) situation reports. 2020.
- 2.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B, von Bergwelt-Baildon MS. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta P, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020. 10.1016/S0140-6736(20)30630-9. [DOI] [PMC free article] [PubMed]
- 4.Fernández-Cuadros ME, Pérez-Moro OS, Albaladejo-Florín MJ. Ozone fundamentals and effectiveness in knee pain: chondromalacia and knee osteoarthritis. Saarbrücken: Lambert Academic Publishing; 2016. [Google Scholar]
- 5.Fernández-Cuadros ME, Pérez-Moro OS, Albaladejo-Florín MJ, Álava-Rabasa S. El ozono intraarticular modula la inflamación, mejora el dolor, la rigidez, la función y tiene un efecto anabólico sobre la artrosis de rodilla: estudio cuasi-experimental prospectivo tipo antes-después, 115 pacientes. Intra-articular Ozone modulates inflammation, ameliorates pain and rigidity, improves function and has anabolic effect on knee osteoarthritis: a prospective quasi-experimental before-and-after study, 115 patients. Rev Soc Esp Dolor. 2020. 10.20986/resed.2020.3775/2019.
- 6.Fernandez-Cuadros, Peña-Lora, Albaladejo-Florín, Álava-Rabasa, Pérez-Moro. Ozone (O3) and SARS-CoV-2: physiological bases and their therapeutic possibilities according to COVID-19 evolutionary stage. SN Comprehensive Clinical Medicine Insights. 2020. 10.1007/s42399-020-003287. [DOI] [PMC free article] [PubMed]
- 7.Taylor E, Haven K, Reed P, Bissielo A, Harvey D, McArthur C, Wilson F. A chest radiograph scoring system in patients with severe acute respiratory infection: a validation study. BMC Med Imaging. 2015;15(1):61. doi: 10.1186/s12880-015-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez-Sánchez G, Schwartz A, Donna VD. Potential cytoprotective activity of ozone therapy in SARS-CoV-2/COVID-19. Antioxidants (Basel) 2020;9(5):389. doi: 10.3390/antiox9050389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conti P, Gallenga CE, Tetè G, Caraffa A, Ronconi G, Younes A, et al. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS- CoV-2) infection and lung inflammation mediated by IL-1. J Biol Regul Homeost Agents. 2020;34(2). 10.23812/Editorial-Conti-2 MID: 32228825. [DOI] [PubMed]
- 10.Marini S, Maggiorotti M, Dardes N, Bonetti M, Martinelli M, Re L, et al. Oxygen-ozone therapy as adjuvant in the current emergency in SARS-COV-2 infection: a clinical study. J Biol Regul Homeost Agents. 2020;34(3). 10.23812/20-250-E-56. [DOI] [PubMed]
- 11.Https://ozonesociety.org First Report – Oxygen Ozone SIOOT in patients recovered with COVID-19. Dr. Antonio Gaspari.
- 12.Https://ozonesociety.org Second Report – Oxygen Ozone SIOOT in patients recovered with COVID-19. Dr. Luigi Valdesani, Dr. Marianno Franzini.
- 13.Wu J, Tan C, Yu H, Wang Y, Tian Y, Shao W, Shen J (2020). Case report: recovery of one Icu-acquired Covid-19 patient via ozonated autohemotherapy. Available at SSRN 3561379. [DOI] [PMC free article] [PubMed]
- 14.Zheng Z, Dong M, Hu K. A preliminary evaluation on the efficacy of ozone therapy in the treatment of COVID-19. J Med Virol. 2020. 10.1002/jmv.26040 PMID: 32437014; PMCID: PMC7280732. [DOI] [PMC free article] [PubMed]
- 15.Hernández A, Papadakos PJ, Torres A, González DA, Vives M, Ferrando C, Baeza J (2020) Dos terapias conocidas podrían ser efectivas como adyuvantes en el paciente crítico infectado por COVID-19. Revista Española de Anestesiología y Reanimación. [DOI] [PMC free article] [PubMed]
- 16.Fernandez-Cuadros ME, Perez-Moro OS, Mirón-Canelo JA. Could ozone be used as a feasible future treatment in osteoarthritis of the knee. Diversity Equal Health Care. 2016;13(3):232–239. [Google Scholar]
- 17.Fernández-Cuadros ME, Pérez-Moro OS, Albaladejo-Florín MJ, Álava-Rabasa S, Tobar-Izquierdo M, Rodriguez-de-Cía J. A new paradigm for the management of knee osteoarthritis: the role of hyaluronic acid, platelet-rich plasma (PRP) and ozone in the modulation of inflammation: a review. Science Repository. 2020;In press. 10.31487/j.JSR.2020.01.01.
- 18.Courville K, Bustamante N, Pecchio M (2020) Cytokine removal and immunomodulation in COVID-19 severe pulmonary respiratory infection. ScienceOpen Preprints.
- 19.Rowen RJ, Robins H. A plausible “penny” costing effective treatment for Corona virus ozone therapy. J Infect Dis Epidemiol. 2020;6:113. [Google Scholar]
- 20.Alonso M, Mirón JA (2013) Sistema de Información Sanitaria. Indicadores de Salud, Bienestar y Calidad de Vida. En: Guía para la elaboración de Trabajos Científicos. Grado, Máster y Posgrado, Salamanca, Gráficas Lope, p. 55-66.